Boronate-Based Inhibitors of Penicillin-Binding Proteins: An Underestimated Avenue for Antibiotic Discovery?
Abstract
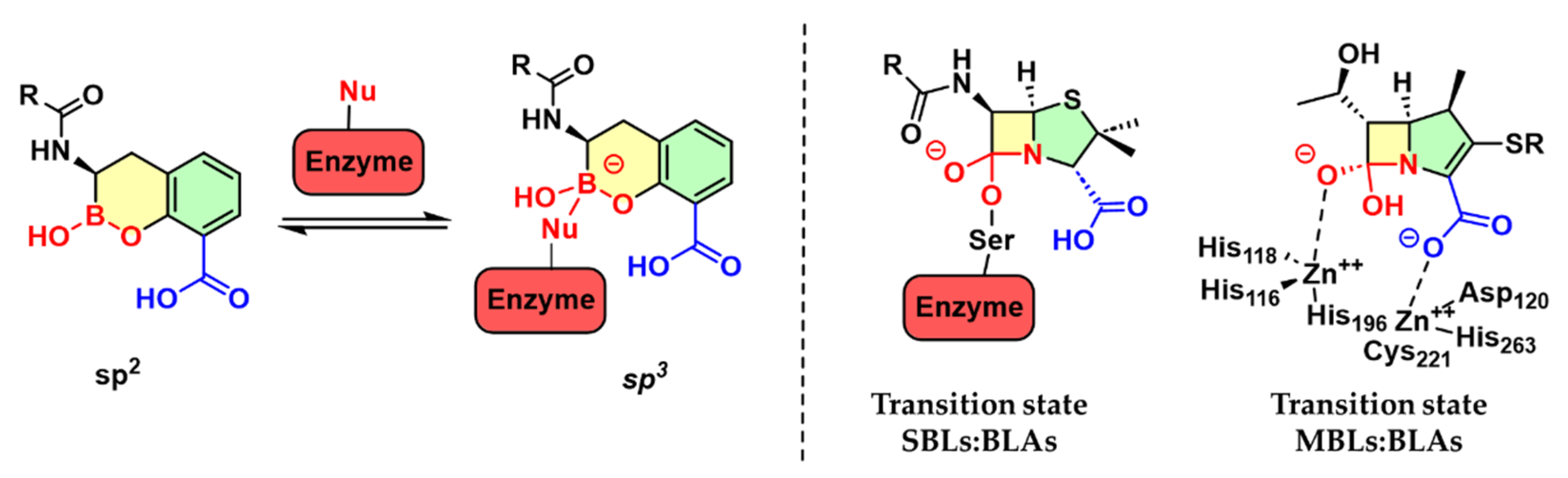
1. Introduction
2. Discussion
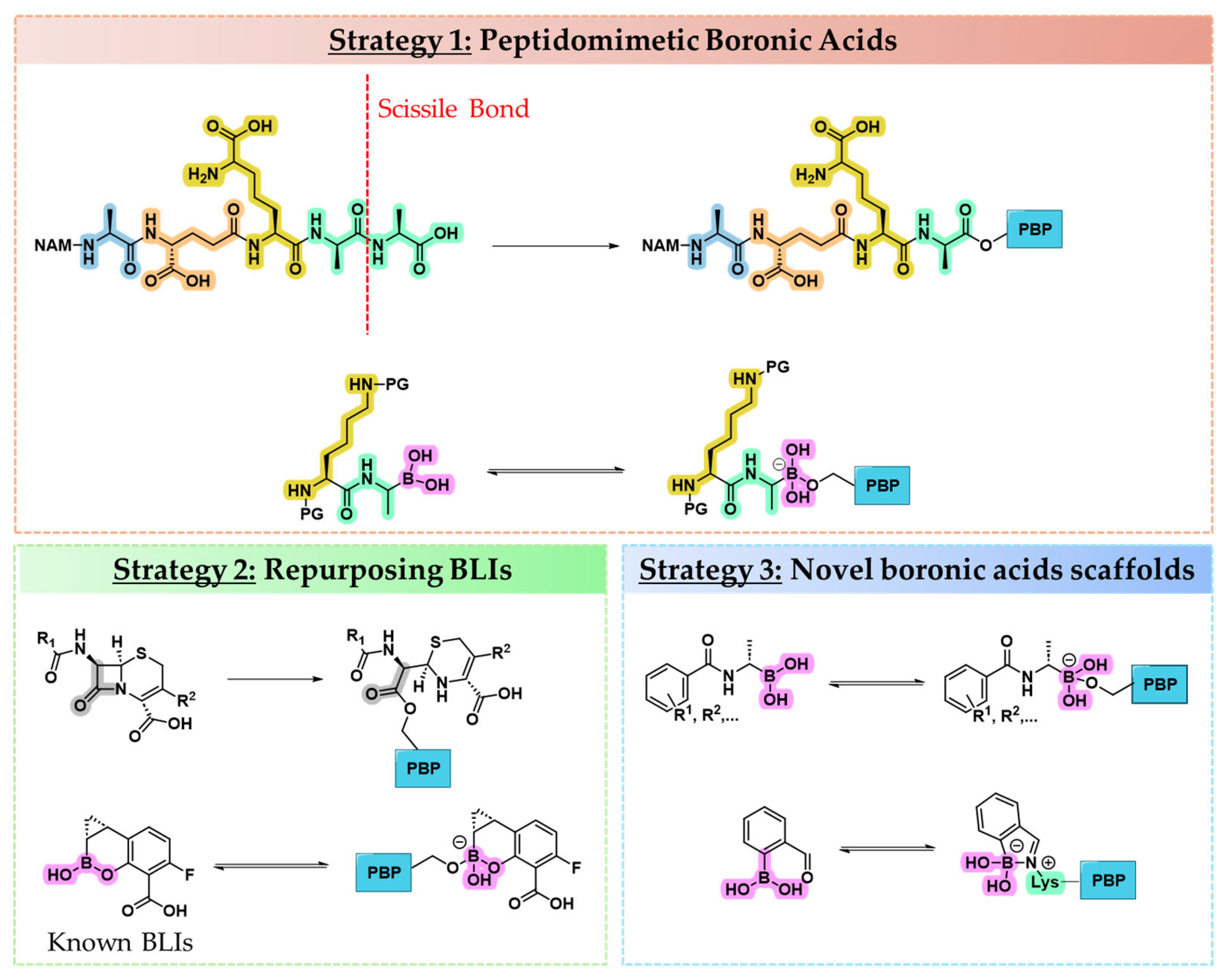
2.1. Strategies for Designing Boronic Acid-Based PBP Inhibitors
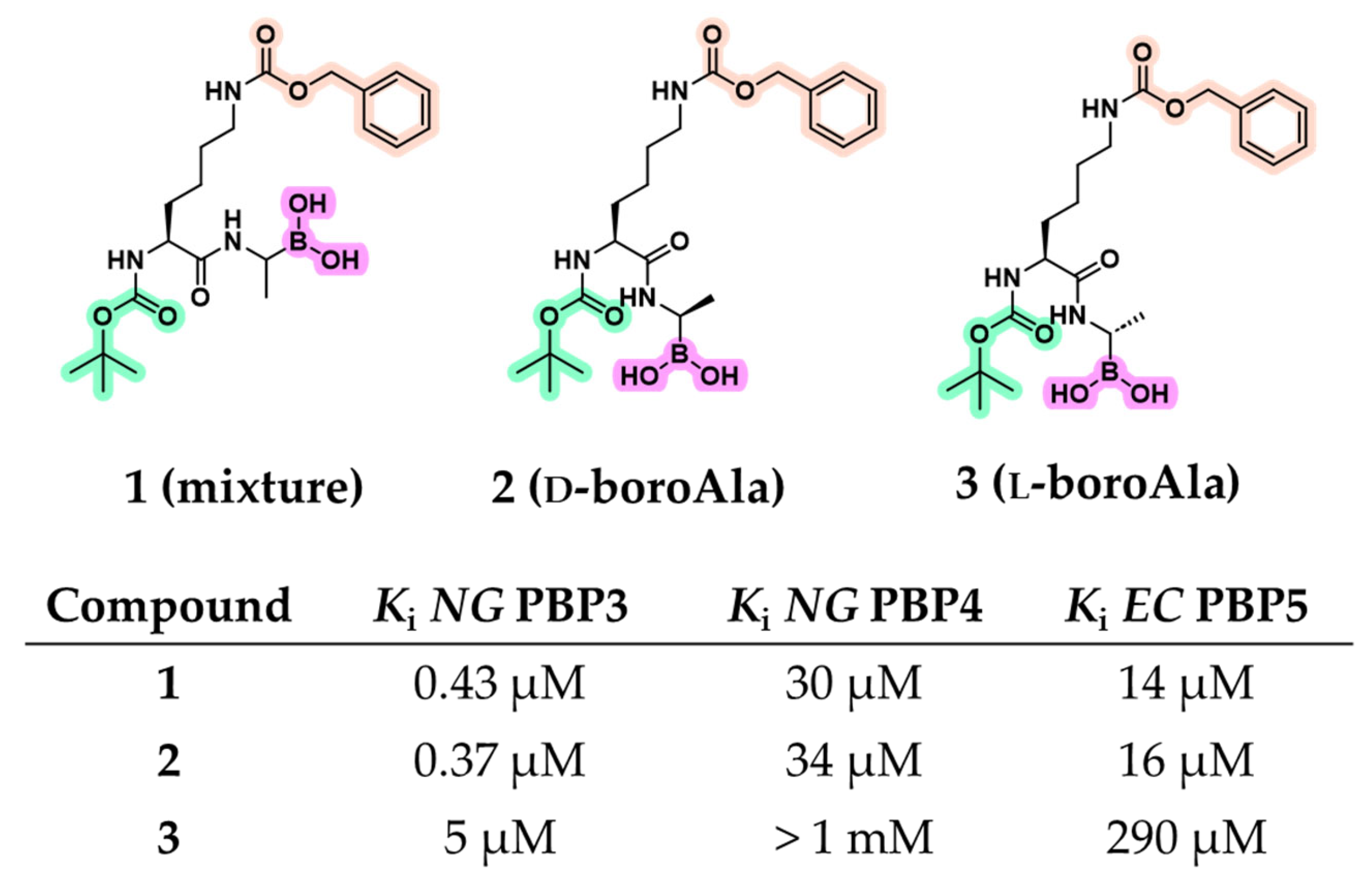
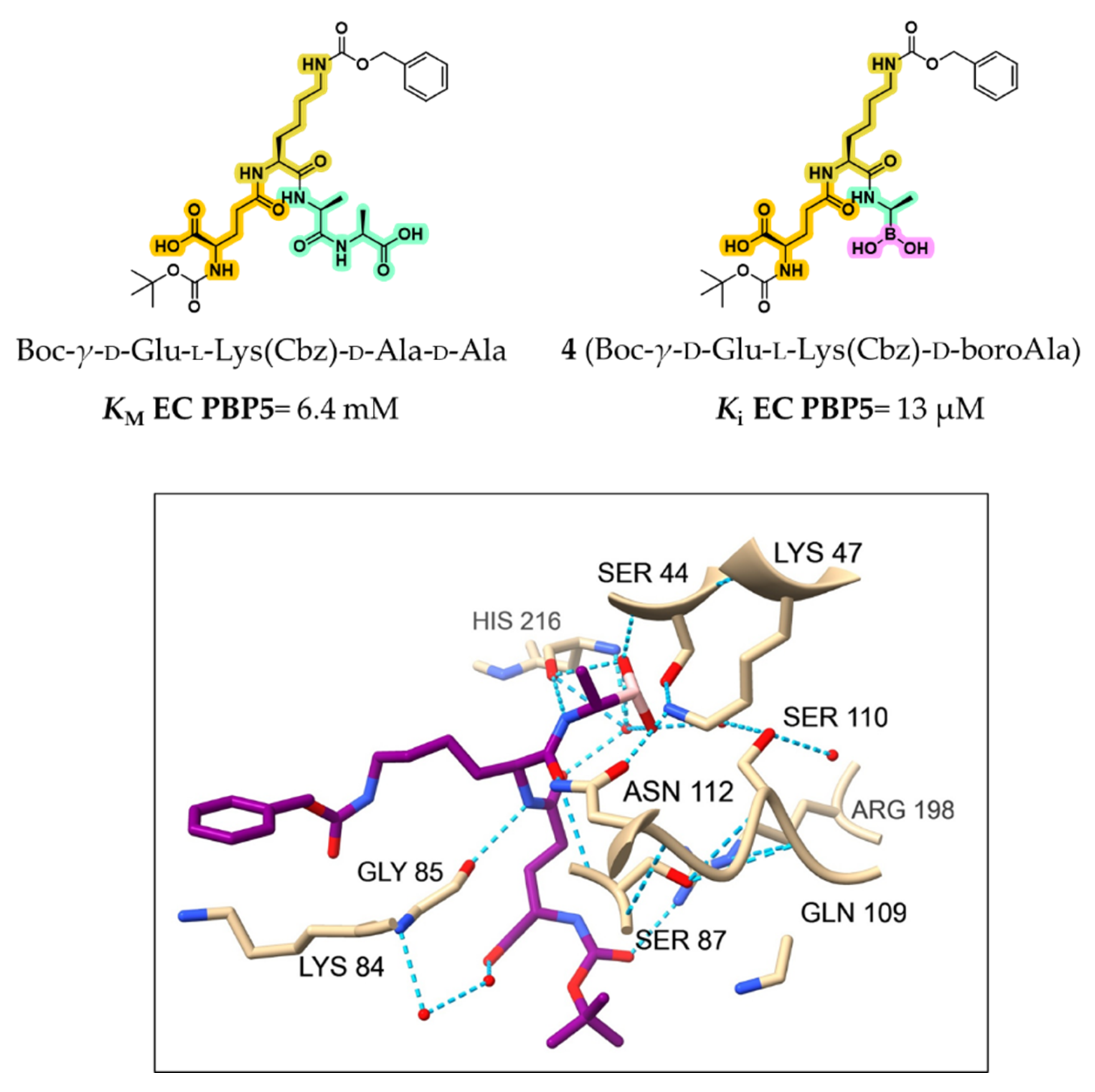
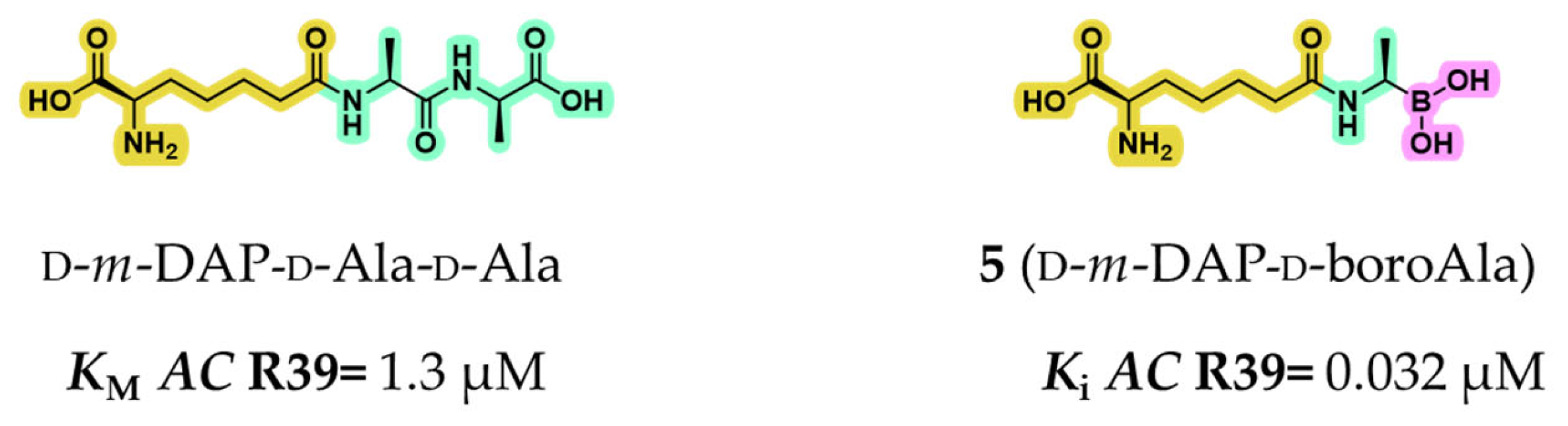
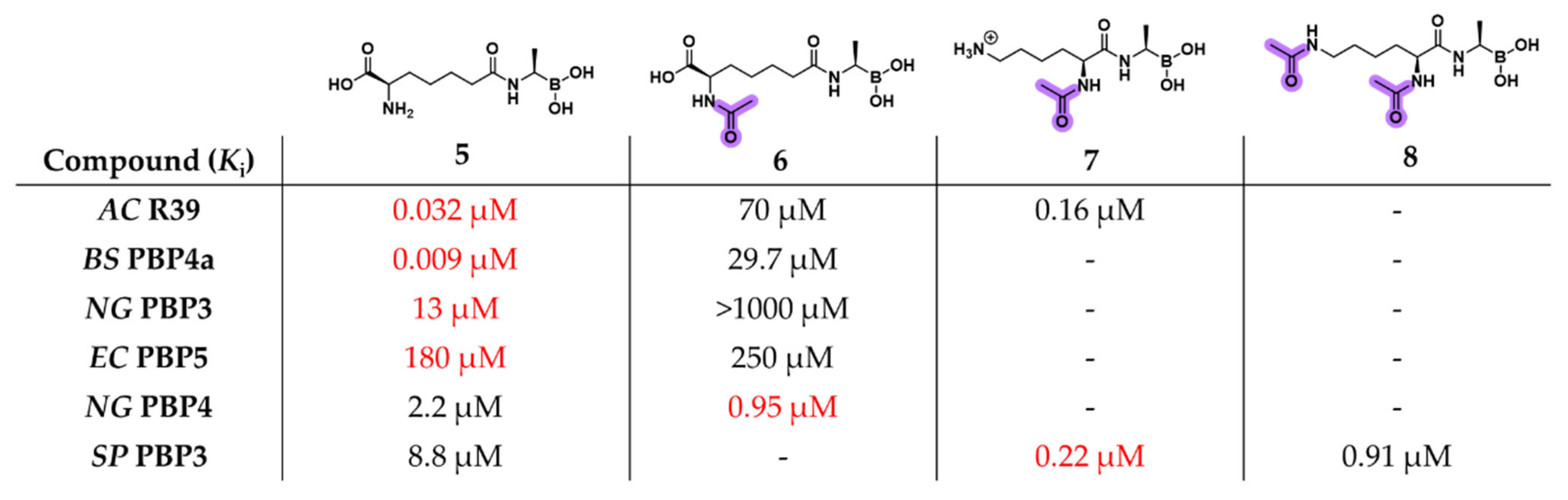
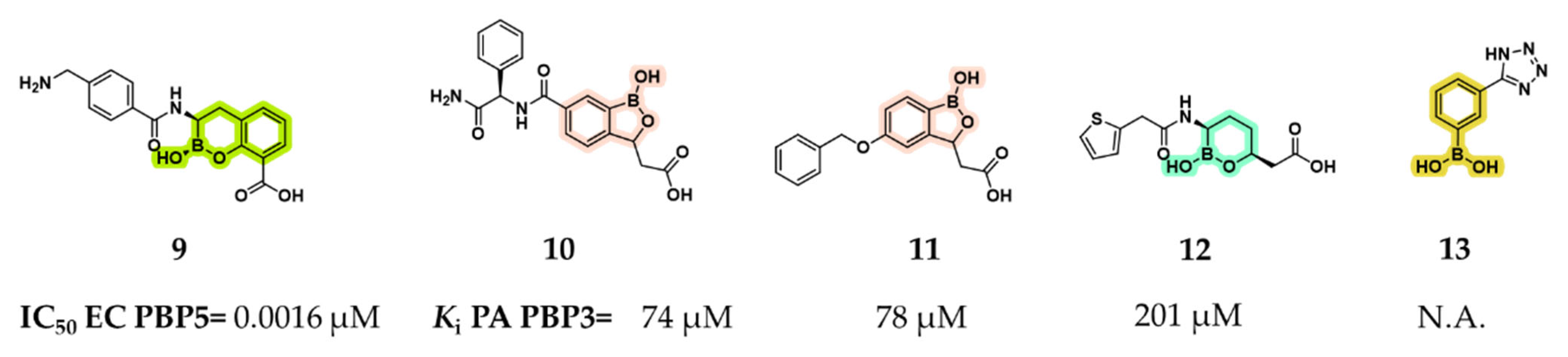
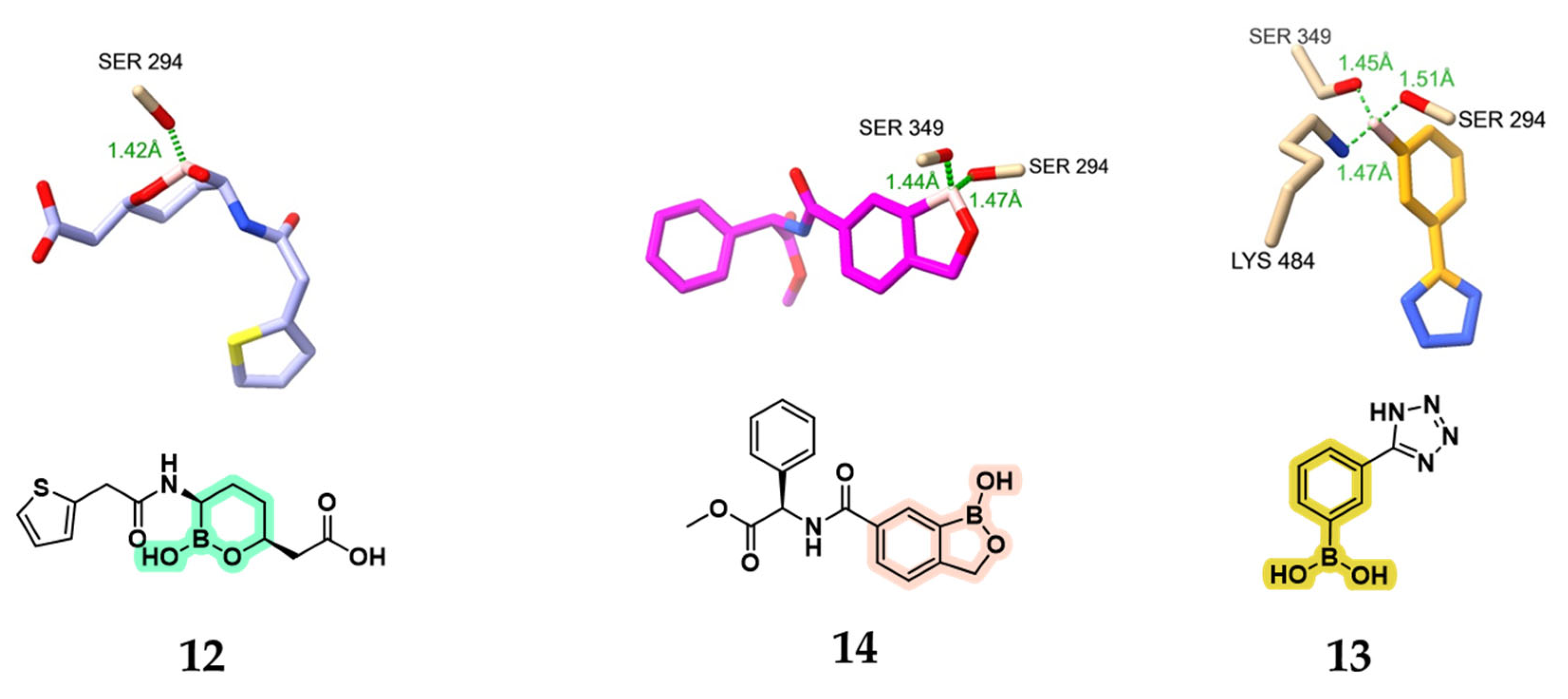
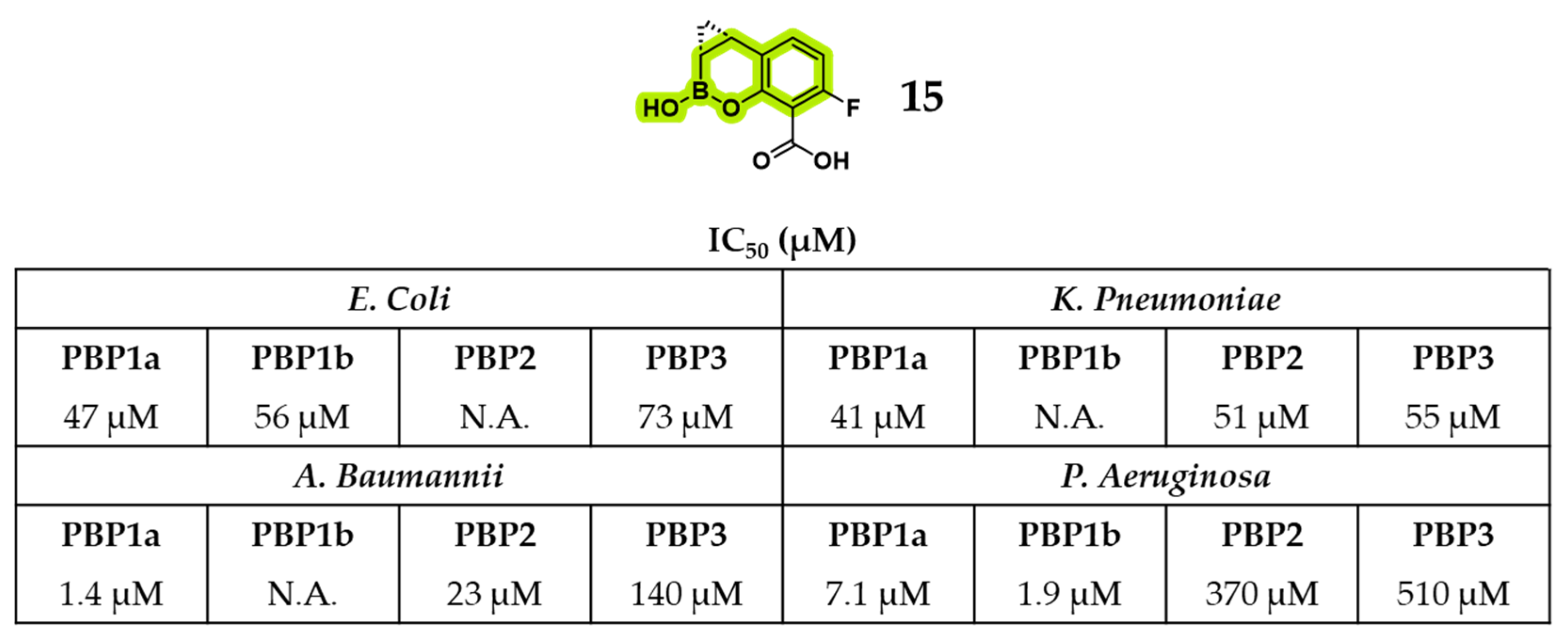
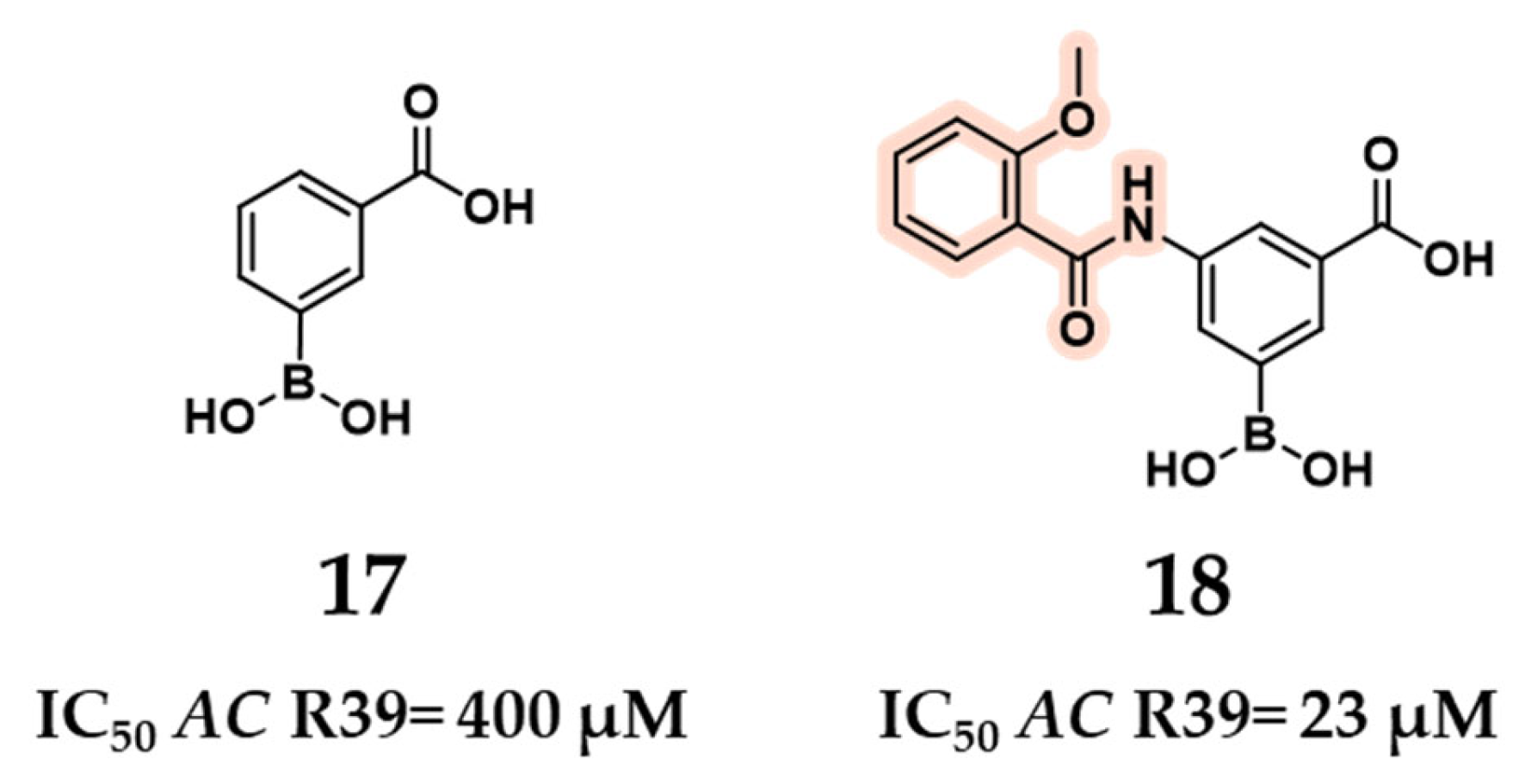
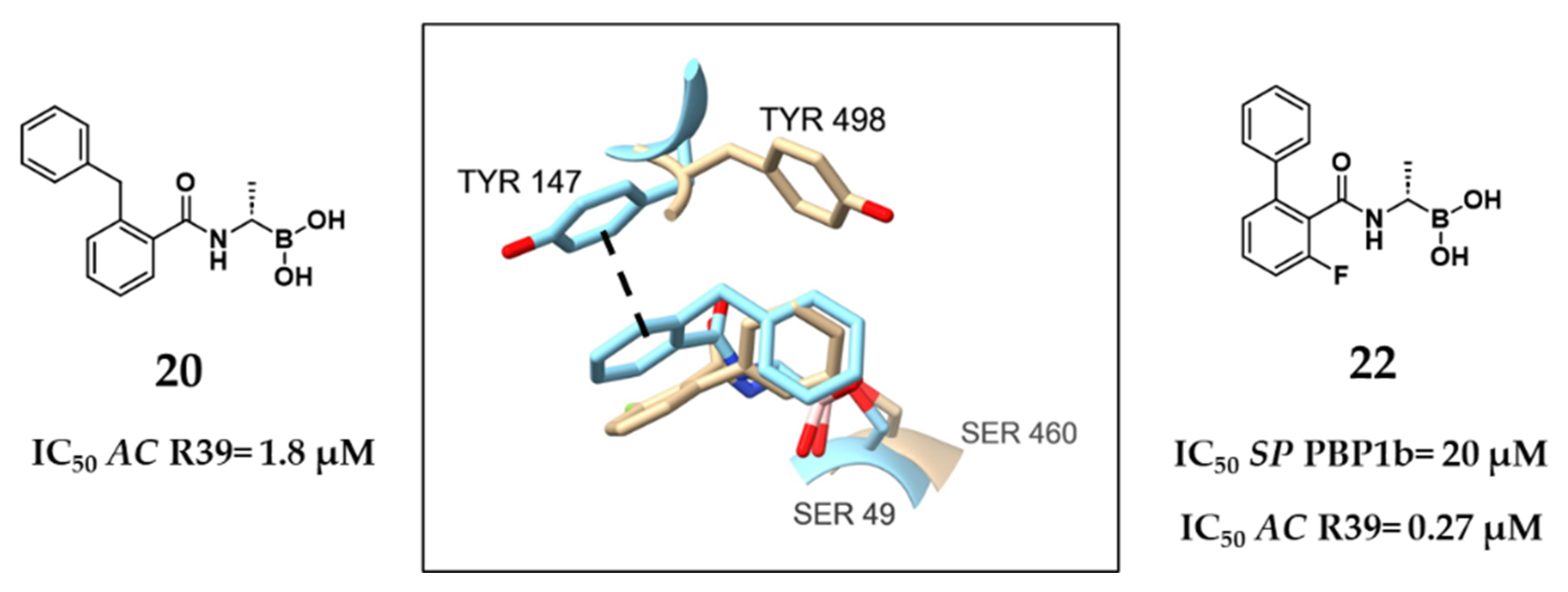
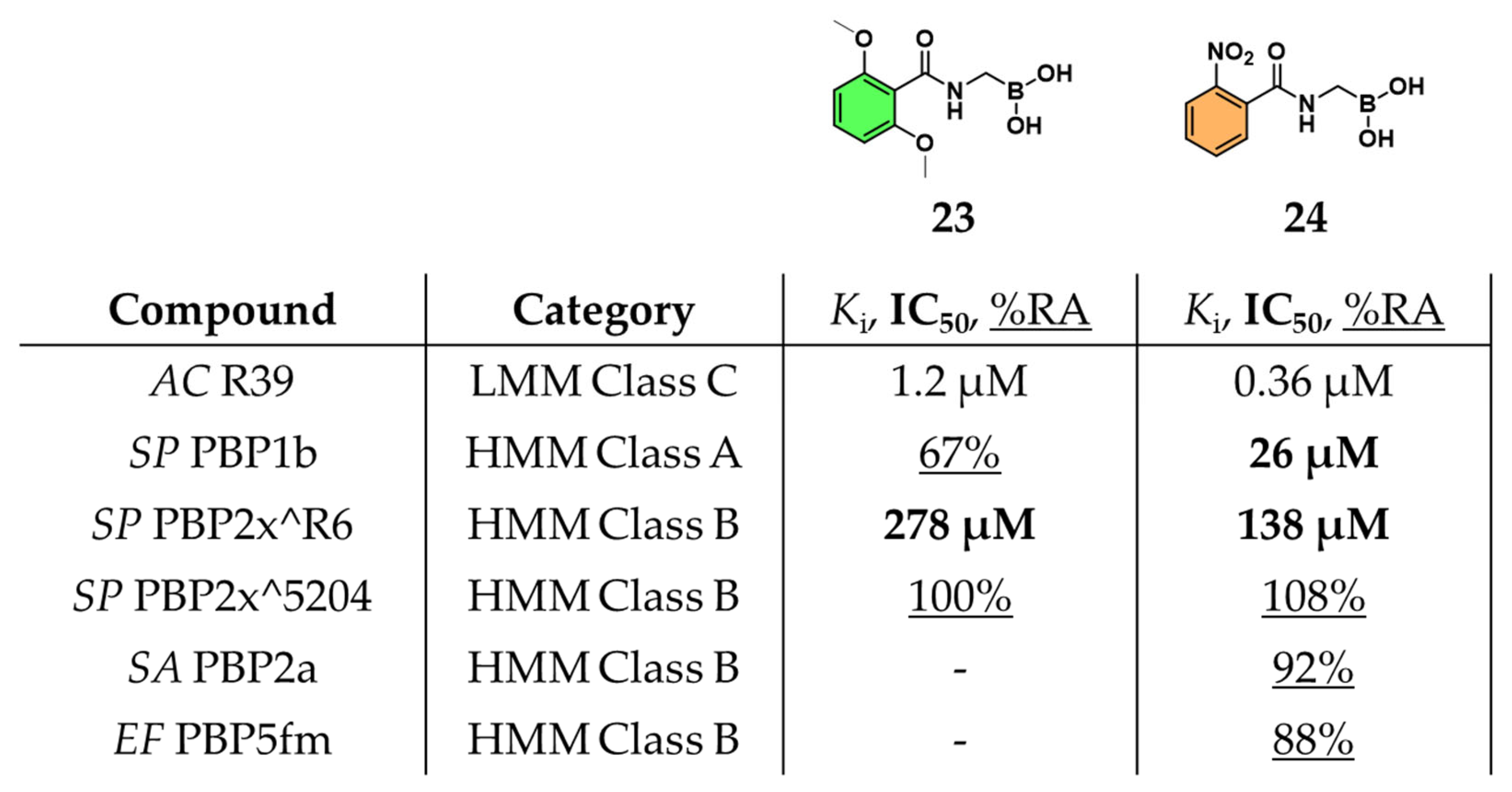
2.1.1. Peptidomimetic Boronic Acid Inhibitors
2.1.2. Repurposing Known BLIs as PBP Inhibitors
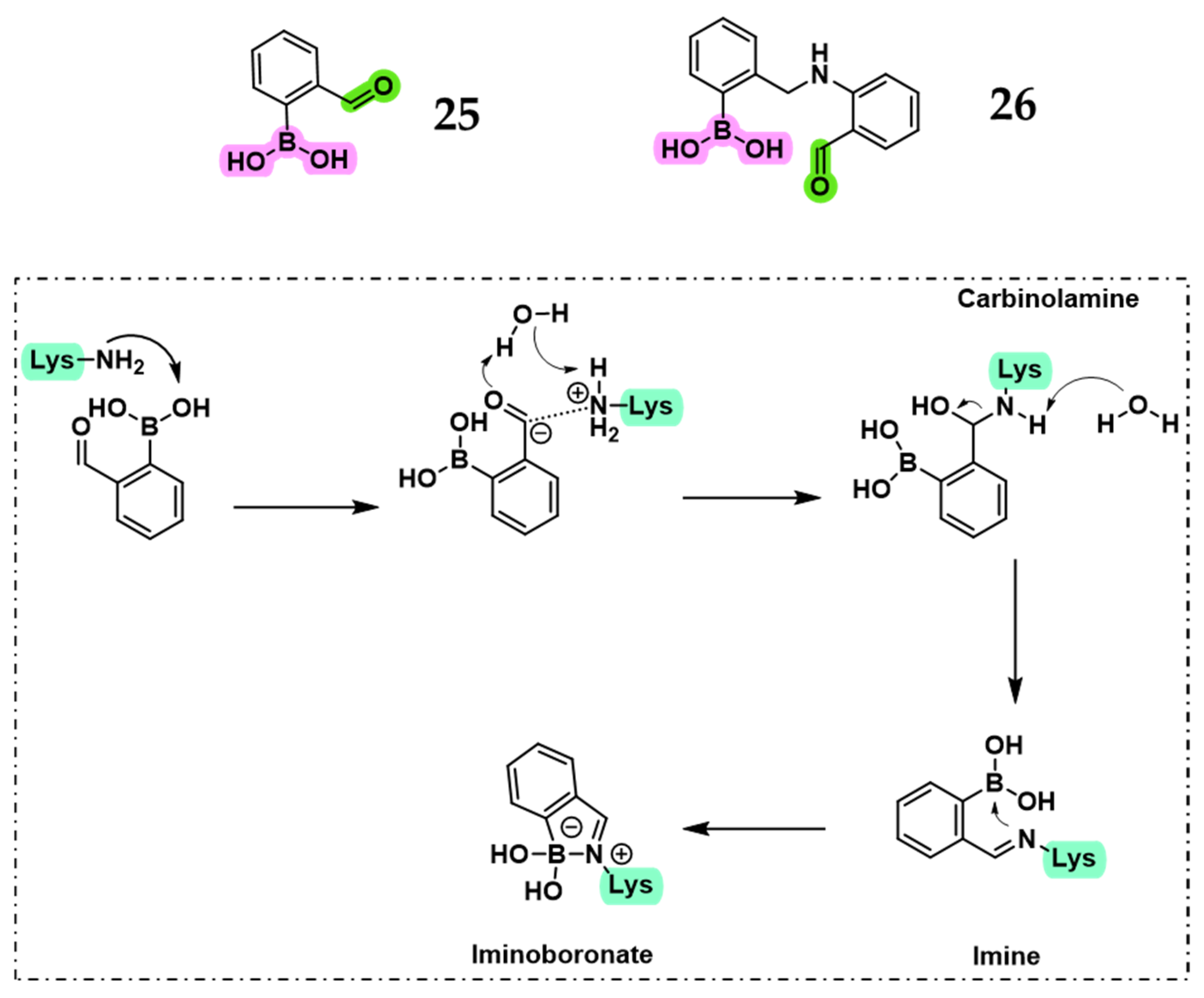
2.1.3. Novel Boron-Based Molecules as PBP Inhibitors
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tomasz, A. Penicillin-Binding Proteins and the Antibacterial Effectiveness of β-Lactam Antibiotics. Rev. Infect. Dis. 1986, 8, 260–278. [Google Scholar] [CrossRef] [PubMed]
- Galinier, A.; Delan-Forino, C.; Foulquier, E.; Lakhal, H.; Pompeo, F. Recent Advances in Peptidoglycan Synthesis and Regulation in Bacteria. Biomolecules 2023, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- Zervosen, A.; Sauvage, E.; Frère, J.M.; Charlier, P.; Luxen, A. Development of New Drugs for an Old Target–The Penicillin Binding Proteins. Molecules 2012, 17, 12478–12505. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, E.; Kerff, F.; Terrak, M.; Ayala, J.A.; Charlier, P. The Penicillin-Binding Proteins: Structure and Role in Peptidoglycan Biosynthesis. FEMS Microbiol. Rev. 2008, 32, 234–258. [Google Scholar] [CrossRef]
- Zapun, A.; Contreras-Martel, C.; Vernet, T. Penicillin-Binding Proteins and β-Lactam Resistance. FEMS Microbiol. Rev. 2008, 32, 361–385. [Google Scholar] [CrossRef]
- Mora-Ochomogo, M.; Lohans, C.T. β-Lactam Antibiotic Targets and Resistance Mechanisms: From Covalent Inhibitors to Substrates. RSC Med. Chem. 2021, 12, 1623–1639. [Google Scholar] [CrossRef]
- Tipper, D.J.; Strominger, J.L. Mechanism of Action of Penicillins: A Proposal Based on Their Structural Similarity to Acyl-D-Alanyl-D-Alanine. Proc. Natl. Acad. Sci. USA 1965, 54, 1133–1141. [Google Scholar] [CrossRef]
- Lima, L.M.; da Silva, B.N.M.; Barbosa, G.; Barreiro, E.J. β-Lactam Antibiotics: An Overview from a Medicinal Chemistry Perspective. Eur. J. Med. Chem. 2020, 208, 112829–112854. [Google Scholar] [CrossRef]
- Binda, E.; Marinelli, F.; Marcone, G.L. Old and New Glycopeptide Antibiotics: Action and Resistance. Antibiotics 2014, 3, 572–594. [Google Scholar] [CrossRef]
- Bush, K.; Bradford, P.A. β-Lactams and β-Lactamase Inhibitors: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247–a025270. [Google Scholar] [CrossRef]
- Pagliero, E.; Chesnel, L.; Hopkins, J.; Croizé, J.; Dideberg, O.; Vernet, T.; Di Guilmi, A.M. Biochemical Characterization of Streptococcus pneumoniae Penicillin-Binding Protein 2b and Its Implication in β-Lactam Resistance. Antimicrob. Agents Chemother. 2004, 48, 1848–1855. [Google Scholar] [CrossRef]
- Antignac, A.; Boneca, I.G.; Rousselle, J.C.; Namane, A.; Carlier, J.P.; Vázquez, J.A.; Fox, A.; Alonso, J.M.; Taha, M.K. Correlation between Alterations of the Penicillin-Binding Protein 2 and Modifications of the Peptidoglycan Structure in Neisseria meingitidis with Reduced Susceptibility to Penicillin G. J. Biol. Chem. 2003, 278, 31529–31535. [Google Scholar] [CrossRef] [PubMed]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-Binding Protein 2a of Methicillin-Resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Van Duin, D.; Paterson, D.L. Multidrug-Resistant Bacteria in the Community: An Update. Infect. Dis. Clin. N. Am. 2020, 34, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.G.; Barlow, M. Evolution of the Serine β-Lactamases: Past, Present and Future. Drug Resist. Updates 2004, 7, 111–123. [Google Scholar] [CrossRef]
- Rodríguez-Baño, J.; Navarro, M.D.; Retamar, P.; Picón, E.; Pascual, Á. β-Lactam/β-Lactam Inhibitor Combinations for the Treatment of Bacteremia Due to Extended-Spectrum β-Lactamase-Producing Escherichia coli: A Post Hoc Analysis of Prospective Cohorts. Clin. Infect. Dis. 2012, 54, 167–174. [Google Scholar] [CrossRef]
- Reading, C.; Cole, M. Clavulanic Acid: A Beta Lactamase Inhibiting Beta Lactam from Streptomyces clavuligerus. Antimicrob. Agents Chemother. 1977, 11, 852–857. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Douzinas, E.E. Ampicillin-Sulbactam: An Update on the Use of Parenteral and Oral Forms in Bacterial Infections. Expert Opin. Drug Metab. Toxicol. 2009, 5, 1099–1112. [Google Scholar] [CrossRef]
- Shlaes, D.M. New β-Lactam-β-Lactamase Inhibitor Combinations in Clinical Development. Ann. N. Y. Acad. Sci. 2013, 1277, 105–114. [Google Scholar] [CrossRef]
- Keam, S.J. Cefepime/Enmetazobactam: First Approval. Drugs 2024, 84, 737–744. [Google Scholar] [CrossRef]
- King, A.M.; King, D.T.; French, S.; Brouillette, E.; Asli, A.; Alexander, J.A.N.; Vuckovic, M.; Maiti, S.N.; Parr, T.R.; Brown, E.D.; et al. Structural and Kinetic Characterization of Diazabicyclooctanes as Dual Inhibitors of Both Serine-β-Lactamases and Penicillin-Binding Proteins. ACS Chem. Biol. 2016, 11, 864–868. [Google Scholar] [CrossRef]
- Heo, Y.A. Imipenem/Cilastatin/Relebactam: A Review in Gram-Negative Bacterial Infections. Drugs 2021, 81, 377–388. [Google Scholar] [CrossRef]
- Durand-Réville, T.F.; Guler, S.; Comita-Prevoir, J.; Chen, B.; Bifulco, N.; Huynh, H.; Lahiri, S.; Shapiro, A.B.; McLeod, S.M.; Carter, N.M.; et al. ETX2514 Is a Broad-Spectrum β-Lactamase Inhibitor for the Treatment of Drug-Resistant Gram-Negative Bacteria Including Acinetobacter baumannii. Nat. Microbiol. 2017, 2, 17104. [Google Scholar] [CrossRef]
- Li, X.; Zhang, J.; Wang, J.; Long, W.; Liang, X.; Yang, Y.; Gong, X.; Li, J.; Liu, L.; Zhang, X. Activities of Aztreonam in Combination with Several Novel β-Lactam-β-Lactamase Inhibitor Combinations against Carbapenem-Resistant Klebsiella Pneumoniae Strains Coproducing KPC and NDM. Front. Microbiol. 2024, 15, 1210313. [Google Scholar] [CrossRef] [PubMed]
- Brem, J.; Cain, R.; Cahill, S.; McDonough, M.A.; Clifton, I.J.; Jiménez-Castellanos, J.C.; Avison, M.B.; Spencer, J.; Fishwick, C.W.G.; Schofield, C.J. Structural Basis of Metallo-β-Lactamase, Serine-β-Lactamase and Penicillin-Binding Protein Inhibition by Cyclic Boronates. Nat. Commun. 2016, 7, 12406. [Google Scholar] [CrossRef] [PubMed]
- Krajnc, A.; Lang, P.A.; Panduwawala, T.D.; Brem, J.; Schofield, C.J. Will Morphing Boron-Based Inhibitors Beat the β-Lactamases? Curr. Opin. Chem. Biol. 2019, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Gaibani, P.; Giani, T.; Bovo, F.; Lombardo, D.; Amadesi, S.; Lazzarotto, T.; Coppi, M.; Rossolini, G.M.; Ambretti, S. Resistance to Ceftazidime/Avibactam, Meropenem/Vaborbactam and Imipenem/Relebactam in Susceptibility Testing. Antibiotics 2022, 11, 628. [Google Scholar] [CrossRef]
- Groft, L.M.; Claeys, K.C.; Heil, E.L. An Evaluation of Meropenem/Vaborbactam for the Treatment of Nosocomial Pneumonia. Expert Opin. Pharmacother. 2021, 22, 265–271. [Google Scholar] [CrossRef]
- Tsivkovski, R.; Lomovskayaa, O. Biochemical Activity of Vaborbactam. Antimicrob. Agents Chemother. 2020, 64, 1–9. [Google Scholar] [CrossRef]
- Liu, B.; Trout, R.E.L.; Chu, G.H.; Mcgarry, D.; Jackson, R.W.; Hamrick, J.C.; Daigle, D.M.; Cusick, S.M.; Pozzi, C.; De Luca, F.; et al. Discovery of Taniborbactam (VNRX-5133): A Broad-Spectrum Serine- and Metallo-β-Lactamase Inhibitor for Carbapenem-Resistant Bacterial Infections. J. Med. Chem. 2020, 63, 2789–2801. [Google Scholar] [CrossRef]
- Lomovskaya, O.; Tsivkovski, R.; Nelson, K.; Rubio-Aparicio, D.; Sun, D.; Totrov, M.; Dudley, M.N. Spectrum of Beta-Lactamase Inhibition by the Cyclic Boronate QPX7728, an Ultrabroad-Spectrum Beta-Lactamase Inhibitor of Serine and Metallo-Beta-Lactamases: Enhancement of Activity of Multiple Antibiotics against Isogenic Strains Expressing Single Beta-Lactamases. Antimicrob. Agents Chemother. 2020, 64, e00212-20. [Google Scholar] [CrossRef]
- Moya, B.; Barcelo, I.M.; Bhagwat, S.; Patel, M.; Bou, G.; Papp-Wallace, K.M.; Bonomo, R.A.; Oliver, A. Potent β-lactam enhancer activity of zidebactam and WCK 5153 against Acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicro. Agents Chemother. 2017, 61, e01238-17. [Google Scholar] [CrossRef]
- Morinaka, A.; Tsutsumi, Y.; Yamada, M.; Suzuki, K.; Watanabe, T.; Abe, T.; Furuuchi, T.; Inamura, S.; Sakamaki, Y.; Mitsuhashi, N.; et al. OP0595, a New Diazabicyclooctane: Mode of Action as a Serine β-Lactamase Inhibitor, Antibiotic and β-Lactam “Enhancer”. J. Antimicrob. Chemother. 2015, 70, 2779–2786. [Google Scholar] [CrossRef]
- Asli, A.; Brouillette, E.; Krause, K.M.; Nichols, W.W.; Malouin, F. Distinctive Binding of Avibactam to Penicillin-Binding Proteins of Gram-Negative and Gram-Positive Bacteria. Antimicrob. Agents Chemother. 2016, 60, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Moya, B.; Bhagwat, S.; Cabot, G.; Bou, G.; Patel, M.; Oliver, A. Effective Inhibition of PBPs by Cefepime and Zidebactam in the Presence of VIM-1 Drives Potent Bactericidal Activity against MBL-Expressing Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2021, 75, 1474–1478. [Google Scholar] [CrossRef] [PubMed]
- Pechenov, A.; Stefanova, M.E.; Nicholas, R.A.; Peddi, S.; Gutheil, W.G. Potential Transition State Analogue Inhibitors for the Penicillin-Binding Proteins. Biochemistry 2003, 42, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Dzhekieva, L.; Kumar, I.; Pratt, R.F. Inhibition of Bacterial D,D-Peptidases (Penicillin-Binding Proteins) in Membranes and in Vivo by Peptidoglycan-Mimetic Boronic Acids. Biochemistry 2012, 51, 2804–2811. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef]
- Ehmann, D.E.; Jahić, H.; Ross, P.L.; Gu, R.F.; Hu, J.; Kern, G.; Walkup, G.K.; Fisher, S.L. Avibactam is a Covalent, Reversible, Non-β-Lactam β-Lactamase Inhibitor. Proc. Natl. Acad. Sci. USA 2012, 109, 11663–11668. [Google Scholar] [CrossRef]
- Woon, E.C.Y.; Zervosen, A.; Sauvage, E.; Simmons, K.J.; Živec, M.; Inglis, S.R.; Fishwick, C.W.G.; Gobec, S.; Charlier, P.; Luxen, A.; et al. Structure Guided Development of Potent Reversibly Binding Penicillin Binding Protein Inhibitors. ACS Med. Chem. Lett. 2011, 2, 219–223. [Google Scholar] [CrossRef]
- Nicola, G.; Peddi, S.; Stefanova, M.; Nicholas, R.A.; Gutheil, W.G.; Davies, C. Crystal Structure of Escherichia coli Penicillin-Binding Protein 5 Bound to a Tripeptide Boronic Acid Inhibitor: A Role for Ser-110 in Deacylation. Biochemistry 2005, 44, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Dzhekieva, L.; Rocaboy, M.; Kerff, F.; Charlier, P.; Sauvage, E.; Pratt, R.F. Crystal Structure of a Complex between the Actinomadura R39 D,D-Peptidase and a Peptidoglycan-Mimetic Boronate Inhibitor: Interpretation of a Transition State Analogue in Terms of Catalytic Mechanism. Biochemistry 2010, 49, 6411–6419. [Google Scholar] [CrossRef] [PubMed]
- Nemmara, V.V.; Dzhekieva, L.; Subarno Sarkar, K.; Adediran, S.A.; Duez, C.; Nicholas, R.A.; Pratt, R.F. Substrate Specificity of Low-Molecular Mass Bacterial D,D-Peptidases. Biochemistry 2011, 50, 10091–10101. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48–75. [Google Scholar] [CrossRef]
- Newman, H.; Krajnc, A.; Bellini, D.; Eyermann, C.J.; Boyle, G.A.; Paterson, N.G.; McAuley, K.E.; Lesniak, R.; Gangar, M.; Von Delft, F.; et al. High-Throughput Crystallography Reveals Boron-Containing Inhibitors of a Penicillin-Binding Protein with Di- and Tricovalent Binding Modes. J. Med. Chem. 2021, 64, 11379–11394. [Google Scholar] [CrossRef]
- Zervosen, A.; Herman, R.; Kerff, F.; Herman, A.; Bouillez, A.; Prati, F.; Pratt, R.F.; Frère, J.M.; Joris, B.; Luxen, A.; et al. Unexpected Tricovalent Binding Mode of Boronic Acids within the Active Site of a Penicillin-Binding Protein. J. Am. Chem. Soc. 2011, 133, 10839–10848. [Google Scholar] [CrossRef]
- Santi, N.; Piccirilli, A.; Corsini, F.; Taracila, M.A.; Perilli, M.; Bonomo, R.A.; Fini, F.; Prati, F.; Caselli, E. Discovery of Boronic Acids-Based β-Lactamase Inhibitors Through In Situ Click Chemistry. Int. J. Mol. Sci. 2025, 26, 4182. [Google Scholar] [CrossRef]
- Alsenani, T. Boronic Acid Transition State Inhibitors as Potent Inactivators of KPC and CTX-M β-Lactamases: Biochemical and Structural Analyses. Antimicrob. Agents Chemother. 2023, 67, e00930–e00940. [Google Scholar] [CrossRef]
- Sun, D.; Tsivkovski, R.; Pogliano, J.; Tsunemoto, H.; Nelson, K.; Rubio-Aparicio, D.; Lomovskaya, O. Intrinsic Antibacterial Activity of Xeruborbactam In Vitro: Assessing Spectrum and Mode of Action. Antimicrob. Agents Chemother. 2022, 66, e00879-22. [Google Scholar] [CrossRef]
- Uehara, T.; Zulli, A.L.; Miller, B.; Avery, L.M.; Boyd, S.A.; Chatwin, C.L.; Chu, G.-H.; Drager, A.S.; Edwards, M.; Emeigh Hart, S.G.; et al. A new class of penicillin-binding protein inhibitors to address drug-resistant Neisseria gonorrhoeae. BioRxiv 2024, 12, 1–49. [Google Scholar] [CrossRef]
- Kumar, V.; Viviani, S.L.; Ismail, J.; Agarwal, S.; Bonomo, R.A.; van den Akker, F. Structural Analysis of the Boronic Acid β-Lactamase Inhibitor Vaborbactam Binding to Pseudomonas aeruginosa Penicillin-Binding Protein 3. PLoS ONE 2021, 16, e0258359. [Google Scholar] [CrossRef]
- Inglis, S.R.; Zervosen, A.; Woon, E.C.Y.; Gerards, T.; Teller, N.; Fischer, D.S.; Luxen, A.; Schofield, C.J. Synthesis and Evaluation of 3-(Dihydroxyboryl)Benzoic Acids as D,D-Carboxypeptidase R39 Inhibitors. J. Med. Chem. 2009, 52, 6097–6106. [Google Scholar] [CrossRef]
- Contreras-Martel, C.; Amoroso, A.; Woon, E.C.Y.; Zervosen, A.; Inglis, S.; Martins, A.; Verlaine, O.; Rydzik, A.M.; Job, V.; Luxen, A.; et al. Structure-Guided Design of Cell Wall Biosynthesis Inhibitors That Overcome β-Lactam Resistance in Staphylococcus aureus (MRSA). ACS Chem. Biol. 2011, 6, 943–951. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villamil, V.; Brusoni, L.S.; Prati, F.; Caselli, E.; Santi, N. Boronate-Based Inhibitors of Penicillin-Binding Proteins: An Underestimated Avenue for Antibiotic Discovery? Pharmaceuticals 2025, 18, 1325. https://doi.org/10.3390/ph18091325
Villamil V, Brusoni LS, Prati F, Caselli E, Santi N. Boronate-Based Inhibitors of Penicillin-Binding Proteins: An Underestimated Avenue for Antibiotic Discovery? Pharmaceuticals. 2025; 18(9):1325. https://doi.org/10.3390/ph18091325
Chicago/Turabian StyleVillamil, Valentina, Luca Svolacchia Brusoni, Fabio Prati, Emilia Caselli, and Nicolò Santi. 2025. "Boronate-Based Inhibitors of Penicillin-Binding Proteins: An Underestimated Avenue for Antibiotic Discovery?" Pharmaceuticals 18, no. 9: 1325. https://doi.org/10.3390/ph18091325
APA StyleVillamil, V., Brusoni, L. S., Prati, F., Caselli, E., & Santi, N. (2025). Boronate-Based Inhibitors of Penicillin-Binding Proteins: An Underestimated Avenue for Antibiotic Discovery? Pharmaceuticals, 18(9), 1325. https://doi.org/10.3390/ph18091325







