Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model
Abstract
1. Introduction
2. Results
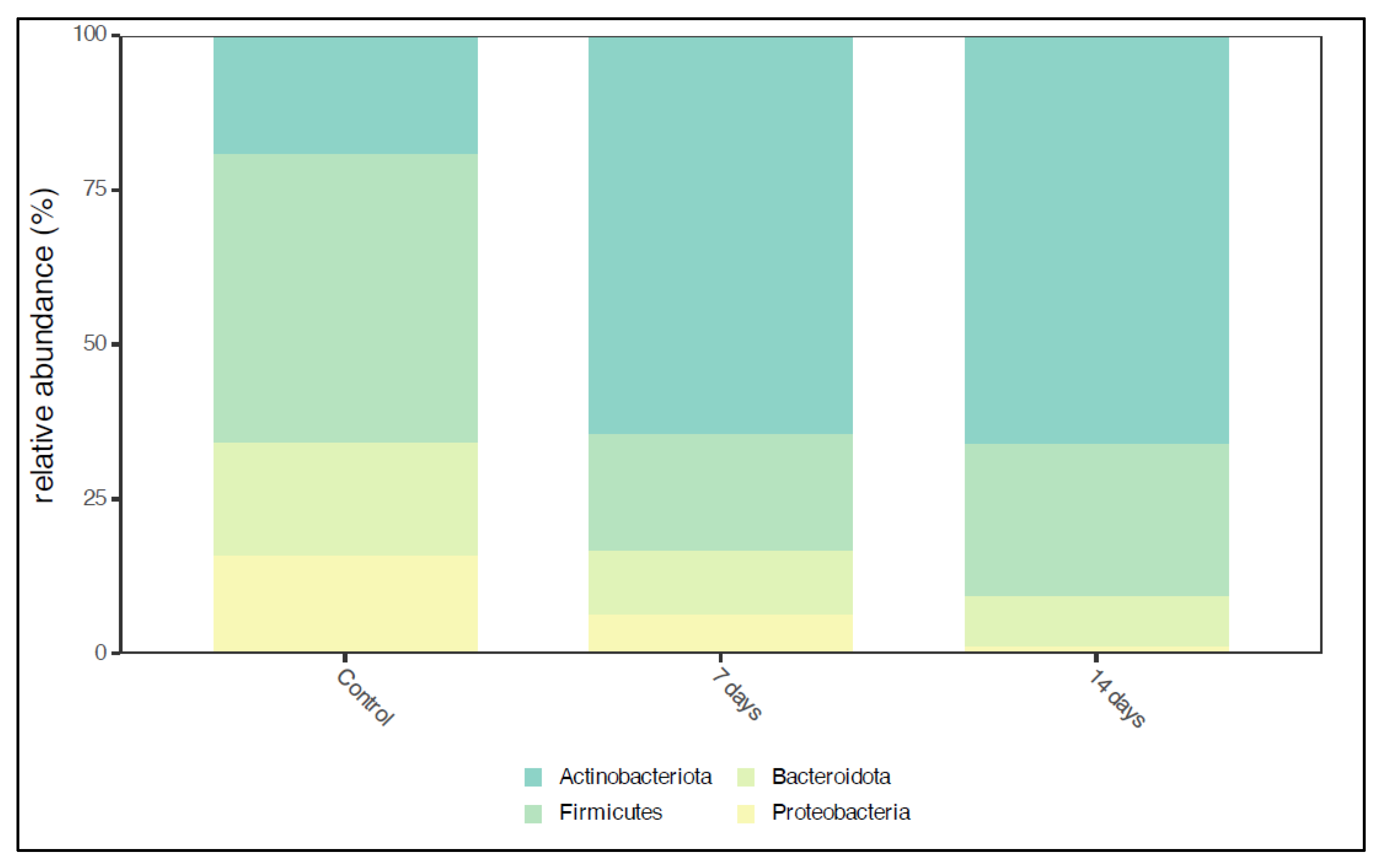
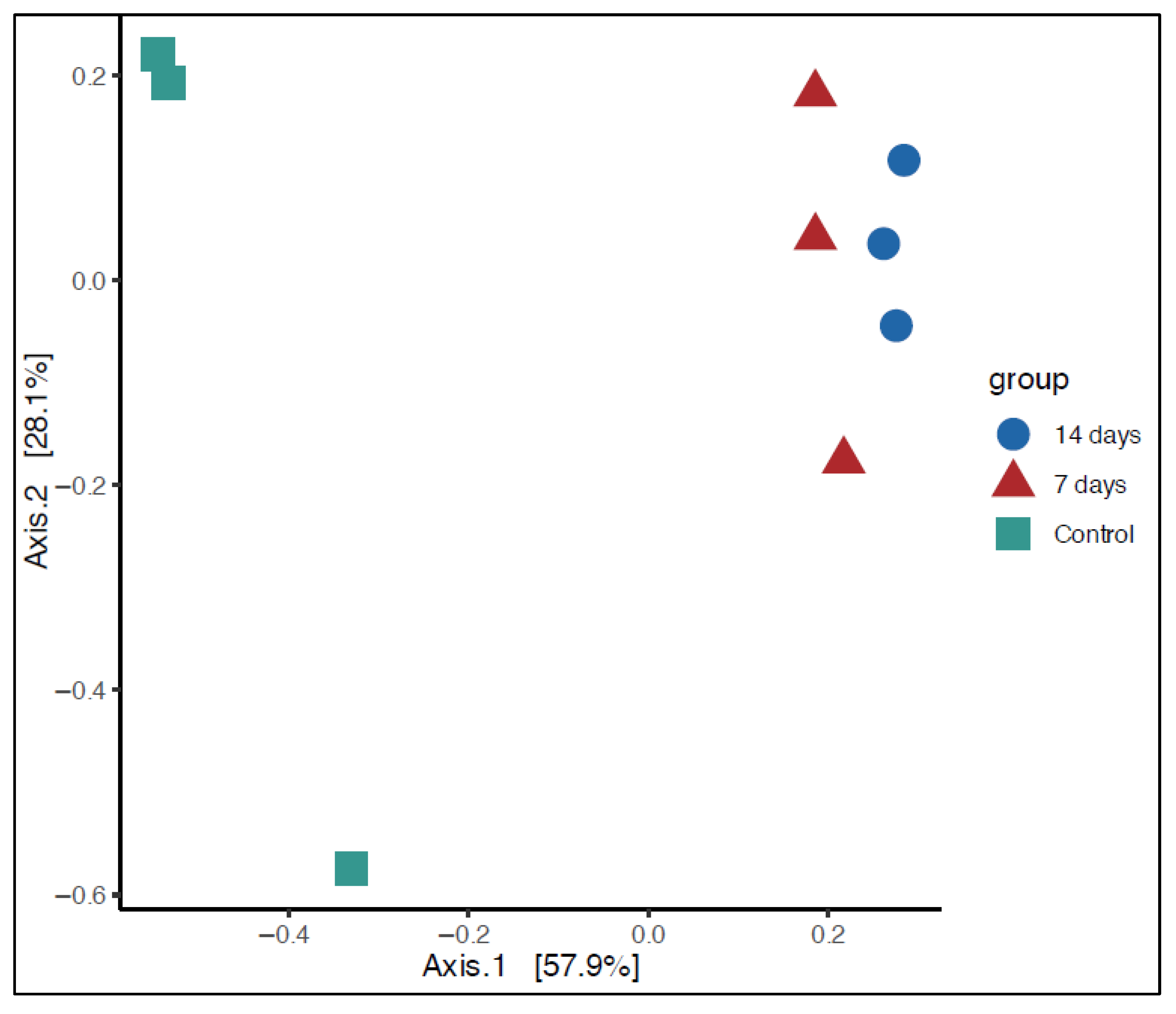
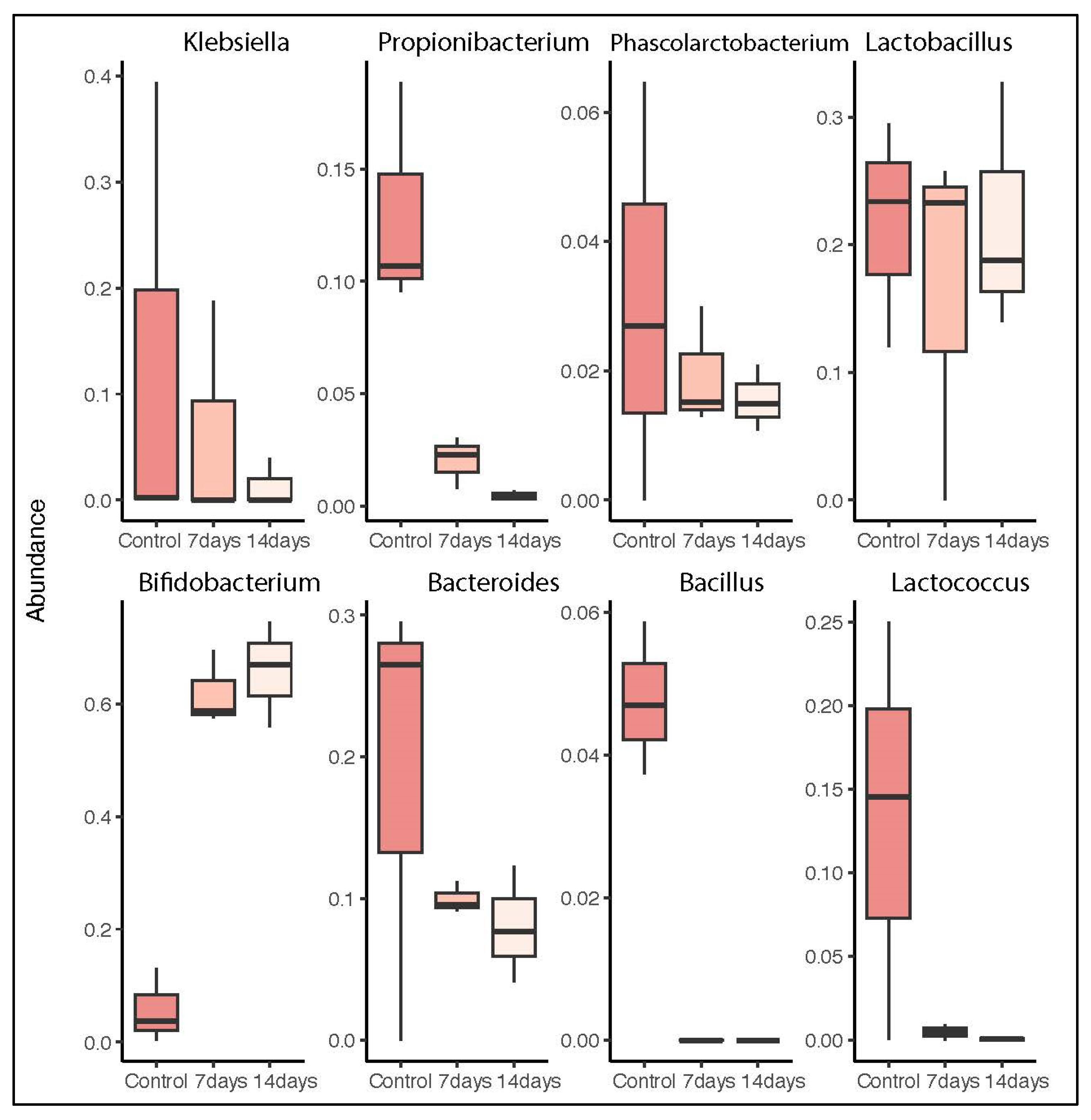
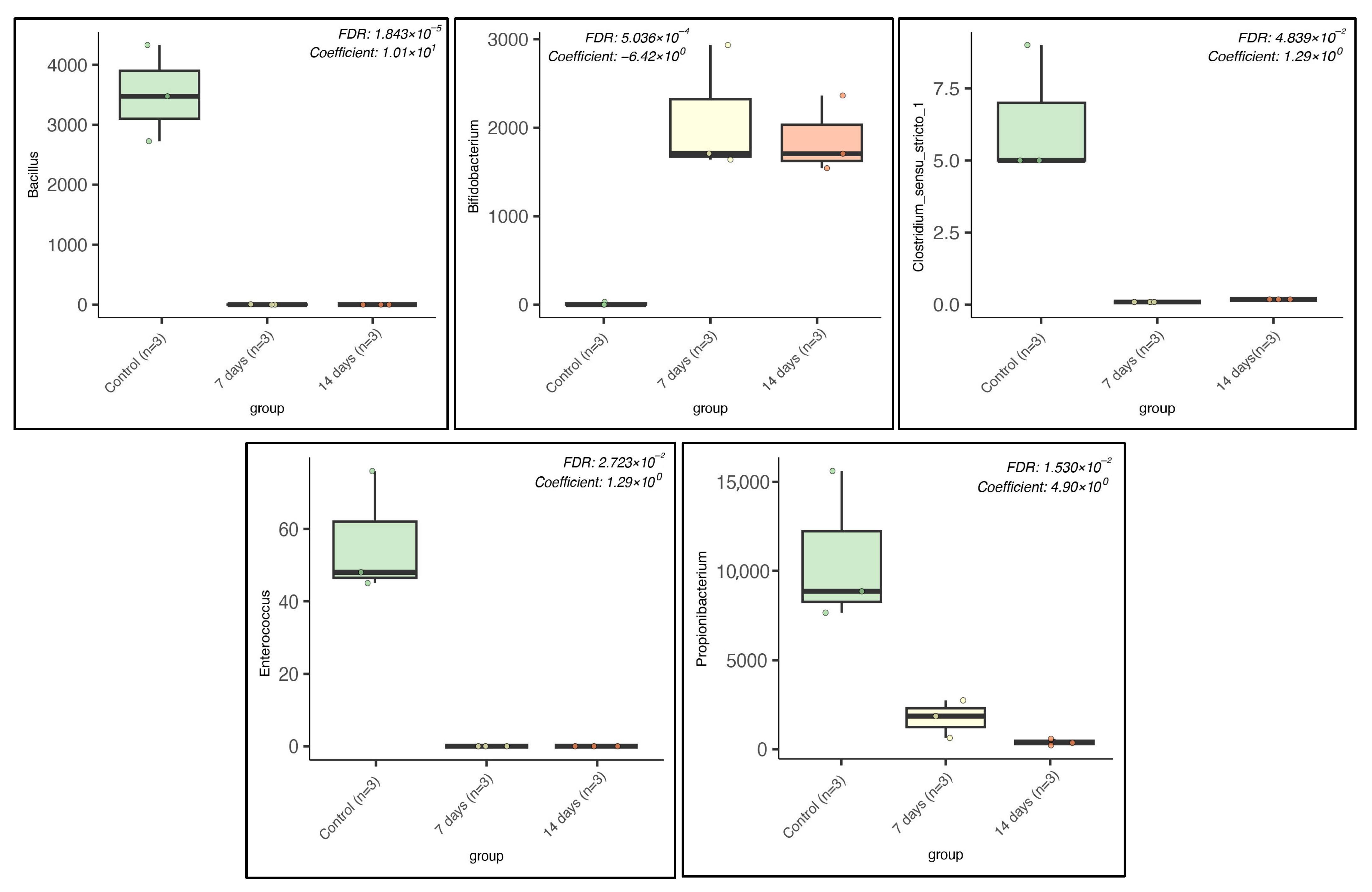
2.1. Microbiota Composition in Long-Term SHIME® Run
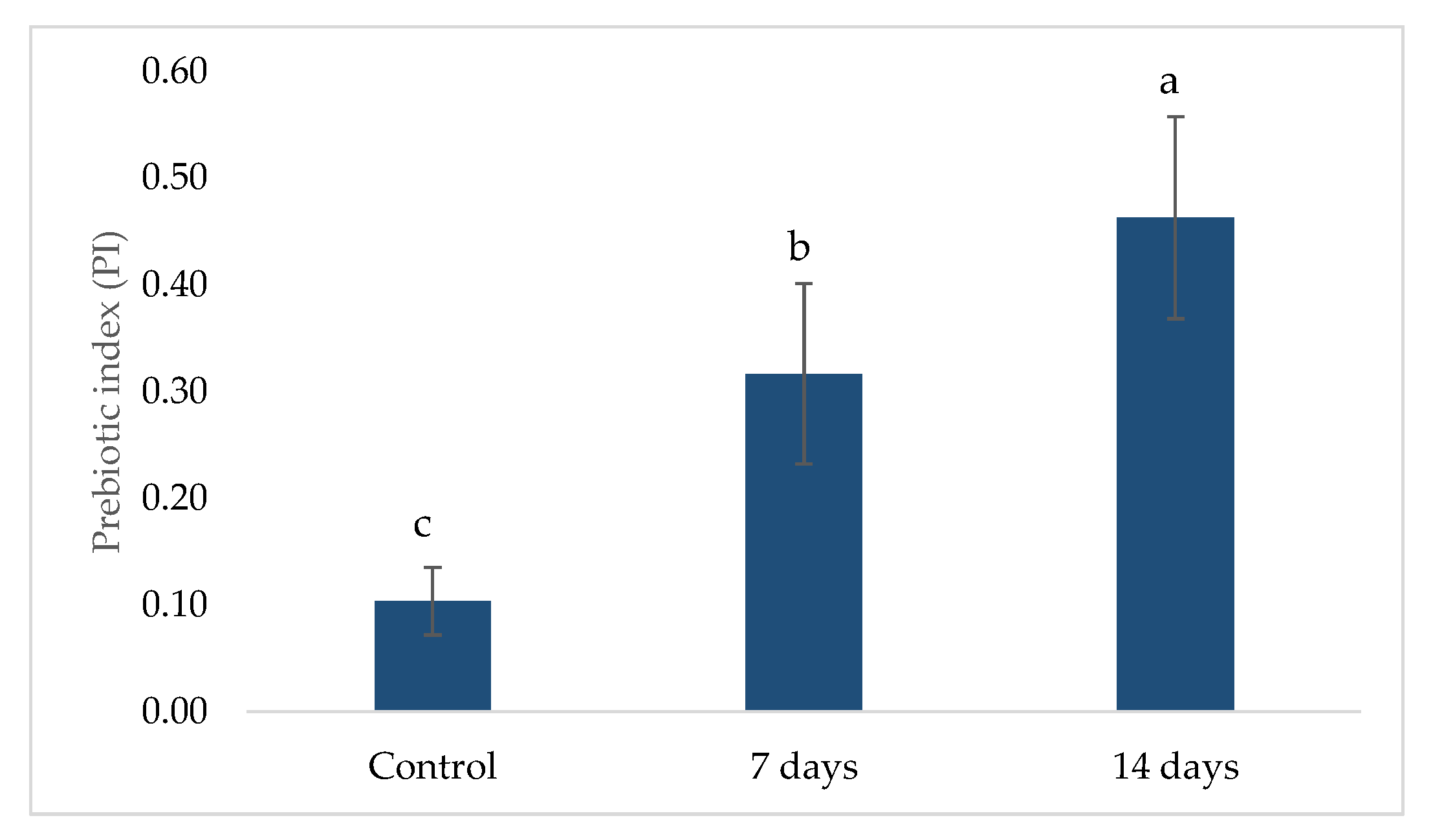
2.2. Prebiotic Index (PI)
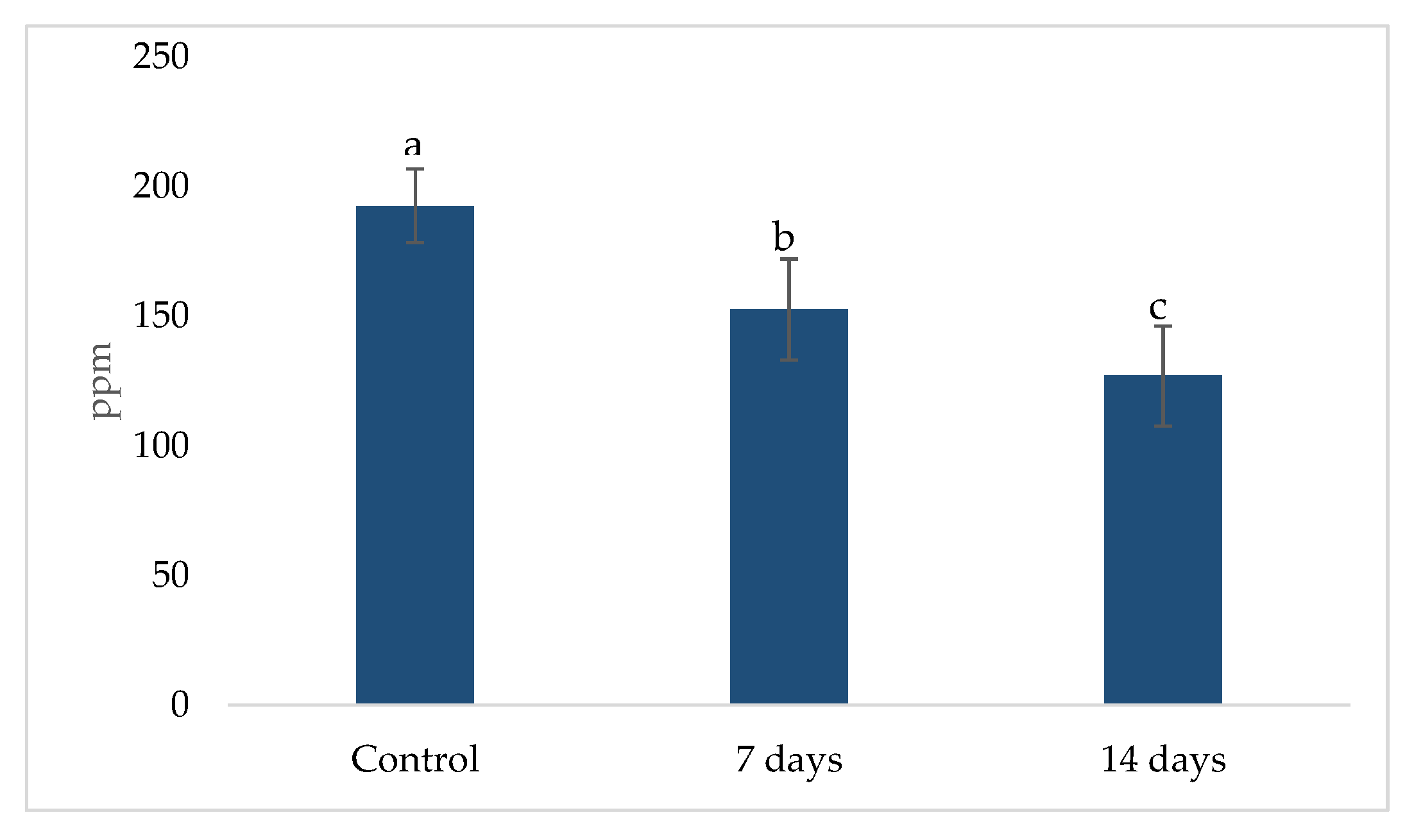
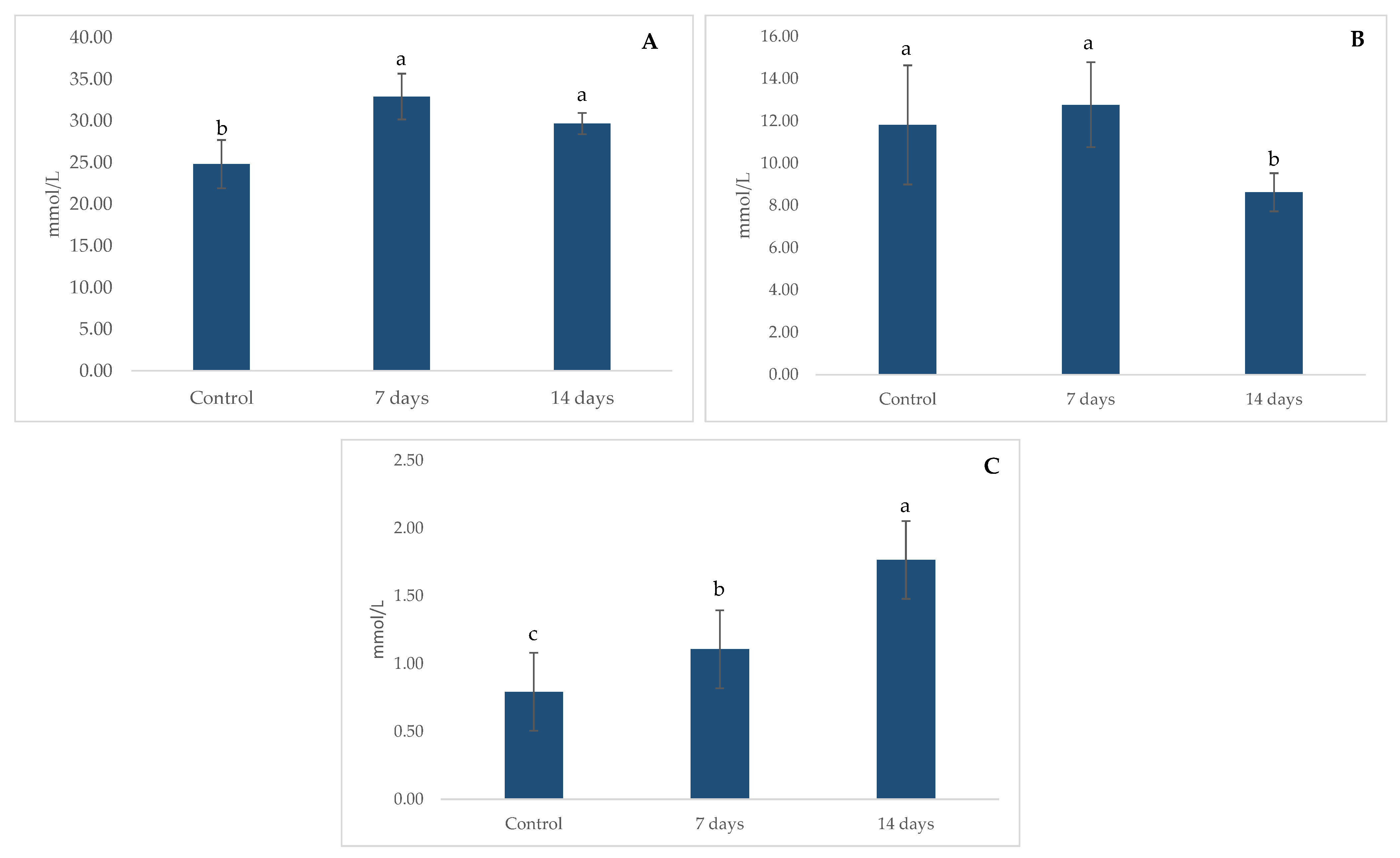
2.3. Metabolic Activity: Ammonia (NH4+) Production, Short Chain Fat Acids (SCFA) and Gamma-Aminobutyric Acid (GABA)
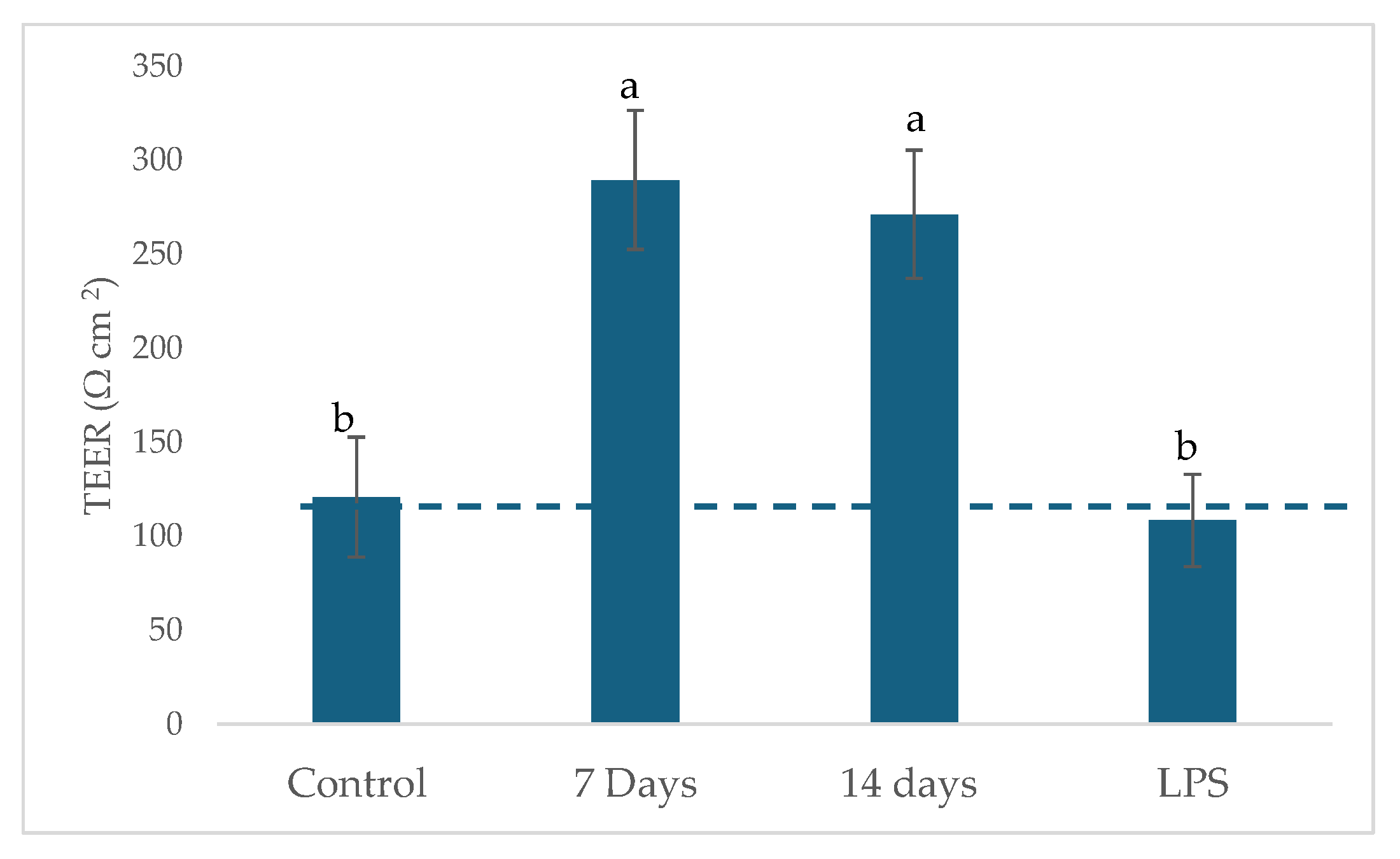
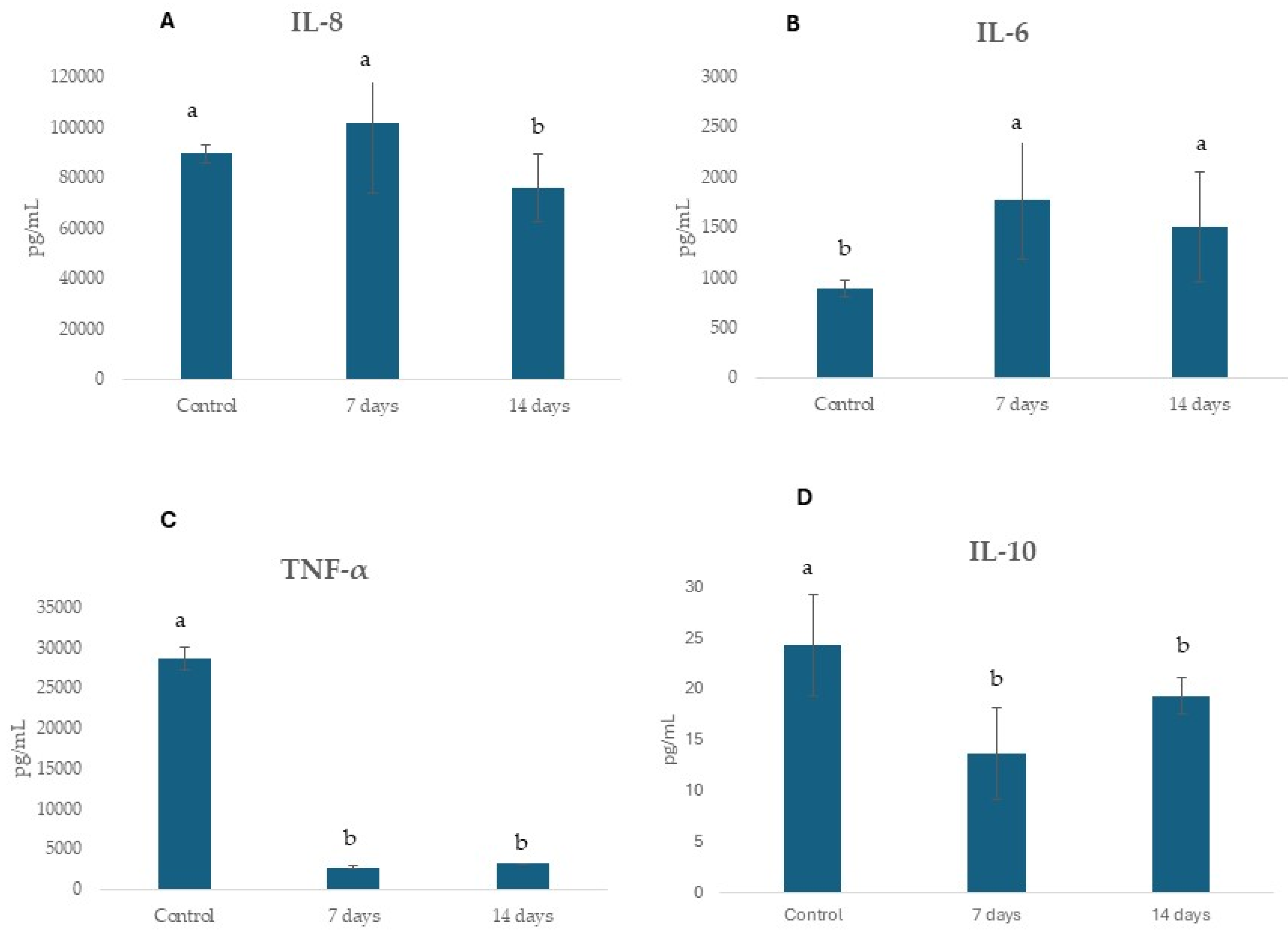
2.4. Potential Regulation of Gut Epithelial Function and Immune Response
3. Discussion
4. Materials and Methods
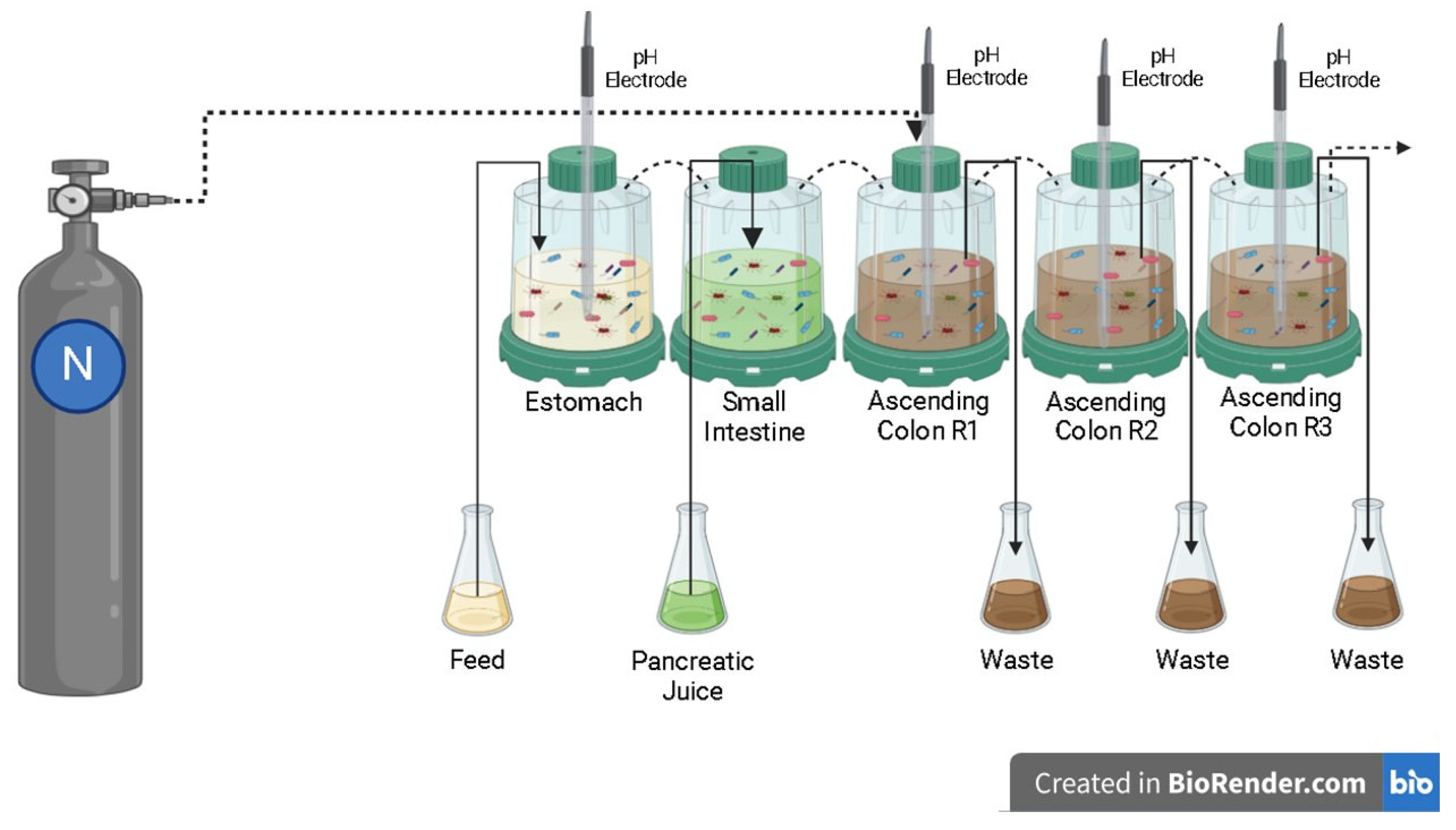
4.1. Simulated Digestion in the Dynamic Colonic Model
4.2. Experimental Protocol
4.3. Metabolic Activity: Ammonia (NH4+), Short-Chain Fatty Acids (SCFAs) and Gamma-Aminobutyric Acid (GABA) Production
4.4. Microbiological Analysis Employing 16S rRNA Gene Sequencing
4.5. Prebiotic Index (PI)
4.6. Co-Culture of Caco-2 and THP 1 Cells
4.7. Statistical Analysis
5. Conclusions
Commentary of Expert (Psychiatrist, Master in Neuropsychiatry and Behavioral Sciences and PhD in Tropical Medicine)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Szuhany, K.L.; Simon, N.M. Anxiety Disorders: A Review. JAMA 2022, 328, 2431–2445. [Google Scholar] [CrossRef]
- Penninx, B.W.; Pine, D.S.; Holmes, E.A.; Reif, A. Anxiety disorders. Lancet 2021, 397, 914–927, Erratum in Lancet 2021, 397, 880. [Google Scholar] [CrossRef]
- Lin, S.K.; Chen, H.C.; Chen, C.H.; Chen, I.M.; Lu, M.L.; Hsu, C.D.; Chiu, Y.H.; Wang, T.Y.; Chen, H.M.; Chung, Y.E.; et al. Exploring the human gut microbiota targets in relation to the use of contemporary antidepressants. J. Affect. Disord. 2024, 344, 473–484. [Google Scholar] [CrossRef]
- Jackson, M.A.; Verdi, S.; Maxan, M.E.; Shin, C.M.; Zierer, J.; Bowyer, R.C.E.; Martin, T.; Williams, F.M.K.; Menni, C.; Bell, J.T.; et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat. Commun. 2018, 9, 2655. [Google Scholar] [CrossRef]
- McGovern, A.S.; Hamlin, A.S.; Winter, G. A review of the antimicrobial side of antidepressants and its putative implications on the gut microbiome. Aust. N. Z. J. Psychiatry 2019, 53, 1151–1166. [Google Scholar] [CrossRef]
- Tomizawa, Y.; Kurokawa, S.; Ishii, D.; Miyaho, K.; Ishii, C.; Sanada, K.; Fukuda, S.; Mimura, M.; Kishimoto, T. Effects of psychotropics on the microbiome in patients with depression and anxiety: Considerations in a naturalistic clinical setting. Int. J. Neuropsychopharmacol. 2021, 24, 97–107. [Google Scholar] [CrossRef]
- Lukić, I.; Getselter, D.; Ziv, O.; Oron, O.; Reuveni, E.; Koren, O.; Elliott, E. Antidepressants affect gut microbiota and Ruminococcus flavefaciens is able to abolish their effects on depressive-like behavior. Transl. Psychiatry 2019, 9, 133. [Google Scholar] [CrossRef]
- Zakaraya, Z.; Abu Assab, M.; Tamimi, L.N.; Karameh, N.; Hailat, M.; Al-Omari, L.; Abu Dayyih, W.; Alasasfeh, O.; Awad, M.; Awad, R. Pharmacokinetics and pharmacodynamics: A comprehensive analysis of the absorption, distribution, metabolism, and excretion of psychiatric drugs. Pharmaceuticals 2024, 17, 280. [Google Scholar] [CrossRef]
- Munoz-Bellido, J.L.; Munoz-Criado, S.; Garcìa-Rodrìguez, J.A. Antimicrobial activity of psychotropic drugs: Selective serotonin reuptake inhibitors. Int. J. Antimicrob. Agents 2000, 14, 177–180. [Google Scholar] [CrossRef]
- Ait Chait, Y.; Mottawea, W.; Tompkins, T.A.; Hammami, R. Unravelling the antimicrobial action of antidepressants on gut commensal microbes. Sci. Rep. 2020, 10, 17878. [Google Scholar] [CrossRef]
- Macedo, D.; Filho, A.J.M.C.; Soares de Sousa, C.N.; Quevedo, J.; Barichello, T.; Júnior, H.V.N.; Freitas de Lucena, D. Antidepressants, antimicrobials or both? Gut microbiota dysbiosis in depression and possible implications of the antimicrobial effects of antidepressant drugs for antidepressant effectiveness. J. Affect. Disord. 2017, 208, 22–32. [Google Scholar] [CrossRef]
- Ayaz, M.; Subhan, F.; Ahmed, J.; Khan, A.U.; Ullah, F.; Ullah, I.; Ali, G.; Syed, N.I.; Hussain, S. Sertraline enhances the activity of antimicrobial agents against pathogens of clinical relevance. J. Biol. Res. 2015, 22, 4. [Google Scholar] [CrossRef]
- Kruszewska, H.; Zareba, T.; Tyski, S. Examination of antimicrobial activity of selected non-antibiotic medicinal preparations. Acta Pol. Pharm. 2012, 69, 1368–1371. [Google Scholar]
- McVey Neufeld, K.A.; Bienenstock, J.; Bharwani, A.; Champagne-Jorgensen, K.; Mao, Y.; West, C.; Liu, Y.; Surette, M.G.; Kunze, W.; Forsythe, P. Oral selective serotonin reuptake inhibitors activate vagus nerve dependent gut-brain signalling. Sci. Rep. 2019, 9, 14290. [Google Scholar] [CrossRef]
- Shen, Y.; Yang, X.; Li, G.; Gao, J.; Liang, Y. The change of gut microbiota in MDD patients under SSRIs treatment. Sci. Rep. 2021, 11, 14918. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Ahmed, A.T.; Arnold, M.; Liu, D.; Luo, C.; Zhu, H.; Mahmoudiandehkordi, S.; Neavin, D.; Louie, G.; Dunlop, B.W.; et al. Metabolomic signature of exposure and response to citalopram/escitalopram in depressed outpatients. Transl. Psychiatry 2019, 9, 173. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Chen, X.; Meng, S.; Yu, Y.; Li, S.; Wu, L.; Zhang, Y. The role of probiotic intervention in regulating gut microbiota, short-chain fatty acids and depression-like behavior in lead-exposed rats. Int. J. Occup. Med. Environ. Health 2022, 35, 95–106. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Palepu, M.S.K.; Satti, S.; Jaiswal, Y.; Singh, A.A.; Dash, S.P.; Gajula, S.N.R.; Sonti, R. Multi-strain probiotic formulation reverses maternal separation and chronic unpredictable mild stress-generated anxiety- and depression-like phenotypes by modulating gut microbiome-brain activity in rats. ACS Chem. Neurosci. 2022, 13, 1948–1965. [Google Scholar] [CrossRef]
- Varesi, A.; Campagnoli, L.I.M.; Chirumbolo, S.; Candiano, B.; Carrara, A.; Ricevuti, G.; Esposito, C.; Pascale, A. The brain-gut-microbiota interplay in depression: A key to design innovative therapeutic approaches. Pharmacol. Res. 2023, 192, 106799. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, W.; Pan, Y.; Wang, J.; Xiao, P.; Wang, Y. Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression-like behavior in stressed mice by modulating the gut microbiota. Food Funct. 2019, 10, 925–937. [Google Scholar] [CrossRef]
- Gao, K.; Farzi, A.; Ke, X.; Yu, Y.; Chen, C.; Chen, S.; Yum, T.; Wang, H.; Li, Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022, 13, 957–969. [Google Scholar] [CrossRef]
- Xu, M.; Tian, P.; Zhu, H.; Zou, R.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. Lactobacillus paracasei CCFM1229 and Lactobacillus rhamnosus CCFM1228 alleviated depression- and anxiety-related symptoms of chronic stress-induced depression in mice by regulating xanthine oxidase activity in the brain. Nutrients 2022, 14, 1294. [Google Scholar] [CrossRef]
- Li, Q.; Li, L.; Niu, X.; Tang, C.; Wang, H.; Gao, J.; Hu, J. Probiotics alleviate depressive behavior in chronic unpredictable mild stress rat models by remodeling intestinal flora. Neuroreport 2021, 32, 686–693. [Google Scholar] [CrossRef]
- Hashikawa-Hobara, N.; Otsuka, A.; Okujima, C.; Hashikawa, N. Lactobacillus paragasseri OLL2809 improves depression-like behavior and increases beneficial gut microbes in mice. Front. Neurosci. 2022, 16, 918953. [Google Scholar] [CrossRef]
- Ding, Y.; Bu, F.; Chen, T.; Shi, G.; Yuan, X.; Feng, Z.; Duan, Z.; Wang, R.; Zhang, S.; Wang, Q.; et al. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl. Microbiol. Biotechnol. 2021, 105, 8411–8426. [Google Scholar] [CrossRef]
- Tian, P.; O’Riordan, K.J.; Lee, Y.K.; Wang, G.; Zhao, J.; Zhang, H.; Cryan, J.F.; Chen, W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol. Stress 2020, 12, 100216. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- De Oliveira, F.L.; Salgaço, M.K.; de Oliveira, M.T.; Mesa, V.; Sartoratto, A.; Peregrino, A.M.; Ramos, W.S.; Sivieri, K. Exploring the potential of Lactobacillus helveticus R0052 and Bifidobacterium longum R0175 as promising psychobiotics using SHIME. Nutrients 2023, 15, 1521. [Google Scholar] [CrossRef]
- Partrick, K.A.; Rosenhauer, A.M.; Auger, J.; Arnold, A.R.; Ronczkowski, N.M.; Jackson, L.M.; Lord, M.N.; Abdulla, S.M.; Chassaing, B.; Huhman, K.L. Ingestion of probiotic (Lactobacillus helveticus and Bifidobacterium longum) alters intestinal microbial structure and behavioral expression following social defeat stress. Sci. Rep. 2021, 11, 3763. [Google Scholar] [CrossRef]
- Bron, P.A.; Catalayud, M.; Marzorati, M.; Pane, M.; Kartal, E.; Dhir, R.; Reid, G. Delivery of metabolically neuroactive probiotics to the human gut. Int. J. Mol. Sci. 2021, 22, 9122. [Google Scholar] [CrossRef]
- Makris, A.P.; Karianaki, M.; Tsamis, K.I.; Paschou, S.A. The role of the gut-brain axis in depression: Endocrine, neural, and immune pathways. Hormones 2021, 1, 1–12, Erratum in Hormones 2021, 20, 223–224. [Google Scholar] [CrossRef]
- Wang, H.; Braun, C.; Murphy, E.F.; Enck, P. Bifidobacterium longum 1714™ strain modulates brain activity of healthy volunteers during social stress. Am. J. Gastroenterol. 2019, 114, 1152–1162. [Google Scholar] [CrossRef]
- Singh, A.; Negi, P.S. Appraising the role of biotics and fermented foods in gut microbiota modulation and sleep regulation. J. Food Sci. 2025, 90, e17634. [Google Scholar] [CrossRef]
- Hotz, J.; Fehlmann, B.; Papassotiropoulos, A.; de Quervain, D.J.; Schicktanz, N.S. Cannabidiol enhances verbal episodic memory in healthy young participants: A randomized clinical trial. J. Psychiatr. Res. 2021, 143, 327–333. [Google Scholar] [CrossRef]
- Jiang, H.; Ling, Z.; Zhang, Y.; Mao, H.; Ma, Z.; Yin, Y.; Wang, W.; Tang, W.; Tan, Z.; Shi, J.; et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav. Immun. 2015, 48, 186–194. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of probiotics on gut microbiota: An overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Peterson, C.T.; Perez Santiago, J.; Iablokov, S.N.; Chopra, D.; Rodionov, D.A.; Peterson, S.N. Short-Chain Fatty Acids modulate healthy gut microbiota composition and functional potential. Curr. Microbiol. 2022, 79, 128. [Google Scholar] [CrossRef]
- Wei, S.; Wang, C.; Zhang, Q.; Yang, H.; Deehan, E.C.; Zong, X.; Wang, Y.; Jin, M. Dynamics of microbial communities during inulin fermentation associated with the temporal response in SCFA production. Carbohydr. Polym. 2022, 298, 120057. [Google Scholar] [CrossRef]
- Fassarella, M.; Blaak, E.E.; Penders, J.; Nauta, A.; Smidt, H.; Zoetendal, E.G. Gut microbiome stability and resilience: Elucidating the response to perturbations in order to modulate gut health. Gut 2021, 70, 595–605. [Google Scholar] [CrossRef]
- Ayob, N.; Muhammad Nawawi, K.N.; Mohamad Nor, M.H.; Raja Ali, R.A.; Ahmad, H.F.; Oon, S.F.; Mohd Mokhtar, N. The effects of probiotics on small intestinal microbiota composition, inflammatory cytokines and intestinal permeability in patients with non-alcoholic fatty liver disease. Biomedicines 2023, 11, 640. [Google Scholar] [CrossRef]
- Torres-Maravilla, E.; Holowacz, S.; Delannoy, J.; Lenoir, L.; Jacouton, E.; Gervason, S.; Meynier, M.; Boucard, A.S.; Carvalho, F.A.; Barbut, F.; et al. Serpin-positive Bifidobacterium breve CNCM I-5644 improves intestinal permeability in two models of irritable bowel syndrome. Sci. Rep. 2022, 1, 19776. [Google Scholar] [CrossRef]
- Abdulqadir, R.; Engers, J.; Al-Sadi, R. Role of Bifidobacterium in modulating the intestinal epithelial tight junction barrier: Current knowledge and perspectives. Curr. Dev. Nutr. 2023, 7, 102026. [Google Scholar] [CrossRef]
- Bakshi, J.; Mishra, K.P. Sodium butyrate prevents lipopolysaccharide induced inflammation and restores the expression of tight junction protein in human epithelial Caco-2 cells. Cell Immunol. 2025, 408, 104912. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef]
- Alhendi, A.; Naser, S.A. The dual role of interleukin-6 in Crohn’s disease pathophysiology. Front. Immunol. 2023, 14, 1295230. [Google Scholar] [CrossRef]
- Waldner, M.J.; Neurath, M.F. Master regulator of intestinal disease: IL-6 in chronic inflammation and cancer development. Semin. Immunol. 2014, 26, 75–79. [Google Scholar] [CrossRef]
- Li, X.Y.; Shang, J.; Wang, X.J.; Ma, H.P.; Ren, L.F.; Zhang, L. Bifidobacterium longum JBLC-141 alleviates hypobaric hypoxia-induced intestinal barrier damage by attenuating inflammatory responses and oxidative stress. Front. Microbiol. 2024, 15, 1501999. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The microbiota-gut-brain axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Bjørklund, G.; Meguid, N.A.; El-Ansary, A. Neuroprotection through probiotic intervention: Lessons from autism research. ARS Med. Tomitana 2024, 29, 100–106. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhu, L.; Li, H.; Chen, Q.; Li, N.; Li, J.; Zhao, Z.; Xiao, D.; Tang, T.; Bi, C.; et al. Insights and progress on the biosynthesis, metabolism, and physiological functions of gamma-aminobutyric acid (GABA): A review. PeerJ 2024, 12, e18712. [Google Scholar] [CrossRef]
- Barros-Santos, T.; Silva, K.S.O.; Libarino-Santos, M.; Cata-Preta, E.G.; Reis, H.S.; Tamura, E.K.; de Oliveira-Lima, A.J.; Berro, L.F.; Uetanabaro, A.P.T.; Marinho, E.A.V. Effects of chronic treatment with new strains of Lactobacillus plantarum on cognitive, anxiety- and depressive-like behaviors in male mice. PLoS ONE 2020, 15, e0234037. [Google Scholar] [CrossRef]
- Rashmi, D.; Zanan, R.; John, S.; Khandagale, K.; Nadaf, A. γ-Aminobutyric acid (GABA): Biosynthesis, role, commercial production, and applications. Stud. Nat. Prod. Chem. 2018, 57, 413–452. [Google Scholar]
- Laroute, V.; Yasaro, C.; Narin, W.; Mazzoli, R.; Pessione, E.; Cocaign-Bousquet, M.; Loubière, P. GABA Production in Lactococcus lactis Is enhanced by arginine and co-addition of malate. Front. Microbiol. 2016, 7, 1050. [Google Scholar] [CrossRef]
- Salami, M.; Soheili, M. The microbiota-gut- hippocampus axis. Front. Neurosci. 2022, 16, 1065995. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Van Hul, M.; Knauf, C.; Cani, P.D. Gut microbiome, endocrine control of gut barrier function and metabolic diseases. J. Endocrinol. 2021, 248, 67–82. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Song, H.; Mun, S.H.; Han, D.W.; Kang, J.H.; An, J.U.; Hwang, C.Y.; Cho, S. Probiotics ameliorate atopic dermatitis by modulating the dysbiosis of the gut microbiota in dogs. BMC Microbiol. 2025, 25, 228. [Google Scholar] [CrossRef]
- Borrego-Ruiz, A.; Borrego, J.J. Early life stress and gut microbiome dysbiosis: A narrative review. Stresses 2025, 5, 38. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, X.; Wang, D.; Yao, Q.; Ma, G.-L.; Fan, X. Simulator of the Human Intestinal Microbial Ecosystem (SHIME®): Current developments, applications, and future prospects. Pharmaceuticals 2024, 17, 1639. [Google Scholar] [CrossRef]
- Salgaço, M.K.; Perina, N.P.; Tomé, T.M.; Mosquera, E.M.B.; Lazarini, T.; Sartoratto, A.; Sivieri, K. Probiotic infant cereal improves children’s gut microbiota: Insights using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®). Food Res. Int. 2021, 143, 110292. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, F.; Larsen, N.; Tieghi, T.M.; Adorno, M.A.T.; Kot, W.; Saad, S.M.I.; Jespersen, L.; Sivieri, K. Modulation of gut microbiota from obese individuals by in vitro fermentation of citrus pectin in combination with Bifidobacterium longum BB-46. Appl. Microbiol. Biotechnol. 2018, 102, 8827–8840. [Google Scholar] [CrossRef]
- Carvalho, N.M.; Oliveira, D.L.; Saleh, M.A.D.; Pintado, M.; Madureira, A.R. Preservation of human gut microbial inoculums for in vitro fermentation studies. Fermentation 2021, 7, 14. [Google Scholar] [CrossRef]
- Herkenhoff, M.E.; de Medeiros, I.U.D.; Garutti, L.H.G.; Salgaço, M.K.; Sivieri, K.; Saad, S.M.I. cashew by-product as a functional substrate for the development of probiotic fermented milk. Foods 2023, 12, 3383. [Google Scholar] [CrossRef] [PubMed]
- Clementino, J.R.; de Oliveira, L.I.G.; Salgaço, M.K.; de Oliveira, F.L.; Mesa, V.; Tavares, J.F.; Silva-Pereira, L.; Raimundo, B.V.B.; Oliveira, K.C.; Medeiros, A.I.; et al. β-Glucan alone or combined with Lactobacillus acidophilus positively influences the bacterial diversity and metabolites in the colonic microbiota of type II diabetic patients. Probiotics Antimicrob. Proteins 2025. [Google Scholar] [CrossRef]
- Daguet, D.; Pinheiro, I.; Verhelst, A.; Possemiers, S.; Marzorati, M. Arabinogalactan and fructooligosaccharides improve the gut barrier function in distinct areas of the colon in the Simulator of the Human Intestinal Microbial Ecosystem. J. Funct. Foods 2016, 20, 369–379. [Google Scholar] [CrossRef]
- Cataldo, P.G.; Villena, J.; Elean, M.; Giori, G.S.; Saavedra, L.; Hebert, M. Immunomodulatory properties of a γ-aminobutyric acid-enriched strawberry juice produced by Levilactobacillus brevis CRL 2013. Front. Microbiol. 2020, 11, 610016. [Google Scholar] [CrossRef]
- Kuczynski, J.; Stombaugh, J.; Walters, W.A.; González, A.; Caporaso, J.G.; Knight, R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr. Protoc. Bioinform. 2011, 10, 10.7.1–10.7.20. [Google Scholar] [CrossRef]
- Hagerty, S.L.; Hutchison, K.E.; Lowry, C.A.; Bryan, A.D. An empirically derived method for measuring hu-man gut microbiome alpha diversity: Demonstrated utility in predicting health-related outcomes among a human clinical sample. PLoS ONE 2020, 15, e0229204. [Google Scholar] [CrossRef]
- Gomes de Oliveira, L.I.; Clementino, J.R.; Salgaço, M.K.; de Oliveira, S.P.A.; Dos Santos Lima, M.; Mesa, V.; de Souza, E.L.; Vinderola, C.G.; Magnani, M.; Sivieri, K. Revealing the beneficial effects of a dairy infant formula on the gut microbiota of early childhood children with autistic spectrum disorder using static and SHIME® fermentation models. Food Funct. 2023, 14, 8964–8974. [Google Scholar] [CrossRef]
- Figueroa-González, I.; Rodríguez-Serrano, G.; Gómez-Ruiz, L.; García-Garibay, M.; Cruz-Guerrero, A. Prebiotic effect of commercial saccharides on probiotic bacteria isolated from commercial products. Food Sci. Technol. 2019, 39, 747–753. [Google Scholar] [CrossRef]
- Liu, L.; Lu, Y.; Xu, C.; Chen, H.; Wang, X.; Wang, Y.; Cai, B.; Li, B.; Verstrepen, L.; Ghyselinck, J.; et al. The Modulation of Chaihu Shugan formula on microbiota composition in the Simulator of the Human Intestinal Microbial Ecosystem Technology platform and its influence on gut barrier and intestinal immunity in Caco-2/THP1-Blue™ Cell Co-Culture model. Front. Pharmacol. 2022, 13, 820543. [Google Scholar] [CrossRef]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Baxter, E.W.; Graham, A.E.; Re, N.A.; Carr, I.M.; Robinson, J.I.; Mackie, S.L.; Morgan, A.W. Standardized protocols for differentiation of THP-1 cells to macrophages with distinct M(IFNγ+LPS), M(IL-4) and M(IL-10) phenotypes. J. Immunol. Methods 2020, 478, 112721. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S.; Watson, M. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
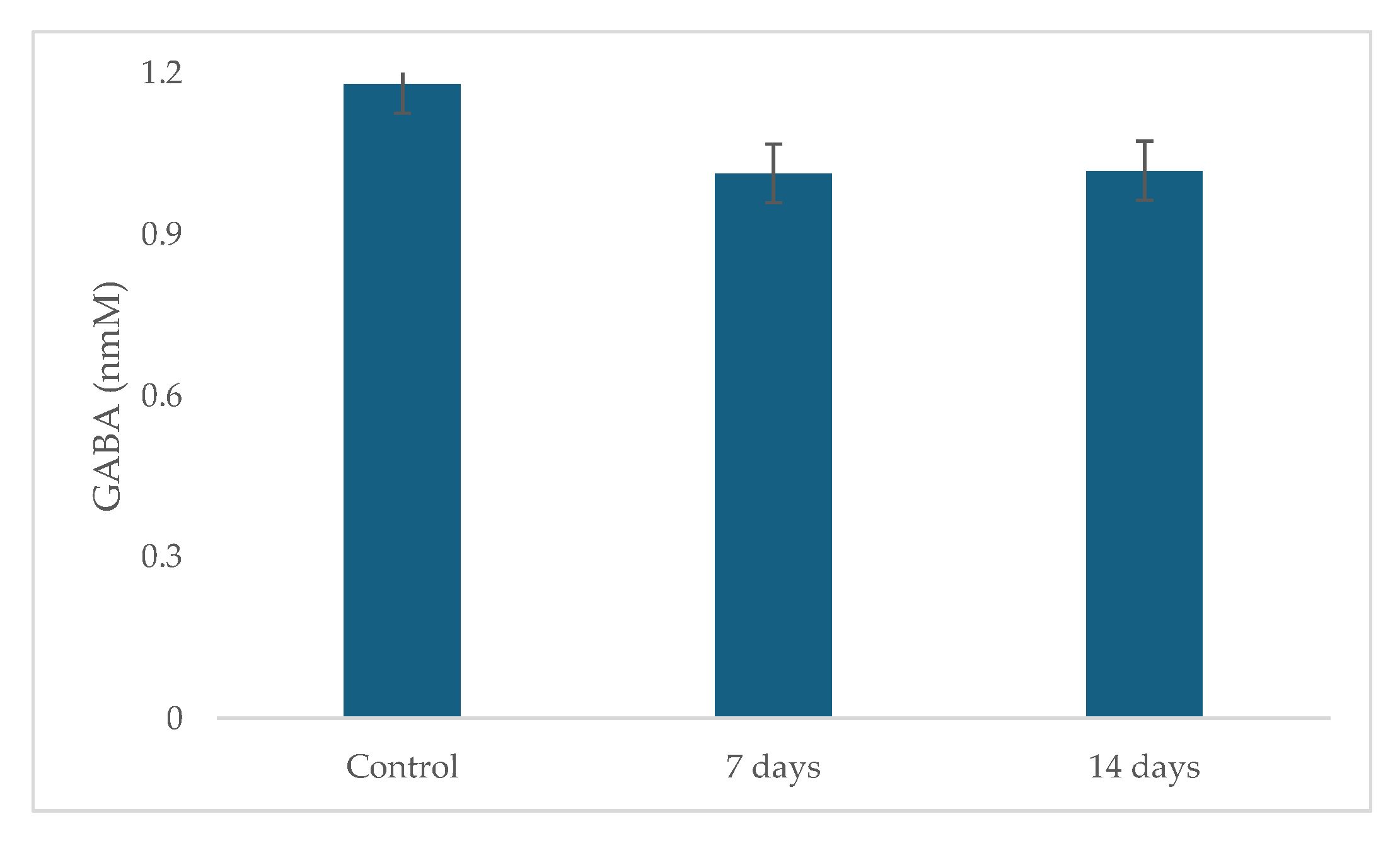
| Evaluated Parameters | 4 Participants |
| Hamilton Scale | Severe anxiety |
| Medications | Escitalopram and Setraline |
| Sex | 2 women and 2 men |
| Age | 36.00 ± 5.41 years old |
| Weight | 64.20 ± 13.20 kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, M.T.; de Oliveira, F.L.; Salgaço, M.K.; Mesa, V.; Sartoratto, A.; Duailibi, K.; Raimundo, B.V.B.; Ramos, W.S.; Sivieri, K. Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model. Pharmaceuticals 2025, 18, 1132. https://doi.org/10.3390/ph18081132
de Oliveira MT, de Oliveira FL, Salgaço MK, Mesa V, Sartoratto A, Duailibi K, Raimundo BVB, Ramos WS, Sivieri K. Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model. Pharmaceuticals. 2025; 18(8):1132. https://doi.org/10.3390/ph18081132
Chicago/Turabian Stylede Oliveira, Marina Toscano, Fellipe Lopes de Oliveira, Mateus Kawata Salgaço, Victoria Mesa, Adilson Sartoratto, Kalil Duailibi, Breno Vilas Boas Raimundo, Williams Santos Ramos, and Katia Sivieri. 2025. "Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model" Pharmaceuticals 18, no. 8: 1132. https://doi.org/10.3390/ph18081132
APA Stylede Oliveira, M. T., de Oliveira, F. L., Salgaço, M. K., Mesa, V., Sartoratto, A., Duailibi, K., Raimundo, B. V. B., Ramos, W. S., & Sivieri, K. (2025). Restoring Balance: Probiotic Modulation of Microbiota, Metabolism, and Inflammation in SSRI-Induced Dysbiosis Using the SHIME® Model. Pharmaceuticals, 18(8), 1132. https://doi.org/10.3390/ph18081132









