Hypolipidemic Effects of Alpinia japonica Extracts: Modulation of PPAR Signaling, Gut Microbiota, and Intestinal Barrier Function in Hyperlipidemic Rats
Abstract
1. Introduction
2. Results
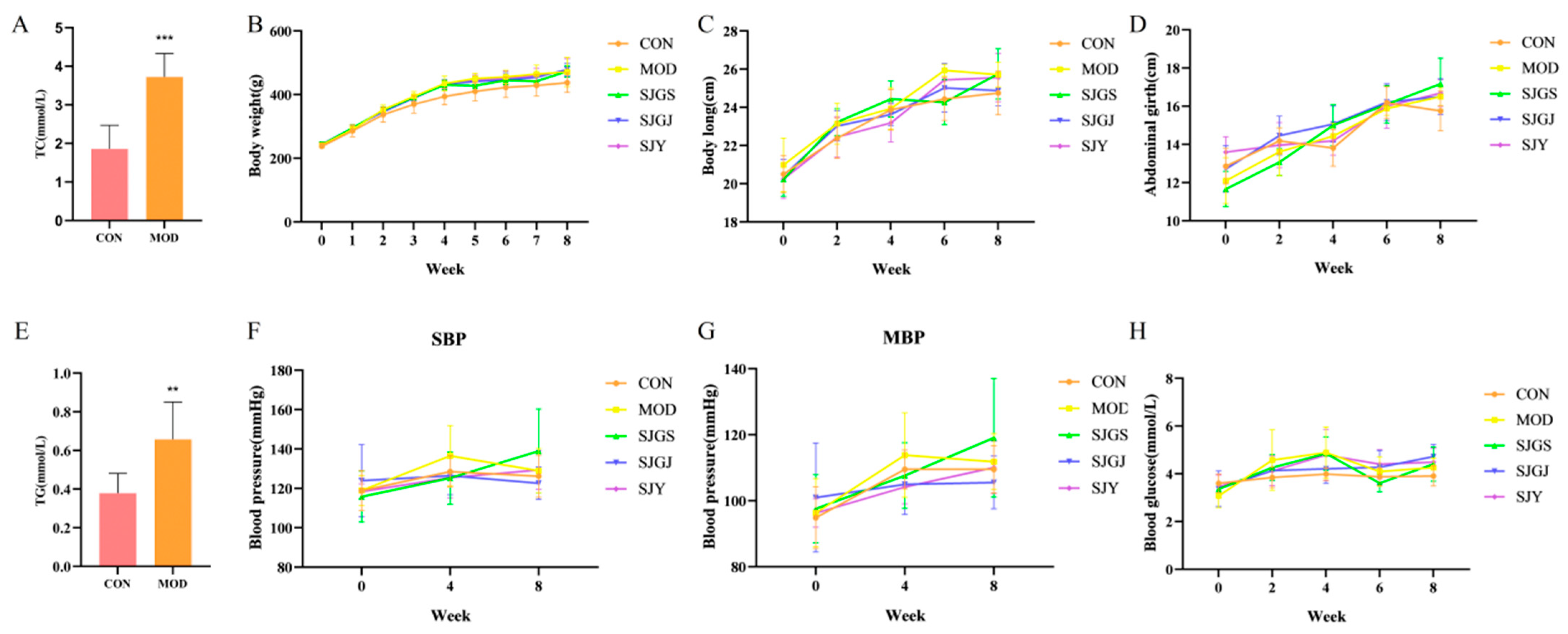
2.1. Effects of A. japonica on Metabolic Parameters in High-Fat Diet-Fed Rats
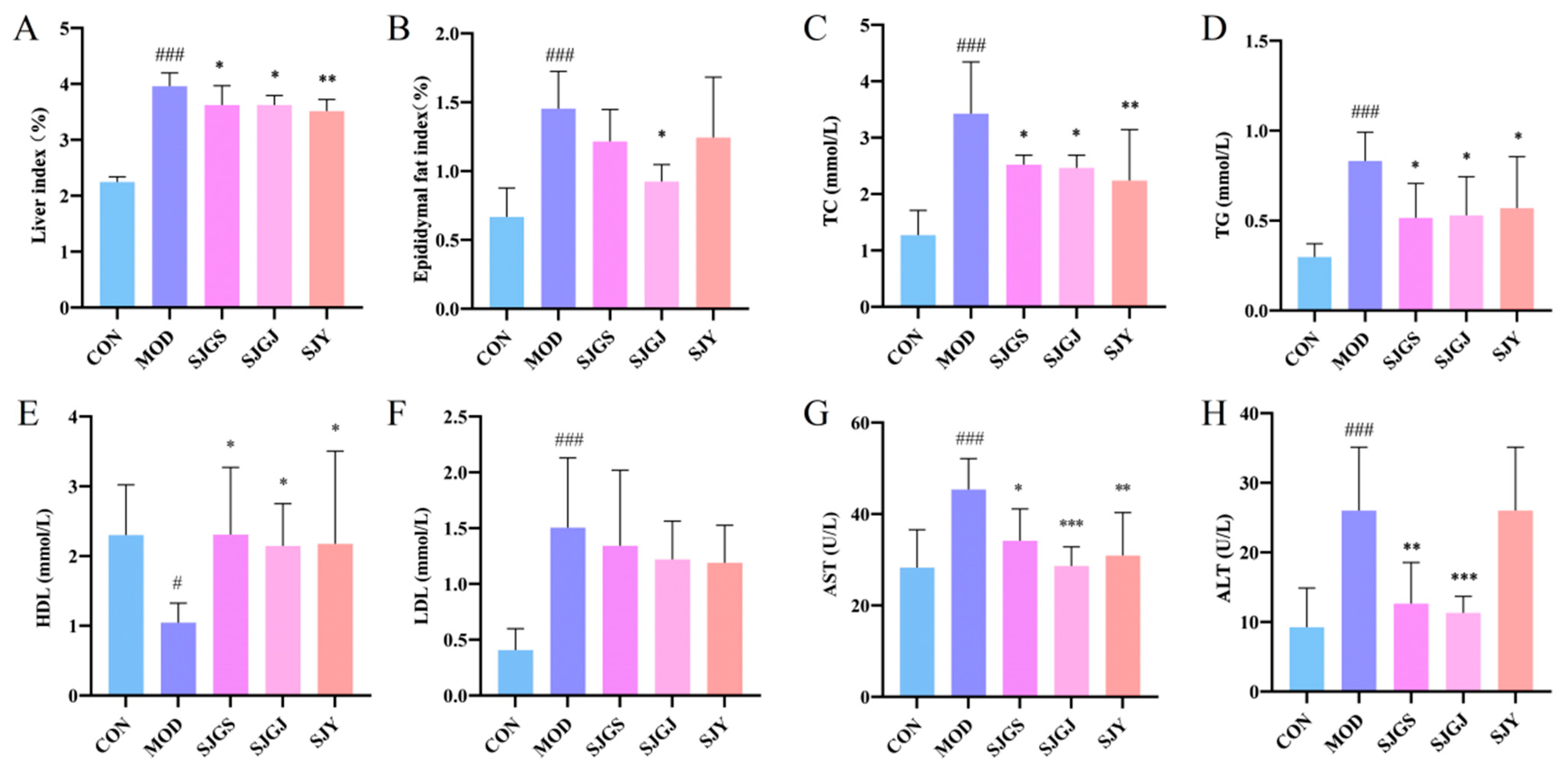
2.2. Hepatoprotective and Lipid-Lowering Effects of A. japonica in High-Fat Diet-Fed Rats
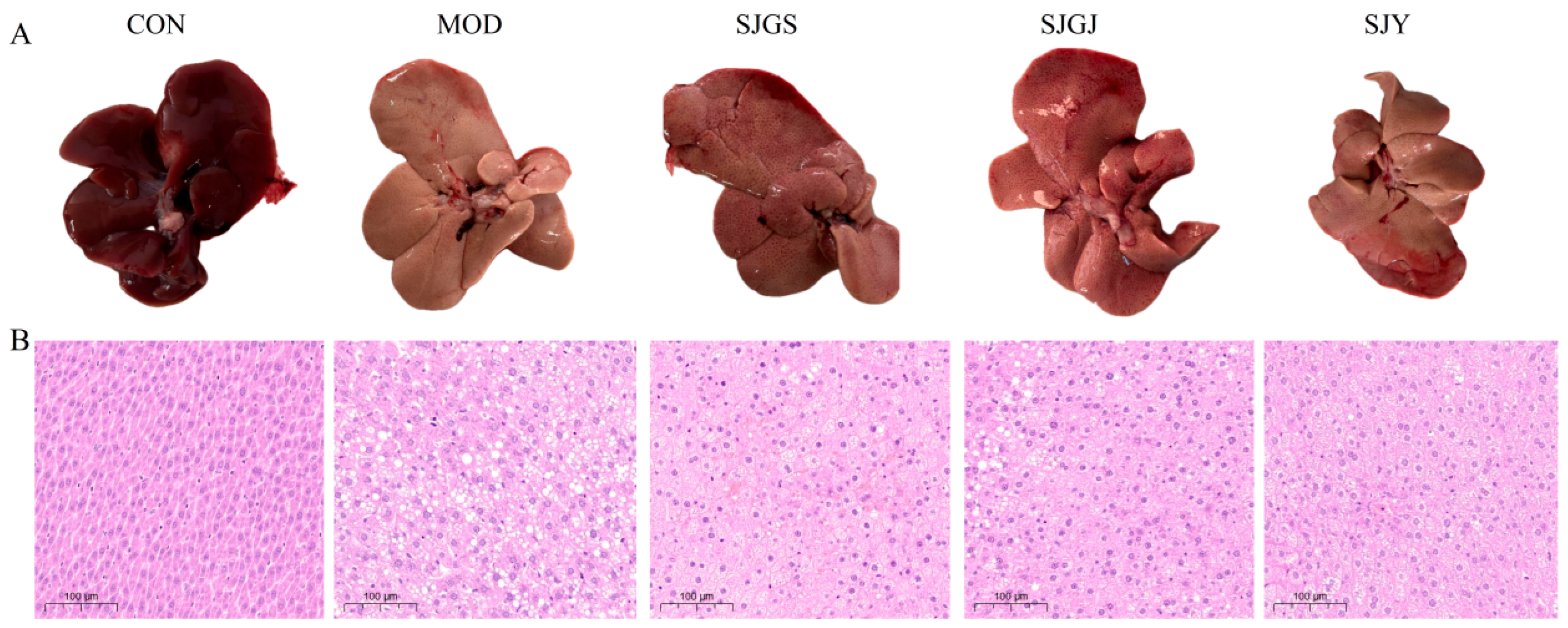
2.3. Histopathological Improvements of Hepatic Steatosis by A. japonica in High-Fat Diet-Fed Rats
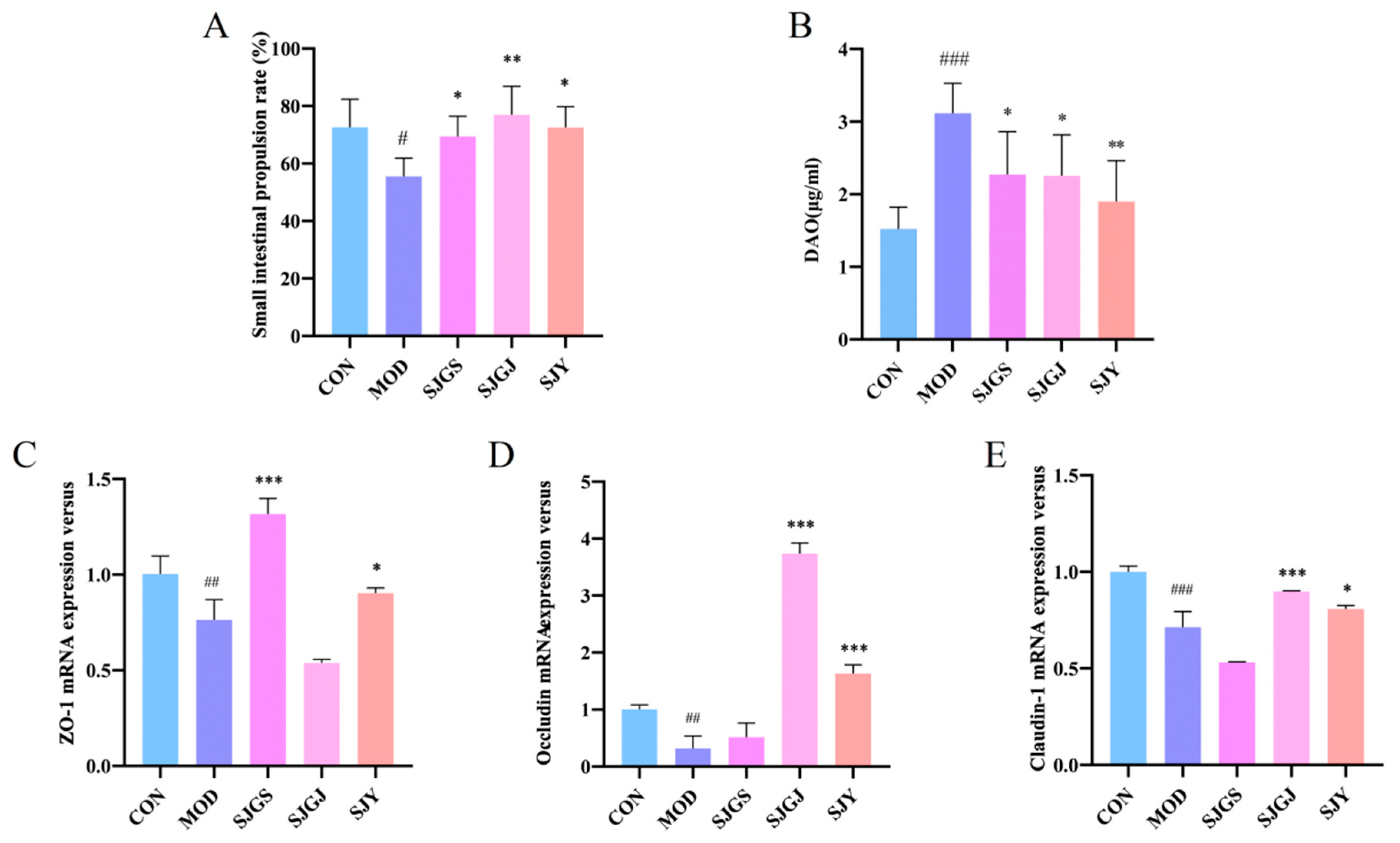
2.4. Effects of A. japonica on Intestinal Motility and Barrier Function in High-Fat Diet-Fed Rats
2.5. Modulation of Intestinal Tight Junction Protein Expression by A. japonica in High-Fat Diet-Fed Rats
2.6. Modulation of Gut Microbiota Composition and Diversity by A. japonica in High-Fat Diet-Fed Rats
2.7. Transcriptomic Profiling Reveals DEGs Regulation by A. japonica in High-Fat Diet-Fed Rat Ileum
2.8. Validation of Lipid Metabolism-Related Gene Expression by A. japonica Extracts in High-Fat Diet-Fed Rats
2.9. Chemical Profiling Reveals Differential Phytochemical Composition Among A. japonica Plant Parts
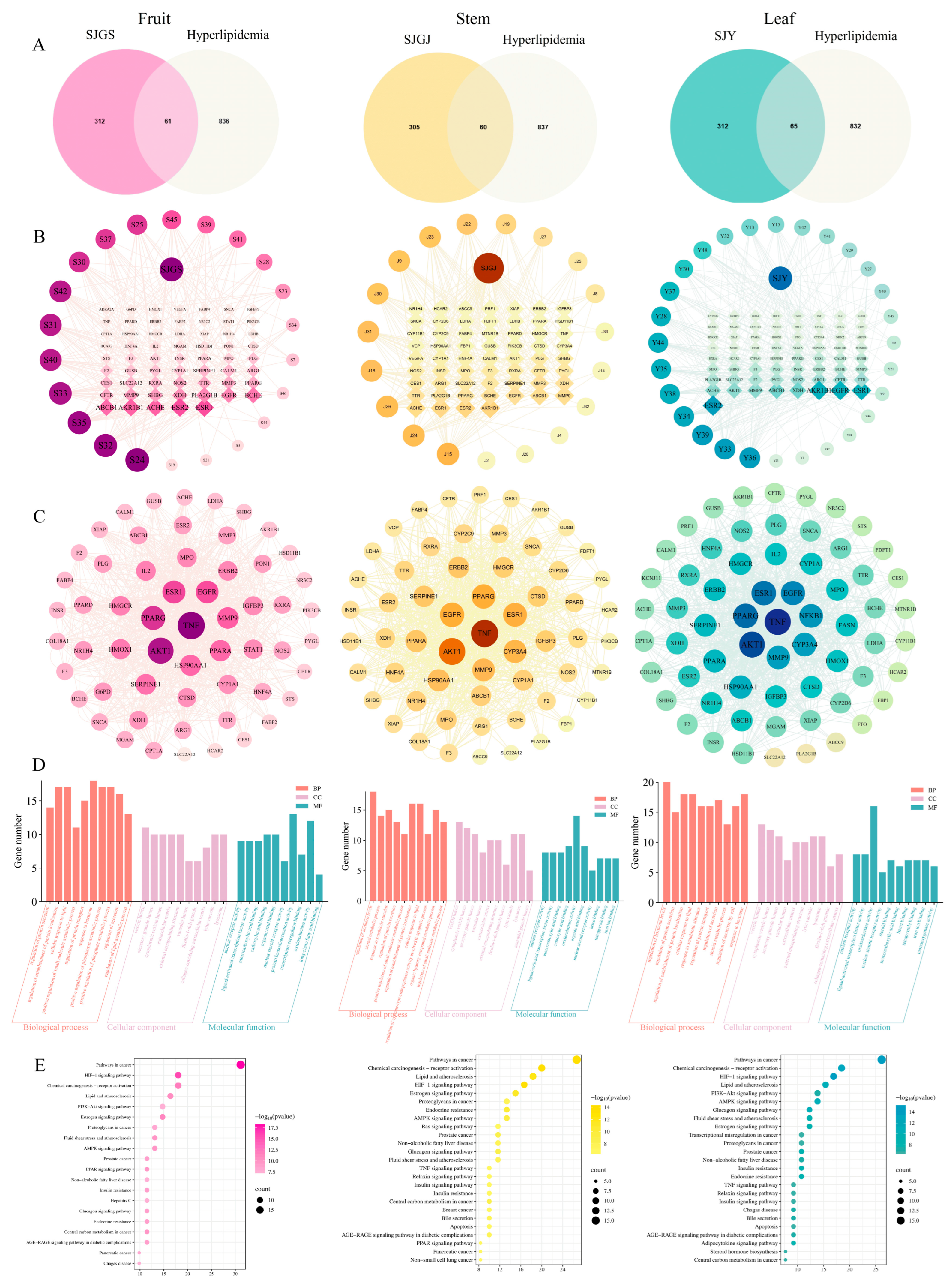
2.10. Network Pharmacological Analysis Reveals Multi-Component Synergistic Mechanisms of A. japonica Against Hyperlipidemia
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Experimental Design and Animal Handling
4.3. Sample Collection and Parameter Analysis
4.4. Analysis of Gut Microbiota Abundance and Diversity
4.5. Ileal Transcriptome Sequencing and Analysis
4.6. Quantitative Real-Time PCR Analysis of Ileal mRNA Expression
4.7. Chemical Profiling by UPLC-Q-Exactive-Orbitrap-MS
4.8. Network Pharmacology Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Editorial Committee of Flora of China. Flora Reipublicae Popularis Sinicae; Science Press: Beijing, China, 1981; Volume 16. [Google Scholar]
- Qiu, S. Textual Research on Medicinal Alpinia (Shanjiang). J. Fujian Coll. Tradit. Chin. Med. 1996, 6, 40–41. [Google Scholar]
- Zhao, J.; Xu, T.; Luo, J.; Peng, Q.; Gao, Z.; Xiang, J. Effects of Volatile Oil of the Alpinia japonica Rhizome on Gastrointestinal Motility of Mice in Tujia Medicine. J. Jishou Univ. (Nat. Sci. Ed.) 2024, 45, 11–15. [Google Scholar] [CrossRef]
- Asakawa, Y.; Ludwiczuk, A.; Sakurai, K.; Tomiyama, K.; Kawakami, Y.; Yaguchi, Y. Comparative Study on Volatile Compounds of Alpinia japonica and Elettaria cardamomum. J. Oleo Sci. 2017, 66, 871–876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kim, J.-G.; Kim, W.; Kim, K.-Y. Alpinia japonica Extract Induces Apoptosis of Hepatocellular Carcinoma Cells through G0/G1 Cell Cycle Arrest and Activation of JNK. Cell. Mol. Biol. 2023, 69, 12–18. [Google Scholar] [CrossRef]
- Li, Q.-M.; Luo, J.-G.; Yang, M.-H.; Kong, L.-Y. Terpenoids from Rhizomes of Alpinia japonica Inhibiting Nitric Oxide Production. Chem. Biodivers. 2015, 12, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.-M.; Luo, J.-G.; Wang, X.-B.; Yang, M.-H.; Kong, L.-Y. Sesquiterpenes from the Rhizomes of Alpinia japonica and Their Inhibitory Effects on Nitric Oxide Production. Fitoterapia 2013, 86, 29–34. [Google Scholar] [CrossRef]
- Kakegawa, T.; Miyazaki, A.; Yasukawa, K. Anti-Inflammatory Effects of Alpinone 3-Acetate from Alpinia japonica Seeds. J. Nat. Med. 2016, 70, 653–660. [Google Scholar] [CrossRef]
- Mei, L.-N.; Wang, Z.-J.; Duan, Y.; Shen, J.-S.; Ye, H.-B.; Zhu, Y.-Y.; Luo, X.-D. 4-Hydroxyboesenbergin B of Alpinia japonica Protected Gastrointestinal Tract by Inhibiting Vancomycin-Resistant Enterococcus and Balancing Intestinal Microbiota. J. Ethnopharmacol. 2025, 347, 119737. [Google Scholar] [CrossRef]
- You, M.; Zhou, L.; Wu, F.; Zhang, L.; Zhu, S.-X.; Zhang, H.-X. Probiotics for the Treatment of Hyperlipidemia: Focus on Gut-Liver Axis and Lipid Metabolism. Pharmacol. Res. 2025, 214, 107694. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Khan, A.; Choudhry, M.W. New Insights Into the Treatment of Hyperlipidemia: Pharmacological Updates and Emerging Treatments. Cureus 2024, 16, e63078. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration. Effects of Statin Therapy on Diagnoses of New-Onset Diabetes and Worsening Glycaemia in Large-Scale Randomised Blinded Statin Trials: An Individual Participant Data Meta-Analysis. Lancet Diabetes Endocrinol. 2024, 12, 306–319. [Google Scholar] [CrossRef]
- He, K.; Hu, Y.; Ma, H.; Zou, Z.; Xiao, Y.; Yang, Y.; Feng, M.; Li, X.; Ye, X. Rhizoma Coptidis Alkaloids Alleviate Hyperlipidemia in B6 Mice by Modulating Gut Microbiota and Bile Acid Pathways. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 1696–1709. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, L.; Zhang, L.; Xu, F.; Zhang, C.; Ren, G.; Chang, K.; He, G.; Du, Z.; Le, Y.; et al. Xie Zhuo Tiao Zhi Formula Modulates Intestinal Microbiota and Liver Purine Metabolism to Suppress Hepatic Steatosis and Pyroptosis in NAFLD Therapy. Phytomedicine 2023, 121, 155111. [Google Scholar] [CrossRef] [PubMed]
- Heidari, H.; Khalaj, A.; Khani, S.; Abdollahi, M.; Farahani, H.; Khani, S. Hypoglycemic, Hypolipidemic and Hepatoprotective Effects of Alpinia officinarum on Nicotinamide/Streptozotocin Induced Type II Diabetic Rats. Horm. Mol. Biol. Clin. Investig. 2022, 43, 289–296. [Google Scholar] [CrossRef]
- Xia, D.-Z.; Yu, X.-F.; Wang, H.-M.; Ren, Q.-Y.; Chen, B.-M. Anti-Obesity and Hypolipidemic Effects of Ethanolic Extract from Alpinia officinarum Hance (Zingiberaceae) in Rats Fed High-Fat Diet. J. Med. Food 2010, 13, 785–791. [Google Scholar] [CrossRef]
- Xiao, T.; Huang, J.; Wang, X.; Wu, L.; Zhou, X.; Jiang, F.; He, Z.; Guo, Q.; Tao, L.; Shen, X. Alpinia zerumbet and Its Potential Use as an Herbal Medication for Atherosclerosis: Mechanistic Insights from Cell and Rodent Studies. Lifestyle Genom. 2020, 13, 138–145. [Google Scholar] [CrossRef]
- Chuang, C.-M.; Wang, H.-E.; Peng, C.-C.; Chen, K.C.; Peng, R.Y. Hypolipidemic Effects of Different Angiocarp Parts of Alpinia zerumbet. Pharm. Biol. 2011, 49, 1257–1264. [Google Scholar] [CrossRef][Green Version]
- Rampanelli, E.; Romp, N.; Troise, A.D.; Ananthasabesan, J.; Wu, H.; Gül, I.S.; De Pascale, S.; Scaloni, A.; Bäckhed, F.; Fogliano, V.; et al. Gut Bacterium Intestinimonas butyriciproducens Improves Host Metabolic Health: Evidence from Cohort and Animal Intervention Studies. Microbiome 2025, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Schoeler, M.; Ellero-Simatos, S.; Birkner, T.; Mayneris-Perxachs, J.; Olsson, L.; Brolin, H.; Loeber, U.; Kraft, J.D.; Polizzi, A.; Martí-Navas, M.; et al. The Interplay between Dietary Fatty Acids and Gut Microbiota Influences Host Metabolism and Hepatic Steatosis. Nat. Commun. 2023, 14, 5329. [Google Scholar] [CrossRef] [PubMed]
- Won, T.H.; Arifuzzaman, M.; Parkhurst, C.N.; Miranda, I.C.; Zhang, B.; Hu, E.; Kashyap, S.; Letourneau, J.; Jin, W.-B.; Fu, Y.; et al. Host Metabolism Balances Microbial Regulation of Bile Acid Signalling. Nature 2025, 638, 216–224. [Google Scholar] [CrossRef]
- Du, H.; Shi, L.; Wang, Q.; Yan, T.; Wang, Y.; Zhang, X.; Yang, C.; Zhao, Y.; Yang, X. Fu Brick Tea Polysaccharides Prevent Obesity via Gut Microbiota-Controlled Promotion of Adipocyte Browning and Thermogenesis. J. Agric. Food Chem. 2022, 70, 13893–13903. [Google Scholar] [CrossRef] [PubMed]
- Manzo, R.; Gallardo-Becerra, L.; Díaz de León-Guerrero, S.; Villaseñor, T.; Cornejo-Granados, F.; Salazar-León, J.; Ochoa-Leyva, A.; Pedraza-Alva, G.; Pérez-Martínez, L. Environmental Enrichment Prevents Gut Dysbiosis Progression and Enhances Glucose Metabolism in High-Fat Diet-Induced Obese Mice. Int. J. Mol. Sci. 2024, 25, 6904. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Zhou, J.; Zhang, H.; Lin, Z.; Khambu, B.; Liu, G.; Ma, M.; Chen, X.; Chalasani, N.; Yin, X.-M. Promotion of Diet-Induced Obesity and Metabolic Syndromes by BID Is Associated with Gut Microbiota. Hepatol. Commun. 2022, 6, 3349–3362. [Google Scholar] [CrossRef]
- Cai, R.; Yue, X.; Wang, Y.; Yang, Y.; Sun, D.; Li, H.; Chen, L. Chemistry and Bioactivity of Plants from the Genus. Amomum. J. Ethnopharmacol. 2021, 281, 114563. [Google Scholar] [CrossRef]
- Cai, W.; Jiang, L.; Zhao, C.; Zhou, X. Advances in Omics Technologies for Traditional Chinese Medicine in the Prevention and Treatment of Metabolic Bone Diseases. Front. Pharmacol. 2025, 16, 1576286. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut Microbiota in Human Metabolic Health and Disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, B.; Zhou, L.; Li, S.; Li, Z.; Luo, H. Multi-Omics Reveals Diet-Induced Metabolic Disorders and Liver Inflammation via Microbiota-Gut-Liver Axis. J. Nutr. Biochem. 2023, 111, 109183. [Google Scholar] [CrossRef]
- Peng, L.; Li, H.; Yang, L.; Liang, Z.; Zhang, X. Exploring the Metabolic and Transcriptomic Profiles of Tetrastigma hemsleyanum for Tissue-Specific Compound Accumulation. Front. Plant Sci. 2025, 16, 1478061. [Google Scholar] [CrossRef] [PubMed]
- Maslahat, M.; Mardinata, D.; Surur, S.M.; Lioe, H.N.; Syafitri, U.D.; Rafi, M.; Rohaeti, E. Untargeted Metabolomics Analysis Using FTIR and LC-HRMS for Differentiating Sonchus Arvensis Plant Parts and Evaluating Their Biological Activity. Chem. Biodivers. 2025, 22, e202401537. [Google Scholar] [CrossRef]
- Yang, W.; Liu, F.; Wu, G.; Liang, S.; Bai, X.; Liu, B.; Zhang, B.; Chen, H.; Yang, J. Widely Targeted Metabolomics Analysis of the Roots, Stems, Leaves, Flowers, and Fruits of Camellia luteoflora, a Species with an Extremely Small Population. Molecules 2024, 29, 4754. [Google Scholar] [CrossRef]
- Farag, M.A.; Baky, M.H.; Morgan, I.; Khalifa, M.R.; Rennert, R.; Mohamed, O.G.; El-Sayed, M.M.; Porzel, A.; Wessjohann, L.A.; Ramadan, N.S. Comparison of Balanites Aegyptiaca Parts: Metabolome Providing Insights into Plant Health Benefits and Valorization Purposes as Analyzed Using Multiplex GC-MS, LC-MS, NMR-Based Metabolomics, and Molecular Networking. RSC Adv. 2023, 13, 21471–21493. [Google Scholar] [CrossRef] [PubMed]
- Amari, N.O.; Missoun, F.; Chaimbault, P.; Berkani, A. Profiling by LC-MS and LC-MS/MS with Electrospray source (ESI) of extracts from three organs of Thymelaea hirsuta L. Ann. Pharm. Fr. 2022, 80, 554–569. [Google Scholar] [CrossRef]
- Curtis, P.J.; Berends, L.; van der Velpen, V.; Jennings, A.; Haag, L.; Chandra, P.; Kay, C.D.; Rimm, E.B.; Cassidy, A. Blueberry Anthocyanin Intake Attenuates the Postprandial Cardiometabolic Effect of an Energy-Dense Food Challenge: Results from a Double Blind, Randomized Controlled Trial in Metabolic Syndrome Participants. Clin. Nutr. 2022, 41, 165–176. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Luo, Y.; Liu, H.; Ling, H.; He, Y.; Lu, Y. PPARα Is One of the Key Targets for Dendrobine to Improve Hepatic Steatosis in NAFLD. J. Ethnopharmacol. 2024, 323, 117684. [Google Scholar] [CrossRef]
- Jung, J.-W.; Wang, F.; Turk, A.; Park, J.-S.; Ma, H.; Ma, Y.; Noh, H.-R.; Sui, G.; Shin, D.-S.; Lee, M.-K.; et al. Zaluzanin C Alleviates Inflammation and Lipid Accumulation in Kupffer Cells and Hepatocytes by Regulating Mitochondrial ROS. Molecules 2023, 28, 7484. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, K.; Zhang, Y.; Zhu, L.; Liao, Y.; Zheng, X.; Chen, Y.; Li, X.; Liu, Z.; Zhang, Z. Fenofibrate Suppresses Corneal Neovascularization by Regulating Lipid Metabolism through PPARα Signaling Pathway. Front. Pharmacol. 2022, 13, 1000254. [Google Scholar] [CrossRef]
- Gerges, S.H.; Wahdan, S.A.; Elsherbiny, D.A.; El-Demerdash, E. Non-Alcoholic Fatty Liver Disease: An Overview of Risk Factors, Pathophysiological Mechanisms, Diagnostic Procedures, and Therapeutic Interventions. Life Sci. 2021, 271, 119220. [Google Scholar] [CrossRef] [PubMed]
- Kuang, J.; Wang, J.; Li, Y.; Li, M.; Zhao, M.; Ge, K.; Zheng, D.; Cheung, K.C.P.; Liao, B.; Wang, S.; et al. Hyodeoxycholic Acid Alleviates Non-Alcoholic Fatty Liver Disease through Modulating the Gut-Liver Axis. Cell Metab. 2023, 35, 1752–1766.e8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, A.; Chauhan, R.S. Computational Dissection through Network Pharmacology and Structure-Based Analysis Unravels Mechanistic Actions of Bioactive Compounds in a Hepatoprotective Herb, Picrorhiza kurroa for the Treatment of NAFLD and NASH. J. Biomol. Struct. Dyn. 2024. [Google Scholar] [CrossRef]
- Boniface, P.K.; Fabrice, F.B.; Paumo, H.K.; Katata-Seru, L.M. Protective Roles and Mechanism of Action of Plant Flavonoids against Hepatic Impairment: Recent Developments. Curr. Drug Targets 2023, 24, 332–360. [Google Scholar] [CrossRef]
- Jinadatta, P.; Rajshekarappa, S.; Sundera Raja Rao, K.; Pasura Subbaiah, S.G.; Shastri, S. In Silico, in Vitro: Antioxidant and Antihepatotoxic Activity of Gnetol from Gnetum ula Brongn. BioImpacts 2019, 9, 239–249. [Google Scholar] [CrossRef]
- Li, S.; Duan, F.; Li, S.; Lu, B. Administration of Silymarin in NAFLD/NASH: A Systematic Review and Meta-Analysis. Ann. Hepatol. 2024, 29, 101174. [Google Scholar] [CrossRef]
- Calle, R.A.; Amin, N.B.; Carvajal-Gonzalez, S.; Ross, T.T.; Bergman, A.; Aggarwal, S.; Crowley, C.; Rinaldi, A.; Mancuso, J.; Aggarwal, N.; et al. ACC Inhibitor Alone or Co-Administered with a DGAT2 Inhibitor in Patients with Non-Alcoholic Fatty Liver Disease: Two Parallel, Placebo-Controlled, Randomized Phase 2a Trials. Nat. Med. 2021, 27, 1836–1848. [Google Scholar] [CrossRef]
- Hadjihambi, A.; Konstantinou, C.; Klohs, J.; Monsorno, K.; Le Guennec, A.; Donnelly, C.; Cox, I.J.; Kusumbe, A.; Hosford, P.S.; Soffientini, U.; et al. Partial MCT1 Invalidation Protects against Diet-Induced Non-Alcoholic Fatty Liver Disease and the Associated Brain Dysfunction. J. Hepatol. 2023, 78, 180–190. [Google Scholar] [CrossRef]
- Kennedy, L.; Meadows, V.; Sybenga, A.; Demieville, J.; Chen, L.; Hargrove, L.; Ekser, B.; Dar, W.; Ceci, L.; Kundu, D.; et al. Mast Cells Promote Nonalcoholic Fatty Liver Disease Phenotypes and Microvesicular Steatosis in Mice Fed a Western Diet. Hepatology 2021, 74, 164–182. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; Lv, X.; Guo, C.; Zhu, H.; Yang, L.; Wang, Y. 2′, 3′, 5′-Tri-O-acetyl-N6-(3-hydroxyphenyl) Adenosine Alleviates Diet-Induced Hyperlipidemia by Modulating Intestinal Gene Expression Profiles and Metabolic Pathway. Life Sci. 2024, 352, 122891. [Google Scholar] [CrossRef] [PubMed]
- Manaer, T.; Sailike, J.; Sun, X.; Yeerjiang, B.; Nabi, X. Therapeutic Effects of Composite Probiotics Derived from Fermented Camel Milk on Metabolic Dysregulation and Intestinal Barrier Integrity in Type 2 Diabetes Rats. Front. Pharmacol. 2024, 15, 1520158. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shen, P.; Li, Q.; Ong, S.S.; Qian, Y.; Lu, H.; Li, M.; Xu, T. Electroacupuncture and Tongbian Decoction Ameliorate CUMS-Induced Depression and Constipation in Mice via TPH2/5-HT Pathway of the Gut-Brain Axis. Brain Res. Bull. 2025, 221, 111207. [Google Scholar] [CrossRef]
- Liu, X.; Luo, M.; Wang, Z.; Yang, S.J.; Su, M.; Wang, Y.; Wang, W.; Sun, Z.; Cai, Y.; Wu, L.; et al. Mind Shift I: Fructus Aurantii–Rhizoma Chuanxiong Synergistically Anchors Stress-Induced Depression-like Behaviours and Gastrointestinal Dysmotility Cluster by Regulating Psycho-Immune-Neuroendocrine Network. Phytomedicine 2024, 128, 155324. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-X.; Zhang, Y.-J.; Li, M.; Tian, J.-X.; Tong, X.-L. Common Pathophysiological Mechanisms and Treatment of Diabetic Gastroparesis. J. Neurogastroenterol. Motil. 2024, 30, 143–155. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Liu, N.; Wu, H.; Cong, K.; Duan, L.; Chen, T.; Zhang, J. Health Benefits of Medicinal Plant Natural Products via Microbiota-Mediated Different Gut Axes. Pharmacol. Res. 2025, 215, 107730. [Google Scholar] [CrossRef]
- Xie, P.; Ma, Z.; Yang, X.; Pan, H.; Shen, P.; Du, H.; Cao, Y.; Xiao, J.; Xiao, H.; Song, M. The Regulatory Effects and Mechanisms of Plant Food-Derived Bioactive Components on Gut Barrier Function and Intestinal Homeostasis: A Comprehensive Review. Crit. Rev. Food Sci. Nutr. 2025. [Google Scholar] [CrossRef]
- Cullen, J.M.A.; Shahzad, S.; Dhillon, J. A Systematic Review on the Effects of Exercise on Gut Microbial Diversity, Taxonomic Composition, and Microbial Metabolites: Identifying Research Gaps and Future Directions. Front. Physiol. 2023, 14, 1292673. [Google Scholar] [CrossRef] [PubMed]
- García-Gamboa, R.; Díaz-Torres, O.; Senés-Guerrero, C.; Gradilla-Hernández, M.S.; Moya, A.; Pérez-Brocal, V.; Garcia-Gonzalez, A.; González-Avila, M. Associations between Bacterial and Fungal Communities in the Human Gut Microbiota and Their Implications for Nutritional Status and Body Weight. Sci. Rep. 2024, 14, 5703. [Google Scholar] [CrossRef] [PubMed]
- Al-Adham, I.S.I.; Agha, A.S.A.A.; Al-Akayleh, F.; Al-Remawi, M.; Jaber, N.; Al Manasur, M.; Collier, P.J. Prebiotics Beyond the Gut: Omics Insights, Artificial Intelligence, and Clinical Trials in Organ-Specific Applications. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Ma, X.-L.; Wang, H.-L.; Li, R.; Cui, J.-J.; Yan, P.-J.; Wang, Y.-N.; Yu, X.-Y.; Du, P.; Yu, H.-Y.; et al. Prebiotic-like Cyclodextrin Assisted Silybin on NAFLD through Restoring Liver and Gut Homeostasis. J. Control. Release 2022, 348, 825–840. [Google Scholar] [CrossRef]
- Cao, P.; Chen, Q.; Shi, C.; Pei, M.; Wang, L.; Gong, Z. Pinocembrin Ameliorates Acute Liver Failure via Activating the Sirt1/PPARα Pathway in Vitro and in Vivo. Eur. J. Pharmacol. 2022, 915, 174610. [Google Scholar] [CrossRef]
- Kumar, S.; Alagawadi, K.R. Anti-Obesity Effects of Galangin, a Pancreatic Lipase Inhibitor in Cafeteria Diet Fed Female Rats. Pharm. Biol. 2013, 51, 607–613. [Google Scholar] [CrossRef]
- Zhou, Y.; Ding, Y.-L.; Zhang, J.-L.; Zhang, P.; Wang, J.-Q.; Li, Z.-H. Alpinetin Improved High Fat Diet-Induced Non-Alcoholic Fatty Liver Disease (NAFLD) through Improving Oxidative Stress, Inflammatory Response and Lipid Metabolism. Biomed. Pharmacother. 2018, 97, 1397–1408. [Google Scholar] [CrossRef]
- Wang, T.; He, C. Pro-Inflammatory Cytokines: The Link between Obesity and Osteoarthritis. Cytokine Growth Factor. Rev. 2018, 44, 38–50. [Google Scholar] [CrossRef]
- Bu, L.; Zhang, Z.; Chen, J.; Fan, Y.; Guo, J.; Su, Y.; Wang, H.; Zhang, X.; Wu, X.; Jiang, Q.; et al. High-Fat Diet Promotes Liver Tumorigenesis via Palmitoylation and Activation of AKT. Gut 2024, 73, 1156–1168. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Q.; Xiang, J.; Zhang, G.-Q.; Fu, L.-Y.; Xu, Y.-N.; Chen, Y.; Tao, L.; Hu, X.-X.; Shen, X.-C. Essential Oil from Fructus Alpinia zerumbet Ameliorates Atherosclerosis by Activating PPARγ-LXRα-ABCA1/G1 Signaling Pathway. Phytomedicine 2024, 123, 155227. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Mu, H.; Chen, J.; Huang, W.; Huang, G.; Deng, M.; Hong, S.; Ai, P.; Gao, C.; Zhou, H. OmicShare Tools: A Zero-code Interactive Online Platform for Biological Data Analysis and Visualization. iMeta 2024, 3, e228. [Google Scholar] [CrossRef] [PubMed]
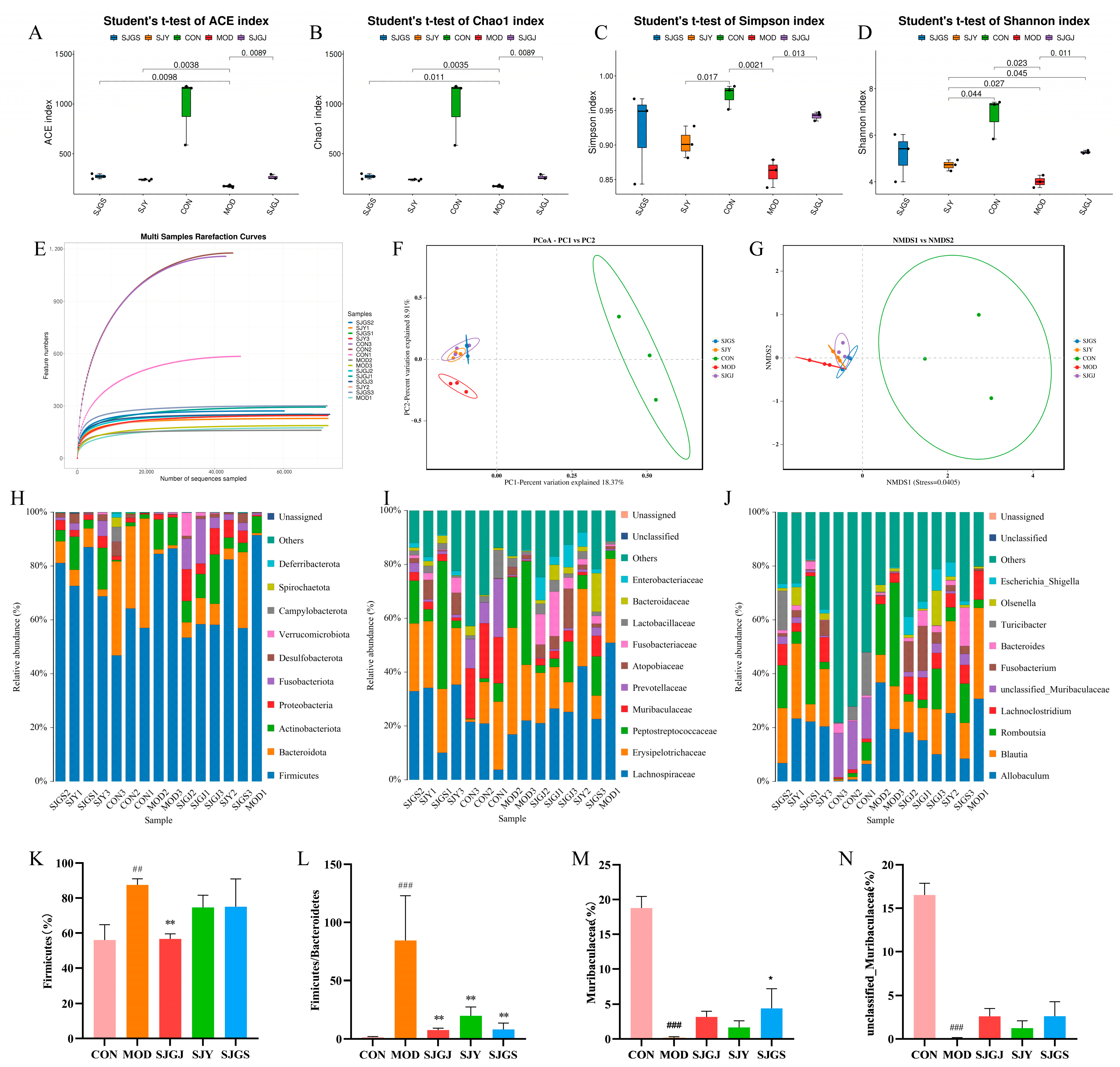
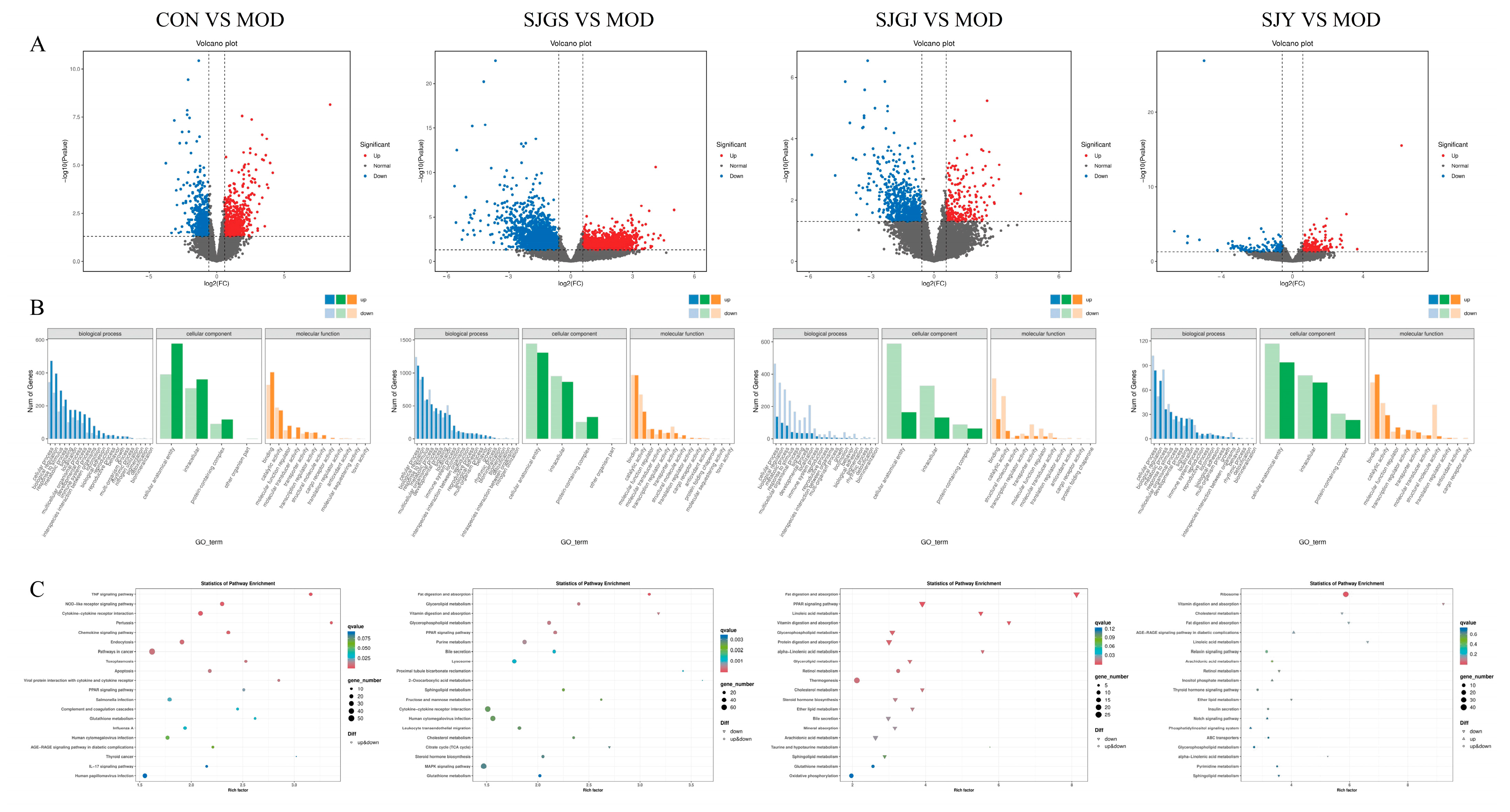

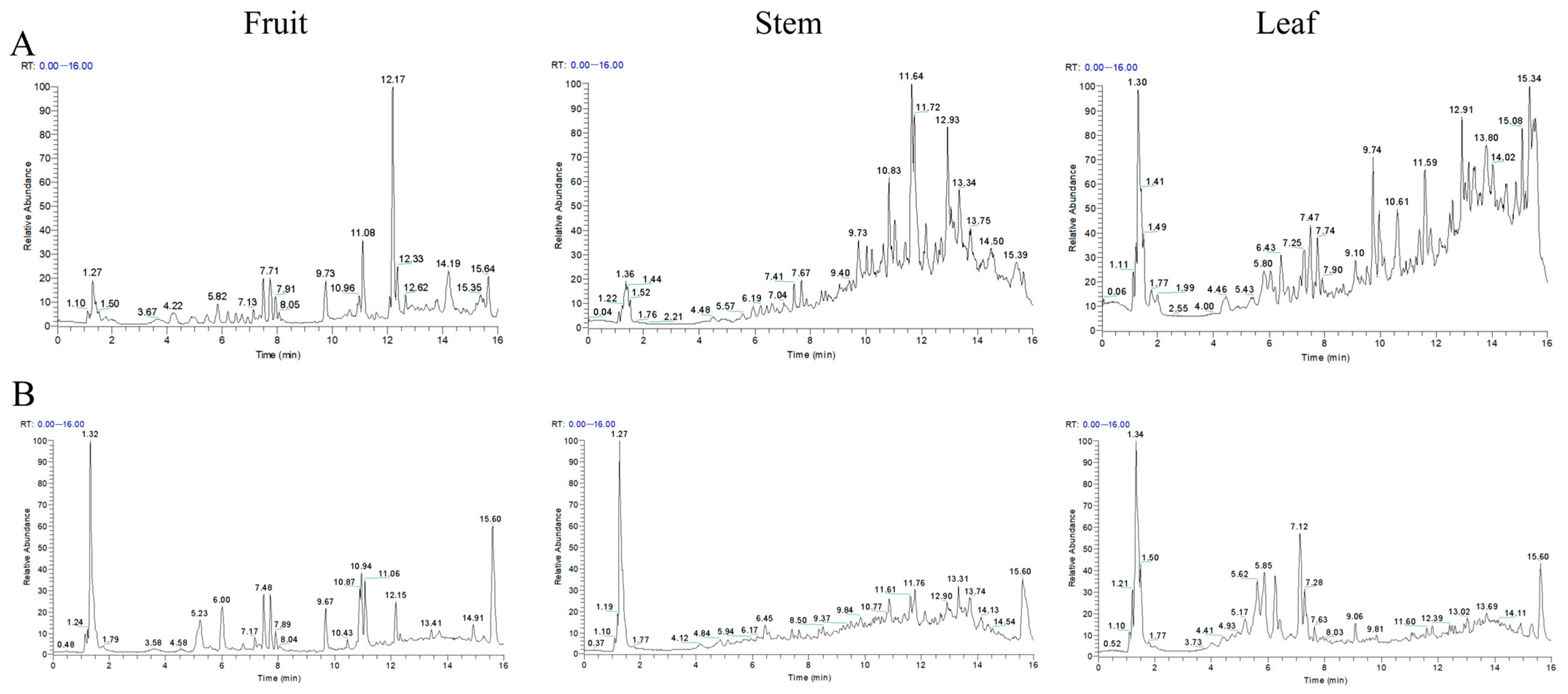
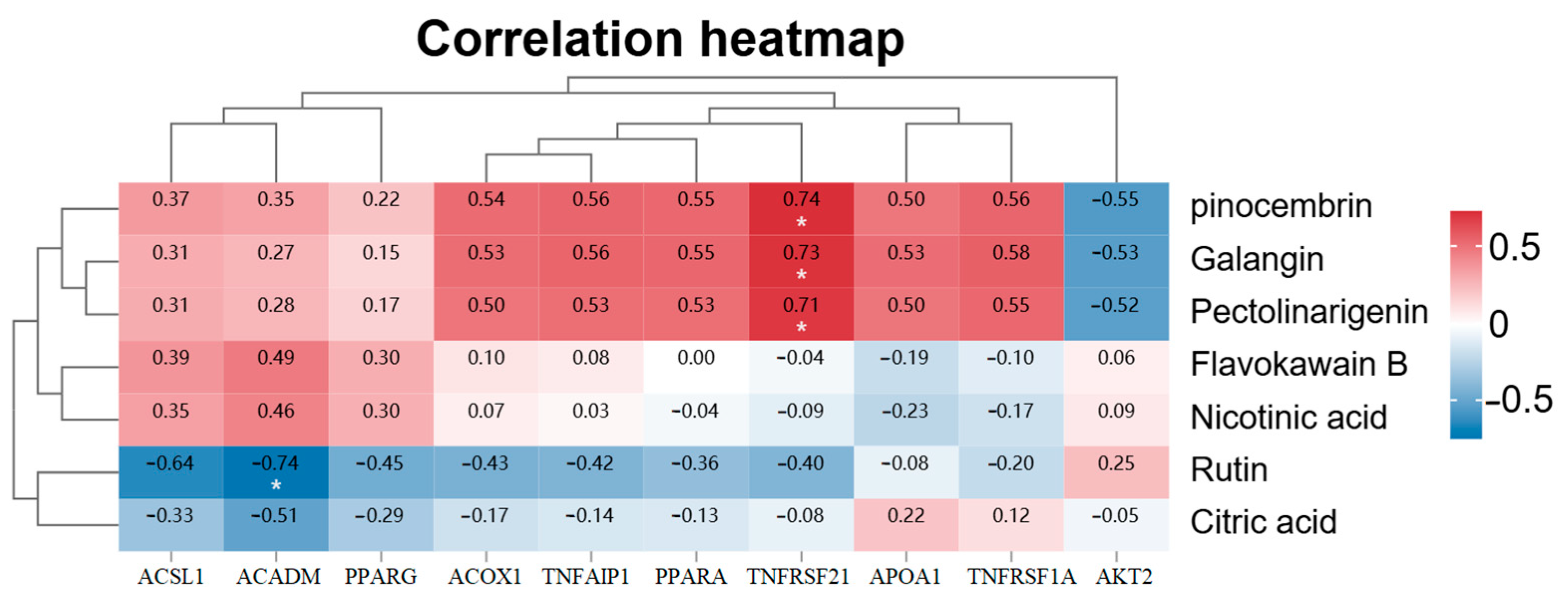
| Groups | Number of DEGs | DEGs in Lipid Metabolic Pathways | |
|---|---|---|---|
| CON vs. MOD | 1310 | ↑719 | SCD2, FAdS2, SCD1, ABCG1, ABCA2, SOCS3, LEPR, PI3K, IL-6R |
| ↓591 | FABP1, PCK1, ACADM, PPARG, FABP2, ACOX1, ACSL1, ACADL PLIN1, ADIPOQ, APOC2 | ||
| SJGJ vs. MOD | 976 | ↑237 | —— |
| ↓739 | FABP1, APOA1, AQP7, PCK1, APOC3, ACADM, ACOX1, FABP2, ACSL1, PLIN1, ADIPOQ, APOC2, GK, PCK1, ACOX3 | ||
| SJY vs. MOD | 319 | ↑176 | —— |
| ↓143 | APOA1, APOC3, CREB3l3, APOC2 | ||
| SJGS vs. MOD | 3587 | ↑1692 | LPL HMGCS2, SCD, ABCG1, SCD2, SCD1, PLTP, AKT3, LEPR, IL-6R, NFKBb1, CPT1C |
| ↓1895 | ACOX1, APOC3, APOA1, PCK1, FABP1, ACSL1, PPARA, AQP7, FABP2, ACADM, SREBF1, PFKFB4, G6PC, PCK1, HNF4A, FBP1, LIPE, RXRA | ||
| No. | Gene Symbol | Primer Sequence (5′→3′) | Length (bp) |
|---|---|---|---|
| 1 | GAPDH-F | ACGGCAAGTTCAACGGCACAG | 21 |
| 2 | GAPDH-R | GAAGACGCCAGTAGACTCCACGAC | 24 |
| 3 | ZO-1-F | AGTCCCTTACCTTTCGCCTG | 20 |
| 4 | ZO-1-R | CAGCTTGTGATACGTGCGAG | 20 |
| 5 | OCCLUDIN-F | CCACCTATCACTTCAGATCAACAG | 24 |
| 6 | OCCLUDIN-R | ATGTACTCTTCGCTCTCCTCTC | 22 |
| 7 | CLAUDIN-F | GGTTGCAGCTTCTGGGTTTC | 20 |
| 8 | CLAUDIN-R | CAGTCACGATGTTGTCCCCA | 20 |
| 9 | APOAL-F | GAGGAGTTTCGAGACCGCAT | 20 |
| 10 | APOAL-R | GTCGCTGGCCTTGGTATGAT | 20 |
| 11 | ACADM-F | TCACCGGATTCATCGTGGAG | 20 |
| 12 | ACADM-R | CGTCGGCCTGGTTCTATCAA | 20 |
| 13 | ACOX1-F | TCACTCGAAGCCAGCGTTAT | 20 |
| 14 | ACOX1-R | AGCAGTGGTGCCCTGATTC | 19 |
| 15 | ACSL1-F | CAAGGTGCTTCAGCCTACCA | 20 |
| 16 | ACSL1-R | ATCCAACAGCCATCGCTTCA | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Fang, C.; Li, H.; Lin, Y.; Que, H.; Liu, H.; Ma, L.; Lin, W. Hypolipidemic Effects of Alpinia japonica Extracts: Modulation of PPAR Signaling, Gut Microbiota, and Intestinal Barrier Function in Hyperlipidemic Rats. Pharmaceuticals 2025, 18, 1320. https://doi.org/10.3390/ph18091320
Zhou L, Fang C, Li H, Lin Y, Que H, Liu H, Ma L, Lin W. Hypolipidemic Effects of Alpinia japonica Extracts: Modulation of PPAR Signaling, Gut Microbiota, and Intestinal Barrier Function in Hyperlipidemic Rats. Pharmaceuticals. 2025; 18(9):1320. https://doi.org/10.3390/ph18091320
Chicago/Turabian StyleZhou, Liqing, Cong Fang, Hongwei Li, Yifan Lin, Huiqing Que, Hongxu Liu, Lihong Ma, and Wenjin Lin. 2025. "Hypolipidemic Effects of Alpinia japonica Extracts: Modulation of PPAR Signaling, Gut Microbiota, and Intestinal Barrier Function in Hyperlipidemic Rats" Pharmaceuticals 18, no. 9: 1320. https://doi.org/10.3390/ph18091320
APA StyleZhou, L., Fang, C., Li, H., Lin, Y., Que, H., Liu, H., Ma, L., & Lin, W. (2025). Hypolipidemic Effects of Alpinia japonica Extracts: Modulation of PPAR Signaling, Gut Microbiota, and Intestinal Barrier Function in Hyperlipidemic Rats. Pharmaceuticals, 18(9), 1320. https://doi.org/10.3390/ph18091320







