Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS
Abstract
1. Introduction
2. Results
2.1. α-Glucosidase Inhibitory Effects of SP Extracts In Vitro
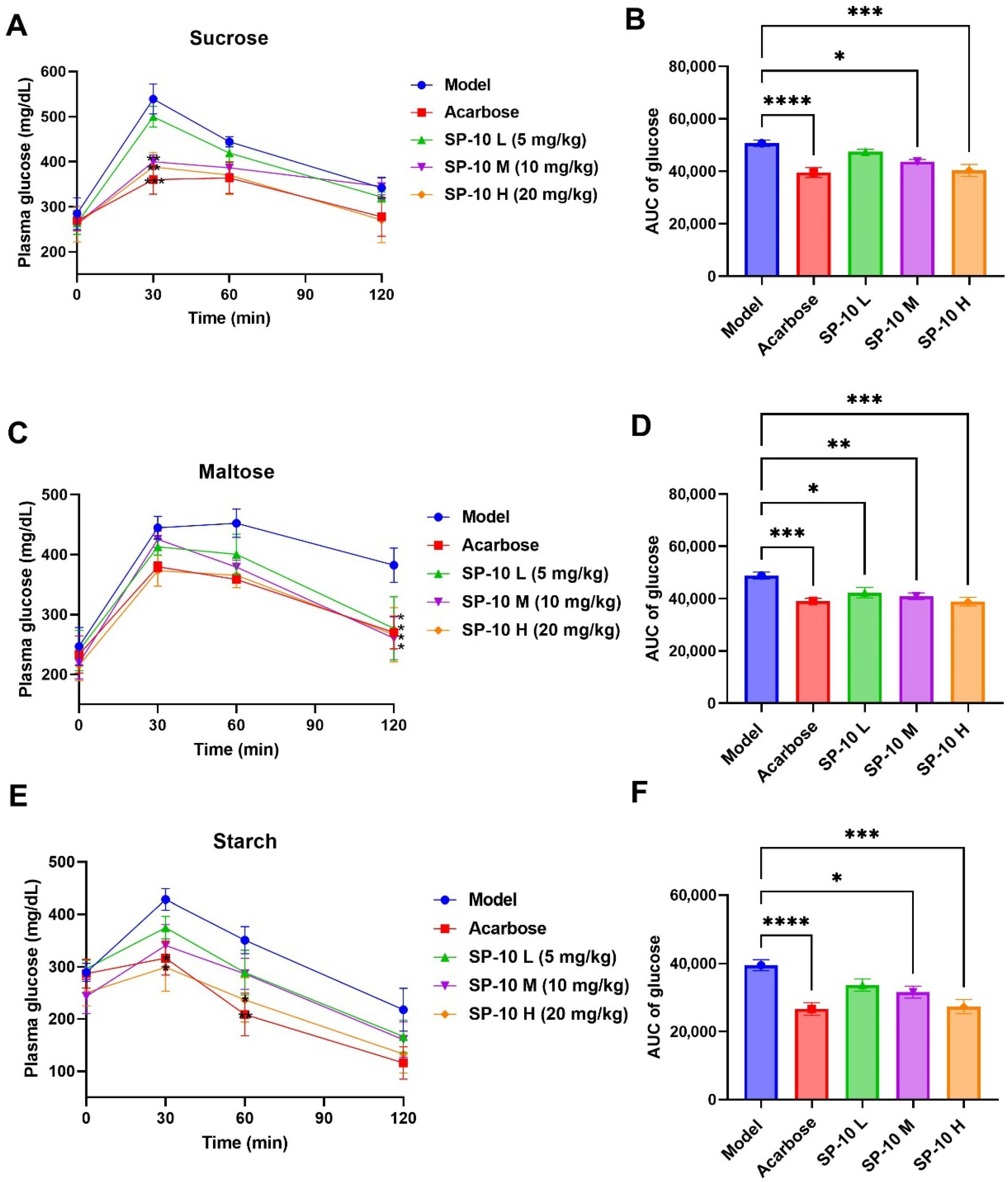
2.2. PBG-Lowering Effects of SP-10 in Diabetic Mice
2.3. Chemical Profiling of SP-10
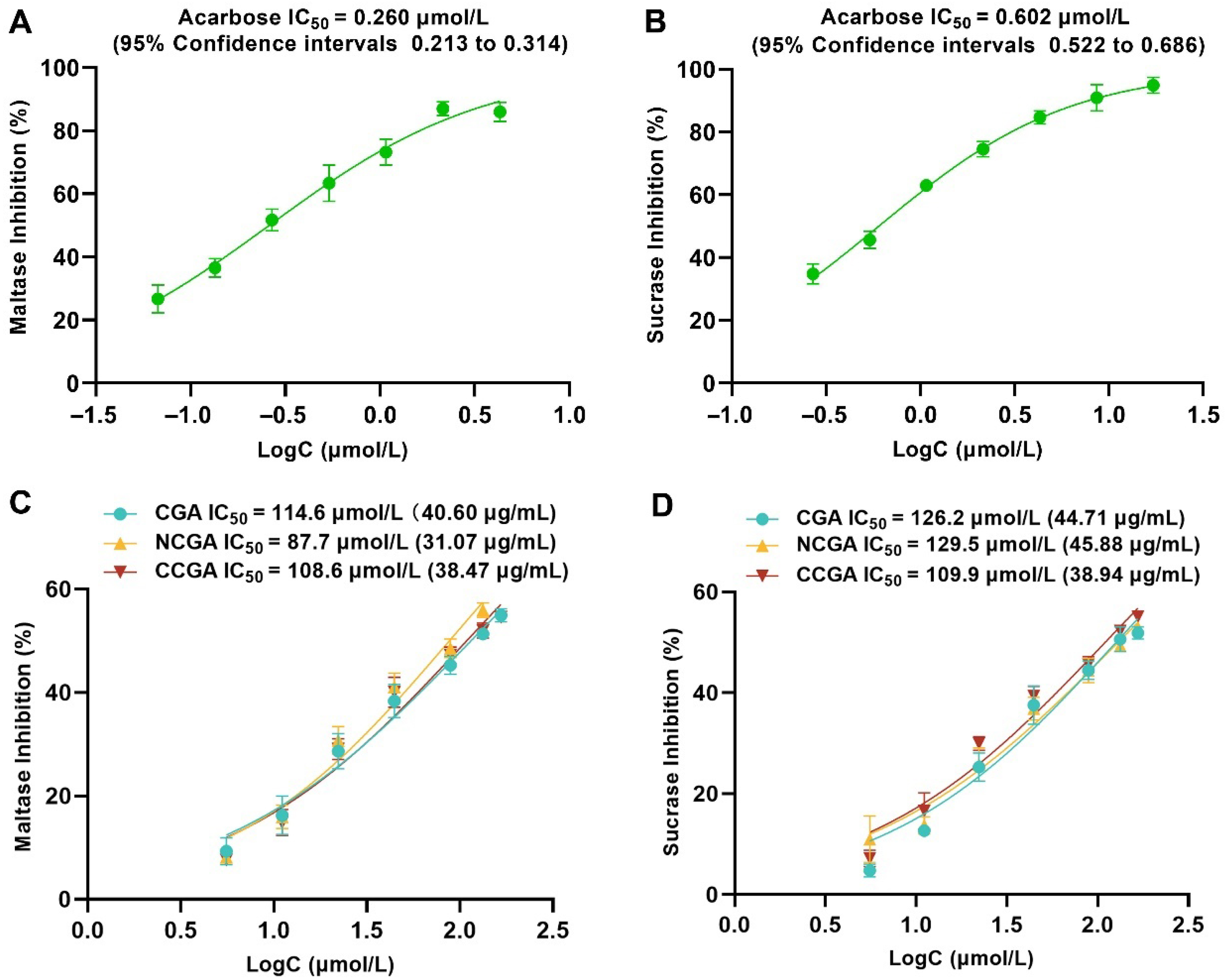
2.4. The Main Compounds of SP-10 Inhibit α-Glucosidase In Vitro
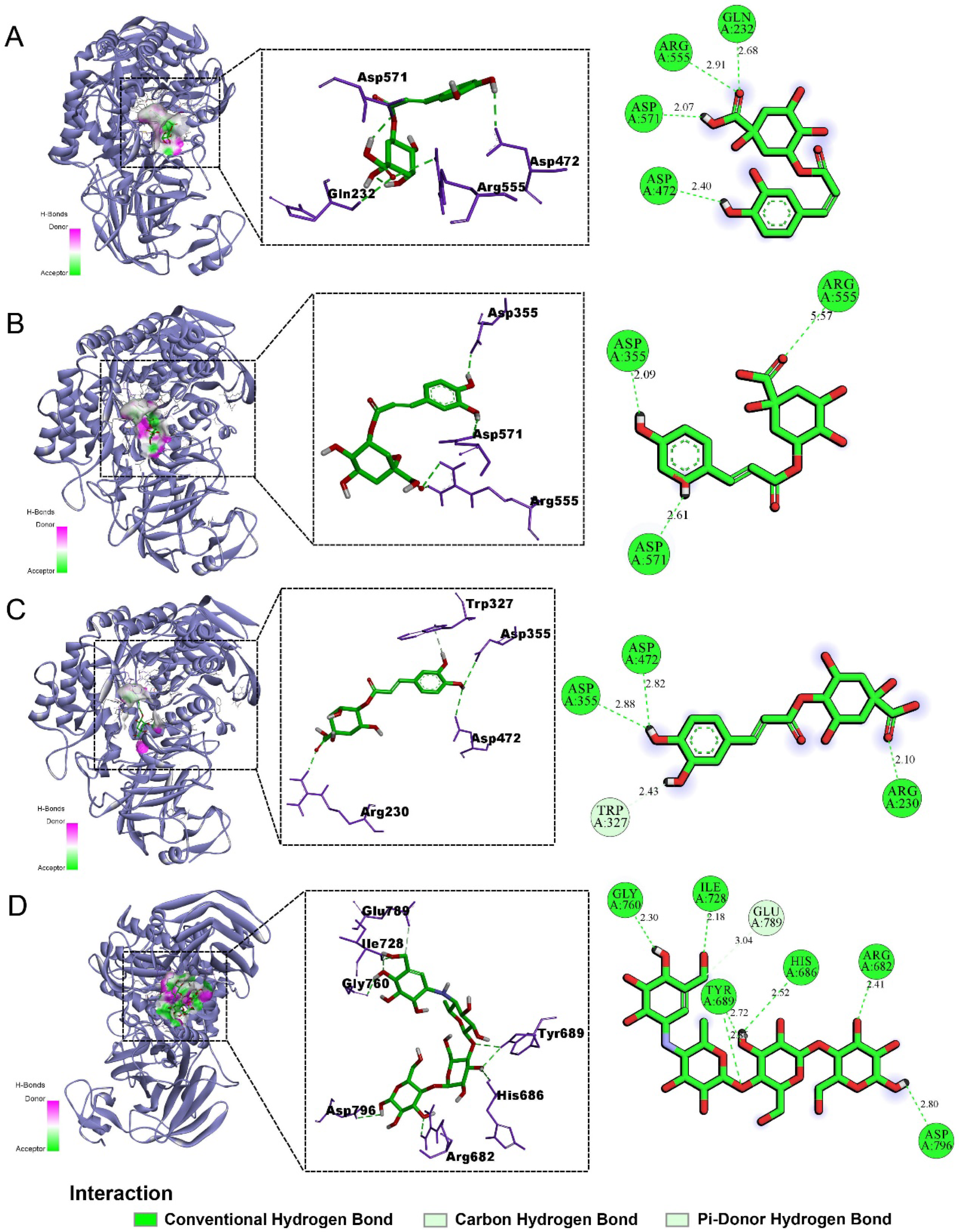
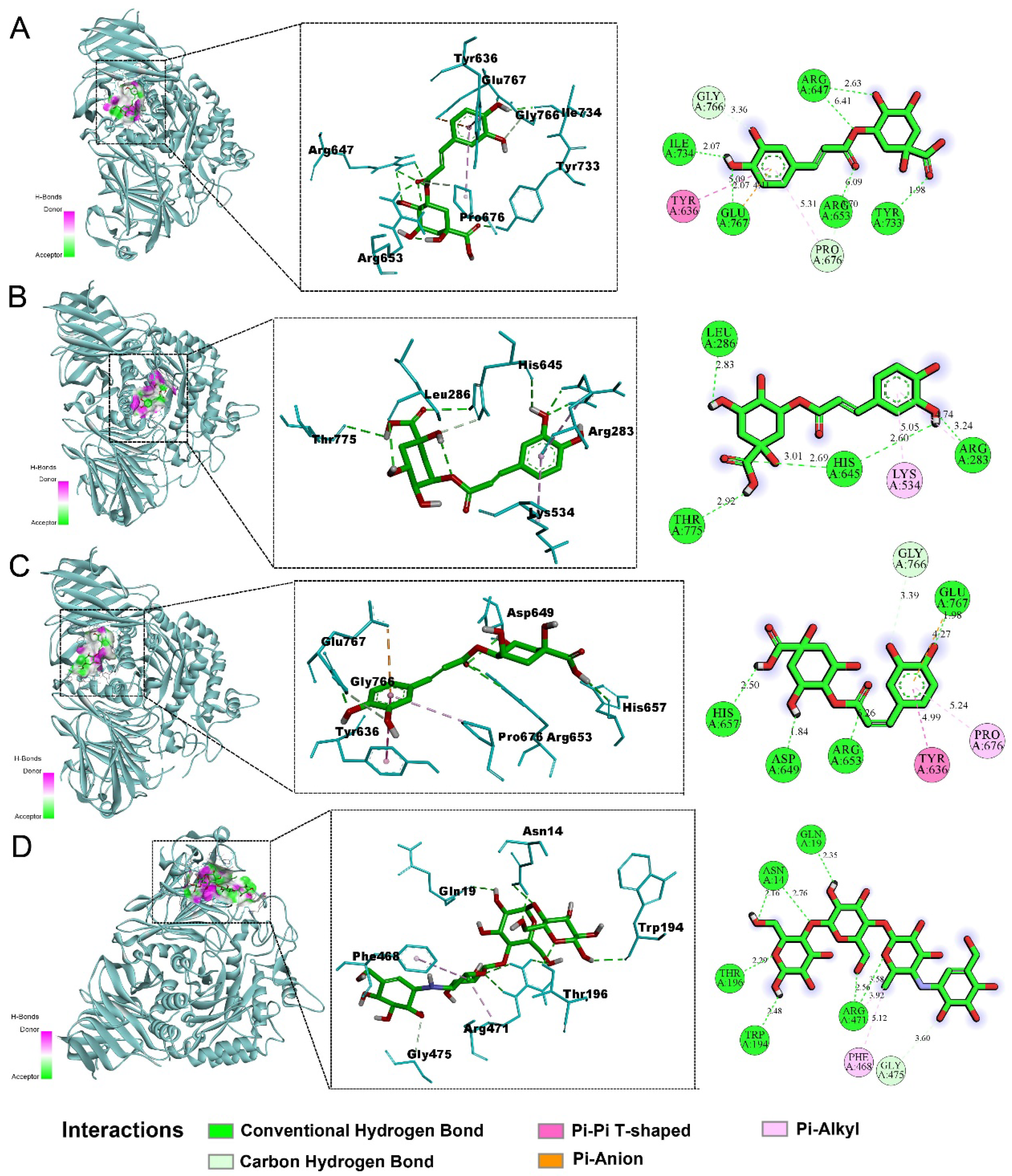
2.5. Molecular Docking Results
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Preparation of Plant Extracts
4.3. Animals
4.4. Preparation and Inhibition Assay of α-Glucosidase
4.5. PBG Detection in Diabetic Mice
4.6. UPLC-Triple-TOF-MS/MS Analysis
4.7. Molecular Docking
4.8. Statistical Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SP | Silphium perfoliatum L. |
| SP-C | SP crude extract |
| DM | Diabetes mellitus |
| AGIs | Alpha-glucosidase inhibitors |
| CI | Confidence intervals |
| AUC | Area under the curve |
| PBG | Postprandial blood glucose |
| CQAs | Caffeoylquinic acid compounds |
| CGA | Chlorogenic acid |
| CCGA | Cryptochlorogenic acid |
| NCGA | Neochlorogenic acid |
References
- Williams, R.; Karuranga, S.; Malanda, B.; Saeedi, P.; Basit, A.; Besançon, S.; Bommer, C.; Esteghamati, A.; Ogurtsova, K.; Zhang, P.; et al. Global and regional estimates and projections of diabetes-related health expenditure: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2020, 162, 108072. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Dirir, A.M.; Daou, M.; Yousef, A.F.; Yousef, L.F. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2022, 21, 1049–1079. [Google Scholar] [CrossRef]
- Agrawal, N.; Sharma, M.; Singh, S.; Goyal, A. Recent Advances of α-Glucosidase Inhibitors: A Comprehensive Review. Curr. Top. Med. Chem. 2022, 22, 2069–2086. [Google Scholar] [CrossRef]
- Hossain, U.; Das, A.K.; Ghosh, S.; Sil, P.C. An overview on the role of bioactive α-glucosidase inhibitors in ameliorating diabetic complications. Food Chem. Toxicol. 2020, 145, 111738. [Google Scholar] [CrossRef] [PubMed]
- Benalla, W.; Bellahcen, S.; Bnouham, M. Antidiabetic Medicinal Plants as a Source of Alpha Glucosidase Inhibitors. Curr. Diabetes Rev. 2010, 6, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Narwal, S.; Kumar, V.; Prakash, O. α-Glucosidase Inhibitors from Plants: A Natural Approach to Treat Diabetes. Pharmacogn. Rev. 2011, 5, 19. [Google Scholar] [CrossRef] [PubMed]
- Tundis, R.; Loizzo, M.R.; Menichini, F. Natural Products as α-Amylase and α-Glucosidase Inhibitors and their Hypoglycaemic Potential in the Treatment of Diabetes: An Update. Mini-Rev. Med. Chem. 2010, 10, 315–331. [Google Scholar] [CrossRef]
- Albrecht, K.A.; Goldstein, W. Silphium perfoliatum: A North American prairie plant with potential as a forage crop. In Proceedings of the International Grassland Congress (IGC), Bolzano, Italy, 26 August–1 September 2024; The Forage and Grassland Foundation: Lexington, KY, USA, 1997; p. 38. [Google Scholar]
- Gilmore, M.R. Uses of Plants by the Indians of the Missouri River Region; Washington Government Printing Office: Washington, DC, USA, 1919. [Google Scholar]
- Densmore, F. How Indians Use Wild Plants for Food, Medicine & Crafts; Courier Dover Publications: New York, NY, USA, 1928. [Google Scholar]
- Kindscher, K. Medicinal Wild Plants of the Prairie: An Ethnobotanical Guide; University Press of Kansas: Lawrence, KY, USA, 1992. [Google Scholar]
- Peni, D.; Stolarski, M.J.; Bordiean, A.; Krzyzaniak, M.; Debowski, M. Silphium perfoliatum—A Herbaceous Crop with Increased Interest in Recent Years for Multi-Purpose Use. Agriculture 2020, 10, 640. [Google Scholar] [CrossRef]
- Guo, Y.; Shang, H.M.; Zhao, J.C.; Zhang, H.X.; Chen, S.L. Enzyme-assisted extraction of a cup plant (Silphium perfoliatum L.) Polysaccharide and its antioxidant and hypoglycemic activities. Process Biochem. 2020, 92, 17–28. [Google Scholar] [CrossRef]
- Kowalski, R.; Wolski, T. The chemical composition of essential oils of Silphium perfoliatum L. Flavour Fragr. J. 2005, 20, 306–310. [Google Scholar]
- Wolski, T.; Kowalski, R.; Mardarowicz, M. Chromatographic analysis of essential oil occurring in inflorescences, leaves and rhizomes of Silphium perfoliatum L. Herba Pol. 2000, 46, 235–242. [Google Scholar]
- El-Sayed, N.H.; Wojcińska, M.; Drost-Karbowsk, K.; Matławska, I.; Williams, J.; Mabry, T.J. Kaempferol triosides from Silphium perfoliatum. Phytochemistry 2002, 60, 835–838. [Google Scholar] [CrossRef]
- Feng, W.S.; Pei, Y.Y.; Zheng, X.K.; Li, C.G.; Ke, Y.Y.; Lv, Y.Y.; Zhang, Y.L. A new kaempferol trioside from Silphium perfoliatum. J. Asian Nat. Prod. Res. 2013, 16, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.D. The Flavonoids and Phenolic Acids of the Genus Silphium and Their Chemosystematic and Medicinal Value. Ph.D. Dissertation, University of Texas, Austin, TX, USA, 2006. [Google Scholar]
- Kowalski, R.; Wolski, T. Evaluation of phenolic acid content in Silphium perfoliatum L. leaves, inflorescences and rhizomes. Electron. J. Pol. Agric. Univ. 2003, 6, 3. [Google Scholar]
- Kowalski, R.; Kędzia, B. Antibacterial activity of Silphium perfoliatum Extracts. Pharm. Biol. 2007, 45, 494–500. [Google Scholar] [CrossRef]
- Zhang, G.Y.; Jia, W.J.; Liu, L.Y.; Wang, L.Y.; Xu, J.Y.; Tao, J.H.; Xu, M.T.; Yue, M.; Luo, H.Q.; Hai, P.; et al. Caffeoylquinic acids from Silphium perfoliatum L. show hepatoprotective effects on cholestatic mice by regulating enterohepatic circulation of bile acids. J. Ethnopharmacol. 2025, 337, 118870. [Google Scholar] [CrossRef]
- Xu, J.Y.; Jia, W.J.; Zhang, G.Y.; Liu, L.Y.; Wang, L.Y.; Wu, D.; Tao, J.H.; Yue, H.L.; Zhang, D.J.; Zhao, X.H. Extract of Silphium perfoliatum L. improve lipid accumulation in NAFLD mice by regulating AMPK/FXR signaling pathway. J. Ethnopharmacol. 2024, 327, 118054. [Google Scholar] [CrossRef]
- Wang, S.; Li, Y.; Huang, D.; Chen, S.; Xia, Y.; Zhu, S. The inhibitory mechanism of chlorogenic acid and its acylated derivatives on α-amylase and α-glucosidase. Food Chem. 2022, 372, 131334. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
- Jaiswal, R.; Müller, H.; Müller, A.; Karar, M.G.E.; Kuhnert, N. Identification and characterization of chlorogenic acids, chlorogenic acid glycosides and flavonoids from Lonicera henryi L. (Caprifoliaceae) leaves by LC-MSn. Phytochemistry 2014, 108, 252–263. [Google Scholar] [CrossRef]
- Li, X.Q.; Sun, X.H.; Cai, S.; Ying, X.X.; Li, F.M. Investigation on the chemical constituents and variation of the flower buds of Lonicera species by UPLC-ESI-MS/MS and principal component analysis. Acta Pharm. Sin. 2009, 44, 895–904. [Google Scholar]
- Zhang, Q. Study of Caffeoylquinic Acids in Qingkailing Injection by LC-MSn. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2013. [Google Scholar]
- Fotirić Akšić, M.; Dabić Zagorac, D.; Sredojević, M.; Milivojević, J.; Gašić, U.; Meland, M.; Natić, M. Chemometric Characterization of Strawberries and Blueberries according to Their Phenolic Profile: Combined Effect of Cultivar and Cultivation System. Molecules 2019, 24, 4310. [Google Scholar] [CrossRef]
- Mizuno, T.; Uchiyama, N.; Tanaka, S.; Nakane, T.; Devkota, H.P.; Fujikawa, K.; Kawahara, N.; Iwashina, T. Flavonoids from Sedum japonicum subsp. oryzifolium (Crassulaceae). Molecules 2022, 27, 7632. [Google Scholar] [CrossRef]
- Stobiecki, M.; Skirycz, A.; Kerhoas, L.; Kachlicki, P.; Muth, D.; Einhorn, J.; Mueller-Roeber, B. Profiling of phenolic glycosidic conjugates in leaves of Arabidopsis thaliana using LC/MS. Metabolomics 2006, 2, 197–219. [Google Scholar] [CrossRef]
- Kiselova-Kaneva, Y.; Galunska, B.; Nikolova, M.; Dincheva, I.; Badjakov, I. High resolution LC-MS/MS characterization of polyphenolic composition and evaluation of antioxidant activity of Sambucus ebulus fruit tea traditionally used in Bulgaria as a functional food. Food Chem. 2022, 367, 130759. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Saini, R.; Bhatnagar, A.; Mishra, A. Exploring plant-based alpha-glucosidase inhibitors: Promising contenders for combatting type-2 diabetes. Arch. Physiol. Biochem. 2024, 130, 694–709. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Lei, M.; Yin, Q.; Nan, H.; Qian, G. Isolation and Characterization of Novel Pueroside B Isomers and Other Bioactive Compounds from Pueraria lobata Roots: Structure Elucidation, α-Glucosidase, and α-Amylase Inhibition Studies. Int. J. Mol. Sci. 2024, 25, 9602. [Google Scholar] [CrossRef]
- Wever, C.; Höller, M.; Becker, L.; Biertümpfel, A.; Köhler, J.; Van Inghelandt, D.; Westhoff, P.; Pude, R.; Pestsova, E. Towards high-biomass yielding bioenergy crop Silphium perfoliatum L.: Phenotypic and genotypic evaluation of five cultivated populations. Biomass Bioenergy 2019, 124, 102–113. [Google Scholar] [CrossRef]
- Šurić, J.; Šic Žlabur, J.; Peter, A.; Brandić, I.; Voća, S.; Dujmović, M.; Leto, J.; Voća, N. Energy vs. Nutritional Potential of Virginia Mallow (Sida hermaphrodita L.) and Cup Plant (Silphium perfoliatum L.). Plants 2022, 11, 2906. [Google Scholar] [CrossRef]
- Kim, G.J.; Jang, Y.; Kwon, K.T.; Kim, J.W.; Kang, S.I.; Ko, H.C.; Lee, J.Y.; Apostolidis, E.; Kwon, Y.I. Jeju Citrus (Citrus unshiu) Leaf Extract and Hesperidin Inhibit Small Intestinal α-Glucosidase Activities In Vitro and Postprandial Hyperglycemia in Animal Model. Int. J. Mol. Sci. 2024, 25, 13721. [Google Scholar] [CrossRef]
- Sarkar, A.; Chakrabarti, A.; Bhaumik, S.; Debnath, B.; Singh, S.S.; Ghosh, R.; Zaki, M.E.A.; Al-Hussain, S.A.; Debnath, S. Parkia javanica Edible Pods Reveal Potential as an Anti-Diabetic Agent: UHPLC-QTOF-MS/MS-Based Chemical Profiling, In Silico, In Vitro, In Vivo, and Oxidative Stress Studies. Pharmaceuticals 2024, 17, 968. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.; Joubert, E. Critical Assessment of In Vitro Screening of α-Glucosidase Inhibitors from Plants with Acarbose as a Reference Standard. Planta Med. 2022, 88, 1078–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.Y.; Jia, W.J.; Jiang, S.R.; Zhang, G.Y.; Zhao, J.; Xu, J.Y.; Wang, L.Y.; Wu, D.; Tao, J.H.; Yue, H.L.; et al. Inhibitory activities and rules of plant gallotannins with different numbers of galloyl moieties on sucrase, maltase and α-amylase in vitro and in vivo. Phytomedicine 2023, 120, 155063. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.J.; Bi, Q.M.; Jiang, S.R.; Tao, J.H.; Liu, L.Y.; Yue, H.L.; Zhao, X.H. Hypoglycemic activity of Codonopsis pilosula (Franch.) Nannf. in vitro and in vivo and its chemical composition identification by UPLC-Triple-TOF-MS/MS. Food Funct. 2022, 13, 2456–2464. [Google Scholar] [CrossRef]
- Jiang, S.R.; Zhao, X.H.; Liu, C.; Dong, Q.; Mei, L.J.; Chen, C.; Shao, Y.; Tao, Y.D.; Yue, H.L. Identification of phenolic compounds in fruits of Ribes stenocarpum Maxim. By UHPLC-QTOF/MS and their hypoglycemic effects in vitro and in vivo. Food Chem. 2021, 344, 128568. [Google Scholar] [CrossRef]
- Jiang, S.R.; Chen, C.; Dong, Q.; Shao, Y.; Zhao, X.H.; Tao, Y.D.; Yue, H.L. Alkaloids and phenolics identification in fruit of Nitraria tangutorum Bobr. by UPLC-Q-TOF-MS/MS and their α-glucosidase inhibitory effects in vivo and in vitro. Food Chem. 2021, 364, 130412. [Google Scholar] [CrossRef]
- Yue, H.L.; Jiang, S.R.; Wang, L.Y.; Banma, C.; Zhou, G.Y.; Shao, Y.; Tao, Y.D.; Zhao, X.H. Hypoglycemic ingredients identification of Rheum tanguticum Maxim. ex Balf. by UHPLC-triple-TOF-MS/MS and interrelationships between ingredients content and glycosidase inhibitory activities. Ind. Crops Prod. 2022, 178, 114595. [Google Scholar] [CrossRef]
- Ćorković, I.; Gašo-Sokač, D.; Pichler, A.; Šimunović, J.; Kopjar, M. Dietary polyphenols as natural inhibitors of α-amylase and α-glucosidase. Life 2022, 12, 1692. [Google Scholar] [CrossRef]
- Han, X.; Wang, P.; Zhang, J.; Lv, Y.; Zhao, Z.; Zhang, F.; Shang, M.; Liu, G.; Wang, X.; Cai, S.; et al. α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects. Pharmaceuticals 2025, 18, 744. [Google Scholar] [CrossRef]
- Ma, S.; Wu, Y.; Min, H.; Ge, L.; Yang, K. Triterpenoid Saponins and Flavonoid Glycosides from the Flower of Camellia flavida and Their Cytotoxic and α-Glycosidase Inhibitory Activities. Int. J. Mol. Sci. 2024, 25, 10977. [Google Scholar] [CrossRef]
- Xu, D.L.; Wang, Q.C.; Zhang, W.Q.; Hu, B.; Zhou, L.; Zeng, X.X.; Sun, Y. Inhibitory activities of caffeoylquinic acid derivatives from IIex kudingcha C.J. Tseng on α-glucosidase from Saccharomyces cerevisiae. J. Agric. Food. Chem. 2015, 63, 3694–3703. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]


| Peak no. | RT (min) | [M-H]- | MS Fragments (m/z) | Proposed Compounds | Molecular Formula | References |
|---|---|---|---|---|---|---|
| 1 | 6.65 | 353.0873 | 707.1815 (2M-H), 191.0550 (M-162), 135.0448 (179-44) | 3-monocaffeoylquinic acid I | C16H18O9 | [26,27,28] |
| 2 | 7.27 | 353.0878 | 707.1818 (2M-H), 191.0546 (M-162), 135.0450 (179-44) | 3-monocaffeoylquinic acid II | C16H18O9 | [26,27,28] |
| 3 | 9.31 | 337.0926 | 191.0541 (M-146), 163.0385 (M-174), 119.0498 (M-174) | 3-p-coumaroylquinic acid | C16H18O8 | [26,27,28] |
| 4 | 9.46 | 353.0876 | 707.1823 (2M-H), 191.0555 (M-162) | 5-monocaffeoylquinic acid I | C16H18O9 | [26,27,28] |
| 5 | 9.88 | 353.0878 | 707.1824 (2M-H), 191.0557 (M-162) | 5-monocaffeoylquinic acid II | C16H18O9 | [26,27,28] |
| 6 | 10.19 | 353.0881 | 707.1823 (2M-H), 191.0554 (M-162), 135.0451 (179-44) | 4-monocaffeoylquinic acid I | C16H18O9 | [26,27,28] |
| 7 | 10.55 | 353.0883 | 707.1823 (2M-H), 191.0557 (M-162), 135.0456 (179-44) | 4-monocaffeoylquinic acid II | C16H18O9 | [26,27,28] |
| 8 | 12.81 | 337.0927 | 191.0559 (M-146), 163.0385 (M-174), 119.0507 (M-174) | 5-p-coumaroylquinic acid | C16H18O8 | [26,27,28] |
| 9 | 12.02 | 337.0924 | unknown | - | ||
| 10 | 15.20 | 741.1953 | 609.1510 (M-132), 301.0359 (M-132-162-146), 300.0277, 299.0203 | quercetin 3-O-rutinoside 7-O-apiofuranoside | C32H38O20 | [29,30,31] |
| 11 | 16.58 | 725.2001 | 593.1544 (M-132), 416.0757, 285.0395 (M-132-162-146), 284.0317, 283.0236 | kaempferol 7-O-rutinoside 3-O-apiofuranoside | C32H38O19 | [29,30,31] |
| 12 | 17.20 | 609.1503 | 301.0350 (M-308), 300.0270 | quercetin 3-O-rhamnosyl-glucoside | C27H30O16 | [32] |
| 13 | 17.58 | 609.1494 | 301.0344 (M-308), 300.0266, 271.0237, 151.0030 | quercetin 3-O-rhamnosyl-galactoside | C27H30O16 | [32] |
| 14 | 17.83 | 401.1822 | 221.1174, 177.1271 | unknown | - | |
| 15 | 18.25 | 463.0895 | 301.0354 (M-162), 271.0235, 255.0290, 243.0285, 151.0028 | quercetin 3-O-glucoside | C21H20O12 | [29] |
| 16 | 18.90 | 593.1549 | 285.0397 (M-308), 284.0321, 255.0295, 227.0346 | kaempferol 3-O-rhamnosyl-glucoside | C27H30O15 | [32] |
| 17 | 19.60 | 515.1243 | 353.0891 (M-162), 191.0558 (M-162-162), 173.0455, 135.0455 (179-44) | dicaffeoylquinic acid I | C25H24O12 | [26,27,28] |
| 18 | 19.76 | 593.1556 | 285.0400 (M-308), 284.0322, 255.0295 | kaempferol 3-O-rhamnosyl-galactoside | C27H30O15 | [32] |
| 19 | 20.26 | 515.1185 | 353.0882 (M-162), 191.0560 (M-162-162), 179.0346, 135.0451 (179-44) | dicaffeoylquinic acid II | C25H24O12 | [26,27,28] |
| 20 | 20.56 | 447.0919 | 285.0383 (M-162), 284.0309, 255.0278, 227.0329 | kaempferol 3-O-glucoside | C21H20O11 | [30] |
| 21 | 21.26 | 903.2303 | 771.1850 (M-132), 609.1495 (M-132-162), 433.0785, 301.0344 | quercetin 3-O-rutinoside 7-O-apiofuranoside derivative | C41H44O23 | [29,30,31] |
| 22 | 21.65 | 515.1188 | 353.0886, 191.0549, 179.0340, 173.0445, 135.0445 | dicaffeoylquinic acid III | C25H24O12 | [26,27,28] |
| 23 | 22.16 | 691.2615 | 335.1244, 317., 273.1241 | unknown | - | |
| 24 | 22.40 | 887.2357 | 755.1897 (M-132), 593.1544, 284.0313, 161.0232 | kaempferol 7-O-rutinoside 3-O-apiofuranoside derivative I | C41H44O22 | [29,30,31] |
| 25 | 22.72 | 887.2371 | 755.1904 (M-132), 469.1354, 417.0828, 285.0396, 284.0320, 161.0237 | kaempferol 7-O-rutinoside 3-O-apiofuranoside derivative II | C41H44O22 | [29,30,31] |
| 26 | 23.84 | 771.1885 | 609.1495 (M-162), 301.0340 (M-162-308), 300.0257, 178.9972 | quercetin 3-O-rhamnosyl-glucoside derivative I | C33H36O19 | [32] |
| 27 | 24.14 | 771.1860 | 609.1507 (M-162), 301.0340 (M-162-308), 300.0264, 151.0026 | quercetin 3-O-rhamnosyl-galactoside derivative II | C33H36O19 | [32] |
| 28 | 25.60 | 755.1908 | 593.1551 (M-162), 469.1362, 285.0397 (M-162-308) | kaempferol 3-O-rhamnosyl-glucoside derivative | C33H36O18 | [31] |
| 29 | 25.97 | 755.1888 | 593.1536 (M-162), 469.1350, 285.0388 (M-162-308) | kaempferol 3-O-rhamnosyl-galactoside derivative | C33H36O18 | [31] |
| Compounds | Affinity (Kcal/mol) | pKi (μmol/L) | H-Bonds | Hydrophobic | Electrostatic |
|---|---|---|---|---|---|
| Chlorogenic acid | −8.13 ± 0.34 | 1.24 ± 0.74 | 4 | 0 | 0 |
| Neochlorogenic acid | −8.03 ± 0.21 | 1.35 ± 0.47 | 3 | 0 | 0 |
| Cryptochlorogenic acid | −7.73 ± 0.15 | 2.20 ± 0.54 | 4 | 0 | 0 |
| Acarbose | −7.30 ± 0.37 | 5.09 ± 2.97 | 8 | 0 | 0 |
| Compounds | Affinity (Kcal/mol) | pKi (μmol/L) | H-Bonds | Hydrophobic | Electrostatic |
|---|---|---|---|---|---|
| Chlorogenic acid | −8.43 ± 0.38 | 0.81 ± 0.69 | 7 | 2 | 1 |
| Neochlorogenic acid | −8.10 ± 0.20 | 1.18 ± 0.40 | 5 | 2 | 0 |
| Cryptochlorogenic acid | −8.53 ± 0.21 | 0.57 ± 0.21 | 5 | 2 | 1 |
| Acarbose | −8.30 ± 0.36 | 0.91 ± 0.47 | 8 | 2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Liu, L.; Jia, W.; Wang, L.; Tao, J.; Zhang, W.; Yue, H.; Zhang, D.; Zhao, X. Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS. Pharmaceuticals 2025, 18, 1087. https://doi.org/10.3390/ph18081087
Zhang G, Liu L, Jia W, Wang L, Tao J, Zhang W, Yue H, Zhang D, Zhao X. Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS. Pharmaceuticals. 2025; 18(8):1087. https://doi.org/10.3390/ph18081087
Chicago/Turabian StyleZhang, Guoying, Liying Liu, Wenjing Jia, Luya Wang, Jihong Tao, Wei Zhang, Huilan Yue, Dejun Zhang, and Xiaohui Zhao. 2025. "Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS" Pharmaceuticals 18, no. 8: 1087. https://doi.org/10.3390/ph18081087
APA StyleZhang, G., Liu, L., Jia, W., Wang, L., Tao, J., Zhang, W., Yue, H., Zhang, D., & Zhao, X. (2025). Hypoglycemic Effects of Silphium perfoliatum L. In Vitro and In Vivo and Its Active Composition Identification by UPLC-Triple-TOF-MS/MS. Pharmaceuticals, 18(8), 1087. https://doi.org/10.3390/ph18081087







