The Therapeutic Potential of Dietary Phytochemicals in Age-Related Neurodegenerative Disorders
Abstract
1. Introduction
2. Relationship Between Aging and Neurodegenerative Disorders
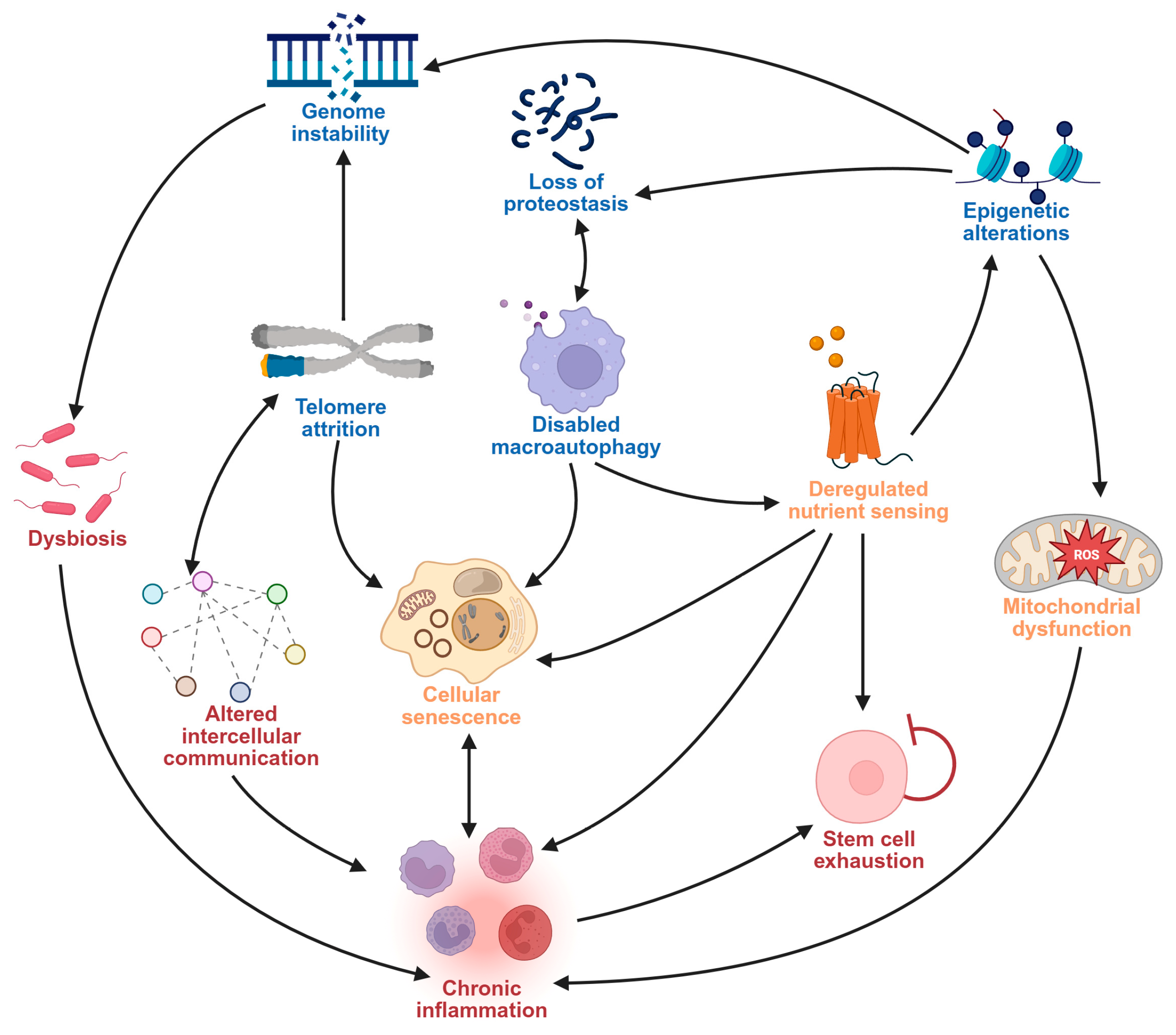
2.1. Hallmarks of Aging as Drivers of Neurodegeneration
2.2. Age-Related Mechanisms in Neurodegenerative Disorders
2.2.1. Major Neurocognitive Disorder
2.2.2. Parkinsonism-Type Diseases (PTD)
2.2.3. Motor Neuron Diseases (MNDs)
2.2.4. Huntington’s Disease (HTTD)
3. Current Treatment and Management Strategies of Age-Related Neurodegenerative Disorders
3.1. Pharmacological Interventions in the Management of Age-Related Neurodegenerative Disorders
3.2. Non-Pharmacological Interventions in the Management of Age-Related Neurodegenerative Disorders
3.3. Limitations in Current Intervention Methods for the Management of Age-Related Neurodegenerative Disorders
4. Dietary Phytochemicals in Age-Related Neurodegenerative Diseases
4.1. Types, Properties, and Anti-Aging Activities of Dietary Phytochemicals
4.2. Bioavailability and Transport Mechanism of Dietary Phytochemicals for Neuroprotection
4.2.1. Bioavailability of Dietary Phytochemicals
4.2.2. Transport of Dietary Phytochemicals to the Brain
Transport of Dietary Phytochemicals Across the BBB
Contribution of the GBA in Dietary Phytochemical-Based Neuroprotection
4.3. Dietary Phytochemical-Based Neuroprotection
4.3.1. Neuroprotective Functions of Dietary Phytochemicals: Evidence from Pre-Clinical Studies
4.3.2. Neuroprotective Functions of Dietary Phytochemicals: Evidence from Clinical Trials
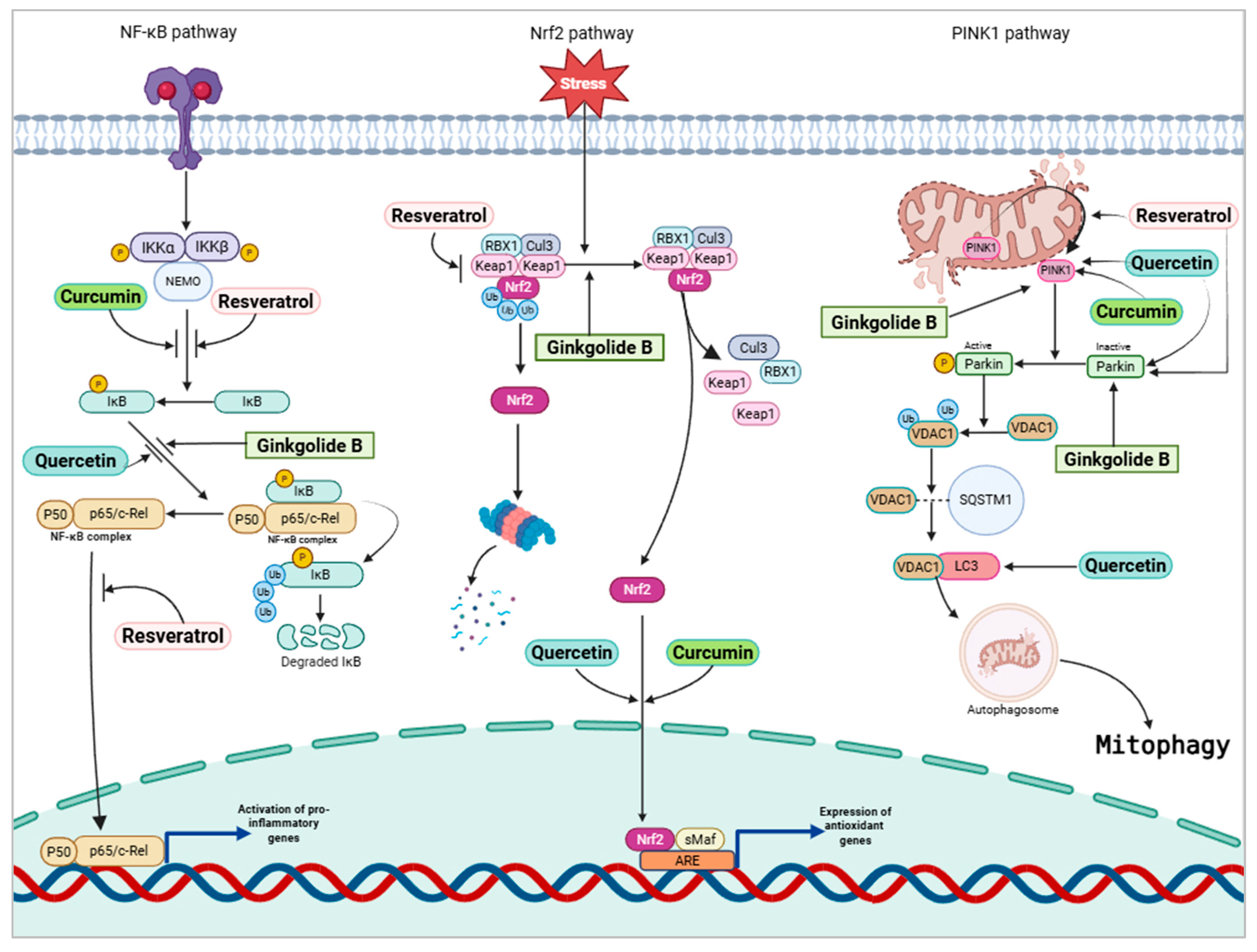
4.3.3. Mechanistic Pathways of Dietary Phytochemical-Based Neuroprotection
4.3.4. Comparing the Therapeutic Potential of Dietary Phytochemicals
5. Challenges and Potential Solutions to Using Dietary Phytochemicals as Remedy for Age-Related Neurodegenerative Disorders
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Yang, Y.; Schwebel, D.C.; Liu, Z.; Li, L.; Cheng, P.; Ning, P.; Hu, G. Population ageing and mortality during 1990–2017: A global decomposition analysis. PLoS Med. 2020, 17, e1003138. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, L. Relationship between aging population, birth rate and disposable income per capita in the context of COVID-19. PLoS ONE 2023, 18, e0289781. [Google Scholar] [CrossRef] [PubMed]
- GBD 2021 Causes of Death Collaborators. Global burden of 288 causes of death and life expectancy decomposition in 204 countries and territories and 811 subnational locations, 1990–2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2024, 403, 2100–2132. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.T.; Addo, K.M.; Findlay, H. Public health challenges and responses to the growing ageing populations. Public Health Chall. 2024, 3, e213. [Google Scholar] [CrossRef]
- Montégut, L.; López-Otín, C.; Kroemer, G. Aging and cancer. Mol. Cancer 2024, 23, 106. [Google Scholar] [CrossRef]
- Bacanoiu, M.V.; Danoiu, M. New strategies to improve the quality of life for normal aging versus pathological aging. J. Clin. Med. 2022, 11, 4207. [Google Scholar] [CrossRef]
- Nabi, M.; Tabassum, N. Role of environmental toxicants on neurodegenerative disorders. Front. Toxicol. 2022, 4, 837579. [Google Scholar] [CrossRef]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E. The worldwide costs of dementia in 2019. Alzheimer Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef]
- Palanisamy, C.P.; Pei, J.; Alugoju, P.; Anthikapalli, N.V.A.; Jayaraman, S.; Veeraraghavan, V.P.; Gopathy, S.; Roy, J.R.; Janaki, C.S.; Thalamati, D. New strategies of neurodegenerative disease treatment with extracellular vesicles (EVs) derived from mesenchymal stem cells (MSCs). Theranostics 2023, 13, 4138. [Google Scholar] [CrossRef]
- Xavier, J.R.; Sameer, B.; Gupta, D.; Mehta, S.; Chauhan, O.P. Bioactive compounds of foods: Phytochemicals and peptides. Food Hum. 2024, 3, 100354. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Muscolo, A.; Mariateresa, O.; Giulio, T.; Mariateresa, R. Oxidative stress: The role of antioxidant phytochemicals in the prevention and treatment of diseases. Int. J. Mol. Sci. 2024, 25, 3264. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.-Y.; Chen, X.; Liu, G.-H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Guerrero, A.; Pallàs, M. Advancing personalized medicine in neurodegenerative diseases: The role of epigenetics and pharmacoepigenomics in pharmacotherapy. Pharmacol. Res. 2024, 205, 107247. [Google Scholar] [CrossRef]
- Solovev, I.; Sergeeva, A.; Geraskina, A.; Shaposhnikov, M.; Vedunova, M.; Borysova, O.; Moskalev, A. Aging and physiological barriers: Mechanisms of barrier integrity changes and implications for age-related diseases. Mol. Biol. Rep. 2024, 51, 917. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Tramutola, A.; Rojo, A.I.; Chondrogianni, N.; Saha, S.; Berry, A.; Giona, L.; Miranda, J.P.; Profumo, E.; Davinelli, S. Proteostasis Decline and Redox Imbalance in Age-Related Diseases: The Therapeutic Potential of NRF2. Biomolecules 2025, 15, 113. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Huang, R.; Zhou, P.-K. DNA damage repair: Historical perspectives, mechanistic pathways and clinical translation for targeted cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 254. [Google Scholar] [CrossRef]
- Chandimali, N.; Bak, S.G.; Park, E.H.; Lim, H.-J.; Won, Y.-S.; Kim, E.-K.; Park, S.-I.; Lee, S.J. Free radicals and their impact on health and antioxidant defenses: A review. Cell Death Discov. 2025, 11, 19. [Google Scholar] [CrossRef]
- Shadfar, S.; Parakh, S.; Jamali, M.S.; Atkin, J.D. Redox dysregulation as a driver for DNA damage and its relationship to neurodegenerative diseases. Transl. Neurodegener. 2023, 12, 18. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tian, X.; Luo, J.; Bao, T.; Wang, S.; Wu, X. Molecular mechanisms of aging and anti-aging strategies. Cell Commun. Signal. 2024, 22, 285. [Google Scholar] [CrossRef]
- Meaza, I.; Cahill, C.R.; Speer, R.M.; Kouokam, J.C.; Wise, J.P., Sr. Particulate hexavalent chromium inhibits global transcription of genes in DNA repair pathways, particularly targeting homologous recombination repair, base excision repair, mismatch repair and microhomology-mediated end-joining. J. Hazard. Mater. 2025, 485, 136892. [Google Scholar] [CrossRef]
- Schumacher, B.; Pothof, J.; Vijg, J.; Hoeijmakers, J.H.J. The central role of DNA damage in the ageing process. Nature 2021, 592, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Panier, S.; Wang, S.; Schumacher, B. Genome instability and DNA repair in somatic and reproductive aging. Annu. Rev. Pathol. 2024, 19, 261–290. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, L. Genome stability from the perspective of telomere length. Trends Genet. 2024, 40, 175–186. [Google Scholar] [CrossRef]
- Wu, Z.; Qu, J.; Liu, G.-H. Roles of chromatin and genome instability in cellular senescence and their relevance to ageing and related diseases. Nat. Rev. Mol. Cell Biol. 2024, 25, 979–1000. [Google Scholar] [CrossRef]
- Wang, K.; Liu, H.; Hu, Q.; Wang, L.; Liu, J.; Zheng, Z.; Zhang, W.; Ren, J.; Zhu, F.; Liu, G.-H. Epigenetic regulation of aging: Implications for interventions of aging and diseases. Sig. Trans. Target. Ther. 2022, 7, 374. [Google Scholar] [CrossRef]
- Hadad, N.; Masser, D.R.; Blanco-Berdugo, L.; Stanford, D.R.; Freeman, W.M. Early-life DNA methylation profiles are indicative of age-related transcriptome changes. Epigenetics Chromatin 2019, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Lettieri-Barbato, D.; Aquilano, K.; Punziano, C.; Minopoli, G.; Faraonio, R. MicroRNAs, long non-coding RNAs, and circular RNAs in the redox control of cell senescence. Antioxidants 2022, 11, 480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tan, Y.; Zhang, Z.; Yi, M.; Zhu, L.; Peng, W. The interaction between ageing and Alzheimer’s disease: Insights from the hallmarks of ageing. Transl. Neurodegener. 2024, 13, 7. [Google Scholar] [CrossRef]
- Gómez-Virgilio, L.; Silva-Lucero, M.-D.-C.; Flores-Morelos, D.-S.; Gallardo-Nieto, J.; Lopez-Toledo, G.; Abarca-Fernandez, A.-M.; Zacapala-Gómez, A.-E.; Luna-Muñoz, J.; Montiel-Sosa, F.; Soto-Rojas, L.O. Autophagy: A key regulator of homeostasis and disease: An overview of molecular mechanisms and modulators. Cells 2022, 11, 2262. [Google Scholar] [CrossRef]
- Lim, S.H.; Hansen, M.; Kumsta, C. Molecular mechanisms of autophagy decline during aging. Cells 2024, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Varela-Eirín, M.; Demaria, M. Cellular senescence. Curr. Biol. 2022, 32, R448–R452. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Fu, Q.; Long, Q.; Liu, S.; Zou, Y.; Fu, D.; Xu, Q.; Jiang, Z.; Ren, X.; Zhang, G.; et al. PDK4-dependent hypercatabolism and lactate production of senescent cells promotes cancer malignancy. Nat. Metab. 2023, 5, 1887–1910. [Google Scholar] [CrossRef] [PubMed]
- Miwa, S.; Kashyap, S.; Chini, E.; von Zglinicki, T. Mitochondrial dysfunction in cell senescence and aging. J. Clin. Investig. 2022, 132, e158447. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Tafaro, L.; Cianferotti, L.; Tramontana, F.; Macchione, I.G.; Caffarelli, C.; Virdis, A.; Ferracci, M.; Rinonapoli, G.; Mecocci, P. Targeting the Hallmarks of Aging with Vitamin D: Starting to Decode the Myth. Nutrients 2024, 16, 906. [Google Scholar] [CrossRef]
- Molinero, N.; Antón-Fernández, A.; Hernández, F.; Ávila, J.; Bartolomé, B.; Moreno-Arribas, M.V. Gut microbiota, an additional hallmark of human aging and neurodegeneration. Neuroscience 2023, 518, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Xiong, M.; Chen, H.; Liu, Y.; Zhou, L.; Hong, Y.; Wang, M.; Wang, C.; Fu, X.; Sun, X. Cellular rejuvenation: Molecular mechanisms and potential therapeutic interventions for diseases. Signal Transduct. Target. Ther. 2023, 8, 116. [Google Scholar] [CrossRef]
- Sachdev, P.S.; Blacker, D.; Blazer, D.G.; Ganguli, M.; Jeste, D.V.; Paulsen, J.S.; Petersen, R.C. Classifying neurocognitive disorders: The DSM-5 approach. Nat. Rev. Neurol. 2014, 10, 634–642. [Google Scholar] [CrossRef]
- Cipriani, G.; Danti, S.; Picchi, L.; Nuti, A.; Fiorino, M.D. Daily functioning and dementia. Dement. Neuropsychol. 2020, 14, 93–102. [Google Scholar] [CrossRef]
- Sharifi, F.; Ghandali, R.; Alimohammadi, M.; Ahmadipour, P. Symptoms and diagnosis of dementia. In Nutrition in Brain Aging and Dementia; Moradikor, N., Chatterjee, I., Mohamed, W., Eds.; Springer: Singapore, 2024; pp. 59–91. [Google Scholar] [CrossRef]
- Bir, S.C.; Khan, M.W.; Javalkar, V.; Toledo, E.G.; Kelley, R.E. Emerging concepts in vascular dementia: A review. J. Stroke Cerebrovasc. Dis. 2021, 30, 105864. [Google Scholar] [CrossRef] [PubMed]
- Kamatham, P.T.; Shukla, R.; Khatri, D.K.; Vora, L.K. Pathogenesis, diagnostics, and therapeutics for Alzheimer’s disease: Breaking the memory barrier. Ageing Res. Rev. 2024, 101, 102481. [Google Scholar] [CrossRef]
- Simon, C.; Soga, T.; Okano, H.J.; Parhar, I. α-Synuclein-mediated neurodegeneration in Dementia with Lewy bodies: The pathobiology of a paradox. Cell Biosci. 2021, 11, 196. [Google Scholar] [CrossRef]
- Latif, S.; Jahangeer, M.; Razia, D.M.; Ashiq, M.; Ghaffar, A.; Akram, M.; Allam, A.E.; Bouyahya, A.; Garipova, L.; Shariati, M.A.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta. 2021, 522, 114–126. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Zhou, J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl. Neurodegener. 2015, 4, 19. [Google Scholar] [CrossRef]
- Salim, S.; Ahmad, F.; Banu, A.; Mohammad, F. Gut microbiome and Parkinson’s disease: Perspective on pathogenesis and treatment. J. Adv. Res. 2023, 50, 83–105. [Google Scholar] [CrossRef] [PubMed]
- Yedavalli, V.S.; Patil, A.; Shah, P. Amyotrophic Lateral Sclerosis and its Mimics/Variants: A Comprehensive Review. J. Clin. Imaging Sci. 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Dashtmian, A.R.; Darvishi, F.B.; Arnold, W.D. Chronological and Biological Aging in Amyotrophic Lateral Sclerosis and the Potential of Senolytic Therapies. Cells 2024, 13, 928. [Google Scholar] [CrossRef]
- Machiela, E.; Southwell, A.L. Biological aging and the Cellular Pathogenesis of Huntington’s Disease. J. Huntingtons Dis. 2020, 9, 115–128. [Google Scholar] [CrossRef]
- Ihunwo, A.O.; Tembo, L.H.; Dzamalala, C. The dynamics of adult neurogenesis in human hippocampus. Neural Regen. Res. 2016, 11, 1869–1883. [Google Scholar] [CrossRef]
- Singh, K.; Gupta, J.K.; Kumar, S.; Soni, U. A Review of the Common Neurodegenerative Disorders: Current Therapeutic Approaches and the Potential Role of Bioactive Peptides. Curr. Protein Pept. Sci. 2024, 25, 507–526. [Google Scholar] [CrossRef]
- Moreira, N.; Lima, J.; Marchiori, M.F.; Carvalho, I.; Sakamoto-Hojo, E.T. Neuroprotective Effects of Cholinesterase Inhibitors: Current Scenario in Therapies for Alzheimer’s Disease and Future Perspectives. J. Alzheimer’s Dis. Rep. 2022, 6, 177–193. [Google Scholar] [CrossRef]
- Tari, P.K.; Parsons, C.G.; Collingridge, G.L.; Rammes, G. Memantine: Updating a rare success story in pro-cognitive therapeutics. Neuropharmacology 2024, 244, 109737. [Google Scholar] [CrossRef]
- Zhuo, C.; Zhu, X.; Jiang, R.; Ji, F.; Su, Z.; Xue, R.; Zhou, Y. Comparison for Efficacy and Tolerability among Ten Drugs for Treatment of Parkinson’s Disease: A Network Meta-Analysis. Sci. Rep. 2017, 8, 45865. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Application of Antipsychotic Drugs in Mood Disorders. Brain Sci. 2023, 13, 414. [Google Scholar] [CrossRef]
- Gurevich, A.; Guller, V.; Berner, Y.N.; Tal, S. Are atypical antipsychotics safer than typical antipsychotics for treating behavioral and psychological symptoms of dementia? J. Nutr. Health Aging 2012, 16, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Kunisawa, N.; Shimizu, S. Antipsychotic Treatment of Behavioral and Psychological Symptoms of Dementia (BPSD): Management of Extrapyramidal Side Effects. Front. Pharmacol. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Durães, F.; Pinto, M.; Sousa, E. Old Drugs as New Treatments for Neurodegenerative Diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef]
- Niidome, T.; Ishikawa, Y.; Ogawa, T.; Nakagawa, M.; Nakamura, Y. Mechanism of action and clinical trial results of Lecanemab (Leqembi(®) 200 mg, 500 mg for Intravenous Infusion), a novel treatment for Alzheimer’s disease. Nihon Yakurigaku Zasshi 2024, 159, 173–181. [Google Scholar] [CrossRef]
- Ono, K.; Tsuji, M. Protofibrils of Amyloid-β are Important Targets of a Disease-Modifying Approach for Alzheimer’s Disease. Int. J. Mol. Sci. 2020, 21, 952. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Chowdhury, N.S. Novel anti-amyloid-beta (Aβ) monoclonal antibody lecanemab for Alzheimer’s disease: A systematic review. Int. J. Immunopathol. Pharmacol. 2023, 37, 3946320231209839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Sig. Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Jayaprakash, N.; Elumalai, K. Translational Medicine in Alzheimer’s Disease: The Journey of Donanemab from Discovery to Clinical Application. Chronic Dis. Transl. Med. 2024, 2024, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Power, D.; Crotty, G.F. Advances in the Pharmacological Management of Parkinson’s Disease. Curr. Treat. Options Neurol. 2025, 27, 22. [Google Scholar] [CrossRef]
- Carbone, F.; Djamshidian, A.; Seppi, K.; Poewe, W. Apomorphine for Parkinson’s Disease: Efficacy and Safety of Current and New Formulations. CNS Drugs 2019, 33, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Zhong, X.B. ASO drug Qalsody (tofersen) targets amyotrophic lateral sclerosis. Trends Pharmacol. Sci. 2023, 44, 1043–1044. [Google Scholar] [CrossRef]
- Kim, A.Y.; Al Jerdi, S.; MacDonald, R.; Triggle, C.R. Alzheimer’s disease and its treatment-yesterday, today, and tomorrow. Front. Pharmacol. 2024, 15, 1399121. [Google Scholar] [CrossRef] [PubMed]
- Raza, M.L.; Bhojani, A.; Batool, S.K.; Zehra, D. Non pharmacological approaches for neurodegenerative diseases: A narrative review. Exp. Gerontol. 2024, 198, 112620. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Huang, B.; Chen, K. The impact of physical exercise on neuroinflammation mechanism in Alzheimer’s disease. Front. Aging Neurosci. 2024, 16, 1444716. [Google Scholar] [CrossRef]
- He, Y.; Wang, Q.; Wu, H.; Dong, Y.; Peng, Z.; Guo, X.; Jiang, N. The role of IGF-1 in exercise to improve obesity-related cognitive dysfunction. Front. Neurosci. 2023, 17, 1229165. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Mattson, M.P.; Maudsley, S. Caloric restriction and intermittent fasting: Two potential diets for successful brain aging. Ageing Res. Rev. 2006, 5, 332–353. [Google Scholar] [CrossRef]
- Sun, J.; Roy, S. Gene-based therapies for neurodegenerative diseases. Nat. Neurosci. 2021, 24, 297–311. [Google Scholar] [CrossRef]
- Sivandzade, F.; Cucullo, L. Regenerative Stem Cell Therapy for Neurodegenerative Diseases: An Overview. Int. J. Mol. Sci. 2021, 22, 2153. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Wei, J.; Feng, C.; Chen, Y.; Chen, T.; Ai, Z.; Zhu, X.; Ji, W.; Li, T. Genetically engineered mesenchymal stem cells with dopamine synthesis for Parkinson’s disease in animal models. NPJ Park. Dis. 2022, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Cha, M.Y.; Kwon, Y.W.; Ahn, H.S.; Jeong, H.; Lee, Y.Y.; Moon, M.; Baik, S.H.; Kim, D.K.; Song, H.; Yi, E.C.; et al. Protein-Induced Pluripotent Stem Cells Ameliorate Cognitive Dysfunction and Reduce Aβ Deposition in a Mouse Model of Alzheimer’s Disease. Stem Cells Transl. Med. 2017, 6, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.A.; Potashkin, J.A. Physical activity and lifestyle modifications in the treatment of neurodegenerative diseases. Front. Aging Neurosci. 2023, 15, 1185671. [Google Scholar] [CrossRef]
- Fritz, N.E.; Rao, A.K.; Kegelmeyer, D.; Kloos, A.; Busse, M.; Hartel, L.; Carrier, J.; Quinn, L. Physical Therapy and Exercise Interventions in Huntington’s Disease: A Mixed Methods Systematic Review. J. Huntingt. Dis. 2017, 6, 217–235. [Google Scholar] [CrossRef]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef]
- Kwon, D.K.; Kwatra, M.; Wang, J.; Ko, H.S. Levodopa-Induced Dyskinesia in Parkinson’s Disease: Pathogenesis and Emerging Treatment Strategies. Cells 2022, 11, 3736. [Google Scholar] [CrossRef] [PubMed]
- Qian, L.; He, X.; Liu, Y.; Gao, F.; Lu, W.; Fan, Y.; Gao, Y.; Wang, W.; Zhu, F.; Wang, Y.; et al. Longitudinal Gut Microbiota Dysbiosis Underlies Olanzapine-Induced Weight Gain. Microbiol. Spectr. 2023, 11, e0005823. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Sig. Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, T.S. Calorie restriction or dietary restriction: How far they can protect the brain against neurodegenerative diseases? Neural Regen. Res. 2022, 17, 1640–1644. [Google Scholar] [CrossRef]
- Rabizadeh, F.; Mirian, M.S.; Doosti, R.; Kiani-Anbouhi, R.; Eftekhari, E. Phytochemical classification of medicinal plants used in the treatment of kidney disease based on traditional Persian medicine. Evid. Based. Complement. Alternat. Med. 2022, 2022, 8022599. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Wazed, M.A.; Asha, S.; Amin, M.R.; Shimul, I.M. Dietary Phytochemicals in Health and Disease: Mechanisms, Clinical Evidence, and Applications—A Comprehensive Review. Food Sci. Nutr. 2025, 13, e70101. [Google Scholar] [CrossRef]
- Jalouli, M.; Rahman, M.A.; Biswas, P.; Rahman, H.; Harrath, A.H.; Lee, I.-S.; Kang, S.; Choi, J.; Park, M.N.; Kim, B. Targeting natural antioxidant polyphenols to protect neuroinflammation and neurodegenerative diseases: A comprehensive review. Front. Pharmacol. 2025, 16, 1492517. [Google Scholar] [CrossRef]
- Rathod, N.B.; Elabed, N.; Punia, S.; Ozogul, F.; Kim, S.K.; Rocha, J.M. Recent Developments in Polyphenol Applications on Human Health: A Review with Current Knowledge. Plants 2023, 12, 1217. [Google Scholar] [CrossRef]
- Shanaida, M.; Mykhailenko, O.; Lysiuk, R.; Hudz, N.; Balwierz, R.; Shulhai, A.; Shapovalova, N.; Shanaida, V.; Bjørklund, G. Carotenoids for Antiaging: Nutraceutical, Pharmaceutical, and Cosmeceutical Applications. Pharmaceuticals 2025, 18, 403. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Connolly, E.L.; Sim, M.; Travica, N.; Marx, W.; Beasy, G.; Lynch, G.S.; Bondonno, C.P.; Lewis, J.R.; Hodgson, J.M.; Blekkenhorst, L.C. Glucosinolates from cruciferous vegetables and their potential role in chronic disease: Investigating the preclinical and clinical evidence. Front. Pharmacol. 2021, 12, 767975. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Egbujor, M.C.; Petrosino, M.; Zuhra, K.; Saso, L. The role of organosulfur compounds as Nrf2 activators and their antioxidant effects. Antioxidants 2022, 11, 1255. [Google Scholar] [CrossRef]
- Naik, R.A.; Rajpoot, R.; Koiri, R.K.; Bhardwaj, R.; Aldairi, A.F.; Johargy, A.K.; Faidah, H.; Babalghith, A.O.; Hjazi, A.; Alsanie, W.F.; et al. Dietary supplementation and the role of phytochemicals against the Alzheimer’s disease: Focus on polyphenolic compounds. J. Prev. Alzheimers Dis. 2025, 12, 100004. [Google Scholar] [CrossRef]
- Guo, J.; Huang, X.; Duo, L.; Yan, M.; Shen, T.; Tang, W.; Li, J. Aging and aging-related diseases: From molecular mechanisms to interventions and treatments. Sig. Trans. Target. Ther. 2022, 7, 391. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Sasso, J.M.; Wang, X.; Zhou, Q.A. Antiaging Strategies and Remedies: A Landscape of Research Progress and Promise. ACS Chem. Neurosci. 2024, 15, 408–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, X.; Ma, J.; Liu, S.; Jin, X.; Liu, B. Unveiling the Longevity Potential of Natural Phytochemicals: A Comprehensive Review of Active Ingredients in Dietary Plants and Herbs. J. Agric. Food Chem. 2024, 72, 24908–24927. [Google Scholar] [CrossRef] [PubMed]
- Cavalier, A.N.; Clayton, Z.S.; Wahl, D.; Hutton, D.A.; McEntee, C.M.; Seals, D.R.; LaRocca, T.J. Protective effects of apigenin on the brain transcriptome with aging. Mech. Ageing Dev. 2024, 217, 111889. [Google Scholar] [CrossRef]
- Kose, T.; Moreno-Fernandez, J.; Vera-Aviles, M.; Sharp, P.A.; Latunde-Dada, G.O. Ferulic acid protects HepG2 cells and mouse liver from iron-induced damage. Biochem. Biophys. Rep. 2023, 35, 101521. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Kumar, P. Piperine in combination with quercetin halt 6-OHDA induced neurodegeneration in experimental rats: Biochemical and neurochemical evidences. Neurosci. Res. 2018, 133, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.-Y.; Qin, Z.-C.; Sun, Y.-Y.; Chen, Y.-S.; Chen, W.-B.; Wang, H.-G.; An, D.; Sun, D.; Liu, Y.-Q. Genistein Promotes Anti-Heat Stress and Antioxidant Effects via the Coordinated Regulation of IIS, HSP, MAPK, DR, and Mitochondrial Pathways in Caenorhabditis elegans. Antioxidants 2023, 12, 125. [Google Scholar] [CrossRef]
- Zhao, R.; Kou, H.; Jiang, D.; Wang, F. Exploring the anti-aging effects of fisetin in telomerase-deficient progeria mouse model. Peer J. 2023, 11, e16463. [Google Scholar] [CrossRef]
- Liu, J.; Liao, H.; Chen, Y.; Zhu, H.; Li, X.; Liu, J.; Xiang, Q.; Zeng, F.; Yang, Q. Resveratrol Inhibits Oxidative Stress and Regulates M1/M2-Type Polarization of Microglia via Mediation of the Nrf2/Shh Signaling Cascade after OGD/R Injury In Vitro. J. Pers. Med. 2022, 12, 2087. [Google Scholar] [CrossRef]
- Fang, E.F.; Waltz, T.B.; Kassahun, H.; Lu, Q.; Kerr, J.S.; Morevati, M.; Fivenson, E.M.; Wollman, B.N.; Marosi, K.; Wilson, M.A.; et al. Tomatidine enhances lifespan and healthspan in C. elegans through mitophagy induction via the SKN-1/Nrf2 pathway. Sci. Rep. 2017, 7, 46208. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.; Luo, T.; Zhang, S.; Liu, K.; Zhang, Y.; Luo, Y.; Ge, P. Lycopene protects human SH-SY5Y neuroblastoma cells against hydrogen peroxide-induced death via inhibition of oxidative stress and mitochondria-associated apoptotic pathways. Mol. Med. Rep. 2016, 13, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Xu, Z.; Sun, Q.; Iniguez, A.B.; Du, M.; Zhu, M.-J. Broccoli-Derived Glucoraphanin Activates AMPK/PGC1α/NRF2 Pathway and Ameliorates Dextran-Sulphate-Sodium-Induced Colitis in Mice. Antioxidants 2022, 11, 2404. [Google Scholar] [CrossRef]
- Camps-Bossacoma, M.; Franch, À.; Pérez-Cano, F.J.; Castell, M. Influence of Hesperidin on the Systemic and Intestinal Rat Immune Response. Nutrients 2017, 9, 580. [Google Scholar] [CrossRef] [PubMed]
- López-Biedma, A.; Sánchez-Quesada, C.; Beltrán, G.; Delgado-Rodríguez, M.; Gaforio, J.J. Phytoestrogen (+)-pinoresinol exerts antitumor activity in breast cancer cells with different oestrogen receptor statuses. BMC Complement. Altern. Med. 2016, 16, 350. [Google Scholar] [CrossRef]
- Hikmawati, V.F.; Alam, F.M.; Ainnayah, J.S.; Fatchiyah, F. Virtual prediction of phenolic and glucosinolate compounds with Keap1 protein as anti-aging by stimulating Nrf2. J. Exp. Life Sci. 2020, 10, 104–112. [Google Scholar] [CrossRef]
- Kim, Y.; Lim, J.; Oh, J. Taming neuroinflammation in Alzheimer’s disease: The protective role of phytochemicals through the gut−brain axis. Biomed. Pharmacother. 2024, 178, 117277. [Google Scholar] [CrossRef]
- Boronat, A.; Rodriguez-Morató, J.; Serreli, G.; Fitó, M.; Tyndale, R.F.; Deiana, M.; de la Torre, R. Contribution of Biotransformations Carried Out by the Microbiota, Drug-Metabolizing Enzymes, and Transport Proteins to the Biological Activities of Phytochemicals Found in the Diet. Adv. Nutr. 2021, 12, 2172–2189. [Google Scholar] [CrossRef]
- Kan, J.; Wu, F.; Wang, F.; Zheng, J.; Cheng, J.; Li, Y.; Yang, Y.; Du, J. Phytonutrients: Sources, bioavailability, interaction with gut microbiota, and their impacts on human health. Front. Nutr. 2022, 9, 960309. [Google Scholar] [CrossRef]
- Chen, T.; Dai, Y.; Hu, C.; Lin, Z.; Wang, S.; Yang, J.; Zeng, L.; Li, S.; Li, W. Cellular and molecular mechanisms of the blood–brain barrier dysfunction in neurodegenerative diseases. Fluids Barriers CNS 2024, 21, 60. [Google Scholar] [CrossRef]
- Isabel, U.-V.; de la Riera, M.; Belén, A.; Dolores, R.S.; Elena, G.-B. A new frontier in neuropharmacology: Recent progress in natural products research for blood–brain barrier crossing. Curr. Res. Biotechnol. 2024, 8, 100235. [Google Scholar] [CrossRef]
- Grabrucker, A.M.; Ruozi, B.; Belletti, D.; Pederzoli, F.; Forni, F.; Vandelli, M.A.; Tosi, G. Nanoparticle transport across the blood brain barrier. Tissue Barriers 2016, 4, e1153568. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier and neurotherapeutics. NeuroRx 2005, 2, 1–2. [Google Scholar] [CrossRef]
- Kılıç, K.D.; Garipoğlu, G.; Çakar, B.; Uyanıkgil, Y.; Erbaş, O. Antioxidant-Effective Quercetin Through Modulation of Brain Interleukin-13 Mitigates Autistic-Like Behaviors in the Propionic Acid-Induced Autism Model in Rats. J. Neuroimmune Pharmacol. 2025, 20, 36. [Google Scholar] [CrossRef]
- Gyawali, A.; Krol, S.; Kang, Y.S. Involvement of a Novel Organic Cation Transporter in Paeonol Transport Across the Blood-Brain Barrier. Biomol. Ther. 2019, 27, 290–301. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar] [PubMed Central]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut-Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef]
- Plamada, D.; Vodnar, D.C. Polyphenols-Gut Microbiota Interrelationship: A Transition to a New Generation of Prebiotics. Nutrients 2021, 14, 137. [Google Scholar] [CrossRef]
- Enayati, A.; Soghi, A.; Butler, A.E.; Rizzo, M.; Sahebkar, A. The Effect of Curcumin on the Gut-Brain Axis: Therapeutic Implications. J. Neurogastroenterol. Motil. 2023, 29, 409–418. [Google Scholar] [CrossRef]
- Abdolmaleky, H.M.; Zhou, J.R. Underlying Mechanisms of Brain Aging and Neurodegenerative Diseases as Potential Targets for Preventive or Therapeutic Strategies Using Phytochemicals. Nutrients 2023, 15, 3456. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Jiang, X.; Liang, Y.; Liu, Q.; Chen, S.; Guo, Y. Berberine improves cognitive impairment by promoting autophagic clearance and inhibiting production of β-amyloid in APP/tau/PS1 mouse model of Alzheimer’s disease. Exp. Gerontol. 2017, 91, 25–33. [Google Scholar] [CrossRef]
- Shi, J.; Chen, J.; Xie, X.; Li, Y.; Ye, W.; Yao, J.; Zhang, X.; Zhang, T.; Gao, J. Baicalein-corrected gut microbiota may underlie the amelioration of memory and cognitive deficits in APP/PS1 mice. Front. Pharmacol. 2023, 14, 1132857. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, R.; del Valle, J.; Modol, L.; Martinez, A.; Granado-Serrano, A.B.; Ramirez-Núñez, O.; Pallás, M.; Portero-Otin, M.; Osta, R.; Navarro, X. Resveratrol Improves Motoneuron Function and Extends Survival in SOD1G93A ALS Mice. Neurotherapeutics 2014, 11, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.; Tao, Y.; Zhang, J.; Wang, J.; Ye, F.; Dan, G.; Zhao, Y.; Cai, Y.; Zhao, J.; Wu, Q.; et al. Resveratrol Regulates Mitochondrial Biogenesis and Fission/Fusion to Attenuate Rotenone-Induced Neurotoxicity. Oxid. Med. Cell. Longev. 2016, 2016, 6705621. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta-Mol. Basis Dis. 2013, 1832, 421–430. [Google Scholar] [CrossRef]
- Li, J.; Han, Y.; Li, M.; Nie, C. Curcumin Promotes Proliferation of Adult Neural Stem Cells and the Birth of Neurons in Alzheimer’s Disease Mice via Notch Signaling Pathway. Cell. Reprogramm. 2019, 21, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.; Wang, Y.-J.; Zhong, J.-H.; Kosaraju, S.; O’Callaghan, N.J.; Zhou, X.-F.; Fenech, M. Grape seed polyphenols and curcumin reduce genomic instability events in a transgenic mouse model for Alzheimer’s disease. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2009, 661, 25–34. [Google Scholar] [CrossRef]
- Cilia, R.; Laguna, J.; Cassani, E.; Cereda, E.; Pozzi, N.G.; Isaias, I.U.; Contin, M.; Barichella, M.; Pezzoli, G. Mucuna pruriens in Parkinson disease: A double-blind, randomized, controlled, crossover study. Neurology 2017, 89, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Gopal, P.M.; Thomas, J.V.; Mohan, M.C.; Thomas, S.C.; Maliakel, B.P.; Krishnakumar, I.M.; Sasidharan, B.C.P. Influence of CurQfen®-curcumin on cognitive impairment: A randomized, double-blinded, placebo-controlled, 3-arm, 3-sequence comparative study. Front. Dement. 2023, 2, 1222708. [Google Scholar] [CrossRef]
- Millar, C.L.; Iloputaife, I.; Baldyga, K.; Norling, A.M.; Boulougoura, A.; Vichos, T.; Tchkonia, T.; Deisinger, A.; Pirtskhalava, T.; Kirkland, J.L.; et al. A pilot study of senolytics to improve cognition and mobility in older adults at risk for Alzheimer’s disease. eBioMedicine 2025, 113, 105612. [Google Scholar] [CrossRef] [PubMed]
- Viña, J.; Escudero, J.; Baquero, M.; Cebrián, M.; Carbonell-Asíns, J.A.; Muñoz, J.E.; Satorres, E.; Meléndez, J.C.; Ferrer-Rebolleda, J.; Cózar-Santiago, M.D.P.; et al. Genistein effect on cognition in prodromal Alzheimer’s disease patients. The GENIAL clinical trial. Alzheimers Res. Ther. 2022, 14, 164. [Google Scholar] [CrossRef] [PubMed]
- Köbe, T.; Witte, A.V.; Schnelle, A.; Tesky, V.A.; Pantel, J.; Schuchardt, J.-P.; Hahn, A.; Bohlken, J.; Grittner, U.; Flöel, A. Impact of resveratrol on glucose control, hippocampal structure and connectivity, and memory performance in patients with mild cognitive impairment. Front. Neurosci. 2017, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Y.; Pei, H.; Wang, H.; Ma, L.; Wang, Z.; Diao, X.; Yang, Y.; Liu, N.; Wei, Y. Shenmayizhi formula combined with ginkgo extract tablets for the treatment of vascular dementia: A randomized, double-blind, controlled trial. Evid. Based Complement. Alternat. Med. 2020, 2020, 8312347. [Google Scholar] [CrossRef]
- Santa, K.; Kumazawa, Y.; Watanabe, K.; Nagaoka, I. The potential use of vitamin D3 and phytochemicals for their anti-ageing effects. Int. J. Mol. Sci. 2024, 25, 2125. [Google Scholar] [CrossRef]
- Fakhri, S.; Piri, S.; Moradi, S.Z.; Khan, H. Phytochemicals targeting oxidative stress, interconnected neuroinflammatory, and neuroapoptotic pathways following radiation. Curr. Neuropharmacol. 2022, 20, 836–856. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, H.; Qu, S. Phytochemicals targeting mitophagy: Therapeutic opportunities and prospects for treating Alzheimer’s disease. Biomed. Pharmacother. 2024, 177, 117144. [Google Scholar] [CrossRef]
- Plano, L.M.D.; Calabrese, G.; Rizzo, M.G.; Oddo, S.; Caccamo, A. The role of the transcription factor Nrf2 in Alzheimer’s disease: Therapeutic opportunities. Biomolecules 2023, 13, 549. [Google Scholar] [CrossRef]
- Heurtaux, T.; Bouvier, D.S.; Benani, A.; Romero, S.H.; Frauenknecht, K.B.; Mittelbronn, M.; Sinkkonen, L. Normal and pathological NRF2 signalling in the central nervous system. Antioxidants 2022, 11, 1426. [Google Scholar] [CrossRef]
- Ngo, V.; Duennwald, M.L. Nrf2 and oxidative stress: A general overview of mechanisms and implications in human disease. Antioxidants 2022, 11, 2345. [Google Scholar] [CrossRef] [PubMed]
- Suzen, S.; Tucci, P.; Profumo, E.; Buttari, B.; Saso, L. A pivotal role of Nrf2 in neurodegenerative disorders: A new way for therapeutic strategies. Pharmaceuticals 2022, 15, 692. [Google Scholar] [CrossRef]
- He, W.-J.; Lv, C.-H.; Chen, Z.; Shi, M.; Zeng, C.-X.; Hou, D.-X.; Qin, S. The regulatory effect of phytochemicals on chronic diseases by targeting Nrf2-ARE signaling pathway. Antioxidants 2023, 12, 236. [Google Scholar] [CrossRef]
- Farkhondeh, T.; Folgado, S.L.; Pourbagher-Shahri, A.M.; Ashrafizadeh, M.; Samarghandian, S. The therapeutic effect of resveratrol: Focusing on the Nrf2 signaling pathway. Biomed. Pharmacother. 2020, 127, 110234. [Google Scholar] [CrossRef]
- Hui, Y.; Chengyong, T.; Cheng, L.; Haixia, H.; Yuanda, Z.; Weihua, Y. Resveratrol Attenuates the Cytotoxicity Induced by Amyloid-β(1-42) in PC12 Cells by Upregulating Heme Oxygenase-1 via the PI3K/Akt/Nrf2 Pathway. Neurochem. Res. 2018, 43, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Anilkumar, S.; Wright-Jin, E. NF-κB as an inducible regulator of inflammation in the central nervous system. Cells 2024, 13, 485. [Google Scholar] [CrossRef]
- Sivamaruthi, B.S.; Raghani, N.; Chorawala, M.; Bhattacharya, S.; Prajapati, B.G.; Elossaily, G.M.; Chaiyasut, C. NF-κB pathway and its inhibitors: A promising frontier in the management of Alzheimer’s disease. Biomedicines 2023, 11, 2587. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, A.; Islam, A.U.; Prakash, H.; Singh, S. Phytochemicals targeting NF-κB signaling: Potential anti-cancer interventions. J. Pharm. Anal. 2022, 12, 394–405. [Google Scholar] [CrossRef]
- Olivera, A.; Moore, T.W.; Hu, F.; Brown, A.P.; Sun, A.; Liotta, D.C.; Snyder, J.P.; Yoon, Y.; Shim, H.; Marcus, A.I.; et al. Inhibition of the NF-κB signaling pathway by the curcumin analog, 3,5-Bis(2-pyridinylmethylidene)-4-piperidone (EF31): Anti-inflammatory and anti-cancer properties. Int. Immunopharmacol. 2012, 12, 368–377. [Google Scholar] [CrossRef]
- Zhang, J.; Zheng, Y.; Luo, Y.; Du, Y.; Zhang, X.; Fu, J. Curcumin inhibits LPS-induced neuroinflammation by promoting microglial M2 polarization via TREM2/TLR4/NF-κB pathways in BV2 cells. Mol. Immunol. 2019, 116, 29–37. [Google Scholar] [CrossRef]
- Su, Z.; Guo, Y.; Huang, X.; Feng, B.; Tang, L.; Zheng, G.; Zhu, Y. Phytochemicals: Targeting mitophagy to treat metabolic disorders. Front. Cell Dev. Biol. 2021, 9, 686820. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Yu, X.; Mahaman, Y.A.R.; Wang, J.-Z.; Liu, R.; Li, Y.; Wang, X. Defective mitophagy and the etiopathogenesis of Alzheimer’s disease. Transl. Neurodegener. 2022, 11, 32. [Google Scholar] [CrossRef]
- Liang, J.H.; Yu, H.; Xia, C.P.; Zheng, Y.H.; Zhang, Z.; Chen, Y.; Raza, M.A.; Wu, L.; Yan, H. Ginkgolide B effectively mitigates neuropathic pain by suppressing the activation of the NLRP3 inflammasome through the induction of mitophagy in rats. Biomed. Pharmacother. 2024, 177, 117006. [Google Scholar] [CrossRef]
- Chen, F. Inhibiting Pink1/Parkin-mediated mitophagy enhances the anticancer effects of quercetin in hepatocellular carcinomaf. Biochem. Biophys. Res. Commun. 2024, 712, 149899. [Google Scholar] [CrossRef]
- Kumar, A.; Nirmal, P.; Kumar, M.; Jose, A.; Tomer, V.; Oz, E.; Proestos, C.; Zeng, M.; Elobeid, T.; Sneha, K. Major phytochemicals: Recent advances in health benefits and extraction method. Molecules 2023, 28, 887. [Google Scholar] [CrossRef]
- Davinelli, S.; Maes, M.; Corbi, G.; Zarrelli, A.; Willcox, D.C.; Scapagnini, G. Dietary phytochemicals and neuro-inflammaging: From mechanistic insights to translational challenges. Immun. Ageing 2016, 13, 16. [Google Scholar] [CrossRef]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in drug discovery—A confluence of tradition and innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
- Paul, J.K.; Azmal, M.; Haque, A.S.N.B.; Talukder, O.F.; Meem, M.; Ghosh, A. Phytochemical-mediated modulation of signaling pathways: A promising avenue for drug discovery. Adv. Redox Res. 2024, 25, 100113. [Google Scholar] [CrossRef]
- Wu, W.; Huang, J.; Han, P.; Zhang, J.; Wang, Y.; Jin, F.; Zhou, Y. Research Progress on Natural Plant Molecules in Regulating the Blood–Brain Barrier in Alzheimer’s Disease. Molecules 2023, 28, 7631. [Google Scholar] [CrossRef] [PubMed]
- Ahlawat, J.; Barroso, G.G.; Asil, S.M.; Alvarado, M.; Armendariz, I.; Bernal, J.; Carabaza, X.; Chavez, S.; Cruz, P.; Escalante, V.; et al. Nanocarriers as Potential Drug Delivery Candidates for Overcoming the Blood–Brain Barrier: Challenges and Possibilities. ACS Omega 2020, 5, 12583–12595. [Google Scholar] [CrossRef] [PubMed]
| Drug Class (Drug) | Mechanism | Side Effects | Limitation | Ref. |
|---|---|---|---|---|
| ChEIs (donepezil, rivastigmine, and galantamine) | Increased levels of acetylcholine by blocking catalytic action of acetylcholinesterase enzymes | Appetite loss, vomiting, nausea, diarrhea, and rhinitis | Limited efficacy in reducing symptoms of neurodegeneration; no effect on AD progression; usage of ChEIs is associated with several gastrointestinal side effects | [54] |
| NMDA (memantine) | Strong voltage-dependent but low potency n-methyl-d-aspartate receptor antagonism | Amyloid-related imaging abnormalities | No evidence for slowing down AD progression long-term; memantine is a non-specific NMDA receptor antagonist | [55] |
| Dopaminergic agents (levodopa) | Dopamine replacement | Motor fluctuations and dyskinesia | Levodopa is linked to long-term motor disorders; requires combination therapy for optimal safety and efficacy | [56] |
| Antipsychotic drugs (aripiprazole, olanzapine, and risperidone) | Dopaminergic and serotonergic blockage | Extrapyramidal symptoms and adverse metabolic and endocrine effects | Use of antipsychotics is limited by severe adverse effects especially when in combination with other medications for neurodegeneration; requires extensive and intensive drug selection and monitoring | [57,58,59] |
| Monoclonal antibodies (lecanemab and donanemab) | Microglial activation via Aβ binding | Dizziness, fatigue, sinusitis, upper respiratory tract infections, headaches, orthostatic hypertension, and amyloid-related imaging abnormalities | Not recommended for people with cerebrovascular diseases or ischemic stroke; requires prolonged treatment to achieve meaningful reduction in amyloid plaques | [63,65] |
| Antisense oligonucleotide (tofersen) | Reduction of dysfunctional SOD1 protein by binding and cleaving SOD1 mRNA | Pain, fatigue, arthralgia, and myalgia | Intrathecal administration; long-term safety and efficacy are still unknown | [68] |
| Phytochemical Class | Dietary Phytochemical | Source | Molecular Structure | Experimental Model | Effects | Ref. |
|---|---|---|---|---|---|---|
| Flavone | Apigenin | Celery and parsley | 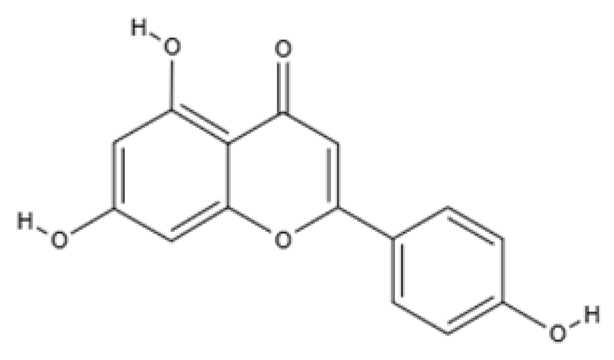 | Male C57BL/6N mice | Normalized IFNγ expression levels reduced in old mice from approximately 2.0 to 1.0; learning and memory in old mice improved compared with control (p < 0.05); recognition index in old mice increased from approximately 40% to 80% compared with controls (p < 0.05) | [99] |
| Phenolic acid | Ferulic acid | Whole grains, fruits, and vegetables | 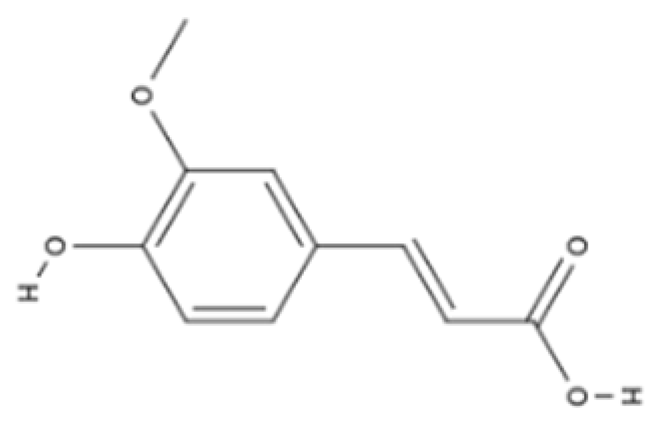 | BALB/c mice and HepG2 hepatoma cells | ROS level in cells treated with ferulic acid decreased from approximately 1.6 to 1.25 fluorescence intensity units compared with iron-treated cells; GSH levels increased from approximately 40 nmol/mL to 65 nmol/mL compared with iron-treated cells | [100] |
| Flavonol | Quercetin | Fruits and vegetables | 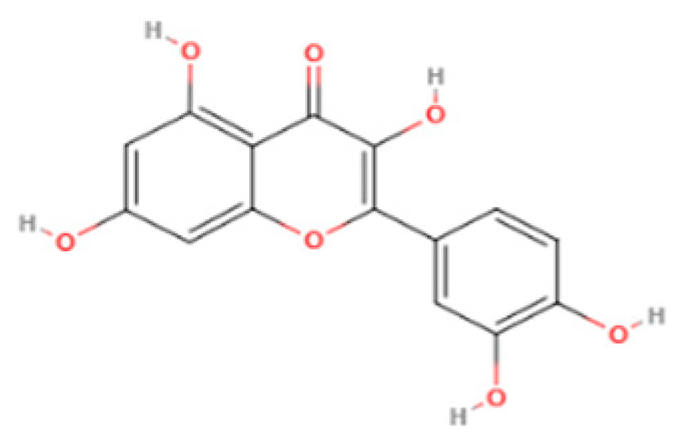 | Male Wistar rats treated with 6-OHDA | Grip strength improved from approximately 0.53 to 0.75 KgF; inflammatory biomarkers were reduced: TNF-α from approximately 200 to 175 pg/mg, IL-1β from approximately 175 to 100 pg/mg, IL-6 from approximately 212.5 to 137.5 pg/mg | [101] |
| Isoflavone | Genistein | Soy and soy-derived products |  | Caenorhabditis elegans | Mean survival rate of oxidative-stress-exposed C. elegans increased by 56.7%; lipofuscin and ROS accumulation reduced by 44.4% and 47.9% respectively; SOD activity increased by 67.5% in H2O2-treated worms | [102] |
| Flavanol | Fisetin | Apples, berries, and vegetables | 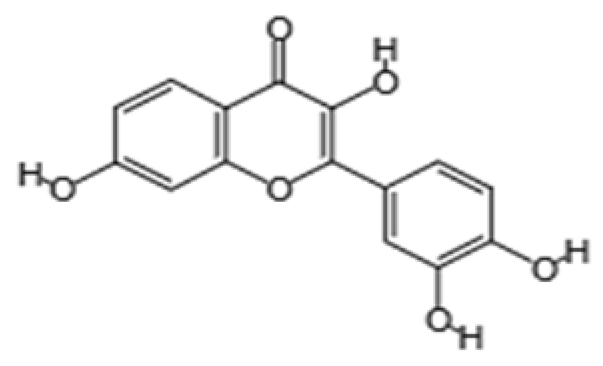 | Male C57BL/6J mice | Upregulation of aging markers was suppressed: relative mRNA expression of CDKN1A decreased from 3.5 to 0.75 (p = 0.018), and relative mRNA expression of CDKN2A decreased from approximately 5 to 1 (p = 0.019) | [103] |
| Stilbene | Resveratrol | Grapes, peanuts, and red wine | 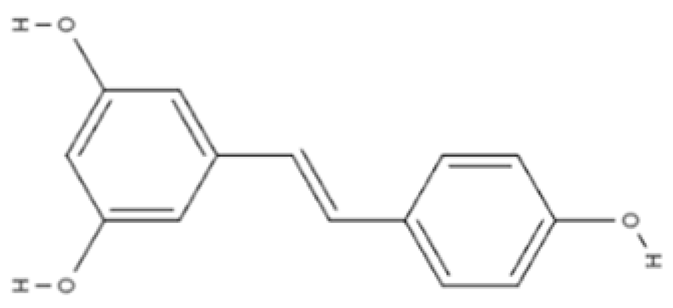 | In vitro N9 microglial cell line OGD/R injury model and in vitro HT22 hippocampal neuronal cell line | SOD levels increased from approximately 27 u/mL to 45 u/mL; MDA levels reduced from 0.8 to 0.6 nmol/mL; mitochondrial function improved via activation of the Nrf2 pathway | [104] |
| Nitrogen-containing phytochemical | Tomatidine | Tomatoes |  | Caenorhabditis elegans | Mitochondrial network morphology score increased from 3.4 in control to 3.9; mt-mKeima signal increased to 561 nm; survival increased by 19% | [105] |
| Carotenoid | Lycopene | Tomatoes, guava, and watermelon | 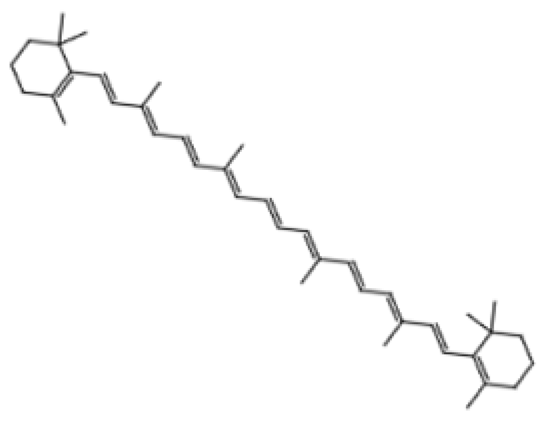 | Human SH-SY5Y neuroblastoma cells | Percentage of cells with apoptotic features decreased from 32.6 ± 4.8% to 25.1 ± 1.9% and 15.2 ± 1.7% (mean ± SD) when treated with 2.0 and 4.0 µmol/l lycopene respectively; apoptosis rate reduced from 42.04% to 26.55% and 17.87% when treated with 2.0 and 4.0 µmol/l lycopene, respectively; lycopene (2.0 and 4.0 µmol/l) increased mitochondrial membrane potentials by 8.71% and 16.42%, respectively | [106] |
| Glucosinolate | Glucoraphanin | Cruciferous vegetables |  | C57BL/6 mice | Relative Nrf2 protein content improved from approximately 1 to 1.125 (p < 0.05); mitochondrial biogenesis increased; macrophage infiltration was inhibited | [107] |
| Flavanone | Hesperidin | Citrus fruits | 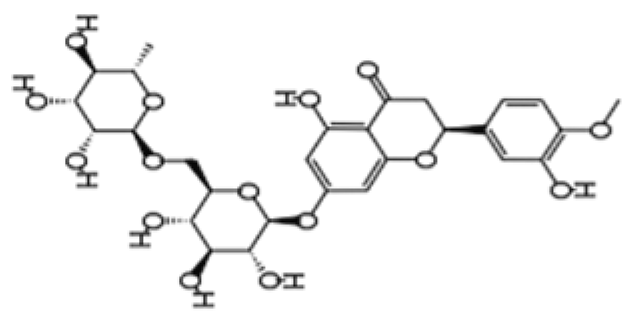 | Lewis rats | Compared with control: IEL TCRγδ+ cells increased to 140%, CD45RA+ increased to 180%, TCRαβ+CD4+ increased to 132%; LPL TCRγδ+ decreased to 35%, NK cells decreased to 29%, TCRαβ+CD8+ decreased to 52%, NKT cells decreased to 42%, CD4+CD103+ decreased to 50%, and CD8+CD103+ decreased to 60% | [108] |
| Lignan | Pinoresinol | Flax seeds and sesame seeds | 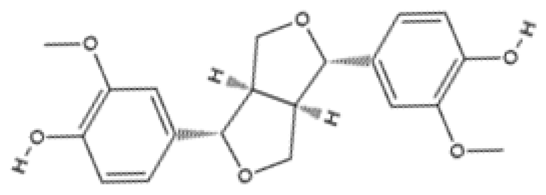 | Human epithelial breast cells (MDA-MB-231 and MCF7) | Weak antioxidant activity (DPPH assay) was observed: 50% RSA at 69 μM compared with 11 μM for α-tocopherol (control) | [109] |
| Phytochemical | Neurodegenerative Disorder | Experimental Model | Aging Hallmarks Targeted | Effects | Ref. |
|---|---|---|---|---|---|
| Berberine | AD | Transgenic APP/tau/PS1 mice | Macroautophagy and disabled proteostasis | Memory impairment was reduced; Aβ concentrations reduced from approximately 850 μg/L to 600 μg/L levels | [125] |
| Baicalein | AD | Transgenic APP/PS1 mice | Dysbiosis | Recognition index improved by about 20% (p < 0.01); gut microbiota was dominated by Bacteroidetes (14.59–67.02%) and Firmicutes (20.19–61.39%). | [126] |
| Resveratrol | ALS | Transgenic SOD1G93A ALS mice | Mitochondrial dysfunction, inflammaion | Microglial reactivity reduced from over 1152% to 649%; motor neuron counts increased from 18.2 ± 1.3 (mean ± SEM) in untreated mice to 35.2 ± 1.1 (mean ± SEM) in resveratrol-treated mice | [127] |
| PD | Male Sprague–Drawley rats and rotenone-induced PC12 cell line | Mitochondrial dysfunction | Improved mitochondrial mass, homeostasis, and neuronal function; ROS fluorescence reduced from approximately 175 to 115 (p < 0.05); ATP concentration (relative to control) increased from approximately 25% to 60% (p < 0.05) | [128] | |
| Quercetin | HTTD | Female Wistar rats | Mitochondrial dyfunction | Mitochondrial function increased by 39.3%; total mitochondrial thiols increased by 37.5%; locomotor function improved from 80 to 150 counts; gait abnormalities were prevented | [129] |
| Curcumin | AD | APP/PS1 transgenic mice | Stem cell exhaustion | Neuronal stem cell proliferation was activated; neurogenesis improved; water maze latency reduced from 37.6 s in controls to 27.3 s in treated mice; hippocampal neuronal apoptosis count decreased from 40 in controls to 30 in treated mice | [130] |
| AD | Transgenic AD mouse model | Genomic instability and telomere attrition | Telomere length increased from 0.47 ± 0.70 kb (mean ± SD) in AD control mice to 1.25 ± 3.15 kb (mean ± SD) in treated mice | [131] |
| Phytochemical | Source | Neurodegenerative Disorder | Study Design | Dose and Duration | Mechanism Targeted | Effects | Ref. |
|---|---|---|---|---|---|---|---|
| Mucuna pruriens powder | Mucuna pruriens | PD | Non-inferiority, phase 2b randomized, double-blind, controlled crossover study (N = 18) | 17.5 mg/kg (high dose), 12.5 mg/kg (low dose), 3.5 mg/kg (with benserazide) for 6 days | Dopamine deficiency | Motor performance improved with MP at 90 min (p = 0.037) and 180 min (p = 0.002); UPDRS-III scores decreased by 16% with MP-LD and by 50% with MP + DDCI at 180 min; mean ‘on’ time increased to 221 min for MP-HD compared with 177 min for LD-DCI (p < 0.001) | [132] |
| Curcumin (unformulated standard curcumin (USC) and curcumin–galactomannan complex (CGM)) | Turmeric | Dementia | Three-arm, randomized, double-blind, parallel-group clinical trial (N = 48) | 400 mg/day twice daily for six months | Inflammation, neurotrophic modulation, proteostasis | Locomotive function increased with CGM compared with placebo (F = 59.95, p = 0.001); GLFS-25 scores decreased by 25.7% from baseline; Aβ42 levels decreased by 23.3% compared with placebo and 16.0% compared with USC; tau proteins reduced by 22.8% | [133] |
| Quercetin | Fruits and vegetables | Mild AD | Single-arm, interventional feasibility study (N = 12) | 100 mg dasatinib + 1250 mg quercetin (DQ) for 2 days every 2 weeks for a total of 12 weeks | Senescence | DQ increased MoCA scores by 2 points (95% CI: 0.1–4.0) in participants with lowest baseline scores | [134] |
| Genistein | Soy and soy-derived products | AD | Double-blind, placebo-controlled bicentric study (N = 27) | 120 mg for 12 months | Proteostasis | Genistein treatment improved cognitive tests (p = 0.002) and stabilized Aβ levels | [135] |
| Resveratrol | Grapes, berries, and peanuts | Mild cognitive impairment | Randomized, double-blind interventional study (N = 40) | 200 mg/day for 26 weeks | Nutrient sensing and neural connectivity | HbA1c reduced by 0.15% (t(17) = 3.3, p = 0.005, Cohen’s d = 1.60); hippocampal volume was preserved; functional connectivity improved between the right anterior hippocampus and right angular cortex | [136] |
| AD | Retrospective study (N = 119) | 500 mg dose once daily, increasing by 500 mg every 13 weeks to 1000 mg twice daily | Neuroinflammation | Decline in cognitive test scores was reduced (p < 0.0001); CSF MMP-9 levels decreased by 48% | [137] | ||
| Ginkgo extract | Ginkgo biloba | VaD | Double-blind, randomized, controlled study (N = 196) | 1 tablet of gingko extract thrice daily and 4.8 g of shenmayizhi formula twice daily for 12 weeks | Vascular endothelial function | Vascular endothelial functions improved compared with control (p < 0.05) | [138] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dareowolabi, B.O.; Moon, E.-Y.; Kim, J.H. The Therapeutic Potential of Dietary Phytochemicals in Age-Related Neurodegenerative Disorders. Pharmaceuticals 2025, 18, 1268. https://doi.org/10.3390/ph18091268
Dareowolabi BO, Moon E-Y, Kim JH. The Therapeutic Potential of Dietary Phytochemicals in Age-Related Neurodegenerative Disorders. Pharmaceuticals. 2025; 18(9):1268. https://doi.org/10.3390/ph18091268
Chicago/Turabian StyleDareowolabi, Boluwatife Olamide, Eun-Yi Moon, and Jin Hee Kim. 2025. "The Therapeutic Potential of Dietary Phytochemicals in Age-Related Neurodegenerative Disorders" Pharmaceuticals 18, no. 9: 1268. https://doi.org/10.3390/ph18091268
APA StyleDareowolabi, B. O., Moon, E.-Y., & Kim, J. H. (2025). The Therapeutic Potential of Dietary Phytochemicals in Age-Related Neurodegenerative Disorders. Pharmaceuticals, 18(9), 1268. https://doi.org/10.3390/ph18091268








