Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways
Abstract
1. Introduction
2. Scientific Nature and Biology of Bacteriophages
2.1. Fundamental Characteristics and Classification
2.2. Differences Between Gram-Positive and Gram-Negative Targeting Phages
2.3. Mechanisms of Bacterial Killing and Specificity
2.4. Phage–Bacteria Evolutionary Dynamics
3. Historical Perspective and Evolution of Phage Therapy
3.1. Early Discovery and Pioneer Era (1915–1940)
3.2. Decline and Overshadowing by Antibiotics (1940–1980)
3.3. Soviet Development and Military Applications (1930–1990)
3.4. Resurgence in the Antibiotic Resistance Era (1990-Present)
3.5. Contemporary Development and Clinical Translation in the Former Soviet States
3.6. Global Expansion and Contemporary Challenges
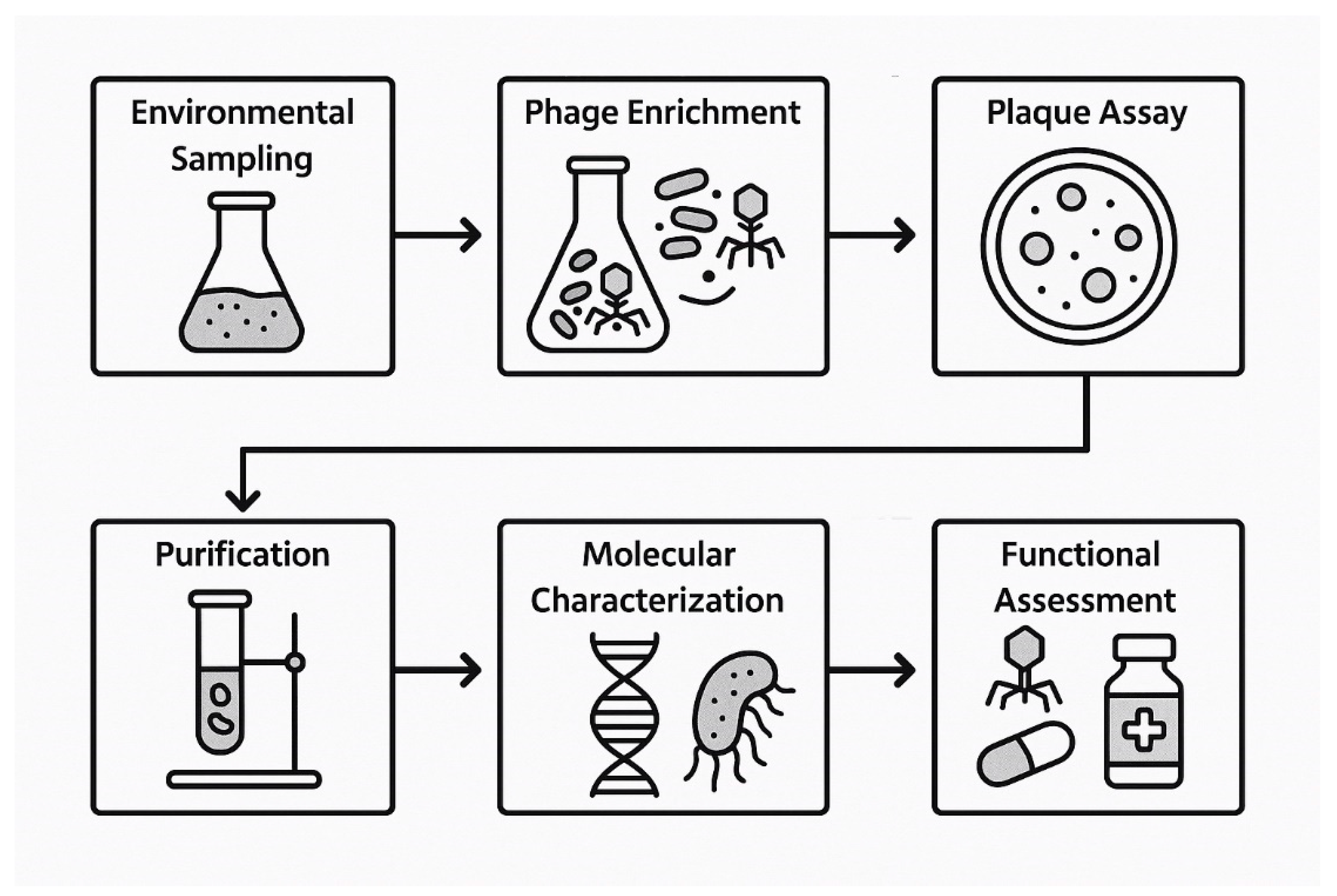
4. Discovery and Identification of Bacteriophages
4.1. Steps of Discovery
4.2. Predictive Approaches
5. Phage Display-Derived Tools
5.1. Peptides
5.2. Nanocarriers
5.3. Engineered Phage
5.4. Chemical Modification
6. Current Regulatory Framework and FDA Pathway
6.1. Regulatory Classification and Oversight Structure
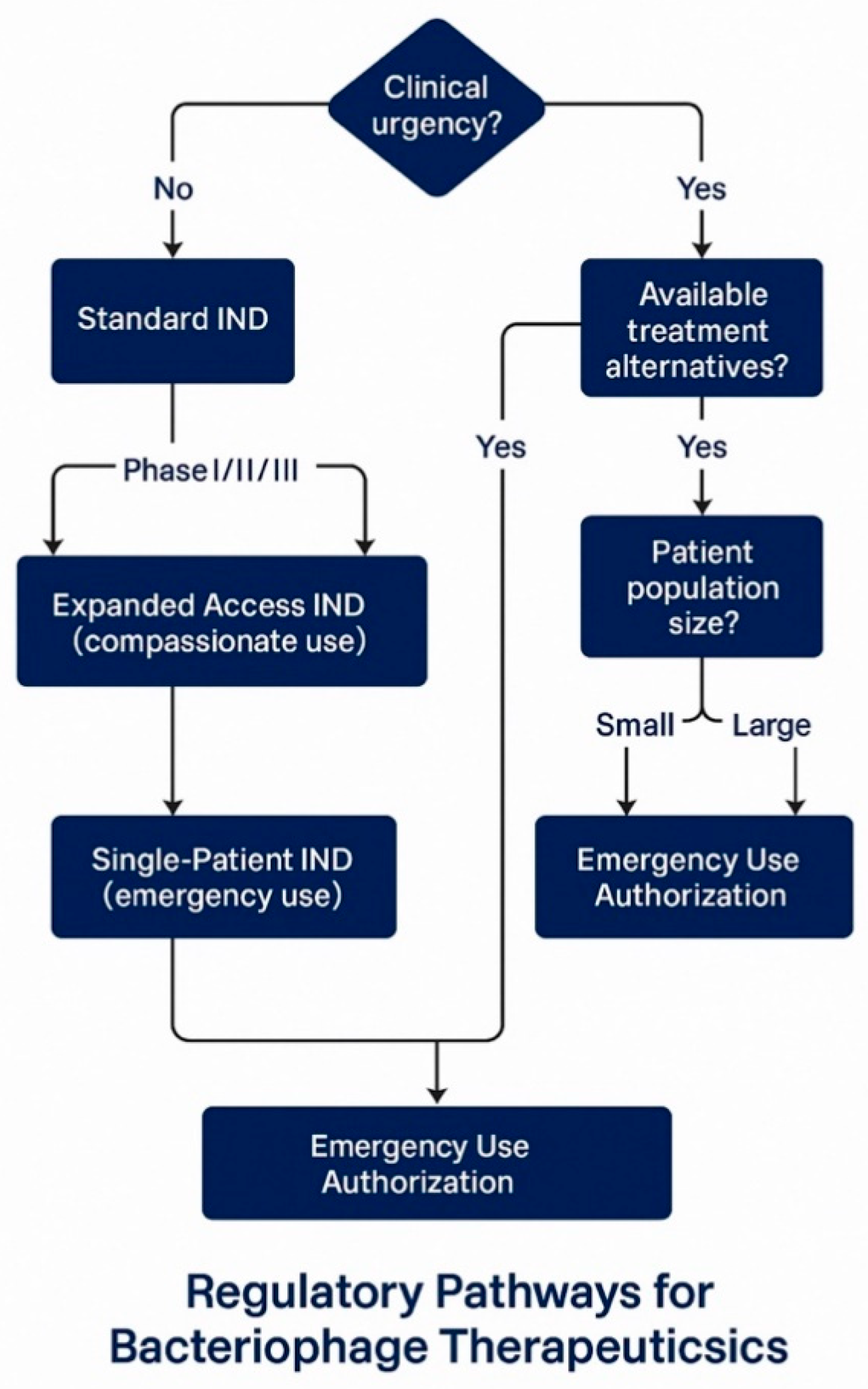
6.2. Investigational New Drug Application Pathways
6.3. FDA Engagement and Collaborative Development
6.4. Regulatory Challenges and Advocacy for Reform
7. Manufacturing and Quality Control Requirements
7.1. Good Manufacturing Practice Compliance Framework
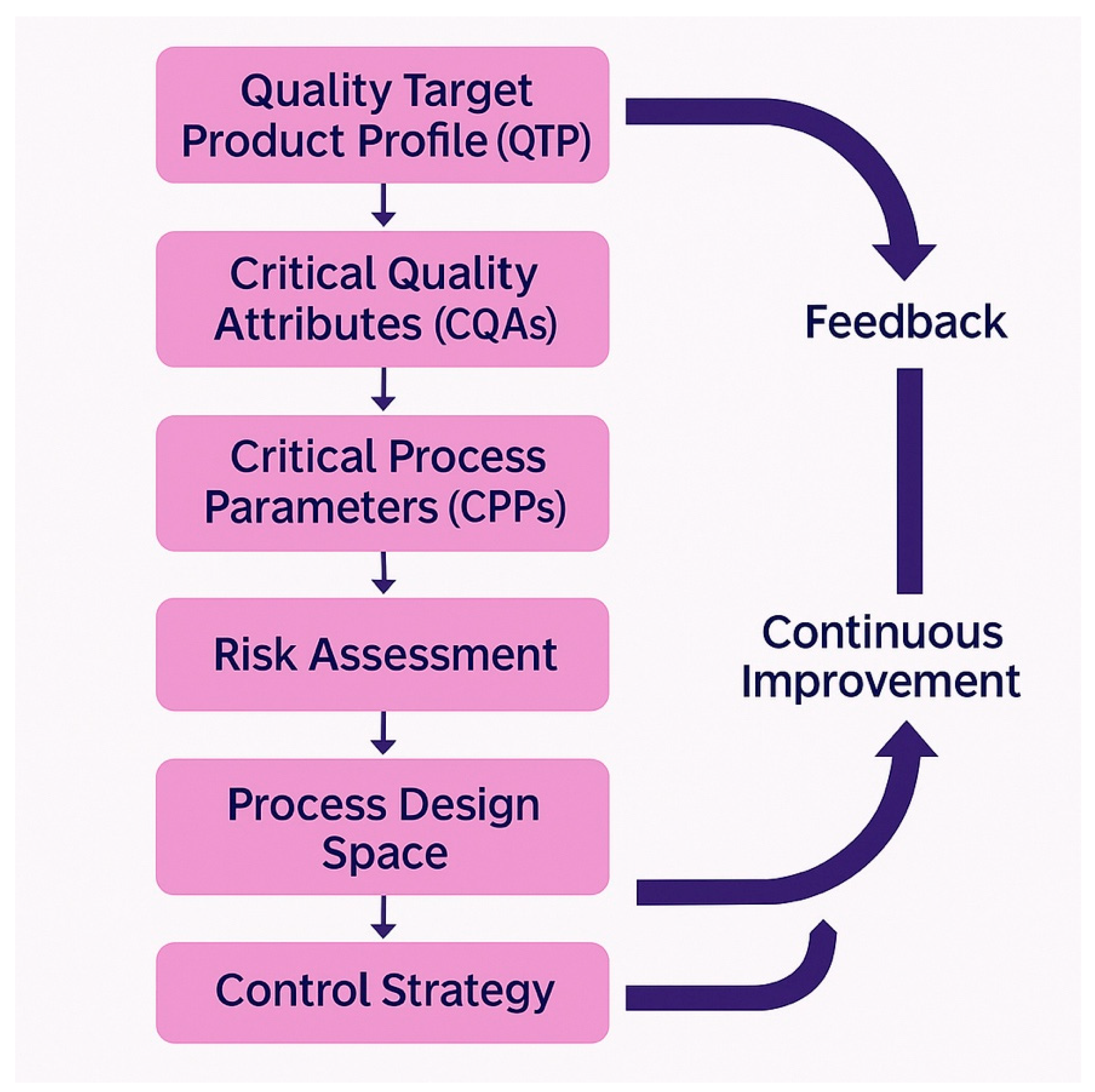
7.2. Quality by Design Implementation
7.3. Manufacturing Process Development and Challenges
7.4. Quality Control Testing and Analytical Development
8. Current Clinical Landscape and Trial Outcomes
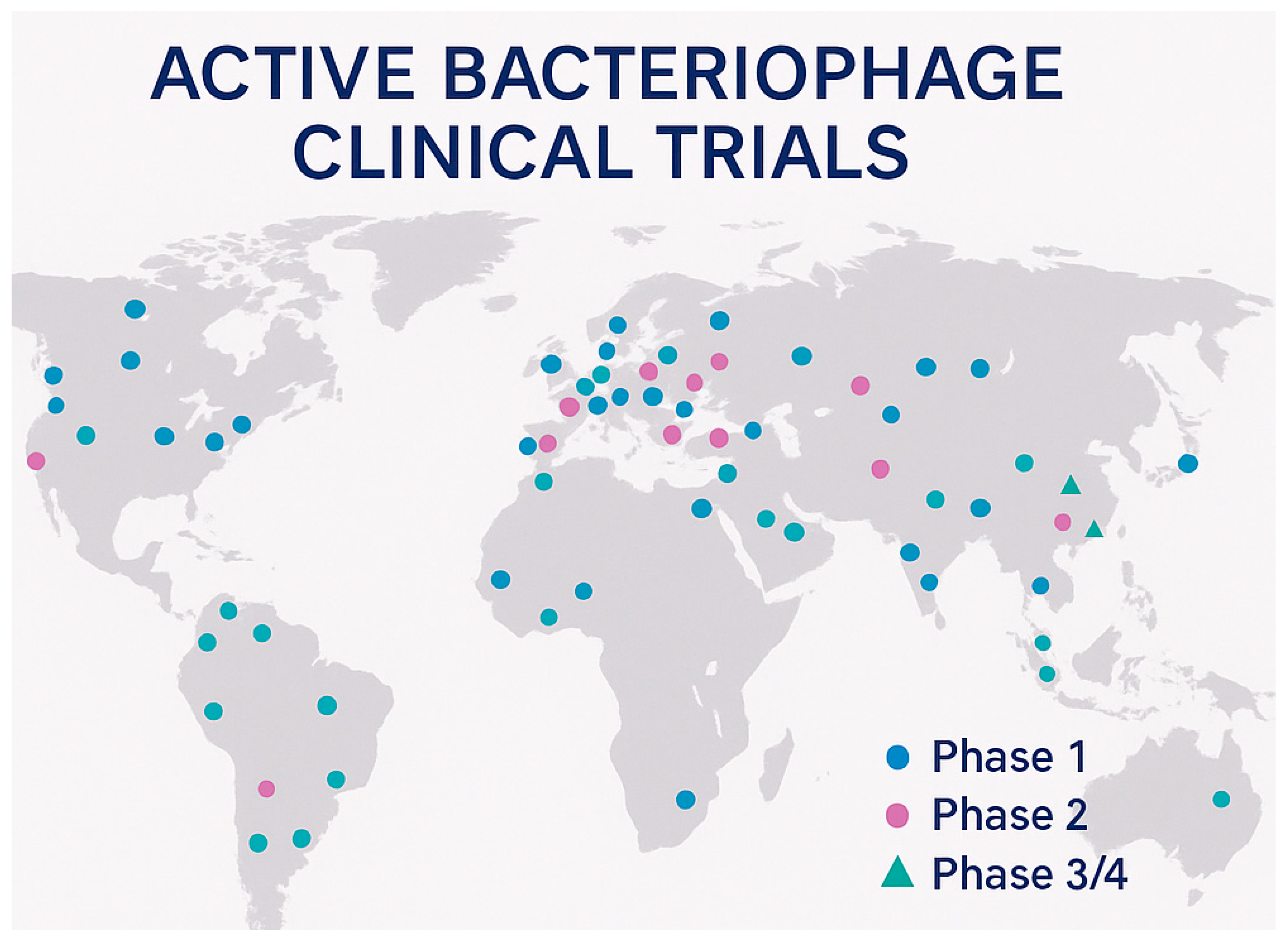
8.1. Global Clinical Trial Portfolio
8.2. Major Industry-Sponsored Clinical Programs
8.3. Academic and Compassionate Use Programs
8.4. International Clinical Experience and Outcomes
8.5. Clinical Studies by Target Bacteria
8.6. Regulatory Differences: Systemic vs. Topical Bacteriophage Products
8.7. Approved Topical Bacteriophage Products
8.7.1. Eastern European Approvals
- Pyophage (Eliava Institute, Georgia): Multi-phage cocktail for topical treatment of purulent infections;
- Intestiphage (Eliava Institute): Oral/rectal administration for gastrointestinal infections;
- Sexthaphage (Eliava Institute): Topical treatment for urogenital infections.
8.7.2. Products in Development
- PP1131 (Pherecydes Pharma): Anti-P. aeruginosa for burn wounds (Phase I/II completed);
- WPP-201 (Adaptive Phage): Topical treatment for diabetic foot ulcers (Preclinical);
- TP-102 (TechnoPhage): Topical formulation for chronic wounds (Phase I planned).
8.7.3. Regulatory Considerations for Topical Products
8.8. Bacteriophage as Food Additives: Regulatory Framework and GRAS Classification
8.8.1. GRAS (Generally Recognized as Safe) Classification Framework
8.8.2. GRAS Notification Process for Bacteriophages
8.8.3. International Harmonization and Global Approvals
8.9. Clinical Trial Challenges and Lessons Learned
9. Strategic Recommendations for FDA Approval
9.1. Pre-Clinical Development Strategy
9.2. Clinical Development Pathway Design
9.3. Regulatory Engagement and Communication Strategy
9.4. Chemistry, Manufacturing, and Controls Strategy
9.5. Commercial and Market Access Considerations
9.6. Bacteriophage Therapy in Developing Countries and Emerging Markets
9.6.1. Regional Development Initiatives
9.6.2. Infrastructure Requirements and Regulatory Capacity Building
9.6.3. Cost Barriers and Public–Private Partnership Opportunities
9.6.4. Regulatory Frameworks in Developing Countries
10. Comparative Analysis of Global Regulatory Guidelines
10.1. United States FDA Framework
10.2. European Medicines Agency (EMA) Framework
10.3. Other Stringent Regulatory Authorities
10.3.1. Health Canada
10.3.2. Japan’s PMDA
10.3.3. Australia’s TGA
10.4. Developing Country Regulatory Frameworks
10.4.1. China’s Regulatory Evolution
10.4.2. India’s Emerging Framework
10.5. Comparative Analysis and Harmonization Opportunities
11. Future Directions and Emerging Opportunities
11.1. Technological Advancements in Phage Engineering
11.2. Combination Therapy Strategies
11.3. Personalized Medicine and Diagnostic Integration
11.4. Regulatory Evolution and Global Harmonization
12. Conclusions
Funding
Conflicts of Interest
References
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- World Health Organization. Antimicrobial Resistance: Global Report on Surveillance; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/9789241564748 (accessed on 7 July 2025).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Review on Antimicrobial Resistance. 2016. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 7 July 2025).
- Kakasis, A.; Panitsa, G. Bacteriophage therapy as an alternative treatment for human infections. A comprehensive review. Int. J. Antimicrob. Agents 2019, 53, 16–21. [Google Scholar] [CrossRef]
- Strathdee, S.A.; Hatfull, G.F.; Mutalik, V.K.; Schooley, R.T. Phage therapy: From biological mechanisms to future directions. Cell 2023, 186, 17–31. [Google Scholar] [CrossRef]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162–173. [Google Scholar] [CrossRef]
- Pelfrene, E.; Willebrand, E.; Cavaleiro Sanches, A.; Sebris, Z.; Cavaleri, M. Bacteriophage therapy: A regulatory perspective. J. Antimicrob. Chemother. 2016, 71, 2071–2074. [Google Scholar] [CrossRef]
- Torres-Barceló, C. The disparate effects of bacteriophages on antibiotic-resistant bacteria. Emerg. Microbes Infect. 2018, 7, 1–12. [Google Scholar] [CrossRef]
- Roach, D.R.; Debarbieux, L. Phage therapy: Awakening a sleeping giant. Emerg. Top. Life Sci. 2017, 1, 93–103. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage therapy-history from Twort and d’Herelle through Soviet experience to current approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar] [CrossRef]
- Eaton, M.D.; Bayne-Jones, S. Bacteriophage therapy: Review of the principles and results of the use of bacteriophage in the treatment of infections. J. Am. Med. Assoc. 1934, 103, 1847–1853. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- FDA. Bacteriophage Products as Drugs Guidance for Industry; U.S. Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research: Silver Spring, MD, USA, 2021. Available online: https://www.fda.gov/media/159400/download (accessed on 7 July 2025).
- ClinicalTrials. gov. Bacteriophage Therapy Clinical Trials Database; National Institutes of Health: Bethesda, MD, USA, 2024. Available online: https://clinicaltrials.gov/ (accessed on 7 July 2025).
- Suttle, C.A. Marine viruses-major players in the global ecosystem. Nat. Rev. Microbiol. 2007, 5, 801–812. [Google Scholar] [CrossRef]
- Ackermann, H.W. Bacteriophage observations and evolution. Res. Microbiol. 2003, 154, 245–251. [Google Scholar] [CrossRef]
- Hatfull, G.F. Bacteriophage genomics. Curr. Opin. Microbiol. 2008, 11, 447–453. [Google Scholar] [CrossRef]
- Rakhuba, D.V.; Kolomiets, E.I.; Dey, E.S.; Novik, G.I. Bacteriophage receptors, mechanisms of phage adsorption and penetration into host cell. Pol. J. Microbiol. 2010, 59, 145–155. [Google Scholar] [CrossRef]
- Wang, J.; Hu, B.; Xu, M.; Yan, Q.; Liu, S.; Zhu, X.; Sun, Z.; Reed, E.; Ding, L.; Gong, J.; et al. Use of bacteriophage in the treatment of experimental animal bacteremia from imipenem-resistant Pseudomonas aeruginosa. Int. J. Mol. Med. 2006, 17, 309–317. [Google Scholar] [CrossRef]
- Leiman, P.G.; Molineux, I.J. Evolution of a new enzyme activity from the same motif fold. Mol. Microbiol. 2008, 69, 287–304. [Google Scholar] [CrossRef]
- Bertozzi Silva, J.; Storms, Z.; Sauvageau, D. Host receptors for bacteriophage adsorption. FEMS Microbiol. Lett. 2016, 363, fnw002. [Google Scholar] [CrossRef]
- Lindberg, A.A. Bacteriophage receptors. Annu. Rev. Microbiol. 1973, 27, 205–241. [Google Scholar] [CrossRef]
- Fischetti, V.A. Bacteriophage lysins as effective antibacterials. Curr. Opin. Microbiol. 2008, 11, 393–400. [Google Scholar] [CrossRef]
- Loessner, M.J. Bacteriophage endolysins-current state of research and applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Kutter, E.; Sulakvelidze, A. (Eds.) Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2005; ISBN 978-0-8493-1336-3. [Google Scholar]
- Young, R. Phage lysis: Three steps, three choices, one outcome. J. Microbiol. 2014, 52, 243–258. [Google Scholar] [CrossRef]
- Brockhurst, M.A.; Koskella, B.; Zhang, Q.G. Coevolution of bacteria and phages: The exceptional nature of the contemporary paradigm. Trends Ecol. Evol. 2007, 22, 185–191. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Merabishvili, M.; Pirnay, J.P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Van Parys, L.; et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Sterile Drug Products Produced by Aseptic Processing—Current Good Manufacturing Practice; U.S. Department of Health and Human Services, Food and Drug Administration: Beltsville, MD, USA, 2004. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/sterile-drug-products-produced-aseptic-processing-current-good-manufacturing-practice (accessed on 7 July 2025).
- FDA. Guidance for Industry: Formal Meetings Between the FDA and Sponsors or Applicants of PDUFA Products; U.S. Department of Health and Human Services, Food and Drug Administration: Beltsville, MD, USA, 2016. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/formal-meetings-between-fda-and-sponsors-or-applicants-pdufa-products (accessed on 7 July 2025).
- Harper, D.; Abedon, S.; Burrowes, B.; McConville, M. (Eds.) Bacteriophages: Biology, Technology, Therapy; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; ISBN 978-3-319-40598-8. [Google Scholar]
- Calendar, R. (Ed.) The Bacteriophages; Oxford University Press: Oxford, UK, 2006; ISBN 978-0-19-514850-3. [Google Scholar]
- Pope, W.H.; Bowman, C.A.; Russell, D.A.; Jacobs-Sera, D.; Asai, D.J.; Cresawn, S.G.; Jacobs, W.R.; Hendrix, R.W.; Lawrence, J.G.; Hatfull, G.F.; et al. Whole genome comparison of a large collection of mycobacteriophages reveals a continuum of phage genetic diversity. eLife 2015, 4, e06416. [Google Scholar] [CrossRef]
- Catalão, M.J.; Gil, F.; Moniz-Pereira, J.; São-José, C.; Pimentel, M. Diversity in bacterial lysis systems: Bacteriophages show the way. FEMS Microbiol. Rev. 2013, 37, 554–571. [Google Scholar] [CrossRef]
- Comeau, A.M.; Tétart, F.; Trojet, S.N.; Prère, M.F.; Krisch, H.M. Phage-antibiotic synergy (PAS): β-lactam and quinolone antibiotics stimulate virulent phage growth. PLoS ONE 2007, 2, e799. [Google Scholar] [CrossRef]
- Pires, D.P.; Melo, L.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and safety requirements for sustainable phage therapy products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef]
- Buckling, A.; Rainey, P.B. Antagonistic coevolution between a bacterium and a bacteriophage. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2002, 269, 931–936. [Google Scholar] [CrossRef]
- Jansen, R.; Embden, J.D.V.; Gaastra, W.; Schouls, L.M. Identification of genes that are associated with DNA repeats in prokaryotes. Mol. Microbiol. 2002, 43, 1565–1575. [Google Scholar] [CrossRef]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Bohannan, B.J.; Lenski, R.E. Linking genetic change to community evolution: Insights from studies of bacteria and bacteriophage. Ecol. Lett. 2000, 3, 362–377. [Google Scholar] [CrossRef]
- Betts, A.; Kaltz, O.; Hochberg, M.E. Contrasted coevolutionary dynamics between a bacterial pathogen and its bacteriophages. Proc. Natl. Acad. Sci. USA 2014, 111, 11109–11114. [Google Scholar] [CrossRef]
- Stern, A.; Sorek, R. The phage-host arms race: Shaping the evolution of microbes. Bioessays 2011, 33, 43–51. [Google Scholar] [CrossRef]
- Cairns, B.J.; Timms, A.R.; Jansen, V.A.; Connerton, I.F.; Payne, R.J. Quantitative models of in vitro bacteriophage-host dynamics and their application to phage therapy. PLoS Pathog. 2009, 5, e1000253. [Google Scholar] [CrossRef]
- Bull, J.J.; Levin, B.R.; DeRouin, T.; Walker, N.; Bloch, C.A. Dynamics of success and failure in phage and antibiotic therapy in experimental infections. BMC Microbiol. 2002, 2, 35. [Google Scholar] [CrossRef]
- Levin, B.R.; Bull, J.J. Population and evolutionary dynamics of phage therapy. Nat. Rev. Microbiol. 2004, 2, 166–173. [Google Scholar] [CrossRef]
- Koskella, B.; Brockhurst, M.A. Bacteria-phage coevolution as a driver of ecological and evolutionary innovation. Nat. Rev. Microbiol. 2014, 12, 44–55. [Google Scholar] [CrossRef]
- Twort, F.W. An investigation on the nature of ultra-microscopic viruses. Lancet 1915, 186, 1241–1243. [Google Scholar] [CrossRef]
- D’Herelle, F. Sur un microbe invisible antagoniste des bacilles dysentériques. Comptes Rendus Hebd. Séances L’académie Sci. 1917, 165, 373–375. [Google Scholar]
- Kutateladze, M.; Adamia, R. Bacteriophages as potential new therapeutics to replace or supplement antibiotics. Trends Biotechnol. 2010, 28, 591–595. [Google Scholar] [CrossRef]
- Conly, J.; Johnston, B. Where are all the new antibiotics? The new antibiotic paradox. Can. J. Infect. Dis. Med. Microbiol. 2005, 16, 159–160. [Google Scholar] [CrossRef]
- Górski, A.; Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Łobocka, M.; Fortuna, W.; Letkiewicz, S.; Zimecki, M.; Filby, G. Bacteriophage therapy for the treatment of infections. Curr. Opin. Investig. Drugs 2009, 10, 766–774. [Google Scholar]
- Myelnikov, D. An alternative cure: The adoption and survival of bacteriophage therapy in the USSR, 1922–1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef]
- D’Herelle, F. Bacteriophage and the Phenomenon of Recovery; Eliava, G., Translator; Tbilisi University Press: Tbilisi, Georgia, 1935. [Google Scholar]
- Sulakvelidze, A.; Alavidze, Z.; Morris, J.G., Jr. Bacteriophage therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Kutter, E.; Borysowski, J.; Międzybrodzki, R.; Górski, A.; Weber-Dąbrowska, B.; Kutateladze, M.; Alavidze, Z.; Gogokhia, L.; Adjei, M.D.; Braff, J. Phage therapy: Bacteriophages as natural, self-replicating antimicrobials. In Antimicrobial Therapies; Humana Press: Totowa, NJ, USA, 2010; pp. 1–75. [Google Scholar] [CrossRef]
- Weber-Dąbrowska, B.; Mulczyk, M.; Górski, A. Bacteriophage therapy of bacterial infections: An update of our institute’s experience. Arch. Immunol. Ther. Exp. 2000, 48, 547–551. [Google Scholar]
- Chanishvili, N.; Sharp, R. Eliava Institute of Bacteriophage, Microbiology and Virology, Georgia. In Bacteriophages; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–13. [Google Scholar] [CrossRef]
- Housby, J.N.; Mann, N.H. Phage therapy. Drug Discov. Today 2009, 14, 536–540. [Google Scholar] [CrossRef]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and use of personalized bacteriophage-based therapeutic cocktails to treat a patient with a disseminated resistant Acinetobacter baumannii infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Borysowski, J.; Weber-Dąbrowska, B.; Fortuna, W.; Letkiewicz, S.; Szufnarowski, K.; Pawełczyk, Z.; Rogóż, P.; Kłak, M.; Wojtasik, E.; et al. Clinical aspects of phage therapy. Adv. Virus Res. 2012, 83, 73–121. [Google Scholar] [CrossRef]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Méd. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- Pirnay, J.P.; De Vos, D.; Verbeken, G.; Merabishvili, M.; Chanishvili, N.; Vaneechoutte, M.; Zizi, M.; Laire, G.; Lavigne, R.; Huys, I.; et al. The phage therapy paradigm: Prêt-à-porter or sur-mesure? Pharm. Res. 2011, 28, 934–937. [Google Scholar] [CrossRef]
- Russell, D.A.; Hatfull, G.F. PhagesDB: The actinobacteriophage database. Bioinformatics 2017, 33, 784–786. [Google Scholar] [CrossRef]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef]
- Phage Australia. National Phage Therapy Network and Clinical Access Pathways; Phage Australia Consortium: Broadway, Australia, 2024; Available online: https://www.phageaustralia.org/our-approach (accessed on 7 July 2025).
- Clokie, M.R.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Jurczak-Kurek, A.; Gąsior, T.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Williamson, K.E.; Radosevich, M.; Wommack, K.E. Abundance and diversity of viruses in six Delaware soils. Appl. Environ. Microbiol. 2005, 71, 3119–3125. [Google Scholar] [CrossRef]
- Samson, J.E.; Magadán, A.H.; Sabri, M.; Moineau, S. Revenge of the phages: Defeating bacterial defences. Nat. Rev. Microbiol. 2013, 11, 675–687. [Google Scholar] [CrossRef]
- Mattila, S.; Ruotsalainen, P.; Jalasvuori, M. On-demand isolation of bacteriophages against drug-resistant bacteria for personalized phage therapy. Front. Microbiol. 2015, 6, 1271. [Google Scholar] [CrossRef]
- Ceyssens, P.J.; Miroshnikov, K.; Mattheus, W.; Krylov, V.; Robben, J.; Noben, J.P.; Vanderschraeghe, S.; Sykilinda, N.; Kropinski, A.M.; Volckaert, G. Comparative analysis of the widespread and conserved PB1-like viruses infecting Pseudomonas aeruginosa. Environ. Microbiol. 2009, 11, 2874–2883. [Google Scholar] [CrossRef]
- Mirzaei, M.K.; Nilsson, A.S. Isolation of phages for phage therapy: A comparison of spot tests and efficiency of plating analyses for determination of host range and efficacy. PLoS ONE 2015, 10, e0118557. [Google Scholar] [CrossRef]
- Kutter, E. Phage host range and efficiency of plating. In Bacteriophages; Humana Press: Totowa, NJ, USA, 2009; pp. 141–149. [Google Scholar] [CrossRef]
- Ackermann, H.W. Phage classification and characterization. In Bacteriophages; Humana Press: Totowa, NJ, USA, 2009; pp. 127–140. [Google Scholar] [CrossRef]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A self-training method for prediction of gene starts in microbial genomes. Implications for finding sequence motifs in regulatory regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Edwards, R.A.; McNair, K.; Faust, K.; Raes, J.; Dutilh, B.E. Computational approaches to predict bacteriophage–host relationships. FEMS Microbiol. Rev. 2016, 40, 258–272. [Google Scholar] [CrossRef]
- Villarroel, J.; Kleinheinz, K.A.; Jurtz, V.I.; Zschach, H.; Lund, O.; Nielsen, M.; Larsen, M.V. HostPhinder: A phage host prediction tool. Viruses 2016, 8, 116. [Google Scholar] [CrossRef]
- Kutter, E.; Gachechiladze, K.; Poglazov, A.; Marusich, E.; Shneider, M.; Aronsson, P.; Napuli, A.; Porter, D.; Mesyanzhinov, V. Evolution of T4-related phages. Virus Genes 1995, 11, 285–297. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Baek, M.; DiMaio, F.; Anishchenko, I.; Dauparas, J.; Ovchinnikov, S.; Lee, G.R.; Wang, J.; Cong, Q.; Kinch, L.N.; Schaeffer, R.D.; et al. Accurate prediction of protein structures and interactions using a three-track neural network. Science 2021, 373, 871–876. [Google Scholar] [CrossRef]
- Varadi, M.; Anyango, S.; Deshpande, M.; Nair, S.; Natassia, C.; Yordanova, G.; Yuan, D.; Stroe, O.; Wood, G.; Laydon, A.; et al. AlphaFold Protein Structure Database: Massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022, 50, D439–D444. [Google Scholar] [CrossRef]
- Arndt, D.; Grant, J.R.; Marcu, A.; Sajed, T.; Pon, A.; Liang, Y.; Wishart, D.S. PHASTER: A better, faster version of the PHAST phage search tool. Nucleic Acids Res. 2016, 44, W16–W21. [Google Scholar] [CrossRef]
- Roux, S.; Enault, F.; Hurwitz, B.L.; Sullivan, M.B. VirSorter: Mining viral signal from microbial genomic data. PeerJ 2015, 3, e985. [Google Scholar] [CrossRef]
- Lima-Mendez, G.; Van Helden, J.; Toussaint, A.; Leplae, R. Reticulate representation of evolutionary and functional relationships between phage genomes. Mol. Biol. Evol. 2008, 25, 762–777. [Google Scholar] [CrossRef]
- Pope, W.H.; Mavrich, T.N.; Garlena, R.A.; Guerrero-Bustamante, C.A.; Jacobs-Sera, D.; Montgomery, M.T.; Russell, D.A.; Warner, M.H.; Hatfull, G.F.; SEA-PHAGES. Bacteriophages of Gordonia spp. display a spectrum of diversity and genetic relationships. mBio 2017, 8, e01069-17. [Google Scholar] [CrossRef]
- Dion, M.B.; Oechslin, F.; Moineau, S. Phage diversity, genomics and phylogeny. Nat. Rev. Microbiol. 2020, 18, 125–138. [Google Scholar] [CrossRef]
- Galiez, C.; Siebert, M.; Enault, F.; Vincent, J.; Söding, J. WIsH: Who is the host? Predicting prokaryotic hosts from metagenomic phage contigs. Bioinformatics 2017, 33, 3113–3114. [Google Scholar] [CrossRef]
- Ahlgren, N.A.; Ren, J.; Lu, Y.Y.; Fuhrman, J.A.; Sun, F. Alignment-free d2* oligonucleotide frequency dissimilarity measure improves prediction of hosts from metagenomically-derived viral sequences. Nucleic Acids Res. 2017, 45, 39–53. [Google Scholar] [CrossRef]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar] [CrossRef]
- Bratkovič, T. Progress in phage display: Evolution of the technique and its applications. Cell. Mol. Life Sci. 2010, 67, 749–767. [Google Scholar] [CrossRef]
- Barbas, C.F.; Kang, A.S.; Lerner, R.A.; Benkovic, S.J. Assembly of combinatorial antibody libraries on phage surfaces: The gene III site. Proc. Natl. Acad. Sci. USA 1991, 88, 7978–7982. [Google Scholar] [CrossRef]
- Kehoe, J.W.; Kay, B.K. Filamentous phage display in the new millennium. Chem. Rev. 2005, 105, 4056–4072. [Google Scholar] [CrossRef]
- Petrenko, V.A. Evolution of phage display: From bioactive peptides to bioselective nanomaterials. Expert Opin. Drug Deliv. 2008, 5, 825–836. [Google Scholar] [CrossRef]
- Moradpour, Z.; Ghasemian, A. Modified phages: Novel antimicrobial agents to combat infectious diseases. Biotechnol. Adv. 2011, 29, 732–738. [Google Scholar] [CrossRef]
- Haq, I.U.; Chaudhry, W.N.; Akhtar, M.N.; Andleeb, S.; Qadri, I. Bacteriophages and their implications on future biotechnology: A review. Virol. J. 2012, 9, 9. [Google Scholar] [CrossRef]
- Sidhu, S.S. Engineering M13 for phage display. Biomol. Eng. 2001, 18, 57–63. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Clackson, T.; Wells, J.A. In vitro selection from protein and peptide libraries. Trends Biotechnol. 1994, 12, 173–184. [Google Scholar] [CrossRef]
- Benhar, I. Biotechnological applications of phage and cell display. Biotechnol. Adv. 2001, 19, 1–33. [Google Scholar] [CrossRef]
- Pasqualini, R.; Ruoslahti, E. Organ targeting in vivo using phage display peptide libraries. Nature 1996, 380, 364–366. [Google Scholar] [CrossRef]
- Arap, W.; Pasqualini, R.; Ruoslahti, E. Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 1998, 279, 377–380. [Google Scholar] [CrossRef]
- Kolonin, M.G.; Saha, P.K.; Chan, L.; Pasqualini, R.; Arap, W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004, 10, 625–632. [Google Scholar] [CrossRef]
- Farokhzad, O.C.; Jon, S.; Khademhosseini, A.; Tran, T.N.; LaVan, D.A.; Langer, R. Nanoparticle-aptamer bioconjugates: A new approach for targeting prostate cancer cells. Cancer Res. 2004, 64, 7668–7672. [Google Scholar] [CrossRef]
- Laakkonen, P.; Porkka, K.; Hoffman, J.A.; Ruoslahti, E. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat. Med. 2002, 8, 751–755. [Google Scholar] [CrossRef]
- Sugahara, K.N.; Teesalu, T.; Karmali, P.P.; Kotamraju, V.R.; Agemy, L.; Girard, O.M.; Hanahan, D.; Mattrey, R.F.; Ruoslahti, E. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell 2009, 16, 510–520. [Google Scholar] [CrossRef]
- Teesalu, T.; Sugahara, K.N.; Kotamraju, V.R.; Ruoslahti, E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl. Acad. Sci. USA 2009, 106, 16157–16162. [Google Scholar] [CrossRef]
- Douglas, T.; Young, M. Viruses: Making friends with old foes. Science 2006, 312, 873–875. [Google Scholar] [CrossRef]
- Larocca, D.; Witte, A.; Johnson, W.; Pierce, G.F.; Baird, A. Targeting bacteriophage to mammalian cell surface receptors for gene delivery. Hum. Gene Ther. 1998, 9, 2393–2399. [Google Scholar] [CrossRef]
- Poul, M.A.; Marks, J.D. Targeted gene delivery to mammalian cells by filamentous bacteriophage. J. Mol. Biol. 1999, 288, 203–211. [Google Scholar] [CrossRef]
- Kassner, P.D.; Burg, M.A.; Baird, A.; Larocca, D. Genetic selection of phage engineered for receptor-mediated gene transfer to mammalian cells. Biochem. Biophys. Res. Commun. 1999, 264, 921–928. [Google Scholar] [CrossRef]
- Hart, S.L.; Knight, A.M.; Harbottle, R.P.; Mistry, A.; Hunger, H.D.; Cutler, D.F.; Williamson, R.; Markham, A.F. Cell binding and internalization by filamentous phage displaying a cyclic Arg-Gly-Asp-containing peptide. J. Biol. Chem. 1994, 269, 12468–12474. [Google Scholar] [CrossRef]
- Sartorius, R.; Pisu, P.; D’Apice, L.; Pizzella, L.; Romano, C.; Cortese, R.; Aurisicchio, L.; Nicosia, A. The use of filamentous bacteriophage fd to deliver MAGE-A10 or MAGE-A3 HLA-A2-restricted peptides and to induce strong antitumor CTL responses. J. Immunol. 2008, 180, 3719–3728. [Google Scholar] [CrossRef]
- Eriksson, F.; Culp, W.D.; Massey, R.; Egevad, L.; Garland, D.; Persson, M.A.; Pisa, P. Tumor specific phage particles promote tumor regression in a mouse melanoma model. Cancer Immunol. Immunother. 2007, 56, 677–687. [Google Scholar] [CrossRef]
- Zamarin, D.; Cadena, R.S.; Deng, L.; Koehne, G.; O’Reilly, E.M. Immune checkpoint modulation: Rational design of combination strategies. Pharmacol. Ther. 2017, 150, 23–32. [Google Scholar] [CrossRef]
- Oyama, H.; Ishii, K.; Maruno, T.; Torisu, T.; Uchiyama, S. Characterization of Adeno-Associated Virus Capsid Proteins with Two Types of VP3-Related Components by Capillary Gel Electrophoresis and Mass Spectrometry. Hum. Gene Ther. 2021, 32, 1403–1416. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hashemi, H.; Pouyanfard, S.; Bandehpour, M.; Noroozbabaei, Z.; Kazemi, B.; Saelens, X.; Mokhtari-Azad, T. Immunization with M2e-displaying T7 bacteriophage nanoparticles protects against influenza A virus challenge. PLoS ONE 2012, 7, e45765. [Google Scholar] [CrossRef]
- Manchester, M.; Singh, P. Virus-based nanoparticles (VNPs): Platform technologies for diagnostic imaging. Adv. Drug Deliv. Rev. 2006, 58, 1505–1522. [Google Scholar] [CrossRef]
- Steinmetz, N.F.; Evans, D.J. Utilisation of plant viruses in bionanotechnology. Org. Biomol. Chem. 2007, 5, 2891–2902. [Google Scholar] [CrossRef]
- Huang, X.; Bronstein, L.M.; Retrum, J.; Dufort, C.; Tsvetkova, I.; Aniagyei, S.; Stein, B.; Stucky, G.; McKenna, B.; Remmes, N.; et al. Self-assembled virus-like particles with magnetic cores. Nano Lett. 2007, 7, 2407–2416. [Google Scholar] [CrossRef]
- Raja, K.S.; Wang, Q.; Gonzalez, M.J.; Manchester, M.; Johnson, J.E.; Finn, M.G. Hybrid virus-polymer materials. 1. Synthesis and properties of PEG-decorated cowpea mosaic virus. Biomacromolecules 2003, 4, 472–476. [Google Scholar] [CrossRef]
- Gillitzer, E.; Willits, D.; Young, M.; Douglas, T. Chemical modification of a viral cage for multivalent presentation. Chem Commun. 2002, 20, 2390–2391. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Destito, G.; Zijlstra, A.; Gonzalez, M.J.; Quigley, J.P.; Manchester, M.; Stuhlmann, H. Viral nanoparticles as tools for intravital vascular imaging. Nat. Med. 2006, 12, 354–360. [Google Scholar] [CrossRef]
- Destito, G.; Yeh, R.; Rae, C.S.; Finn, M.G.; Manchester, M. Folic acid-mediated targeting of cowpea mosaic virus particles to tumor cells. Chem. Biol. 2007, 14, 1152–1162. [Google Scholar] [CrossRef]
- Koonin, E.V.; Senkevich, T.G.; Dolja, V.V. The ancient virus world and evolution of cells. Biol. Direct 2006, 1, 29. [Google Scholar] [CrossRef]
- Forterre, P. The origin of viruses and their possible roles in major evolutionary transitions. Virus Res. 2006, 117, 5–16. [Google Scholar] [CrossRef]
- Roossinck, M.J. The good viruses: Viral mutualistic symbioses. Nat. Rev. Microbiol. 2011, 9, 99–108. [Google Scholar] [CrossRef]
- Wommack, K.E.; Colwell, R.R. Virioplankton: Viruses in aquatic ecosystems. Microbiol. Mol. Biol. Rev. 2000, 64, 69–114. [Google Scholar] [CrossRef]
- Suttle, C.A. Viruses in the sea. Nature 2005, 437, 356–361. [Google Scholar] [CrossRef]
- Rohwer, F.; Edwards, R. The phage proteomic tree: A genome-based taxonomy for phage. J. Bacteriol. 2002, 184, 4529–4535. [Google Scholar] [CrossRef]
- Devoto, A.E.; Santini, J.M.; Olm, M.R.; Anantharaman, K.; Burstein, D.; Keren, R.; Shechter, N.; Burgin, J.; Douglas, T.; Guo, R.; et al. Megaphages infect Prevotella and variants are widespread in gut microbiomes. Nat. Microbiol. 2019, 4, 693–700. [Google Scholar] [CrossRef]
- Brüssow, H.; Hendrix, R.W. Phage genomics: Small is beautiful. Cell 2002, 108, 13–16. [Google Scholar] [CrossRef]
- Lawrence, J.G.; Hatfull, G.F.; Hendrix, R.W. Imbroglios of viral taxonomy: Genetic exchange and failings of phenetic approaches. J. Bacteriol. 2002, 184, 4891–4905. [Google Scholar] [CrossRef]
- ICH. General Considerations for Clinical Trials E8(R1). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2016. Available online: https://www.ich.org/page/efficacy-guidelines (accessed on 7 July 2025).
- STAT: Phage therapy: Researchers Sharpen Another Arrow in the Quiver Against Antibiotic Resistance. Available online: https://www.statnews.com/2024/02/20/phage-therapy-research-antibiotic-resistance/ (accessed on 7 July 2025).
- FDA. Agency Response Letter GRAS Notice No. GRN 000170; U.S. Food and Drug Administration: Rockville, MD, USA, 2006. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/gras-notice-inventory (accessed on 7 July 2025).
- Sulakvelidze, A.; Barrow, P. Phage therapy in animals and agribusiness. In Bacteriophages: Biology and Applications; CRC Press: Boca Raton, FL, USA, 2005; pp. 335–380. ISBN 978-0-8493-1336-3. [Google Scholar]
- FDA. Guidance for Industry: Biologics License Applications (BLA) Process; U.S. Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2018. Available online: https://www.fda.gov/vaccines-blood-biologics/development-approval-process-cber/biologics-license-applications-bla-process-cber (accessed on 7 July 2025).
- Locus Biosciences. ELIMINATE Phase 2/3 Clinical Trial Announcement; Locus Biosciences, Inc.: Durham, NC, USA, 2022; Available online: https://www.locus-bio.com/locus-biosciences-announces-first-patient-treated-in-the-eliminate-registrational-phase-2-3-trial-of-lbp-ec01-for-urinary-tract-infections/ (accessed on 7 July 2025).
- Armata Pharmaceuticals. Clinical Development Pipeline; Armata Pharmaceuticals, Inc.: Marina del Rey, CA, USA, 2024; Available online: https://www.armatapharma.com/pipeline (accessed on 7 July 2025).
- FDA. Guidance for Industry: Expanded Access to Investigational Drugs for Treatment Use; U.S. Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2013. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/expanded-access-investigational-drugs-treatment-use-qs (accessed on 7 July 2025).
- Aitken, S.L.; Dilley, J.J.; Wang, X.; Hanisch, B.; Wanger, A.; Aslam, S.; Pankey, G.; Doernberg, S.; Biswas, B.; Henry, M. Bacteriophage therapy for treatment of patients with drug-resistant bacterial infections: A review of challenges and considerations for clinical practice. Clin. Infect. Dis. 2023, 77, 1259–1269. [Google Scholar] [CrossRef]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current status of bacteriophage therapy for severe bacterial infections. J. Intensive Care 2024, 12, 44. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Individual Patient Expanded Access Applications: Form FDA 3926; U.S. Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2017. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/individual-patient-expanded-access-applications-form-fda-3926 (accessed on 7 July 2025).
- Young, R.; Gill, J.J.; Steele, A. Phage therapy redux–what is to be done? Trends Microbiol. 2021, 29, 350–360. [Google Scholar] [CrossRef]
- FDA. Guidance for Industry: Antibacterial Therapies for Patients with an Unmet Medical Need for the Treatment of Serious Bacterial Diseases; U.S. Department of Health and Human Services, Food and Drug Administration: Rockville, MD, USA, 2019. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/antibacterial-therapies-patients-unmet-medical-need-treatment-serious-bacterial-diseases (accessed on 7 July 2025).
- Luong, T.; Salabarria, A.C.; Roach, D.R. Phage therapy in the era of resistance: Where do we stand and where are we going? Clin. Ther. 2020, 42, 1659–1680. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage therapy: Clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 2018, 8, 376. [Google Scholar] [CrossRef]
- ASM. Phage Therapy: Past, Present and Future; American Society for Microbiology: Washington, DC, USA, 2022; Available online: https://asm.org/articles/2022/august/phage-therapy-past,-present-and-future (accessed on 7 July 2025).
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]
- Rohde, C.; Resch, G.; Pirnay, J.P.; Blasdel, B.G.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E.; et al. Expert opinion on three phage therapy related topics: Bacterial phage resistance, phage training and prophages in bacterial production strains. Viruses 2018, 10, 178. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.P.; De Vos, D.; Jennes, S.; Zizi, M.; Lavigne, R.; Casteels, M.; Huys, I. Optimizing the European regulatory framework for sustainable bacteriophage therapy. Arch. Immunol. Ther. Exp. 2014, 62, 31–39. [Google Scholar] [CrossRef]
- ICH. Pharmaceutical Development Q8(R2). International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2009. Available online: https://www.ich.org/page/quality-guidelines (accessed on 7 July 2025).
- ICH. Quality Risk Management Q9. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2005. Available online: https://www.ich.org/page/quality-guidelines (accessed on 7 July 2025).
- NIH. National Institutes of Health Funding for Phage Therapy Research; National Institutes of Health: Bethesda, MD, USA, 2024. Available online: https://www.nih.gov/ (accessed on 7 July 2025).
- BARDA. Biomedical Advanced Research and Development Authority Funding Announcements; U.S. Department of Health and Human Services: Washington, DC, USA, 2024. Available online: https://aspr.hhs.gov/AboutASPR/ProgramOffices/BARDA/Pages/default.aspx (accessed on 7 July 2025).
- TechnoPhage. TP-122A Clinical Development Program; TechnoPhage, S.A.: Cantanhede, Portugal, 2024; Available online: https://www.technophage.pt/ (accessed on 7 July 2025).
- Maddocks, S.; Fabijan, A.P.; Ho, J.; Lin, R.C.; Ben Zakour, N.L.; Dugan, C.; Kliman, I.; Branston, S.; Morales, S.; Iredell, J.R. Real-world evidence for the use of bacteriophage therapy in treating drug-resistant bacterial infections. J. Antimicrob. Chemother. 2019, 74, 3720–3726. [Google Scholar] [CrossRef]
- Duplessis, C.; Biswas, B.; Hanisch, B.; Perkins, M.; Henry, M.; Quinones, J.; Harmon, D.; Gomez, A.; Ganem, C.; Knox, J. Refractory Pseudomonas bacteremia in a 2-year-old sterilized by bacteriophage therapy. J. Pediatr. Infect. Dis. Soc. 2018, 7, 253–256. [Google Scholar] [CrossRef]
- Chaudhry, W.N.; Concepción-Acevedo, J.; Park, T.; Andleeb, S.; Bull, J.J.; Levin, B.R. Synergy and order effects of antibiotics and phages in killing Pseudomonas aeruginosa biofilms. PLoS ONE 2017, 12, e0168615. [Google Scholar] [CrossRef]
- EMA. Guideline on Quality, Non-Clinical and Clinical Aspects of Medicinal Products Containing Genetically Modified Cells; European Medicines Agency: Amsterdam, The Netherlands, 2015; Available online: https://www.ema.europa.eu/en/human-regulatory/overview/advanced-therapy-medicinal-products-overview (accessed on 7 July 2025).
- TGA. Therapeutic Goods Administration Special Access Scheme Guidance; Australian Government Department of Health: Woden, Australia, 2023. Available online: https://www.tga.gov.au/sites/default/files/2024-10/special-access-scheme-sas-guidance.pdf (accessed on 7 July 2025).
- Tan, D.; Zhang, Y.; Cheng, M.; Le, S.; Gu, J.; Bao, J.; Qin, J.; Guo, X.; Zhu, T. Characterization of Klebsiella pneumoniae ST11 isolates and their interactions with lytic phages. Viruses 2018, 10, 110. [Google Scholar] [CrossRef]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef]
- World Bank. World Bank Country and Lending Groups. The World Bank Group. 2024. Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519 (accessed on 7 July 2025).
- Zhang, L.; Bao, J.; Deng, X.; Liu, K.; Ji, H.; Chen, J.; Feng, T.; Gu, J. Global trends and research hotspots of bacteriophage therapy: A bibliometric analysis. Front. Microbiol. 2022, 13, 886803. [Google Scholar]
- Liang, S.; Qi, Y.; Yu, H.; Sun, W.; Raza, S.H.A.; Alkhorayef, N.; Alkhalil, S.S.; Salama, E.E.A.; Zhang, L. Bacteriophage Therapy as an Application for Bacterial Infection in China. Antibiotics 2023, 12, 417. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- ICMR. Guidelines for Phage Therapy Research and Clinical Implementation in India; Indian Council of Medical Research: New Delhi, India, 2023. Available online: https://www.icmr.gov.in/ (accessed on 7 July 2025).
- NMPA. National Medical Products Administration Regulatory Guidelines for Novel Therapeutics; China National Medical Products Administration: Beijing, China, 2020. Available online: https://www.nmpa.gov.cn/ (accessed on 7 July 2025).
- Yao, J.; Zou, P.; Cui, Y.; Quan, L.; Gao, C.; Li, Z.; Gong, W.; Yang, M. Recent Advances in Strategies to Combat Bacterial Drug Resistance: Antimicrobial Materials and Drug Delivery Systems. Pharmaceutics 2023, 15, 1188. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Grand View Research. Bacteriophage Market Size, Share & Trends Analysis Report; Grand View Research, Inc.: San Francisco, CA, USA, 2021; Available online: https://www.grandviewresearch.com/industry-analysis/bacteriophage-market (accessed on 7 July 2025).
- APAC Healthcare Market Research. Asia Pacific Bacteriophage Therapy Market Outlook; APAC Healthcare Analytics: North Sydney, Australia, 2023. [Google Scholar]
- Hatfull, G.F.; Dedrick, R.M.; Schooley, R.T. Phage Therapy for Antibiotic-Resistant Bacterial Infections. Annu. Rev. Med. 2022, 73, 197–211. [Google Scholar] [CrossRef] [PubMed]
- EMA. Reflection Paper on the Therapeutic Use of Bacteriophages; European Medicines Agency: Amsterdam, The Netherlands, 2011; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/reflection-paper-promoting-authorisation-alternatives-antimicrobial-veterinary-medicinal-products-eu_en.pdf (accessed on 7 July 2025).
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dąbrowska, B.; Bagińska, N.; et al. Phage therapy: What have we learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef]
- Pirnay, J.P.; Verbeken, G.; Rose, T.; Jennes, S.; Zizi, M.; Huys, I.; Piessen, G.; Ameloot, C.; Fauconnier, A. Introducing yesterday’s phage therapy in today’s medicine. Future Virol. 2012, 7, 379–390. [Google Scholar] [CrossRef]
- Uyama, Y.; Shibata, T.; Nagai, N.; Hanaoka, H.; Toyoshima, S.; Mori, K. Successful bridging strategy based on ICH E5 guideline for drugs approved in Japan. Clin. Pharmacol. Ther. 2005, 78, 112–116. [Google Scholar] [CrossRef]
- PMDA. Pharmaceuticals and Medical Devices Agency Guidance on Regenerative Medicine Products; Pharmaceuticals and Medical Devices Agency: Tokyo, Japan, 2020; Available online: https://www.pmda.go.jp/english/ (accessed on 7 July 2025).
- Tominaga, T.; Kawakami, K.; Ushijima, K.; Kawano, K.; Funakoshi, K.; Ando, H.; Hirai, T.; Teramachi, H. Regulatory harmonization for innovative therapies: Perspectives from Japan. Regen. Ther. 2021, 16, 30–37. [Google Scholar] [CrossRef]
- Wu, L.; Ding, Q.; Jiang, H.; Liu, J.; Yang, H.; Zhang, J.; Yu, W.; Zhang, Q.; Zhu, B.; Kang, M.; et al. Personalized phage therapy against multidrug-resistant Acinetobacter baumannii lung infection in a patient with chronic obstructive pulmonary disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef]
- ICH. Emerging Guidance on Novel Biological Therapeutics. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2024. Available online: https://www.ich.org/ (accessed on 7 July 2025).
- Yehl, K.; Lemire, S.; Yang, A.C.; Ando, H.; Mimee, M.; Torres, M.D.; de la Fuente-Nunez, C.; Lu, T.K. Engineering phage host-range and suppressing bacterial resistance through phage tail fiber mutagenesis. Cell 2019, 179, 459–469. [Google Scholar] [CrossRef]
- EMA. Reflection Paper on the Therapeutic Use of Bacteriophages—Updated Considerations; European Medicines Agency: Amsterdam, The Netherlands, 2019; Available online: https://www.ema.europa.eu/en/quality-safety-and-efficacy-bacteriophages-veterinary-medicines-scientific-guideline (accessed on 7 July 2025).
- Berryhill, B.A.; Gil-Gil, T.; Smith, A.P.; Levin, B.R. The future of phage therapy in the USA. Trends Mol. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Bacteriophages | Conventional Antibiotics | References |
|---|---|---|---|
| Spectrum of Activity | Narrow (strain/species level) | Broad spectrum (multiple species) | [26,27] |
| Mechanism of Action | Host-specific lysis and replication | Metabolic inhibition or cell wall disruption | [24,28] |
| Resistance Development | Co-evolution with host; reversible | Often irreversible, cross-resistance is common | [9,29] |
| Effect on Microbiome | Minimal impact on non-target bacteria | Significant disruption of commensal flora | [9,30] |
| Pharmacokinetics | Self-amplifying at the infection site | Traditional dose–response relationship | [6,13] |
| Manufacturing | Live biological agent; batch variability | Chemical synthesis; consistent batches | [8,31] |
| Stability | Temperature and pH sensitive | Generally stable under ambient conditions | [8,32] |
| Regulatory Classification | Biological product (CBER) | Drug product (CDER) | [14,33] |
| Development Timeline | 5–10 years (with regulatory support) | 10–15 years (traditional pathway) | [30,34] |
| Pathway Type | Clinical Scenario | Timeline | Key Requirements | Success Examples | References |
|---|---|---|---|---|---|
| Standard IND | Systematic development | 5–10 years | Complete preclinical package, GMP manufacturing, controlled trials | Locus Biosciences LBP-EC01, Armata AP-PA02 | [143,144,145] |
| Expanded Access IND | Serious/life-threatening conditions | 30–60 days | Treatment IND application, safety data, no comparable therapy | TAILΦR program (12/50 patients treated) | [146,147,148] |
| Single-Patient IND | Emergency treatment | 24–48 h | Emergency IND application, physician justification, informed consent | Tom Patterson case (2016), multiple IPATH cases | [64,69,149] |
| Emergency Use Authorization | Public health emergency | Days to weeks | EUA request, safety/efficacy data, risk–benefit analysis | Available but not yet utilized for phages | [149,150] |
| Quality Attribute | Test Method | Acceptance Criteria | Regulatory Rationale | References |
|---|---|---|---|---|
| Identity | PCR, sequencing, restriction analysis | Match the reference standard | Ensure correct phage product | [68,80] |
| Phage Titer | Plaque-forming units (PFU) | ≥108 PFU/mL (typical) | Therapeutic potency | [77,78] |
| Purity–Host Proteins | Bradford assay, SDS-PAGE | ≤10% total protein | Product quality and safety | [8,31] |
| Purity–Bacterial DNA | qPCR quantification | ≤10 ng/dose | Prevent immune reactions | [8,160] |
| Endotoxin Level | LAL assay | ≤5 EU/kg body weight | Pyrogenicity prevention | [32,159] |
| Sterility | USP <71> method | No growth | Patient safety | [32,159] |
| pH | pH meter | 6.5–8.0 (typical) | Stability and biocompatibility | [8,31] |
| Osmolality | Osmometer | 280–320 mOsm/kg | Biocompatibility | [32,160] |
| Particulate Matter | Light obscuration | USP <788> limits | Injectable safety | [32,159] |
| Container Closure Integrity | Helium leak test | No detectable leaks | Sterility maintenance | [32,159] |
| Company/Institution | Product | Target Pathogen | Indication | Phase | Status | Primary Endpoint | References |
|---|---|---|---|---|---|---|---|
| Locus Biosciences | LBP-EC01 | E. coli | Uncomplicated UTI | II/III | Active | Clinical cure rate | [15,144] |
| Armata Pharmaceuticals | AP-PA02 | P. aeruginosa | CF lung infection | Ib/IIa | Completed | Safety/tolerability | [15,145] |
| Armata Pharmaceuticals | AP-PA02 | P. aeruginosa | Non-CF bronchiectasis | II | Active | Bacterial load reduction | [15,145] |
| Armata Pharmaceuticals | AP-SA01 | S. aureus | Bacteremia | Ib/IIa | Active | Safety/PK profile | [15,145] |
| TechnoPhage | TP-122A | P. aeruginosa | Ventilator pneumonia | I/IIa | Active | Safety/tolerability | [15,163] |
| Adaptive Phage Therapeutics | AdAPT-001 | Various MDR | Prosthetic joint infection | I | Planning | Safety assessment | [15] |
| Pherecydes Pharma | PP1131 | P. aeruginosa | Burn wound infection | I/II | Terminated | Bacterial load reduction | [153] |
| TAILΦR/Baylor | Custom cocktails | Patient-specific | Compassionate use | N/A | Ongoing | Clinical improvement | [147,148] |
| Study/Program | Patient Population | Treatment Approach | Clinical Improvement | Bacterial Eradication | Key Findings | References |
|---|---|---|---|---|---|---|
| Belgian Consortium (n = 100) | Multi-country severe infections | Individualized phage therapy | 77.2% | 61.3% | Combination with antibiotics improved outcomes | [164] |
| TAILΦR Program (n = 12) | Device-related/systemic infections | Customized phage cocktails | 75% | 58% | 38/50 requests could not be accommodated | [147,148] |
| PhagoBurn Trial (n = 220) | P. aeruginosa burn infections | Standardized phage PP1131 | No significant benefit | Slower than control | Trial terminated for insufficient efficacy | [153] |
| IPATH Program (n = 20+) | Compassionate use cases | Personalized treatments | 70% | 50% | Variable outcomes based on infection type | [69] |
| Locus ELIMINATE (interim) | E. coli UTI | Engineered phage cocktail | 100% (small cohort) | 85% | Symptoms resolved by day 10 | [144] |
| Target Bacteria | Completed Studies | Ongoing Studies | Planned Studies | Primary Indications | Key Sponsors | References |
|---|---|---|---|---|---|---|
| Pseudomonas aeruginosa | 8 | 12 | 6 | CF lung infections, VAP, burn wounds, chronic wounds | Armata, TechnoPhage, Pherecydes | [15,145,153] |
| Escherichia coli | 3 | 8 | 4 | UTI, bloodstream infections, ESBL infections | Locus Biosciences, BiomX | [15,144] |
| Staphylococcus aureus | 4 | 6 | 3 | Bacteremia, MRSA infections, prosthetic infections | Armata, Adaptive Phage | [15,145] |
| Acinetobacter baumannii | 2 | 4 | 2 | VAP, burn infections, MDR infections | IPATH, TAILΦR | [15,64,147] |
| Enterococcus spp. | 1 | 3 | 2 | VRE infections, UTI | Academic centers | [15] |
| Clostridium difficile | 2 | 2 | 1 | CDI, recurrent CDI | BiomX | [15] |
| Mixed Gram-negative | 5 | 8 | 4 | Sepsis, wound infections | Multiple | [15] |
| Total | 25 | 43 | 22 | Various | 90 Global Studies | [15,155] |
| Regulatory Aspect | Systemic Products | Topical Products | References |
|---|---|---|---|
| FDA Classification | Biological product (CBER) | Biological product (CBER) | [14,33] |
| Phase I Requirements | Full dose escalation, extensive safety monitoring | Limited dose escalation, local tolerability focus | [139,151] |
| Pharmacokinetics | Comprehensive PK/PD studies required | Limited systemic exposure assessment | [6,151] |
| Manufacturing Standards | Full GMP compliance from Phase I | GMP compliance with potential relaxed standards | [8,32] |
| Sterility Requirements | Terminal sterilization or aseptic processing | Bioburden control, antimicrobial effectiveness | [32,159] |
| Endotoxin Limits | ≤5 EU/kg body weight | ≤20 EU/g product (topical limit) | [32,160] |
| Clinical Endpoints | Microbiological and clinical cure | Local bacterial reduction, wound healing | [151,153] |
| Safety Database | 300–600 patients for approval | 100–300 patients typically sufficient | [143,151] |
| Container Closure | Parenteral packaging standards | Topical packaging, stability considerations | [32,159] |
| Labeling Requirements | Comprehensive systemic safety warnings | Local application warnings, skin sensitivity | [14,151] |
| Product Name | Manufacturer | Target Bacteria | Approval Year | Application | GRAS Status | References |
|---|---|---|---|---|---|---|
| ListShield™ | Intralytix (Columbia, MD, USA) | Listeria monocytogenes | 2006 | Ready-to-eat foods | GRN 000170 | [141,142] |
| EcoShield™ | Intralytix | E. coli O157:H7 | 2007 | Ground beef, fresh produce | GRN 000218 | [141,142] |
| SalmoFresh™ | Intralytix | Salmonella spp. | 2009 | Poultry, eggs | GRN 000275 | [141,142] |
| ShigaShield™ | Intralytix | Shigella spp. | 2010 | Fresh produce | GRN 000320 | [141,142] |
| PhageGuard S | Micreos (Zug, Switzerland) | Salmonella spp. | 2013 | Processed foods | GRN 000435 | [141,142] |
| PhageGuard E | Micreos | E. coli | 2015 | Meat products | GRN 000510 | [141,142] |
| FoodShield™ | Intralytix | Multi-pathogen | 2018 | Various applications | GRN 000745 | [141,142] |
| AquaShield™ | Intralytix | Aquaculture pathogens | 2020 | Fish farming | GRN 000855 | [141,142] |
| Region/Country | Regulatory Authority | Approval Pathway | Approved Products | Market Status | References |
|---|---|---|---|---|---|
| United States | FDA/CFSAN | GRAS notification | 8+ products | Commercial | [141,142] |
| European Union | EFSA | Novel food assessment | 3 products | Limited commercial | [158,167] |
| Canada | Health Canada | Food additive petition | 2 products | Commercial | [158] |
| Australia | FSANZ | Food additive application | 1 product | Commercial | [70,168] |
| New Zealand | FSANZ | Food additive application | 1 product | Commercial | [70,168] |
| Israel | Ministry of Health | Food safety approval | 4 products | Commercial | [158] |
| South Korea | K-FDA | Food additive approval | 1 product | Limited | [158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niazi, S.K. Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways. Pharmaceuticals 2025, 18, 1115. https://doi.org/10.3390/ph18081115
Niazi SK. Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways. Pharmaceuticals. 2025; 18(8):1115. https://doi.org/10.3390/ph18081115
Chicago/Turabian StyleNiazi, Sarfaraz K. 2025. "Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways" Pharmaceuticals 18, no. 8: 1115. https://doi.org/10.3390/ph18081115
APA StyleNiazi, S. K. (2025). Bacteriophage Therapy: Discovery, Development, and FDA Approval Pathways. Pharmaceuticals, 18(8), 1115. https://doi.org/10.3390/ph18081115







