Exercise-Induced Muscle–Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging
Abstract
1. Introduction
2. Dynamic Regulation of Energy Substrate Use: Metabolic Flexibility Paradigm
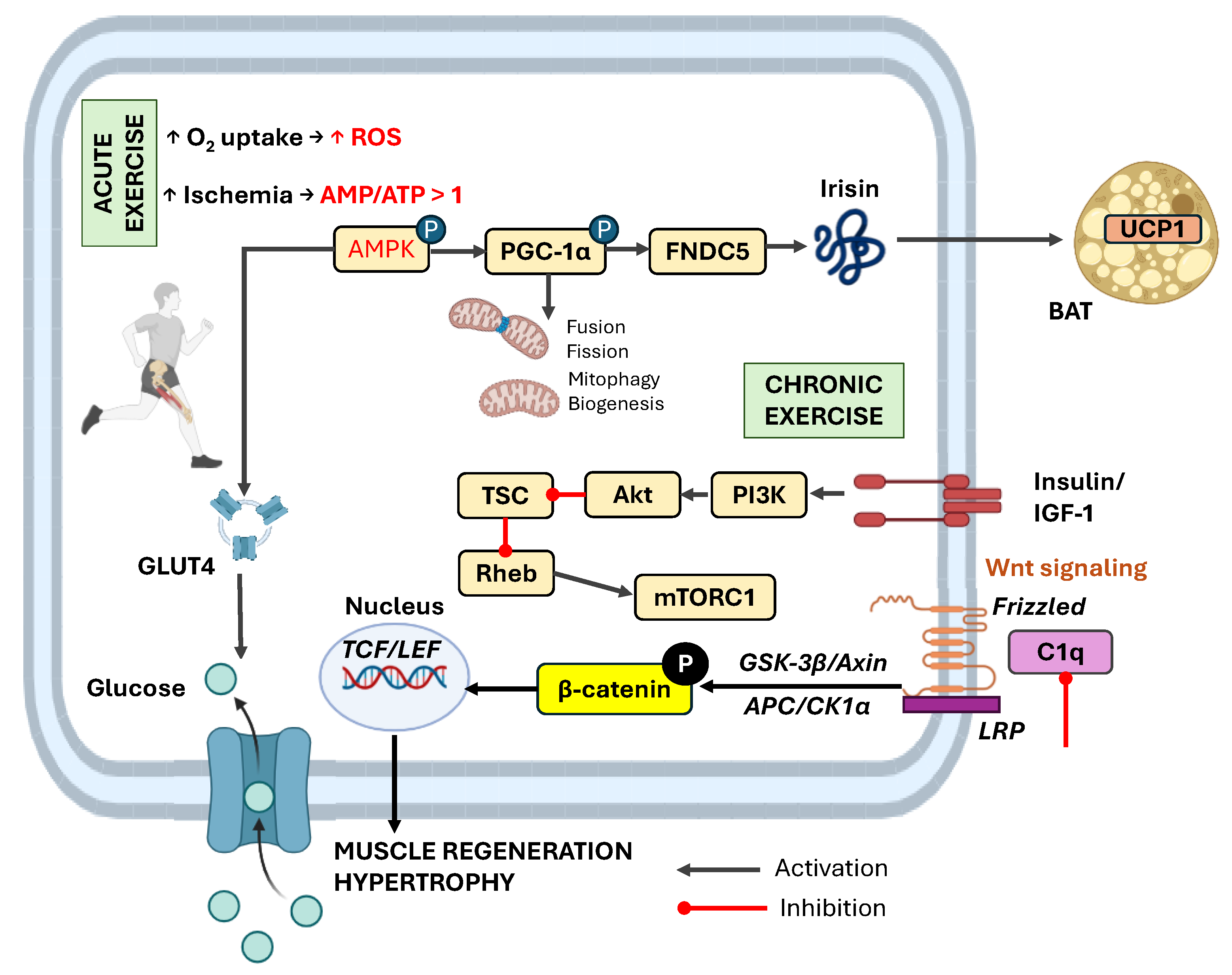
3. Muscle-Derived Signals During Acute vs. Chronic Exercise
4. Key Myokines Regulating Muscle–Adipose Tissue Crosstalk and Metabolic Flexibility
4.1. IL-6: Sensors and Mediators of Energy Status
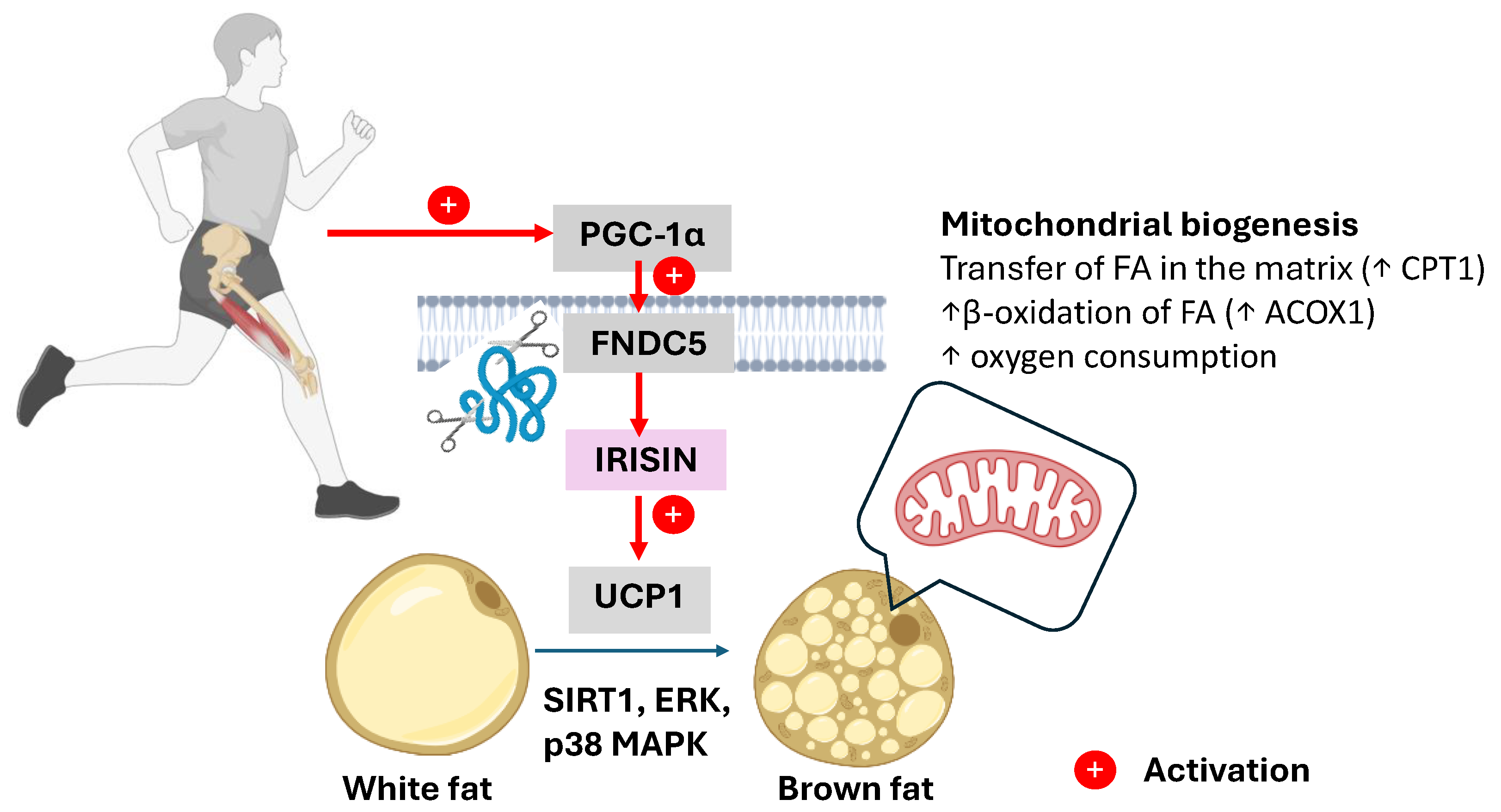
4.2. Irisin: Mediator of Browning and Metabolic Reprogramming
4.3. Myostatin: Brake of Muscle–Fat Metabolic Plasticity
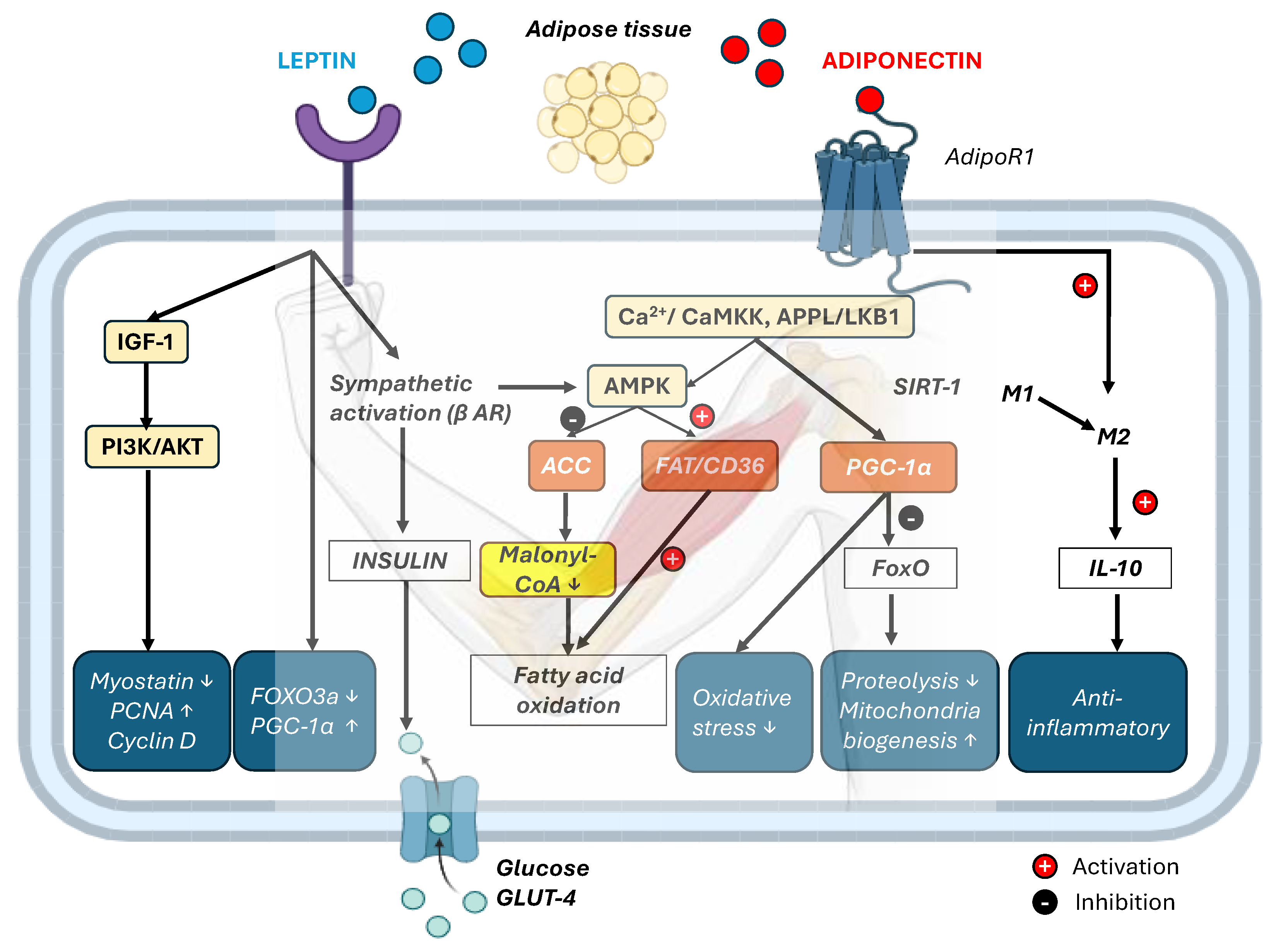
5. Adipokine Signaling Dynamics in Response to Acute and Chronic Exercise
6. Crosstalk Outcomes: Lipid Oxidation, Mitochondrial Biogenesis, and Glucose Uptake
7. Therapeutic Consequences and Pharmacological Management of Metabolic Flexibility in Aging
8. Conclusions
9. Limitations and Future Research Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAS | Acetylsalicylic acid |
| ACC | Acetyl-CoA carboxylase |
| ACOX1 | Acyl-CoA oxidase 1 |
| AdipoR1 | Adiponectin Receptor 1 |
| Akt | Protein kinase B |
| AMPK | AMP-activated protein kinase |
| AOX | α-Ketoglutarate dehydrogenase |
| APC | Adenomatous Polyposis Coli |
| APPL1 | Protein containing PH domain, PTB domain, and leucine zipper motif-1 |
| ATGL | Adipose triglyceride lipase |
| BAT | Brown adipose tissue |
| BDNF | Brain-derived neurotrophic factor |
| C1q | Complement component 1q |
| CACH | Catabolic–anabolic cycling hormesis |
| CaMKK | Ca2+/calmodulin-dependent protein kinase kinase |
| CCL2 | C-C motif chemokine ligand 2 |
| CD137 (TNFRSF9) | Tumor necrosis factor receptor superfamily member 9 |
| CD36 | Cluster of differentiation 36 |
| CK1α | Casein Kinase 1 alpha |
| CPT1 | Carnitine palmitoyltransferase 1 |
| CTSB | Cathepsin B |
| CX3CL1 | C-X3-C motif chemokine ligand 1 |
| eGFR | Estimated glomerular filtration rate. |
| ERK | Extracellular signal-regulated kinase |
| ERRα | Estrogen-related receptor alpha |
| ETC | Electron transport chain |
| FAs | Fatty acids |
| FAS | Fatty acid synthase |
| FAT | Fatty acid translocase |
| FGF21 | Fibroblast growth factor 21 |
| FNDC5 | Fibronectin type III domain-containing protein 5 |
| FoxO1 | Forkhead box protein O1 |
| G-6-P-ase | Glucose-6-phosphatase |
| gApN | Globular adiponectin |
| GSK-3β | Glycogen Synthase Kinase-3 Beta |
| HDLs | High-density lipoproteins |
| HFD | High-fat diet |
| HIIT | High-intensity interval training |
| HSL | Hormone-sensitive lipase |
| IGF-1 | Insulin-like growth factor 1 |
| IL-10 | Interleukin-10 |
| IL-1Ra | IL-1 receptor antagonist |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| JAK | Janus kinase |
| KC | Keratinocyte chemoattractant |
| LDLs | Low-density lipoproteins |
| LEF | Lymphoid Enhancer-binding Factor |
| LepRb | Leptin long-form receptor |
| LIF | Leukemia inhibitory factor |
| LKB1 | Liver kinase B1 |
| LPS | Lipopolysaccharide |
| MCI | Mild cognitive impairment |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MOTS-c | Mitochondrial open reading frame of the 12S rRNA type-c |
| mtDNA | Mitochondrial DNA |
| mTOR | Mechanistic target of rapamycin |
| mTORC1 | Rapamycin complex 1 |
| MuRF1 | Muscle RING-finger protein-1 |
| NAD+ | Nicotinamide adenine dinucleotide |
| NF-κB | Nuclear factor-κB |
| NMN | Nicotinamide mononucleotide |
| NR | Nicotinamide riboside |
| NRF-1 and 2 | Nuclear respiratory factor 1 and 2 |
| p38 MAPK | p38 Mitogen-activated protein kinase |
| PCNA | Proliferating cell nuclear antigen |
| PEPCK | Phosphoenolpyruvate carboxykinase |
| PGC-1α | Peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| PI3K | Phosphoinositide 3-kinase |
| PKC-β | Protein kinase C beta |
| POMC | Pro-opiomelanocortin |
| PPARα | Peroxisome Proliferator-Activated Receptor Alpha |
| PRDM16 | PR domain-containing 16 |
| Rheb | Ras homolog enriched in brain |
| ROS | Reactive oxygen species |
| SCD5 | Stearoyl-CoA desaturase 5 |
| SIRT1 | Sirtuin 1 |
| SOCS3 | Suppressor of Cytokine Signaling 3 |
| STAT | Signal transducer and activator of transcription |
| T2DM | Type 2 diabetes mellitus |
| TCA | Tricarboxylic Acid Cycle |
| TCF | T-cell Factor |
| TFAM | Mitochondrial transcription factor A |
| TLR4 | Toll-like receptor 4 |
| TMEM26 | Transmembrane protein 26 |
| TNFα | Tumor necrosis factor-α |
| TSC | Tuberous sclerosis complex |
| UCP1 | Uncoupling protein 1 |
| WAT | White adipose tissue |
| β-AR | β-adrenergic receptor |
References
- Smith, J.A.B.; Murach, K.A.; Dyar, K.A.; Zierath, J.R. Exercise Metabolism and Adaptation in Skeletal Muscle. Nat. Rev. Mol. Cell Biol. 2023, 24, 607–632. [Google Scholar] [CrossRef]
- Joseph, A.; Adhihetty, P.J.; Buford, T.W.; Wohlgemuth, S.E.; Lees, H.A.; Nguyen, L.M.-D.; Aranda, J.M.; Sandesara, B.D.; Pahor, M.; Manini, T.M.; et al. The Impact of Aging on Mitochondrial Function and Biogenesis Pathways in Skeletal Muscle of Sedentary High- and Low-functioning Elderly Individuals. Aging Cell 2012, 11, 801–809. [Google Scholar] [CrossRef]
- López-Lluch, G. Mitochondrial Activity and Dynamics Changes Regarding Metabolism in Ageing and Obesity. Mech. Ageing Dev. 2017, 162, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Laurens, C.; Bergouignan, A.; Moro, C. Exercise-Released Myokines in the Control of Energy Metabolism. Front. Physiol. 2020, 11, 91. [Google Scholar] [CrossRef]
- Whitham, M.; Febbraio, M.A. The Ever-Expanding Myokinome: Discovery Challenges and Therapeutic Implications. Nat. Rev. Drug Discov. 2016, 15, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Leal, L.G.; Lopes, M.A.; Batista, M.L. Physical Exercise-Induced Myokines and Muscle-Adipose Tissue Crosstalk: A Review of Current Knowledge and the Implications for Health and Metabolic Diseases. Front. Physiol. 2018, 9, 1307. [Google Scholar] [CrossRef]
- Lu, C.; Zhao, H.; Liu, Y.; Yang, Z.; Yao, H.; Liu, T.; Gou, T.; Wang, L.; Zhang, J.; Tian, Y.; et al. Novel Role of the SIRT1 in Endocrine and Metabolic Diseases. Int. J. Biol. Sci. 2023, 19, 484–501. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK Activators: Mechanisms of Action and Physiological Activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef]
- Li, F.; Li, Y.; Duan, Y.; Hu, C.-A.A.; Tang, Y.; Yin, Y. Myokines and Adipokines: Involvement in the Crosstalk between Skeletal Muscle and Adipose Tissue. Cytokine Growth Factor Rev. 2017, 33, 73–82. [Google Scholar] [CrossRef]
- Cornish, S.M.; Bugera, E.M.; Duhamel, T.A.; Peeler, J.D.; Anderson, J.E. A Focused Review of Myokines as a Potential Contributor to Muscle Hypertrophy from Resistance-Based Exercise. Eur. J. Appl. Physiol. 2020, 120, 941–959. [Google Scholar] [CrossRef]
- Fan, W.; Evans, R.M. Exercise Mimetics: Impact on Health and Performance. Cell Metab. 2017, 25, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Hargreaves, M.; Joyner, M.J.; Zierath, J.R. Integrative Biology of Exercise. Cell 2014, 159, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Magdy Aly Hassan ElMeligie, M. Exercise Mimetics: An Emerging and Controversial Topic in Sport and Exercise Physiology. In Exercise Physiology; Ferraz, R., Neiva, H., Marinho, D.A., Teixeira, J.E., Forte, P., Branquinho, L., Eds.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Smith, R.L.; Soeters, M.R.; Wüst, R.C.I.; Houtkooper, R.H. Metabolic Flexibility as an Adaptation to Energy Resources and Requirements in Health and Disease. Endocrine Rev. 2018, 39, 489–517. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, E.J.; Mattson, M.P. The Catabolic—Anabolic Cycling Hormesis Model of Health and Resilience. Ageing Res. Rev. 2024, 102, 102588. [Google Scholar] [CrossRef]
- Tetlow, N.; Whittle, J. Prehabilitation- Do We Need Metabolic Flexibility. Ann. Nutr. Metab. 2025, 81, 223–233. [Google Scholar] [CrossRef]
- Schüttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef]
- Russ, D.W.; Manickam, R.; Tipparaju, S.M. Targeting Intramyocellular Lipids to Improve Aging Muscle Function. Lipids Health Dis. 2025, 24, 197. [Google Scholar] [CrossRef] [PubMed]
- Egan, B.; Sharples, A.P. Molecular Responses to Acute Exercise and Their Relevance for Adaptations in Skeletal Muscle to Exercise Training. Physiol. Rev. 2023, 103, 2057–2170. [Google Scholar] [CrossRef]
- Viollet, B. The Energy Sensor AMPK: Adaptations to Exercise, Nutritional and Hormonal Signals. In Hormones, Metabolism and the Benefits of Exercise; Spiegelman, B., Ed.; Springer: Cham, Switzerland, 2017; pp. 13–24. [Google Scholar]
- Almuraikhy, S.; Doudin, A.; Domling, A.; Althani, A.A.J.F.; Elrayess, M.A. Molecular Regulators of Exercise-mediated Insulin Sensitivity in Non-obese Individuals. J. Cell. Mol. Med. 2024, 28, e18015. [Google Scholar] [CrossRef]
- Richter, E.A.; Ruderman, N.B. AMPK and the Biochemistry of Exercise: Implications for Human Health and Disease. Biochem. J. 2009, 418, 261–275. [Google Scholar] [CrossRef]
- Vainshtein, A.; Tryon, L.D.; Pauly, M.; Hood, D.A. Role of PGC-1α during Acute Exercise-Induced Autophagy and Mitophagy in Skeletal Muscle. Am. J. Physiol.-Cell Physiol. 2015, 308, C710–C719. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, P.H.C.; Vann, C.G.; Phillips, S.M.; McKendry, J.; Young, K.C.; Kavazis, A.N.; Roberts, M.D. Skeletal Muscle Ribosome and Mitochondrial Biogenesis in Response to Different Exercise Training Modalities. Front. Physiol. 2021, 12, 725866. [Google Scholar] [CrossRef] [PubMed]
- Cobley, J.N.; Bartlett, J.D.; Kayani, A.; Murray, S.W.; Louhelainen, J.; Donovan, T.; Waldron, S.; Gregson, W.; Burniston, J.G.; Morton, J.P.; et al. PGC-1α Transcriptional Response and Mitochondrial Adaptation to Acute Exercise Is Maintained in Skeletal Muscle of Sedentary Elderly Males. Biogerontology 2012, 13, 621–631. [Google Scholar] [CrossRef]
- Batatinha, H.P.; Lira, F.S.; Kruger, K.; Rosa Neto, J.C. Physical Exercise and Metabolic Reprogramming. In Essential Aspects of Immunometabolism in Health and Disease; Springer: Cham, Switzerland, 2022; pp. 235–256. [Google Scholar]
- Lu, Z.; Qian, P.; Chang, J.; He, X.; Zhang, H.; Wu, J.; Zhang, T.; Wu, J. Multi-Omics Analysis Explores the Effect of Chronic Exercise on Liver Metabolic Reprogramming in Mice. Front. Cell Dev. Biol. 2023, 11, 1199902. [Google Scholar] [CrossRef] [PubMed]
- Abu Shelbayeh, O.; Arroum, T.; Morris, S.; Busch, K.B. PGC-1α Is a Master Regulator of Mitochondrial Lifecycle and ROS Stress Response. Antioxidants 2023, 12, 1075. [Google Scholar] [CrossRef]
- Powers, S.K.; Jackson, M.J. Exercise-Induced Oxidative Stress: Cellular Mechanisms and Impact on Muscle Force Production. Physiol. Rev. 2008, 88, 1243–1276. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial Uncoupling: A Key Controller of Biological Processes in Physiology and Diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox Control of Skeletal Muscle Atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef]
- Geng, T.; Li, P.; Okutsu, M.; Yin, X.; Kwek, J.; Zhang, M.; Yan, Z. PGC-1α Plays a Functional Role in Exercise-Induced Mitochondrial Biogenesis and Angiogenesis but Not Fiber-Type Transformation in Mouse Skeletal Muscle. Am. J. Physiol.-Cell Physiol. 2010, 298, C572–C579. [Google Scholar] [CrossRef]
- Jacques, M.; Landen, S.; Sharples, A.P.; Garnham, A.; Schittenhelm, R.; Steele, J.; Heikkinen, A.; Sillanpää, E.; Ollikainen, M.; Broatch, J.; et al. Molecular Landscape of Sex- and Modality-Specific Exercise Adaptation in Human Skeletal Muscle through Large-Scale Multi-Omics Integration. Cell Rep. 2025, 44, 115750. [Google Scholar] [CrossRef]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Åkerström, T.C.A.; Nielsen, A.R.; Fischer, C.P. Role of Myokines in Exercise and Metabolism. J. Appl. Physiol. 2007, 103, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Toft, A.D.; Falahati, A.; Steensberg, A. Source and Kinetics of Interleukin-6 in Humans during Exercise Demonstrated by a Minimally Invasive Model. Eur. J. Appl. Physiol. 2011, 111, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Moienneia, N.; Attarzadeh Hosseini, S.R. Acute and Chronic Responses of Metabolic Myokine to Different Intensities of Exercise in Sedentary Young Women. Obes. Med. 2016, 1, 15–20. [Google Scholar] [CrossRef]
- Xu, D.; Yin, C.; Wang, S.; Xiao, Y. JAK-STAT in Lipid Metabolism of Adipocytes. JAK-STAT 2013, 2, e27203. [Google Scholar] [CrossRef]
- Rose-John, S. IL-6 Trans-Signaling via the Soluble IL-6 Receptor: Importance for the Pro-Inflammatory Activities of IL-6. Int. J. Biol. Sci. 2012, 8, 1237–1247. [Google Scholar] [CrossRef]
- Jensen-Urstad, A.P.L.; Semenkovich, C.F. Fatty Acid Synthase and Liver Triglyceride Metabolism: Housekeeper or Messenger? Biochim. Biophys. Acta (BBA)—Mol. Cell Biol. Lipids 2012, 1821, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Steensberg, A.; Van Hall, G.; Osada, T.; Sacchetti, M.; Saltin, B.; Pedersen, B.K. Production of Interleukin-6 in Contracting Human Skeletal Muscles Can Account for the Exercise-induced Increase in Plasma Interleukin-6. J. Physiol. 2000, 529, 237–242. [Google Scholar] [CrossRef]
- Ostrowski, K.; Schjerling, P.; Pedersen, B.K. Physical Activity and Plasma Interleukin-6 in Humans—Effect of Intensity of Exercise. Eur. J. Appl. Physiol. 2000, 83, 512–515. [Google Scholar] [CrossRef]
- Nash, D.; Hughes, M.G.; Butcher, L.; Aicheler, R.; Smith, P.; Cullen, T.; Webb, R. IL -6 Signaling in Acute Exercise and Chronic Training: Potential Consequences for Health and Athletic Performance. Scand. Med. Sci. Sports 2023, 33, 4–19. [Google Scholar] [CrossRef]
- Wolsk, E.; Mygind, H.; Grøndahl, T.S.; Pedersen, B.K.; Van Hall, G. IL-6 Selectively Stimulates Fat Metabolism in Human Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2010, 299, E832–E840. [Google Scholar] [CrossRef]
- Wedell-Neergaard, A.-S.; Lang Lehrskov, L.; Christensen, R.H.; Legaard, G.E.; Dorph, E.; Larsen, M.K.; Launbo, N.; Fagerlind, S.R.; Seide, S.K.; Nymand, S.; et al. Exercise-Induced Changes in Visceral Adipose Tissue Mass Are Regulated by IL-6 Signaling: A Randomized Controlled Trial. Cell Metab. 2019, 29, 844–855.e3. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.Q.; Duque-Guimaraes, D.E.; Machado, U.F.; Zierath, J.R.; Krook, A. Altered Response of Skeletal Muscle to IL-6 in Type 2 Diabetic Patients. Diabetes 2013, 62, 355–361. [Google Scholar] [CrossRef]
- Sriwijitkamol, A.; Coletta, D.K.; Wajcberg, E.; Balbontin, G.B.; Reyna, S.M.; Barrientes, J.; Eagan, P.A.; Jenkinson, C.P.; Cersosimo, E.; DeFronzo, R.A.; et al. Effect of Acute Exercise on AMPK Signaling in Skeletal Muscle of Subjects with Type 2 Diabetes. Diabetes 2007, 56, 836–848. [Google Scholar] [CrossRef]
- Kistner, T.M.; Pedersen, B.K.; Lieberman, D.E. Interleukin 6 as an Energy Allocator in Muscle Tissue. Nat. Metab. 2022, 4, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.L.; Steinberg, G.R.; Macaulay, S.L.; Thomas, W.G.; Holmes, A.G.; Ramm, G.; Prelovsek, O.; Hohnen-Behrens, C.; Watt, M.J.; James, D.E.; et al. Interleukin-6 Increases Insulin-Stimulated Glucose Disposal in Humans and Glucose Uptake and Fatty Acid Oxidation In Vitro via AMP-Activated Protein Kinase. Diabetes 2006, 55, 2688–2697. [Google Scholar] [CrossRef] [PubMed]
- Petersen, E.W.; Carey, A.L.; Sacchetti, M.; Steinberg, G.R.; Macaulay, S.L.; Febbraio, M.A.; Pedersen, B.K. Acute IL-6 Treatment Increases Fatty Acid Turnover in Elderly Humans in Vivo and in Tissue Culture in Vitro. Am. J. Physiol.-Endocrinol. Metab. 2005, 288, E155–E162. [Google Scholar] [CrossRef]
- Ghesmati, Z.; Rashid, M.; Fayezi, S.; Gieseler, F.; Alizadeh, E.; Darabi, M. An Update on the Secretory Functions of Brown, White, and Beige Adipose Tissue: Towards Therapeutic Applications. Rev. Endocr. Metab. Disord. 2024, 25, 279–308. [Google Scholar] [CrossRef]
- Han, M.S.; White, A.; Perry, R.J.; Camporez, J.-P.; Hidalgo, J.; Shulman, G.I.; Davis, R.J. Regulation of Adipose Tissue Inflammation by Interleukin 6. Proc. Natl. Acad. Sci. USA 2020, 117, 2751–2760. [Google Scholar] [CrossRef]
- Waseem, R.; Shamsi, A.; Mohammad, T.; Hassan, M.I.; Kazim, S.N.; Chaudhary, A.A.; Rudayni, H.A.; Al-Zharani, M.; Ahmad, F.; Islam, A. FNDC5/Irisin: Physiology and Pathophysiology. Molecules 2022, 27, 1118. [Google Scholar] [CrossRef]
- Archundia-Herrera, C.; Macias-Cervantes, M.; Ruiz-Muñoz, B.; Vargas-Ortiz, K.; Kornhauser, C.; Perez-Vazquez, V. Muscle Irisin Response to Aerobic vs HIIT in Overweight Female Adolescents. Diabetol. Metab. Syndr. 2017, 9, 101. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, R.; Meng, Y.; Li, S.; Donelan, W.; Zhao, Y.; Qi, L.; Zhang, M.; Wang, X.; Cui, T.; et al. Irisin Stimulates Browning of White Adipocytes Through Mitogen-Activated Protein Kinase P38 MAP Kinase and ERK MAP Kinase Signaling. Diabetes 2014, 63, 514–525. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Y.; Wang, F.; Donelan, W.; Zona, M.C.; Li, S.; Reeves, W.; Ding, Y.; Tang, D.; Yang, L. Effects of Irisin on the Differentiation and Browning of Human Visceral White Adipocytes. Am. J. Transl. Res. 2019, 11, 7410–7421. [Google Scholar] [PubMed]
- Degens, H.; Patel, K.; Matsakas, A. Myostatin Knockout Mice Have Larger Muscle Fibers With Normal Function and Morphology. Muscle Nerve 2025, 71, 1122–1131. [Google Scholar] [CrossRef]
- Wang, J.; Jia, D.; Zhang, Z.; Wang, D. Exerkines and Sarcopenia: Unveiling the Mechanism Behind Exercise-Induced Mitochondrial Homeostasis. Metabolites 2025, 15, 59. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and Irisin in Humans: I. Predictors of Circulating Concentrations in Serum and Plasma and II. mRNA Expression and Circulating Concentrations in Response to Weight Loss and Exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Herouvi, D.; Vlachopapadopoulou, E.A.; Vakaki, M.; Gouriotis, D.; Marmarinos, A.; Kalpia, C.; Kossiva, L.; Tsolia, M.; Karavanaki, K. Relation of Serum Irisin Levels with Adiposity, Components of Metabolic Syndrome and Carotid Intima Media Thickness in Prepubertal Children with Obesity: A Cross-Sectional Study. Endocrine 2024, 87, 1031–1040. [Google Scholar] [CrossRef]
- Guo, T.; Jou, W.; Chanturiya, T.; Portas, J.; Gavrilova, O.; McPherron, A.C. Myostatin Inhibition in Muscle, but Not Adipose Tissue, Decreases Fat Mass and Improves Insulin Sensitivity. PLoS ONE 2009, 4, e4937. [Google Scholar] [CrossRef]
- Baumgartner, M.; Lischka, J.; Schanzer, A.; De Gier, C.; Walleczek, N.-K.; Greber-Platzer, S.; Zeyda, M. Plasma Myostatin Increases with Age in Male Youth and Negatively Correlates with Vitamin D in Severe Pediatric Obesity. Nutrients 2022, 14, 2133. [Google Scholar] [CrossRef]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.P.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef]
- Deng, B.; Zhang, F.; Wen, J.; Ye, S.; Wang, L.; Yang, Y.; Gong, P.; Jiang, S. The Function of Myostatin in the Regulation of Fat Mass in Mammals. Nutr. Metab. 2017, 14, 29. [Google Scholar] [CrossRef]
- Lin, J.; Arnold, H.B.; Della-Fera, M.A.; Azain, M.J.; Hartzell, D.L.; Baile, C.A. Myostatin Knockout in Mice Increases Myogenesis and Decreases Adipogenesis. Biochem. Biophys. Res. Commun. 2002, 291, 701–706. [Google Scholar] [CrossRef]
- WADA. 2025. Available online: https://www.wada-ama.org/sites/default/files/2024-09/2025list_en_final_clean_12_september_2024.pdf (accessed on 18 August 2025).
- Braga, M.; Reddy, S.T.; Vergnes, L.; Pervin, S.; Grijalva, V.; Stout, D.; David, J.; Li, X.; Tomasian, V.; Reid, C.B.; et al. Follistatin Promotes Adipocyte Differentiation, Browning, and Energy Metabolism. J. Lipid Res. 2014, 55, 375–384. [Google Scholar] [CrossRef]
- Cheng, J.; Lee, J.; Liu, Y.; Wang, Y.; Duan, M.; Zeng, Z. Effects of Myostatin Gene Knockout on White Fat Browning and Related Gene Expression in Type 2 Diabetic Mice. Adv. Clin. Exp. Med. 2023, 33, 609–617. [Google Scholar] [CrossRef]
- Shan, T.; Liang, X.; Bi, P.; Kuang, S. Myostatin Knockout Drives Browning of White Adipose Tissue through Activating the AMPK-PGC1α-Fndc5 Pathway in Muscle. FASEB J. 2013, 27, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.; Xiao, W.; Qin, X.; Cai, G.; Chen, H.; Hua, Z.; Cheng, C.; Li, X.; Hua, W.; Xiao, H.; et al. Myostatin Regulates Fatty Acid Desaturation and Fat Deposition through MEF2C/miR222/SCD5 Cascade in Pigs. Commun. Biol. 2020, 3, s42003–s42020. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, J.J.; Lloyd, D.J.; Gekakis, N. Loss-of-Function Mutation in Myostatin Reduces Tumor Necrosis Factor α Production and Protects Liver Against Obesity-Induced Insulin Resistance. Diabetes 2009, 58, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Z.; Gu, M.; Zhu, L.; Hai, C.; Di, A.; Wu, D.; Bai, C.; Su, G.; Liu, X.; et al. Loss of Myostatin Alters Mitochondrial Oxidative Phosphorylation, TCA Cycle Activity, and ATP Production in Skeletal Muscle. Int. J. Mol. Sci. 2022, 23, 15707. [Google Scholar] [CrossRef]
- Baati, N.; Feillet-Coudray, C.; Fouret, G.; Vernus, B.; Goustard, B.; Jollet, M.; Bertrand-Gaday, C.; Coudray, C.; Lecomte, J.; Bonnieu, A.; et al. New Evidence of Exercise Training Benefits in Myostatin-Deficient Mice: Effect on Lipidomic Abnormalities. Biochem. Biophys. Res. Commun. 2019, 516, 89–95. [Google Scholar] [CrossRef]
- Keshel, T.E. Exercise Training and Insulin Resistance: A Current Review. J. Obes. Weight. Loss. Ther. 2015, s5, 003. [Google Scholar] [CrossRef]
- Babaei, P.; Hoseini, R. Exercise Training Modulates Adipokine Dysregulations in Metabolic Syndrome. Sports Med. Health Sci. 2022, 4, 18–28. [Google Scholar] [CrossRef]
- Becic, T.; Studenik, C.; Hoffmann, G. Exercise Increases Adiponectin and Reduces Leptin Levels in Prediabetic and Diabetic Individuals: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Sci. 2018, 6, 97. [Google Scholar] [CrossRef]
- Bouassida, A.; Chamari, K.; Zaouali, M.; Feki, Y.; Zbidi, A.; Tabka, Z. Review on Leptin and Adiponectin Responses and Adaptations to Acute and Chronic Exercise. Br. J. Sports Med. 2010, 44, 620–630. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Hathaway, E.D.; Ward-Ritacco, C.L.; Williams, T.D.; Dobbs, W.C. The Effect of Chronic Exercise Training on Leptin: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Sports Med. 2018, 48, 1437–1450. [Google Scholar] [CrossRef]
- Fontana, A.; Vieira, J.G.; Vianna, J.M.; Bichowska, M.; Krzysztofik, M.; Wilk, M.; Reis, V.M. Reduction of Leptin Levels during Acute Exercise Is Dependent on Fasting but Not on Caloric Restriction during Chronic Exercise: A Systematic Review and Meta-Analysis. PLoS ONE 2023, 18, e0288730. [Google Scholar] [CrossRef]
- Rabaglia, M.E.; Gray-Keller, M.P.; Frey, B.L.; Shortreed, M.R.; Smith, L.M.; Attie, A.D. α-Ketoisocaproate-Induced Hypersecretion of Insulin by Islets from Diabetes-Susceptible Mice. Am. J. Physiol.-Endocrinol. Metab. 2005, 289, E218–E224. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, F. Natural Products From Plants Targeting Leptin Resistance for the Future Development of Anti-Obesity Agents. Phytother. Res. 2025, 39, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, H.; Meng, Q.; Lee, W.Y.; Li, Z.; Sun, S. Recent Advances in Osteonecrosis of the Femoral Head: A Focus on Mesenchymal Stem Cells and Adipocytes. J. Transl. Med. 2025, 23, 592. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.M.; Arnaldo, L.; Milhomem, L.P.; Aguiar, S.S.; Franco, O.L. The Intricate Relationship between Circadian Rhythms and Gastrointestinal Peptides in Obesity. Peptides 2025, 185, 171356. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Yu, C.; Tang, Z.; Sun, J.; Wang, Y.; Zhao, Z.; Lin, B.; Li, C. Leptin Induces Altered Differentiation of Keratinocytes by Inducing Insulin Resistance: Implications for Metabolic Syndrome-Induced Resistance of Psoriatic Therapy. J. Dermatol. Treat. 2024, 35, 2309305. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Rehman, S.U.; Khan, A.; Badshah, H.; Abid, N.B.; Kim, M.W.; Jo, M.H.; Chung, S.S.; Lee, H.; Rutten, B.P.F.; et al. Adiponectin-Mimetic Novel Nonapeptide Rescues Aberrant Neuronal Metabolic-Associated Memory Deficits in Alzheimer’s Disease. Mol. Neurodegener. 2021, 16, 23. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Febbraio, M.A. Muscles, Exercise and Obesity: Skeletal Muscle as a Secretory Organ. Nat. Rev. Endocrinol. 2012, 8, 457–465. [Google Scholar] [CrossRef]
- Lee, J.H.; Jun, H.-S. Role of Myokines in Regulating Skeletal Muscle Mass and Function. Front. Physiol. 2019, 10, 42. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Mambrini, S.P.; Grillo, A.; Colosimo, S.; Zarpellon, F.; Pozzi, G.; Furlan, D.; Amodeo, G.; Bertoli, S. Diet and Physical Exercise as Key Players to Tackle MASLD through Improvement of Insulin Resistance and Metabolic Flexibility. Front. Nutr. 2024, 11, 1426551. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Gillen, Z.M.; Fukuda, D.H.; Cramer, J.T. Metabolic Flexibility and Inflexibility: Pathology Underlying Metabolism Dysfunction. J. Clin. Mol. 2023, 12, 4453. [Google Scholar] [CrossRef]
- Fang, P.; She, Y.; Yu, M.; Min, W.; Shang, W.; Zhang, Z. Adipose–Muscle Crosstalk in Age-Related Metabolic Disorders: The Emerging Roles of Adipo-Myokines. Ageing Res. Rev. 2023, 84, 101829. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, Q.; Xiao, J.; Niu, C.; Li, Y.; Zhang, X.; Zhou, Z.; Shu, G.; Yin, G. Wnt/β-Catenin Signalling: Function, Biological Mechanisms, and Therapeutic Opportunities. Signal Transduct. Target. Ther. 2022, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Newmire, D.; Willoughby, D.S. Wnt and β-Catenin Signaling and Skeletal Muscle Myogenesis in Response to Muscle Damage and Resistance Exercise and Training. Int. J. Kinesiol. Sports Sci. 2015, 3, 40–50. [Google Scholar] [CrossRef]
- Gao, X.; Chen, Y.; Cheng, P. Unlocking the Potential of Exercise: Harnessing Myokines to Delay Musculoskeletal Aging and Improve Cognitive Health. Front. Physiol. 2024, 15, 1338875. [Google Scholar] [CrossRef]
- Benavente, C.; Padial, P.; Scott, B.R.; Almeida, F.; Olcina, G.; Pérez-Regalado, S.; Feriche, B. Strength and Muscle Mass Development after a Resistance-Training Period at Terrestrial and Normobaric Intermittent Hypoxia. Pflug. Arch.—Eur. J. Physiol. 2024, 476, 1221–1233. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Feng, W.; Lai, J.; Yuan, D.; Xiao, W.; Li, Y. Role of Adipokines in Sarcopenia. Chin. Med. J. 2023, 136, 1794–1804. [Google Scholar] [CrossRef]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin Stimulates Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Momken, I.; Chabowski, A.; Dirkx, E.; Nabben, M.; Jain, S.S.; McFarlan, J.T.; Glatz, J.F.C.; Luiken, J.J.F.P.; Bonen, A. A New Leptin-Mediated Mechanism for Stimulating Fatty Acid Oxidation: A Pivotal Role for Sarcolemmal FAT/CD36. Biochem. J. 2017, 474, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Sáinz, N.; Rodríguez, A.; Catalán, V.; Becerril, S.; Ramírez, B.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin Administration Downregulates the Increased Expression Levels of Genes Related to Oxidative Stress and Inflammation in the Skeletal Muscle of Ob/ObMice. Mediat. Inflamm. 2010, 2010, 784343. [Google Scholar] [CrossRef]
- Sáinz, N.; Rodríguez, A.; Catalán, V.; Becerril, S.; Ramírez, B.; Gómez-Ambrosi, J.; Frühbeck, G. Leptin Administration Favors Muscle Mass Accretion by Decreasing FoxO3a and Increasing PGC-1α in Ob/Ob Mice. PLoS ONE 2009, 4, e6808. [Google Scholar] [CrossRef]
- Zhou, L.; Deepa, S.S.; Etzler, J.C.; Ryu, J.; Mao, X.; Fang, Q.; Liu, D.D.; Torres, J.M.; Jia, W.; Lechleiter, J.D.; et al. Adiponectin Activates AMP-Activated Protein Kinase in Muscle Cells via APPL1/LKB1-Dependent and Phospholipase C/Ca2+/Ca2+/Calmodulin-Dependent Protein Kinase Kinase-Dependent Pathways. J. Biol. Chem. 2009, 284, 22426–22435. [Google Scholar] [CrossRef]
- Kang, C.; Li Ji, L. Role of PGC-1α Signaling in Skeletal Muscle Health and Disease. Ann. N. Y. Acad. Sci. 2012, 1271, 110–117. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 Regulate PGC-1α and Mitochondria by Ca2+ and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef]
- Ye, Y.; Liu, X.; Wu, N.; Han, Y.; Wang, J.; Yu, Y.; Chen, Q. Efficacy and Safety of Berberine Alone for Several Metabolic Disorders: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Pharmacol. 2021, 12, 653887. [Google Scholar] [CrossRef]
- Wu, S.; Zou, M.-H. AMPK, Mitochondrial Function, and Cardiovascular Disease. Int. J. Mol. Sci. 2020, 21, 4987. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ye, J. Mitochondrial Inhibitor as a New Class of Insulin Sensitizer. Acta Pharm. Sin. B 2012, 2, 341–349. [Google Scholar] [CrossRef]
- Chen, L.; Su, X.; Hu, Y. Berberine Down-Regulated Myostatin Expression and Facilitated Metabolism via Smad Pathway in Insulin Resistant Mice. Diabetes Metab. Syndr. Obes. 2020, 13, 4561–4569. [Google Scholar] [CrossRef]
- Yadawa, A.K.; Srivastava, P.; Singh, A.; Kumar, R.; Arya, J.K.; Rizvi, S.I. Berberine Attenuates Brain Aging via Stabilizing Redox Homeostasis and Inflammation in an Accelerated Senescence Model of Wistar Rats. Metab. Brain. Dis. 2024, 39, 649–659. [Google Scholar] [CrossRef]
- Nagashima, K.; Lopez, C.; Donovan, D.; Ngai, C.; Fontanez, N.; Bensadoun, A.; Fruchart-Najib, J.; Holleran, S.; Cohn, J.S.; Ramakrishnan, R.; et al. Effects of the PPARγ Agonist Pioglitazone on Lipoprotein Metabolism in Patients with Type 2 Diabetes Mellitus. J. Clin. Investig. 2005, 115, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Krämer, D.K.; Al-Khalili, L.; Guigas, B.; Leng, Y.; Garcia-Roves, P.M.; Krook, A. Role of AMP Kinase and PPARδ in the Regulation of Lipid and Glucose Metabolism in Human Skeletal Muscle. J. Biol. Chem. 2007, 282, 19313–19320. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Yamamoto, J.; Iwasaki, S.; Asaba, H.; Hamura, H.; Ikeda, Y.; Watanabe, M.; Magoori, K.; Ioka, R.X.; Tachibana, K.; et al. Activation of Peroxisome Proliferator-Activated Receptor δ Induces Fatty Acid β-Oxidation in Skeletal Muscle and Attenuates Metabolic Syndrome. Proc. Natl. Acad. Sci. USA 2003, 100, 15924–15929. [Google Scholar] [CrossRef] [PubMed]
- Tontonoz, P.; Spiegelman, B.M. Fat and Beyond: The Diverse Biology of PPARγ. Annu. Rev. Biochem. 2008, 77, 289–312. [Google Scholar] [CrossRef]
- Erol, A. The Functions of PPARs in Aging and Longevity. PPAR Res. 2007, 2007, 39654. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; McCarty, M.F.; O’Keefe, J.H. Nutraceutical Activation of Sirt1: A Review. Open Heart 2022, 9, e002171. [Google Scholar] [CrossRef]
- Fuku, N.; Pareja-Galeano, H.; Zempo, H.; Alis, R.; Arai, Y.; Lucia, A.; Hirose, N. The Mitochondrial-derived Peptide MOTS-c: A Player in Exceptional Longevity? Aging Cell 2015, 14, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Zeng, J.; Drew, B.G.; Sallam, T.; Martin-Montalvo, A.; Wan, J.; Kim, S.-J.; Mehta, H.; Hevener, A.L.; de Cabo, R.; et al. The Mitochondrial-Derived Peptide MOTS-c Promotes Metabolic Homeostasis and Reduces Obesity and Insulin Resistance. Cell Metab. 2015, 21, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.; Zhang, L.; Lin, Y.; Rao, X.; Wang, X.; Hua, F.; Ying, J. Mitochondria-Derived Peptide MOTS-c: Effects and Mechanisms Related to Stress, Metabolism and Aging. J. Transl. Med. 2023, 21, 36. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c Is an Exercise-Induced Mitochondrial-Encoded Regulator of Age-Dependent Physical Decline and Muscle Homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef]
- D’Souza, R.F.; Woodhead, J.S.T.; Hedges, C.P.; Zeng, N.; Wan, J.; Kumagai, H.; Lee, C.; Cohen, P.; Cameron-Smith, D.; Mitchell, C.J.; et al. Increased Expression of the Mitochondrial Derived Peptide, MOTS-c, in Skeletal Muscle of Healthy Aging Men Is Associated with Myofiber Composition. Aging 2020, 12, 5244–5258. [Google Scholar] [CrossRef] [PubMed]
- Cognitive Vitality Reports® CB4211. Available online: https://www.alzdiscovery.org/uploads/cognitive_vitality_media/mots-c.pdf?utm_source=chatgpt.com 2021 (accessed on 21 June 2025).
- Chavda, V.P.; Balar, P.C.; Vaghela, D.A.; Dodiya, P. Unlocking Longevity with GLP-1: A Key to Turn Back the Clock? Maturitas 2024, 186, 108028. [Google Scholar] [CrossRef]
- Serhan, C.N.; Levy, B.D. Resolvins in Inflammation: Emergence of the pro-Resolving Superfamily of Mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Gängler, S.; Wieczorek, M.; Belsky, D.W.; Ryan, J.; Kressig, R.W.; Stähelin, H.B.; Theiler, R.; Dawson-Hughes, B.; Rizzoli, R.; et al. Individual and Additive Effects of Vitamin D, Omega-3 and Exercise on DNA Methylation Clocks of Biological Aging in Older Adults from the DO-HEALTH Trial. Nat. Aging 2025, 5, 376–385. [Google Scholar] [CrossRef]
- Espinoza, S.E.; Woods, R.L.; Ekram, A.R.M.S.; Ernst, M.E.; Polekhina, G.; Wolfe, R.; Shah, R.C.; Ward, S.A.; Storey, E.; Nelson, M.R.; et al. The Effect of Low-Dose Aspirin on Frailty Phenotype and Frailty Index in Community-Dwelling Older Adults in the ASPirin in Reducing Events in the Elderly Study. J. Gerontol. Ser. A 2022, 77, 2007–2014. [Google Scholar] [CrossRef]
- Membrez, M.; Migliavacca, E.; Christen, S.; Yaku, K.; Trieu, J.; Lee, A.K.; Morandini, F.; Giner, M.P.; Stiner, J.; Makarov, M.V.; et al. Trigonelline Is an NAD+ Precursor That Improves Muscle Function during Ageing and Is Reduced in Human Sarcopenia. Nat. Metab. 2024, 6, 433–447. [Google Scholar] [CrossRef]
- Barinda, A.J.; Hardi, H.; Louisa, M.; Khatimah, N.G.; Marliau, R.M.; Felix, I.; Fadhillah, M.R.; Jamal, A.K. Repurposing Effect of Cardiovascular-Metabolic Drug to Increase Lifespan: A Systematic Review of Animal Studies and Current Clinical Trial Progress. Front. Pharmacol. 2024, 15, 1373458. [Google Scholar] [CrossRef]
- Liu, S.-H.; Chiu, C.-Y.; Wang, L.-P.; Chiang, M.-T. Omega-3 Fatty Acids-Enriched Fish Oil Activates AMPK/PGC-1α Signaling and Prevents Obesity-Related Skeletal Muscle Wasting. Mar. Drugs 2019, 17, 380. [Google Scholar] [CrossRef]
- Bu, Y.; Peng, M.; Tang, X.; Xu, X.; Wu, Y.; Chen, A.F.; Yang, X. Protective Effects of Metformin in Various Cardiovascular Diseases: Clinical Evidence and AMPK-dependent Mechanisms. J. Cell. Mol. Med. 2022, 26, 4886–4903. [Google Scholar] [CrossRef]
- Juan, C.G.; Matchett, K.B.; Davison, G.W. A Systematic Review and Meta-Analysis of the SIRT1 Response to Exercise. Sci. Rep. 2023, 13, 14752. [Google Scholar] [CrossRef]
- Elisia, I.; Yeung, M.; Kowalski, S.; Wong, J.; Rafiei, H.; Dyer, R.A.; Atkar-Khattra, S.; Lam, S.; Krystal, G. Omega 3 Supplementation Reduces C-Reactive Protein, Prostaglandin E2 and the Granulocyte/Lymphocyte Ratio in Heavy Smokers: An Open-Label Randomized Crossover Trial. Front. Nutr. 2022, 9, 1051418. [Google Scholar] [CrossRef]
- Beba, M.; Djafarian, K.; Shab-Bidar, S. Effect of Berberine on C-Reactive Protein: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 2019, 46, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Saedmocheshi, S.; Saed, L.; Saiedi, A.; Vahabzade, Z. Effect of Aerobic Exercise Training along with Omega-3 Supplementation on CRP and IL-6 in Obese Older Women. Med. J. 2019, 41, 49–55. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Li, Y.; Yu, S.; Zhao, Y. SIRT1/PGC-1α Signaling Promotes Mitochondrial Functional Recovery and Reduces Apoptosis after Intracerebral Hemorrhage in Rats. Front. Mol. Neurosci. 2018, 10, 443. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Ortiz, K.; Pérez-Vázquez, V.; Macías-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2717. [Google Scholar] [CrossRef]
- Neto, I.V.D.S.; Pinto, A.P.; Muñoz, V.R.; De Cássia Marqueti, R.; Pauli, J.R.; Ropelle, E.R.; Silva, A.S.R.D. Pleiotropic and Multi-Systemic Actions of Physical Exercise on PGC-1α Signaling during the Aging Process. Ageing Res. Rev. 2023, 87, 101935. [Google Scholar] [CrossRef]
- Osman, I.; Segar, L. Pioglitazone, a PPARγ Agonist, Attenuates PDGF-Induced Vascular Smooth Muscle Cell Proliferation through AMPK-Dependent and AMPK-Independent Inhibition of mTOR/p70S6K and ERK Signaling. Biochem. Pharmacol. 2016, 101, 54–70. [Google Scholar] [CrossRef]
- Torma, F.; Gombos, Z.; Jokai, M.; Takeda, M.; Mimura, T.; Radak, Z. High Intensity Interval Training and Molecular Adaptive Response of Skeletal Muscle. Sports Med. Health Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Dun, R.; Yao, D.; Wu, F.; Qian, Y.; Zhou, Y.; Zhan, T.; Shao, M.; Gao, J.; Wang, C. Effects of Resveratrol on Renal Ischemia-Reperfusion Injury: A Systematic Review and Meta-Analysis. Front. Nutr. 2023, 9, 1064507. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Maggio, M.; Pizzarelli, F.; Michelassi, S.; Ruggiero, C.; Ceda, G.P.; Bandinelli, S.; Ferrucci, L. Omega-3 and Renal Function in Older Adults. Curr. Pharm. Des. 2009, 15, 4149–4156. [Google Scholar] [CrossRef] [PubMed]
- Arfanda, P.E.; Wiriawan, O.; Setijono, H.; Kusnanik, N.W.; Muhammad, H.N.; Puspodari, P.; Ayubi, N.; Aprilo, I.; Arimbi, A. The Effect of Low-Impact Aerobic Dance Exercise Video on Cardiovascular Endurance, Flexibility, and Concentration in Females With Sedentary Lifestyle. Phys. Educ. Theory Methodol. 2022, 22, 303–308. [Google Scholar] [CrossRef]
- Sharma, A.; Chabloz, S.; Lapides, R.A.; Roider, E.; Ewald, C.Y. Potential Synergistic Supplementation of NAD+ Promoting Compounds as a Strategy for Increasing Healthspan. Nutrients 2023, 15, 445. [Google Scholar] [CrossRef]
- Rabassa, M.; Zamora-Ros, R.; Urpi-Sarda, M.; Bandinelli, S.; Ferrucci, L.; Andres-Lacueva, C.; Cherubini, A. Association of Habitual Dietary Resveratrol Exposure with the Development of Frailty in Older Age: The Invecchiare in Chianti Study. Am. J. Clin. Nutr. 2015, 102, 1534–1542. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.; Wang, J. Effect of Blood Flow Restriction Combined with Low-Intensity Training on the Lower Limbs Muscle Strength and Function in Older Adults: A Meta-Analysis. Exp. Gerontol. 2022, 164, 111827. [Google Scholar] [CrossRef]
- Irving, B.A.; Lanza, I.R.; Henderson, G.C.; Rao, R.R.; Spiegelman, B.M.; Nair, K.S. Combined Training Enhances Skeletal Muscle Mitochondrial Oxidative Capacity Independent of Age. J. Clin. Endocrinol. Metab. 2015, 100, 1654–1663. [Google Scholar] [CrossRef]


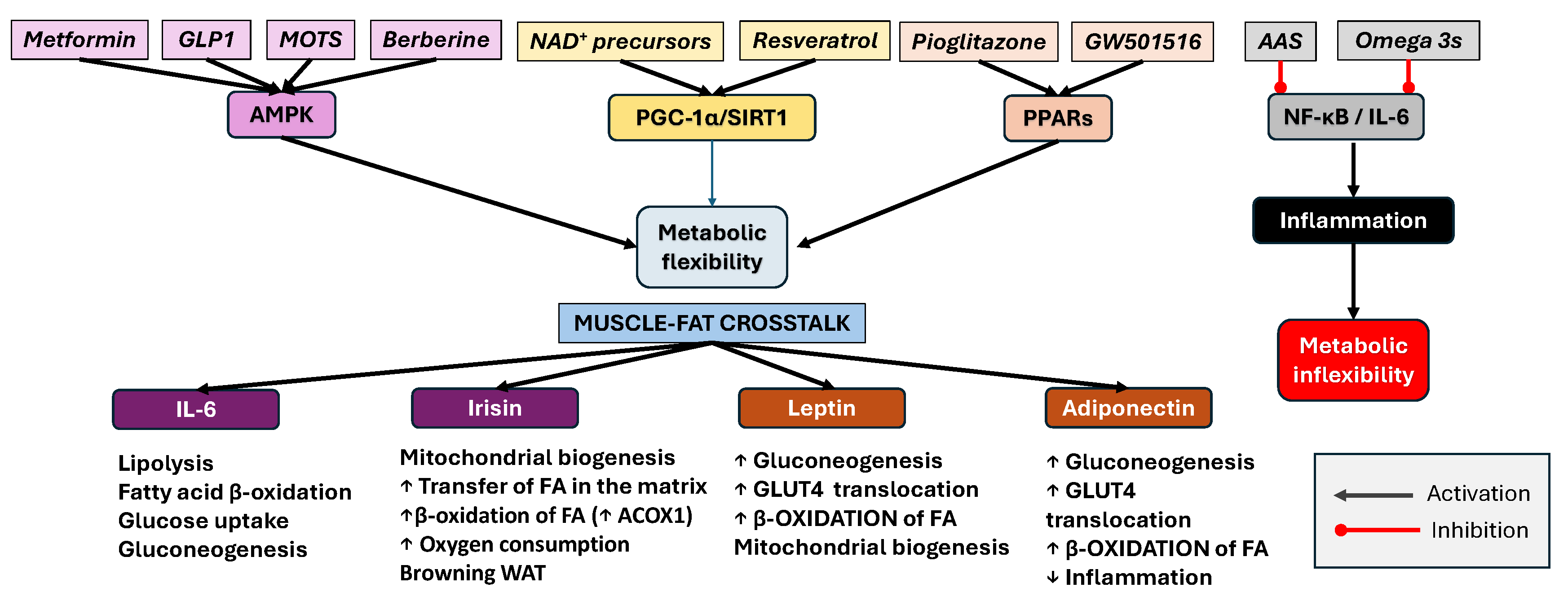
| Clinical Condition or Functional/Metabolic Trait | Recommended Agents | Mechanism/Target | Recommended Exercise Protocol | Notes | Ref. |
|---|---|---|---|---|---|
| Cardiovascular disease | Metformin, Omega 3s, Low-dose AAS | AMPK activation, anti-inflammatory, antiplatelet | Moderate-intensity aerobic exercise (e.g., 30–40 min, 4–5 days/week) | Bleeding risk of AAS | [127,128,129,130] |
| Chronic inflammation (↑CRP/IL-6) | Berberine, Omega 3s | AMPK activation, NF-κB inhibition | Combination of resistance (2–3 days/week) and aerobic exercise (3–4 days/week) | CRP-guided personalization | [131,132,133] |
| Mitochondrial disfunction/Sarcopenia | NAD+ precursors (NR/NMN), Resveratrol | SIRT1-PGC-1α axis, Oxidative phosphorilation | Progressive resistance training (2–3 days/week) with balance/flexibility exercises | Exercise mimetics (like MOTS) to be considered | [118,134,135,136] |
| Poor glycemic control | Metformin, Pioglitazone, GLP1 analogs | AMPK + PPARγ insulin sensitization | HIIT or moderate-intensity continuous training (≥150 min/week) | Fluid retention and edema with pioglitazone | [137,138] |
| Renal impairment (* eGFR < 30) | Resveratrol, Omega 3s | Antioxidant, mitochondrial protection | Low-impact aerobic training (walking, cycling) 20–30 min/day | Avoid metformin and pioglitazone | [139,140,141] |
| Frailty | NAD+ precursors, Resveratrol, MOTS | Mitochondrial protection, muscle maintenance | Low-intensity combined training (resistance, balance, and aerobic exercise 2–3 days/week) | Experimental options, in early human trials | [142,143,144,145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tero-Vescan, A.; Degens, H.; Matsakas, A.; Ștefănescu, R.; Ősz, B.E.; Slevin, M. Exercise-Induced Muscle–Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging. Pharmaceuticals 2025, 18, 1222. https://doi.org/10.3390/ph18081222
Tero-Vescan A, Degens H, Matsakas A, Ștefănescu R, Ősz BE, Slevin M. Exercise-Induced Muscle–Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging. Pharmaceuticals. 2025; 18(8):1222. https://doi.org/10.3390/ph18081222
Chicago/Turabian StyleTero-Vescan, Amelia, Hans Degens, Antonios Matsakas, Ruxandra Ștefănescu, Bianca Eugenia Ősz, and Mark Slevin. 2025. "Exercise-Induced Muscle–Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging" Pharmaceuticals 18, no. 8: 1222. https://doi.org/10.3390/ph18081222
APA StyleTero-Vescan, A., Degens, H., Matsakas, A., Ștefănescu, R., Ősz, B. E., & Slevin, M. (2025). Exercise-Induced Muscle–Fat Crosstalk: Molecular Mediators and Their Pharmacological Modulation for the Maintenance of Metabolic Flexibility in Aging. Pharmaceuticals, 18(8), 1222. https://doi.org/10.3390/ph18081222






