Anti-Inflammatory and Antioxidant Effects of Topical Formulations Containing Plant Extracts, Methylsulfonylmethane, and Peptiskin® in In Vitro Models of Arthritis
Abstract
1. Introduction
2. Results
2.1. Particle Sizes and Zeta Potentials of AS632 and AS633
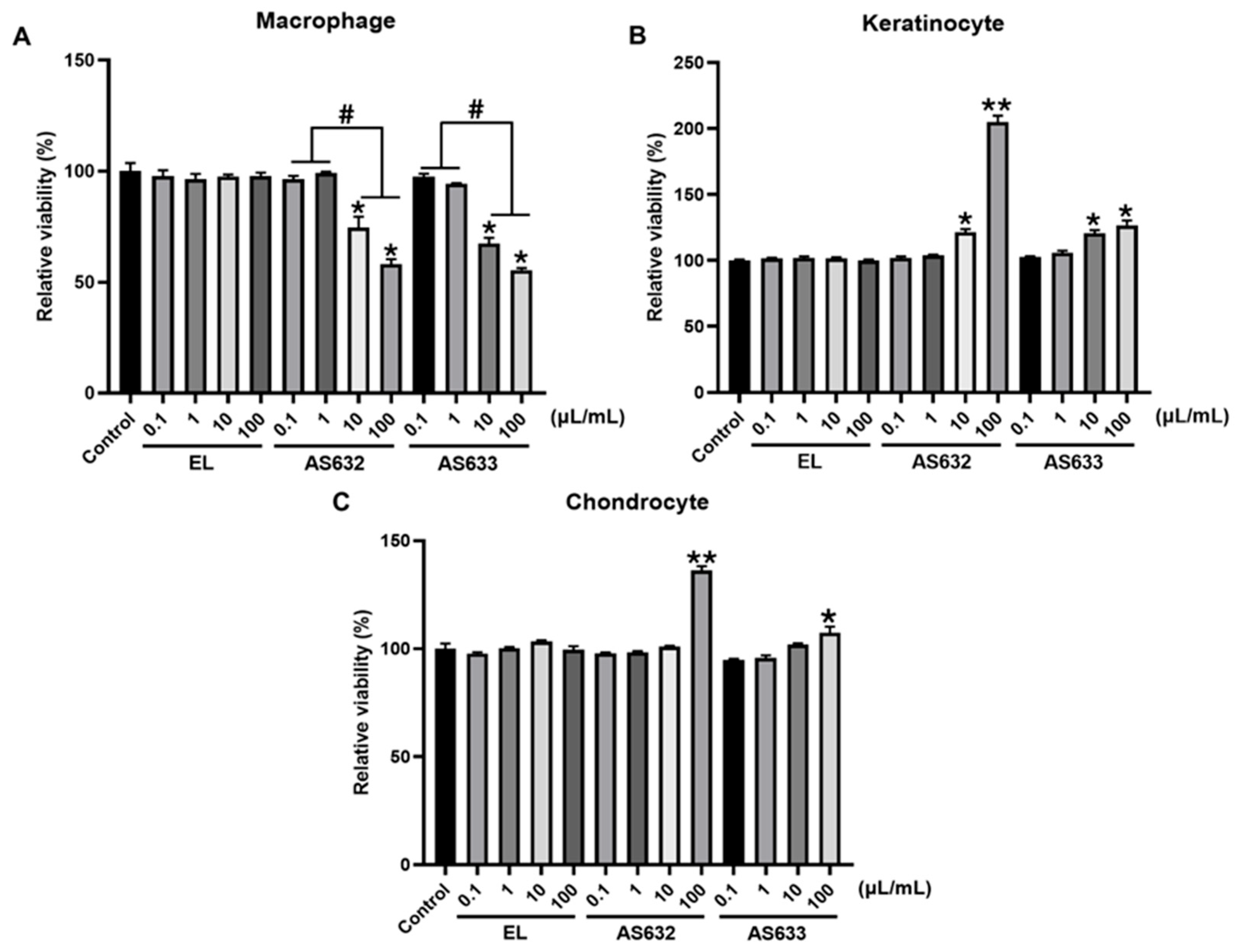
2.2. Cytotoxicity Analysis of AS632 and AS633
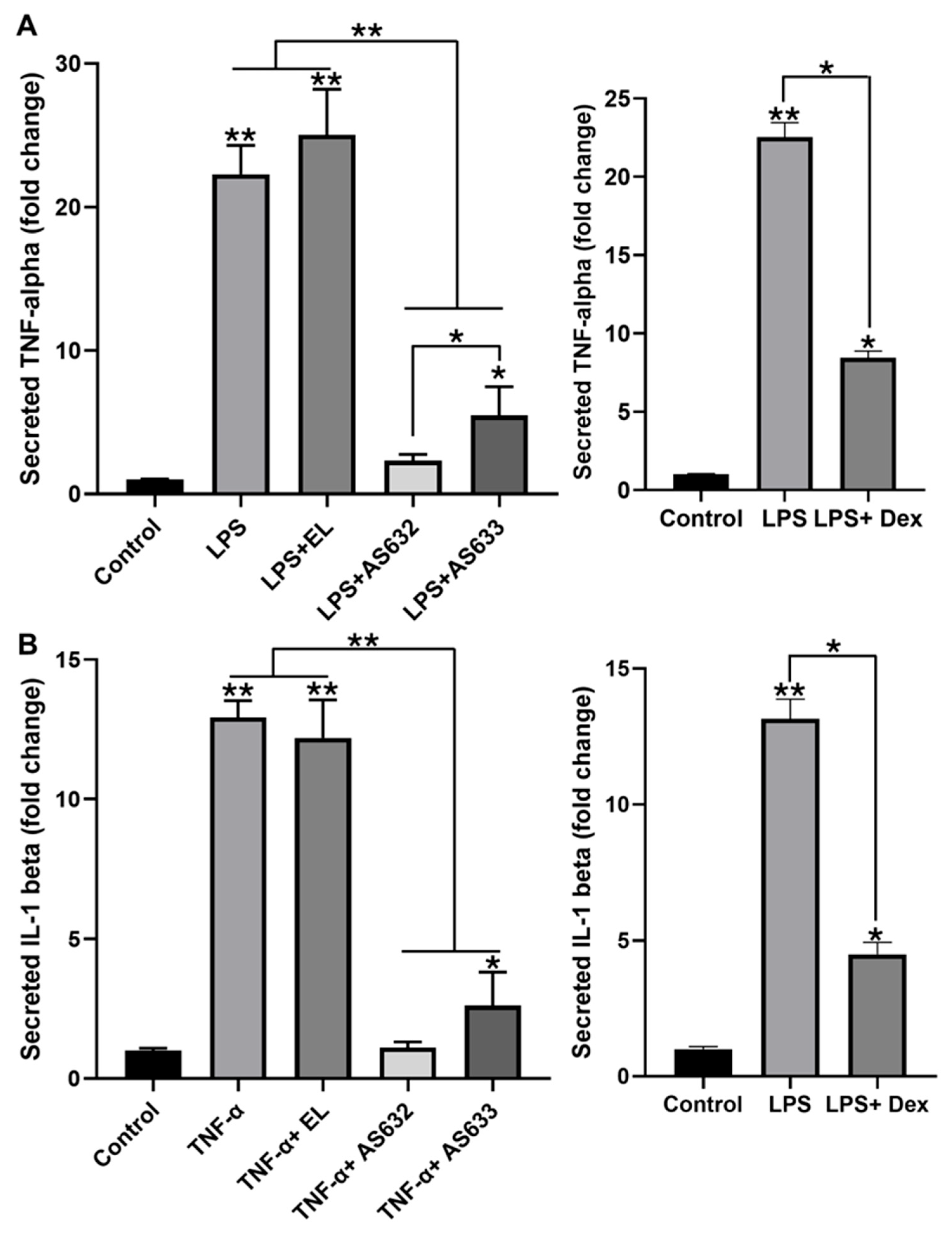
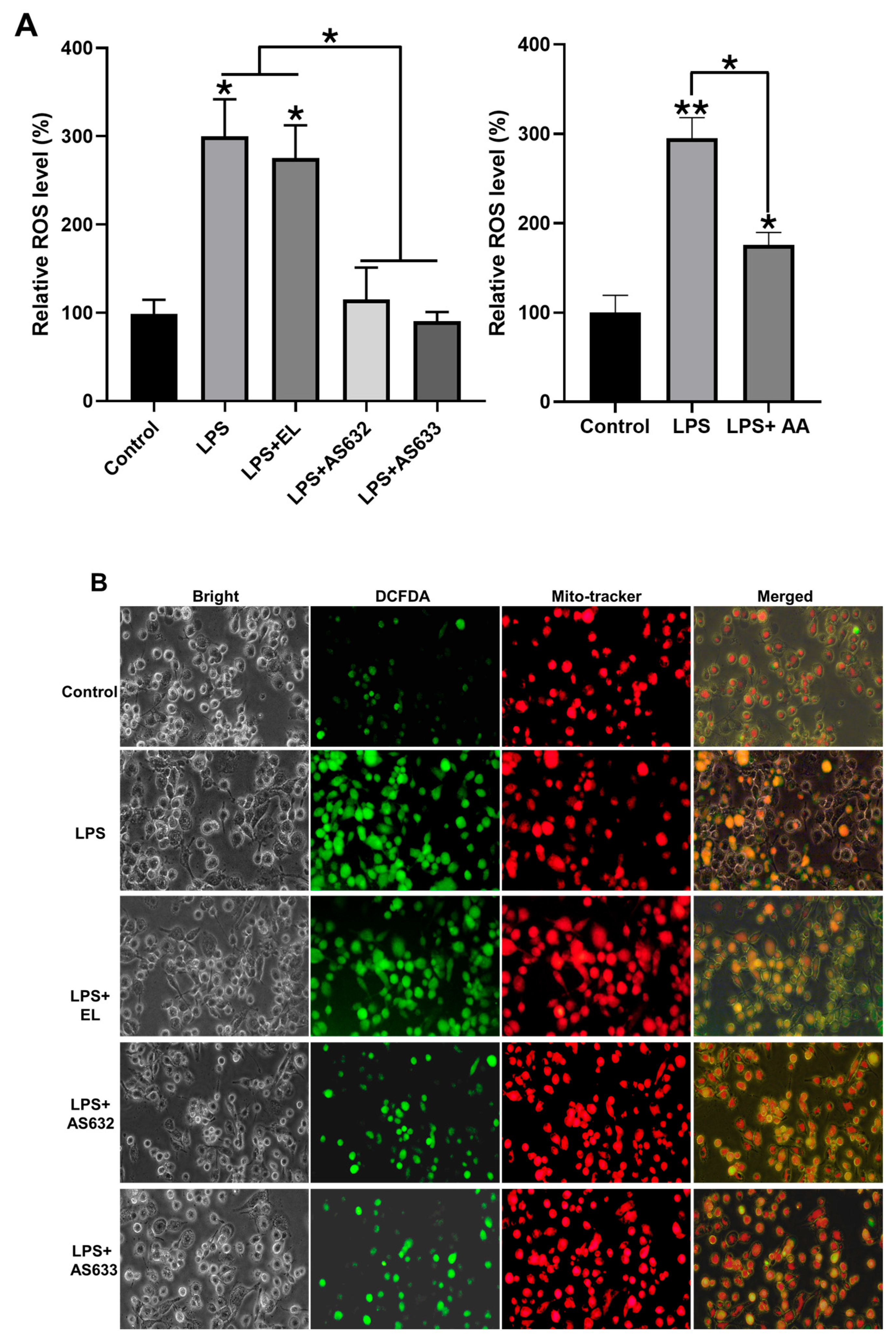
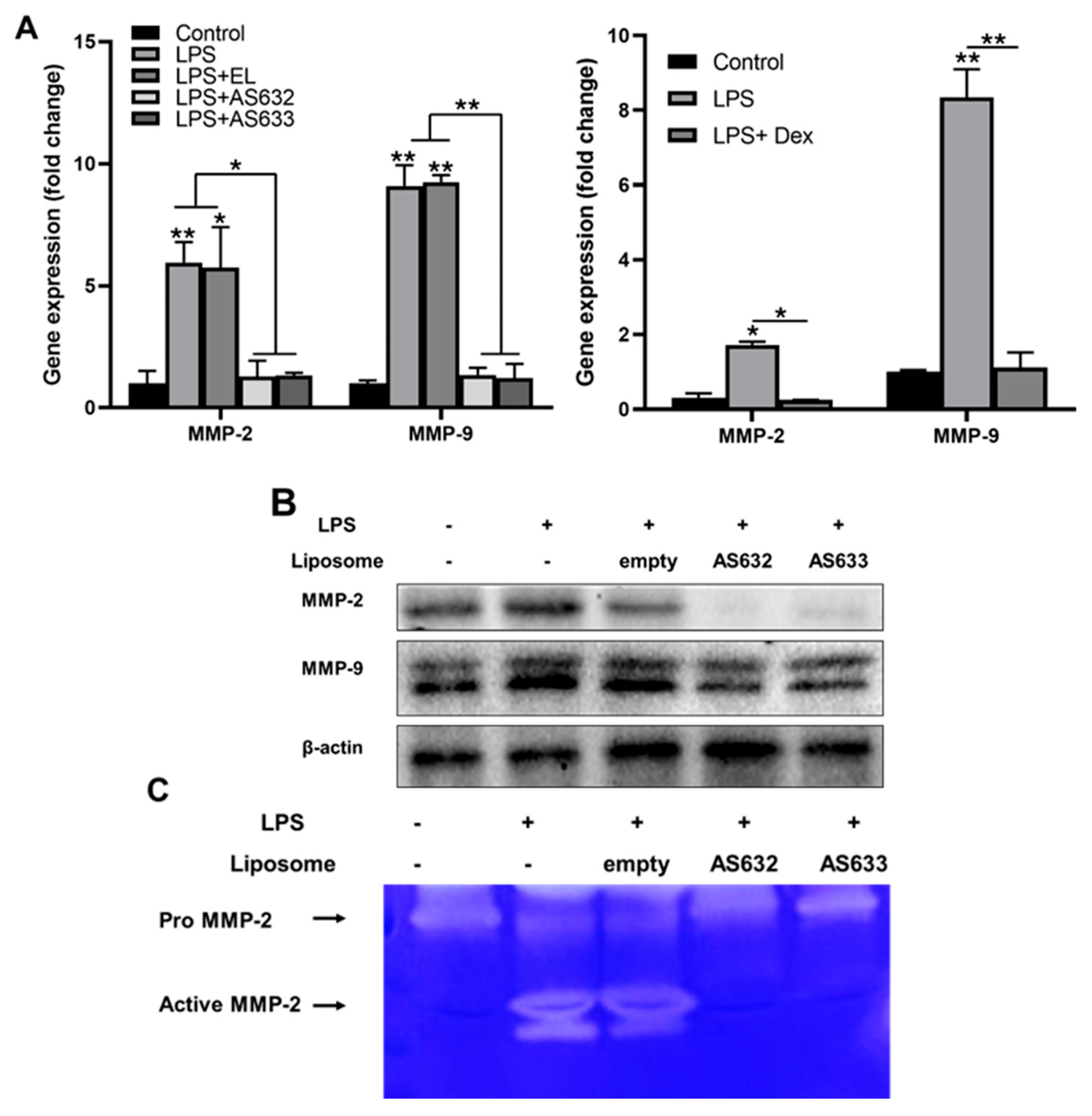
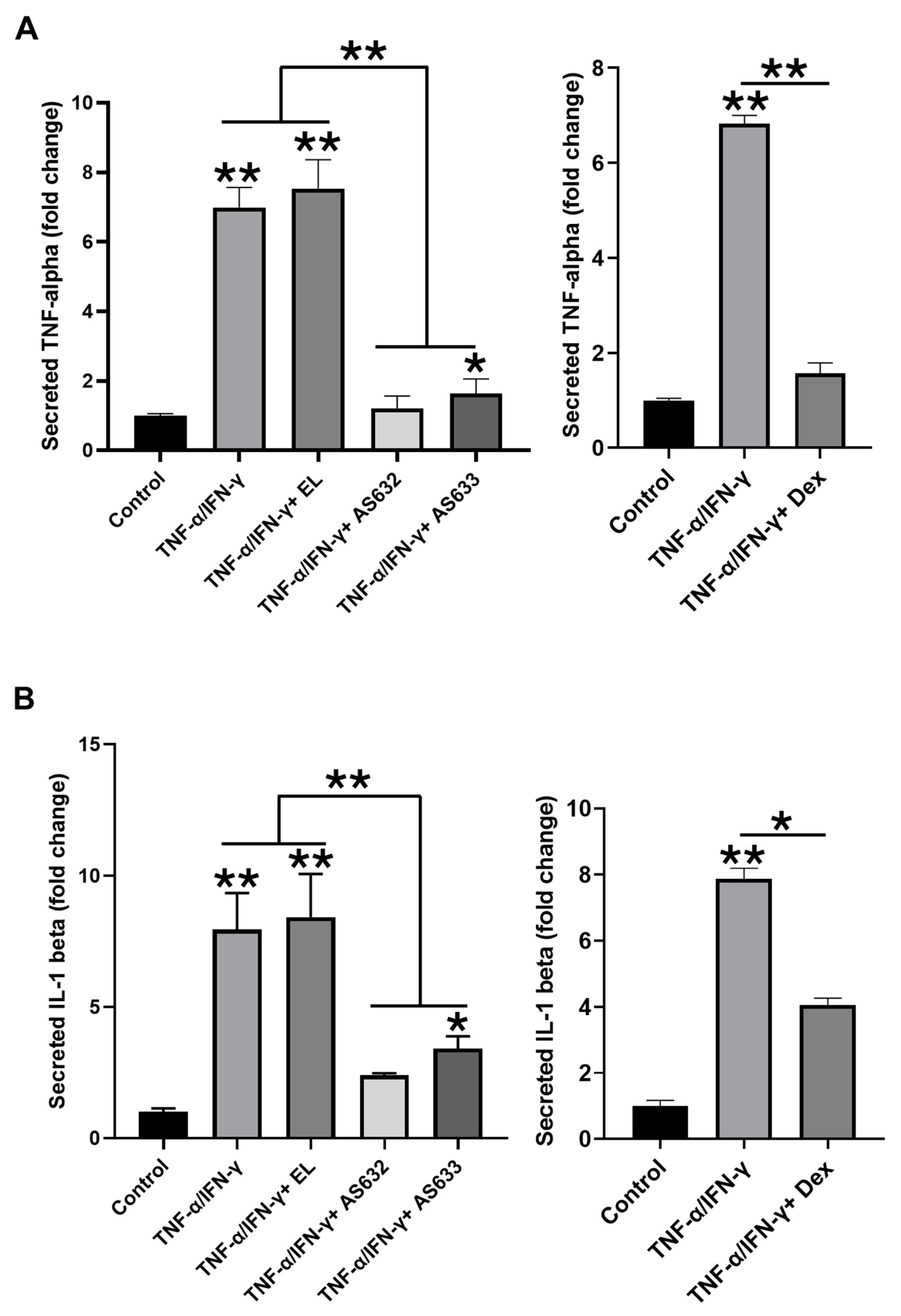
2.3. Anti-Inflammatory and Antioxidant Effects of AS632 in Macrophages
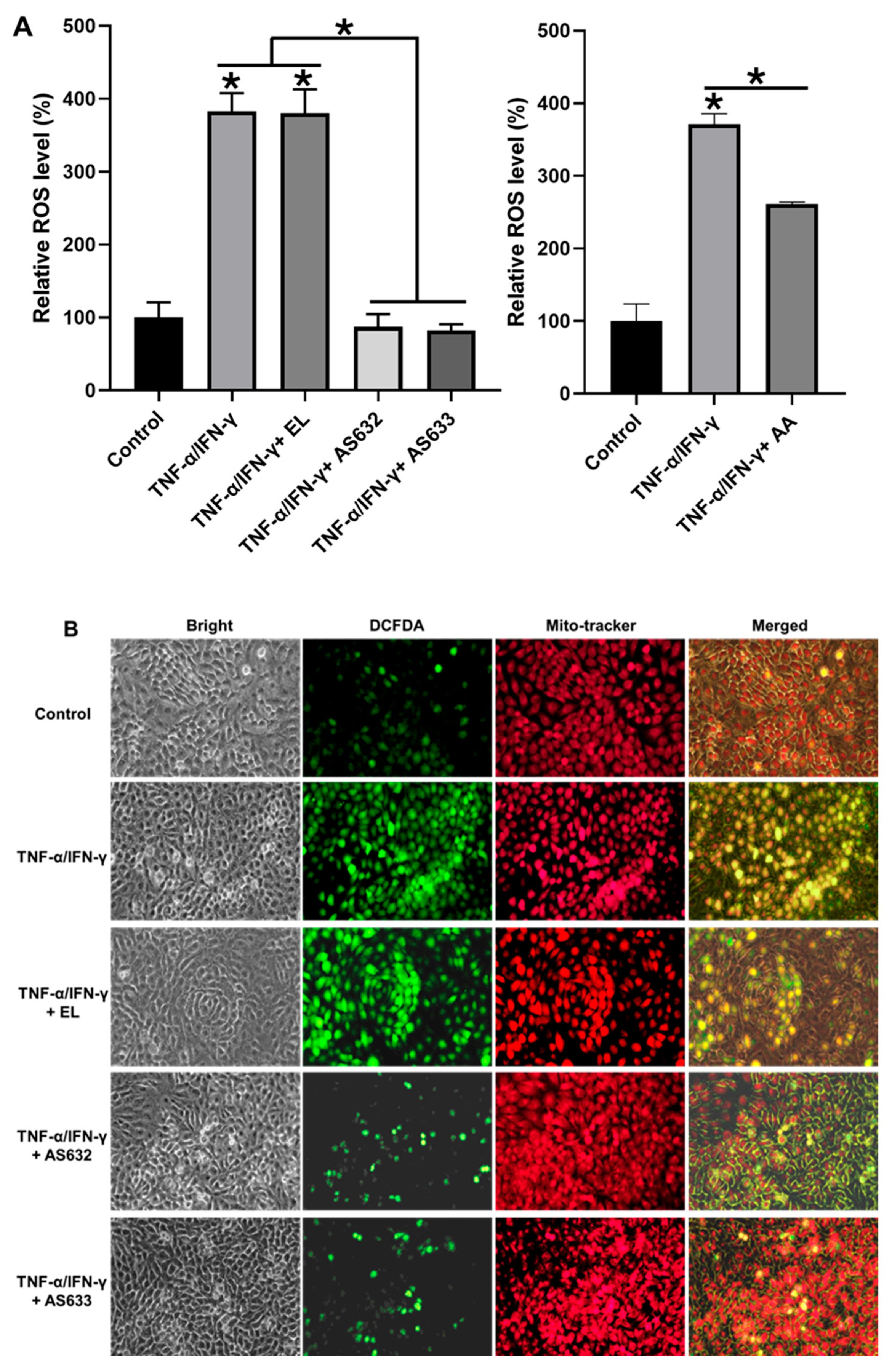
2.4. Anti-Inflammatory and Antioxidant Effects of AS632 in Keratinocytes
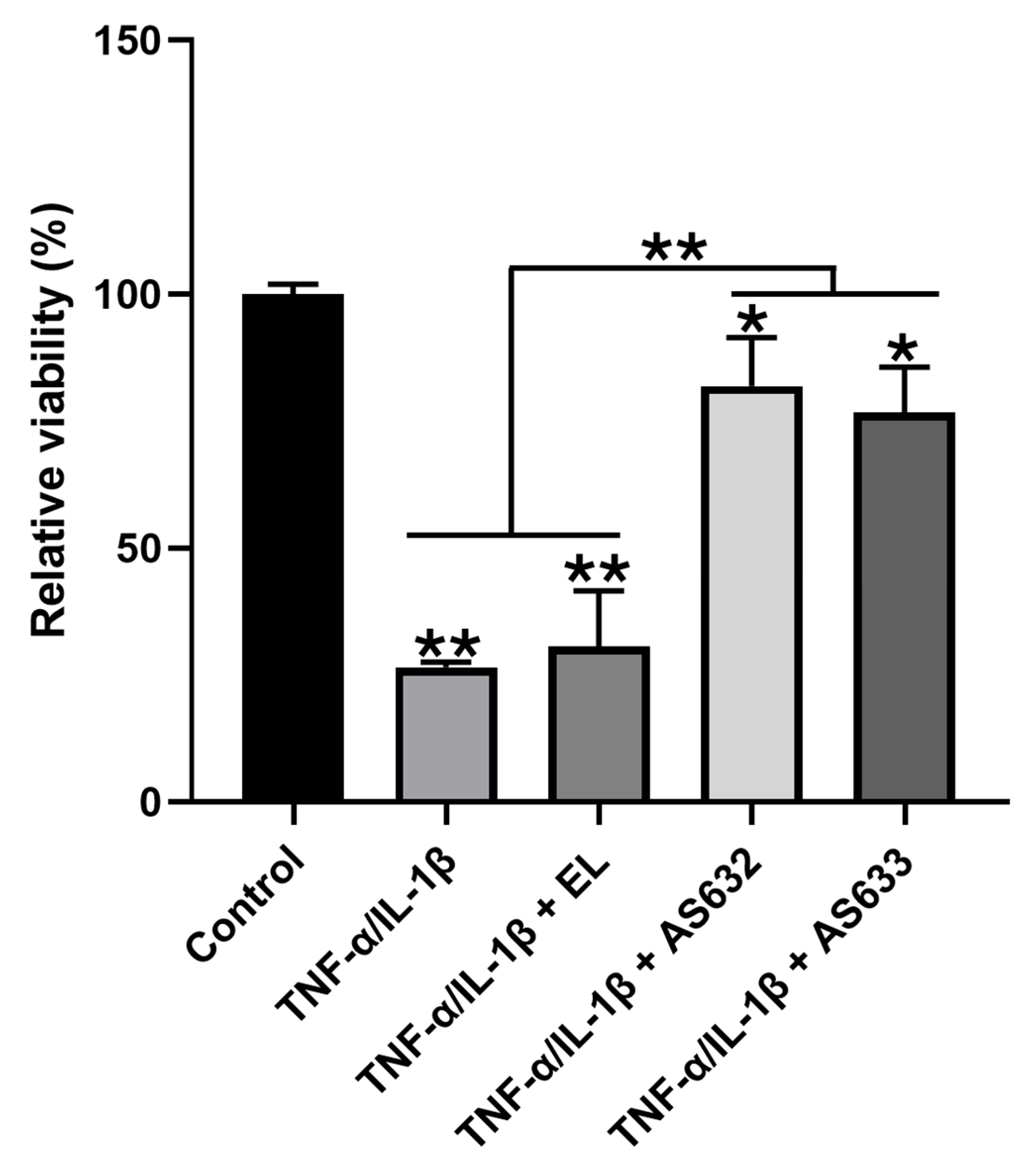
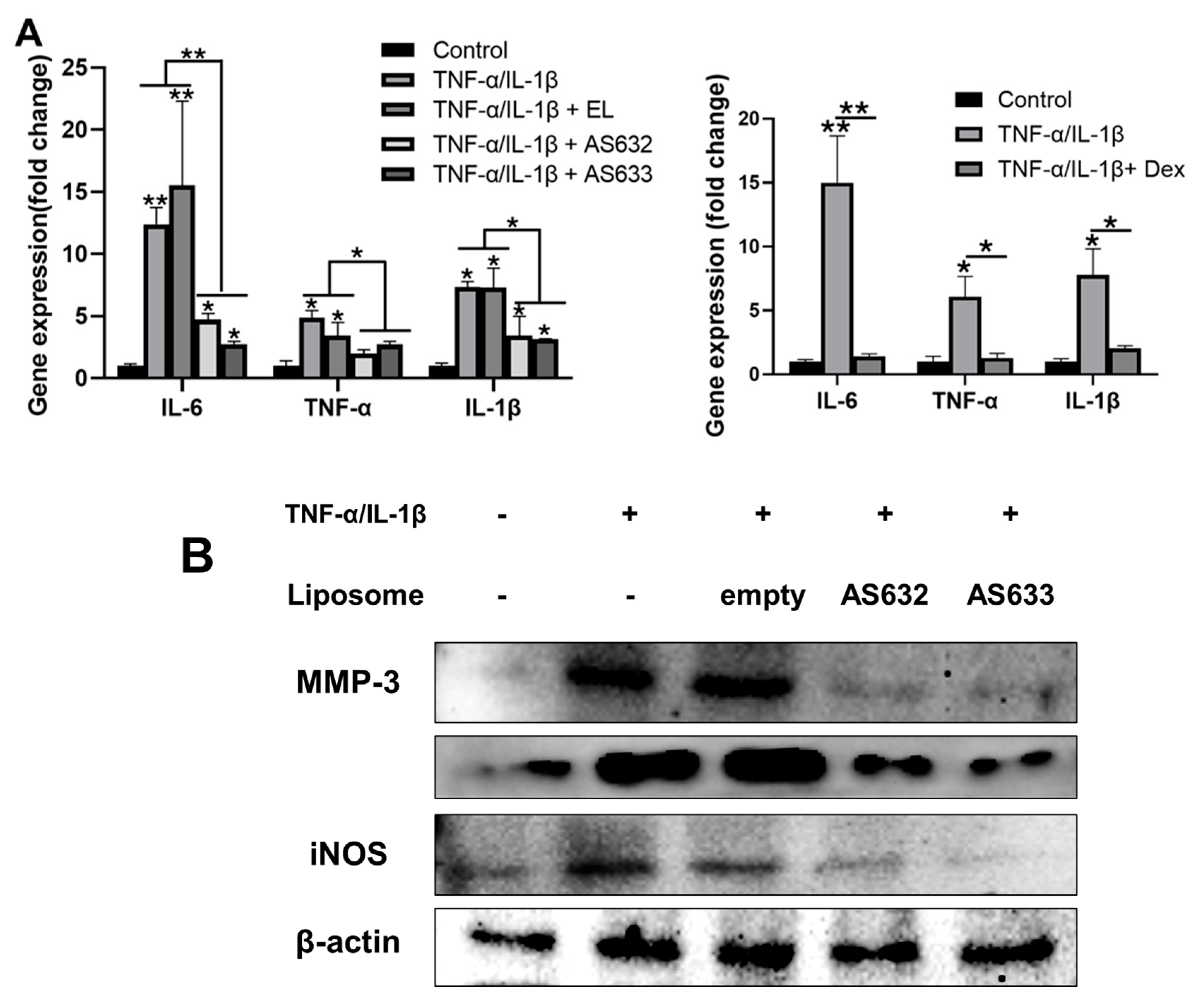
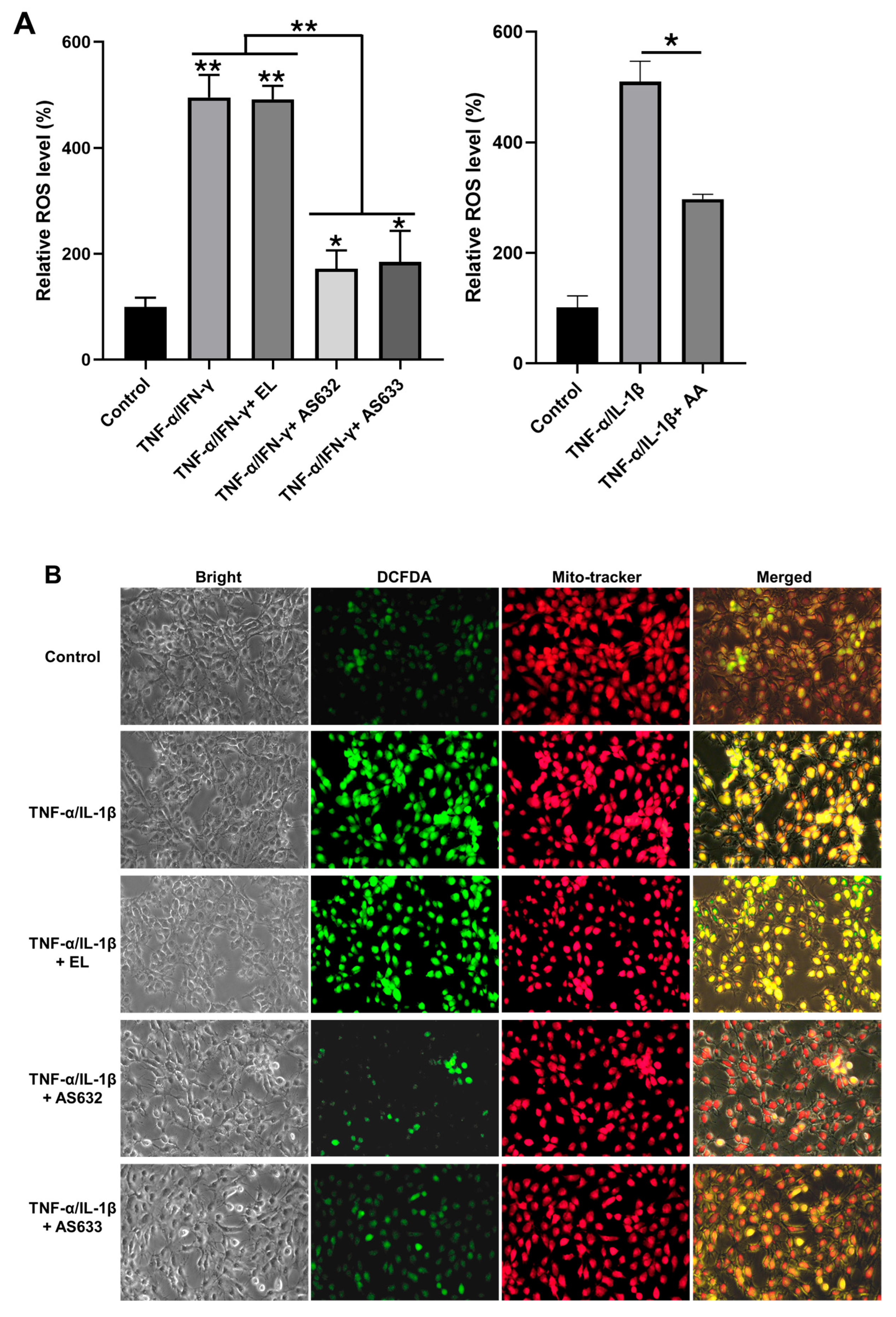
2.5. Anti-Inflammatory and Antioxidant Effects of AS632 in Chondrocytes
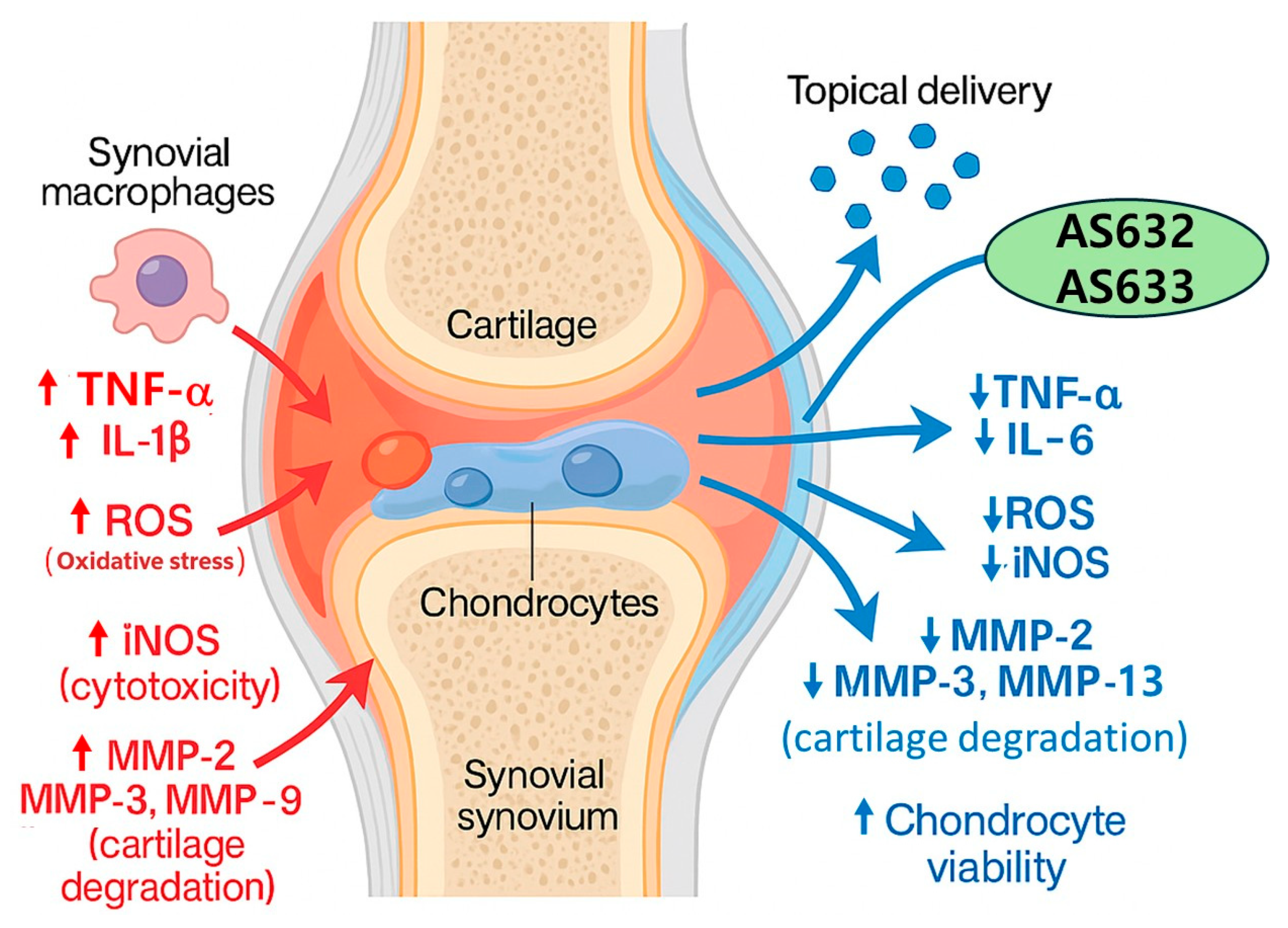
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Plant Extract Preparation
4.3. Preparation of AS632 and AS633
4.4. Particle Size and Zeta Potential Analysis
4.5. Cell Culture and Treatment
4.6. Cytotoxicity Testing by MTT Assay
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. RNA Preparation and Real-Time Quantitative PCR
4.9. Intracellular ROS Detection
4.10. Western Blot
4.11. Zymography
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| BSA | Bovine serum albumin |
| ELISA | Enzyme-linked immunosorbent assay |
| iNOS | Inducible nitric oxide synthase |
| LPS | Lipopolysaccharide |
| MMP | Matrix metalloproteinase |
| MSM | Methylsulfonylmethane |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| Peptiskin® | Synthetic peptides |
| ROS | Reactive oxygen species |
| TNF | Tumor necrosis factor |
References
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.P.; Fahmi, H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Malemud, C.J. The role of the JAK/STAT signal pathway in rheumatoid arthritis. Ther. Adv. Musculoskelet. Dis. 2019, 11, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- Saeed, M.; Naveed, M.; Bibi, J.; Kamboh, A.A.; Arain, M.A.; Shah, Q.A.; Alagawany, M.; El-Hack, M.E.A.; Abdel-Latif, M.A.; Yatoo, M.I.; et al. The promising pharmacological effects and therapeutic/medicinal applications of Punica Granatum L. (Pomegranate) as a functional food in humans and animals. Recent Pat. Inflamm. Allergy Drug Discov. 2018, 12, 24–38. [Google Scholar] [CrossRef]
- Rakhshandeh, H.; Rahimi, V.B.; Habibi, Z.; Sirousi, Z.; Askari, V.R. Punica Granatum seed oil detracts peritoneal adhesion: Perusing antioxidant, anti-inflammatory, antifibrotic, and antiangiogenic impacts. Physiol. Rep. 2022, 10, e15545. [Google Scholar] [CrossRef]
- Michel, P.; Wajs-Bonikowska, A.; Magiera, A.; Wosiak, A.; Balcerczak, E.; Czerwińska, M.E.; Olszewska, M.A. Anti-Inflammatory and antioxidant effects of (6S,9R)-vomifoliol from Gaultheria procumbens L.: In vitro and ex vivo study in human immune cell models. Int. J. Mol. Sci. 2025, 26, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Anti-inflammatory and anti-oxidative effects of Centella asiatica extract in lipopolysaccharide-stimulated BV2 microglial cells. Pharmacogn. Mag. 2019, 15, 140–146. [Google Scholar] [CrossRef]
- Kim, M.J.; Yang, Y.J.; Min, G.Y.; Heo, J.W.; Son, J.D.; You, Y.Z.; Kim, H.H.; Kim, G.S.; Lee, H.J.; Yang, J.H.; et al. Anti-inflammatory and antioxidant properties of Camellia sinensis L. extract as a potential therapeutic for atopic dermatitis through NF-κB pathway inhibition. Sci. Rep. 2025, 15, 2371. [Google Scholar] [CrossRef]
- Colletti, A.; Cicero, A.F.G. Nutraceutical approach to chronic osteoarthritis: From molecular research to clinical evidence. Int. J. Mol. Sci. 2021, 22, 12920. [Google Scholar] [CrossRef]
- Solabia Group. Peptiskin®—Active Ingredient—Cosmetics. Solabia Website. 2022. Available online: https://www.solabia.com/cosmetics/product/peptiskin/ (accessed on 19 August 2025).
- Goldring, M.B.; Goldring, S.R. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann. N. Y. Acad. Sci. 2010, 1192, 230–237. [Google Scholar] [CrossRef]
- Michel, P.; Żbikowska, H.M.; Rudnicka, K.; Gonciarz, W.; Krupa, A.; Gajewski, A.; Machała, P.; Olszewska, M.A. Anti-inflammatory, antioxidant and photoprotective activity of standardised Gaultheria procumbens L. leaf, stem, and fruit extracts in UVA-irradiated human dermal fibroblasts. J. Ethnopharmacol. 2024, 319, 117219. [Google Scholar] [CrossRef]
- Buranasudja, V.; Rani, D.; Malla, A.; Kobtrakul, K.; Vimolmangkang, S. Insights into antioxidant activities and anti-skin-aging potential of callus extract from Centella asiatica (L.). Sci. Rep. 2021, 11, 13459. [Google Scholar] [CrossRef]
- Kumari, S.; Deori, M.; Elancheran, R.; Kotoky, J.; Devi, R. In Vitro and in vivo antioxidant, anti-hyperlipidemic properties and chemical characterization of Centella asiatica (L.) extract. Front. Pharmacol. 2016, 7, 400. [Google Scholar] [CrossRef] [PubMed]
- Labouta, H.I.; Schneider, M. Interaction of inorganic nanoparticles with the skin barrier: Current status and critical review. Nanomed. Nanotechnol. Biol. Med. 2013, 9, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Davarani, F.H.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Romão, V.C.; Fonseca, J.E. Disease mechanisms in preclinical rheumatoid arthritis: A narrative review. Front. Med. 2022, 9, 689711. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage heterogeneity in the context of rheumatoid arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, Y.; Qiao, Z.; Hu, Y.; Zhang, H.; Zhang, X.; Huang, Y.; Xiang, Q. Efficacy of a Novel Lyophilized Arginine Lysine Polypeptide in the Treatment of Acne: In Vitro Experiments and a Split Face Controlled Clinical Trial. J. Biomed. Sci. Res. 2021, 3, 132. [Google Scholar]
- Avcil, M.; Akman, G.; Klokkers, J.; Jeong, D.; Çelik, A. Efficacy of bioactive peptides loaded on hyaluronic acid microneedle patches: A monocentric clinical study. J. Cosmet. Dermatol. 2020, 19, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hamdan, N.; Nyakuma, B.B.; Wong, S.L.; Wong, K.Y.; Tan, H.; Jamaluddin, H.; Lee, T.H. Purification, identification and molecular docking studies of antioxidant and anti-inflammatory peptides from Edible Bird’s Nest. Food Chem. 2024, 454, 139797. [Google Scholar] [CrossRef]
- Hamanaka, R.B.; Shin, K.W.D.; Atalay, M.V.; Cetin-Atalay, R.; Shah, H.; Szafran, J.C.H.; Woods, P.S.; Meliton, A.Y.; Shamaa, O.R.; Tian, Y.; et al. Role of arginine and its metabolism in TGF-β-induced activation of lung fibroblasts. bioRxiv 2024. [Google Scholar] [CrossRef]
- Han, H.; Yin, J.; Wang, B.; Huang, X.; Yao, J.; Zheng, J.; Fan, W.; Li, T.; Yin, Y. Effects of dietary lysine restriction on inflammatory responses in piglets. Sci. Rep. 2018, 8, 2451. [Google Scholar] [CrossRef]
- Lan, J.; Dou, X.; Li, J.; Yang, Y.; Xue, C.; Wang, C.; Gao, N.; Shan, A. L-arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-κB and MAPK pathways and stimulating β-defensin expression in vivo and in vitro. J. Agric. Food Chem. 2020, 68, 2648–2663. [Google Scholar] [CrossRef]
- Martins, L.; Leme, A.F.P.; Kantovitz, K.R.; de Luciane Martins En Sallum, E.A.; Casati, M.Z.; Nociti, F.H., Jr. Leucine-Rich Amelogenin Peptide (LRAP) Uptake by Cementoblast Requires Flotillin-1 Mediated Endocytosis. J. Cell. Physiol. 2017, 232, 556–565. [Google Scholar] [CrossRef]
- Burrage, P.S.; Mix, K.S.; Brinckerhoff, C.E. Matrix metalloproteinases: Role in arthritis. Front. Biosci. 2006, 11, 529–543. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. Ther. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef] [PubMed]
- Víteček, J.; Lojek, A.; Valacchi, G.; Kubala, L. Arginine-based inhibitors of nitric oxide synthase: Therapeutic potential and challenges. Mediat. Inflamm. 2012, 2012, 318087. [Google Scholar] [CrossRef] [PubMed]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
| Sample | Measurement Type | Replicate 1 | Replicate 2 | Replicate 3 |
|---|---|---|---|---|
| AS632 | Particle size (nm) | 238.6 | 236.4 | 241.9 |
| Zeta potential (mV) | −42.7 | −48.7 | −47.5 | |
| AS633 | Particle size (nm) | 217.0 | 235.8 | 227.4 |
| Zeta potential (mV) | −52.8 | −50.2 | −47.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang, T.X.; Dang, N.M.; Bae, K.G.; Kim, J.Y. Anti-Inflammatory and Antioxidant Effects of Topical Formulations Containing Plant Extracts, Methylsulfonylmethane, and Peptiskin® in In Vitro Models of Arthritis. Pharmaceuticals 2025, 18, 1270. https://doi.org/10.3390/ph18091270
Hoang TX, Dang NM, Bae KG, Kim JY. Anti-Inflammatory and Antioxidant Effects of Topical Formulations Containing Plant Extracts, Methylsulfonylmethane, and Peptiskin® in In Vitro Models of Arthritis. Pharmaceuticals. 2025; 18(9):1270. https://doi.org/10.3390/ph18091270
Chicago/Turabian StyleHoang, Thi Xoan, Nhat Minh Dang, Kang Gyu Bae, and Jae Young Kim. 2025. "Anti-Inflammatory and Antioxidant Effects of Topical Formulations Containing Plant Extracts, Methylsulfonylmethane, and Peptiskin® in In Vitro Models of Arthritis" Pharmaceuticals 18, no. 9: 1270. https://doi.org/10.3390/ph18091270
APA StyleHoang, T. X., Dang, N. M., Bae, K. G., & Kim, J. Y. (2025). Anti-Inflammatory and Antioxidant Effects of Topical Formulations Containing Plant Extracts, Methylsulfonylmethane, and Peptiskin® in In Vitro Models of Arthritis. Pharmaceuticals, 18(9), 1270. https://doi.org/10.3390/ph18091270






