Systems Network Integration of Transcriptomic, Proteomic, and Bioinformatic Analyses Reveals the Mechanism of XuanYunNing Tablets in Meniere’s Disease via JAK-STAT Pathway Modulation
Abstract
1. Introduction
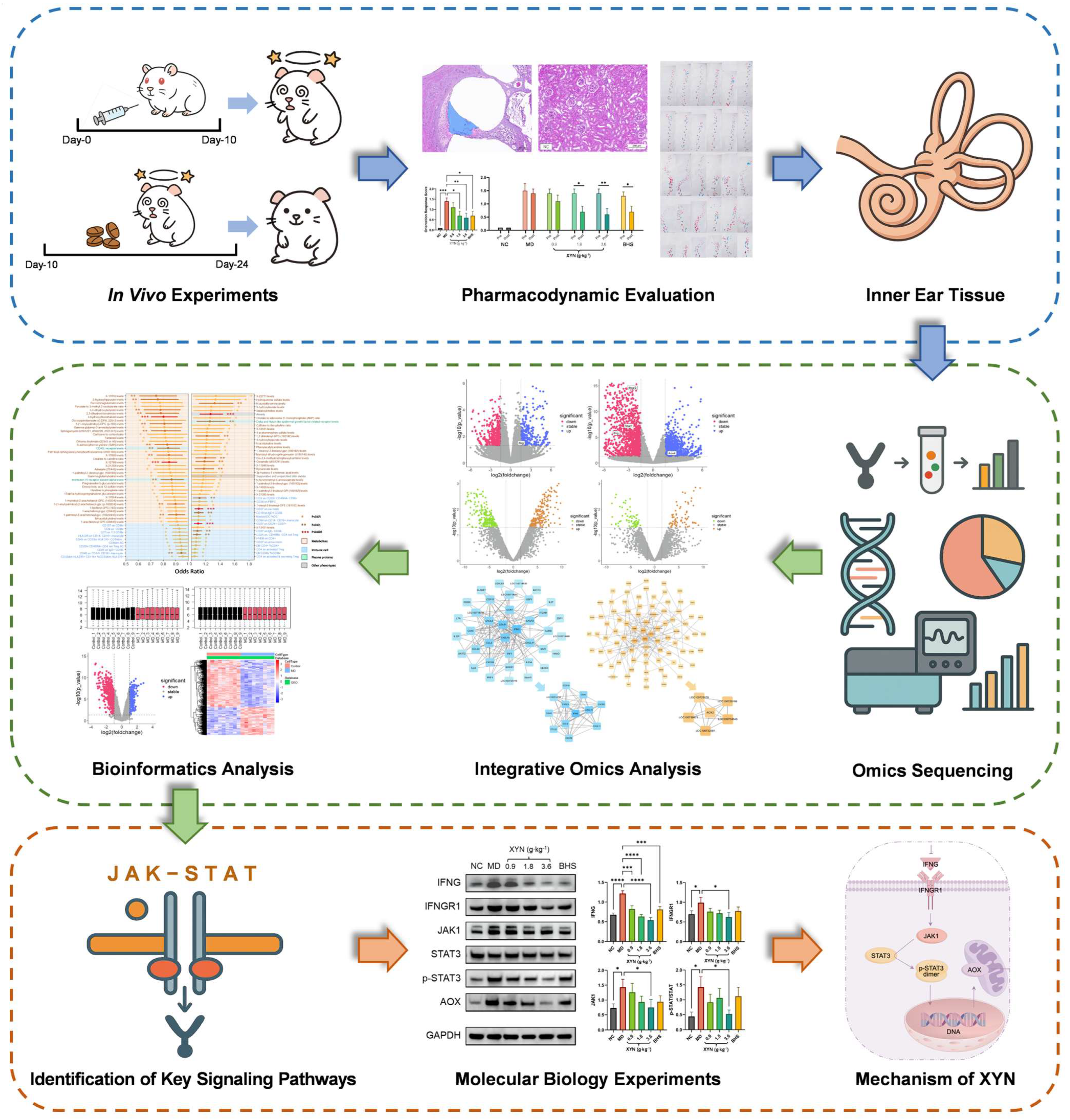
2. Results
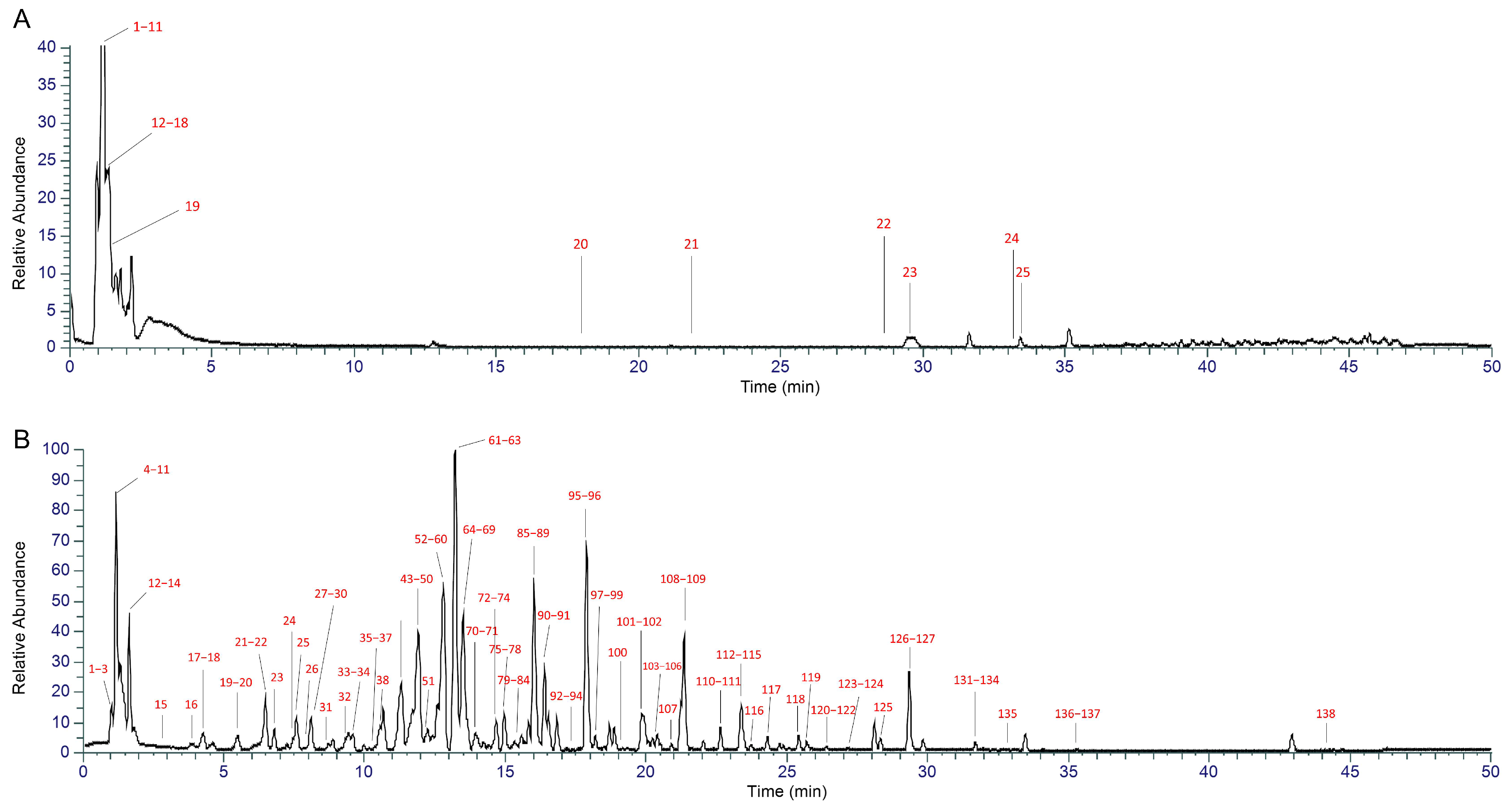
2.1. UHPLC-MS-Based Chemical Profiling of XYN
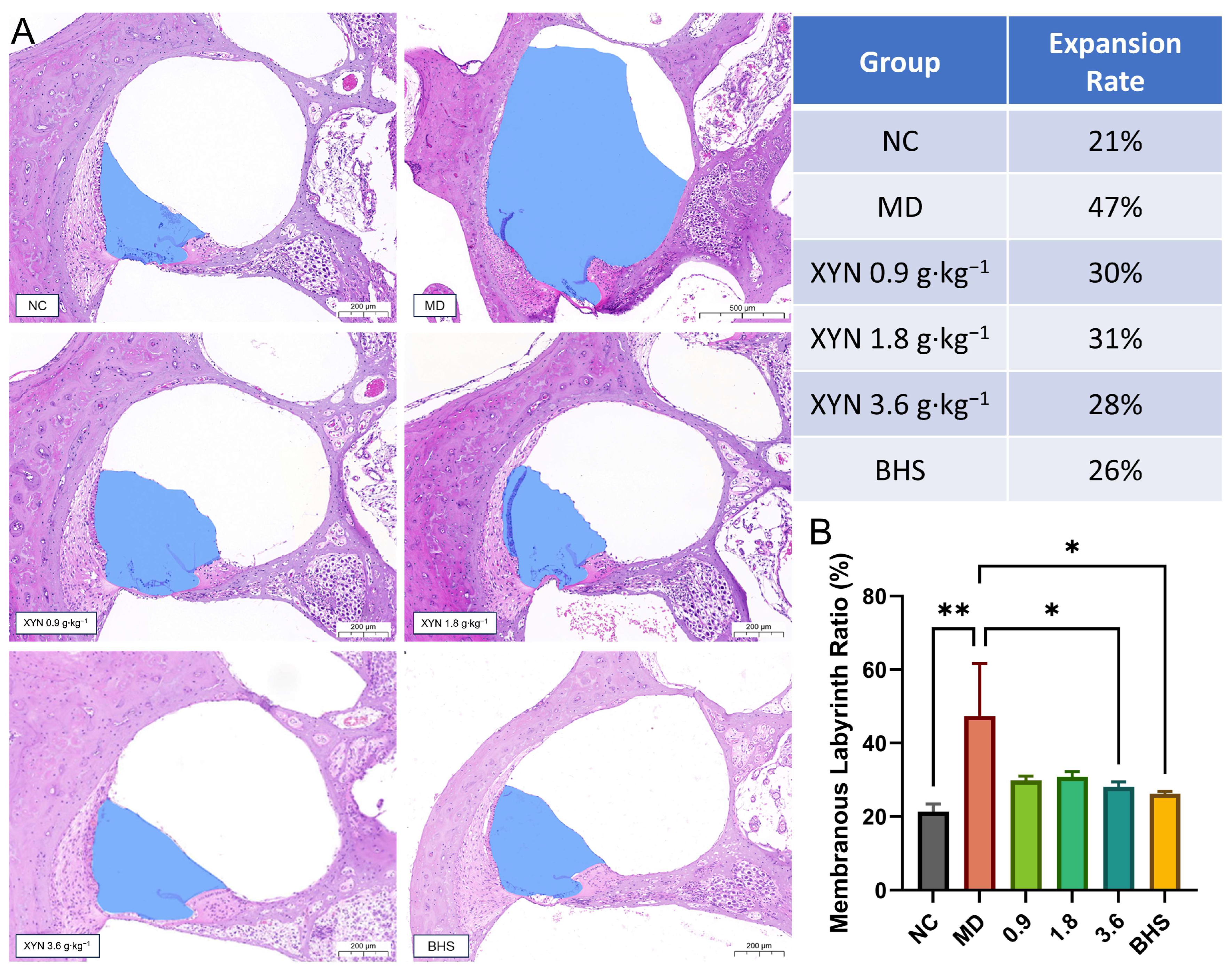
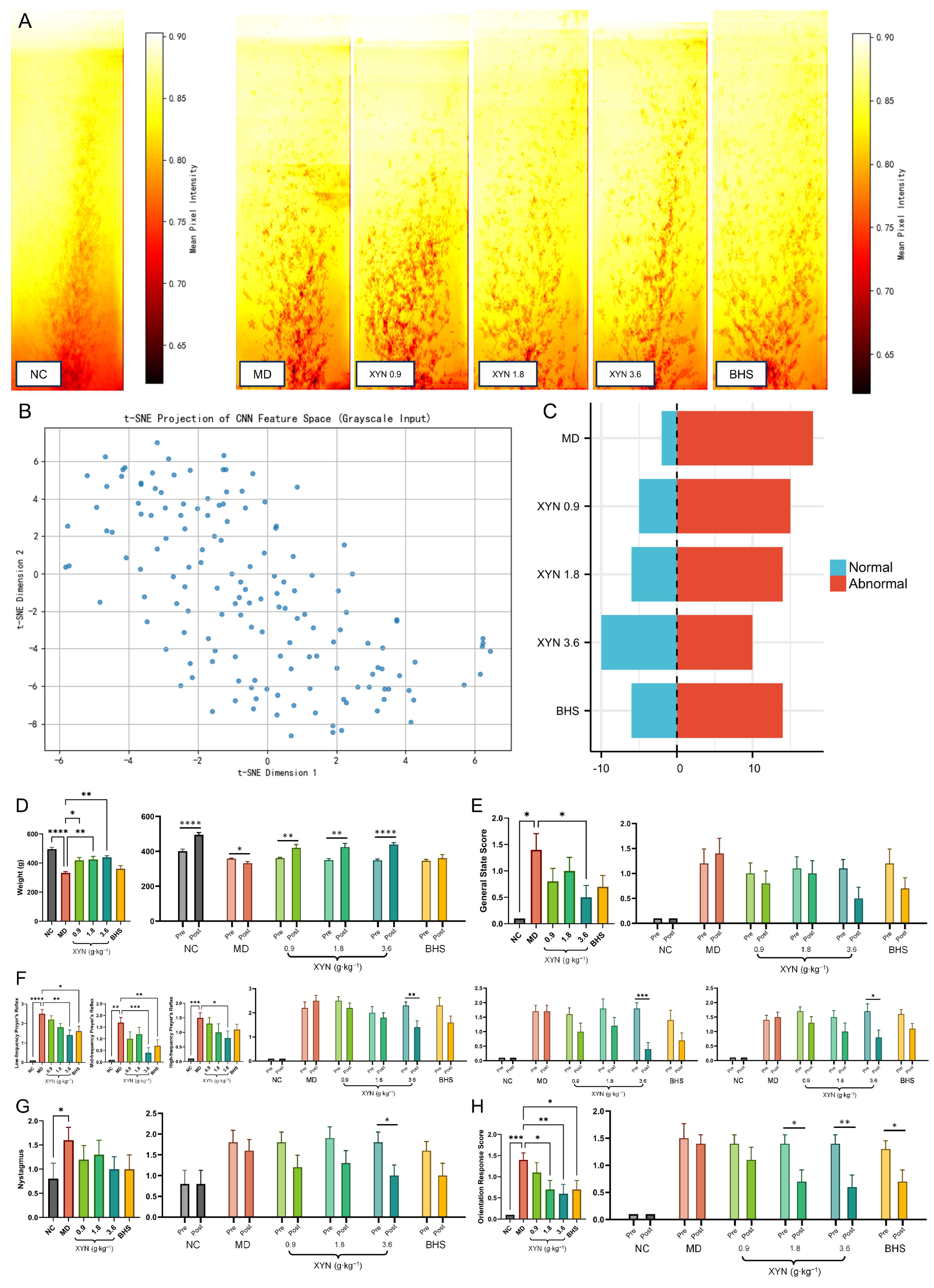
2.2. XYN Administration Alleviates Endolymphatic Hydrops and Improves Associated Symptoms
2.2.1. Validation of the Endolymphatic Hydrops Model and Post-Modeling Re-Grouping
2.2.2. XYN Attenuates Endolymphatic Hydrops in the Cochlea
2.2.3. XYN Improves Gait Trajectories in Guinea Pigs
2.2.4. XYN Improves Behavioral Indices in Guinea Pigs Across Groups
2.2.5. XYN Improves Behavioral Indicators Before and After Treatment Within the Same Guinea Pigs
2.3. The JAK-STAT Signaling Pathway as a Core Axis in the Pathogenesis of Endolymphatic Hydrops and the Therapeutic Action of XYN
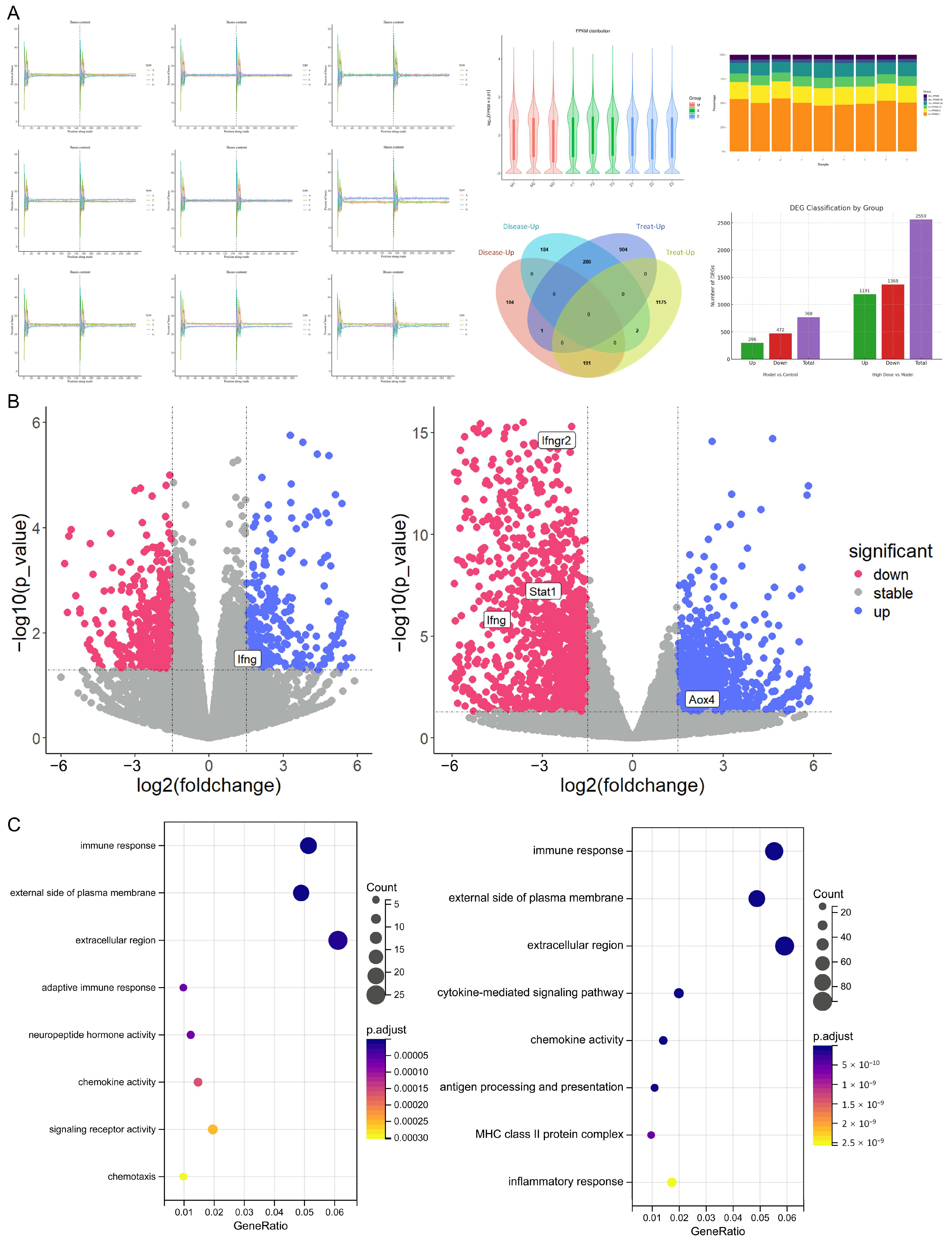
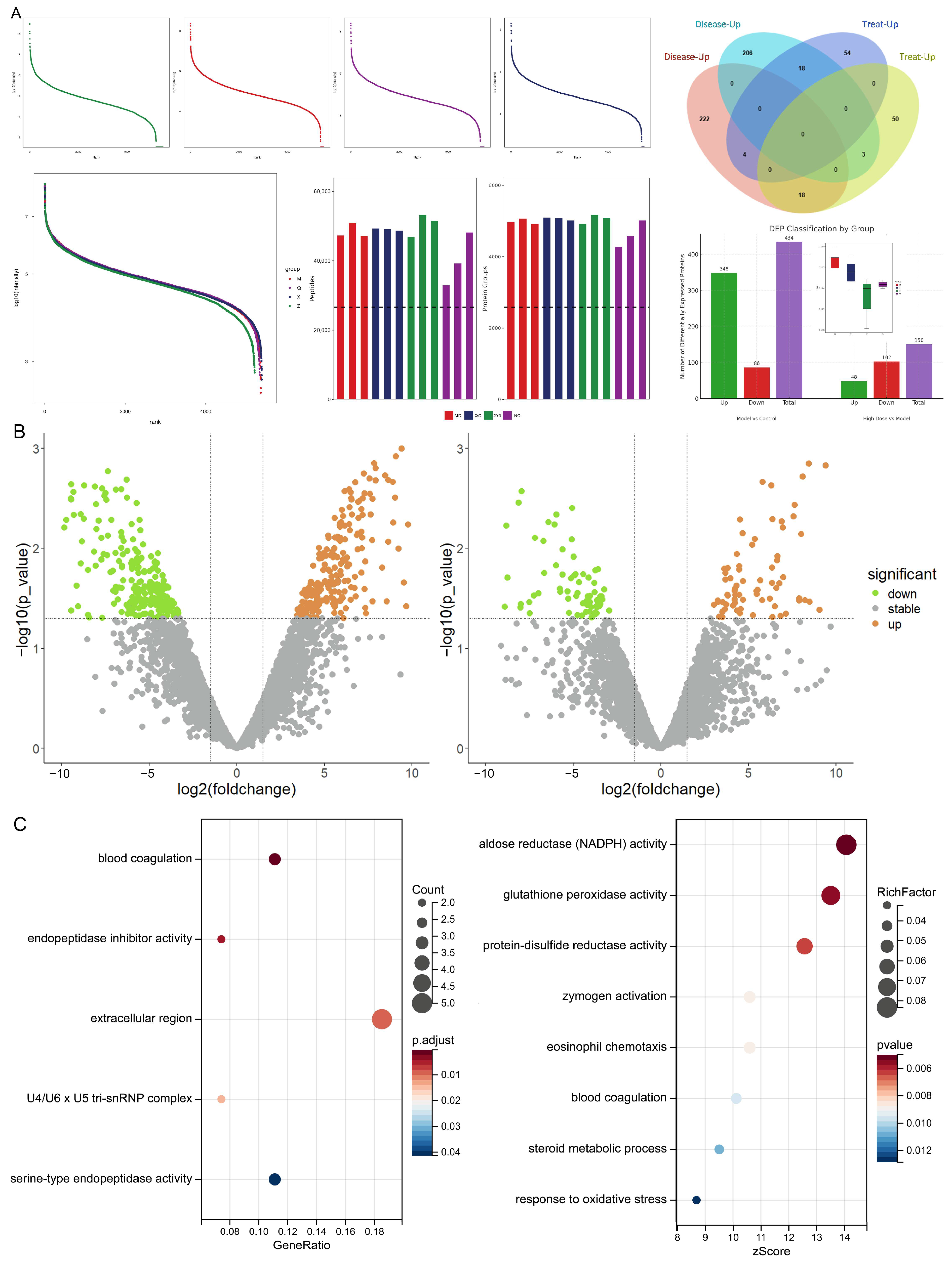
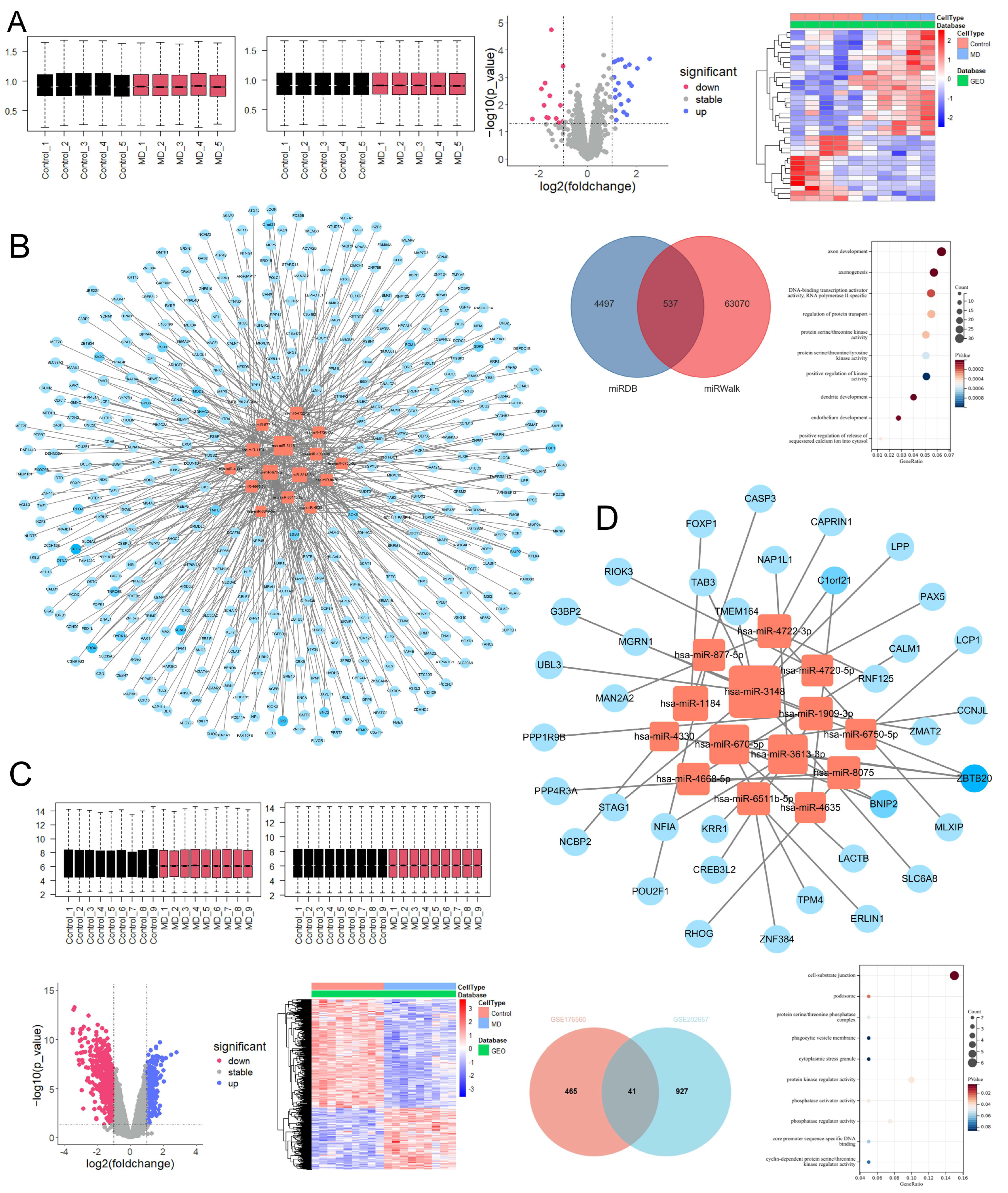
2.3.1. Differentially Expressed mRNAs and Proteins Associated with Endolymphatic Hydrops and XYN Intervention
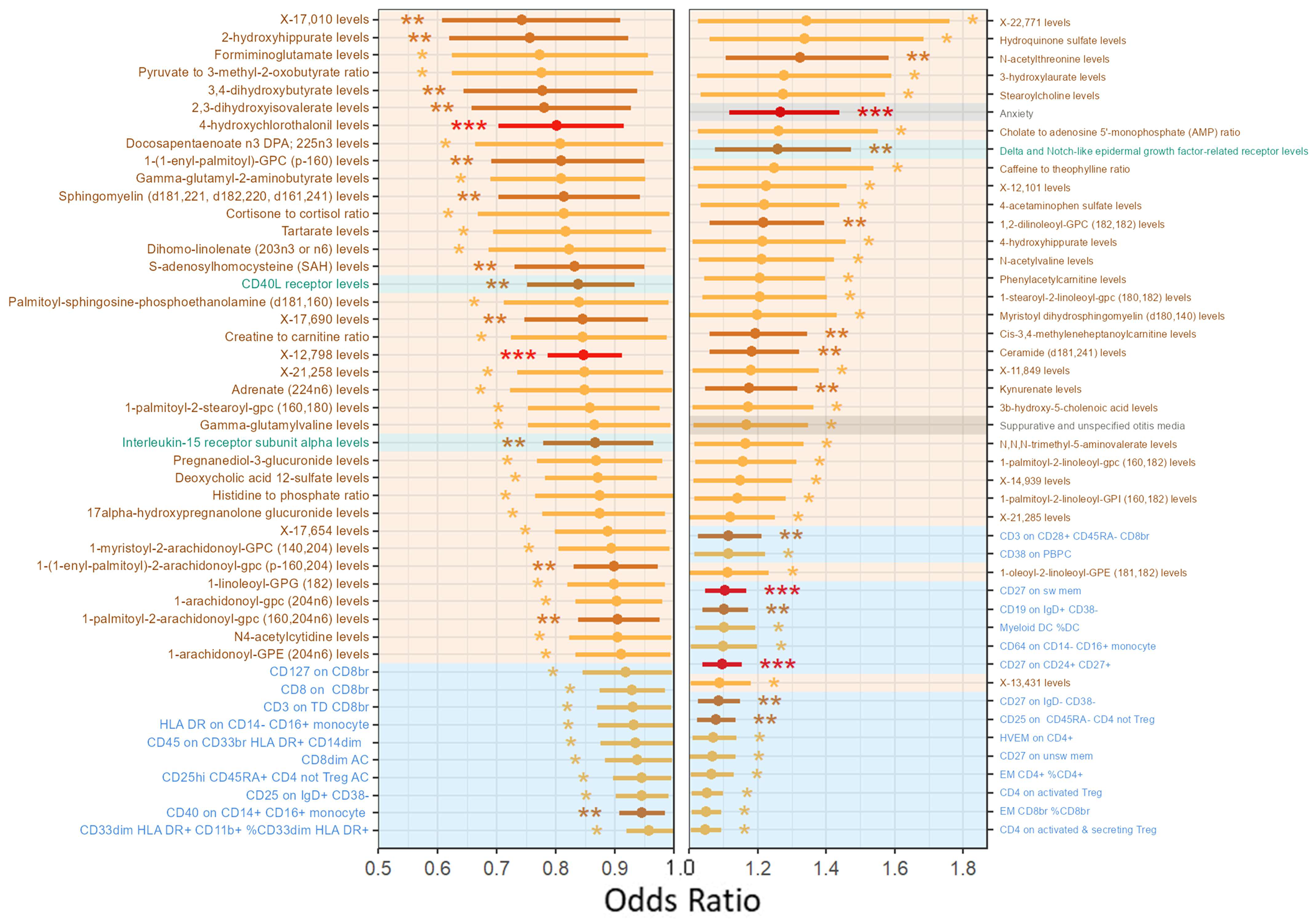
2.3.2. Metabolism, Inflammation, and Immunity-Related Phenotypes Are Associated with Meniere’s Disease Onset
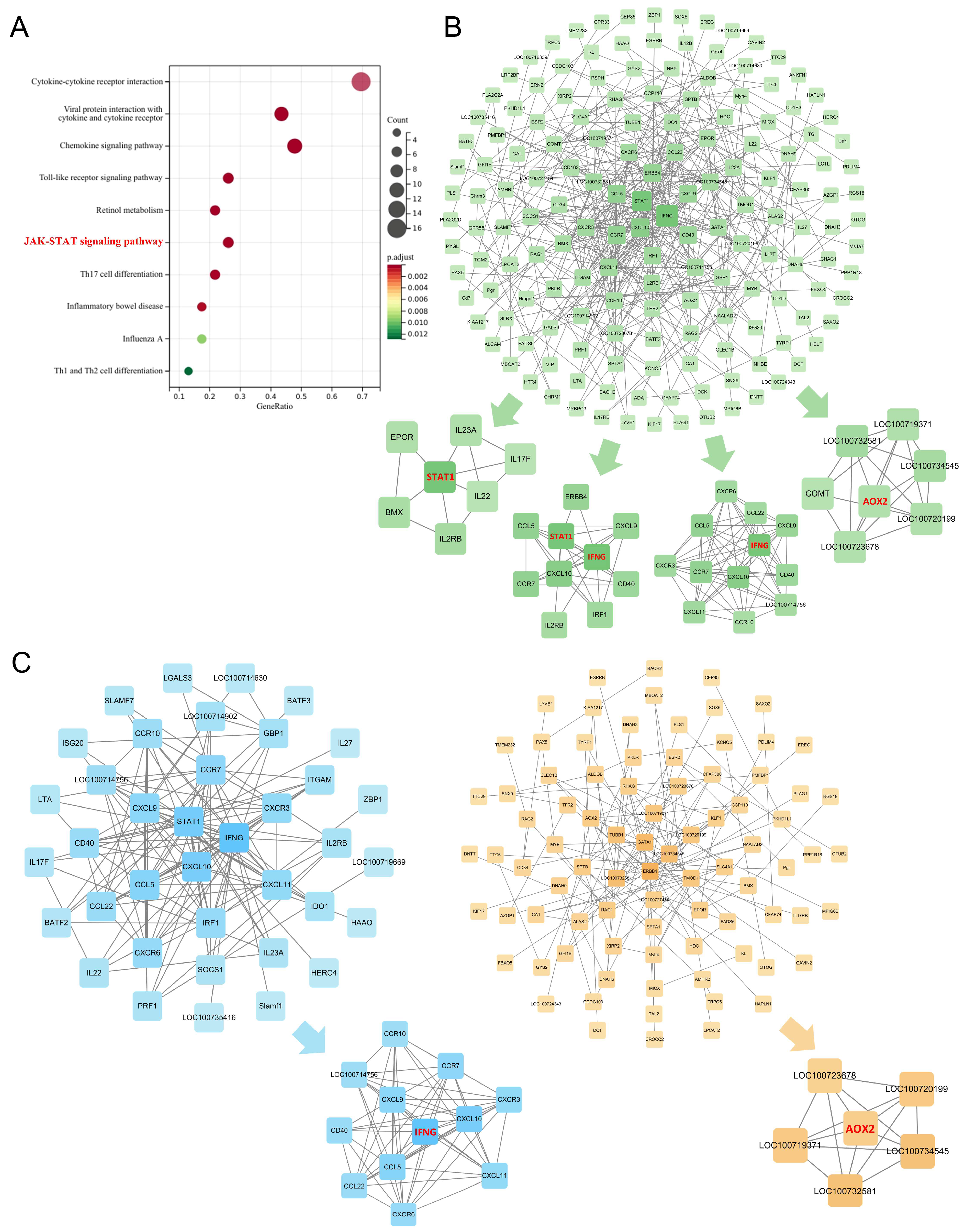
2.3.3. The JAK-STAT Signaling Pathway as a Core Axis in Endolymphatic Hydrops Pathogenesis and XYN Intervention
2.3.4. The hsa-miR-3148/ZBTB20 Regulatory Axis as a Potential Blood Biomarker for Meniere’s Disease
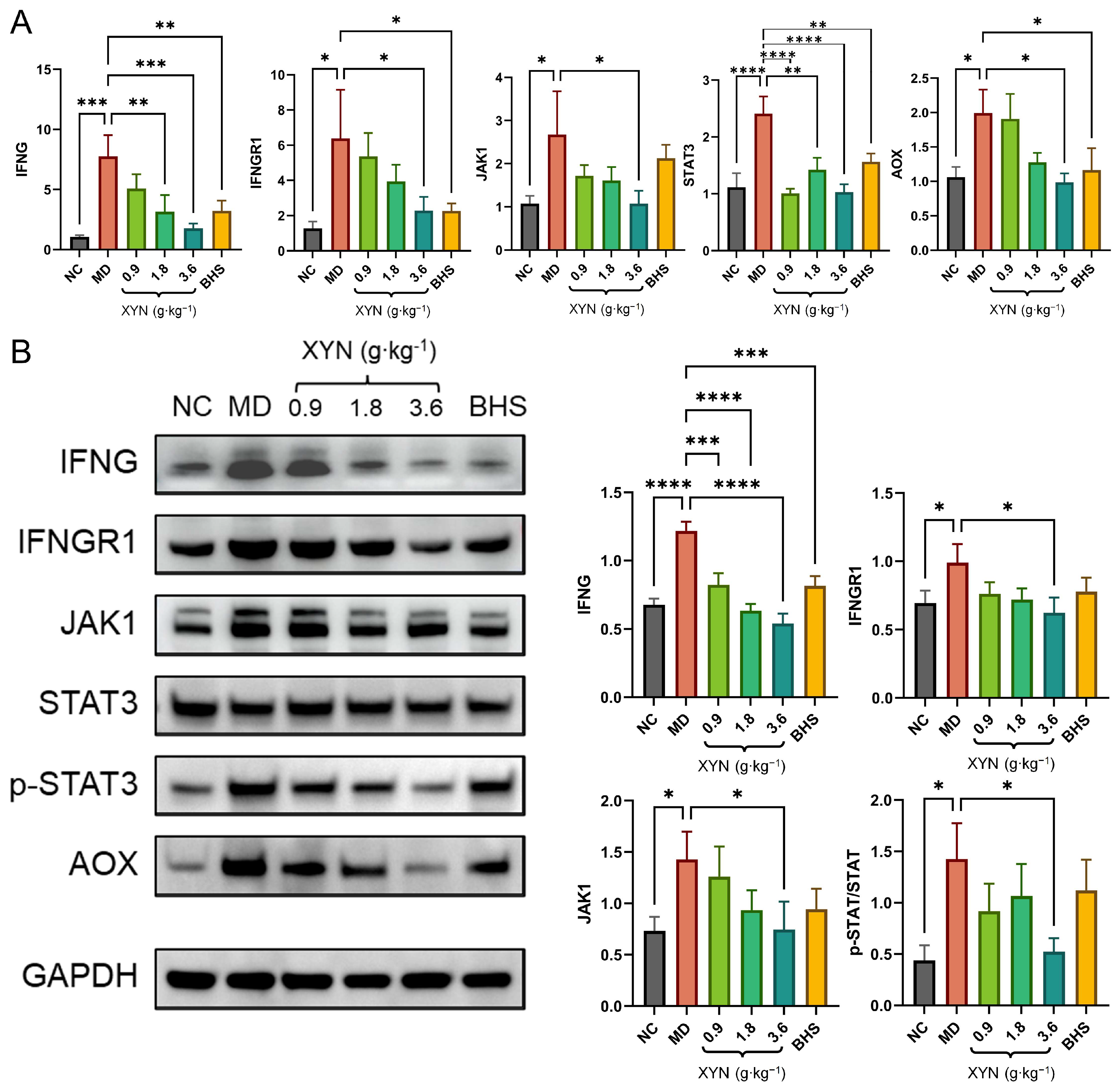
2.4. Overactivation of the JAK-STAT Signaling Pathway in MD and Its Suppression by XYN Treatment
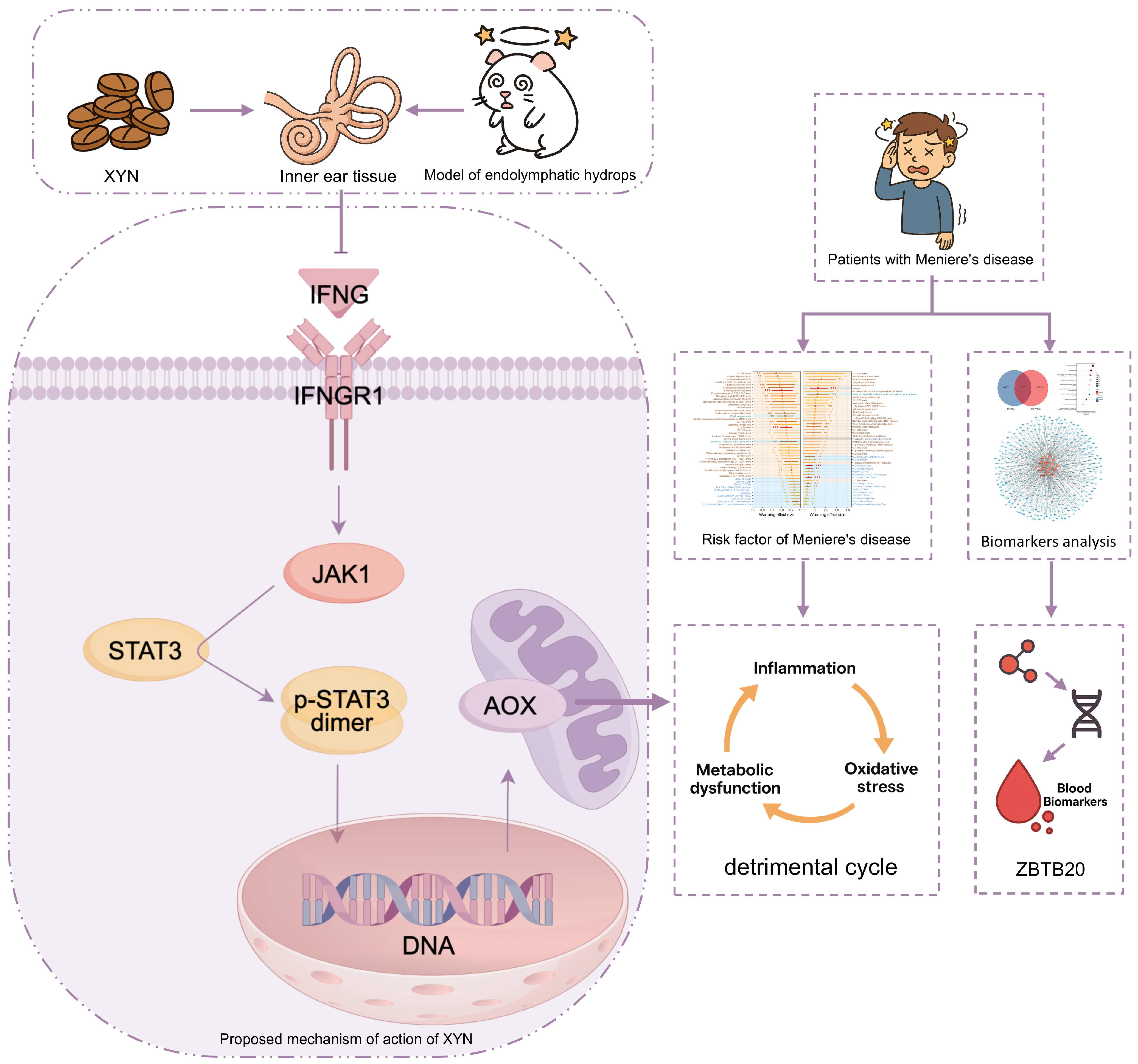
3. Discussion
4. Materials and Methods
4.1. Component Identification Results of XYN
4.2. Experimental Animals
4.3. Pharmacodynamic Evaluation Based on Endolymphatic Hydrops Model
4.3.1. Establishment of the Guinea Pig Endolymphatic Hydrops Model
4.3.2. Behavioral Assessments
4.3.3. Re-Grouping and Drug Administration
4.4. High-Throughput Sequencing and Bioinformatics Analysis
4.4.1. Transcriptomic and Proteomic Analysis
4.4.2. Integrated Omics Analysis
4.4.3. Analysis of Risk Factors for Meniere’s Disease
4.4.4. Analysis of Blood Biomarkers in Meniere’s Disease Patients
4.5. Molecular Biology Experiments
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| AVP | Arginine vasopressin |
| BHS | Betahistine |
| CNN | Convolutional neural network |
| dDAVP | Diamino-Cys1, D-Arg8-vasopressin |
| DEGs | Differentially expressed genes |
| DEPs | Differentially expressed proteins |
| GWAS | Genome-wide association studies |
| IV | Instrumental variable |
| IVW | Inverse-variance weighted |
| LD | Linkage disequilibrium |
| MD | Ménière disease |
| MCODE | Molecular complex detection |
| MR | Mendelian randomization |
| OCSVM | One-class support vector machine |
| PBS | Phosphate-buffered saline |
| RT-qPCR | Real-time quantitative PCR |
| SNP | Single nucleotide polymorphism |
| TCM | Traditional Chinese medicine |
| WB | Western blot |
References
- “Meniere’s Disease—Symptoms and Causes.” Mayo Clinic. Available online: https://www.mayoclinic.org/diseases-conditions/menieres-disease/symptoms-causes/syc-20374910 (accessed on 22 November 2023).
- “Meniere’s Disease.” American Hearing Research Foundation. Available online: https://www.american-hearing.org/disease/menieres-disease/ (accessed on 22 November 2023).
- Ma, H.; Zhong, S.; Wen, Y. Regulatory Effect of Acetic Desmopressin on the Expression of Aquaporin-2 in the Cochlea of Guinea Pigs. J. Audiol. Speech Pathol. 2014, 22, 286–289. [Google Scholar]
- Perez-Carpena, P.; Lopez-Escamez, J.A. Current Understanding and Clinical Management of Meniere’s Disease: A Systematic Review. Semin. Neurol. 2019, 40, 138–150. [Google Scholar] [CrossRef]
- Kwon, M.J.; Kang, H.S.; Kim, J.; Kim, J.H.; Bang, W.J.; Yoo, D.M.; Lee, N.-E.; Han, K.M.; Kim, N.Y.; Choi, H.G.; et al. Epidemiological Evidence for Upper Respiratory Infections as a Potential Risk Factor for Meniere’s Disease: A Korean National Health Sample Cohort Study. Microorganisms 2024, 12, 2047. [Google Scholar] [CrossRef]
- Alexander, T.H.; Harris, J.P. Current Epidemiology of Meniere’s Syndrome. Otolaryngol. Clin. N. Am. 2010, 43, 965–970. [Google Scholar] [CrossRef]
- Koenen, L.; Andaloro, C. Meniere Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Basura, G.J.; Adams, M.E.; Monfared, A.; Schwartz, S.R.; Antonelli, P.J.; Burkard, R.; Bush, M.L.; Bykowski, J.; Colandrea, M.; Derebery, J.; et al. Clinical Practice Guideline: Ménière’s Disease. Otolaryngol. —Head Neck Surg. 2020, 162 (Suppl. S2), S1–S55. [Google Scholar] [CrossRef]
- Adrion, C.; Fischer, C.S.; Wagner, J.; Gürkov, R.; Mansmann, U.; Strupp, M.; BEMED Study Group. Efficacy and Safety of Betahistine Treatment in Patients with Meniere’s Disease: Primary Results of a Long-Term, Multicentre, Double-Blind, Randomised, Placebo-Controlled, Dose-Defining Trial (BEMED Trial). Br. Med. Assoc. 2016, 352, h6816. [Google Scholar] [CrossRef]
- Esch, B.V.; Zaag-Loonen, H.V.D.; Bruintjes, T.; van Benthem, P.P. Betahistine in Ménière’s Disease or Syndrome: A Systematic Review. Audiol. Neurotol. 2022, 27, 1–33. [Google Scholar] [CrossRef]
- Rosenbaum, A.; Winter, M. Is Betahistine Effective for Ménière’s Disease? Medwave 2017, 17, e7068. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Zhang, D.; Zhou, W.; Wang, L.; Wang, B.; Zhang, T.; Li, S. Network pharmacology: Towards the artificial intelligence-based precision traditional Chinese medicine. Brief. Bioinform. 2023, 25, bbad518. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ren, Y.; Song, P. Traditional Chinese medicine for intractable and rare diseases: Research progress and future strategies. Intractable Rare Dis. Res. 2025, 14, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Ning, J.; Liu, D.; Yang, M. Clinical Study on Treating 120 Cases of Meniere’s Disease with XYN. China Med. Her. 2012, 10, 611–613. [Google Scholar]
- Wang, Z. Efficacy Analysis of XYN Decoction in Treating 200 Cases of Meniere’s Disease. Hebei J. Tradit. Chin. Med. 1996, 7–8. Available online: https://kns.cnki.net/kcms2/article/abstract?v=QUtgg5W7F1-o_g7KacxiiNnCXc20HBS_LCLGP4hYvJnmpdqV0UlYmLh-5qgZpMxOheNkzmC0Zn6FipoyiJfU68dZChYEz4pFsV8v124MbjKd6SGYn9V8DzM7QyGihFesk0iU_5EuVv44bRCwXgiROHVIhRPf1nVpfwYfbl2h2iM=&uniplatform=NZKPT&language=CHS (accessed on 1 May 2025).
- Shi, Y. Observation of the Efficacy of XYN in Treating Vertigo Caused by Meniere’s Disease. Chin. Community Dr. Med. 2010, 12, 124. [Google Scholar]
- Wu, Z.; Wang, X.; Zhang, Y.; Yuan, J.J.; Wu, W.; Dong, Z.J.; Hong, Q.Y.; Su, Y.T.; Xin, Z.H.; Yan, Z.F.; et al. Research Review on the Advantages of Traditional Chinese Medicine in Treating Aural Vertigo and Meniere’s Disease. Chin. J. Exp. Tradit. Med. Formulae 2023, 29, 196–203. [Google Scholar]
- Zhai, Y. Treatment of 36 Cases of Meniere’s Disease with XYN Tablets. Guangming J. Chin. Med. 2009, 24, 862–863. [Google Scholar]
- Gu, H. Treatment of 72 Cases of Meniere’s Syndrome with XYN Tablets. Clin. Res. Tradit. Chin. Med. 2011, 3, 97–99. [Google Scholar]
- Luo, Y.; Tang, X.; Zhou, H.; Liu, T.; Zhong, S.J. Analysis of TCM Syndrome Patterns in 632 Emergency Patients with Vertigo. New Chin. Med. 2002, 29–30. [Google Scholar]
- Fu, M.; He, W. Exploring the Treatment of Meniere’s Disease from the Perspective of Spleen and Stomach Theory. China Med. Her. 2020, 17, 149–152, 172. [Google Scholar]
- Zhang, W.; Zhang, Y.; Huo, D. TCM Differentiation and Treatment During the Attack Phase of Meniere’s Disease. Jilin J. Tradit. Chin. Med. 2009, 29, 571–572. [Google Scholar]
- Li, T.; Leng, H. Exploring the Etiology and Treatment of Meniere’s Disease Based on the “Phlegm-Retention Causing Dizziness” Theory. Pract. J. Tradit. Chin. Intern. Med. 2022, 36, 88–91. [Google Scholar]
- Wang, Y.; Zhao, Y. Overview of Commonly Used Oral Chinese Patent Medicines for Treating Vertigo. Mod. Distance Educ. Chin. Tradit. Med. 2016, 14, 143–145. [Google Scholar]
- Ding, Y. Clinical Study on XYN in Treating Meniere’s Disease. China Mod. Dr. 2010, 48, 149–150. [Google Scholar]
- Li, Y.; Li, Q.; Zhu, Y.; Huang, C.; Li, H.; Deng, Z.; Xu, C.; Wang, W.; Chen, L.; Zhang, S.; et al. Medicinal Plant-Derived Carbon Dots Nanozymes Ameliorate Ulcerative Colitis via Anti-inflammatory, Antioxidant, and Gut Barrier-Protective Effects. ACS Appl. Mater. Interfaces 2025, 17, 42751–42766. [Google Scholar] [CrossRef]
- Ullah, A.; Sun, Q.; Li, J.; Li, J.; Khatun, P.; Kou, G.; Lyu, Q. Bioactive Compounds in Citrus reticulata Peel Are Potential Candidates for Alleviating Physical Fatigue through a Triad Approach of Network Pharmacology, Molecular Docking, and Molecular Dynamics Modeling. Nutrients 2024, 16, 1934. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, C.; Huang, H.; Yao, S.; Xu, C.; Ye, Y.; Gui, S.; Li, G. A lipid-soluble extract of Pinellia pedatisecta Schott orchestrates intratumoral dendritic cell-driven immune activation through SOCS1 signaling in cervical cancer. J. Ethnopharmacol. 2021, 267, 112837. [Google Scholar] [CrossRef]
- Huang, Q.; Yin, S. Research Progress on the Relationship Between Sodium Channels, Aquaporins in Inner Ear Epithelium and Endolymph Metabolism. J. Otolaryngol. Ophthalmol. Shandong Univ. 2019, 33, 145–148. [Google Scholar]
- Tan, C.; Cao, Y.; Liu, H.; Zhu, J.; He, X.; Du, C.; Zhou, M. Study on Immune-Related Factors in Meniere’s Disease. J. Clin. Otorhinolaryngol. 1998, 208–210. Available online: https://kns.cnki.net/kcms2/article/abstract?v=QUtgg5W7F18cQxpAgp1u6W3b1cob5s2YJC8bkhFPYQ9sNNnsSBjfQiOd6jNKHCoGksBX33yUl-qawNWB3AAu06uFhX2sXvFbYNcixIVIrx-VnMBdg7r8BlHbDn-TIgh1vumY6dXNeFa79xTgzdjVuavRxP1dfCOG_Z-41nbcNbTz2K1Et3AN85lNDCNj5ySxHJFrZmcHMMhad7rusOwf62ePm-ndVK5S-wk7q0599b4=&uniplatform=NZKPT (accessed on 1 May 2025).
- Lee, A.; Webster, K.E.; George, B.; Harrington-Benton, N.A.; Judd, O.; Kaski, D.; Maarsingh, O.R.; MacKeith, S.; Ray, J.; Van Vugt, V.A. Surgical Interventions for Ménière’s Disease. Cochrane Database Syst. Rev. 2023, 2, CD015249. [Google Scholar] [CrossRef]
- Mohseni-Dargah, M.; Falahati, Z.; Pastras, C.; Khajeh, K.; Mukherjee, P.; Razmjou, A.; Stefani, S.; Asadnia, M. Meniere’s Disease: Pathogenesis, Treatments, and Emerging Approaches for an Idiopathic Bioenvironmental Disorder. Environ. Res. 2023, 238 Pt 1, 116972. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, H.; Paparella, M.M. Meniere’s Disease. Lancet 2008, 372, 406–414. [Google Scholar] [CrossRef]
- Burgess, S.; Butterworth, A.; Malarstig, A.; Thompson, S.G. Use of Mendelian Randomisation to Assess Potential Benefit of Clinical Intervention. BMJ Clin. Res. Ed. 2012, 345, e7325. [Google Scholar]
- Skrivankova, V.W.; Richmond, R.C.; Woolf, B.A.R.; Davies, N.M.; Swanson, S.A.; VanderWeele, T.J.; Timpson, N.J.; Higgins, J.P.T.; Dimou, N.; Langenberg, C.; et al. Strengthening the Reporting of Observational Studies in Epidemiology Using Mendelian Randomisation (STROBE-MR): Explanation and Elaboration. BMJ Clin. Res. Ed. 2021, 375, n2233. [Google Scholar] [CrossRef]
- Darnell, J.E.; Kerr, I.M.; Stark, G.R. Jak-STAT Pathways and Transcriptional Activation in Response to IFNs and Other Extracellular Signaling Proteins. Science 1994, 264, 1415–1421. [Google Scholar] [CrossRef]
- Xue, C.; Yao, Q.; Gu, X.; Shi, Q.; Yuan, X.; Chu, Q.; Bao, Z.; Lu, J.; Li, L. Evolving Cognition of the JAK-STAT Signaling Pathway: Autoimmune Disorders and Cancer. Signal Transduct. Target. Ther. 2023, 8, 204. [Google Scholar] [CrossRef]
- Mahjoor, M.; Mahmoudvand, G.; Farokhi, S.; Shadab, A.; Kashfi, M.; Afkhami, H. Double-Edged Sword of JAK/STAT Signaling Pathway in Viral Infections: Novel Insights into Virotherapy. Cell Commun. Signal. 2023, 21, 272. [Google Scholar] [CrossRef]
- Xia, T.; Fu, S.; Yang, R.; Yang, K.; Lei, W.; Yang, Y.; Zhang, Q.; Zhao, Y.; Yu, J.; Yu, L.; et al. Advances in the Study of Macrophage Polarization in Inflammatory Immune Skin Diseases. J. Inflamm. 2023, 20, 33. [Google Scholar] [CrossRef]
- Sarapultsev, A.; Gusev, E.; Komelkova, M.; Utepova, I.; Luo, S.; Hu, D. JAK-STAT Signaling in Inflammation and Stress-Related Diseases: Implications for Therapeutic Interventions. Mol. Biomed. 2023, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Hu, W.; Wang, R.; Yang, Q.; Zhu, M.; Li, M.; Cai, J.; Rose, P.; Mao, J.; Zhu, Y.Z. Signaling Pathways in Rheumatoid Arthritis: Implications for Targeted Therapy. Signal Transduct. Target. Ther. 2023, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Mu, X.; Wu, X.; Liu, Y.; Deng, J.; Liu, Y.; Han, F.; Nie, X. The cGAS-STING Pathway: A Therapeutic Target in Diabetes and Its Complications. Burn. Trauma 2024, 12, tkad050. [Google Scholar] [CrossRef] [PubMed]
- Decout, A.; Katz, J.D.; Venkatraman, S.; Ablasser, A. The cGAS–STING Pathway as a Therapeutic Target in Inflammatory Diseases. Nat. Rev. Immunol. 2021, 21, 548–569. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019, 10, 1607. [Google Scholar] [CrossRef]
- Lu, D.; He, A.; Tan, M.; Mrad, M.; El Daibani, A.; Hu, D.; Liu, X.; Kleiboeker, B.; Che, T.; Hsu, F.F.; et al. Liver ACOX1 Regulates Levels of Circulating Lipids That Promote Metabolic Health Through Adipose Remodeling. Nat. Commun. 2024, 15, 4214. [Google Scholar] [CrossRef]
- Albernaz, P.L.M. Menière’s Disease and Disorders of the Carbohydrate Metabolism Involving the Inner Ear. Int. Arch. Otorhinolaryngol. 2019, 23, 218–220. [Google Scholar] [CrossRef]
- Rossmeisl, M.; Jilkova, Z.M.; Kuda, O.; Jelenik, T.; Medrikova, D.; Stankova, B.; Kristinsson, B.; Haraldsson, G.G.; Svensen, H.; Stoknes, I.; et al. Metabolic Effects of n-3 PUFA as Phospholipids Are Superior to Triglycerides in Mice Fed a High-Fat Diet: Possible Role of Endocannabinoids. PLoS ONE 2012, 7, e38834. [Google Scholar] [CrossRef]
- Marchitti, S.A.; Brocker, C.; Stagos, D.; Vasiliou, V. Non-P450 Aldehyde Oxidizing Enzymes: The Aldehyde Dehydrogenase Superfamily. Expert Opin. Drug Metab. Toxicol. 2008, 4, 697–720. [Google Scholar] [CrossRef]
- Terao, M.; Garattini, E.; Romão, M.J.; Leimkühler, S. Evolution, Expression, and Substrate Specificities of Aldehyde Oxidase Enzymes in Eukaryotes. J. Biol. Chem. 2020, 295, 5377–5389. [Google Scholar] [CrossRef]
- Yang, M.; Wu, C.; Lin, Y.; Tsai, M.; Hwang, C.; Yang, C. Dissecting the Circadian Clock and Toll-like Receptor Gene Alterations in Meniere’s Disease and Vestibular Migraine. Otolaryngol.—Head Neck Surg. 2025, 172, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Frejo, L.; Lopez-Escamez, J.A. Cytokines and Inflammation in Meniere Disease. Clin. Exp. Otorhinolaryngol. 2022, 15, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Teraoka, M.; Hato, N.; Inufusa, H.; You, F. Role of Oxidative Stress in Sensorineural Hearing Loss. Int. J. Mol. Sci. 2024, 25, 4146. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Ma, X.H.; Bai, Q.F.; Tang, J.; Sun, J.H.; Jiang, F.; Guo, W.; Wang, C.M.; Yang, R.; Wen, Y.C.; et al. ZBTB20 is Essential for Cochlear Maturation and Hearing in Mice. Proc. Natl. Acad. Sci. USA 2023, 120, e2220867120. [Google Scholar] [CrossRef]
- Salt, A.N.; Plontke, S.K. Endolymphatic Hydrops: Pathophysiology and Experimental Models. Otolaryngol. Clin. N. Am. 2010, 43, 971–983. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, P.; Bao, Y.; Han, Y.; Wang, Y.; Zhang, Q.; Zhan, Z.; Meng, J.; Li, Y.; Li, N. Zinc Finger Protein ZBTB20 Promotes Toll-like Receptor-Triggered Innate Immune Responses by Repressing IκBα Gene Transcription. Proc. Natl. Acad. Sci. USA 2013, 110, 11097–11102. [Google Scholar] [CrossRef]
- Seo, Y.J.; Brown, D. Experimental Animal Models for Meniere’s Disease: A Mini-Review. J. Audiol. Otol. 2020, 24, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kakigi, A.; Egami, N.; Uehara, N.; Fujita, T.; Nibu, K.I.; Yamashita, S.; Yamasoba, T. Live imaging and functional changes of the inner ear in an animal model of Meniere’s disease. Sci. Rep. 2020, 10, 12271. [Google Scholar] [CrossRef] [PubMed]
- Meddens, S.F.W.; de Vlaming, R.; Bowers, P.; Burik, C.A.P.; Linnér, R.K.; Lee, C.; Okbay, A.; Turley, P.; Rietveld, C.A.; Fontana, M.A.; et al. Genomic Analysis of Diet Composition Finds Novel Loci and Associations with Health and Lifestyle. Mol. Psychiatry 2021, 26, 2056–2069. [Google Scholar] [CrossRef] [PubMed]
| Gene | Primer Direction | Sequence (5′-3′) | Length |
|---|---|---|---|
| GAPDH | Forward | ATCAAGTGGGGTGATGCTGG | 20 |
| Reverse | AAGATGCCTTTGAGGGAGCC | 20 | |
| IFNG | Forward | AAGACAACAGCAGCAACAAGG | 21 |
| Reverse | ACATGCTCGTCATTGACCGAAA | 20 | |
| IFNGR1 | Forward | GTGAACAGAAGTGAGACCCGA | 21 |
| Reverse | AGAATACAGCAAGGGCACCG | 20 | |
| JAK1 | Forward | CCATGATGCGGAACATGCAG | 20 |
| Reverse | GTTAATGCTGACCAGGGCCT | 20 | |
| STAT3 | Forward | CACCACAAAAGTCAGGCTGC | 20 |
| Reverse | ACAGCCTGAGTAGTTCACGC | 20 | |
| AOX | Forward | TCCCAGCTGGTGATCGAGTA | 20 |
| Reverse | ACGGTGCTGGTTTCAGAGAG | 20 |
| Antibody | Catalog No. | Manufacturer | Dilution Ratio |
|---|---|---|---|
| HRP-conjugated Goat Anti-Rabbit IgG(H + L) | SA00001-2 | Proteintech (Rosemont, IL, USA) | 1:50,000 |
| HRP-conjugated Goat Anti-Mouse IgG(H + L) | SA00001-1 | Proteintech | 1:10,000 |
| STAT3 Rabbit Monoclonal antibody | A19566 | ABclonal Technology (Wuhan, China) | 1:10,000 |
| Phospho-STAT3-Y705 Rabbit Monoclonal antibody | AP1468 | ABclonal Technology | 1:5000 |
| IFN-gamma Rabbit Monoclonal antibody | A25684 | ABclonal Technology | 1:1000 |
| JAK1 Monoclonal antibody | 66466-1-Ig | Proteintech | 1:3000 |
| Aldehyde oxidase Polyclonal antibody | 19495-1-AP | Proteintech | 1:300 |
| GAPDH Polyclonal antibody | 10494-1-AP | Proteintech | 1:20,000 |
| IFN-gamma R1 Polyclonal antibody | 10808-1-AP | Proteintech | 1:600 |
| Gene | Comparison | p-Value | Significance | Confidence Interval |
|---|---|---|---|---|
| IFNG | NC vs. MD | 0.0002 | *** | −9.91 to −3.50 |
| MD vs. XYN-1.8 | 0.0064 | ** | 1.39 to 7.81 | |
| MD vs. XYN-3.6 | 0.0006 | *** | 2.78 to 9.19 | |
| MD vs. BHS | 0.0072 | ** | 1.32 to 7.73 | |
| IFNGR1 | NC vs. MD | 0.013 | * | −9.08 to −1.16 |
| MD vs. XYN-3.6 | 0.0429 | * | 0.14 to 8.06 | |
| MD vs. BHS | 0.0421 | * | 0.16 to 8.08 | |
| JAK1 | NC vs. MD | 0.0252 | * | −2.99 to −0.21 |
| MD vs. XYN-3.6 | 0.0254 | * | 0.21 to 2.99 | |
| STAT3 | NC vs. MD | <0.0001 | **** | −1.88 to −0.71 |
| MD vs. XYN-0.9 | <0.0001 | **** | 0.82 to 1.99 | |
| MD vs. XYN-1.8 | 0.0017 | ** | 0.40 to 1.57 | |
| MD vs. XYN-3.6 | <0.0001 | **** | 0.79 to 1.96 | |
| MD vs. BHS | 0.0061 | ** | 0.26 to 1.43 | |
| AOX | NC vs. MD | 0.0168 | * | −1.69 to −0.18 |
| MD vs. XYN-3.6 | 0.0104 | * | 0.26 to 1.76 | |
| MD vs. BHS | 0.0324 | * | 0.07 to 1.58 |
| Protein | Comparison | p-Value | Significance | Confidence Interval |
|---|---|---|---|---|
| IFNG | NC vs. MD | <0.0001 | **** | −0.7333 to −0.3485 |
| MD vs. 0.9 | 0.0002 | *** | 0.2048 to 0.5896 | |
| MD vs. 1.8 | <0.0001 | **** | 0.3922 to 0.7771 | |
| MD vs. 3.6 | <0.0001 | **** | 0.4851 to 0.8699 | |
| MD vs. BHS | 0.0002 | *** | 0.2115 to 0.5963 | |
| IFNGR1 | NC vs. MD | 0.0482 | * | −0.5889 to −0.002520 |
| MD vs. 3.6 | 0.0161 | * | 0.07293 to 0.6593 | |
| JAK1 | NC vs. MD | 0.0431 | * | −1.372 to −0.02326 |
| MD vs. 3.6 | 0.0468 | * | 0.01032 to 1.359 | |
| p-STAT3/STAT3 | NC vs. MD | 0.013 | * | −1.751 to −0.2238 |
| MD vs. 3.6 | 0.0222 | * | 0.1385 to 1.665 | |
| AOX | NC vs. MD | <0.0001 | **** | −1.857 to −0.9269 |
| NC vs. 0.9 | 0.0009 | *** | −1.302 to −0.3711 | |
| MD vs. 0.9 | 0.0208 | * | 0.09051 to 1.021 | |
| MD vs. 1.8 | <0.0001 | **** | 0.6883 to 1.619 | |
| MD vs. 3.6 | <0.0001 | **** | 0.8443 to 1.775 | |
| MD vs. BHS | 0.0002 | *** | 0.5183 to 1.449 |
| Score | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| General condition | Normal | Mild gait instability | Gait instability with weight loss | Severe gait instability, inability to move normally, weight loss |
| Auricular reflex | Strong | Marked | Mild | Absent |
| Nystagmus | Absent | Mild | Moderate-frequency, moderate-amplitude | High-frequency, large-amplitude |
| Righting reflex | Rapid | Impaired righting response | Difficulty righting | No righting response |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Wang, C.; Gao, Y.; Tao, X.; Wu, C.; Guo, S.; Huang, J.; Zhou, J.; Qiao, C.; Chai, K.; et al. Systems Network Integration of Transcriptomic, Proteomic, and Bioinformatic Analyses Reveals the Mechanism of XuanYunNing Tablets in Meniere’s Disease via JAK-STAT Pathway Modulation. Pharmaceuticals 2025, 18, 1266. https://doi.org/10.3390/ph18091266
Jin Z, Wang C, Gao Y, Tao X, Wu C, Guo S, Huang J, Zhou J, Qiao C, Chai K, et al. Systems Network Integration of Transcriptomic, Proteomic, and Bioinformatic Analyses Reveals the Mechanism of XuanYunNing Tablets in Meniere’s Disease via JAK-STAT Pathway Modulation. Pharmaceuticals. 2025; 18(9):1266. https://doi.org/10.3390/ph18091266
Chicago/Turabian StyleJin, Zhengsen, Chunguo Wang, Yifei Gao, Xiaoyu Tao, Chao Wu, Siyu Guo, Jiaqi Huang, Jiying Zhou, Chuanqi Qiao, Keyan Chai, and et al. 2025. "Systems Network Integration of Transcriptomic, Proteomic, and Bioinformatic Analyses Reveals the Mechanism of XuanYunNing Tablets in Meniere’s Disease via JAK-STAT Pathway Modulation" Pharmaceuticals 18, no. 9: 1266. https://doi.org/10.3390/ph18091266
APA StyleJin, Z., Wang, C., Gao, Y., Tao, X., Wu, C., Guo, S., Huang, J., Zhou, J., Qiao, C., Chai, K., Chang, H., Li, C., Zou, X., & Wu, J. (2025). Systems Network Integration of Transcriptomic, Proteomic, and Bioinformatic Analyses Reveals the Mechanism of XuanYunNing Tablets in Meniere’s Disease via JAK-STAT Pathway Modulation. Pharmaceuticals, 18(9), 1266. https://doi.org/10.3390/ph18091266






