Reply to Guidolin et al. Comment on “Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633”
- Materials and Methods
- Experimental procedure of retention study using frontal TDA
- Experimental procedure of recovery study after ultrafiltration using ion chromatography coupled to ICP-MS (courtesy of Prof. Uwe Karst [4])
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Somnin, C.; Chamieh, J.; Leclercq, L.; Medina, C.; Rousseaux, O.; Cottet, H. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633. [Google Scholar] [CrossRef] [PubMed]
- Guidolin, N.; Maisano, F.; Tedoldi, F.; Baranyai, Z. Comment on Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633. Pharmaceuticals 2025, 18, 1233. [Google Scholar] [CrossRef]
- Toma, C.-M.; Imre, S.; Vari, C.-E.; Muntean, D.-L.; Tero-Vescan, A. Ultrafiltration Method for Plasma Protein Binding Studies and Its Limitations. Processes 2021, 9, 382. [Google Scholar] [CrossRef]
- Maas, T.J.; Factor, C.; Strzeminska, I.; Karst, U. Advanced methodological framework for speciation analysis of gadolinium in skin tissue following contrast agent exposure. Metallomics 2025. submitted on 21 June 2025. [Google Scholar]
- Guidolin, N.; Travagin, F.; Giovenzana, G.B.; Vágner, A.; Lotti, S.; Chianale, F.; Brücher, E.; Maisano, F.; Kirchin, M.A.; Tedoldi, F.; et al. Interaction of macrocyclic gadolinium-based MR contrast agents with type I collagen. Equilibrium and kinetic studies. Dalton Trans. 2020, 49, 14863–14870. [Google Scholar] [CrossRef] [PubMed]
- Somnin, C.; Leclercq, L.; Chamieh, J.; Le Menedeu, M.; Medina, C.; Rousseaux, O.; Tripier, R.; Platas Iglesias, C.; Cottet, H. Taylor dispersion analysis for measurement of diffusivity and size of gadolinium-based contrast agents. Eur. J. Pharm. Sci. 2024, 200, 106831. [Google Scholar] [CrossRef] [PubMed]
- Somnin, C.; Chamieh, J.; Leclercq, L.; Medina, C.; Rousseaux, O.; Cottet, H. Interaction study between gadolinium-based contrast agents and lysozyme using frontal analysis continuous capillary electrophoresis. Anal. Chim. Acta 2025, 1372, 344433. [Google Scholar] [CrossRef]
- Macke, M.; Quarles, C.D.; Sperling, M.; Karst, U. Fast and automated monitoring of gadolinium-based contrast agents in surface waters. Water Res. 2021, 207, 117836. [Google Scholar] [CrossRef] [PubMed]
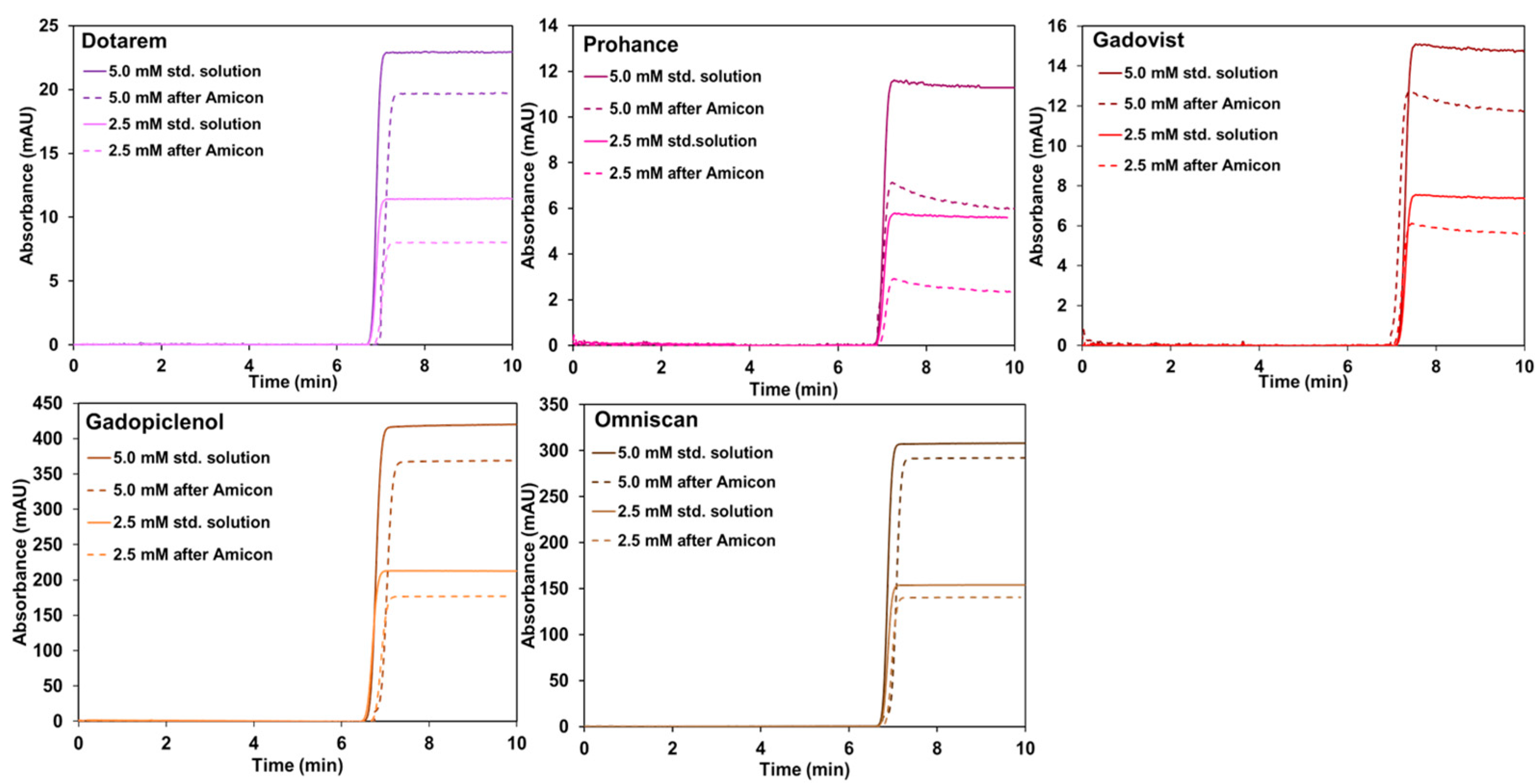
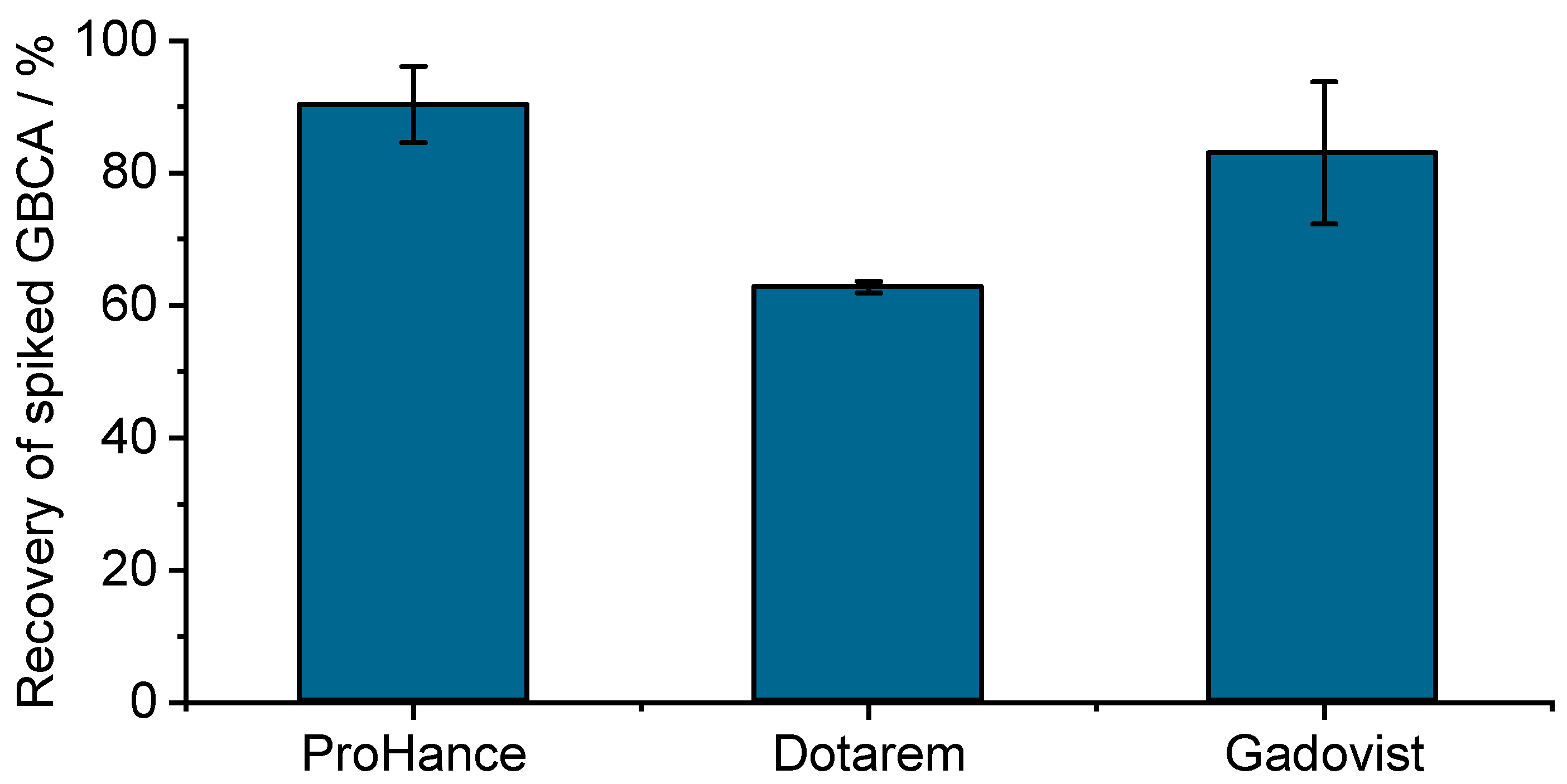

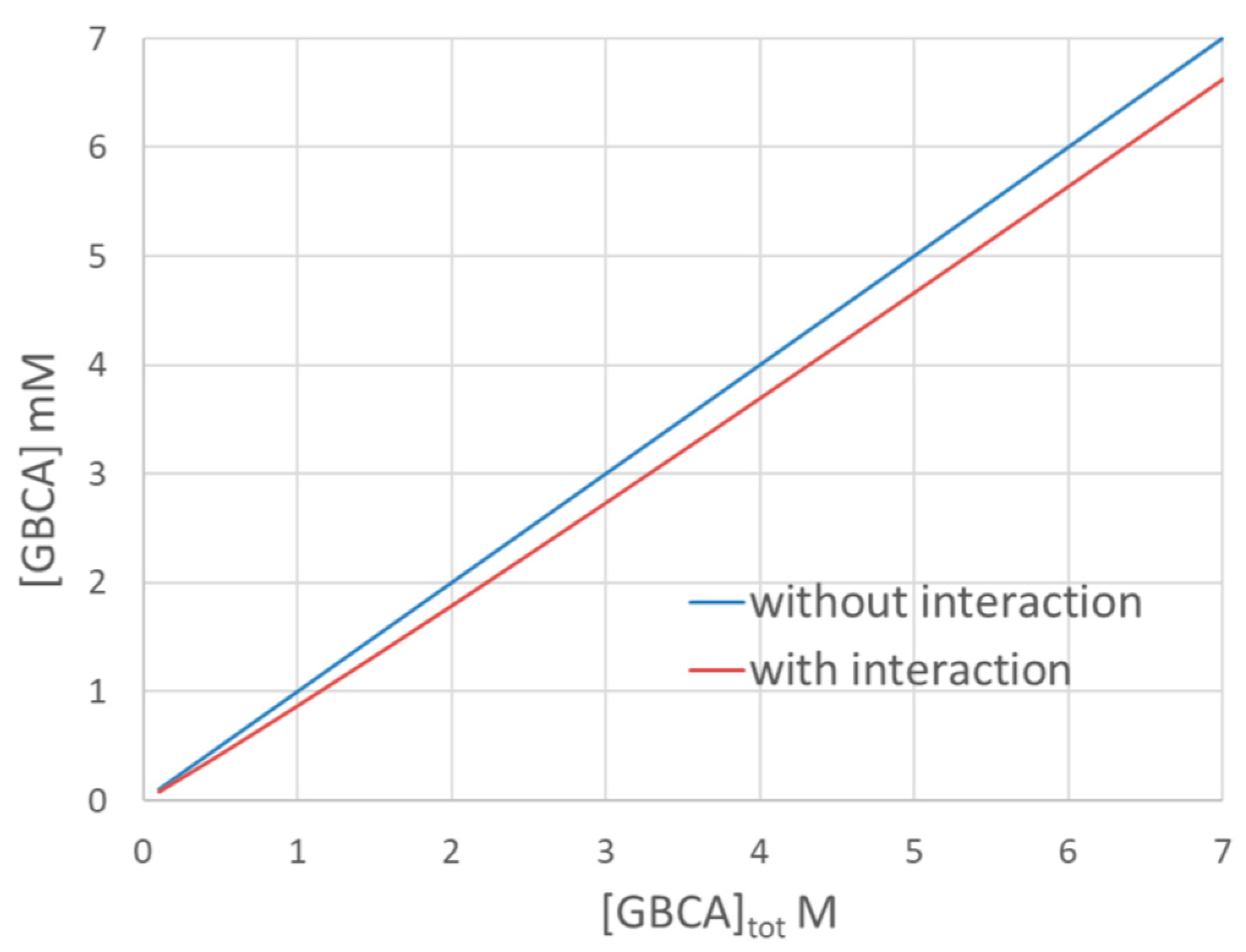
| Sample | BGE | UV Detection at 200 nm and 270 nm | LEDIF λex 275 nm λcollection 300–450 nm and λcollection 300–330 nm | ||||
|---|---|---|---|---|---|---|---|
| Slope (AU M−1) | LOD e (mM) | LOQ e (mM) | Slope (RFU mM−1) | LOD e (mM) | LOQ e (mM) | ||
| Gd-DTPA-BMA (Omniscan®) | Water | 57.7 a n.d. b | 0.10 a n.d. b | 0.32 a n.d. b | 0.058 c 0.033 d | 0.71 c 1.72 d | 2.36 c 5.72 d |
| Gd-BOPTA (MultiHance®) | Water | 51.2 a n.d. b | 0.18 a n.d. b | 0.61 a n.d. b | n.d. c n.d. d | n.d. c n.d. d | n.d. c n.d. d |
| Gd-HP-DO3A (Prohance®) | Tris-HCl | 1.96 a n.d. b | 0.84 a n.d. b | 2.81 a n.d. b | 0.171 c 0.075 d | 0.29 c 0.76 d | 0.97 c 2.52 d |
| Gd-BT-DO3A (Gadovist®) | Tris-HCl | 2.65 a n.d. b | 0.70 a n.d. b | 2.35 a n.d. b | 0.102 c 0.053 d | 1.50 c 0.39 d | 5.00 c 1.28 d |
| Gd-DOTA (Dotarem®) | Tris-HCl | 2.63 a n.d. b | 0.22 a n.d. b | 0.72 a n.d. b | 0.202 c 0.094 d | 0.56 c 1.06 d | 1.87 c 3.52 d |
| Gd-PCTA D2 (Elucirem®) | Tris-HCl | 85.6 a 16.3 b | 0.12 a 0.06 b | 0.40 a 0.20 b | 0.129 c 0.063 d | 0.43 c 0.88 d | 1.38 c 2.94 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somnin, C.; Chamieh, J.; Leclercq, L.; Medina, C.; Rousseaux, O.; Cottet, H. Reply to Guidolin et al. Comment on “Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633”. Pharmaceuticals 2025, 18, 1284. https://doi.org/10.3390/ph18091284
Somnin C, Chamieh J, Leclercq L, Medina C, Rousseaux O, Cottet H. Reply to Guidolin et al. Comment on “Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633”. Pharmaceuticals. 2025; 18(9):1284. https://doi.org/10.3390/ph18091284
Chicago/Turabian StyleSomnin, Chutintorn, Joseph Chamieh, Laurent Leclercq, Christelle Medina, Olivier Rousseaux, and Hervé Cottet. 2025. "Reply to Guidolin et al. Comment on “Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633”" Pharmaceuticals 18, no. 9: 1284. https://doi.org/10.3390/ph18091284
APA StyleSomnin, C., Chamieh, J., Leclercq, L., Medina, C., Rousseaux, O., & Cottet, H. (2025). Reply to Guidolin et al. Comment on “Somnin et al. Study of Interactions Between Gadolinium-Based Contrast Agents and Collagen by Taylor Dispersion Analysis and Frontal Analysis Continuous Capillary Electrophoresis. Pharmaceuticals 2024, 17, 1633”. Pharmaceuticals, 18(9), 1284. https://doi.org/10.3390/ph18091284







