Pharmacological Potential of Cinnamic Acid and Derivatives: A Comprehensive Review
Abstract
1. Introduction
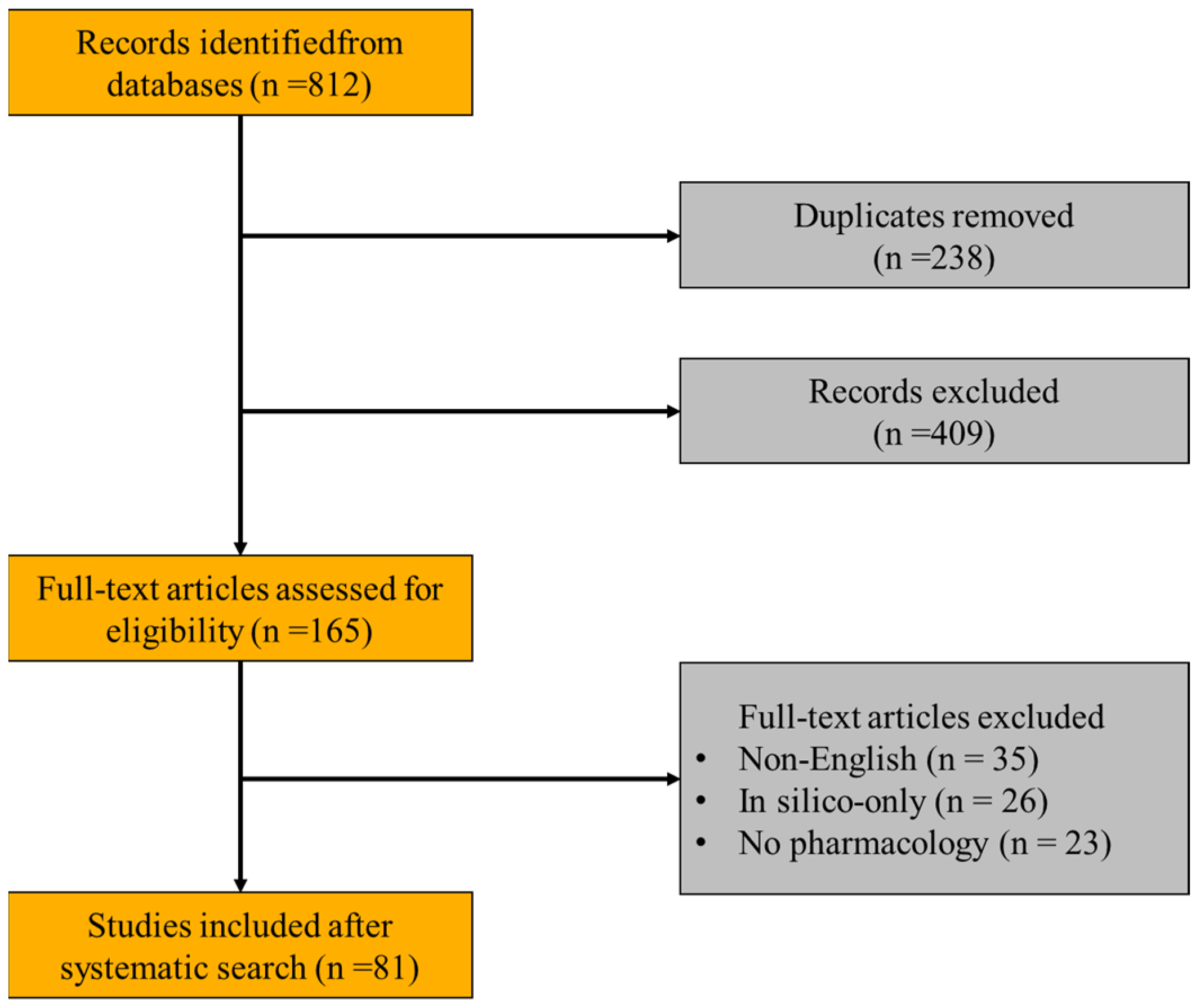
2. Review Methodology
3. Chemical Composition
4. Anti-Inflammatory Effect
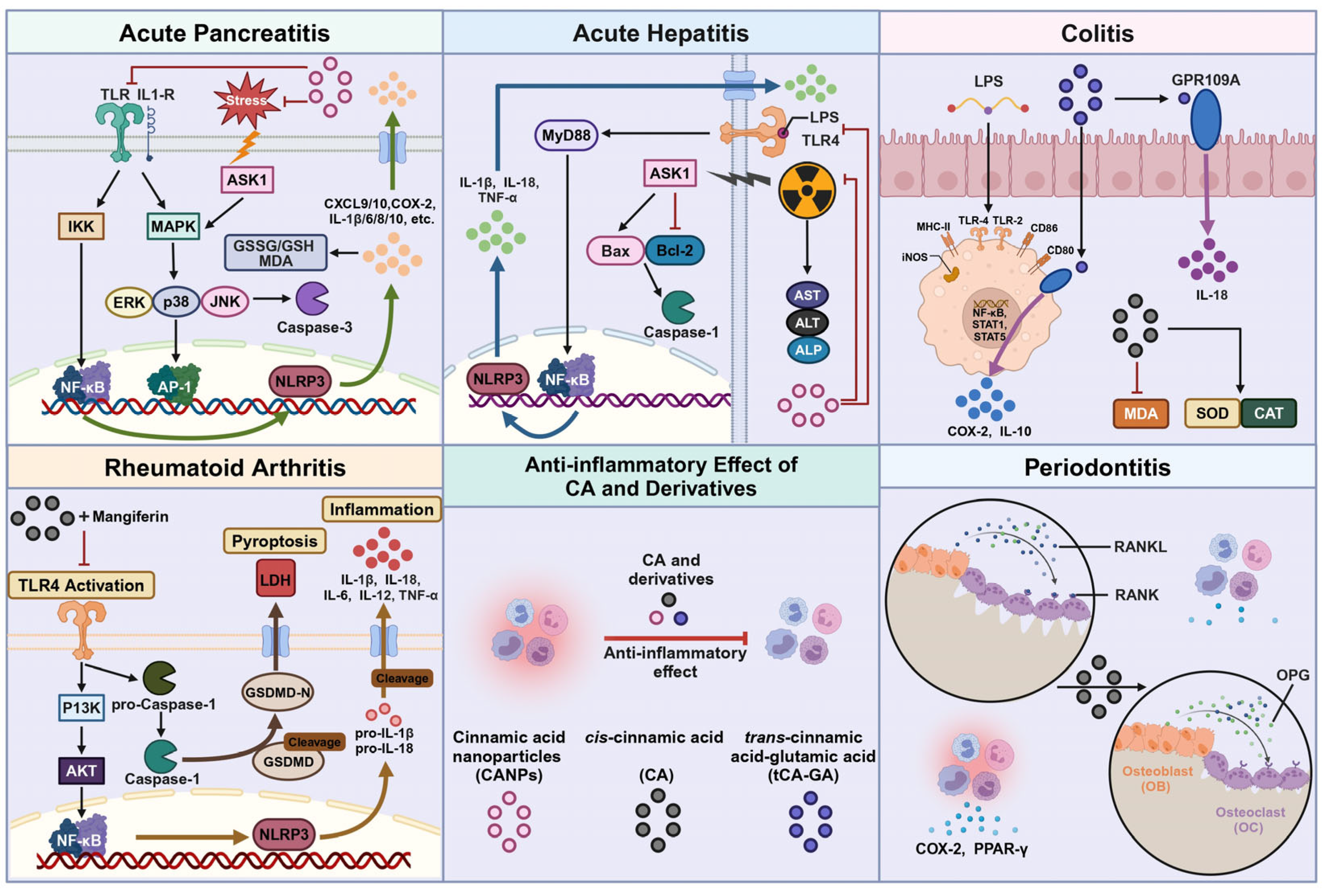
4.1. Anti-Acute Pancreatitis
4.2. Anti-Acute Hepatitis
4.3. Anti-Colitis
4.4. Anti-Rheumatoid Arthritis
4.5. Anti-Periodontitis
5. Antibacterial Effect
5.1. Anti-Staphylococcus aureus
5.2. Anti-Pseudomonas aeruginosa
5.3. Anti-Foodborne Pseudomonas
5.4. Antifungal
6. Anti-Tumor Effect
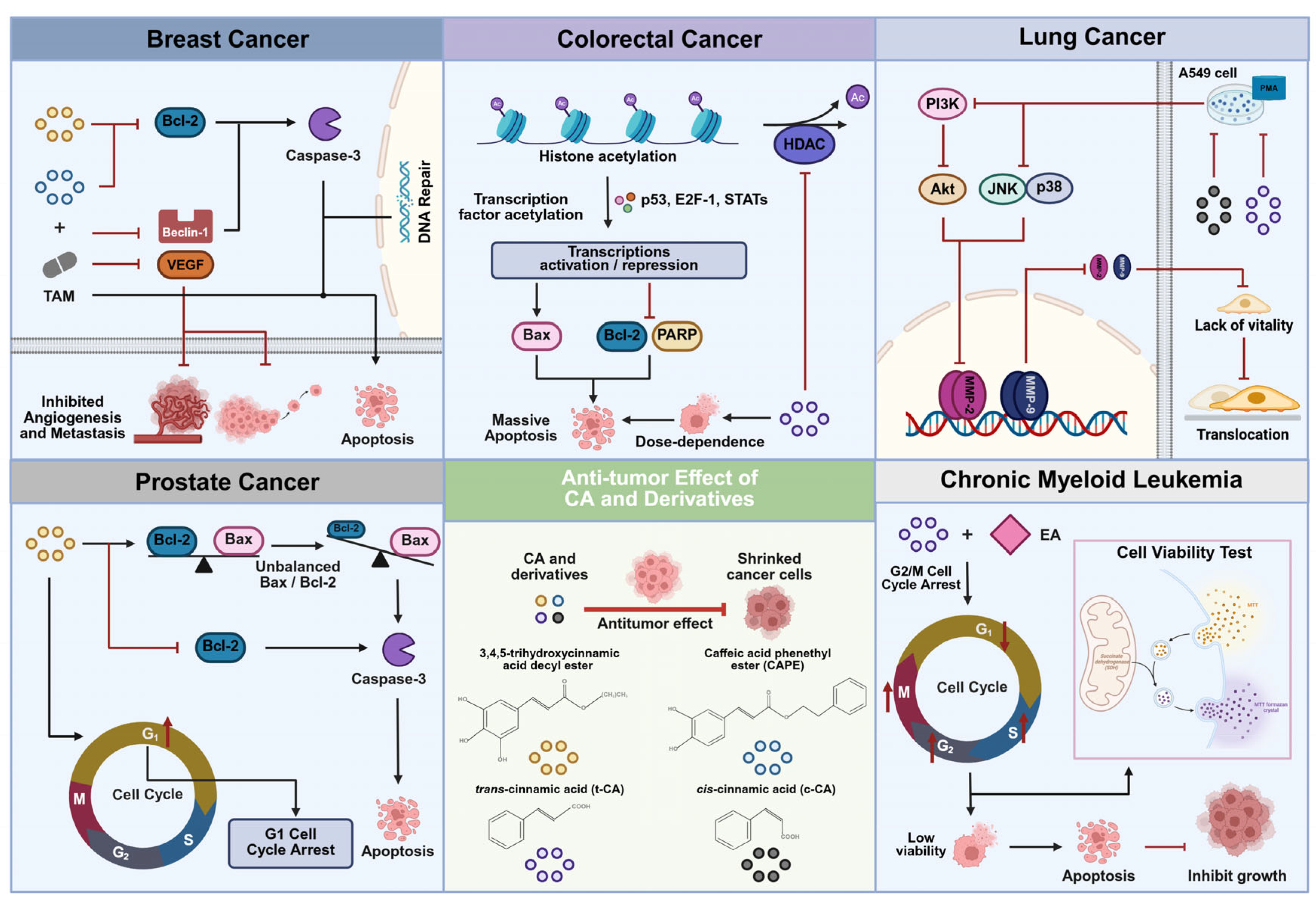
6.1. Breast Cancer
6.2. Colorectal Cancer
6.3. Lung Cancer
6.4. Prostate Cancer
6.5. Chronic Myeloid Leukemia
7. Anti-Diabetic
8. Anti-Depressant
9. Other Pharmacological Effects
10. Toxicological Evaluation
11. Current Application Status and Development Prospects
12. Summary
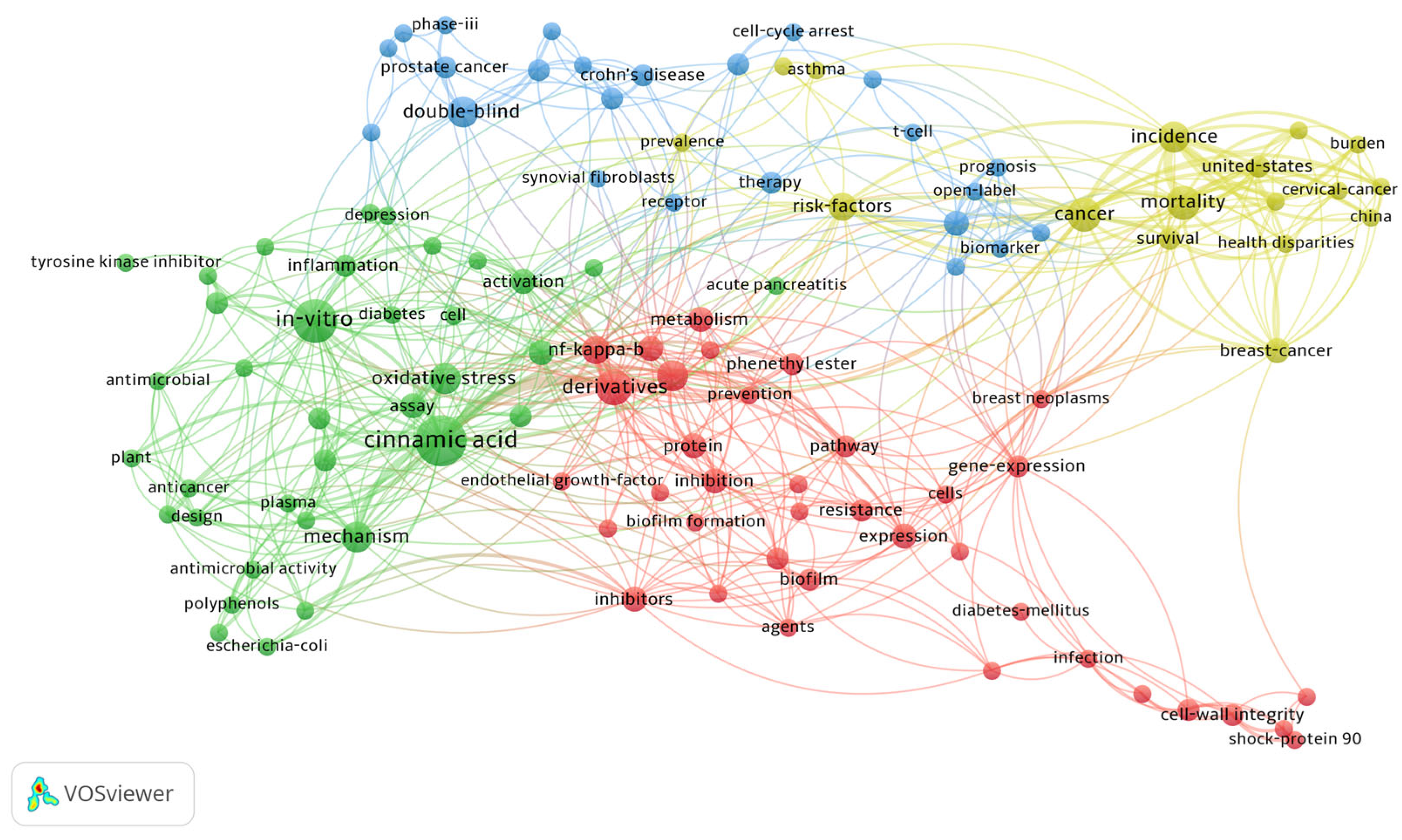
13. VOSviewer
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme | LB | Luria–Bertani |
| AD | Alzheimer’s disease | LD50 | median lethal dose |
| AH | Acute hepatitis | LDH | Lactate dehydrogenase |
| AIA-M | adjuvant-induced arthritis-modified rat model | LDL | low-density lipoprotein |
| AIF | apoptosis-inducing factor | LPS | Lipopolysaccharide |
| ALP | Alkaline phosphatase | LUAD | Lung Adenocarcinoma |
| ALT | Alanine aminotransferase | LQM755 | 3-(4-phenoxy)phenyl-N-[(3,4-dichlorophenyl)methyl]prop-2-enamide |
| ANP | Atrial Natriuretic Peptide | MAOIs | monoamine oxidase inhibitors |
| AP | alkaline phosphatase | MAPK | Mitogen-activated protein kinase |
| ASK1 | Apoptosis Signal-regulating Kinase 1 | MDA | malondialdehyde |
| AST | Aspartate aminotransferase | MDCK | Madin-Darby canine kidney |
| ATACL | 4-hydroxy-3,5-di-tert-butylcinnamic acid | MG | mangiferin |
| ATP | Adenosine Triphosphate | MIC | minimum inhibitory concentration |
| Aβ | Bate-Amyloid | Micro-CT | Quantitative micro-computed tomography |
| BAX | BCL-2-associated X protein | MMP-2 | Matrix metalloproteinase-2 |
| BBR | berberine | MMP-9 | Matrix metalloproteinase-9 |
| Bcl-2 | B-cell lymphoma-2 | MPO | Myeloperoxidase |
| BDNF | brain-derived neurotrophic factor | MPZ | mepenzolate |
| BMD | bone mineral density | MRSA | Methicillin-resistant Staphylococcus aureus |
| BV/TV | bone volume/tissue volume | MTP | Microtitre plates |
| CA | Cinnamic acid | MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| CA-NPs | cinnamic acid nanoparticles | MTX | methotrexate |
| CAPE | caffeic acid phenethyl ester | MyD88 | Myeloid Differentiation Primary Response 88 |
| CAT | catalase | NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| c-CA | cis-Cinnamic acid | NLRP3 | NOD-like receptor protein 3 |
| CINC-3 | neutrophil chemoattractant-3 | NPs | nanoparticles |
| CKMB | Creatine Kinase-MB | NSAIDs | nonsteroidal anti-inflammatory drugs |
| CLSM | confocal laser scanning microscopic | NSCLC | non-small cell lung cancer |
| CML | Chronic myeloid leukemia | Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| COX-2 | Cyclooxygenase-2 | –OCH3 | methoxy |
| CRC | Colorectal cancer | OGTT | oral glucose tolerance test |
| CRP | C-Reactive Protein | –OH | hydroxy |
| CSH | cell surface hydrophobicity | OPG | osteoprotegerin |
| DCM | diabetic cardiomyopathy | OS | overall surviva |
| D-Gal | d-galactosamine | p38 | p38 mitogen-activated protein kinase |
| DMARDs | disease-induced anti-rheumarthritis drugs | PA | Pseudomonas aeruginosa |
| DNBS | dinitrobenzene sulfonic acid | PARP | Poly ADP-ribose polymerase |
| DSS | dextran sodium sulfate | PI3K | Phosphatidylinositol 3-kinase |
| EA | ethacrylic acid | PMA | phorbol-12-myristate-13-acetat |
| eDNA | exogenous DNA | PPAR-γ | Peroxisome proliferator–activated receptor-γ |
| EPS | polysaccharide substances in Pseudomonas aeruginosa | QS | quorum sensing |
| ERK1/2 | extracellular signal-regulated kinase 1/2 | RA | Rheumatoid arthritis |
| FESEM | field-emission scanning electron microscopy | RANKL | receptor activator of nuclear factor κ-B |
| FLS | fibroblast-like synoviocytes | SEM | scanning electron microscopy |
| FST | forced swimming test | SERM | selective estrogen receptor modulator |
| GGT | gamma-glutamyl transferase | SOD | superoxide dismutase |
| GPR109A | G-protein-coupled receptor 109A | Sp | separation |
| GPx | glutathione peroxidase | SPT | sucrose preference test |
| GRAS | Generally Recognized as Safe | SSRIs | selective serotonin reuptake inhibitors |
| GSH | total glutathione | Tb | trabecular |
| GSH-Px | glutathione peroxidase | t-CA | trans-Cinnamic acid |
| GSSG | oxidized glutathione | tCA-GA | trans-Cinnamic acid and synthesized its conjugates with glutamic acid |
| H&E | hematoxylin and eosin | T-Ch | total cholesterol |
| HDAC | histone deacetylase | TEM | transmission electron microscop |
| HFD | high-fat diet | TG | triglycerides |
| IBD | Inflammatory bowel disease | TGF-β | Transforming growth factor-beta |
| IC30 | Inhibitory Concentration for 30% effect | Th | thickness |
| IC50 | half maximal inhibitory concentration | TKIs | Tyrosine kinase inhibitors |
| IL-10 | Interleukin-10 | TLR4 | Toll-like receptor 4 |
| IL-12 | Interleukin-12 | TMD | tissue mineral density |
| IL-16 | Interleukin-16 | TNF-α | Tumor necrosis factor alpha |
| IL-18 | Interleukin-18 | TSA | trichostatin A |
| IL-1β | Interleukin-1 beta | TST | tail suspension test |
| IL-23 | Interleukin-23 | TTC | 2,3,5 Triphenyltetrazolium chloride |
| IL-6 | Interleukin-6 | UC | Ulcerative colitis |
| iNOS | Inducible Nitric Oxide Synthase | UVB | ultraviolet radiation b |
| JNK | c-Jun N-terminal kinase | VEGF | Vascular endothelial growth factor |
| β-MHC | beta-myosin heavy chain |
References
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis. N. Engl. J. Med. 2023, 388, 1919–1920. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Hao, E.; Qin, J.; Wei, J.; Jiao, Y.; Yi, X.; Huang, L.; Xie, J.; Luo, H.; Zhang, Z.; et al. Prediction and analysis of chemical composition, pharmacological action and quality marker (Q-marker) of cinnamon. Chin. Herb. Med. 2018, 49, 20–34. [Google Scholar]
- Feng, L.S.; Cheng, J.B.; Su, W.Q.; Li, H.Z.; Xiao, T.; Chen, D.A.; Zhang, Z.L. Cinnamic acid hybrids as anticancer agents: A mini-review. Arch. Pharm. 2022, 355, e2200052. [Google Scholar] [CrossRef]
- Hussein, M.A.; Abdulrazzaq, M.H. The Protective Effect of Cinnamic Acid against Ulcerative Colitis in Mice. Int. J. Drug Deliv. Technol. 2023, 13, 143–149. [Google Scholar] [CrossRef]
- Huang, Z.; Pang, D.; Liao, S.; Zou, Y.; Zhou, P.; Li, E.; Wang, W. Synergistic effects of cinnamaldehyde and cinnamic acid in cinnamon essential oil against S. pullorum. Ind. Crops Prod. 2021, 162, 113296. [Google Scholar] [CrossRef]
- Anlar, H.G.; Bacanlı, M.; Çal, T.; Aydın, S.; Arı, N.; Ündeğer Bucurgat, Ü.; Başaran, A.A.; Başaran, A.N. Effects of cinnamic acid on complications of diabetes. Turk. J. Med. Sci. 2018, 48, 168–177. [Google Scholar] [CrossRef]
- Olawale, H.O.; Aniekeme, E.E.; Arthur, J.S.; Taiwo, E.A. Evaluation of Antioxidant Activity of Cinnamic Acid and Some of its Derivatives. Eur. Chem. Bull. 2019, 8, 224–226. [Google Scholar] [CrossRef]
- Meirelles, L.E.F.; Souza, M.V.F.; Carobeli, L.R.; Morelli, F.; Mari, N.L.; Damke, E.; Shinobu Mesquita, C.S.; Teixeira, J.J.V.; Consolaro, M.E.L.; Silva, V. Combination of Conventional Drugs with Biocompounds Derived from Cinnamic Acid: A Promising Option for Breast Cancer Therapy. Biomedicines 2023, 11, 275. [Google Scholar] [CrossRef]
- Zhu, B.; Shang, B.; Li, Y.; Zhen, Y. Inhibition of histone deacetylases by trans-cinnamic acid and its antitumor effect against colon cancer xenografts in athymic mice. Mol. Med. Rep. 2016, 13, 4159–4166. [Google Scholar] [CrossRef]
- Yen, G.-C.; Chen, Y.-L.; Sun, F.-M.; Chiang, Y.-L.; Lu, S.-H.; Weng, C.-J. A comparative study on the effectiveness of cis- and trans-form of cinnamic acid treatments for inhibiting invasive activity of human lung adenocarcinoma cells. Eur. J. Pharm. Sci. 2011, 44, 281–287. [Google Scholar] [CrossRef]
- Imai, M.; Yokoe, H.; Tsubuki, M.; Takahashi, N. Growth Inhibition of Human Breast and Prostate Cancer Cells by Cinnamic Acid Derivatives and Their Mechanism of Action. Biol. Pharm. Bull. 2019, 42, 1134–1139. [Google Scholar] [CrossRef] [PubMed]
- Yenigül, M.; Akçok, İ.; Gencer Akçok, E.B. Ethacrynic acid and cinnamic acid combination exhibits selective anticancer effects on K562 chronic myeloid leukemia cells. Mol. Biol. Rep. 2022, 49, 7521–7530. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Amaliyah, N.; Sarjono, P.R.; Ngadiwiyana, N.; Ismiyarto, I. Antibacterial Activity of Cinnamic Acid—Chitosan Encapsulation. J. Kim. Sains Apl. 2018, 21, 8–12. [Google Scholar] [CrossRef]
- He, X.; Wang, L.; Xia, B.; Cao, X.; Hu, N.; Huang, J.; Yi, Y. Antifungal effect of cinnamic acid and induced resistance of cinnamic acid-protocatechuic acid-CaCl2-NaCl-pullulan composite preservative to Trichoderma harzianum in postharvest Hypsizygus marmoreus. LWT 2023, 184, 11. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, J.; Guo, H.; Niu, C.; Yuan, Y.; Yue, T. Phenotypic and Transcriptomic Analyses Reveal the Cell Membrane Damage of Pseudomonas fragi Induced by Cinnamic Acid. Front. Microbiol. 2021, 12, 796754. [Google Scholar] [CrossRef] [PubMed]
- De Vita, D.; Simonetti, G.; Pandolfi, F.; Costi, R.; Di Santo, R.; D’Auria, F.D.; Scipione, L. Exploring the anti-biofilm activity of cinnamic acid derivatives in Candida albicans. Bioorganic Med. Chem. Lett. 2016, 26, 5931–5935. [Google Scholar] [CrossRef]
- Li, C. Hormone Metabolism and Signaling in Plants; Academic Press: Cambridge, MA, USA, 2017. [Google Scholar]
- Ruwizhi, N.; Aderibigbe, B.A. Cinnamic Acid Derivatives and Their Biological Efficacy. Int. J. Mol. Sci. 2020, 21, 5712. [Google Scholar] [CrossRef]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis essential oil: A review of its phytochemistry, anti-inflammatory activity, and mechanisms of action involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Boyunegmez Tumer, T.; Ozleyen, A.; Rodríguez-Pérez, C.; Ezzat, S.M.; Azzini, E.; Hosseinabadi, T.; Butnariu, M.; Sarac, I.; et al. Symphytum Species: A Comprehensive Review on Chemical Composition, Food Applications and Phytopharmacology. Molecules 2019, 24, 2272. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Yu, Y.; Zhu, Y.; Tong, R. A Mini-Review of Diagnostic and Therapeutic Nano-Tools for Pancreatitis. Int. J. Nanomed. 2022, 17, 4367–4381. [Google Scholar] [CrossRef]
- Ammer-Herrmenau, C.; Asendorf, T.; Beyer, G.; Buchholz, S.M.; Cameron, S.; Damm, M.; Frost, F.; Henker, R.; Jaster, R.; Phillip, V.; et al. Study protocol P-MAPS: Microbiome as predictor of severity in acute pancreatitis-a prospective multicentre translational study. BMC Gastroenterol. 2021, 21, 304. [Google Scholar] [CrossRef]
- Wydmanski, J.; Polanowski, P.; Tukiendorf, A.; Maslyk, B. Radiation-induced injury of the exocrine pancreas after chemoradiotherapy for gastric cancer. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2016, 118, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Abozaid, O.A.R.; Moawed, F.S.M.; Ahmed, E.S.A.; Ibrahim, Z.A. Cinnamic acid nanoparticles modulate redox signal and inflammatory response in gamma irradiated rats suffering from acute pancreatitis. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165904. [Google Scholar] [CrossRef] [PubMed]
- Heneghan, M.A.; Mcfarlane, I.G. Current and novel immunosuppressive therapy for autoimmune hepatitis (p7–13). Hepatology 2010, 35, 7–13. [Google Scholar] [CrossRef]
- Stan, S.D.; Singh, S.V.; Brand, R.E. Chemoprevention strategies for pancreatic cancer. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 347–356. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; Moawed, F.S.M.; Moustafa, E.M. Suppression of inflammatory cascades via novel cinnamic acid nanoparticles in acute hepatitis rat model. Arch. Biochem. Biophys. 2020, 696, 108658. [Google Scholar] [CrossRef]
- Liu, T.C.; Stappenbeck, T.S. Genetics and Pathogenesis of Inflammatory Bowel Disease. Annu. Rev. Pathol. 2016, 11, 127–148. [Google Scholar] [CrossRef] [PubMed]
- Löfberg, R. Review article: Medical treatment of mild to moderately active Crohn’s disease. Aliment. Pharmacol. Ther. 2003, 17 (Suppl. S2), 18–22. [Google Scholar] [CrossRef]
- Blander, J.M. A new approach for inflammatory bowel disease therapy. Nat. Med. 2019, 25, 545–546. [Google Scholar] [CrossRef]
- Randall, C.W.; Vizuete, J.A.; Martinez, N.; Alvarez, J.J.; Garapati, K.V.; Malakouti, M.; Taboada, C.M. From historical perspectives to modern therapy: A review of current and future biological treatments for Crohn’s disease. Ther. Adv. Gastroenterol. 2015, 8, 143–159. [Google Scholar] [CrossRef]
- Crowe, J.S.; Roberts, K.J.; Carlton, T.M.; Maggiore, L.; Cubitt, M.F.; Clare, S.; Harcourt, K.; Reckless, J.; MacDonald, T.T.; Ray, K.P.; et al. Preclinical Development of a Novel, Orally-Administered Anti-Tumour Necrosis Factor Domain Antibody for the Treatment of Inflammatory Bowel Disease. Sci. Rep. 2018, 8, 4941. [Google Scholar] [CrossRef]
- Ekin, S.; Yildiz, H.; Alp, H.H. NOX4, MDA, IMA and oxidative DNA damage: Can these parameters be used to estimate the presence and severity of OSA? Sleep. Breath.=Schlaf Atm. 2021, 25, 529–536. [Google Scholar] [CrossRef]
- Kang, C.; Kim, J.; Ju, S.; Cho, H.; Kim, H.Y.; Yoon, I.S.; Yoo, J.W.; Jung, Y. Colon-Targeted Trans-Cinnamic Acid Ameliorates Rat Colitis by Activating GPR109A. Pharmaceutics 2022, 15, 41. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, M.F.; Garcia-Carbonell, R.; Whisenant, K.D.; Guma, M. Fibroblast-like synoviocyte metabolism in the pathogenesis of rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 110. [Google Scholar] [CrossRef]
- Tak, P.P.; Kalden, J.R. Advances in rheumatology: New targeted therapeutics. Arthritis Res. Ther. 2011, 13 (Suppl. S1), S5. [Google Scholar] [CrossRef] [PubMed]
- Burmester, G.R.; Pope, J.E. Novel treatment strategies in rheumatoid arthritis. Lancet 2017, 389, 2338–2348. [Google Scholar] [CrossRef]
- Li, W.; Wang, K.; Liu, Y.; Wu, H.; He, Y.; Li, C.; Wang, Q.; Su, X.; Yan, S.; Su, W.; et al. A Novel Drug Combination of Mangiferin and Cinnamic Acid Alleviates Rheumatoid Arthritis by Inhibiting TLR4/NFκB/NLRP3 Activation-Induced Pyroptosis. Front. Immunol. 2022, 13, 912933. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Chavakis, T. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat. Rev. Immunol. 2021, 21, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.D.; Xia-Juan, X.; Crump, K.E.; Abe, T.; Hajishengallis, G.; Sahingur, S.E. Toll-Like Receptor 9-Mediated Inflammation Triggers Alveolar Bone Loss in Experimental Murine Periodontitis. Infect. Immun. 2015, 83, 2992–3002. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque-induced gingival conditions. J. Periodontol. 2018, 89 (Suppl. S1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Zou, T.; Jiang, X.; Lin, X.; Cai, W. Cinnamic Acid Reduces Inflammation and Apoptosis in Necrotizing Enterocolitis. Curr. Top. Nutraceutical Res. 2022, 20, 70–75. [Google Scholar] [CrossRef]
- Karatas, O.; Yuce, H.B.; Taskan, M.M.; Gevrek, F.; Temiz, C. Cinnamic acid decreases periodontal inflammation and alveolar bone loss in experimental periodontitis. J. Periodontal Res. 2020, 55, 676–685. [Google Scholar] [CrossRef]
- Yilmaz, S.; Sova, M.; Ergün, S. Antimicrobial activity of trans-cinnamic acid and commonly used antibiotics against important fish pathogens and nonpathogenic isolates. J. Appl. Microbiol. 2018, 125, 1714–1727. [Google Scholar] [CrossRef]
- Lucas-González, R.; Yilmaz, B.; Mousavi Khaneghah, A.; Hano, C.; Shariati, M.A.; Bangar, S.P.; Goksen, G.; Dhama, K.; Lorenzo, J.M. Cinnamon: An antimicrobial ingredient for active packaging. Food Packag. Shelf Life 2023, 35, 101026. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Wong Fok Lung, T.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus host interactions and adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- Viçosa, G.N.; Botta, C.; Ferrocino, I.; Bertolino, M.; Ventura, M.; Nero, L.A.; Cocolin, L. Staphylococcus aureus undergoes major transcriptional reorganization during growth with Enterococcus faecalis in milk. Food Microbiol. 2018, 73, 17–28. [Google Scholar] [CrossRef]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef]
- Tang, Z.; Liu, M.; Zhao, Y. Research Activities in Advanced Materials at National Center for Nanoscience and Technology of China. Adv. Mater. 2019, 31, e1901327. [Google Scholar] [CrossRef]
- Huang, X.; Wang, P.; Li, T.; Tian, X.; Guo, W.; Xu, B.; Huang, G.; Cai, D.; Zhou, F.; Zhang, H.; et al. Self-Assemblies Based on Traditional Medicine Berberine and Cinnamic Acid for Adhesion-Induced Inhibition Multidrug-Resistant Staphylococcus aureus. ACS Appl. Mater. Interfaces 2020, 12, 227–237. [Google Scholar] [CrossRef]
- Vishwakarma, A.; Dang, F.; Ferrell, A.; Barton, H.A.; Joy, A. Peptidomimetic Polyurethanes Inhibit Bacterial Biofilm Formation and Disrupt Surface Established Biofilms. J. Am. Chem. Soc. 2021, 143, 9440–9449. [Google Scholar] [CrossRef]
- Azam, M.W.; Khan, A.U. Updates on the pathogenicity status of Pseudomonas aeruginosa. Drug Discov. Today 2019, 24, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2022 Guidance on the Treatment of Extended-Spectrum β-lactamase Producing Enterobacterales (ESBL-E), Carbapenem-Resistant Enterobacterales (CRE), and Pseudomonas aeruginosa with Difficult-to-Treat Resistance (DTR-P. aeruginosa). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2022, 75, 187–212. [Google Scholar] [CrossRef]
- Fatemeh, F.; Kambiz, D.; Jafar, K.; Shohreh Afshar, Y. Silver resistance in Pseudomonas aeruginosa and Acinetobacter baumannii isolated from burn patients. Immunopathol. Persa 2023, 9, 39498. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet. Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Rahim, K.; Saleha, S.; Zhu, X.; Huo, L.; Basit, A.; Franco, O.L. Bacterial Contribution in Chronicity of Wounds. Microb. Ecol. 2017, 73, 710–721. [Google Scholar] [CrossRef]
- Rajkumari, J.; Borkotoky, S.; Murali, A.; Suchiang, K.; Mohanty, S.K.; Busi, S. Cinnamic acid attenuates quorum sensing associated virulence factors and biofilm formation in Pseudomonas aeruginosa PAO1. Biotechnol. Lett. 2018, 40, 1087–1100. [Google Scholar] [CrossRef]
- Leitão, M.M.; Gonçalves, A.S.C.; Sousa, S.F.; Borges, F.; Simões, M.; Borges, A. Two cinnamic acid derivatives as inhibitors of Pseudomonas aeruginosa las and pqs quorum-sensing systems: Impact on biofilm formation and virulence factors. Biomed. Pharmacother.=Biomed. Pharmacother. 2025, 187, 118090. [Google Scholar] [CrossRef]
- Ercolini, D.; Casaburi, A.; Nasi, A.; Ferrocino, I.; Di Monaco, R.; Ferranti, P.; Mauriello, G.; Villani, F. Different molecular types of Pseudomonas fragi have the same overall behaviour as meat spoilers. Int. J. Food Microbiol. 2010, 142, 120–131. [Google Scholar] [CrossRef]
- Damdam, A.N.; Alzahrani, A.; Salah, L.; Salama, K.N. Effects of UV-C Irradiation and Vacuum Sealing on the Shelf-Life of Beef, Chicken and Salmon Fillets. Foods 2023, 12, 606. [Google Scholar] [CrossRef]
- Quintieri, L.; Fanelli, F.; Zühlke, D.; Caputo, L.; Logrieco, A.F.; Albrecht, D.; Riedel, K. Biofilm and Pathogenesis-Related Proteins in the Foodborne P. fluorescens ITEM 17298 with Distinctive Phenotypes During Cold Storage. Front. Microbiol. 2020, 11, 991. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Gao, Z.; Li, G.; Fu, F.; Shan, Y. Antimicrobial and antibiofilm efficacy and mechanism of essential oil from Citrus Changshan-huyou Y. B. chang against Listeria monocytogenes. Food Control 2019, 105, 256–264. [Google Scholar] [CrossRef]
- Niu, J.; Shang, M.; Li, X.; Sang, S.; Chen, L.; Long, J.; Jiao, A.; Ji, H.; Jin, Z.; Qiu, C. Health benefits, mechanisms of interaction with food components, and delivery of tea polyphenols: A review. Crit. Rev. Food Sci. Nutr. 2024, 64, 12487–12499. [Google Scholar] [CrossRef]
- Salci, T.P.; Negri, M.; Abadio, A.K.R.; Svidzinski, T.I.E.; Kioshima, É.S. Targeting Candida spp. To develop antifungal agents. Drug Discov. Today 2018, 23, 802–814. [Google Scholar] [CrossRef]
- Danion, F.; Duréault, A.; Gautier, C.; Senechal, A.; Persat, F.; Bougnoux, M.E.; Givel, C.; Couderc, L.J.; Lortholary, O.; Garcia-Hermoso, D.; et al. Emergence of azole resistant-Aspergillus fumigatus infections during STAT3-deficiency. J. Med. Microbiol. 2020, 69, 844–849. [Google Scholar] [CrossRef]
- Lima, T.C.; Ferreira, A.R.; Silva, D.F.; Lima, E.O.; de Sousa, D.P. Antifungal activity of cinnamic acid and benzoic acid esters against Candida albicans strains. Nat. Prod. Res. 2018, 32, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chan, K.L.; Cheng, L.W. Cinnamic Acid Analogs as Intervention Catalysts for Overcoming Antifungal Tolerance. Molecules 2017, 22, 1783. [Google Scholar] [CrossRef] [PubMed]
- Lovly, C.M. Expanding Horizons for Treatment of Early-Stage Lung Cancer. N. Engl. J. Med. 2022, 386, 2050–2051. [Google Scholar] [CrossRef]
- Coussens, N.P.; Braisted, J.C.; Peryea, T.; Sittampalam, G.S.; Simeonov, A.; Hall, M.D. Small-Molecule Screens: A Gateway to Cancer Therapeutic Agents with Case Studies of Food and Drug Administration-Approved Drugs. Pharmacol. Rev. 2017, 69, 479–496. [Google Scholar] [CrossRef]
- Garraway, L.A.; Jänne, P.A. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012, 2, 214–226. [Google Scholar] [CrossRef]
- Giaquinto, A.N.; Sung, H.; Newman, L.A.; Freedman, R.A.; Smith, R.A.; Star, J.; Jemal, A.; Siegel, R.L. Breast cancer statistics 2024. CA Cancer J. Clin. 2024, 74, 477–495. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Davidson, N.E.; Kensler, T.W. “MAPping” the course of chemoprevention in breast cancer. N. Engl. J. Med. 2011, 364, 2463–2464. [Google Scholar] [CrossRef] [PubMed]
- Motawi, T.K.; Abdelazim, S.A.; Darwish, H.A.; Elbaz, E.M.; Shouman, S.A. Could Caffeic Acid Phenethyl Ester Expand the Antitumor Effect of Tamoxifen in Breast Carcinoma? Nutr. Cancer 2016, 68, 435–445. [Google Scholar] [CrossRef]
- Islam, M.R.; Akash, S.; Rahman, M.M.; Nowrin, F.T.; Akter, T.; Shohag, S.; Rauf, A.; Aljohani, A.S.M.; Simal-Gandara, J. Colon cancer and colorectal cancer: Prevention and treatment by potential natural products. Chem.-Biol. Interact. 2022, 368, 110170. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N.; et al. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Wang, L.T.; Liou, J.P.; Li, Y.H.; Liu, Y.M.; Pan, S.L.; Teng, C.M. A novel class I HDAC inhibitor, MPT0G030, induces cell apoptosis and differentiation in human colorectal cancer cells via HDAC1/PKCδ and E-cadherin. Oncotarget 2014, 5, 5651–5662. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Z.; McLeod, H.L.; He, F.Z.; Chen, X.P.; Zhou, H.H.; Shu, Y.; Zhang, W. Epigenetic perspectives on cancer chemotherapy response. Pharmacogenomics 2014, 15, 699–715. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Shan, G.; Yang, H.; Gu, J.; Lu, C.; Xu, F.; Ge, D. Development of a ferroptosis-based model to predict prognosis, tumor microenvironment, and drug response for lung adenocarcinoma with weighted genes co-expression network analysis. Front. Pharmacol. 2022, 13, 1072589. [Google Scholar] [CrossRef]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef]
- Zhao, J.; Guo, C.; Ma, Z.; Liu, H.; Yang, C.; Li, S. Identification of a novel gene expression signature associated with overall survival in patients with lung adenocarcinoma: A comprehensive analysis based on TCGA and GEO databases. Lung Cancer 2020, 149, 90–96. [Google Scholar] [CrossRef]
- Wu, P.; Zheng, Y.; Wang, Y.; Wang, Y.; Liang, N. Development and validation of a robust immune-related prognostic signature in early-stage lung adenocarcinoma. J. Transl. Med. 2020, 18, 380. [Google Scholar] [CrossRef]
- Isoda, K.; Nozawa, T.; Taira, Y.; Taira, I.; Shimizu, Y.; Ishida, I. Effects of surface charge and palladium on hepatic and kidney injury induced by polystyrene nanoparticles co-administered to mice with paraquat and cisplatin. Die Pharm. 2018, 73, 165–168. [Google Scholar] [CrossRef]
- Kilic, K.; Sakat, M.S.; Akdemir, F.N.E.; Yildirim, S.; Saglam, Y.S.; Askin, S. Protective effect of gallic acid against cisplatin-induced ototoxicity in rats. Braz. J. Otorhinolaryngol. 2019, 85, 267–274. [Google Scholar] [CrossRef]
- Fillon, M. Rates of advanced prostate cancer continue to increase. CA Cancer J. Clin. 2020, 70, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Mohile, S.G.; Mustian, K.; Bylow, K.; Hall, W.; Dale, W. Management of complications of androgen deprivation therapy in the older man. Crit. Rev. Oncol./Hematol. 2009, 70, 235–255. [Google Scholar] [CrossRef] [PubMed]
- Minciacchi, V.R.; Kumar, R.; Krause, D.S. Chronic Myeloid Leukemia: A Model Disease of the Past, Present and Future. Cells 2021, 10, 117. [Google Scholar] [CrossRef]
- Deininger, M.W.; Shah, N.P.; Altman, J.K.; Berman, E.; Bhatia, R.; Bhatnagar, B.; DeAngelo, D.J.; Gotlib, J.; Hobbs, G.; Maness, L.; et al. Chronic Myeloid Leukemia, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 1385–1415. [Google Scholar] [CrossRef] [PubMed]
- Shadman, M. Diagnosis and Treatment of Chronic Lymphocytic Leukemia: A Review. JAMA 2023, 329, 918–932. [Google Scholar] [CrossRef]
- The, L. Diabetes: An ounce of prevention is worth a pound of cure. Lancet 2015, 386, 932. [Google Scholar] [CrossRef]
- Armstrong, D.G.; Tan, T.W.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers: A Review. JAMA 2023, 330, 62–75. [Google Scholar] [CrossRef]
- Gray, S.P.; Jandeleit-Dahm, K. The pathobiology of diabetic vascular complications—Cardiovascular and kidney disease. J. Mol. Med. 2014, 92, 441–452. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Hameed, A.; Shukrana, M.; Raza, S.A.; Chishti, S.; Kabir, N.; Siddiqui, R.A. Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro. Phytomedicine Int. J. Phytother. Phytopharm. 2015, 22, 297–300. [Google Scholar] [CrossRef]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef]
- Nair, A.; Preetha Rani, M.R.; Salin Raj, P.; Ranjit, S.; Rajankutty, K.; Raghu, K.G. Cinnamic acid is beneficial to diabetic cardiomyopathy via its cardioprotective, anti-inflammatory, anti-dyslipidemia, and antidiabetic properties. J. Biochem. Mol. Toxicol. 2022, 36, e23215. [Google Scholar] [CrossRef] [PubMed]
- Thapar, A.; Eyre, O.; Patel, V.; Brent, D. Depression in young people. Lancet 2022, 400, 617–631. [Google Scholar] [CrossRef]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef]
- Ishtiak-Ahmed, K.; Musliner, K.L.; Christensen, K.S.; Mortensen, E.L.; Nierenberg, A.A.; Gasse, C. Real-World Evidence on Clinical Outcomes of Commonly Used Antidepressants in Older Adults Initiating Antidepressants for Depression: A Nationwide Cohort Study in Denmark. Am. J. Psychiatry 2024, 181, 47–56. [Google Scholar] [CrossRef]
- Fagiolini, A.; Florea, I.; Loft, H.; Christensen, M.C. Effectiveness of Vortioxetine on Emotional Blunting in Patients with Major Depressive Disorder with inadequate response to SSRI/SNRI treatment. J. Affect. Disord. 2021, 283, 472–479. [Google Scholar] [CrossRef]
- Zhuo, R.; Cheng, X.; Luo, L.; Yang, L.; Zhao, Y.; Zhou, Y.; Peng, L.; Jin, X.; Cui, L.; Liu, F.; et al. Cinnamic Acid Improved Lipopolysaccharide-Induced Depressive-Like Behaviors by Inhibiting Neuroinflammation and Oxidative Stress in Mice. Pharmacology 2022, 107, 281–289. [Google Scholar] [CrossRef]
- Subedi, L.; Gaire, B.P. Neuroprotective Effects of Curcumin in Cerebral Ischemia: Cellular and Molecular Mechanisms. ACS Chem. Neurosci. 2021, 12, 2562–2572. [Google Scholar] [CrossRef] [PubMed]
- Pozdnyakov, D.I.; Andrey, V. Correction of Mitochondrial Dysfunction by 4-Hydroxy-3,5-Ditretbutyl Cinnamic Acid in Experimental Alzheimer’s Disease Induced by Aβ Injection in Rats. Pharm. Sci. 2020, 27, 313–325. [Google Scholar] [CrossRef]
- Pozdnyakov, D.I. 4-Hydroxy-3,5-di-tret-butyl cinnamic acid restores the activity of the hippocampal mitochondria in rats under permanent focal cerebral ischemia. Iran. J. Basic Med. Sci. 2021, 24, 1590–1601. [Google Scholar] [CrossRef]
- Cheli, F.; Baldi, A. Nutrition-based health: Cell-based bioassays for food antioxidant activity evaluation. J. Food Sci. 2011, 76, R197–R205. [Google Scholar] [CrossRef]
- Anik, M.I.; Mahmud, N.; Masud, A.A.; Khan, M.I.; Islam, M.N.; Uddin, S.; Hossain, M.K. Role of Reactive Oxygen Species in Aging and Age-Related Diseases: A Review. ACS Appl. Bio Mater. 2022, 5, 4028–4054. [Google Scholar] [CrossRef]
- Babaeenezhad, E.; Nouryazdan, N.; Nasri, M.; Ahmadvand, H.; Moradi Sarabi, M. Cinnamic acid ameliorate gentamicin-induced liver dysfunctions and nephrotoxicity in rats through induction of antioxidant activities. Heliyon 2021, 7, e07465. [Google Scholar] [CrossRef]
- Aldaba-Muruato, L.R.; Escalante-Hipólito, B.; Alarcón-López, A.Y.; Martínez-Soriano, P.A.; Angeles, E.; Macías-Pérez, J.R. Preclinical Research on Cinnamic Acid Derivatives for the Prevention of Liver Damage: Promising Therapies for Liver Diseases. Biomedicines 2025, 13, 1094. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Mnafgui, K.; Derbali, A.; Sayadi, S.; Gharsallah, N.; Elfeki, A.; Allouche, N. Anti-obesity and cardioprotective effects of cinnamic acid in high fat diet-induced obese rats. J. Food Sci. Technol. 2015, 52, 4369–4377. [Google Scholar] [CrossRef]
- von Mutius, E.; Smits, H.H. Primary prevention of asthma: From risk and protective factors to targeted strategies for prevention. Lancet 2020, 396, 854–866. [Google Scholar] [CrossRef] [PubMed]
- Pakkasela, J.; Ilmarinen, P.; Honkamäki, J.; Tuomisto, L.E.; Andersén, H.; Piirilä, P.; Hisinger-Mölkänen, H.; Sovijärvi, A.; Backman, H.; Lundbäck, B.; et al. Age-specific incidence of allergic and non-allergic asthma. BMC Pulm. Med. 2020, 20, 9. [Google Scholar] [CrossRef]
- Pierangeli, I.; Nieuwenhuijsen, M.J.; Cirach, M.; Rojas-Rueda, D. Health equity and burden of childhood asthma—Related to air pollution in Barcelona. Environ. Res. 2020, 186, 109067. [Google Scholar] [CrossRef] [PubMed]
- Haidar, A.L.S.; Munaf, H.Z. The Possible Protective Effect of Cinnamic Acid on Ovalbumin-Induced Asthma in Mice. Iraqi J. Pharm. Sci. 2023, 32, 133–138. [Google Scholar] [CrossRef]
- Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.L.; Bories, G.; Cocconcelli, P.S.; Flachowsky, G.; Gropp, J.; Kolar, B.; et al. Safety and efficacy of aryl-substituted primary alcohol, aldehyde, acid, ester and acetal derivatives belonging to chemical group 22 when used as flavourings for all animal species. EFSA J. Eur. Food Saf. Auth. 2017, 15, e04672. [Google Scholar] [CrossRef]
- Olivieri, D.; Verboni, M.; Benedetti, S.; Paderni, D.; Carfagna, C.; Duranti, A.; Lucarini, S. New cinnamic acid sugar esters as potential UVB filters: Synthesis, cytotoxicity, and physicochemical properties. Carbohydr. Res. 2025, 550, 109405. [Google Scholar] [CrossRef]
- Kong, Y.H.; Jo, Y.O.; Cho, C.W.; Son, D.; Park, S.; Rho, J.; Choi, S.Y. Inhibitory effects of cinnamic acid on melanin biosynthesis in skin. Biol. Pharm. Bull. 2008, 31, 946–948. [Google Scholar] [CrossRef]
- Niero, E.L.; Machado-Santelli, G.M. Cinnamic acid induces apoptotic cell death and cytoskeleton disruption in human melanoma cells. J. Exp. Clin. Cancer Res. CR 2013, 32, 31. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Subhan, N.; Hossain, H.; Hossain, M.; Reza, H.M.; Rahman, M.M.; Ullah, M.O. Hydroxycinnamic acid derivatives: A potential class of natural compounds for the management of lipid metabolism and obesity. Nutr. Metab. 2016, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Zawiła, T.; Swolana, D.; Rok, J.; Rzepka, Z.; Wojtyczka, R.D. Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules 2025, 30, 660. [Google Scholar] [CrossRef] [PubMed]

| Compound | Molecular Weight | pKa | Notable Substituents/Configuration | Water Solubility | Key Properties |
|---|---|---|---|---|---|
| Cinnamic acid (CA) | 148.16 | ~4.44 | Basic scaffold: benzene ring + propenoic | 0.46–0.50 g/L (25 degrees) | Parent compound, moderate acidity, forms basis for multiple derivatives |
| Trans-cinnamic acid (t-CA) | 148.16 | ~4.44 | Trans config around C=C | ~0.47 g/L (25 degrees) | More stable vs. cis isomer, reported HDAC inhibition, anticancer activities |
| Caffeic acid phenethyl ester (CAPE) | 284.29 | ~4.25 | Additional –OH groups on ring + phenethyl group | Very low (~0.1 mg/mL) | Potent antioxidant, anti-inflammatory, anti-tumor; poor water solubility |
| 3,4,5-trihydroxycinnamic acid decyl ester | 338.37 | ~4.0 | 3 –OH groups on benzene + decyl ester | Very low | Enhanced lipophilicity, significant cytotoxic effects in some cancer cell lines |
| p-Coumaric acid (4-hydroxycinnamic acid) | 164.16 | ~4.34 | Hydroxy at para-position | 2.5 g/L (25 degrees) | Greater hydrophilicity vs. cinnamic acid, antioxidant and anti-inflammatory activities |
| Derivative | Key Structural Motif | Main Molecular Targets | Reported Clinical/Preclinical Significance |
|---|---|---|---|
| Caffeic acid phenethyl ester (CAPE) | 2 phenolic –OH + phenethyl tail | NF-κB, VEGF, COX-2 | Synergizes with tamoxifen in ER+ breast cancer; anti-inflammatory |
| Trans-cinnamic acid (t-CA) | Trans-C=C | HDAC I/II, MMP-2/-9 | HDAC-dependent apoptosis in CRC; anti-invasive in NSCLC |
| Cis-cinnamic acid (c-CA) | Cis-C=C | MMP-2/-9 | Co-inhibits invasion with t-CA in NSCLC |
| 3,4,5-Trihydroxy-cinnamic acid decyl ester | Tri-OH ring + decyl ester | Caspase-3, Bax/Bcl-2 | Potent apoptosis in breast and prostate cancer cells |
| Methyl caffeate | Methoxy + catechol | Ergosterol biosynthesis (fungi) | Inhibits Candida albicans growth |
| Methyl 2-nitrocinnamate | Nitro substitution | Ergosterol biosynthesis | Synergistic antifungal against C. albicans |
| p-Coumaric acid | Para–OH | NF-κB, MAPK | Antioxidant/anti-inflammatory prototype |
| Pharmacological Effects | Aimed Subtype | Main Targets | Potential Mechanisms | Reference |
|---|---|---|---|---|
| Anti-breast cancer | Breast cancer | MCF-7 cells | Inhibition of cell growth, cell cycle arrest, apoptosis induction via caspase-3 activation, downregulation of Bcl-2 mRNA | Masahiko Imai et al. [11], Motawi et al. [79] |
| Anti-colorectal cancer | Colorectal cancer (CRC) | HT29, MIA PaCa-2 cells | HDAC inhibition, increased acetylated H3 and H4 proteins, apoptosis induction through Bax expression, reduced PARP and Bcl-2 | Bingyan Zhu [9] |
| Anti-lung cancer | Non-small cell lung cancer (NSCLC) | A549 cells | Inhibition of cell viability, reduction in MMP-2 and MMP-9 activities, decreased cell migration | Gow-Chin Yen [10] |
| Anti-prostate cancer | Prostate cancer | PC-3 cells | Inhibition of cell growth, cell cycle arrest, apoptosis induction via caspase-3 activation, decreased Bcl-2 mRNA | Masahiko Imai et al. [11] |
| Anti-chronic myeloid leukemia | Chronic myeloid leukemia (CML) | K562 cells | Cytotoxic effect, cell cycle arrest, apoptosis induction, synergistic effect with ethacrylic acid | Münevver Yenigül et al. [12] |
| Anti-acute pancreatitis | Acute pancreatitis | Rats with acute pancreatitis | Reduction of oxidative stress, inhibition of ERK1/2, JNK, and p38 in MAPK signaling pathways, downregulation of NF-κB and NLRP3, reduced apoptosis via caspase activation | Omayma AR Abozaid [25] |
| Anti-acute hepatitis | Acute hepatitis | Rats with acute hepatitis | Reduction of serum ALT, AST, and ALP activities, decrease in TNF-α, IL-1β, and IL-18 levels, inhibition of TLR4 and MyD88, reduced NLRP3 and NF-κB, decreased apoptosis | Ehab A. Ibrahim et al. [28] |
| Anti-colitis | Ulcerative colitis, colitis | Mice with DSS-induced colitis, rats with DNBS-induced colitis | Reduction in MDA levels, increase in SOD and CAT levels, activation of GPR109A, reduction in MPO activity, inflammatory mediators, and IL-10 levels | Maysam A Hussein et al. [4], Changyu Kang et al. [35] |
| Anti-rheumatoid arthritis | Rheumatoid arthritis | AIA-M rat models | Inhibition of TLR4/PI3K/AKT/NFkB/NLRP3 pathway, reduction in inflammatory cytokines TNF-a, IL-6, IL-12, decreased caspase-1 expression, reduced IL-1β release | Weijie Li [41] |
| Anti-periodontitis | Periodontitis | Wistar rats with ligation-induced periodontitis | Decrease in RANKL expression, inflammation, osteoclast count, increase in OPG expression, osteoblast count, reduction in PPAR-γ and COX-2 levels | Ozkan Karatas [46] |
| Antibacterial effect | Staphylococcus aureus | MRSA | Inhibition of bacterial growth, biofilm formation, cell membrane damage, and nutrient intake; enhanced antibacterial activity with CA-BBR NPs | Huang Xuemei et al. [53] |
| Antibacterial effect | Pseudomonas aeruginosa | PAO1 strain | Reduction in exovirulence factor production, biofilm formation, cell surface hydrophobicity, exogenous DNA, and EPS; decreased biofilm thickness | Rajkumari et al. [61] |
| Antibacterial effect | Foodborne Pseudomonas | Psychrophilic Pseudomonas | Disruption of cell membrane homeostasis, reduced ATPase activity, cell membrane depolarization, decreased intracellular pH, increased bacterial mortality | Yuxiang Zhang et al. [16] |
| Anti-diabetic | Type II diabetes | Diabetic rats | Reduction in blood glucose levels, improved glucose tolerance, enhanced insulin secretion, reduced oxidative stress, decreased liver enzyme levels | Rahman M Hafizur et al. [100], Hatice Gül Anlar et al. [6], Anupama Nair et al. [102] |
| Anti-depressant | Depression | LPS-induced mice | Reduction in depressive-like behaviors, dampening pro-inflammatory responses, enhancing oxidative stress parameters, reversing decrease in BDNF levels | Rengong Zhuo et al. [107] |
| Antioxidant | Nephrotoxicity | Rats | Improved oxidative stress, reduced transaminase activity | Esmaeel Babaeenezhad et al. [113] |
| Anti-obesity | Obesity | HFD-fed rats | Normalization of blood lipid levels, reduced lipase, and ACE activities enhanced aortic diameter, prevention of vasoconstriction, amelioration of hepatic steatosis | Kais Mnafgui et al. [116] |
| Anti-asthma | Asthma | Asthma patients | Reduction in leukocyte infiltration, suppression of pro-inflammatory cytokine production, mitigation of inflammatory response and tissue damage in the lungs | Haidar AL-Saffar et al. [120] |
| Chemical Compound | Model | Duration | Dose | Reference |
|---|---|---|---|---|
| Cinnamic acid nanoparticles (CA-NPs) | L-arginine- and gamma ray-induced acute pancreatitis rat model | 21 d | 60 mg/kg | Omayma AR Abozaid’s [25] |
| D-galactosamine and gamma radiation-induced acute hepatitis rat model | 30 d | 60 mg/kg | Ehab A. Ibrahim et al. [28] | |
| Cinnamic acid (CA) | Mouse model of ulcerative colitis induced by dextran sodium sulfate | 7 d | 25~50 mg/kg | Maysam A Hussein et al. [4] |
| Induction of periodontitis rat model by ligating the first mandibular tooth around the left and right mandibles with 4-0 silk thread | 30 d | 7 mg/kg | Ozkan Karatas [46] | |
| STZ-induced non-obese type II diabetes mellitus rat model | 60 min | 5~10 mg/kg | Rahman M Hafizur et al. [100] | |
| Rat model of type 1 diabetes induced by streptozotocin (STZ) | 28 d | 50 mg/kg | Hatice Gül Anlar et al. [6] | |
| High-fat high-fructose and streptozotocin-induced diabetes rat model | 60 d | 5~10 mg/kg | Anupama Nair et al. [102] | |
| LPS-induced depression mouse model | 14 d | 100~200 mg/kg | Rengong Zhuo et al. [107] | |
| Rats on a high-fat diet | 7 weeks | 30 mg/kg | Kais Mnafgui et al. [116] | |
| trans-Cinnamic acid (t-CA) | 2,4-dinitrobenzenesulfonic acid (DNBS) induced colitis rat model | 6 d | 15~30 mg/kg | Changyu Kang et al. [35] |
| Cinnamic acid and mangiferin | AIA-M rat models | 30 d | 46.652 mg/kg cinnamic acid 600.912 mg/kg mangiferin | Weijie Li [41] |
| Synthesized cinnamic acid and berberine into organic nanostructures (CA-BBR NPs) | Zebrafish larvae | 72 h | 2.5~80 μM | Huang Xuemei et al. [53] |
| 4-Hydroxy-3,5-di-tret-butyl cinnamic acid | Brain ischemia in rats | 3 d | 25~100 mg/kg | Dmitry I Pozdnyakov et al. [110] |
| Aβ1-42-induced AD rat model. | 60 d | 100 mg/kg | Dmitry I Pozdnyakov et al. [109] | |
| 3-(4-Phenoxy)phenyl-N-[(3,4-dichlorophenyl)methyl]prop-2-enamide | CCl4-induced acute liver injury rat model. | 2 d | 20 mg/kg | Liseth Rubí Aldaba-Muruato et al. [114] |
| Chemical Compound | Model | Duration | Dose | Reference |
| Synthesized cinnamic acid and berberine into organic nanostructures (CA-BBR NPs) | Multidrug-resistant S. aureus Madin–Darby canine kidney (MDCK) cells | 16 h 24–48 h | 0.0325~0.2 μmol/mL 1.5625~50 μM | Huang Xuemei et al. [53] |
| Cinnamic acid (CA) | Strain, P. aeruginosa PAO1 | 24 h | 100~500 μg/mL | Rajkumari et al. [61] |
| Pseudomonas fragi 38-8 | 30 min | 0~1 mg/mL | Yuxiang Zhang et al. [16] | |
| Ferulic acid and caprylic acid | Wild-type P. aeruginosa PA14 Wild-type P. aeruginosa PAO1 Biosensor strain P. aeruginosa PAO1 pqsA CTXluxHpqsA Reporter strain PA14-R3 developed by Massai et al. | 0–24 h | 6.25~1000 µg mL−1 | Ariana S.C. Gonçalves et al. [62] |
| Methyl caffeate and methyl 2-nitro cinnamate | Candida albicans ATCC-76645, LM-106, LM-23 | 128~256 μg/mL | Tamires C. Lima et al. [70] | |
| Chloro-α-methylcinnamic acid 4-methylcinnamic acid | Aspergillus fumigatus | 5~7 d | 0.1~1.0 mM | Jong H. Kim et al. [71] |
| 3,4,5-trihydroxycinnamate decyl ester | MCF-7 breast cancer cells | 72 h | 0.4~20 µM | Masahiko Imai et al. [11] |
| Prostate cancer PC-3 cells | ||||
| Caffeic acid phenethyl ester (CAPE) | MCF-7 breast cancer cells | 48 h | 0.1~200 mM | Motawi et al. [79] |
| trans-Cinnamic acid (t-CA) | HT-29 human colon cancer cells MIA PaCa-2 human pancreatic cancer cells | 48 h | 0.09~2.72 mM | Bingyan Zhu [9] |
| cis-Cinnamic acid (c-CA) and trans-cinnamic acid (t-CA) | A549 human lung adenocarcinoma cells | 24–48 h | 0~200 μM | Gow-Chin Yen [10] |
| Ethacrylic acid (EA) and cinnamic acid (CA) | K562 chronic myeloid leukemia cells | 48 h | 50~500 µM | Münevver Yenigül et al. [12] |
| Compound | Clinical Condition | Dose | Duration | Clinical Outcome/Effects | Reference |
|---|---|---|---|---|---|
| Cinnamic acid | Asthma patients | 200 mg/day | 4 weeks | Improved pulmonary function, reduced inflammatory markers | Haidar AL-Saffar et al. [120] |
| CAPE | Breast cancer patients | 20 mg/day (combined with Tamoxifen) | 3 months | Enhanced apoptotic activity, reduced angiogenesis markers (VEGF), potential risk of drug interaction noted | Motawi et al. [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Jiang, X.; Guo, J.; Lu, H.; Xie, J.; Zhang, F.; Yao, C.; Hao, E. Pharmacological Potential of Cinnamic Acid and Derivatives: A Comprehensive Review. Pharmaceuticals 2025, 18, 1141. https://doi.org/10.3390/ph18081141
Tian Y, Jiang X, Guo J, Lu H, Xie J, Zhang F, Yao C, Hao E. Pharmacological Potential of Cinnamic Acid and Derivatives: A Comprehensive Review. Pharmaceuticals. 2025; 18(8):1141. https://doi.org/10.3390/ph18081141
Chicago/Turabian StyleTian, Yu, Xinya Jiang, Jiageng Guo, Hongyu Lu, Jinling Xie, Fan Zhang, Chun Yao, and Erwei Hao. 2025. "Pharmacological Potential of Cinnamic Acid and Derivatives: A Comprehensive Review" Pharmaceuticals 18, no. 8: 1141. https://doi.org/10.3390/ph18081141
APA StyleTian, Y., Jiang, X., Guo, J., Lu, H., Xie, J., Zhang, F., Yao, C., & Hao, E. (2025). Pharmacological Potential of Cinnamic Acid and Derivatives: A Comprehensive Review. Pharmaceuticals, 18(8), 1141. https://doi.org/10.3390/ph18081141






