Structure–Function Insights into Quinuclidine-3-One BisQACs: Synthesis, Modulation of Bacterial Resistance, Structure–Activity Relationship, and Biological Profiling
Abstract
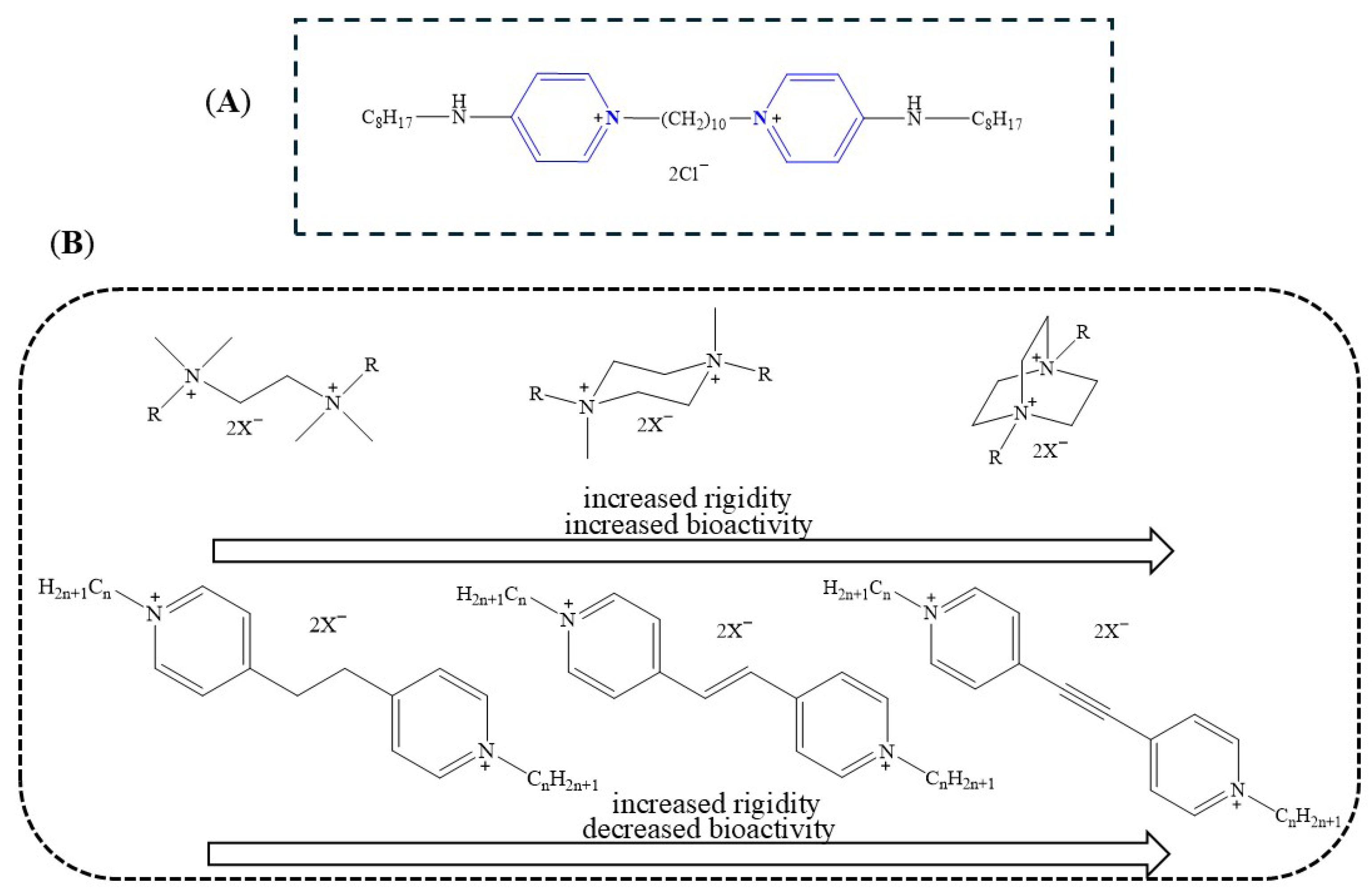
1. Introduction
2. Results and Discussion
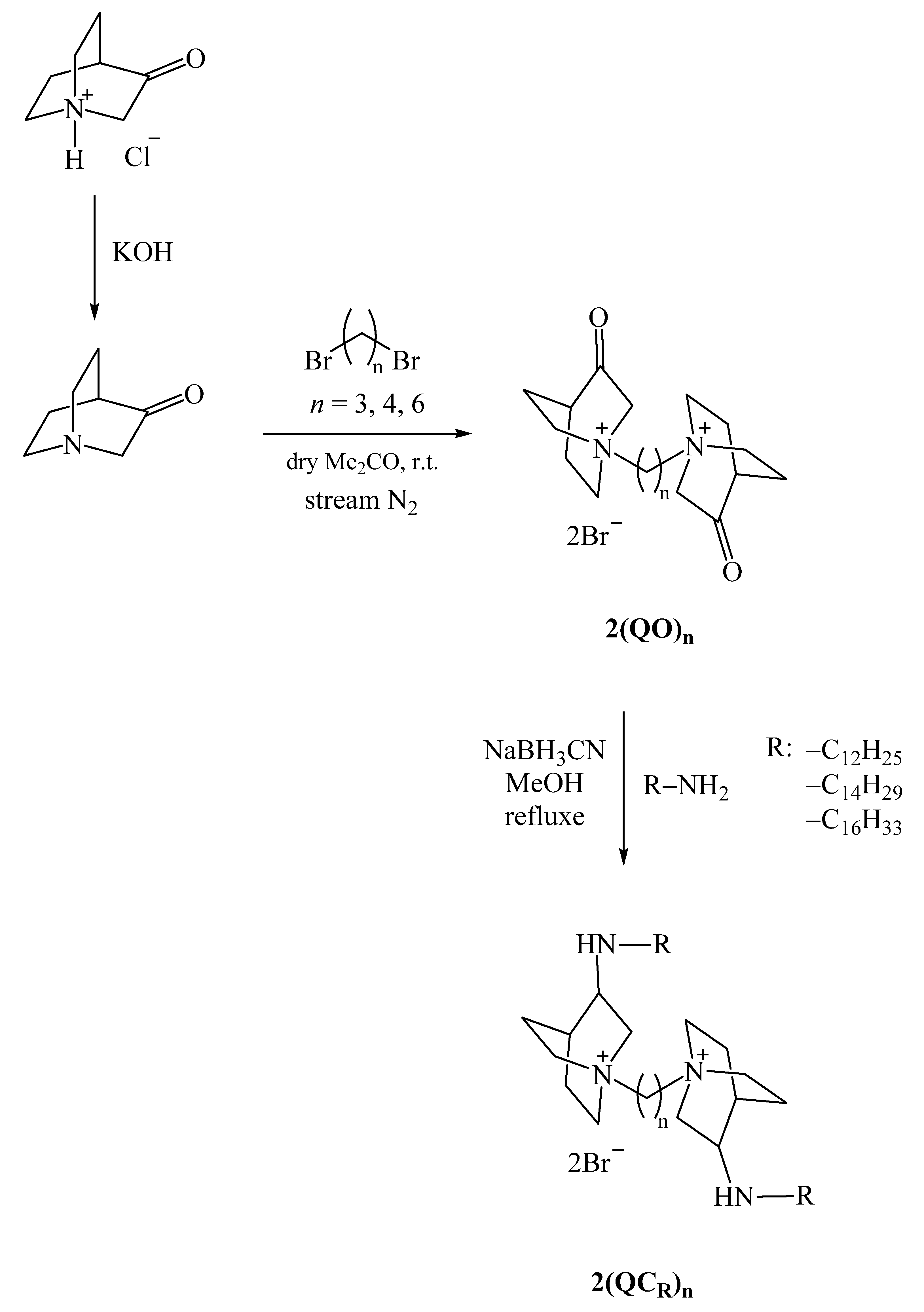
2.1. Synthesis of BisQAC
2.2. Antibacterial Evaluation (MICs)
2.3. Time-Resolved Analysis of Bacterial Growth Kinetics
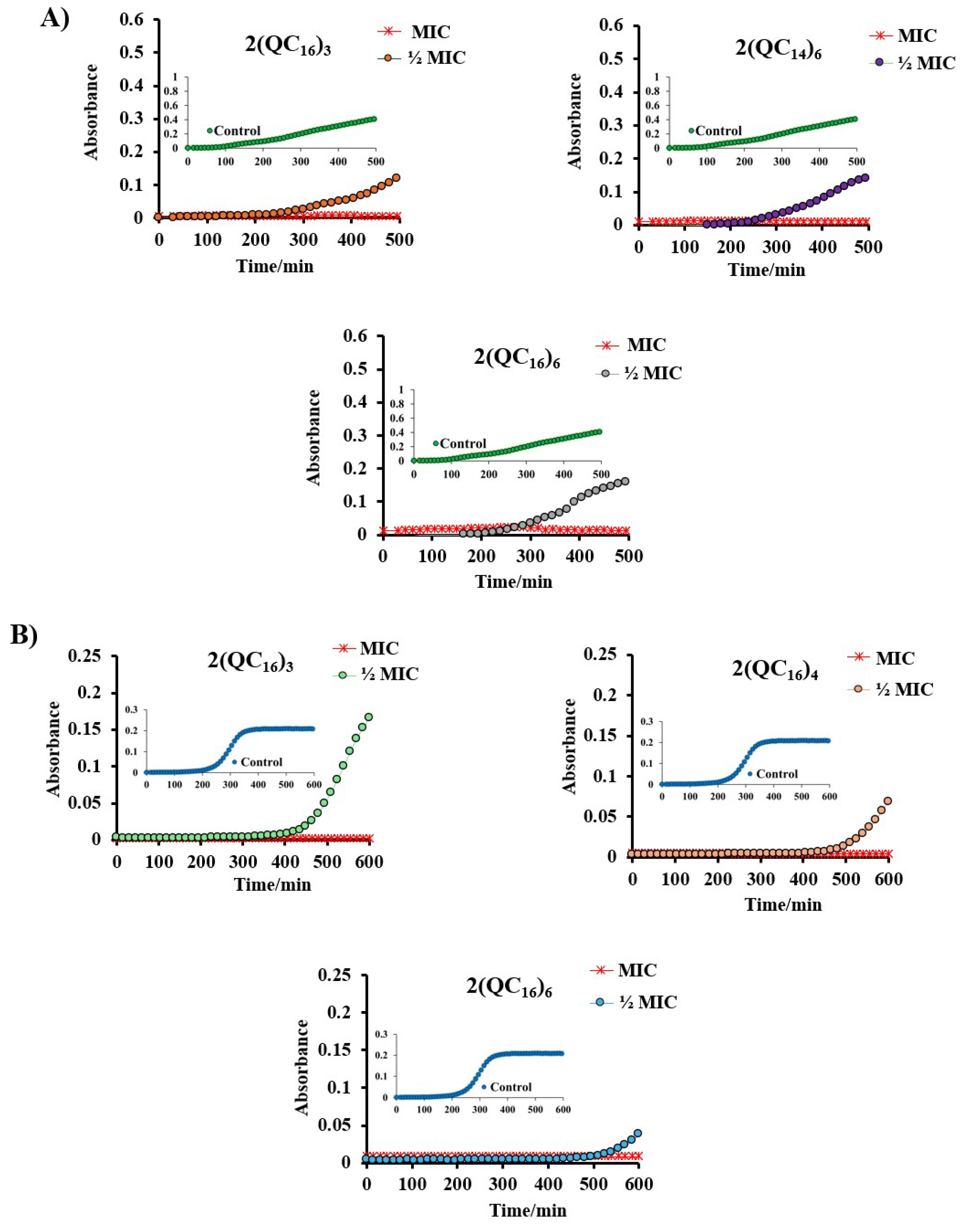
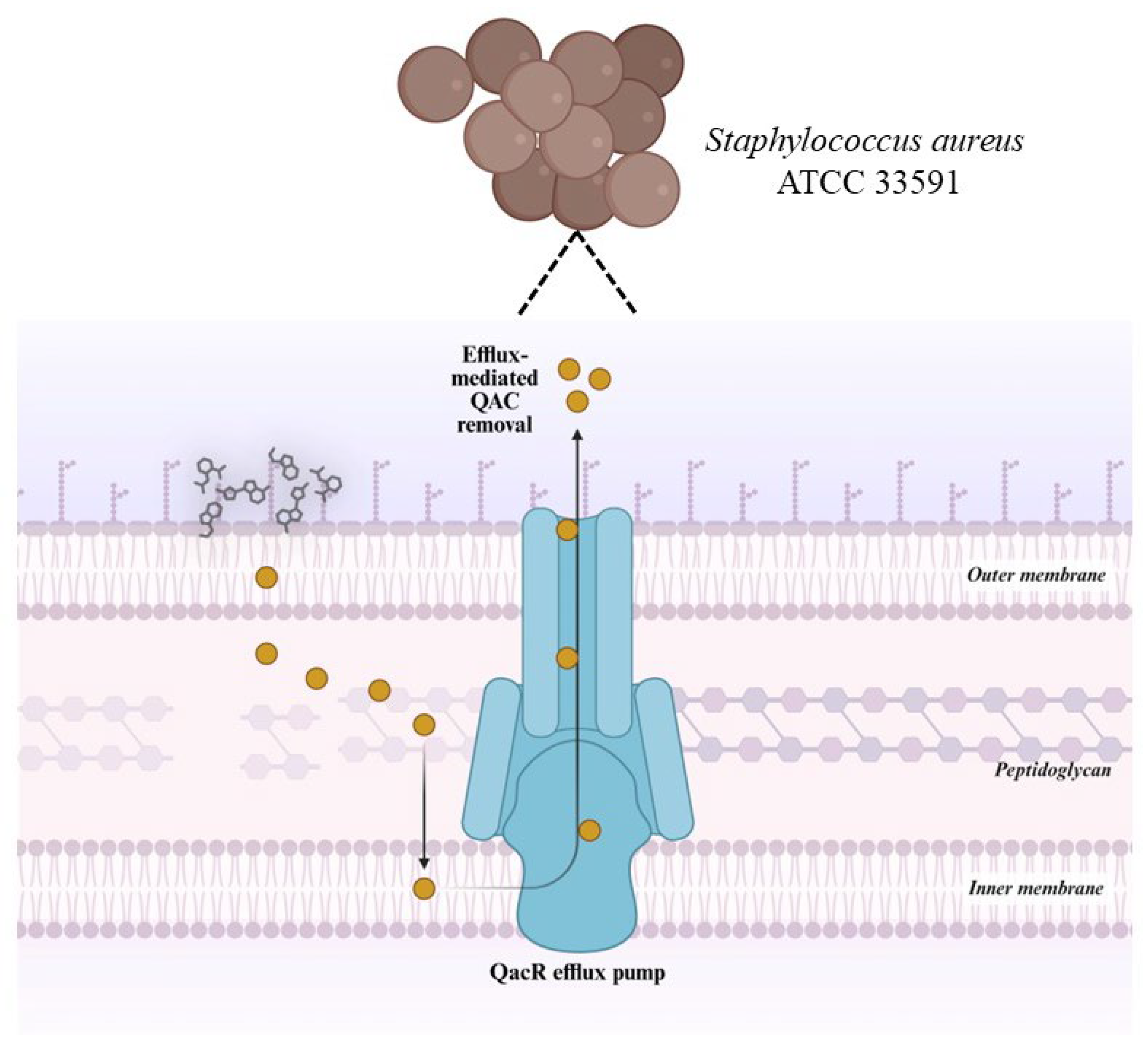
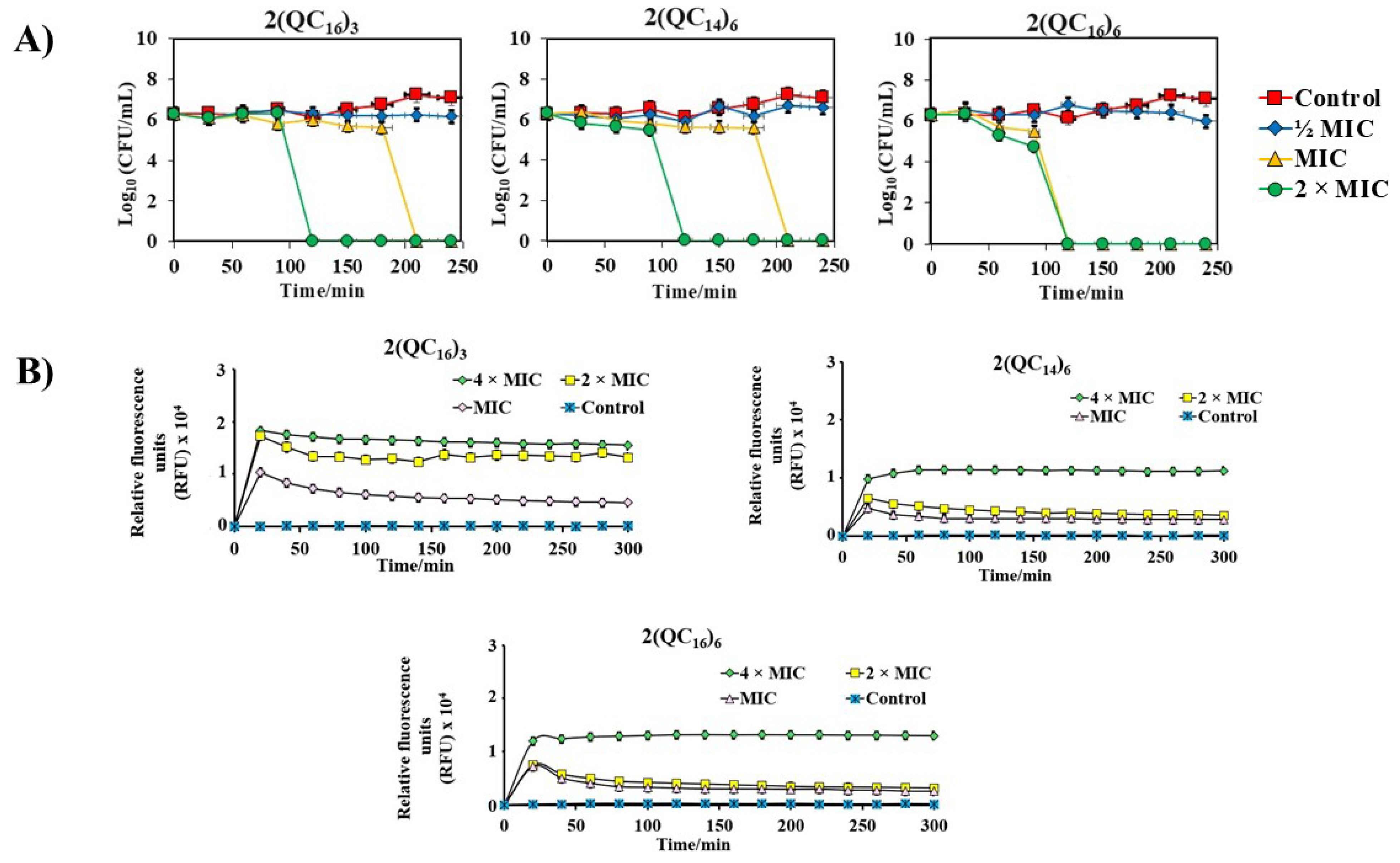
2.4. Potential for the Development of Bacterial Resistance
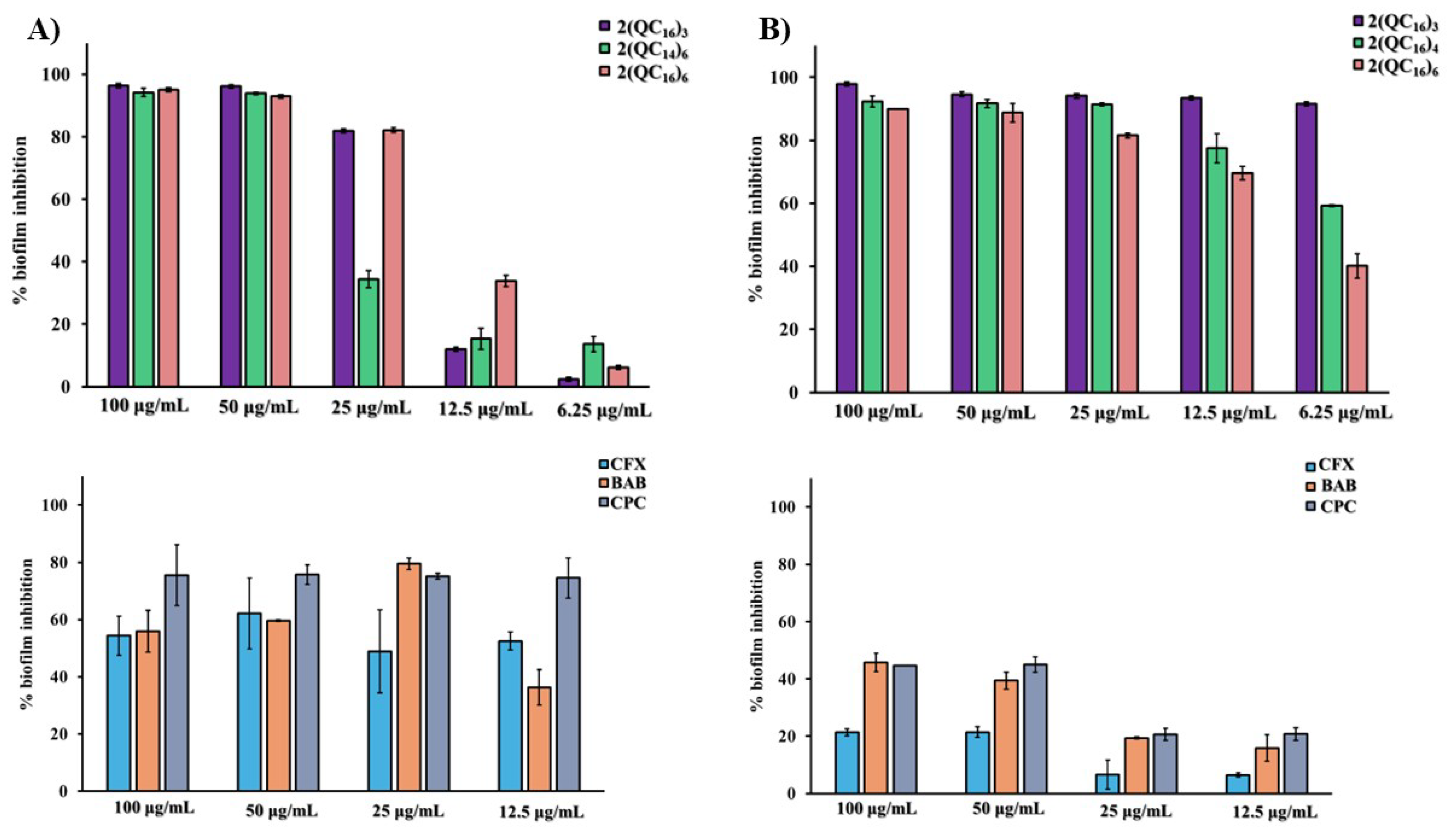
2.5. Minimal Biofilm Inhibition Concentrations (MBICs)
2.6. Time-Dependent Activity of bisQACs
2.7. Spectrofluorimetric Analysis of Propidium Iodide (PI) Uptake Assay
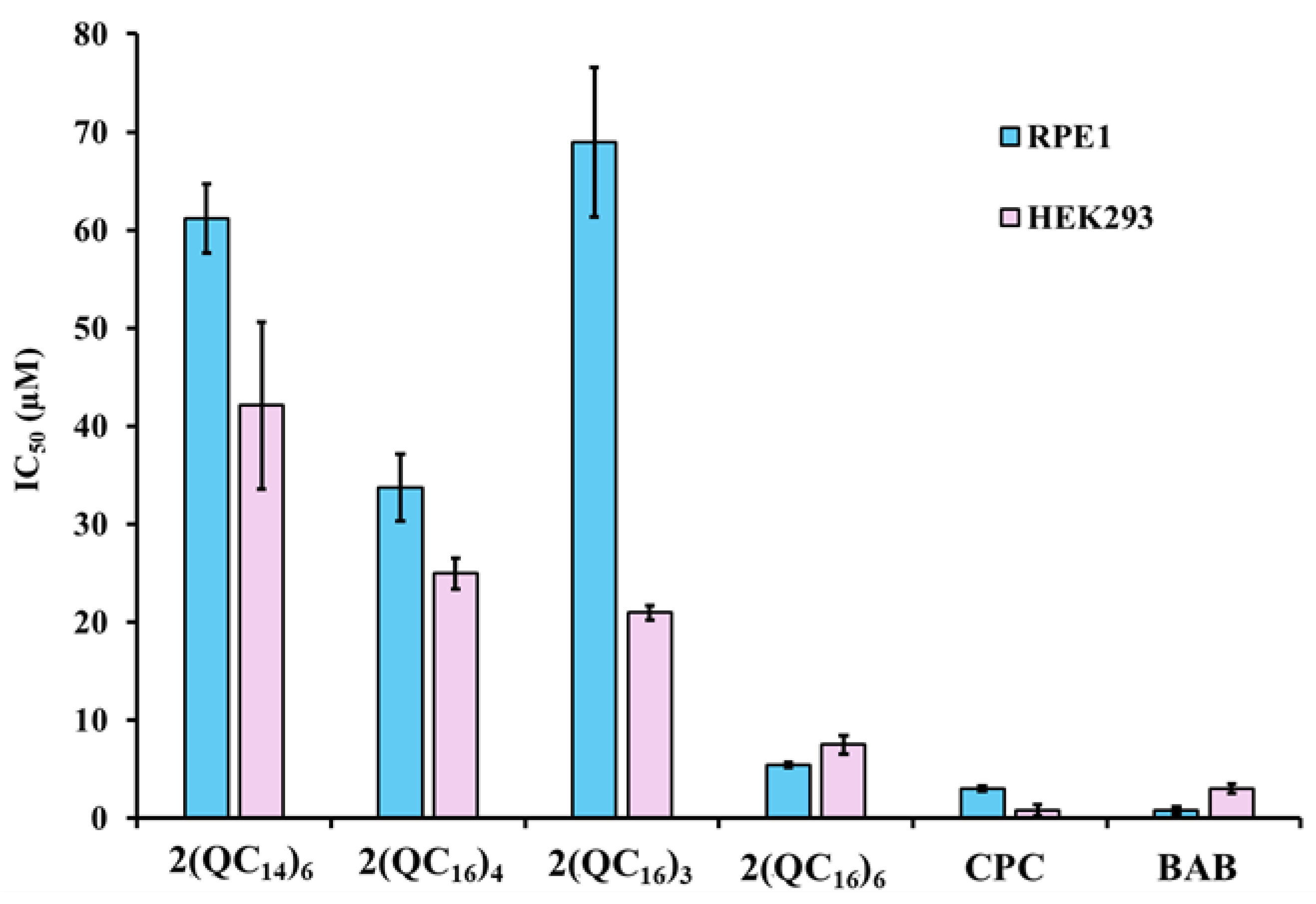
2.8. Cytotoxicity of BisQACs
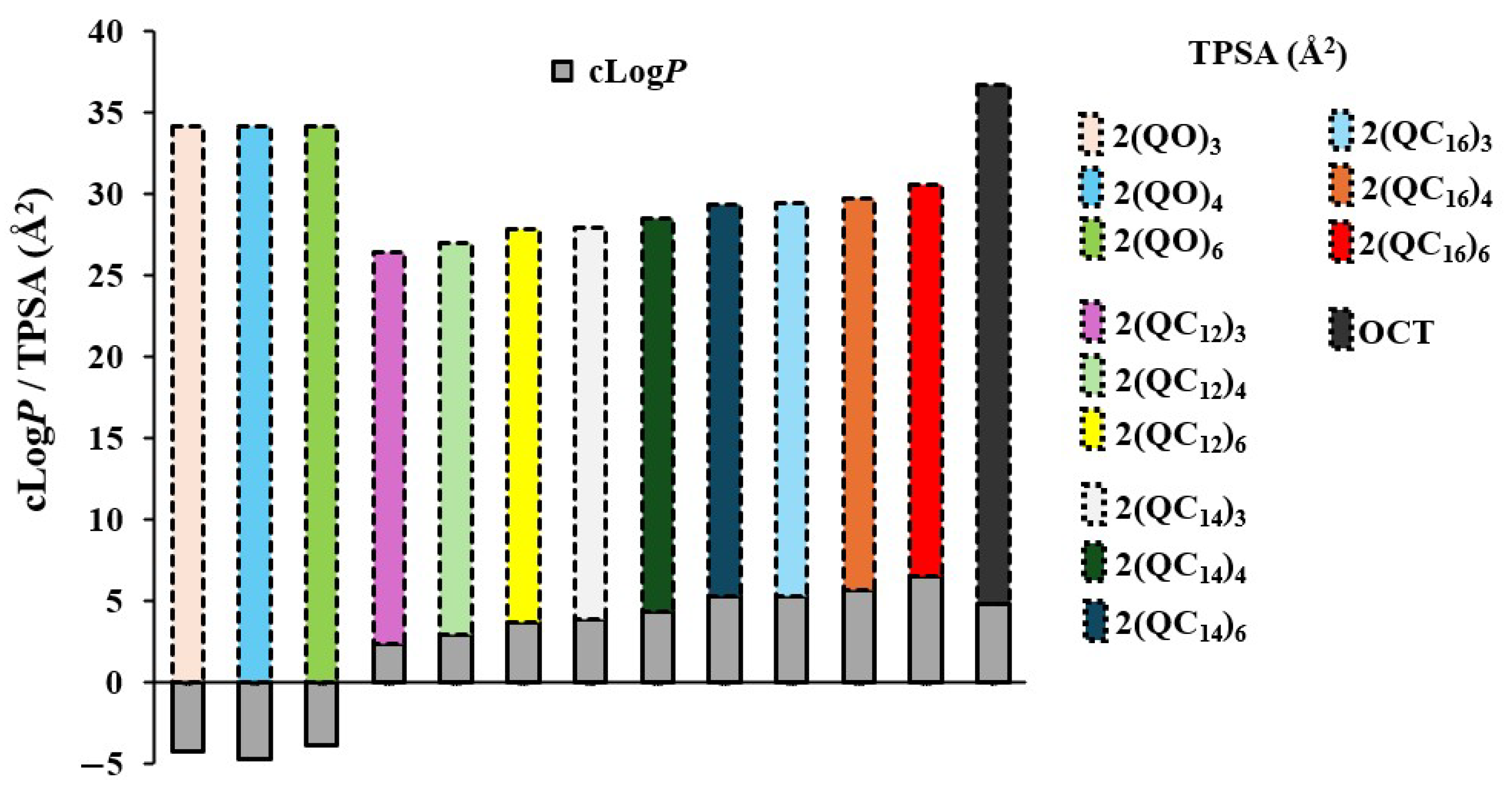
3. Structure-Activity Relationship (SAR)
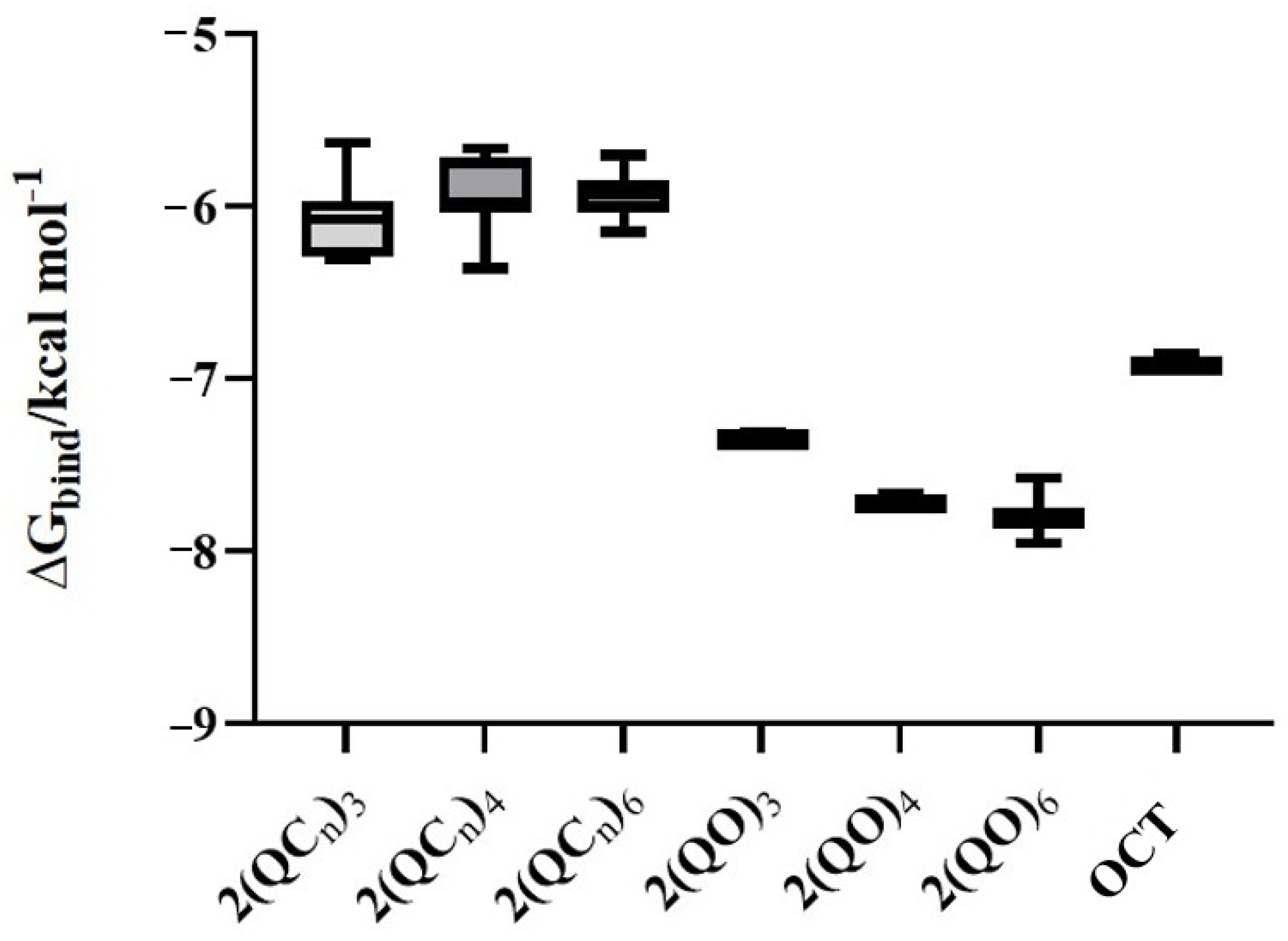
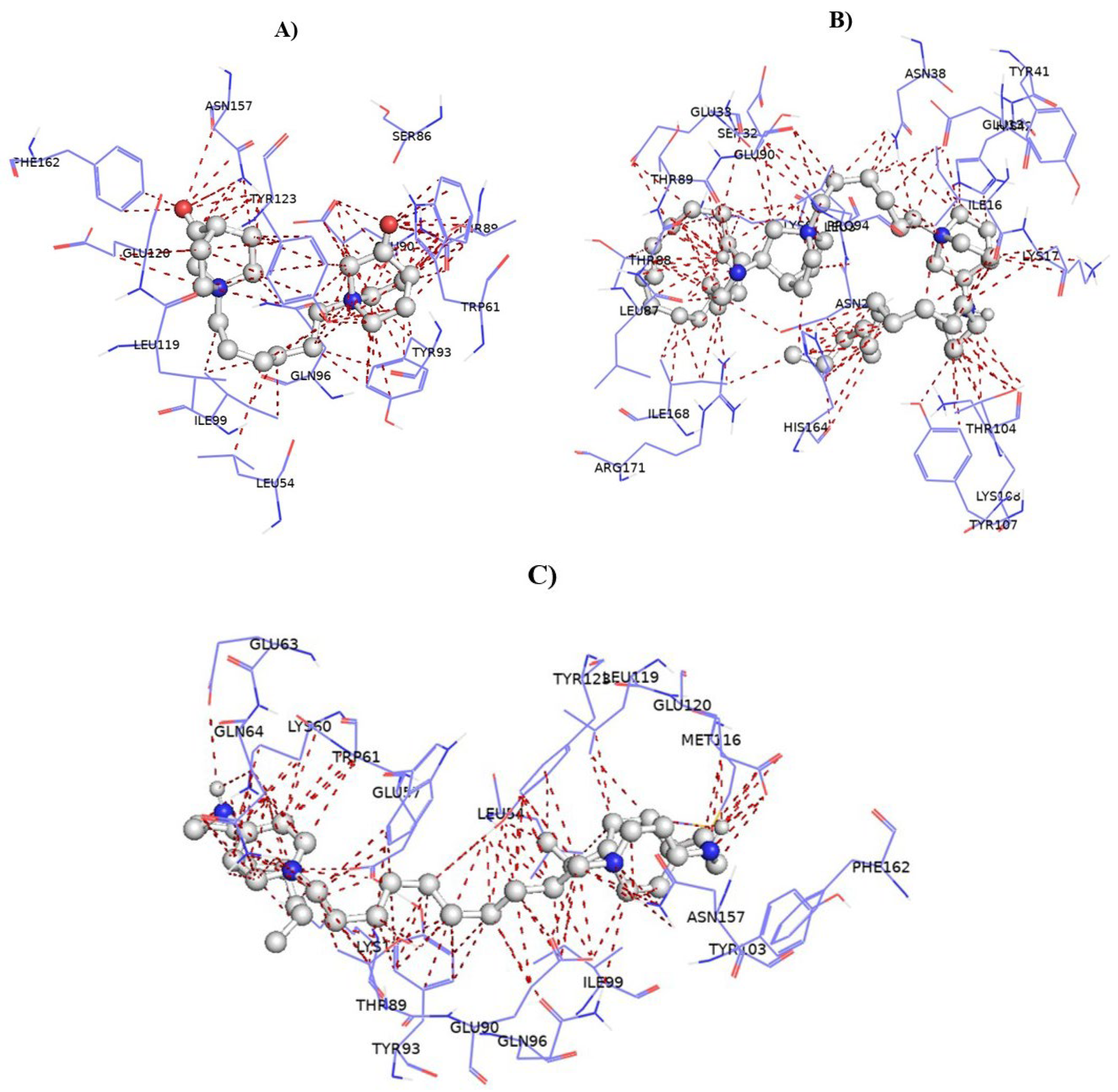
In Silico Molecular Docking Study of bisQACs Binding to the QacR Regulatory Protein
4. Materials and Methods
4.1. Synthesis
4.1.1. Deprotonation of Quinuclidin-3-One Hydrochloride
4.1.2. Synthesis of bisQACs of Quinuclidin-3-One
4.2. Broth Microdilution Assays
4.2.1. Minimum Inhibitory Concentration (MIC)
4.2.2. Time-Resolved Growth Analysis
4.2.3. Potential of Bacterial Resistance Development
4.2.4. Biofilm Inhibition Assay
4.2.5. Time-Dependent Bactericidal Activity Assessment
4.2.6. Assessment of Cell Viability via Flow Cytometry
4.2.7. Propidium Iodide (PI) Uptake Assay
4.2.8. Cytotoxicity
4.3. ADME Drug Properties
Quantitative Assessment of Molecular Descriptors
4.4. Molecular Docking of bisQAC Compounds with QacR Protein
Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lu, Z.; Mahony, A.K.; Arnold, W.A.; Marshall, C.W.; McNamara, P.J. Quaternary ammonia compounds in disinfectant products: Evaluating the potential for promoting antibiotic resistance and disrupting wastewater treatment plant performance. Environ. Sci. Adv. 2024, 3, 208–226. [Google Scholar] [CrossRef]
- Guo, X.; Chen, Y.; Wang, L.; Wu, X.; Fan, J.; Li, F.; Zeng, X.; Ge, Y.; Chi, Y.; Cui, L.; et al. In vitro inactivation of SARS-CoV-2 by commonly used disinfection products and methods. Sci. Rep. 2021, 11, 2418. [Google Scholar] [CrossRef]
- Hoque, J.; Akkapeddi, P.; Yarlagadda, V.; Uppu, D.S.S.M.; Kumar, P.; Haldar, J. Cleavable cationic antibacterial amphiphiles: Synthesis, mechanism of action, and cytotoxicities. Langmuir 2012, 28, 12225–12234. [Google Scholar] [CrossRef]
- Richter, M.F.; Drown, B.S.; Riley, A.P.; Garcia, A.; Shirai, T.; Svec, R.L.; Hergenrother, P.J. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 2017, 545, 299–304. [Google Scholar] [CrossRef]
- Mao, X.; Aue, D.L.; Buchalla, W.; Hiller, K.A.; Maisch, T.; Hellwig, E.; Al-Ahmad, A.; Cieplik, F. Cetylpyridinium chloride: Mechanism of action, antimicrobial efficacy in biofilms, and potential risks of resistance. Antimicrob. Agents Chemother. 2020, 64, e00576-20. [Google Scholar] [CrossRef]
- Zhang, C.; Cui, F.; Zeng, G.; Jiang, M.; Yang, Z.; Yu, Z.; Zhu, M.; Shen, L. Quaternary ammonium compounds (QACs): A review on occurrence, fate and toxicity in the environment. Sci. Total Environ. 2015, 518–519, 352–362. [Google Scholar] [CrossRef]
- Boyce, J.M. Quaternary ammonium disinfectants and antiseptics: Tolerance, resistance and potential impact on antibiotic resistance. Antimicrob. Resist. Infect. Control 2023, 12, 32. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Lu, H.; Zhu, L. Diversified antibiotic resistance and sensitivity patterns induced by quaternary ammonium compounds. Front. Environ. Sci. Eng. 2025, 19, 103. [Google Scholar] [CrossRef]
- Crnčević, D.; Odžak, R.; Šprung, M. A Short Review on Quaternary Ammonium Compounds (QACs): From Antibacterial Action to Next-Generation Design. Croat. Chem. Acta 2025, 98, P1–P10. [Google Scholar] [CrossRef]
- Forman, M.E.; Jennings, M.C.; Wuest, W.M.; Minbiole, K.P.C. Building a Better Quaternary Ammonium Compound (QAC): Branched Tetracationic Antiseptic Amphiphiles. ChemMedChem 2016, 11, 1401–1405. [Google Scholar] [CrossRef] [PubMed]
- Lainioti, G.C.; Druvari, D. Designing Antibacterial-Based Quaternary Ammonium Coatings (Surfaces) or Films for Biomedical Applications: Recent Advances. Int. J. Mol. Sci. 2024, 25, 12264. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Denkel, L.; Feßler, A.T.; Eicker, R.; Mellmann, A.; Schwarz, S.; Geffers, C.; Hübner, N.O.; Leistner, R. Clinical Evidence for the Use of Octenidine Dihydrochloride to Prevent Healthcare-Associated Infections and Decrease Staphylococcus aureus Carriage or Transmission—A Review. Pathogens 2023, 12, 612. [Google Scholar] [CrossRef] [PubMed]
- Denkel, L.A.; Kramer, T.S.; Schwab, F.; Golembus, J.; Wolke, S.; Gastmeier, P.; Geffers, C. Chlorhexidine and octenidine susceptibility of bacterial isolates from clinical samples in a three-armed cluster randomised decolonisation trial. PLoS ONE 2022, 17, e0278569. [Google Scholar] [CrossRef]
- Malanovic, N.; Ön, A.; Pabst, G.; Zellner, A.; Lohner, K. Octenidine: Novel insights into the detailed killing mechanism of Gram-negative bacteria at a cellular and molecular level. Int. J. Antimicrob. Agents 2020, 56, 106146. [Google Scholar] [CrossRef]
- Kwaśniewska, D.; Chen, Y.L.; Wieczorek, D. Biological activity of quaternary ammonium salts and their derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef]
- Brayton, S.R.; Toles, Z.E.A.; Sanchez, C.A.; Michaud, M.E.; Thierer, L.M.; Keller, T.M.; Risener, C.J.; Quave, C.L.; Wuest, W.M.; Minbiole, K.P.C. Soft QPCs: Biscationic Quaternary Phosphonium Compounds as Soft Antimicrobial Agents. ACS Infect. Dis. 2023, 9, 943–951. [Google Scholar] [CrossRef]
- Zhou, F.; Maeda, T.; Nagamune, H.; Kourai, H. Synthesis and antimicrobial characteristics of novel biocides, 1,1′-(decanedioyl)bis(4-methyl-4-alkylpiperazinium iodide)s with a gemini structure. Biocontrol Sci. 2004, 9, 61–67. [Google Scholar] [CrossRef]
- Liang, Y.; Li, H.; Ji, J.; Wang, J.; Ji, Y. Self-Aggregation, Antimicrobial Activity and Cytotoxicity of Ester-Bonded Gemini Quaternary Ammonium Salts: The Role of the Spacer. Molecules 2023, 28, 5469. [Google Scholar] [CrossRef] [PubMed]
- Brycki, B.E.; Szulc, A.; Kowalczyk, I.; Koziróg, A.; Sobolewska, E. Antimicrobial Activity of Gemini Surfactants with Ether Group in the Spacer Part. Molecules 2021, 26, 5759. [Google Scholar] [CrossRef] [PubMed]
- Kontos, R.C.; Schallenhammer, S.A.; Bentley, B.S.; Morrison, K.R.; Feliciano, J.A.; Tasca, J.A.; Kaplan, A.R.; Bezpalko, M.W.; Kassel, W.S.; Wuest, W.M.; et al. An Investigation into Rigidity–Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2019, 14, 83–87. [Google Scholar] [CrossRef]
- Leitgeb, A.J.; Feliciano, J.A.; Sanchez, H.A.; Allen, R.A.; Morrison, K.R.; Sommers, K.J.; Carden, R.G.; Wuest, W.M.; Minbiole, K.P.C. Further Investigations into Rigidity-Activity Relationships in BisQAC Amphiphilic Antiseptics. ChemMedChem 2020, 15, 667–670. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Saverina, E.A.; Son, E.; Akchurin, R.N.; Kartseva, A.S.; Firstova, V.V.; Vereshchagin, A.N. Exploring the correlation of linker structure and antimicrobial activities of pyridinium-based cationic biocides: Aromatic versus aliphatic architectures. Eur. J. Med. Chem. 2025, 292, 117673. [Google Scholar] [CrossRef]
- Hao, J.; Qin, T.; Zhang, Y.; Li, Y.; Zhang, Y. Synthesis, surface properties and antimicrobial performance of novel gemini pyridinium surfactants. Colloids Surf. B Biointerfaces 2019, 181, 814–821. [Google Scholar] [CrossRef]
- Obando, D.; Koda, Y.; Pantarat, N.; Lev, S.; Zuo, X.; Bijosono Oei, J.; Widmer, F.; Djordjevic, J.T.; Sorrell, T.C.; Jolliffe, K.A. Synthesis and Evaluation of a Series of Bis(pentylpyridinium) Compounds as Antifungal Agents. ChemMedChem 2018, 13, 1421–1436. [Google Scholar] [CrossRef]
- Bayrakal, G.M.; Aydin, A.; Issa, G.; Dumen, E.; Sudagidan, M. Investigation of quaternary ammonium compound resistance in Staphylococcus aureus strains isolated from several foods and food production facilities. Acta Aliment. 2025, 54, 145–152. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Ahn, J. Advances in the Discovery of Efflux Pump Inhibitors as Novel Potentiators to Control Antimicrobial-Resistant Pathogens. Antibiotics 2023, 12, 1417. [Google Scholar] [CrossRef]
- Grkovic, S.; Hardie, K.M.; Brown, M.H.; Skurray, R.A. Interactions of the QacR Multidrug-Binding Protein with Structurally Diverse Ligands: Implications for the Evolution of the Binding Pocket. Biochemistry 2003, 42, 15226–15236. [Google Scholar] [CrossRef]
- Yamamoto, M.; Takami, T.; Matsumura, R.; Dorofeev, A.; Hirata, Y.; Nagamune, H. In vitro evaluation of the biocompatibility of newly synthesized bis-quaternary ammonium compounds with spacer structures derived from pentaerythritol or hydroquinone. Biocontrol Sci. 2016, 21, 231–241. [Google Scholar] [CrossRef]
- Schallenhammer, S.A.; Duggan, S.M.; Morrison, K.R.; Bentley, B.S.; Wuest, W.M.; Minbiole, K.P.C. Hybrid BisQACs: Potent Biscationic Quaternary Ammonium Compounds Merging the Structures of Two Commercial Antiseptics. ChemMedChem 2017, 12, 1931–1934. [Google Scholar] [CrossRef]
- Frolov, N.; Detusheva, E.; Fursova, N.; Ostashevskaya, I.; Vereshchagin, A. Microbiological Evaluation of Novel Bis-Quaternary Ammonium Compounds: Clinical Strains, Biofilms, and Resistance Study. Pharmaceuticals 2022, 15, 514. [Google Scholar] [CrossRef]
- Frolov, N.A.; Seferyan, M.A.; Detusheva, E.V.; Son, E.; Kolmakov, I.G.; Kartseva, A.S.; Firstova, V.V.; Vereshchagin, A.N.; Elinson, M.N. Development of Naphthalene-Derivative Bis-QACs as Potent Antimicrobials: Unraveling Structure–Activity Relationship and Microbiological Properties. Molecules 2024, 29, 5526. [Google Scholar] [CrossRef]
- Hübner, N.O.; Siebert, J.; Kramer, A. Octenidine dihydrochloride, a modern antiseptic for skin, mucous membranes and wounds. Skin Pharmacol. Physiol. 2010, 23, 244–258. [Google Scholar] [CrossRef]
- Nowacki, A.; Sikora, K.; Dmochowska, B.; Wiśniewski, A. Studies of the formation of N-substituted pyridinium mesylates: A theoretical approach. Comput. Theor. Chem. 2012, 1000, 33–41. [Google Scholar] [CrossRef]
- Spahr, A.C.; Michaud, M.E.; Amoo, L.E.; Sanchez, C.A.; Hogue, C.E.; Thierer, L.M.; Gau, M.R.; Wuest, W.M.; Minbiole, K.P.C. Rigidity-Activity Relationships of bisQPC Scaffolds against Pathogenic Bacteria. ChemMedChem 2022, 17, e202200224. [Google Scholar] [CrossRef]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary Ammonium Compounds: An Antimicrobial Mainstay and Platform for Innovation to Address Bacterial Resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef]
- Song, Z.; Wang, H.; Wu, Y.; Gu, J.; Li, S.; Han, H. Fabrication of Bis-Quaternary Ammonium Salt as an Efficient Bactericidal Weapon Against Escherichia coli and Staphylococcus aureus. ACS Omega 2018, 3, 14517–14525. [Google Scholar] [CrossRef]
- Bessa, L.J.; Ferreira, M.; Gameiro, P. Evaluation of membrane fluidity of multidrug-resistant isolates of Escherichia coli and Staphylococcus aureus in presence and absence of antibiotics. J. Photochem. Photobiol. B Biol. 2018, 181, 150–156. [Google Scholar] [CrossRef]
- Theophel, K.; Schacht, V.J.; Schlüter, M.; Schnell, S.; Stingu, C.S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef]
- Bertranda, R.L. Lag phase is a dynamic, organized, adaptive, and evolvable period that prepares bacteria for cell division. J. Bacteriol. 2019, 201, 1–3. [Google Scholar] [CrossRef]
- Rastin, M.; Mahmoudi, M.; Sahebari, M.; Tabasi, N. Clinical & immunological characteristics in systemic lupus erythematosus patients. Indian J. Med. Res. 2017, 146, 224–229. [Google Scholar] [CrossRef]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of bacterial efflux pumps in antibiotic resistance, virulence, and strategies to discover novel efflux pump inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Paulsen, I.T.; Brown, M.H.; Littlejohn, T.G.; Mitchell, B.A.; Skurray, R.A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: Membrane topology and identification of residues involved in substrate specificity. Proc. Natl. Acad. Sci. USA 1996, 93, 3630–3635. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Blanco, P.; Alcalde-Rico, M.; Corona, F.; Reales-Calderón, J.A.; Sánchez, M.B.; Martínez, J.L. Multidrug efflux pumps as main players in intrinsic and acquired resistance to antimicrobials. Drug Resist. Updat. 2016, 28, 13–27. [Google Scholar] [CrossRef]
- Alkhalifa, S.; Jennings, M.C.; Granata, D.; Klein, M.; Wuest, W.M.; Minbiole, K.P.C.; Carnevale, V. Analysis of the Destabilization of Bacterial Membranes by Quaternary Ammonium Compounds: A Combined Experimental and Computational Study. ChemBioChem 2020, 21, 1510–1516. [Google Scholar] [CrossRef]
- Gravel, J.; Paradis-Bleau, C.; Schmitzer, A.R. Adaptation of a bacterial membrane permeabilization assay for quantitative evaluation of benzalkonium chloride as a membrane-disrupting agent. MedChemComm 2017, 8, 1408–1413. [Google Scholar] [CrossRef]
- Forman, M.E.; Fletcher, M.H.; Jennings, M.C.; Duggan, S.M.; Minbiole, K.P.C.; Wuest, W.M. Structure–Resistance Relationships: Interrogating Antiseptic Resistance in Bacteria with Multicationic Quaternary Ammonium Dyes. ChemMedChem 2016, 11, 958–962. [Google Scholar] [CrossRef]
- Liu, X.; Testa, B.; Fahr, A. Lipophilicity and its relationship with passive drug permeation. Pharm. Res. 2011, 28, 962–977. [Google Scholar] [CrossRef]
- Avdeef, A.; Nielsen, P.E.; Tsinman, O. PAMPA—A drug absorption in vitro model 11. Matching the in vivo unstirred water layer thickness by individual-well stirring in microtitre plates. Eur. J. Pharm. Sci. 2004, 22, 365–374. [Google Scholar] [CrossRef]
- Lou, L.L.; Martin, J.C. Selected thoughts on hydrophobicity in drug design. Molecules 2021, 26, 875. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Römling, U.; Balsalobre, C. Biofilm infections, their resilience to therapy and innovative treatment strategies. J. Intern. Med. 2012, 272, 541–561. [Google Scholar] [CrossRef] [PubMed]
- Rhee, K.Y.; Gardiner, D.F. Clinical relevance of bacteriostatic versus bactericidal activity in the treatment of gram-positive bacterial infections. Clin. Infect. Dis. 2004, 39, 755–756. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.B.; Frier, M. Mode of action of the antibacterial compound dequalinium acetate. Appl. Microbiol. 1969, 17, 118–127. [Google Scholar] [CrossRef]
- Tischer, M.; Pradel, G.; Ohlsen, K.; Holzgrabe, U. Quaternary Ammonium Salts and Their Antimicrobial Potential: Targets or Nonspecific Interactions? ChemMedChem 2012, 7, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Niu, L.-N.; Ma, S.; Li, J.; Tay, F.R.; Chen, J.-H. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef]
- Wang, G.; Yang, L.; Jiang, L.; Chen, J.; Jing, Q.; Mai, Y.; Deng, L.; Lin, Y.; Chen, L.; Chen, Z.; et al. A new class of quaternary ammonium compounds as potent and environmental friendly disinfectants. J. Clean. Prod. 2022, 379, 134632. [Google Scholar] [CrossRef]
- Marzullo, P.; Gruttadauria, M.; D’Anna, F. Quaternary Ammonium Salts-Based Materials: A Review on Environmental Toxicity, Anti-Fouling Mechanisms and Applications in Marine and Water Treatment Industries. Biomolecules 2024, 14, 957. [Google Scholar] [CrossRef]
- Arnold, W.A.; Blum, A.; Branyan, J.; Bruton, T.A.; Carignan, C.C.; Cortopassi, G.; Datta, S.; Dewitt, J.; Doherty, A.; Halden, R.U.; et al. Quaternary Ammonium Compounds: A Chemical Class of Emerging Concern. Environ. Sci. Technol. 2023, 57, 7645–7665. [Google Scholar] [CrossRef]
- Weller, S.R.; Burnell, J.E.; Aho, B.M.; Obeng, B.; Ledue, E.L.; Shim, J.K.; Hess, S.T.; Gosse, J.A. Antimicrobial cetylpyridinium chloride causes functional inhibition of mitochondria as potently as canonical mitotoxicants, nanostructural disruption of mitochondria, and mitochondrial Ca2+ efflux in living rodent and primary human cells. Food Chem. Toxicol. 2024, 186, 114547. [Google Scholar] [CrossRef]
- Ng, M.K.; Mont, M.A.; Bonutti, P.M. Clinical and Environmental Harms of Quaternary Ammonium Disinfectants and the Promise of Ultraviolet-C (UV-C) Alternatives: A Narrative Review. Cureus 2025, 17, e84022. [Google Scholar] [CrossRef]
- Odžak, R.; Crnčević, D.; Sabljić, A.; Primožič, I.; Šprung, M. Synthesis and Biological Evaluation of 3-Amidoquinuclidine Quaternary Ammonium Compounds as New Soft Antibacterial Agents. Pharmaceuticals 2023, 16, 187. [Google Scholar] [CrossRef]
- Grkovic, S.; Brown, M.H.; Roberts, N.J.; Paulsen, I.T.; Skurray, R.A. QacR is a repressor protein that regulates expression of the Staphylococcus aureus multidrug efflux pump QacA. J. Biol. Chem. 1998, 273, 18665–18673. [Google Scholar] [CrossRef]
- Takeuchi, K.; Imai, M.; Shimada, I. Conformational equilibrium defines the variable induction of the multidrug-binding transcriptional repressor QacR. Proc. Natl. Acad. Sci. USA 2019, 116, 19963–19972. [Google Scholar] [CrossRef]
- Morrison, K.R.; Allen, R.A.; Minbiole, K.P.C.; Wuest, W.M. More QACs, more questions: Recent advances in structure activity relationships and hurdles in understanding resistance mechanisms. Tetrahedron Lett. 2019, 60, 150935. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural mechanisms of QacR induction and multidrug recognition. Science 2001, 294, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.A.; Brennan, R.G. Structural mechanisms of multidrug recognition and regulation by bacterial multidrug transcription factors. Mol. Microbiol. 2002, 45, 885–893. [Google Scholar] [CrossRef]
- Schumacher, M.A.; Miller, M.C.; Grkovic, S.; Brown, M.H.; Skurray, R.A.; Brennan, R.G. Structural basis for cooperative DNA binding by two dimers of the multidrug-binding protein QacR. EMBO J. 2002, 21, 1210–1218. [Google Scholar] [CrossRef]
- Peters, K.M.; Brooks, B.E.; Schumacher, M.A.; Skurray, R.A.; Brennan, R.G.; Brown, M.H. A single acidic residue can guide binding site selection but does not govern QaCR cationic-drug affinity. PLoS ONE 2011, 6, e15974. [Google Scholar] [CrossRef] [PubMed]
- Barratt, E.; Bingham, R.J.; Warner, D.J.; Laughton, C.A.; Phillips, S.E.V.; Homans, S.W. Van der Waals interactions dominate ligand−protein association in a protein binding site occluded from solvent water. J. Am. Chem. Soc. 2005, 127, 11827–11834. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, 9th ed.; CLSI Document M07-A9; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Stiefel, P.; Schmidt-Emrich, S.; Maniura-Weber, K.; Ren, Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. [Google Scholar] [CrossRef]

| Compounds | Mr | Gram-Positive Bacteria | Gram-Negative Bacteria | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Staphylococcus aureus ATCC 25923 | Staphylococcus aureus ATCC 33591 | Staphylococcus aureus Clinical/MRSA | Bacillus cereus ATCC 14579 | Listeria monocytogenes ATCC 7644 | Enterococcus faecalis ATCC 29212 | Escherichia coli ATTC 25922 | Salmonella enterica Food Isolate | Pseudomonas aeruginosa ATTC 27853 | ||
| Minimum Inhibitory Concentrations (MIC)/μM | ||||||||||
| 2(QO)3 | 452.23 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 |
| 2(QO)4 | 466.26 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 |
| 2(QO)6 | 494.31 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 |
| 2(QC12)3 | 790.94 | 75 | 75 | >75 | >75 | 38 | >75 | >75 | 75 | >75 |
| 2(QC12)4 | 804.97 | >75 | 75 | >75 | >75 | 75 | >75 | >75 | >75 | >75 |
| 2(QC12)6 | 833.02 | 75 | 19 | >75 | >75 | 38 | 75 | 38 | >75 | >75 |
| 2(QC14)3 | 847.05 | 75 | 75 | >75 | >75 | 38 | >75 | >75 | >75 | >75 |
| 2(QC14)4 | 861.08 | >75 | 75 | >75 | >75 | >75 | >75 | >75 | >75 | >75 |
| 2(QC14)6 | 889.32 | 38 | 19 | 75 | 75 | 19 | 38 | 19 | 75 | >75 |
| 2(QC16)3 | 903.16 | 38 | 19 | >75 | >75 | 9 | >75 | >75 | >75 | >75 |
| 2(QC16)4 | 917.19 | 75 | 9 | 75 | >75 | 9 | 38 | 19 | 75 | >75 |
| 2(QC16)6 | 945.24 | 9 | 5 | 38 | 75 | 19 | 38 | 19 | 75 | >75 |
| BAB | 384.40 | 10 | 25 | 25 | 25 | 10 | 15 | 63 | 50 | >125 |
| Compounds | IC50 (μM) | MIC (μM) | SI (IC50/MIC) | ||
|---|---|---|---|---|---|
| RPE1 | HEK293 | S. aureus | RPE1 | HEK293 | |
| 2(QC14)6 | 61 | 42 | 38 | 1.61 | 1.11 |
| 2(QC16)3 | 69 | 21 | 38 | 1.82 | 0.55 |
| 2(QC16)4 | 34 | 25 | 75 | 0.45 | 0.33 |
| 2(QC16)6 | 5 | 7 | 9 | 0.56 | 0.78 |
| CPC | 3 | 0.80 | 4 | 0.75 | 0.20 |
| BAB | 0.82 | 3 | 10 | 0.08 | 0.30 |
| Compound | SMILES |
|---|---|
| 2(QO)3 | O=C(C1)C2CC[N+]1(CCC[N+]34CCC(C(C4)=O)CC3)CC2.[Br−].[Br−] |
| 2(QO)4 | O=C(C1)C2CC[N+]1(CCCC[N+]34CCC(C(C4)=O)CC3)CC2.[Br−].[Br−] |
| 2(QO)6 | O=C(C1)C2CC[N+]1(CCCCCC[N+]34CCC(C(C4)=O)CC3)CC2.[Br−].[Br−] |
| 2(QC12)3 | CCCCCCCCCCCNCC(C1)C2CC[N+]1(CCC[N+]34CCC(C(NCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC12)4 | CCCCCCCCCCCNCC(C1)C2CC[N+]1(CCCC[N+]34CCC(C(NCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC12)6 | CCCCCCCCCCCNCC(C1)C2CC[N+]1(CCCCCC[N+]34CCC(C(NCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC14)3 | CCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCC[N+]34CCC(C(NCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC14)4 | CCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCCC[N+]34CCC(C(NCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC14)6 | CCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCCCCC[N+]34CCC(C(NCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC16)3 | CCCCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCC[N+]34CCC(C(NCCCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC16)4 | CCCCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCCC[N+]34CCC(C(NCCCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| 2(QC16)6 | CCCCCCCCCCCCCCCCNC(C1)C2CC[N+]1(CCCCCC[N+]34CCC(C(NCCCCCCCCCCCCCCCC)C4)CC3)CC2.[Br−].[Br−] |
| OCT | CCCCCCCCNC1=CC=[N+](CCCCCCCCCC[N+]2=CC=C(NCCCCCCCC)C=C2)C=C1.[Cl−].[Cl−] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabljić, A.; Čarija, D.; Ramić, A.; Šprung, M.; Odžak, R. Structure–Function Insights into Quinuclidine-3-One BisQACs: Synthesis, Modulation of Bacterial Resistance, Structure–Activity Relationship, and Biological Profiling. Pharmaceuticals 2025, 18, 1286. https://doi.org/10.3390/ph18091286
Sabljić A, Čarija D, Ramić A, Šprung M, Odžak R. Structure–Function Insights into Quinuclidine-3-One BisQACs: Synthesis, Modulation of Bacterial Resistance, Structure–Activity Relationship, and Biological Profiling. Pharmaceuticals. 2025; 18(9):1286. https://doi.org/10.3390/ph18091286
Chicago/Turabian StyleSabljić, Antonio, Doris Čarija, Alma Ramić, Matilda Šprung, and Renata Odžak. 2025. "Structure–Function Insights into Quinuclidine-3-One BisQACs: Synthesis, Modulation of Bacterial Resistance, Structure–Activity Relationship, and Biological Profiling" Pharmaceuticals 18, no. 9: 1286. https://doi.org/10.3390/ph18091286
APA StyleSabljić, A., Čarija, D., Ramić, A., Šprung, M., & Odžak, R. (2025). Structure–Function Insights into Quinuclidine-3-One BisQACs: Synthesis, Modulation of Bacterial Resistance, Structure–Activity Relationship, and Biological Profiling. Pharmaceuticals, 18(9), 1286. https://doi.org/10.3390/ph18091286