Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain
Abstract
1. Introduction
2. Results
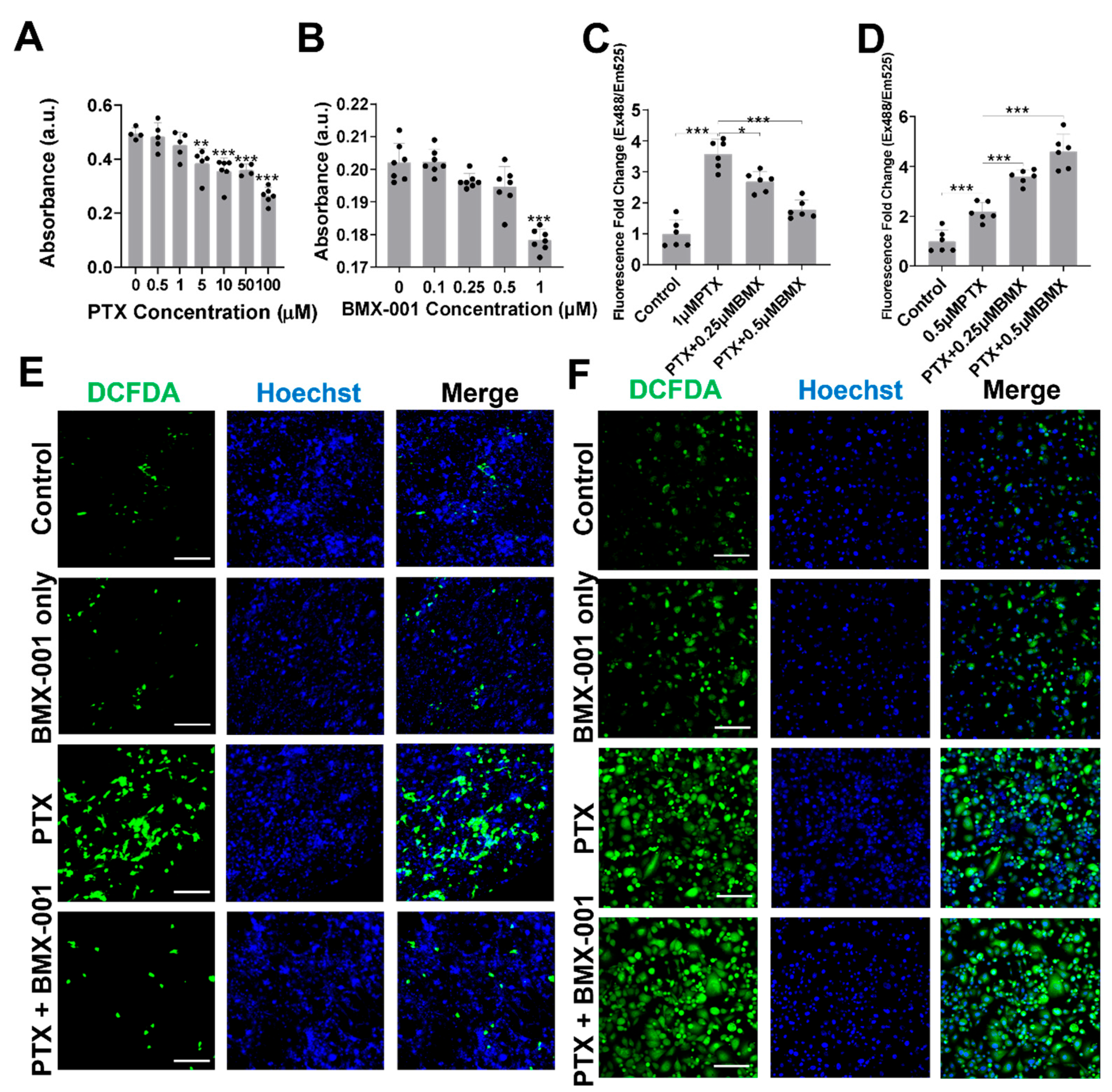
2.1. BMX-001 Reduced Total ROS Elevated by PTX in Satellite Glial Cells (SGCs) but Increased ROS in MDA-MB-231 Cells In Vitro
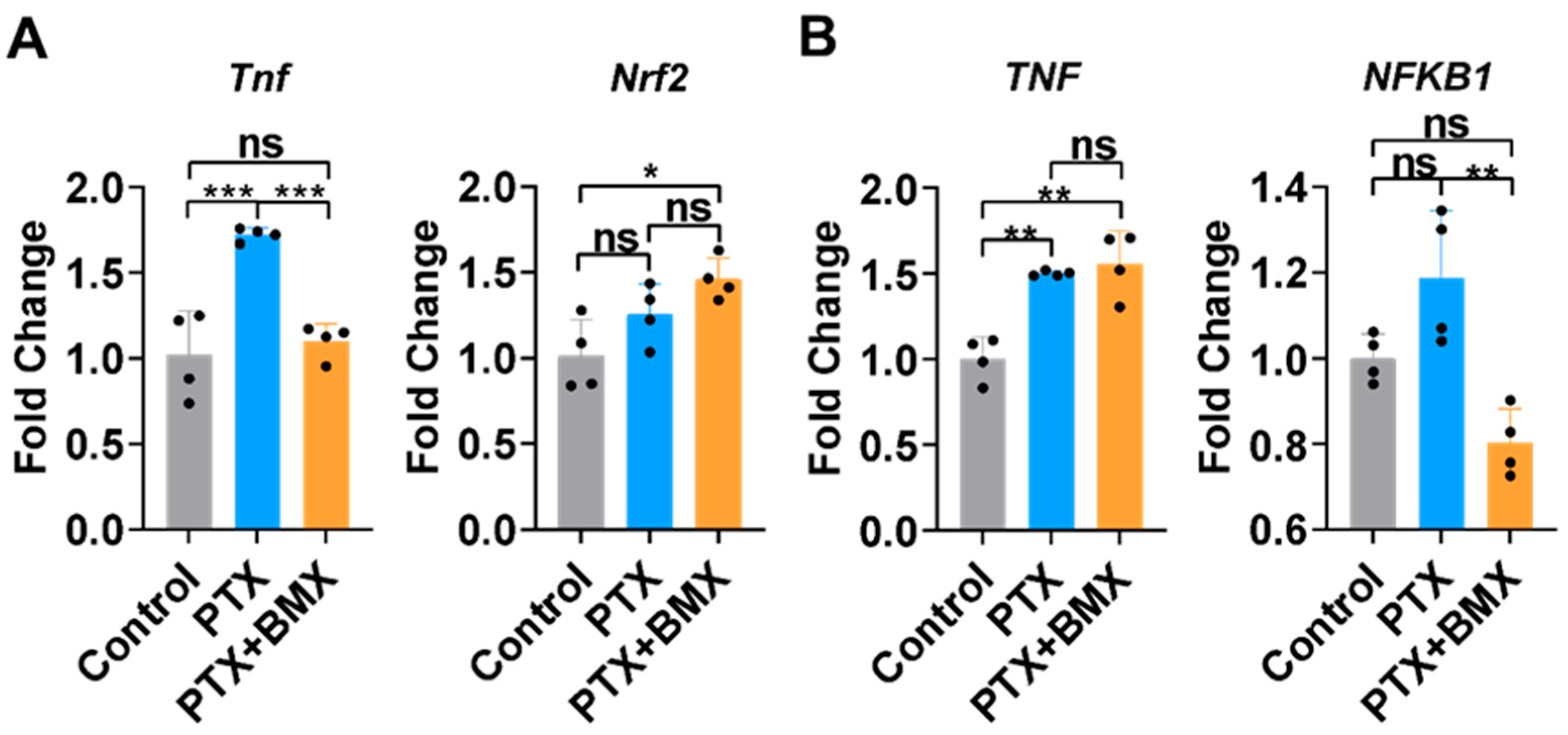
2.2. BMX-001 Regulated Inflammation and Antioxidants Differently in SGCs and MDA-MB-231
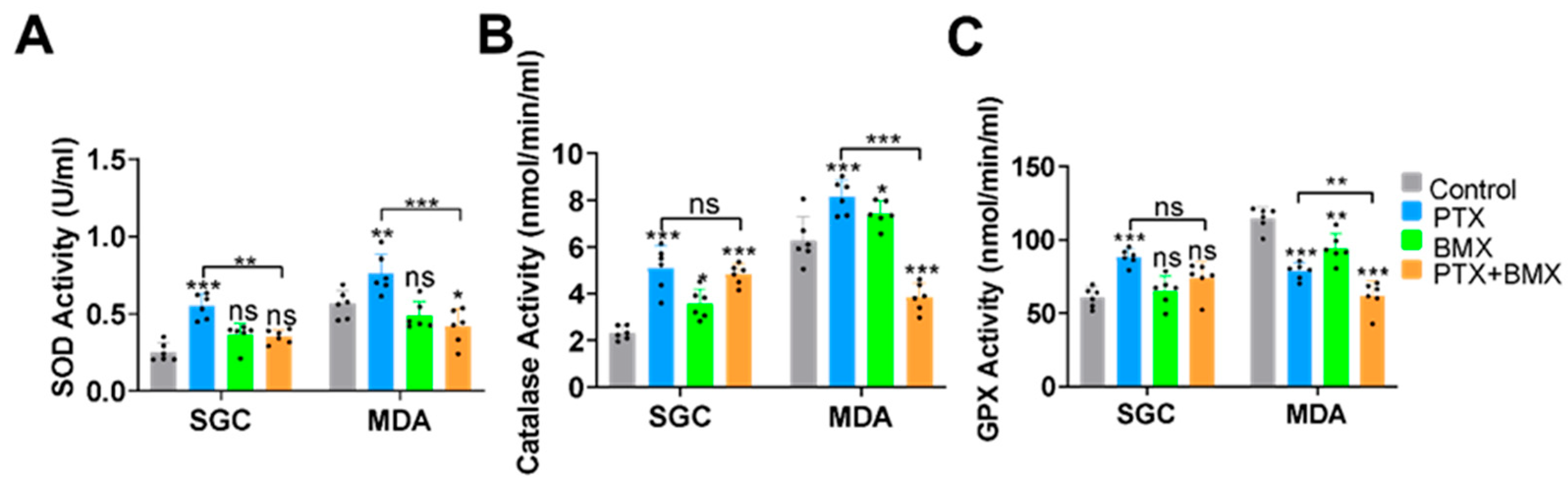
2.3. BMX-001 Regulated Antioxidant Enzyme Activity Differently in SGCs and MDA-MB-231
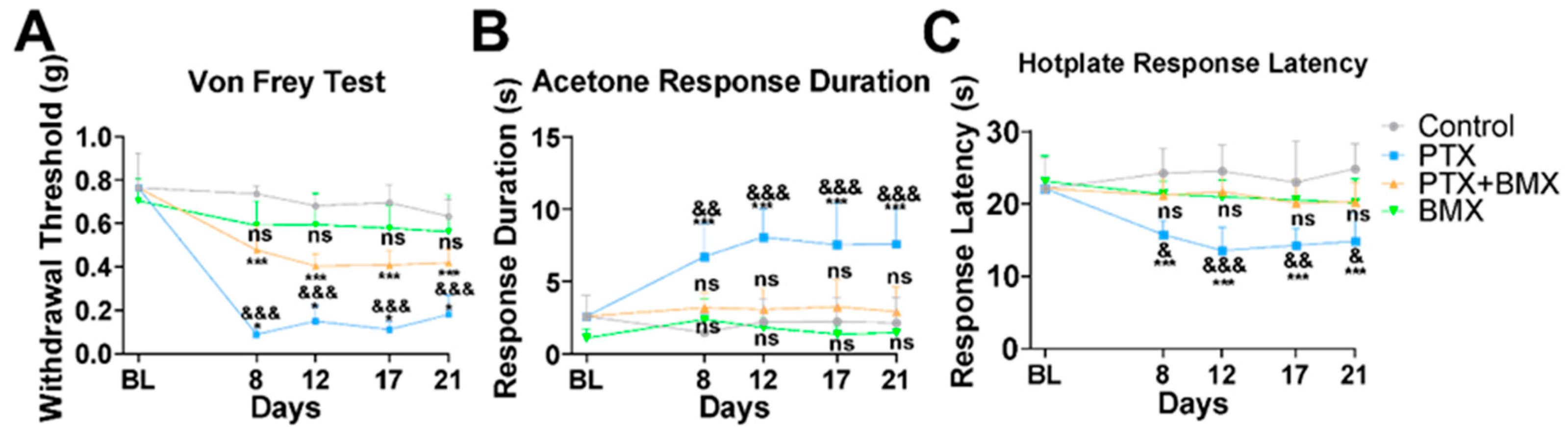
2.4. BMX-001 Alleviated Mechanical and Thermal Allodynia in a Mouse Model of CINP
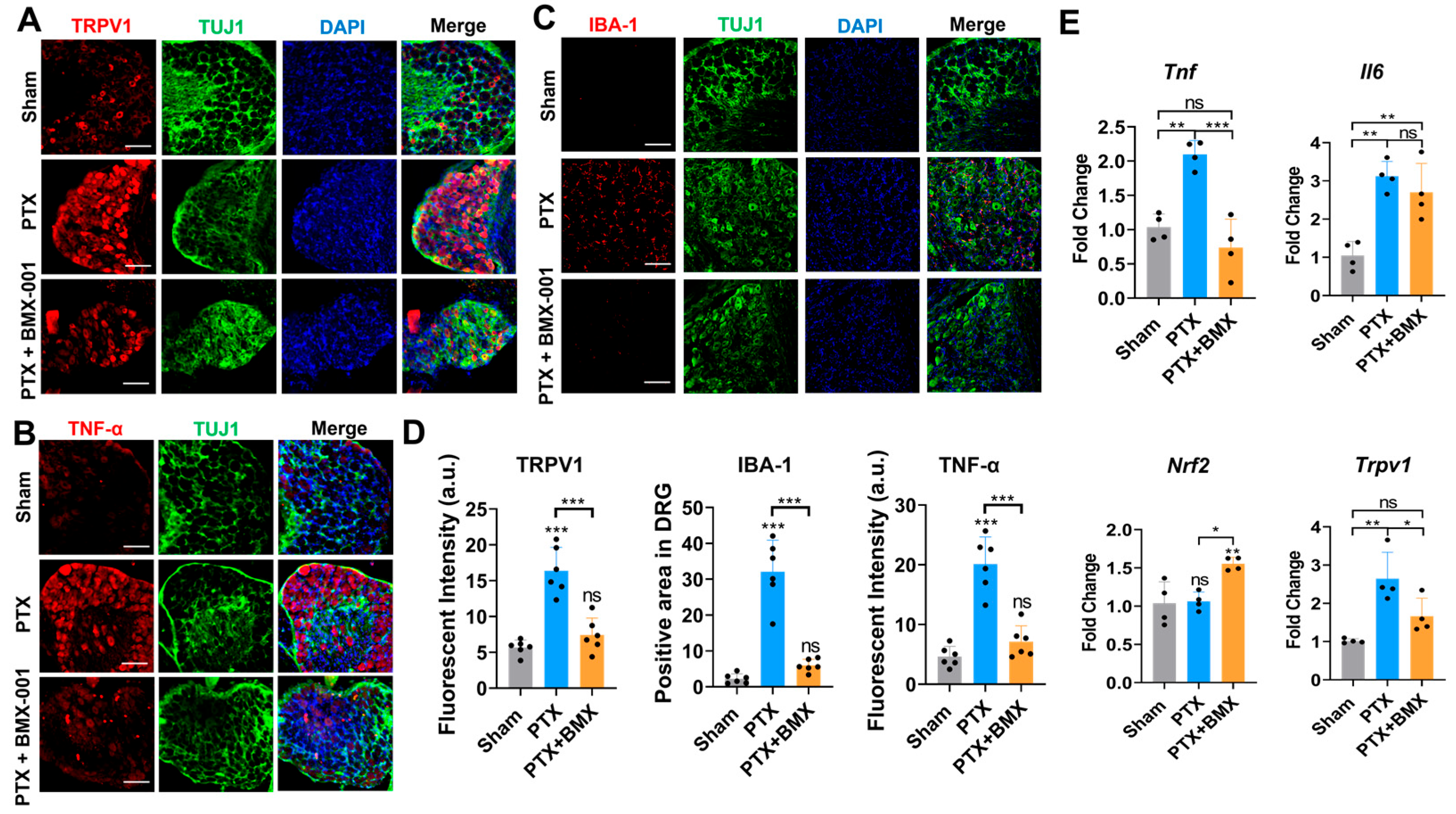
2.5. BMX-001 Ameliorated Inflammation, ROS, and Hyposensitivity in Peripheral Nerves of Mice with CINP
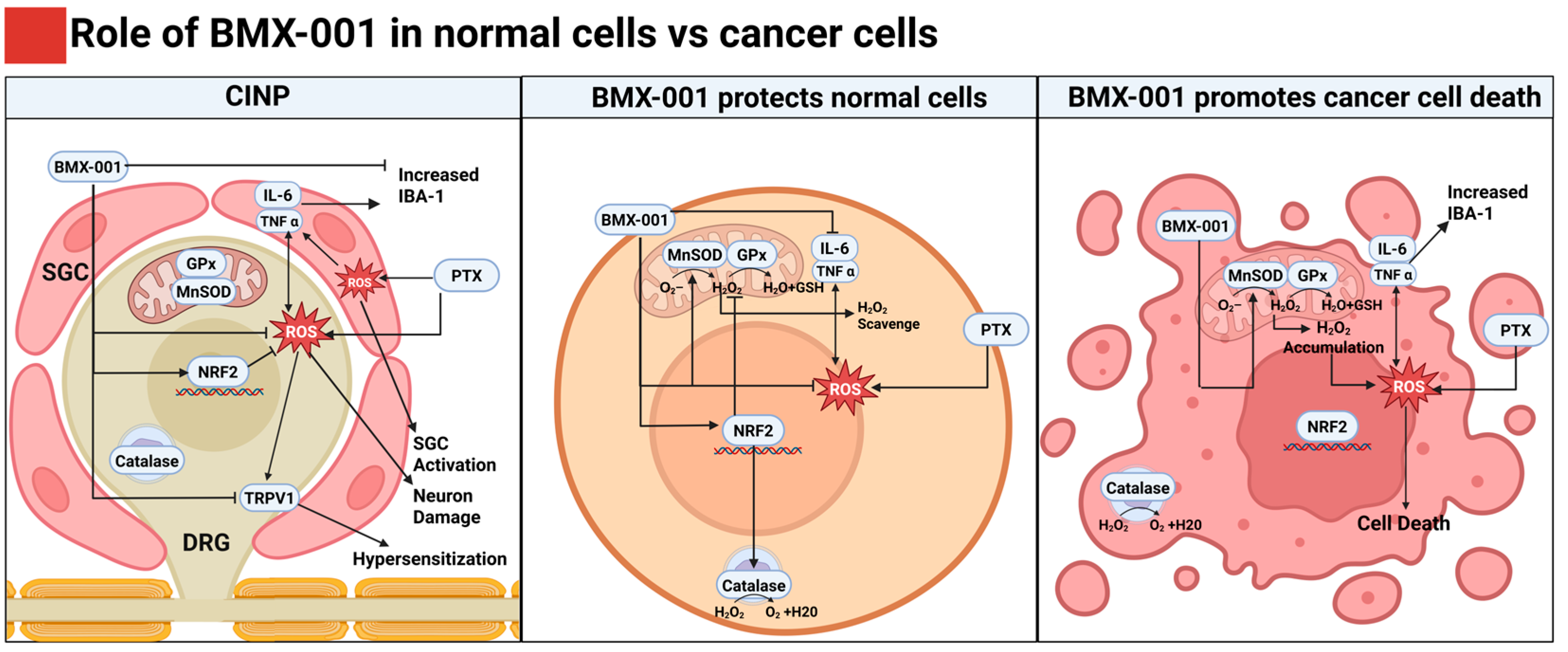
3. Discussion
4. Materials and Methods
4.1. Mouse SGCs Isolation and Culture
4.2. In Vitro Cytotoxicity Assay for PTX and BMX-001
4.3. In Vitro ROS Evaluation
4.4. In Vitro SOD, Catalase, and GPx Activity Assay
4.5. CINP Mouse Model and Treatments
4.6. Behavioral Tests
4.7. Immunofluorescence (IF) Imaging
4.8. RNA Isolation and Quantitative PCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CINP | Chemotherapy-induced neuropathic pain |
| DRG | Dorsal root ganglion |
| GFAP | Glial fibrillary acidic protein |
| GPx | Glutathione peroxidase |
| H2O2 | Hydrogen peroxide |
| H2DCFDA | 2′,7′-dichlorofluorescin diacetate |
| IBA | Ionized calcium-binding adaptor molecule |
| IF | Immunofluorescence |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| PTX | Paclitaxel |
| ROS | Reactive oxygen species |
| SGC | Satellite glial cell |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| TRPV | Transient receptor potential vanilloid |
References
- Staff, N.P.; Grisold, A.; Grisold, W.; Windebank, A.J. Chemotherapy-induced peripheral neuropathy: A current review. Ann. Neurol. 2017, 81, 772–781. [Google Scholar] [CrossRef]
- Starobova, H.; Vetter, I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front. Mol. Neurosci. 2017, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Flatters, S.J.L.; Dougherty, P.M.; Colvin, L.A. Clinical and preclinical perspectives on chemotherapy-induced peripheral neuropathy (CIPN): A narrative review. Br. J. Anaesth. 2017, 119, 737–749. [Google Scholar] [CrossRef]
- Sun, L.; Yu, X.; Guo, L.; Zhou, L.; Du, R.; Yao, H.; Zhao, C.; Yuan, F. Risk factors of paclitaxel-induced peripheral neuropathy in patients with breast cancer: A prospective cohort study. Front. Oncol. 2024, 14, 1327318. [Google Scholar] [CrossRef]
- Hiramoto, S.; Asano, H.; Miyamoto, T.; Takegami, M.; Kawabata, A. Risk factors and pharmacotherapy for chemotherapy-induced peripheral neuropathy in paclitaxel-treated female cancer survivors: A retrospective study in Japan. PLoS ONE 2021, 16, e0261473. [Google Scholar] [CrossRef]
- Preti, K.; Davis, M.E. Chemotherapy-Induced Peripheral Neuropathy: Assessment and Treatment Strategies for Advanced Practice Providers. Clin. J. Oncol. Nurs. 2024, 28, 351–357. [Google Scholar] [CrossRef]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. Pain 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Bae, E.H.; Greenwald, M.K.; Schwartz, A.G. Chemotherapy-Induced Peripheral Neuropathy: Mechanisms and Therapeutic Avenues. Neurotherapeutics 2021, 18, 2384–2396. [Google Scholar] [CrossRef]
- Freynhagen, R.; Baron, R.; Huygen, F.; Perrot, S. Narrative review of the efficacy and safety of the high-concentration (179 mg) capsaicin patch in peripheral neuropathic pain with recommendations for clinical practice and future research. Pain Rep. 2025, 10, e1235. [Google Scholar] [CrossRef] [PubMed]
- Mattar, M.; Umutoni, F.; Hassan, M.A.; Wamburu, M.W.; Turner, R.; Patton, J.S.; Chen, X.; Lei, W. Chemotherapy-Induced Peripheral Neuropathy: A Recent Update on Pathophysiology and Treatment. Life 2024, 14, 991. [Google Scholar] [CrossRef] [PubMed]
- Desforges, A.D.; Hebert, C.M.; Spence, A.L.; Reid, B.; Dhaibar, H.A.; Cruz-Topete, D.; Cornett, E.M.; Kaye, A.D.; Urits, I.; Viswanath, O. Treatment and diagnosis of chemotherapy-induced peripheral neuropathy: An update. Biomed. Pharmacother. 2022, 147, 112671. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, F.; Mantawy, E.M.; Said, R.S.; Azab, S.S.; El-Demerdash, E. Captopril attenuates oxidative stress and neuroinflammation implicated in cisplatin-induced cognitive deficits in rats. Psychopharmacology 2025, 242, 563–578. [Google Scholar] [CrossRef]
- Utkina-Sosunova, I.; Chiorazzi, A.; de Planell-Saguer, M.; Li, H.; Meregalli, C.; Pozzi, E.; Carozzi, V.A.; Canta, A.; Monza, L.; Alberti, P.; et al. Molsidomine provides neuroprotection against vincristine-induced peripheral neurotoxicity through soluble guanylyl cyclase activation. Sci. Rep. 2024, 14, 19341. [Google Scholar] [CrossRef] [PubMed]
- Kerckhove, N.; Collin, A.; Conde, S.; Chaleteix, C.; Pezet, D.; Balayssac, D. Long-Term Effects, Pathophysiological Mechanisms, and Risk Factors of Chemotherapy-Induced Peripheral Neuropathies: A Comprehensive Literature Review. Front. Pharmacol. 2017, 8, 86. [Google Scholar] [CrossRef]
- Bozdaganyan, M.; Fedorov, V.; Kholina, E.; Kovalenko, I.; Gudimchuk, N.; Orekhov, P. Exploring tubulin-paclitaxel binding modes through extensive molecular dynamics simulations. Sci. Rep. 2025, 15, 8378. [Google Scholar] [CrossRef]
- Shemesh, O.A.; Spira, M.E. Paclitaxel induces axonal microtubules polar reconfiguration and impaired organelle transport: Implications for the pathogenesis of paclitaxel-induced polyneuropathy. Acta Neuropathol. 2010, 119, 235–248. [Google Scholar] [CrossRef]
- Qin, P.; Sun, Y.; Li, L. Mitochondrial dysfunction in chronic neuroinflammatory diseases (Review). Int. J. Mol. Med. 2024, 53, 47. [Google Scholar] [CrossRef]
- Rodrigues, H.C.N.; Martins, T.F.P.; Santana, N.; Braga, C.C.; Silva, M.A.C.; Cunha, L.C.D.; Sugizaki, C.S.A.; Freitas, A.; Costa, N.A.; Peixoto, M. Antioxidant and anti-inflammatory response to curcumin supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled clinical trial. Clin. Nutr. ESPEN 2021, 44, 136–142. [Google Scholar] [CrossRef]
- Elblehi, S.S.; El Euony, O.I.; El-Sayed, Y.S. Apoptosis and astrogliosis perturbations and expression of regulatory inflammatory factors and neurotransmitters in acrylamide-induced neurotoxicity under omega3 fatty acids protection in rats. Neurotoxicology 2020, 76, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Hershman, D.L.; Unger, J.M.; Crew, K.D.; Minasian, L.M.; Awad, D.; Moinpour, C.M.; Hansen, L.; Lew, D.L.; Greenlee, H.; Fehrenbacher, L.; et al. Randomized double-blind placebo-controlled trial of acetyl-L-carnitine for the prevention of taxane-induced neuropathy in women undergoing adjuvant breast cancer therapy. J. Clin. Oncol. 2013, 31, 2627–2633. [Google Scholar] [CrossRef]
- De Grandis, D. Acetyl-L-carnitine for the treatment of chemotherapy-induced peripheral neuropathy: A short review. CNS Drugs 2007, 21 (Suppl. S1), 39–43; discussion 45–46. [Google Scholar] [CrossRef]
- Meng, J.; Zhang, Q.; Yang, C.; Xiao, L.; Xue, Z.; Zhu, J. Duloxetine, a Balanced Serotonin-Norepinephrine Reuptake Inhibitor, Improves Painful Chemotherapy-Induced Peripheral Neuropathy by Inhibiting Activation of p38 MAPK and NF-kappaB. Front. Pharmacol. 2019, 10, 365. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Spasojevic, I. Mn Porphyrin-Based Redox-Active Drugs: Differential Effects as Cancer Therapeutics and Protectors of Normal Tissue Against Oxidative Injury. Antioxid Redox Signal. 2018, 29, 1691–1724. [Google Scholar] [CrossRef] [PubMed]
- Tovmasyan, A.; Bueno-Janice, J.C.; Jaramillo, M.C.; Sampaio, R.S.; Reboucas, J.S.; Kyui, N.; Benov, L.; Deng, B.; Huang, T.T.; Tome, M.E.; et al. Radiation-Mediated Tumor Growth Inhibition Is Significantly Enhanced with Redox-Active Compounds That Cycle with Ascorbate. Antioxid Redox Signal. 2018, 29, 1196–1214. [Google Scholar] [CrossRef]
- Li, X.; Duan, W.; Du, L.; Chu, D.; Wang, P.; Yang, Z.; Qu, X.; Yang, Z.; Batinic-Haberle, I.; Spasojevic, I.; et al. Intracarotid Infusion of Redox-Active Manganese Porphyrin, MnTnBuOE-2-PyP(5+), following Reperfusion Improves Long-Term, 28-Day Post-Stroke Outcomes in Rats. Antioxidants 2023, 12, 1861. [Google Scholar] [CrossRef] [PubMed]
- Brizel, D.M.; Mowery, Y.M.; Zhen, W.; Chan, J.; Macleod, D.; Yom, S.S. A Phase 1-2 Trial of Concurrent Radiation Therapy, Cisplatin and BMX-001 in Locally Advanced Head and Neck Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, S145. [Google Scholar] [CrossRef]
- Zakeri, K.; Narayanan, D.; Vikram, B.; Evans, G.; Coleman, C.N.; Prasanna, P.G.S. Decreasing the Toxicity of Radiation Therapy: Radioprotectors and Radiomitigators Being Developed by the National Cancer Institute Through Small Business Innovation Research Contracts. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.U.; Lim, J.; Shin, S.W.; Kim, Y.; Park, W.Y.; Batinic-Haberle, I.; Choi, C.; Park, W. Single-Cell Profiling Reveals Immune-Based Mechanisms Underlying Tumor Radiosensitization by a Novel Mn Porphyrin Clinical Candidate, MnTnBuOE-2-PyP(5+) (BMX-001). Antioxidants 2024, 13, 477. [Google Scholar] [CrossRef]
- Warpsinski, G.; Smith, M.J.; Srivastava, S.; Keeley, T.P.; Siow, R.C.M.; Fraser, P.A.; Mann, G.E. Nrf2-regulated redox signaling in brain endothelial cells adapted to physiological oxygen levels: Consequences for sulforaphane mediated protection against hypoxia-reoxygenation. Redox Biol. 2020, 37, 101708. [Google Scholar] [CrossRef]
- Kosmacek, E.A.; Oberley-Deegan, R.E.; Buettner, G.R.; Spitz, D.R.; Allen, B.G. Redox-active Mn porphyrin BMX-001 selectively protects normal cells while enhancing cancer cell death with radiation. Redox Biol. 2021, 48, 102202. [Google Scholar]
- Miriyala, S.; Zhao, Y.; St Clair, D.K.; Batinic-Haberle, I. MnTnBuOE-2-PyP as a radioprotector and cancer therapeutic: Differential effects in normal vs. tumor cells. Antioxidants 2019, 8, 313. [Google Scholar]
- Batinic-Haberle, I.; Tovmasyan, A.; Huang, Z.; Duan, W.; Du, L.; Siamakpour-Reihani, S.; Cao, Z.; Sheng, H.; Spasojevic, I.; Secord, A.A. H2O2-driven anticancer activity of Mn porphyrins and the underlying molecular pathways. Oxidative Med. Cell. Longev. 2021, 2021, 6653790. [Google Scholar] [CrossRef]
- Chatterjee, A.; Spasojevic, I.; Batinic-Haberle, I.; Tse, H.M. Tumor-specific targeting of reactive oxygen species by redox-active metalloporphyrins. Free. Radic. Biol. Med. 2020, 160, 479–491. [Google Scholar]
- Zhang, S.; Cao, J.; Spasojevic, I.; Treggiari, M.; Sheng, H. Treatment with Manganese Porphyrin, MnTnBuOE-2-PyP(5+), Suppressed the Activation of Macrophages in a Mouse Intracerebral Hemorrhage. Pharmaceuticals 2025, 18, 547. [Google Scholar] [CrossRef]
- Peters, K.B.; Kirkpatrick, J.P.; Batinic-Haberle, I.; Affronti, M.L.; Woodring, S.; Iden, D.; Lipp, E.S.; Boyd, K.; Healy, P.; Herndon, J.; et al. First in Human Clinical Trial of a Metalloporphyrin Dual Radioprotectant and Radiosensitizer, BMX-001, in Newly Diagnosed High-Grade Glioma Undergoing Concurrent Chemoradiation. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, E106. [Google Scholar] [CrossRef]
- Hanani, M.; Spray, D.C. Emerging importance of satellite glia in nervous system function and dysfunction. Nat. Rev. Neurosci. 2020, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Avraham, O.; Deng, P.Y.; Jones, S.; Kuruvilla, R.; Semenkovich, C.F.; Klyachko, V.A.; Cavalli, V. Satellite glial cells promote regenerative growth in sensory neurons. Nat. Commun. 2020, 11, 4891. [Google Scholar] [CrossRef] [PubMed]
- Pozzi, E.; Ballarini, E.; Rodriguez-Menendez, V.; Canta, A.; Chiorazzi, A.; Monza, L.; Bossi, M.; Alberti, P.; Malacrida, A.; Meregalli, C.; et al. Paclitaxel, but Not Cisplatin, Affects Satellite Glial Cells in Dorsal Root Ganglia of Rats with Chemotherapy-Induced Peripheral Neurotoxicity. Toxics 2023, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.H.; Hankerd, K.M.; Kim, H.K.; Chung, J.M.; La, J.H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15, 1744806919840098. [Google Scholar] [CrossRef]
- Fumagalli, G.; Monza, L.; Cavaletti, G.; Rigolio, R.; Meregalli, C. Neuroinflammatory Process Involved in Different Preclinical Models of Chemotherapy-Induced Peripheral Neuropathy. Front. Immunol. 2020, 11, 626687. [Google Scholar] [CrossRef]
- Duggett, N.A.; Griffiths, L.A.; McKenna, O.E.; de Santis, V.; Yongsanguanchai, N.; Mokori, E.B.; Flatters, S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience 2016, 333, 13–26. [Google Scholar] [CrossRef]
- Klein, I.; Lehmann, H.C. Pathomechanisms of Paclitaxel-Induced Peripheral Neuropathy. Toxics 2021, 9, 229. [Google Scholar] [CrossRef]
- Kober, K.M.; Mazor, M.; Abrams, G.; Olshen, A.; Conley, Y.P.; Hammer, M.; Schumacher, M.; Chesney, M.; Smoot, B.; Mastick, J.; et al. Phenotypic Characterization of Paclitaxel-Induced Peripheral Neuropathy in Cancer Survivors. J. Pain Symptom Manag. 2018, 56, 908–919.e3. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, Y.; de Carvalho-Barbosa, M.; Kavelaars, A.; Heijnen, C.J.; Albrecht, P.J.; Dougherty, P.M. Dorsal Root Ganglion Infiltration by Macrophages Contributes to Paclitaxel Chemotherapy-Induced Peripheral Neuropathy. J. Pain 2016, 17, 775–786. [Google Scholar] [CrossRef]
- Peters, K.; Cohen, A.; Butowski, N.; Villano, J.; Mendez, J.; Giglio, P.; McGranahan, T.; Zhang, C.; Smeltzer, J.; Raza, S.; et al. LTBK-09. Results of Bmx-Hgg Study: A Multi-Institutional, Randomized Phase 2 Clinical Trial of Concurrent Chemoradiation with or Without Bmx-001 in Patients With Newly Diagnosed High Grade Glioma. Neuro-Oncology 2023, 25 (Suppl. S5), v311. [Google Scholar] [CrossRef]
- Soares, R.B.; Manguinhas, R.; Costa, J.G.; Saraiva, N.; Gil, N.; Rosell, R.; Camões, S.P.; Batinic-Haberle, I.; Spasojevic, I.; Castro, M.; et al. The redox-active manganese (III) porphyrin, MnTnBuOE-2-PyP5+, impairs the migration and invasion of non-small cell lung cancer cells, either alone or combined with cisplatin. Cancers 2023, 15, 3814. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Sharma, P.; Subedi, U.; Bhattarai, S.; Miller, C.; Manikandan, S.; Batinic-Haberle, I.; Spasojevic, I.; Sun, H.; Panchatcharam, M.; et al. Mn(III) Porphyrin, MnTnBuOE-2-PyP(5+), Commonly Known as a Mimic of Superoxide Dismutase Enzyme, Protects Cardiomyocytes from Hypoxia/Reoxygenation Induced Injury via Reducing Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 6159. [Google Scholar] [CrossRef]
- Zhao, Y.; Carroll, D.W.; You, Y.; Chaiswing, L.; Wen, R.; Batinic-Haberle, I.; Bondada, S.; Liang, Y.; Clair, D.K.S. A novel redox regulator, MnTnBuOE-2-PyP(5+), enhances normal hematopoietic stem/progenitor cell function. Redox Biol. 2017, 12, 129–138. [Google Scholar] [CrossRef]
- Batinic-Haberle, I.; Tovmasyan, A.; Veade, A.; Spasojevic, I.; Reihani, S.; Secord, A. Mn porphyrin/ascorbate sensitizes serous epithelial ovarian cancer to chemotherapy. Free. Radic. Biol. Med. 2018, 128, S74–S75. [Google Scholar] [CrossRef]
- Gad, S.C.; Sullivan, D.W., Jr.; Spasojevic, I.; Mujer, C.V.; Spainhour, C.B.; Crapo, J.D. Nonclinical safety and toxicokinetics of MnTnBuOE 2 PyP5+ (BMX 001). Int. J. Toxicol. 2016, 35, 438–453. [Google Scholar] [CrossRef]
- Peters, K.; Kirkpatrick, J.; Batinic-Haberle, I.; Affronti, M.; Woodring, S.; Iden, D.; Panta, S.; Lipp, E.; Healy, P.; Herndon, J.; et al. ACTR-28. Phase 1 dose escalation trial of the safety of BMX-001 concurrent with radiation therapy and temozolomide in newly diagnosed patients with high-grade gliomas. Neuro-Oncology 2018, 20 (Suppl. S6), vi17. [Google Scholar] [CrossRef][Green Version]
- Shin, S.W.; Choi, C.; Kim, H.; Kim, Y.; Park, S.; Kim, S.Y.; Batinic-Haberle, I.; Park, W. MnTnHex-2-PyP(5+), Coupled to Radiation, Suppresses Metastasis of 4T1 and MDA-MB-231 Breast Cancer via AKT/Snail/EMT Pathways. Antioxidants 2021, 10, 1769. [Google Scholar] [CrossRef]
- Hadzic, T.; Aykin-Burns, N.; Zhu, Y.; Coleman, M.C.; Leick, K.; Jacobson, G.M.; Spitz, D.R. Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radic. Biol. Med. 2010, 48, 1024–1033. [Google Scholar] [CrossRef]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Pigeolet, E.; Corbisier, P.; Houbion, A.; Lambert, D.; Michiels, C.; Raes, M.; Zachary, M.D.; Remacle, J. Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech. Ageing Dev. 1990, 51, 283–297. [Google Scholar] [CrossRef] [PubMed]
- Baud, O.; Greene, A.E.; Li, J.; Wang, H.; Volpe, J.J.; Rosenberg, P.A. Glutathione peroxidase-catalase cooperativity is required for resistance to hydrogen peroxide by mature rat oligodendrocytes. J. Neurosci. 2004, 24, 1531–1540. [Google Scholar] [CrossRef] [PubMed]
- Mapuskar, K.A.; Anderson, C.M.; Spitz, D.R.; Batinic-Haberle, I.; Allen, B.G.; Oberley-Deegan, R.E. Utilizing Superoxide Dismutase Mimetics to Enhance Radiation Therapy Response While Protecting Normal Tissues. Semin. Radiat. Oncol. 2019, 29, 72–80. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, D.; Tu, H.; Alimi, O.A.; Kong, Y.; Satyanarayana, R.; Kuss, M.; Li, Y.; Duan, B. Red blood cell membrane-camouflaged poly(lactic-co-glycolic acid) microparticles as a potential controlled release drug delivery system for local stellate ganglion microinjection. Acta Biomater. 2023, 161, 201–212. [Google Scholar] [CrossRef]
- Birer, S.R.; Lee, C.T.; Choudhury, K.R.; Young, K.H.; Spasojevic, I.; Batinic Haberle, I.; Crapo, J.D.; Dewhirst, M.W.; Ashcraft, K.A. Inhibition of the continuum of radiation induced normal tissue injury by a redox active Mn porphyrin. Radiat. Res. 2017, 188, 94–104. [Google Scholar] [CrossRef]
- Bacalhau, C.; Costa-Pereira, J.T.; Tavares, I. Preclinical research in paclitaxel-induced neuropathic pain: A systematic review. Front. Vet. Sci. 2023, 10, 1264668. [Google Scholar] [CrossRef]
- Cristiano, C.; Avagliano, C.; Cuozzo, M.; Liguori, F.M.; Calignano, A.; Russo, R. The beneficial effects of ultramicronized palmitoylethanolamide in the management of neuropathic pain and associated mood disorders induced by paclitaxel in mice. Biomedicines 2022, 12, 1155. [Google Scholar] [CrossRef] [PubMed]
- Caillaud, M.; Patel, N.H.; Toma, W.; White, A.; Thompson, D.; Mann, J.; Salvemini, D. A fenofibrate diet prevents paclitaxel-induced peripheral neuropathy in mice. Cancers 2021, 13, 69. [Google Scholar] [CrossRef]
- Cuozzo, M.; Castelli, V.; Avagliano, C.; Cimini, A.; D’Angelo, M.; Cristiano, C.; Calignano, A. Effects of chronic oral probiotic treatment in paclitaxel-induced neuropathic pain. Biomedicines 2021, 9, 346. [Google Scholar] [CrossRef]
- Kong, Y.; Pan, T.; Liu, B.; Kuss, M.; Krishnan, M.A.; Alimi, O.A.; Shi, W.; Duan, B. Double-Layer Microneedle Patch Loaded with HA-PBA-QCT for Management of Paclitaxel-Induced Peripheral Neuropathic Pain. Small 2025, 21, e2409748. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, T.; Alimi, O.A.; Liu, B.; Krishnan, M.A.; Kuss, M.; Shi, W.; Krishnamurthy, J.; Dong, J.J.; Oberley-Deegan, R.E.; Duan, B. Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain. Pharmaceuticals 2025, 18, 1159. https://doi.org/10.3390/ph18081159
Pan T, Alimi OA, Liu B, Krishnan MA, Kuss M, Shi W, Krishnamurthy J, Dong JJ, Oberley-Deegan RE, Duan B. Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain. Pharmaceuticals. 2025; 18(8):1159. https://doi.org/10.3390/ph18081159
Chicago/Turabian StylePan, Tianshu, Olawale A. Alimi, Bo Liu, Mena A. Krishnan, Mitchell Kuss, Wei Shi, Jairam Krishnamurthy, Jianghu James Dong, Rebecca E. Oberley-Deegan, and Bin Duan. 2025. "Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain" Pharmaceuticals 18, no. 8: 1159. https://doi.org/10.3390/ph18081159
APA StylePan, T., Alimi, O. A., Liu, B., Krishnan, M. A., Kuss, M., Shi, W., Krishnamurthy, J., Dong, J. J., Oberley-Deegan, R. E., & Duan, B. (2025). Therapeutic Potential of BMX-001 for Preventing Chemotherapy-Induced Peripheral Neuropathic Pain. Pharmaceuticals, 18(8), 1159. https://doi.org/10.3390/ph18081159








