1. Introduction
Acute lung injury (ALI) and its severe form, acute respiratory distress syndrome (ARDS), represent critical respiratory emergencies frequently encountered in clinical practice and are characterized by diffuse alveolar damage, pulmonary edema, and refractory hypoxemia [
1,
2,
3]. Acute respiratory distress syndrome (ARDS) is characterized by noncardiogenic pulmonary edema. It has a high mortality rate and lacks effective pharmacotherapy [
4]. In COVID-19, cytokine release syndrome can cause severe lung tissue damage leading to acute respiratory distress syndrome (ARDS) [
5]. With the outbreak of COVID-19 worldwide, the mortality of ARDS has increased correspondingly, which makes it urgent to find effective targets and strategies for the treatment of ARDS. Recent clinical trials of Janus kinase (JAK) inhibitors in treating COVID-19-induced ARDS have shown a positive outcome, which makes the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway a potential therapeutic target for treating ARDS [
4].
ALI has a rapid onset and represents an early stage of ARDS. Without timely and effective treatment, ALI may progress to ARDS, resulting in irreversible lung injury and, potentially, death [
2]. Despite substantial advances in ventilatory support techniques and intensive care management in recent years, ALI/ARDS mortality remains alarmingly high at approximately 40%, imposing a significant epidemiological burden on global public health systems [
6,
7,
8]. ALI is pathologically characterized by the infiltration of inflammatory cells and an increase in pro-inflammatory mediators, leading to disruption of the alveolar–capillary barrier and pulmonary edema, which ultimately results in severe impairment of gas exchange [
9,
10]. Hence, anti-inflammatory interventions are critical for preventing ALI progression to ARDS. Although aberrant pulmonary inflammation underlies the pathophysiology of ALI, there are currently no targeted pharmacotherapies available. Clinical management relies on supportive care such as mechanical ventilation combined with corticosteroids. This approach has limited efficacy and significant adverse effects [
11,
12]. Therefore, there is an urgent need to identify novel, highly effective anti-inflammatory agents with reduced adverse effects.
Podocarpus is the most representative genus within the family
Podocarpaceae and is phylogenetically related to the
Taxaceae family. Traditional medicine systems in diverse regions, including China (notably Yao medicine), Africa, and New Zealand, utilize plants of
Podocarpus species, such as
P. nagi,
P. sensu latissimo (s. l.), and
P. totara, for their therapeutic properties [
13]. Phytochemical investigations in recent decades have revealed that terpenoids, flavonoids, steroids, and phenols constitute the predominant classes of compounds isolated from various
Podocarpus species, with terpenoids being the most abundant [
13,
14,
15]. Notably, norditerpene dilactones, a subclass of terpenoids, have received the greatest amount of pharmacological research attention [
15]. These phytochemicals were reported to exhibit diverse bioactivities, including anti-inflammatory, anticancer, antimicrobial, and antioxidant activities, with the anti-inflammatory activity being particularly notable [
13,
14,
15,
16,
17,
18,
19]. Evidence from cytokine-based inflammation models has indicated that
Podocarpus extracts exhibited significant anti-inflammatory activity, mediated by their inhibitory effects on various signal transduction pathways [
13]. However, previous phytochemical investigations have been limited in number and have disproportionately focused on
P. nagi and
P. macrophyllus, with many congeners receiving minimal attention.
P. nakaii is present sporadically in broadleaved (angiosperm) forests in the central mountains of Taiwan [
20]. It was rated as endangered according to the IUCN Red List criteria. Hence, phytochemical studies on this species are limited. Our preliminary screening revealed that the ethanol extract of the seeds of
P.
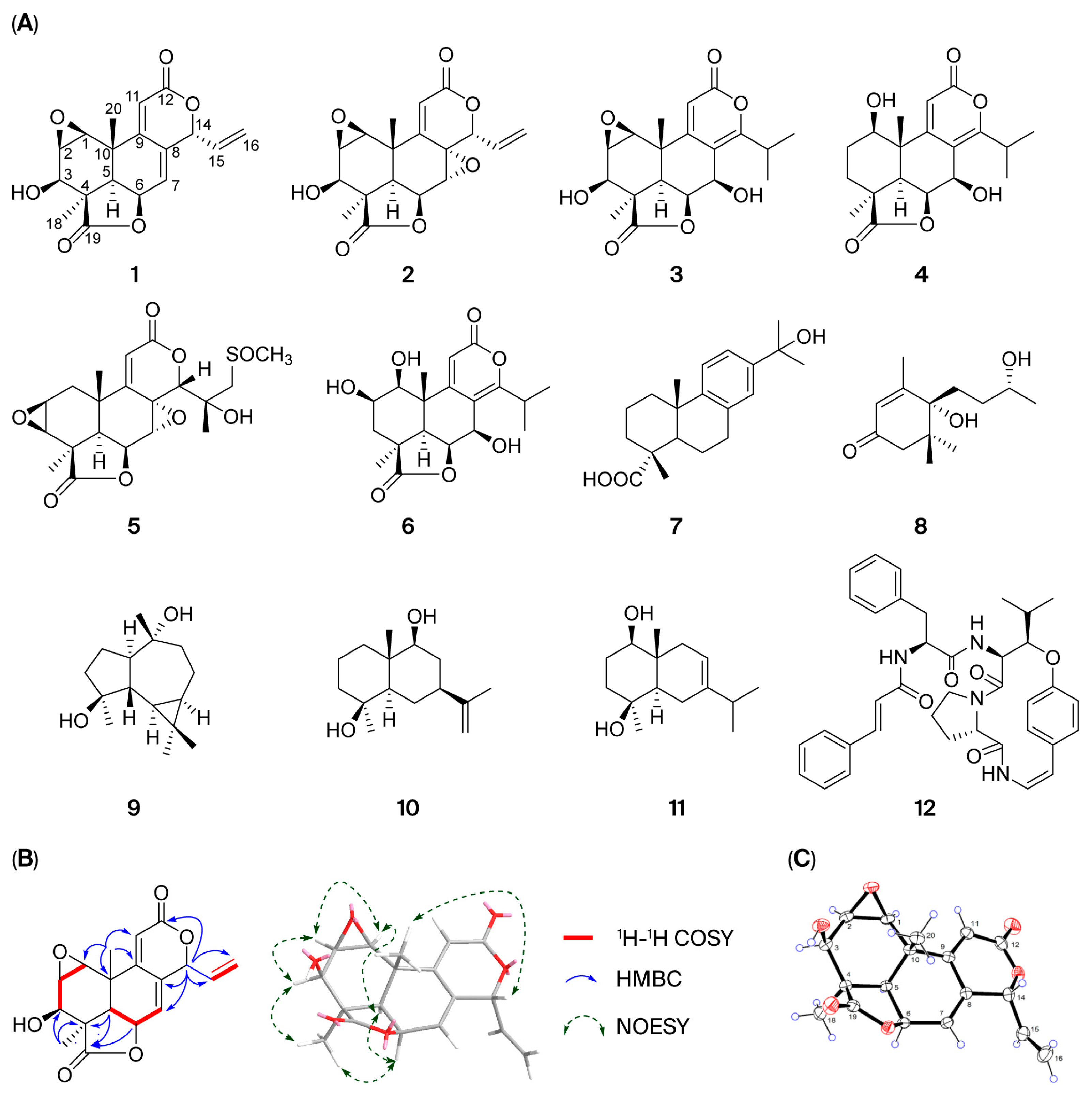
nakaii (EESPN) alleviated the symptoms of ALI in mice. In this study, EESPN was subjected to extensive chemical investigation, which led to the isolation and characterization of twelve compounds
1–
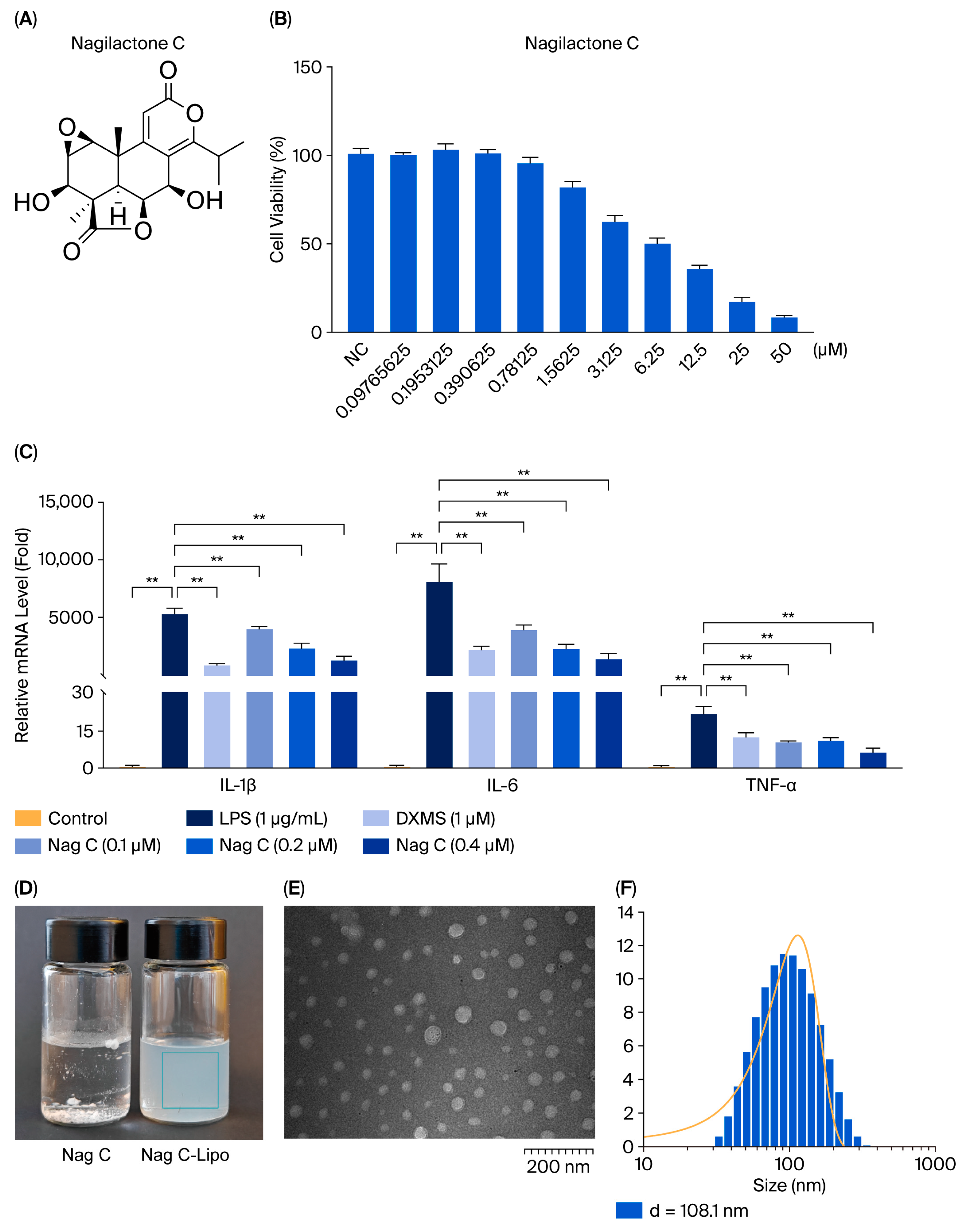
12. In the in vitro bioassay, nagilactone C (
3, Nag C) showed potent anti-inflammatory activity, which was further systematically investigated for in vivo efficacy and mechanisms of action. Simultaneously, a three-stage research strategy—”transcriptome-wide profiling → targeted pathway validation → causal cellular verification”—was implemented to identify and validate molecular targets.
3. Discussion
ALI is a life-threatening clinical syndrome caused by various etiological factors and is characterized by pathological features, including inflammatory cell infiltration, pulmonary edema, and alveolar epithelial apoptosis [
2]. ALI is pathologically characterized by the infiltration of inflammatory cells and an increase in pro-inflammatory mediators, leading to disruption of the alveolar–capillary barrier and pulmonary edema, which ultimately results in severe impairment of gas exchange [
1,
9]. Hence, modulating inflammation is pivotal for preventing ALI progression to ARDS.
Natural products serve as reliable sources of anti-inflammatory agents [
39,
40]. Numerous natural products, e.g., flavonoids, alkaloids, and polyphenols, have been reported to exert protective effects against ALI [
41]. These compounds exert therapeutic effects against ALI through multiple mechanisms, including inhibition of classical inflammatory pathways (e.g., NF-κB and MAPK signaling), suppression of neutrophil infiltration, promotion of macrophage polarization toward anti-inflammatory phenotypes, and upregulation of tight junction proteins to maintain epithelial barrier integrity [
42]. Additionally, many natural products exert cytoprotective effects by suppressing mitochondrial apoptotic pathways, scavenging oxygen free radicals, and promoting cellular damage repair, which also contributes to the management of ALI [
43]. Terpenoids are recognized as a class of highly promising natural anti-inflammatory agents because of their broad natural occurrence, structural diversity, and pronounced anti-inflammatory effects. Studies have revealed that terpenoids exert anti-inflammatory effects through multiple mechanisms, including the suppression of inflammatory mediator production (e.g., prostaglandins, leukotrienes, and cytokines), the modulation of inflammation-related signaling pathways (e.g., the NF-κB, MAPK, and JAK–STAT pathways), and the inhibition of inflammatory cell activation [
44,
45,
46]. These findings provide crucial scientific evidence for further research and development of terpenoid-based therapeutics.
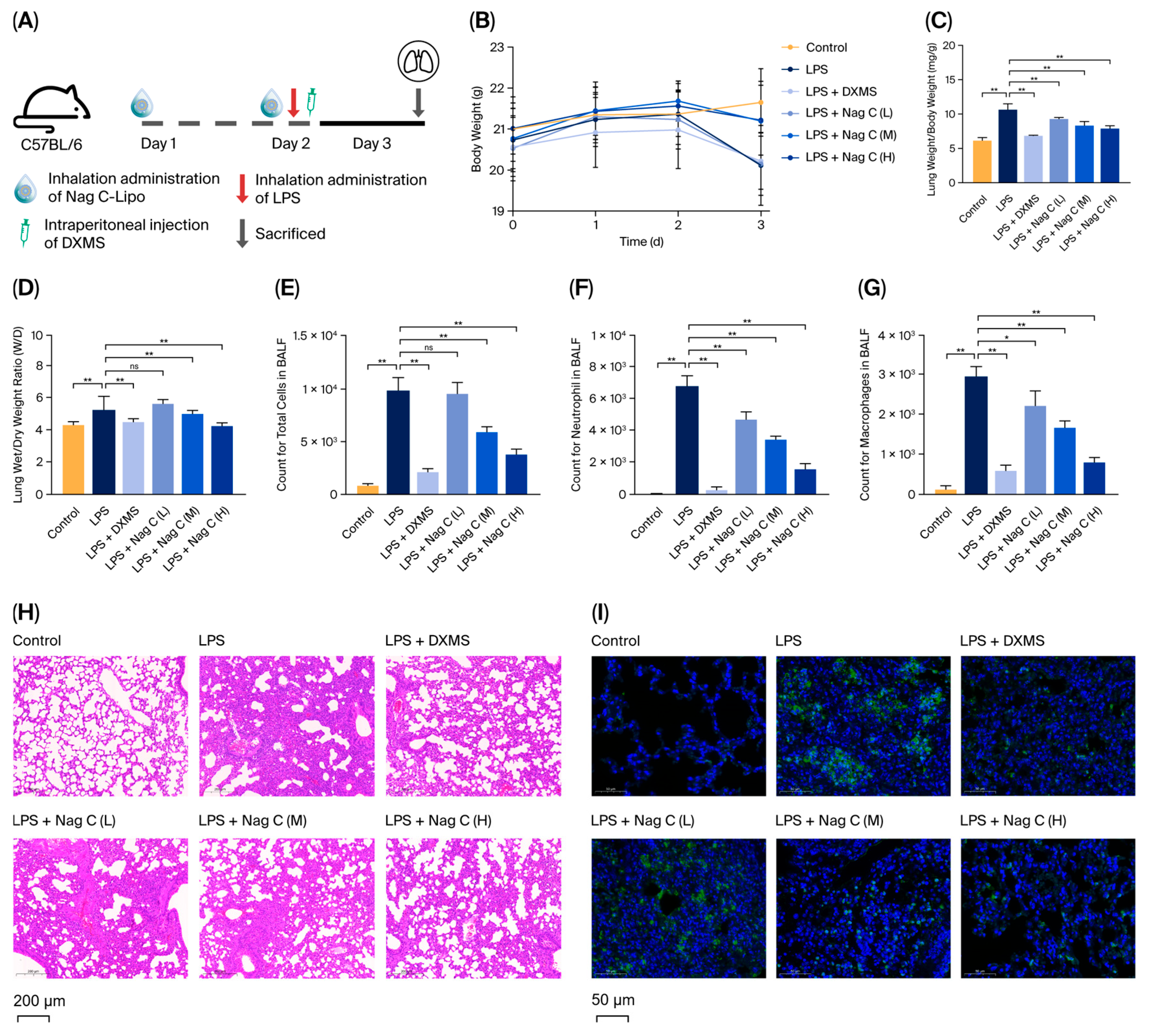
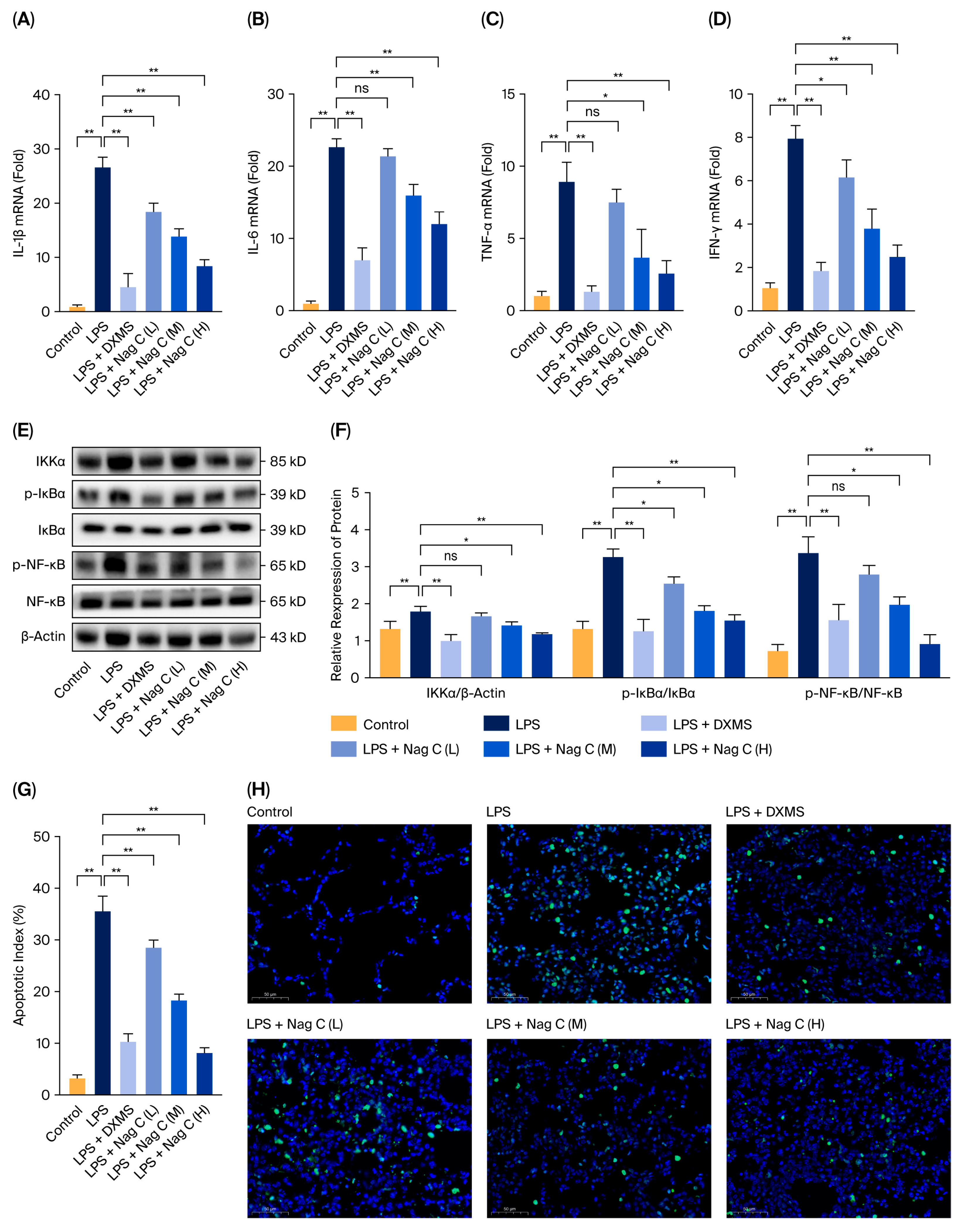
In this study, the chemical constituents of the seeds of P. nakaii, a terpenoid-rich material, were investigated, leading to the isolation and characterization of twelve compounds (1–12). Preliminary screening revealed that Nag C exhibited potent anti-inflammatory activity. The anti-inflammatory mechanisms of Nag C were systematically investigated in an LPS-induced murine ALI model. To address the poor solubility of Nag C and increase its bioavailability and therapeutic efficacy against ALI, Nag C liposomes were prepared for inhalation administration. The therapeutic potential of this inhalable formulation in ALI treatment was systematically evaluated. The results demonstrated that the Nag C liposome formulation significantly ameliorated LPS-induced histopathological damage in lung tissues, reduced neutrophil infiltration and pro-inflammatory cytokine levels, and increased the levels of key biomarkers, including myeloperoxidase (MPO) activity and apoptotic indices. Mechanistically, Nag C exerts therapeutic effects on ALI via the modulation of the canonical NF-κB pathway. The findings of this study provide experimental evidence to support the development of Nag C as a therapeutic agent.
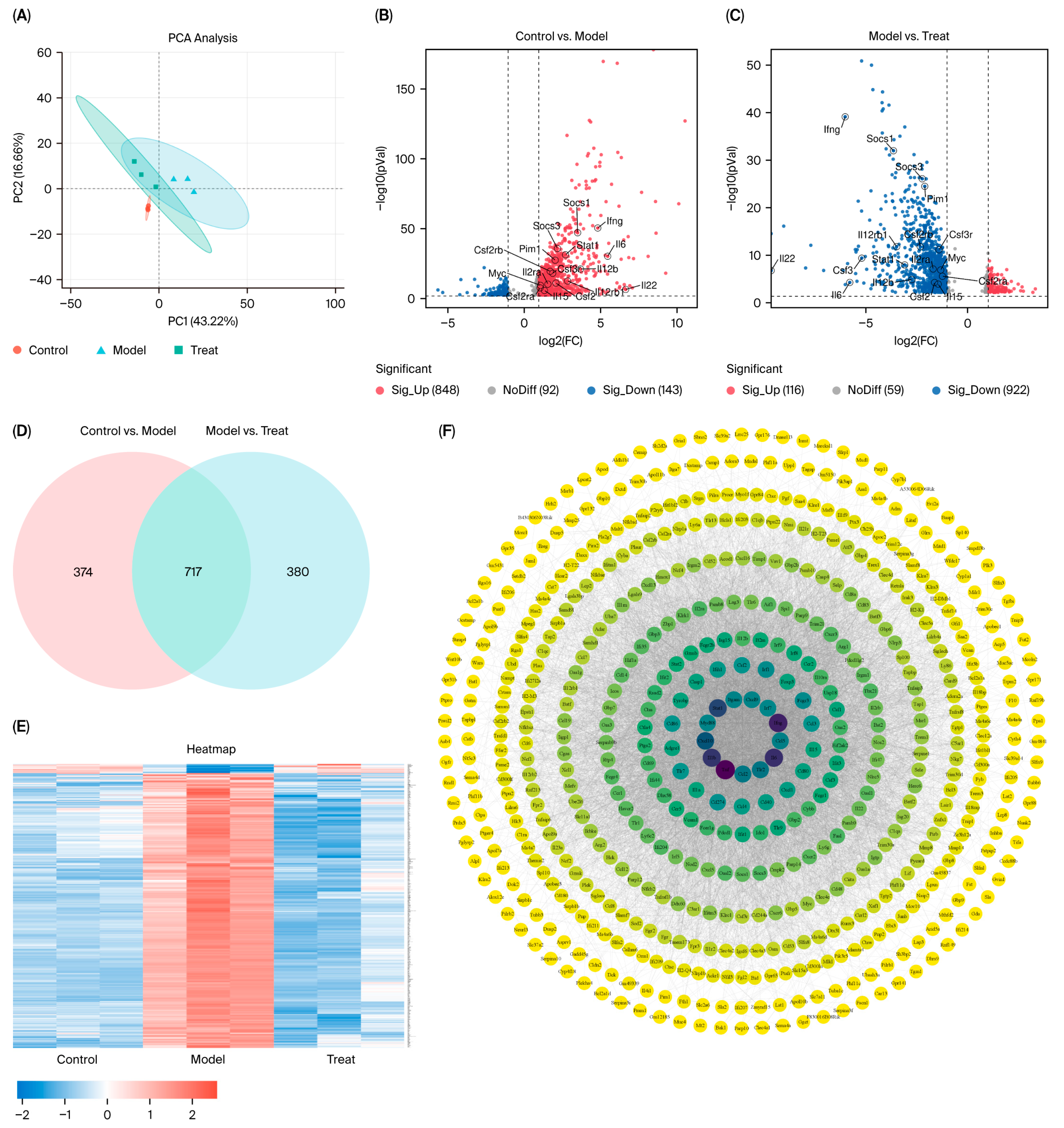
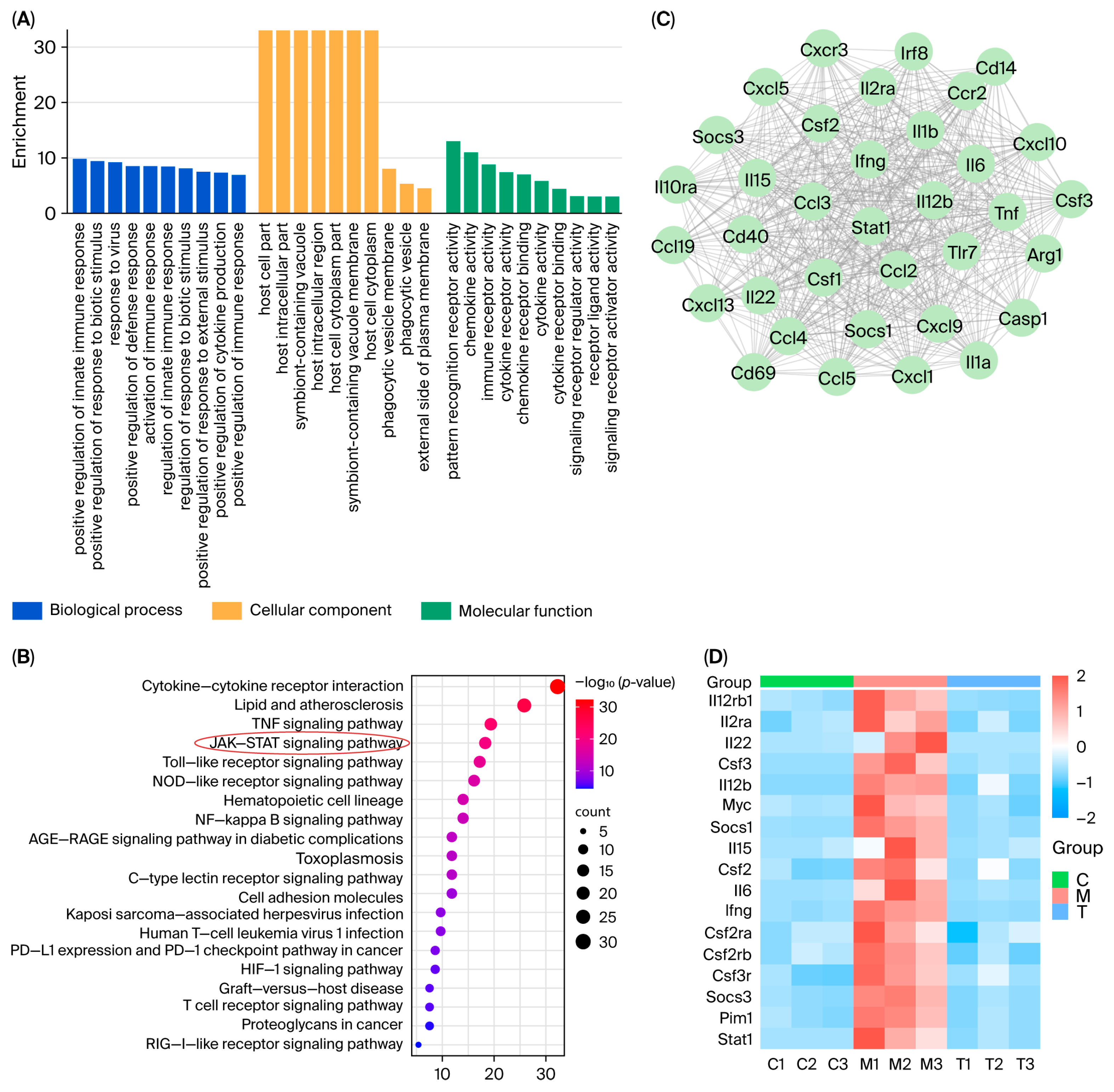
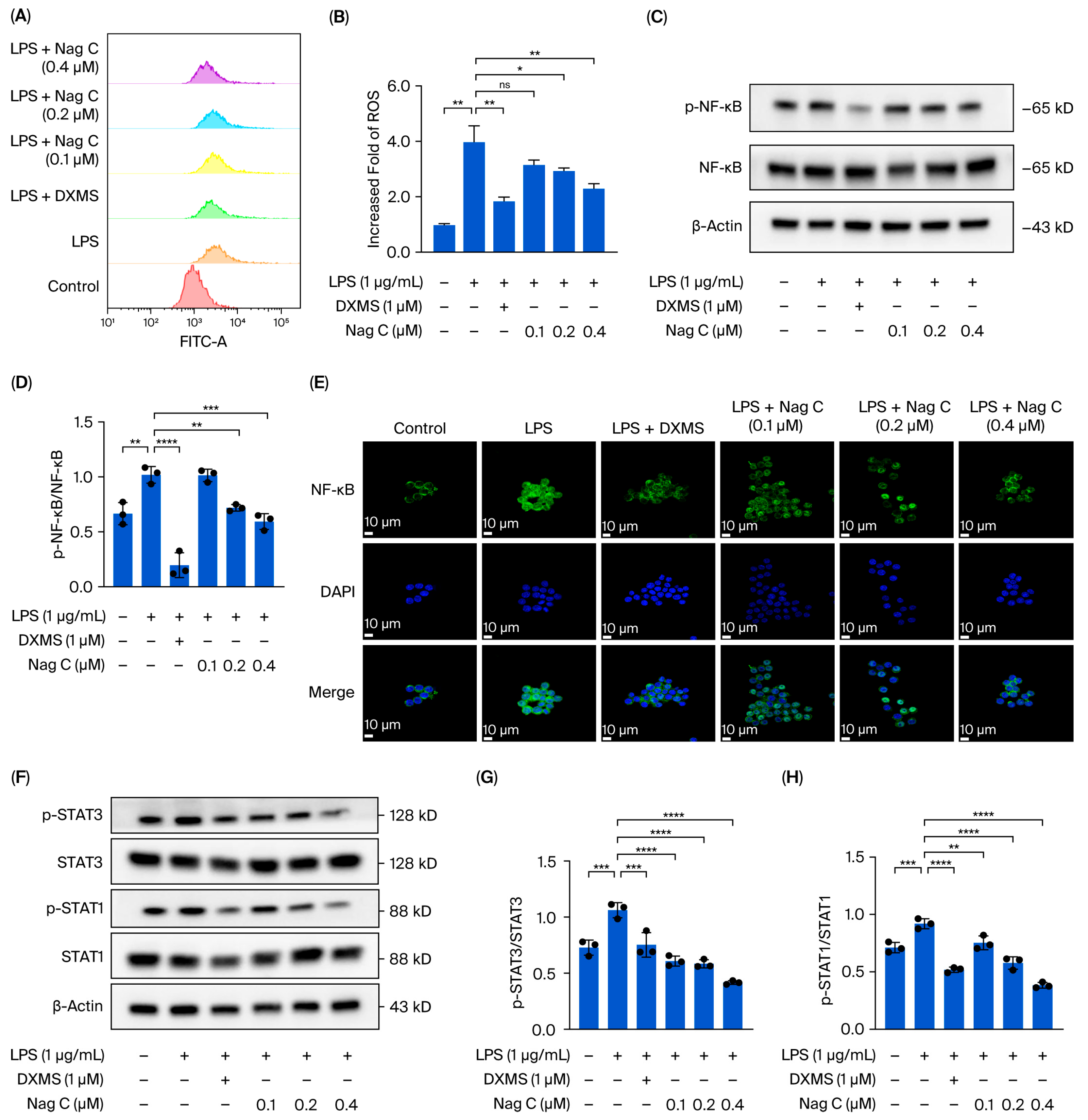
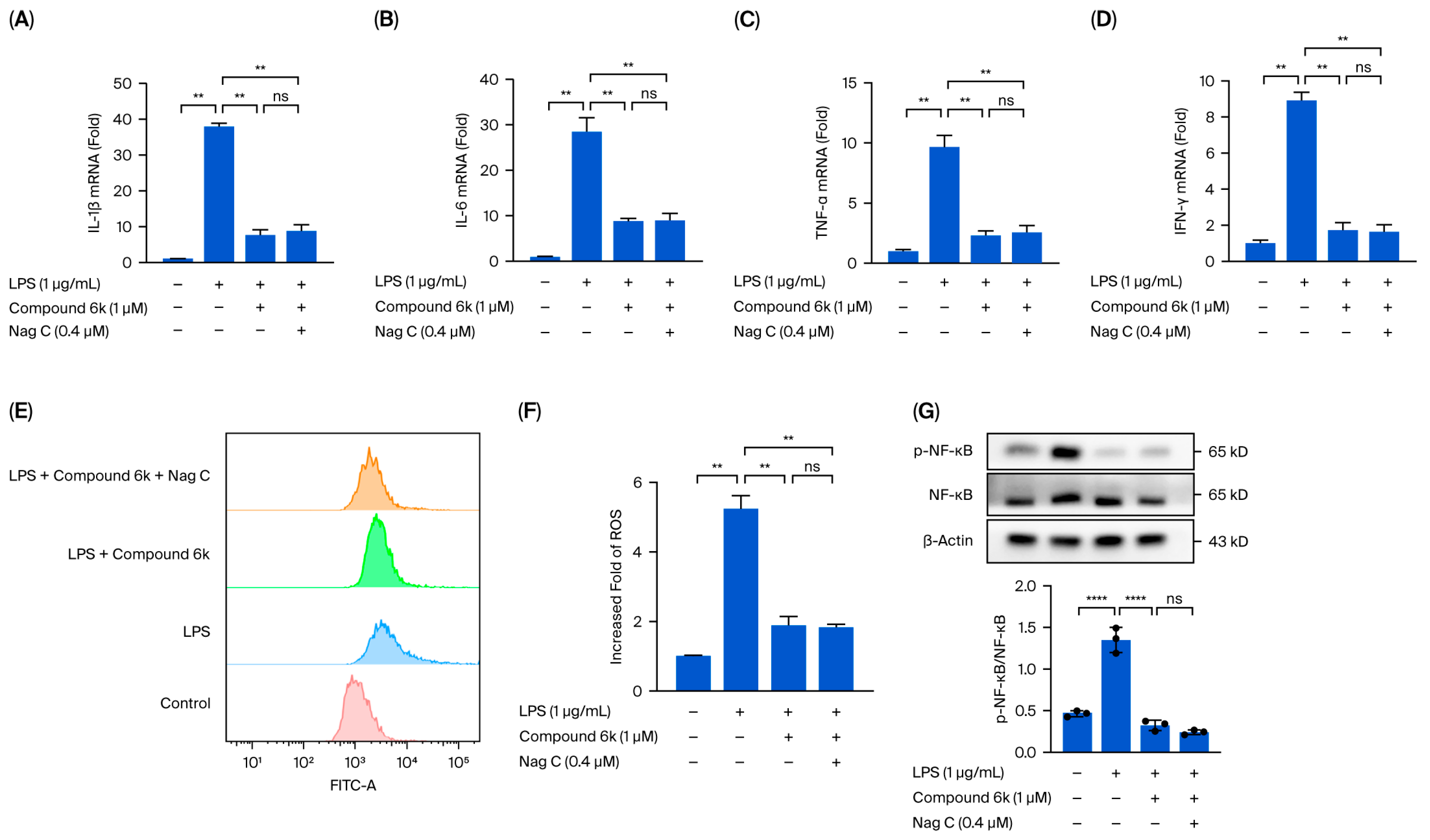
This study focused on the systematic mechanistic investigation of Nag C for the treatment of ALI. Initial transcriptomic profiling and analysis of lung tissues from Nag C-treated mice revealed the significant involvement of the JAK–STAT signaling pathway. To validate the hypothesis that Nag C exerts its anti-inflammatory effects via STAT pathway inhibition, we first examined its ability to modulate LPS-induced inflammation in RAW264.7 macrophages in relation to STAT signaling. These results demonstrated that Nag C concurrently suppressed inflammatory responses and significantly downregulated the phosphorylation levels of the NF-κB, STAT3, and STAT1 proteins, supporting its crucial involvement in the modulation of the STAT signaling pathway. Subsequent pharmacological inhibition of STAT3/STAT1 in RAW264.7 cells significantly reduced inflammatory cytokine levels, ROS levels, and NF-κB activity, confirming that targeted STAT blockade is a viable anti-inflammatory strategy. Notably, when Nag C was administered following STAT3/STAT1 inhibition, no additional suppression of these inflammatory markers was observed, providing compelling evidence that Nag C exerted its anti-inflammatory effects primarily through STAT pathway modulation. Finally, assessment of STAT pathway activation in murine lung tissues demonstrated that Nag C similarly suppressed the phosphorylation of STAT3 and STAT1 proteins in vivo, confirming that STAT pathway inhibition is the mechanistic basis for the therapeutic efficacy of Nag C against ALI.
This study revealed that Nag C is a bioactive component with a protective effect against ALI from the seeds of P. nakaii. Mechanistic experiments demonstrated that Nag C exerts its therapeutic effects by inhibiting the STAT signaling pathway. These results highlight a promising lead compound in the development of novel anti-inflammatory therapeutics targeting this pathway. Notably, this study pioneers the association between STAT3/STAT1 dual-inhibition strategies and the anti-inflammatory mechanisms of natural products. These findings not only advance the understanding of terpenoid pharmacological activities but also establish an innovative therapeutic paradigm for precise modulation of STAT signaling networks.
4. Materials and Methods
4.1. Reagents
RAW264.7 cells were obtained from Meilun Biotechnology Co., Ltd. (Dalian, China). High-glucose DMEM and trypsin were purchased from Zhejiang Senrui Biotechnology Co., Ltd. (Huzhou, China). Serum-free cell freezing medium was purchased from Wuhan San Ying Biotechnology Co., Ltd. (Wuhan, China). Phosphate-buffered saline (PBS), hematoxylin and eosin (H&E) stain, and neutral balsam were purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China). Thiazolyl blue tetrazolium bromide (MTT) was purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). TRIzol reagent was purchased from Tiangen Biotech (Beijing) Co., Ltd. (Beijing, China). An RNA reverse transcription kit was purchased from Takara Bio, Inc. (Kusatsu, Shiga, Japan). Quantitative real-time PCR (qPCR) reagents were purchased from Yeasen Biotechnology (Shanghai) Co., Ltd. (Shanghai, China). A TUNEL assay kit was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The protease inhibitor, phosphatase inhibitor, loading buffer, bicinchoninic acid (BCA) protein assay kit, bovine serum albumin (BSA), and enhanced chemiluminescence (ECL) detection reagents were purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China). DAB chromogenic solution was purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). The protein molecular weight marker was purchased from Absin Bioscience, Inc. (Shanghai, China). The 12% precast polyacrylamide gels and antibody dilution buffer were purchased from Yeasen Biotechnology Co., Ltd. (Shanghai, China). The polyvinylidene fluoride (PVDF) membrane was purchased from Merck KGaA (Darmstadt, Germany). Antibodies against NF-κB (8242S), p-NF-κB (3039S), IκBα (4812S), p-IκBα (2859S), STAT3 (4113T), p-STAT3 (9139T), and β-actin (4967S) were obtained from Cell Signaling Technology Co., Ltd. (Waltham, MA, USA). Anti-STAT1 (ab31369), anti-p-STAT1 (ab109461), and antimyeloperoxidase (MPO) (ab90810) primary antibodies were purchased from Abcam (Waltham, MA, USA). Horseradish peroxidase (HRP)-conjugated goat anti-rabbit secondary antibody, Alexa Fluor 488-conjugated goat anti-rabbit secondary antibody, and Alexa Fluor 647-conjugated goat anti-mouse IgG (H + L) secondary antibody were purchased from Shanghai Beyotime Biotechnology Co., Ltd. (Shanghai, China).
All the solvents used for chromatography were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). TLC plates (GF254) and silica gel (200–300 mesh) were purchased from Qingdao Haiyang Chemical Factory (Qingdao, Shandong, China). MCI CHP20P resin (75–150 μm) was purchased from Mitsubishi Chemical Corporation (Tokyo, Japan). The ODS-C18 gel (50 μm) was purchased from YMC Co., Ltd. (Kyoto, Japan). Chromatography gels (HW40 and HW60) were purchased from TOSOH Corporation (Tokyo, Japan).
4.2. Plant Material
The plant material was collected from Wuxi, Jiangsu Province, China, in October 2017. They were identified as seeds of P. nakaii by Prof. Yong-Hong Zhang from Fujian Medical University, China. A sample (No. P20171008) was deposited at Zhejiang University of Technology.
4.3. Cells
RAW264.7 cells (mouse monocyte/macrophage leukemia cells) were obtained from Meilun Biotechnology Co., Ltd. (Dalian, China).
4.4. Animals
Eight-week-old male C57BL/6 mice were obtained from the Experimental Animal Center of Hangzhou Medical College (Hangzhou, Zhejiang, China) and maintained in specific pathogen-free (SPF) facilities. The housing conditions included a temperature range of 20–26 °C, a relative humidity of 40–70%, and a 12 h light/dark cycle. All animal experiments and procedures were conducted in compliance with the international standards of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accreditation program and were reviewed and approved by the Animal Ethics Committee of Hangzhou Medical College (Approval No. 2021–029).
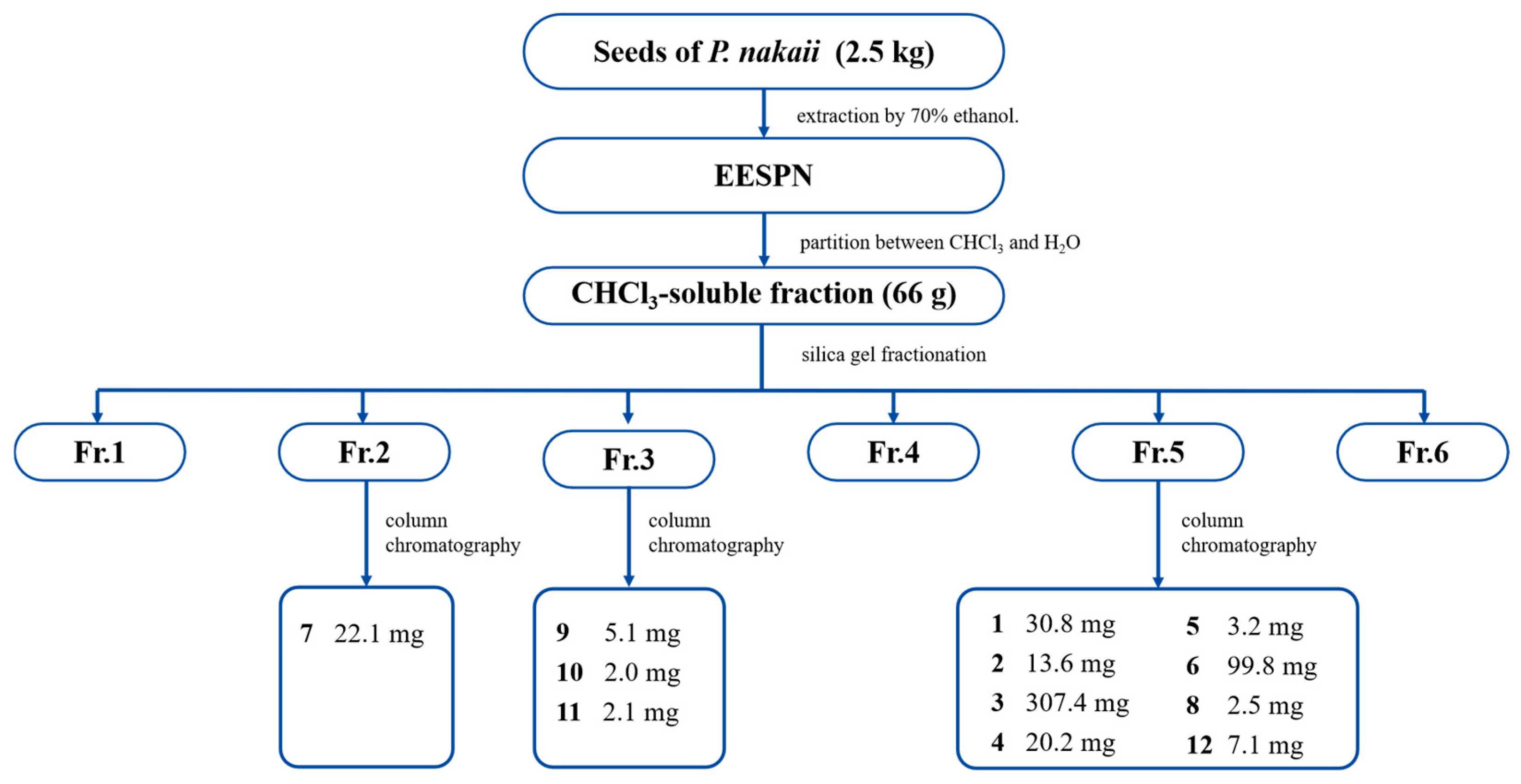
4.5. Extraction and Isolation of Compounds 1–12
Dry seeds of P. nakaii (2.5 kg) were ground into a coarse powder and extracted by reflux with 70% ethanol (3 times, 2 h each). The ethanol extract (316 g) was then partitioned between chloroform and water. The resulting chloroform-soluble residue (66 g) was subjected to silica gel column chromatography (CC) (CHCl3/MeOH 20:1 → 0:1, v/v) to obtain six fractions, Fr.1–Fr.6. Fr. 2 (1.1 g) was purified by MCI gel CC (MeOH/H2O 20:80 → 90:10, v/v) to yield three subfractions, 2A–2C. Fr. 2A was purified by silica gel CC (petroleum ether/acetone 8:1 → 3:1, v/v) to afford 7 (22.1 mg). Fr. 3 (2.3 g) was fractionated into five fractions, 3A–3E, by CC on MCI gel (MeOH/H2O 20:80 → 90:10, v/v). Fr. 3A was purified by silica gel CC (petroleum ether/acetone 8:1 → 5:1, v/v) to yield 9 (5.1 mg). Fr. 3C was purified using an LH-20 gel (MeOH) to afford 10 (2.0 mg). Fr. 3D was purified by ODS C18 CC (MeOH/H2O 50:50 → 70:10, v/v) to produce 11 (2.1 mg). Fr. 5 (5.8 g) was first divided into eight fractions, 5A–5F, by MCI gel CC (MeOH/H2O 50:50 → 90:10, v/v). Compound 3 (307.4 mg) was obtained by crystallization from Fr. 5A. Compound 8 (2.5 mg) was obtained from Fr. 5B by silica gel CC (CHCl3/MeOH 20:1, v/v). Fr. 5D was purified by silica gel CC (CHCl3/MeOH 20:1 → 15:1, v/v) to yield 4 (20.2 mg) and 5 (3.2 mg). Fr. 5E was separated by silica gel CC (CHCl3/MeOH (80:1 → 60:1, v/v)) to afford 1 (30.8 mg) and 2 (13.6 mg). Compound 6 (99.8 mg) was obtained from Fr. 5F by silica gel CC (CHCl3/MeOH (60:1 → 20:1, v/v)). Fr. 5H was separated by CC on silica gel (petroleum ether/acetone (8:1 → 3:1, v/v) to give 12 (7.1 mg). The structures of compound 1 were confirmed via spectroscopic methods (1H-NMR, 13C-NMR, DEPT, HMBC, 1H-1H-COSY, NOESY, HSQC, HRESIMS, and single-crystal X-ray diffraction (Cu-Kα)). Compounds 2–12 were identified by comparison of the 1H- and 13C-NMR data with those reported in the literature.
4.6. In Vitro Anti-Inflammatory Activity Evaluation of Compounds 1–12
The effects of compounds 1–12 on the viability of RAW264.7 cells were initially assessed via the MTT method. RAW264.7 cells in the logarithmic growth phase were selected, trypsinized with 0.25% trypsin, and prepared as single-cell suspensions. The suspension was evenly seeded into a 96-well plate (100 μL per well) and incubated at 37 °C in a humidified atmosphere with 5% CO2 for 12 h to allow partial adhesion. Following incubation, the original culture medium was removed and replaced with experimental medium containing compounds 1–12 at concentrations ranging from 0.097 to 50 μM. The final concentration of dimethyl sulfoxide (DMSO) used was 0.1‰ (v/v) in all the treatment groups. A control group containing only 0.1‰ DMSO was included. Each experimental condition was assayed in quintuplicate to minimize experimental error. The absorbance (OD value) of each well was measured at a wavelength of 490 nm using a microplate reader after 48 h, and the IC50 was calculated.
For the anti-inflammatory assay, RAW264.7 cells were allocated into six groups, namely, the control group (DMSO, 1‰), the LPS-induced model group, the dexamethasone (DXMS)-positive control (LPS + 50 μg/mL DXMS), and the treatment groups (high-dose, medium-dose, and low-dose). All groups except the control group were stimulated with LPS at a final concentration of 1 μg/mL. After 2 h of LPS induction, the treatment groups were exposed to compounds 1–12. The positive control received DXMS at a final concentration of 50 μg/mL. Following 12 h of treatment, the cells from all the groups were harvested for subsequent analyses. Total RNA was extracted from cells using TRIzol reagent and subsequently reverse-transcribed into cDNA using a reverse transcription kit. The cDNA was then subjected to RT–qPCR analysis using a fluorescent quantitative PCR reagent. The expression levels of the inflammatory factors IL-1β, IL-6, and TNF-α were determined; GAPDH served as the internal reference gene.
4.7. Preparation of Nag C Liposomes
Initially, 4 mg of Nag C, 40 mg of soybean phospholipid (drug-to-lipid ratio = 1:10), and 8 mg of cholesterol (phospholipid-to-cholesterol ratio = 5:1) were weighed and dissolved in 2 mL of anhydrous ethanol, followed by sonication until a clear solution was obtained. The solution was then slowly and uniformly dripped into 10 mL of phosphate-buffered saline (PBS) under magnetic stirring at 45 °C. Finally, the ethanol was evaporated to obtain Nag C liposomes. The physicochemical properties of Nag C liposomes were characterized. The average diameter was determined by transmission electron microscopy (TEM), whereas the zeta potential and encapsulation efficiency were quantified using dynamic light scattering (DLS) and an ultrafiltration/HPLC method, respectively. Then encapsulation efficiency was analyzed on the prepared Nag C liposomes formulation following one week of storage at 4 °C under controlled conditions.
4.8. Animals and Experimental Protocol
Forty-eight male C57BL/6 mice (8 weeks old) were randomly divided into six groups (n = 8 per group): the control group (PBS only), the LPS model group (LPS alone), the DXMS group (LPS + DXMS (10 mg/kg)), the Nag C low-dose (L) group (LPS + Nag C (0.25 mg/kg)), the Nag C medium-dose (M) group (LPS + Nag C (0.5 mg/kg)), and the Nag C high-dose (H) group (LPS + Nag C (1 mg/kg)). After a 2-day acclimatization period, the mice in the Nag C treatment groups received the corresponding doses via intranasal inhalation (50 μL per administration, twice daily). Twenty-four hours post-treatment, all groups except the control group were anesthetized with 1% sodium pentobarbital (0.1 mL per mouse) and subsequently administered 50 μL of LPS (20 μg per mouse) via intranasal instillation. Four hours after LPS induction, the DXMS group received an intraperitoneal injection of dexamethasone (10 mg/kg), while the Nag C treatment groups were administered their respective doses via intranasal inhalation. The control group received PBS only through intranasal instillation. Twenty-four hours after LPS induction, the mice were euthanized, and samples were collected for further analysis.
4.9. Body Weight and Lung Index Measurements
Body weights were recorded daily throughout this study to monitor changes. At the experimental endpoint, eight mice per group were randomly selected using a random number table. The selected animals were anesthetized with 1% sodium pentobarbital and euthanized via orbital exsanguination, followed by necropsy. Harvested lung tissues were immediately processed as follows: they were immersed in ice-cold normal saline (4 °C), rinsed three times to remove residual blood, and gently blotted on filter paper to remove surface moisture. Wet weights were recorded, and the organ coefficient (lung weight/body weight ratio) was calculated to evaluate pulmonary edema.
4.10. Collection and Processing of BALF
Three mice per group, selected randomly, were deeply anesthetized via intraperitoneal injection of 1% sodium pentobarbital. Following tracheal exposure through dissection, an intravenous indwelling needle was used for intubation. Lung lavage was performed by instilling and withdrawing 1 mL of ice-cold sterile saline (0.9% NaCl) three times. The collected BALF was immediately centrifuged (1500× g, 10 min, 4 °C). The supernatants were aliquoted into sterile microcentrifuge tubes and stored at −80 °C for subsequent analysis. The resulting cell pellet was resuspended in phosphate-buffered saline (PBS), and total cells, macrophages, and neutrophils were enumerated using a hemocytometer.
4.11. Real-Time Quantitative PCR
The PCR methodology is detailed in the referenced literature [
47] and can be briefly described as follows. The tissue samples (20 mg) were weighed into prechilled nuclease-free microcentrifuge tubes and immediately homogenized in 1 mL of TRIzol reagent (Waltham, MA, USA) on ice until complete lysis was achieved. High-purity total RNA was isolated following the manufacturer’s protocol, including chloroform phase separation, isopropanol precipitation, and ethanol washing steps. The mRNA expression levels of pro-inflammatory cytokines (IL-1β, IL-6, and TNF-α) in the lung tissues were quantified via RT–qPCR as described in
Section 4.6.
4.12. Western Blotting
Mouse lung tissues and RAW264.7 cells were placed in microcentrifuge tubes and homogenized in RIPA lysis buffer supplemented with protease and phosphatase inhibitors. The tissues were thoroughly disrupted on ice using a mechanical homogenizer, followed by a 20 min incubation on ice with intermittent vortexing every 5 min to ensure complete lysis. The lysates were subsequently centrifuged at 12,000×
g for 15 min at 4 °C in a precooled centrifuge. The resulting supernatant was collected as the total protein extract. Subsequent procedures, including protein quantification, denaturation, SDS–PAGE, membrane transfer, antibody incubation, and chemiluminescent detection, were performed as previously described [
47].
4.13. Histological and Imaging Tests of the Lungs
The lung tissue samples were collected from sacrificed mice, fixed with 4% paraformaldehyde for 24 h, embedded in paraffin, and sectioned at a thickness of 5 μm. The inflammatory status of the lung tissues was subsequently evaluated via HE staining, and MPO levels and apoptosis (TUNEL assay) were subsequently assessed via immunofluorescence analysis. The expression levels of Dectin-1 and Syk in lung tissues were determined using immunohistochemical analysis.
4.14. RNA Sequencing
Following a one-week acclimatization period, C57BL/6 mice were allocated into groups according to the experimental design detailed in
Section 4.8. Upon completion of the experiment, lung tissues were collected from the mice in the control group, model control group (LPS-induced), and high-dose treatment group (
n = 3 per group). The collected tissues were immediately snap-frozen in liquid nitrogen and subsequently stored at −80 °C. All samples were then transported under continuous cold chain conditions to the biobank for subsequent analysis. Initially, quality control was performed on the raw sequencing expression data obtained from mouse lung tissue samples. Principal component analysis (PCA) and differential transcriptome expression analysis were subsequently conducted. This analysis identified sets of DEGs for comparisons between the control and model groups and between the model and treatment groups. Finally, a Venn diagram was constructed to visualize the intersecting relationships among the DEG sets derived from these intergroup comparisons. Gene ontology (GO) enrichment analysis was subsequently conducted on the identified sets of DEGs. KEGG enrichment analysis was subsequently performed on the DEGs in the top-ranked (most significantly enriched) GO terms/pathways identified in the prior analysis. This comprehensive approach serves to investigate the functional roles and interactions of genes within metabolic pathways, signal transduction cascades, and disease-associated pathways, with the goal of identifying therapeutically relevant targets within these pathways. All target genes derived from the enrichment analyses were imported into the STRING database to construct a PPI network. The core targets within the network were subsequently predicted and visualized on the basis of their PPIs.
4.15. Flow Cytometry
The dichlorodihydrofluorescein diacetate (DCFH-DA) probe was diluted to a working concentration of 10 μM in serum-free medium, and all procedures were performed under light-protected conditions. Following aspiration of the culture media, the RAW264.7 macrophages in the 6-well plates were detached using gentle pipetting and centrifuged at 1000× g (4 °C, 5 min), and the resulting pellet was resuspended in 10 μM DCFH-DA working solution prepared in serum-free medium. The cells were incubated at 37 °C under 5% CO2 with light protection for 20 min and subjected to gentle agitation at 5 min intervals, followed by three washes with ice-cold PBS to remove insufficiently internalized probes after incubation. The cells were analyzed using the FITC channel on a flow cytometer, where the voltage and gain parameters were optimized to position the fluorescence signals within the linear detection range. The cellular fluorescence distribution was recorded. The flow cytometry data were analyzed using FlowJo software 10.8.1 to quantify the intracellular ROS levels.
4.16. Immunofluorescence
Adherent cells seeded in 6-well glass-bottom plates were centrifuged at 1000× g for 10 min. After media removal, the samples were dried at 30 °C for 2 h. The cells were then fixed with freshly prepared 4% paraformaldehyde for 15 min at room temperature (RT), followed by three 5 min washes with 0.1 M PBS buffer (pH 7.4). Permeabilization was performed with 0.5% Triton X-100 (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China) for 15 min. Nonspecific binding was blocked with 10% goat serum for 1 h at RT prior to overnight incubation at 4 °C with a primary antibody against NF-κB p65 (1:200 dilution). After three 10 min PBST washes (with PBS containing 0.05% Tween-20), the samples were incubated with an Alexa Fluor 488-conjugated secondary antibody (1:500) for 1 h under light-protected conditions. The nuclei were counterstained with DAPI (1 μg/mL) for 5 min in darkness. Finally, the coverslips were mounted with ProLong Gold antifade reagent and imaged by confocal laser scanning microscopy.
4.17. Statistical Analysis
All data were obtained from at least three independent experiments and expressed as the mean ± standard deviation (mean ± SD). Statistical analysis was performed using SPSS 23.0. One-way analysis of variance (ANOVA) was used to assess intergroup differences, with a threshold of p < 0.05 considered statistically significant. Figures were generated using GraphPad Prism 10.0.