VDR Decrease Enhances the Efficacy of 1,25-Dihydroxyvitamin D3 Inhibiting Gefitinib Resistance by Regulating EGFR/FASN Loop in NSCLC Cells
Abstract
1. Introduction
2. Results
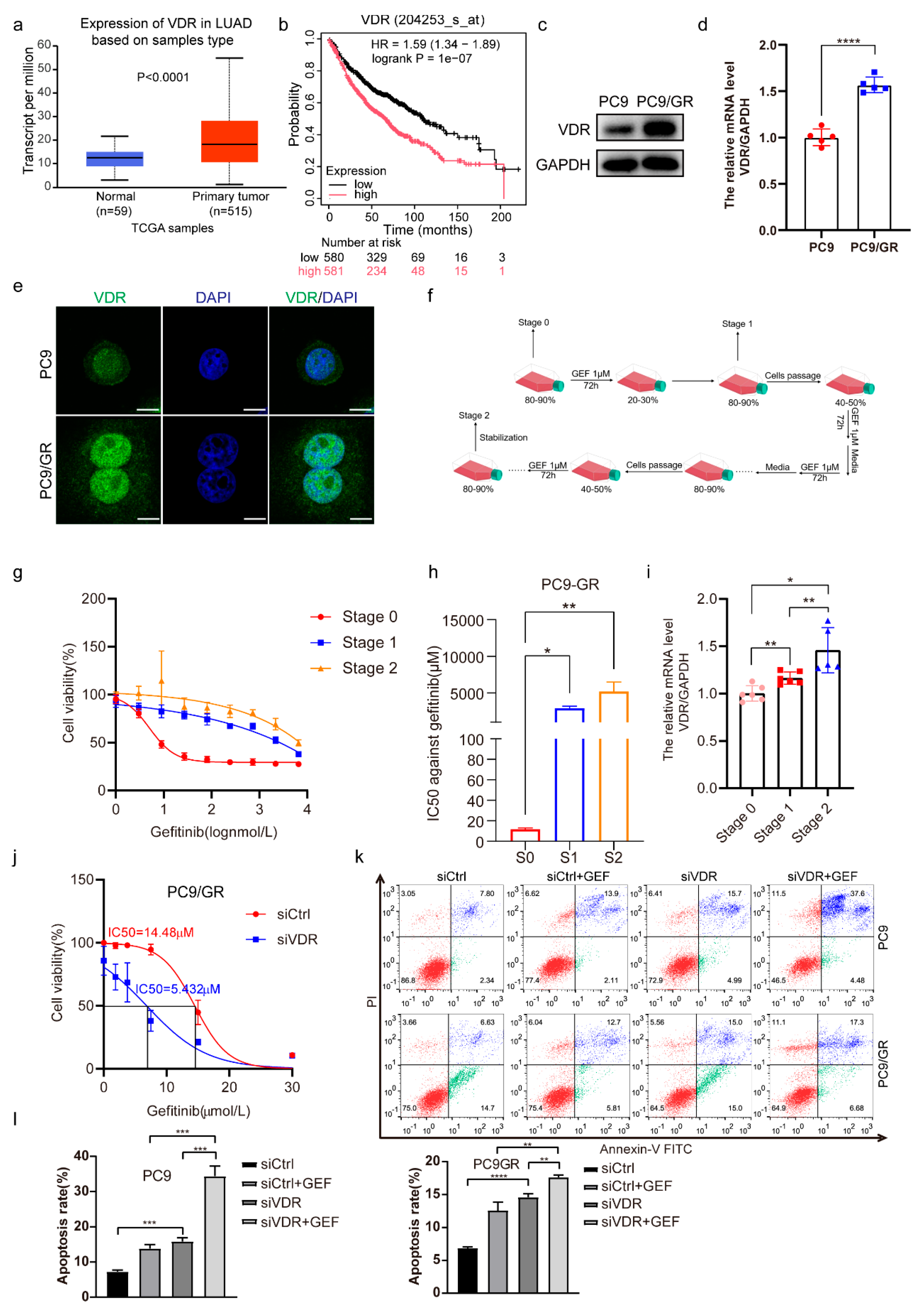
2.1. Downregulation of VDR Expression Enhances the Gefitinib Cytotoxicity of NSCLC Cells
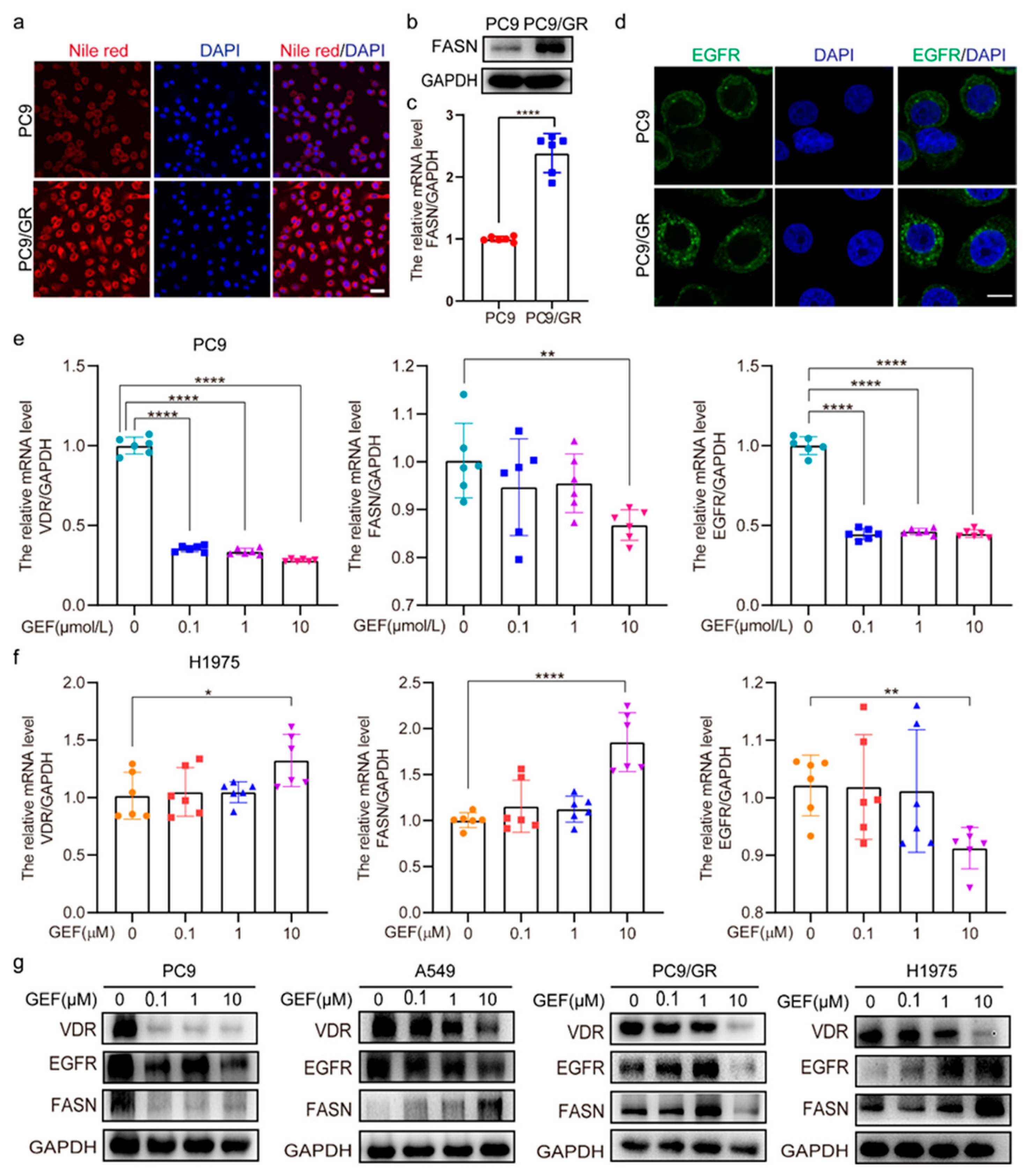
2.2. VDR Expression Correlates with EGFR and FASN
2.3. The Interaction Among VDR, EGFR, FASN, and AKT Regulates Gefitinib Sensitivity
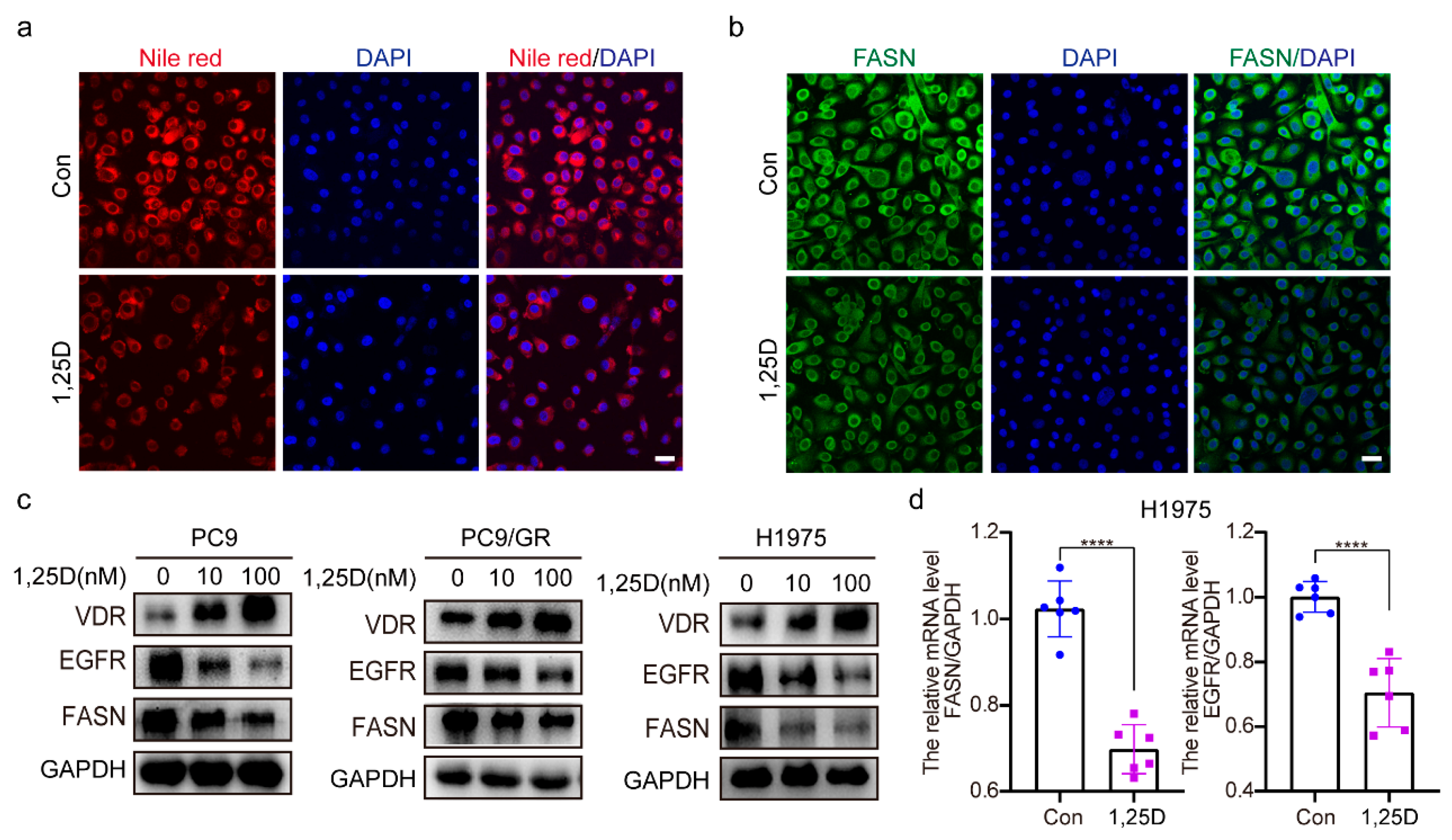
2.4. 1,25(OH)2D3 Increases VDR Expression and Decreases Expression of EGFR and FASN
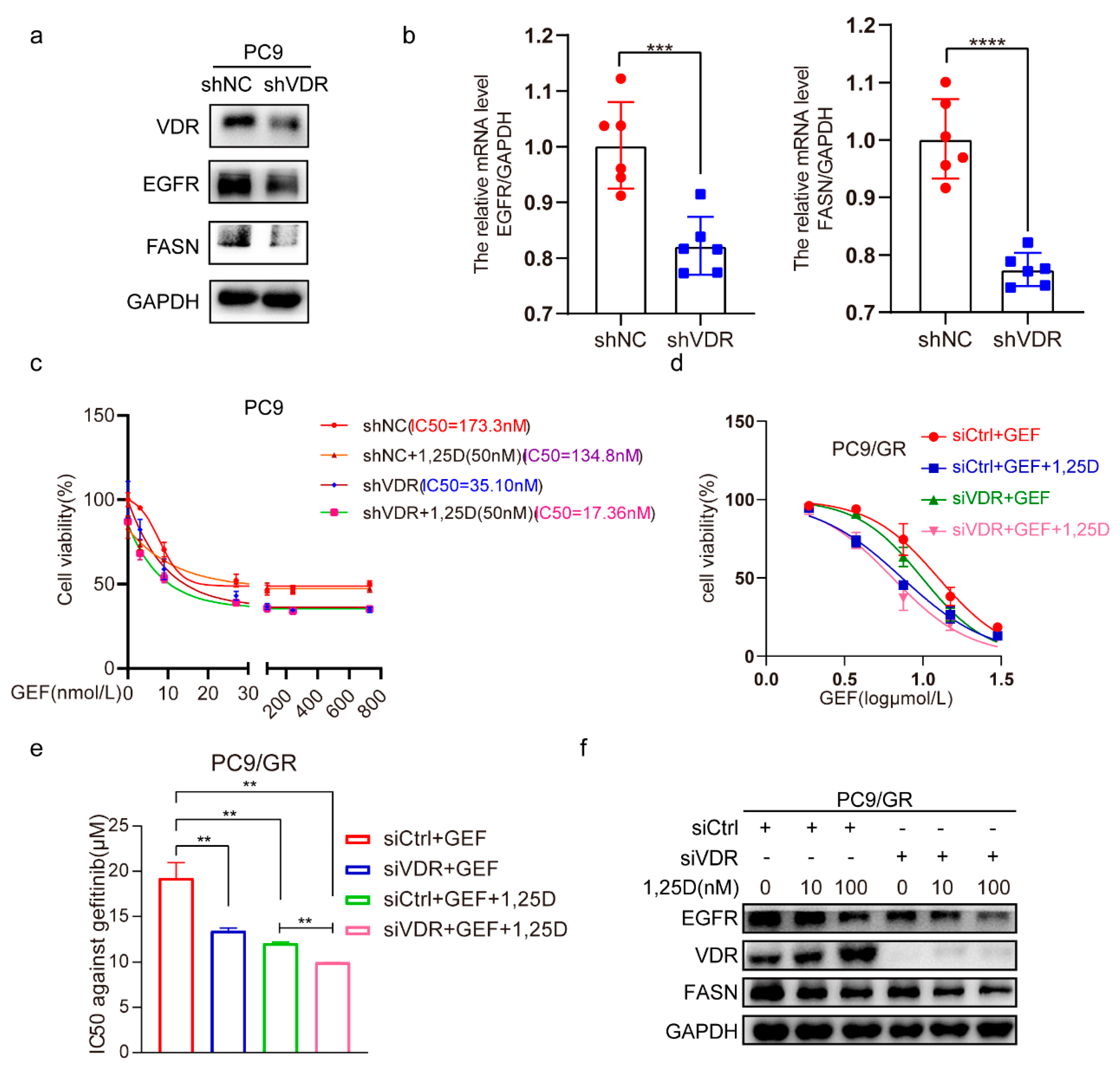
2.5. Knockdown of VDR Combined with 1,25(OH)2D3 Increases Gefitinib Cytotoxicity by Inhibiting EGFR and FASN in NSCLC Cells
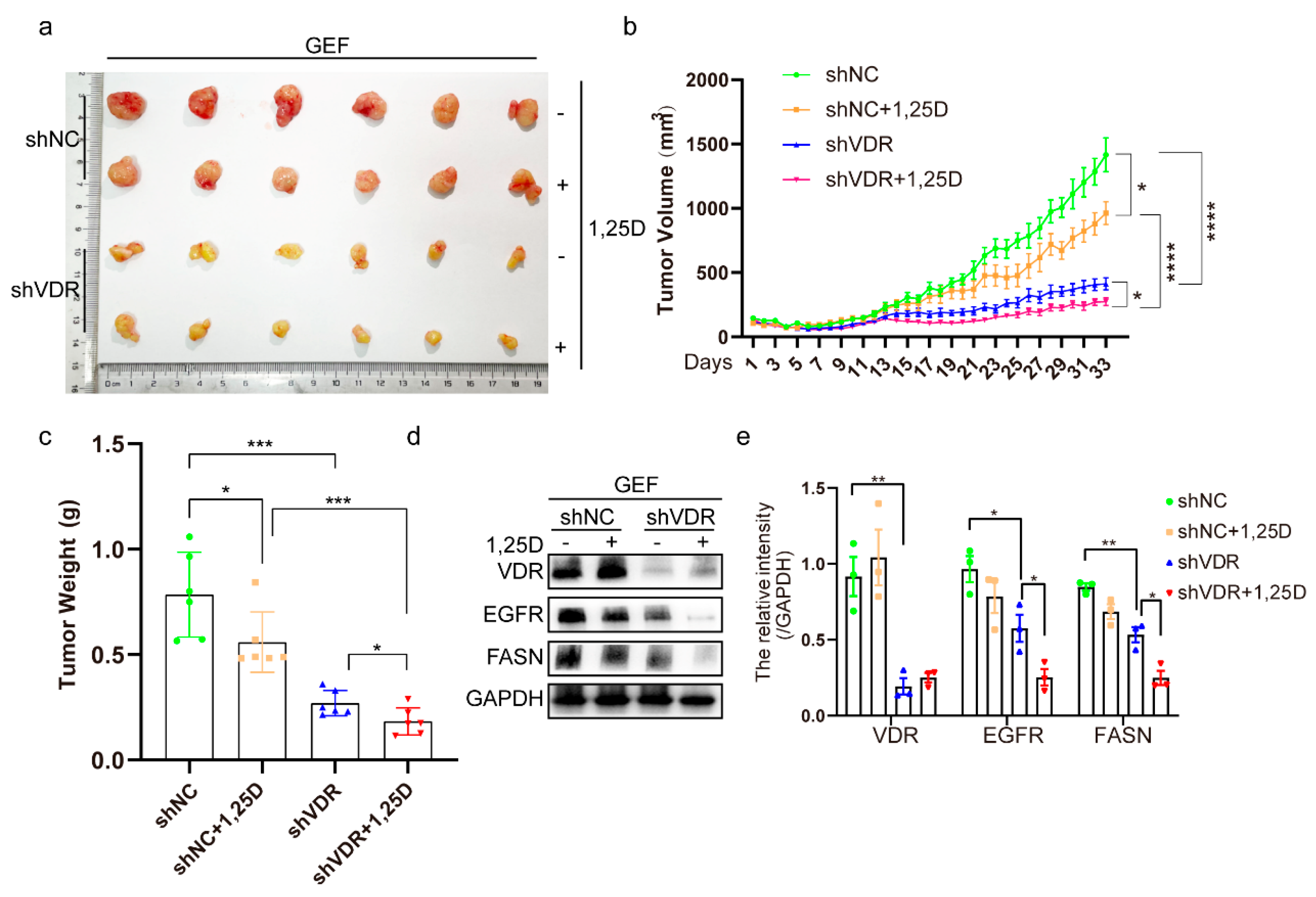
2.6. Knockdown of VDR Combined with 1,25(OH)2D3 Inhibits Tumor Growth by Suppressing EGFR and FASN In Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Lines
4.2. Reagents and Chemicals
4.3. Western Blot Analysis
4.4. Quantitative Real-Time PCR
4.5. Cell Viability Assays
4.6. Colony-Formation Assay
4.7. siRNA and Plasmid Transfection
4.8. Flow Cytometry for the Detection of Apoptosis
4.9. The Steps of Inducing Gefitinib-Resistant PC9 Cells
4.10. Immunofluorescence
4.11. Nile Red Staining
4.12. Lentivirus Infection
4.13. In Vivo Mouse Model
4.14. TCGA and Kaplan–Meier Analysis
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EGFR | epidermal growth factor receptor |
| EGFR-TKIs | epidermal growth factor receptor tyrosine kinase inhibitors |
| NSCLC | non-small cell lung cancer |
| TCGA | The Cancer Genome Atlas |
| 1,25(OH)2D3 | 1,25-dihydroxyvitamin D3 |
| VDR | vitamin D receptor |
| VDREs | vitamin D response elements |
| FASN | fatty acid synthase |
| LUAD | lung adenocarcinoma |
| PC9/GR | PC9/gefitinib-resistant strain (gefitinib-resistant PC9) |
| SREBP | sterol regulatory element-binding protein |
| SCAP | SREBP cleavage-activating protein |
| IC50 | half maximal inhibitory concentration |
References
- Zheng, R.S.; Chen, R.; Han, B.F.; Wang, S.M.; Li, L.; Sun, K.X.; Zeng, H.M.; Wei, W.W.; He, J. Cancer incidence and mortality in China, 2022. Zhonghua Zhong Liu Za Zhi 2024, 46, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, F.; Hirsch, F.R.; Pirker, R.; Felip, E.; Valencia, C.; Smit, E.F. The role of anti-EGFR therapies in EGFR-TKI-resistant advanced non-small cell lung cancer. Cancer Treat. Rev. 2024, 122, 102664. [Google Scholar] [CrossRef] [PubMed]
- Blaquier, J.B.; Ortiz-Cuaran, S.; Ricciuti, B.; Mezquita, L.; Cardona, A.F.; Recondo, G. Tackling Osimertinib Resistance in EGFR-Mutant Non-Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 3579–3591. [Google Scholar] [CrossRef]
- Dimitrakopoulou, V.I.; Tsilidis, K.K.; Haycock, P.C.; Dimou, N.L.; Al-Dabhani, K.; Martin, R.M.; Lewis, S.J.; Gunter, M.J.; Mondul, A.; Shui, I.M.; et al. Circulating vitamin D concentration and risk of seven cancers: Mendelian randomisation study. BMJ 2017, 359, j4761. [Google Scholar] [CrossRef]
- Heist, R.S.; Zhou, W.; Wang, Z.; Liu, G.; Neuberg, D.; Su, L.; Asomaning, K.; Hollis, B.W.; Lynch, T.J.; Wain, J.C.; et al. Circulating 25-hydroxyvitamin D, VDR polymorphisms, and survival in advanced non-small-cell lung cancer. J. Clin. Oncol. 2008, 26, 5596–5602. [Google Scholar] [CrossRef]
- Akiba, T.; Morikawa, T.; Odaka, M.; Nakada, T.; Kamiya, N.; Yamashita, M.; Yabe, M.; Inagaki, T.; Asano, H.; Mori, S.; et al. Vitamin D Supplementation and Survival of Patients with Non-small Cell Lung Cancer: A Randomized, Double-Blind, Placebo-Controlled Trial. Clin. Cancer Res. 2018, 24, 4089–4097. [Google Scholar] [CrossRef]
- Jia, Z.; Zhang, Y.; Yan, A.; Wang, M.; Han, Q.; Wang, K.; Wang, J.; Qiao, C.; Pan, Z.; Chen, C.; et al. 1,25-dihydroxyvitamin D3 signaling-induced decreases in IRX4 inhibits NANOG-mediated cancer stem-like properties and gefitinib resistance in NSCLC cells. Cell Death Dis. 2020, 11, 670. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, K.; Duan, Y.; Hu, K.; Zhang, Y.; Wang, M.; Xiao, K.; Liu, S.; Pan, Z.; Ding, X. Claudin1 decrease induced by 1,25-dihydroxy-vitamin D3 potentiates gefitinib resistance therapy through inhibiting AKT activation-mediated cancer stem-like properties in NSCLC cells. Cell Death Dis. 2022, 8, 122. [Google Scholar] [CrossRef]
- Shaurova, T.; Dy, G.K.; Battaglia, S.; Hutson, A.; Zhang, L.; Zhang, Y.; Lovly, C.M.; Seshadri, M.; Goodrich, D.W.; Johnson, C.S.; et al. Vitamin D3 Metabolites Demonstrate Prognostic Value in EGFR-Mutant Lung Adenocarcinoma and Can be Deployed to Oppose Acquired Therapeutic Resistance. Cancers 2020, 12, 675. [Google Scholar] [CrossRef]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef]
- Fathi, N.; Ahmadian, E.; Shahi, S.; Roshangar, L.; Khan, H.; Kouhsoltani, M.; Dizaj, S.M.; Sharifi, S. Role of vitamin D and vitamin D receptor (VDR) in oral cancer. Biomed. Pharmacother. 2019, 109, 391–401. [Google Scholar] [CrossRef]
- Deuster, E.; Jeschke, U.; Ye, Y.; Mahner, S.; Czogalla, B. Vitamin D and VDR in Gynecological Cancers-A Systematic Review. Int. J. Mol. Sci. 2017, 18, 2328. [Google Scholar] [CrossRef] [PubMed]
- Khazan, N.; Kim, K.K.; Hansen, J.N.; Singh, N.A.; Moore, T.; Snyder, C.W.A.; Pandita, R.; Strawderman, M.; Fujihara, M.; Takamura, Y.; et al. Identification of a Vitamin-D Receptor Antagonist, MeTC7, which Inhibits the Growth of Xenograft and Transgenic Tumors In Vivo. J. Med. Chem. 2022, 65, 6039–6055. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Vallega, K.A.; Boda, V.K.; Quan, Z.; Wang, D.; Fan, S.; Wang, Q.; Ramalingam, S.S.; Li, W.; Sun, S.Y. Targeting Transient Receptor Potential Melastatin-2 (TRPM2) Enhances Therapeutic Efficacy of Third Generation EGFR Inhibitors against EGFR Mutant Lung Cancer. Adv. Sci. 2024, 11, e2310126. [Google Scholar] [CrossRef] [PubMed]
- Passaro, A.; Janne, P.A.; Mok, T.; Peters, S. Overcoming therapy resistance in EGFR-mutant lung cancer. Nat. Cancer 2021, 2, 377–391. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Guo, G.; Panchani, N.; Bender, M.E.; Gerber, D.E.; Minna, J.D.; Fattah, F.; Gao, B.; Peyton, M.; Kernstine, K.; et al. EGFR inhibition triggers an adaptive response by co-opting antiviral signaling pathways in lung cancer. Nat. Cancer 2020, 1, 394–409. [Google Scholar] [CrossRef]
- Niu, M.; Xu, J.; Liu, Y.; Li, Y.; He, T.; Ding, L.; He, Y.; Yi, Y.; Li, F.; Guo, R.; et al. FBXL2 counteracts Grp94 to destabilize EGFR and inhibit EGFR-driven NSCLC growth. Nat. Commun. 2021, 12, 5919. [Google Scholar] [CrossRef]
- McGaffin, K.R.; Chrysogelos, S.A. Identification and characterization of a response element in the EGFR promoter that mediates transcriptional repression by 1,25-dihydroxyvitamin D3 in breast cancer cells. J. Mol. Endocrinol. 2005, 35, 117–133. [Google Scholar] [CrossRef]
- Cordero, J.B.; Cozzolino, M.; Lu, Y.; Vidal, M.; Slatopolsky, E.; Stahl, P.D.; Barbieri, M.A.; Dusso, A. 1,25-dihydroxyvitamin D down-regulates cell membrane growth- and nuclear growth-promoting signals by the epidermal growth factor receptor. J. Biol. Chem. 2002, 277, 38965–38971. [Google Scholar] [CrossRef]
- González, E.A.; Disthabanchong, S.; Kowalewski, R.; Martin, K.J. Mechanisms of the regulation of EGF receptor gene expression by calcitriol and parathyroid hormone in UMR 106-01 cells. Kidney Int. 2002, 61, 1627–1634. [Google Scholar] [CrossRef]
- Dougherty, U.; Mustafi, R.; Sadiq, F.; Almoghrabi, A.; Mustafi, D.; Kreisheh, M.; Sundaramurthy, S.; Liu, W.C.; Konda, V.J.; Pekow, J.; et al. The Renin-Angiotensin System Mediates EGF Receptor-Vitamin D Receptor Cross-Talk in Colitis-Associated Colon Cancer. Clin. Cancer Res. 2014, 20, 5848–5859. [Google Scholar] [CrossRef]
- Wang, G.; Li, T.; Wan, Y.; Li, Q. MYC expression and fatty acid oxidation in EGFR-TKI acquired resistance. Drug Resist. Updat. 2024, 72, 101019. [Google Scholar] [CrossRef]
- Bach, D.H.; Luu, T.T.; Kim, D.; An, Y.J.; Park, S.; Park, H.J.; Lee, S.K. BMP4 Upregulation Is Associated with Acquired Drug Resistance and Fatty Acid Metabolism in EGFR-Mutant Non-Small-Cell Lung Cancer Cells. Mol. Ther. Nucleic. Acids. 2018, 12, 817–828. [Google Scholar] [CrossRef]
- Vazquez-Martin, A.; Colomer, R.; Brunet, J.; Lupu, R.; Menendez, J.A. Overexpression of fatty acid synthase gene activates HER1/HER2 tyrosine kinase receptors in human breast epithelial cells. Cell Prolif. 2008, 41, 59–85. [Google Scholar] [CrossRef]
- Bollu, L.R.; Ren, J.; Blessing, A.M.; Katreddy, R.R.; Gao, G.; Xu, L.; Wang, J.; Su, F.; Weihua, Z. Involvement of de novo synthesized palmitate and mitochondrial EGFR in EGF induced mitochondrial fusion of cancer cells. Cell Cycle 2014, 13, 2415–2430. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Dong, Y.; Zi, R.; Wang, Y.; Chen, Y.; Liu, C.; Wang, J.; Wang, X.; Li, J.; et al. Non-alcoholic fatty liver disease promotes liver metastasis of colorectal cancer via fatty acid synthase dependent EGFR palmitoylation. Cell Death Discov. 2024, 10, 41. [Google Scholar] [CrossRef]
- Ali, A.; Levantini, E.; Teo, J.T.; Goggi, J.; Clohessy, J.G.; Wu, C.S.; Chen, L.; Yang, H.; Krishnan, I.; Kocher, O.; et al. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 2018, 10, e8313. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Song, F.; Zhao, X.J.; Jiang, H.; Wu, X.Q.; Wang, B.; Zhou, M.; Tian, M.; Shi, B.Z.; Wang, H.M.; et al. EGFR modulates monounsaturated fatty acid synthesis through phosphorylation of SCD1 in lung cancer. Mol. Cancer 2017, 16, 127. [Google Scholar] [CrossRef] [PubMed]
- Polonio-Alcala, E.; Porta, R.; Ruiz-Martinez, S.; Vasquez-Dongo, C.; Relat, J.; Bosch-Barrera, J.; Ciurana, J.; Puig, T. AZ12756122, a novel fatty acid synthase inhibitor, decreases resistance features in EGFR-TKI resistant EGFR-mutated NSCLC cell models. Biomed. Pharmacother. 2022, 156, 113942. [Google Scholar] [CrossRef] [PubMed]
- Koll, L.; Gül, D.; Elnouaem, M.I.; Raslan, H.; Ramadan, O.R.; Knauer, S.K.; Strieth, S.; Hagemann, J.; Stauber, R.H.; Khamis, A. Exploiting Vitamin D Receptor and Its Ligands to Target Squamous Cell Carcinomas of the Head and Neck. Int. J. Mol. Sci. 2023, 24, 4675. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, K.; Yuan, W.; Xu, Y.; Li, P.; Wu, R.; Han, J.; Yin, Z.; Lu, L.; Gao, Y. 1α,25(OH)(2)D(3) ameliorates insulin resistance by alleviating γδ T cell inflammation via enhancing fructose-1,6-bisphosphatase 1 expression. Theranostics 2023, 13, 5290–5304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, S.; Liu, J.; Chen, Y.; Zhong, S.; Lian, D.; Liang, J.; Huang, S.; Hou, S. Vitexin Regulates Angiogenesis and Osteogenesis in Ovariectomy-Induced Osteoporosis of Rats via the VDR/PI3K/AKT/eNOS Signaling Pathway. J. Agric. Food. Chem. 2023, 71, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Menendez, J.A.; Lupu, R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat. Rev. Cancer 2007, 7, 763–777. [Google Scholar] [CrossRef]
- Furuta, E.; Pai, S.K.; Zhan, R.; Bandyopadhyay, S.; Watabe, M.; Mo, Y.Y.; Hirota, S.; Hosobe, S.; Tsukada, T.; Miura, K.; et al. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008, 68, 1003–1011. [Google Scholar] [CrossRef]
- Li, J.; Huang, Q.; Long, X.; Zhang, J.; Huang, X.; Aa, J.; Yang, H.; Chen, Z.; Xing, J. CD147 reprograms fatty acid metabolism in hepatocellular carcinoma cells through Akt/mTOR/SREBP1c and P38/PPARα pathways. J. Hepatol. 2015, 63, 1378–1389. [Google Scholar] [CrossRef]
- Lamming, D.W.; Sabatini, D.M. A Central role for mTOR in lipid homeostasis. Cell. Metab. 2013, 18, 465–469. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. mTOR signaling in growth control and disease. Cell 2012, 149, 274–293. [Google Scholar] [CrossRef]
- Zhao, G.; Dong, L.; Shi, H.; Li, H.; Lu, X.; Guo, X.; Wang, J. MicroRNA-1207-5p inhibits hepatocellular carcinoma cell growth and invasion through the fatty acid synthase-mediated Akt/mTOR signalling pathway. Oncol. Rep. 2016, 36, 1709–1716. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Wang, C.; Ho, C.; Ladu, S.; Lee, S.A.; Mattu, S.; Destefanis, G.; Delogu, S.; Zimmermann, A.; Ericsson, J.; et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 2011, 140, 1071–1083. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, Y.; Ren, R.; Chen, Y.; Lei, J.; Li, Y. Harnessing lipid metabolism modulation for improved immunotherapy outcomes in lung adenocarcinoma. J. Immunother. Cancer 2024, 12, e008811. [Google Scholar] [CrossRef]
- Qiao, S.; Tuohimaa, P. Vitamin D3 inhibits fatty acid synthase expression by stimulating the expression of long-chain fatty-acid-CoA ligase 3 in prostate cancer cells. FEBS Lett. 2004, 577, 451–454. [Google Scholar] [CrossRef]
- Wang, W.L.; Welsh, J.; Tenniswood, M. 1,25-Dihydroxyvitamin D3 modulates lipid metabolism in prostate cancer cells through miRNA mediated regulation of PPARA. J. Steroid. Biochem. Mol. Biol. 2013, 136, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.G.; Lange, T.S.; Robinson, K.; Kim, K.K.; Uzun, A.; Horan, T.C.; Kawar, N.; Yano, N.; Chu, S.R.; Mao, Q.; et al. Efficacy of a non-hypercalcemic vitamin-D2 derived anti-cancer agent (MT19c) and inhibition of fatty acid synthesis in an ovarian cancer xenograft model. PloS ONE 2012, 7, e34443. [Google Scholar] [CrossRef] [PubMed]
- Leyssens, C.; Marien, E.; Verlinden, L.; Derua, R.; Waelkens, E.; Swinnen, J.V.; Verstuyf, A. Remodeling of phospholipid composition in colon cancer cells by 1alpha,25(OH)2D3 and its analogs. J. Steroid. Biochem. Mol. Biol. 2015, 148, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.; Varambally, S. UALCAN: A Portal for Facilitating Tumor Subgroup Gene Expression and Survival Analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Nishimura, T.; Nakata, A.; Chen, X.; Nishi, K.; Meguro-Horike, M.; Sasaki, S.; Kita, K.; Horike, S.I.; Saitoh, K.; Kato, K.; et al. Cancer stem-like properties and gefitinib resistance are dependent on purine synthetic metabolism mediated by the mitochondrial enzyme MTHFD2. Oncogene 2019, 38, 2464–2481. [Google Scholar] [CrossRef]
- Hu, P.S.; Li, T.; Lin, J.F.; Qiu, M.Z.; Wang, D.S.; Liu, Z.X.; Chen, Z.H.; Yang, L.P.; Zhang, X.L.; Zhao, Q.; et al. VDR-SOX2 signaling promotes colorectal cancer stemness and malignancy in an acidic microenvironment. Signal Transduct. Target. Ther. 2020, 5, 183. [Google Scholar] [CrossRef]
- Healy, K.D.; Frahm, M.A.; DeLuca, H.F. 1,25-Dihydroxyvitamin D3 up-regulates the renal vitamin D receptor through indirect gene activation and receptor stabilization. Arch. Biochem. Biophys. 2005, 433, 466–473. [Google Scholar] [CrossRef]
- Kim, S.; Shevde, N.K.; Pike, J.W. 1,25-Dihydroxyvitamin D3 stimulates cyclic vitamin D receptor/retinoid X receptor DNA-binding, co-activator recruitment, and histone acetylation in intact osteoblasts. J. Bone. Miner. Res. 2005, 20, 305–317. [Google Scholar] [CrossRef]
- Maj, E.; Trynda, J.; Maj, B.; Gębura, K.; Bogunia-Kubik, K.; Chodyński, M.; Kutner, A.; Wietrzyk, J. Differential response of lung cancer cell lines to vitamin D derivatives depending on EGFR, KRAS, p53 mutation status and VDR polymorphism. J. Steroid. Biochem. Mol. Biol. 2019, 193, 105431. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Kawaura, A.; Kato, S.; Takeda, E.; Okano, T. Metastatic growth of lung cancer cells is extremely reduced in Vitamin D receptor knockout mice. J. Steroid. Biochem. Mol. Biol. 2004, 89–90, 545–547. [Google Scholar] [CrossRef] [PubMed]
- Gkotinakou, I.M.; Kechagia, E.; Pazaitou-Panayiotou, K.; Mylonis, I.; Liakos, P.; Tsakalof, A. Calcitriol Suppresses HIF-1 and HIF-2 Transcriptional Activity by Reducing HIF-1/2α Protein Levels via a VDR-Independent Mechanism. Cells 2020, 9, 2440. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Ferri, I.; Sidoni, A.; Traina, G.; Fettucciari, K.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Curcio, F.; Ceccarini, M.R.; et al. VDR independent induction of acid-sphingomyelinase by 1,23(OH)(2) D(3) in gastric cancer cells: Impact on apoptosis and cell morphology. Biochimie 2018, 146, 35–42. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, L.; Li, M.X.; Shen, J.; Liu, X.D.; Xiao, Z.G.; Wu, D.L.; Ho, I.H.T.; Wu, J.C.Y.; Cheung, C.K.Y.; et al. Vitamin D3 activates the autolysosomal degradation function against Helicobacter pylori through the PDIA3 receptor in gastric epithelial cells. Autophagy 2019, 15, 707–725. [Google Scholar] [CrossRef]
- Kawagoe, F.; Mendoza, A.; Hayata, Y.; Asano, L.; Kotake, K.; Mototani, S.; Kawamura, S.; Kurosaki, S.; Akagi, Y.; Takemoto, Y.; et al. Discovery of a Vitamin D Receptor-Silent Vitamin D Derivative That Impairs Sterol Regulatory Element-Binding Protein In Vivo. J. Med. Chem. 2021, 64, 5689–5709. [Google Scholar] [CrossRef]
- Yao, Z.; Fenoglio, S.; Gao, D.C.; Camiolo, M.; Stiles, B.; Lindsted, T.; Schlederer, M.; Johns, C.; Altorki, N.; Mittal, V.; et al. TGF-beta IL-6 axis mediates selective and adaptive mechanisms of resistance to molecular targeted therapy in lung cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 15535–15540. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, Q.; Li, D.; Wei, X.; Jia, Y.; Zhang, Z.; Ai, B.; Cao, X.; Guo, T.; Liao, Y. Co-administration of 20(S)-protopanaxatriol (g-PPT) and EGFR-TKI overcomes EGFR-TKI resistance by decreasing SCD1 induced lipid accumulation in non-small cell lung cancer. J. Exp. Clin. Cancer Res. 2019, 38, 129. [Google Scholar] [CrossRef]
- Li, L.; Han, R.; Xiao, H.; Lin, C.; Wang, Y.; Liu, H.; Li, K.; Chen, H.; Sun, F.; Yang, Z.; et al. Metformin sensitizes EGFR-TKI-resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin. Cancer Res. 2014, 20, 2714–2726. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Fang, M.; Hou, M.; Duan, Y.; Wang, J.; Hu, K.; Liu, S.; Liu, X.; Peng, X.; Ding, X.; et al. VDR Decrease Enhances the Efficacy of 1,25-Dihydroxyvitamin D3 Inhibiting Gefitinib Resistance by Regulating EGFR/FASN Loop in NSCLC Cells. Pharmaceuticals 2025, 18, 1238. https://doi.org/10.3390/ph18081238
Yang J, Fang M, Hou M, Duan Y, Wang J, Hu K, Liu S, Liu X, Peng X, Ding X, et al. VDR Decrease Enhances the Efficacy of 1,25-Dihydroxyvitamin D3 Inhibiting Gefitinib Resistance by Regulating EGFR/FASN Loop in NSCLC Cells. Pharmaceuticals. 2025; 18(8):1238. https://doi.org/10.3390/ph18081238
Chicago/Turabian StyleYang, Junqing, Mingyu Fang, Mengjun Hou, Yalei Duan, Jiali Wang, Kaiyong Hu, Shuo Liu, Xiaoying Liu, Xiaohan Peng, Xuansheng Ding, and et al. 2025. "VDR Decrease Enhances the Efficacy of 1,25-Dihydroxyvitamin D3 Inhibiting Gefitinib Resistance by Regulating EGFR/FASN Loop in NSCLC Cells" Pharmaceuticals 18, no. 8: 1238. https://doi.org/10.3390/ph18081238
APA StyleYang, J., Fang, M., Hou, M., Duan, Y., Wang, J., Hu, K., Liu, S., Liu, X., Peng, X., Ding, X., & Jia, Z. (2025). VDR Decrease Enhances the Efficacy of 1,25-Dihydroxyvitamin D3 Inhibiting Gefitinib Resistance by Regulating EGFR/FASN Loop in NSCLC Cells. Pharmaceuticals, 18(8), 1238. https://doi.org/10.3390/ph18081238





