Phenotypic Screening of H1-Antihistamines Identifies Promethazine and Rupatadine as Active Compounds Against Toxocara canis Infective Larvae
Abstract
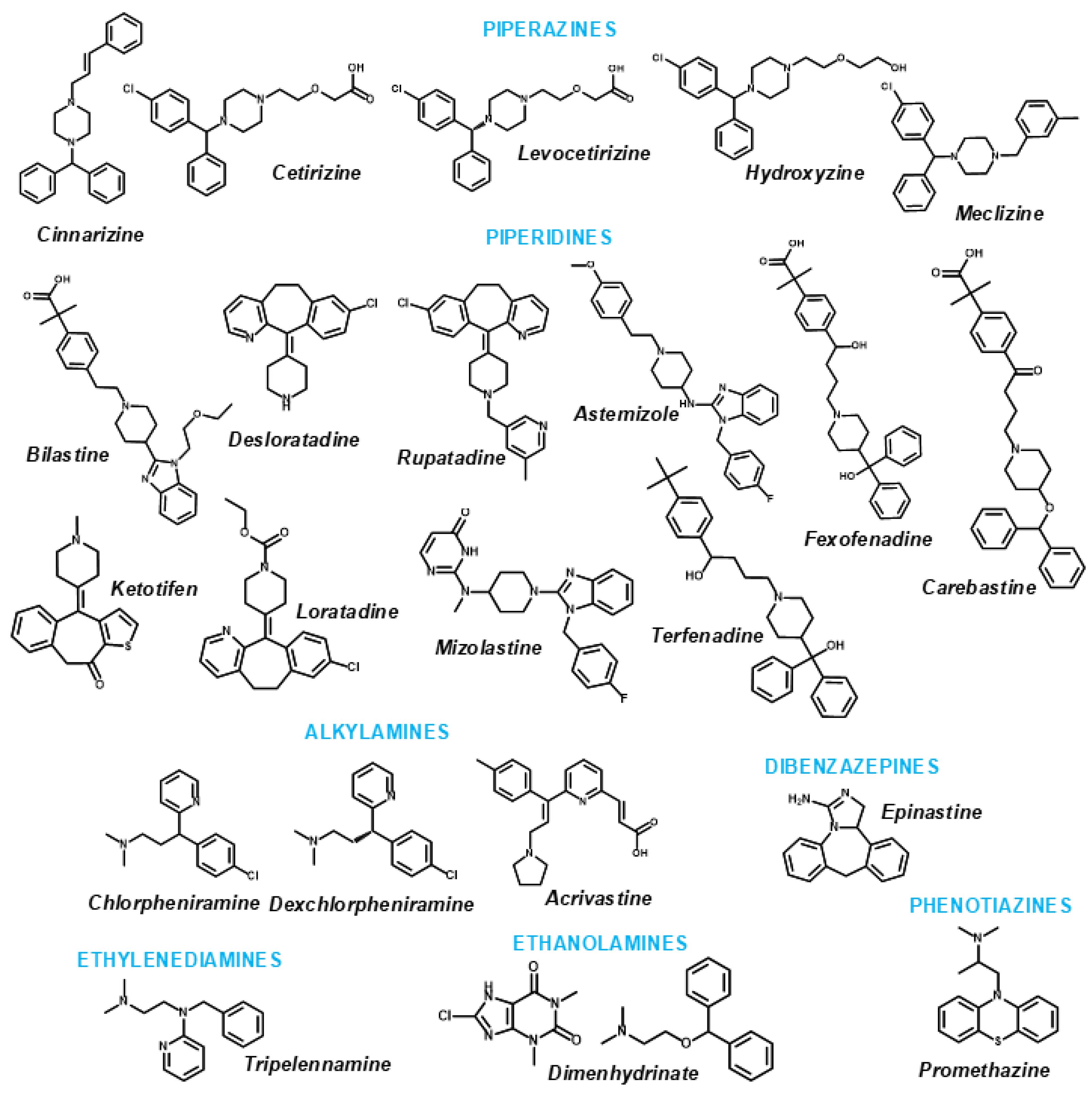
1. Introduction
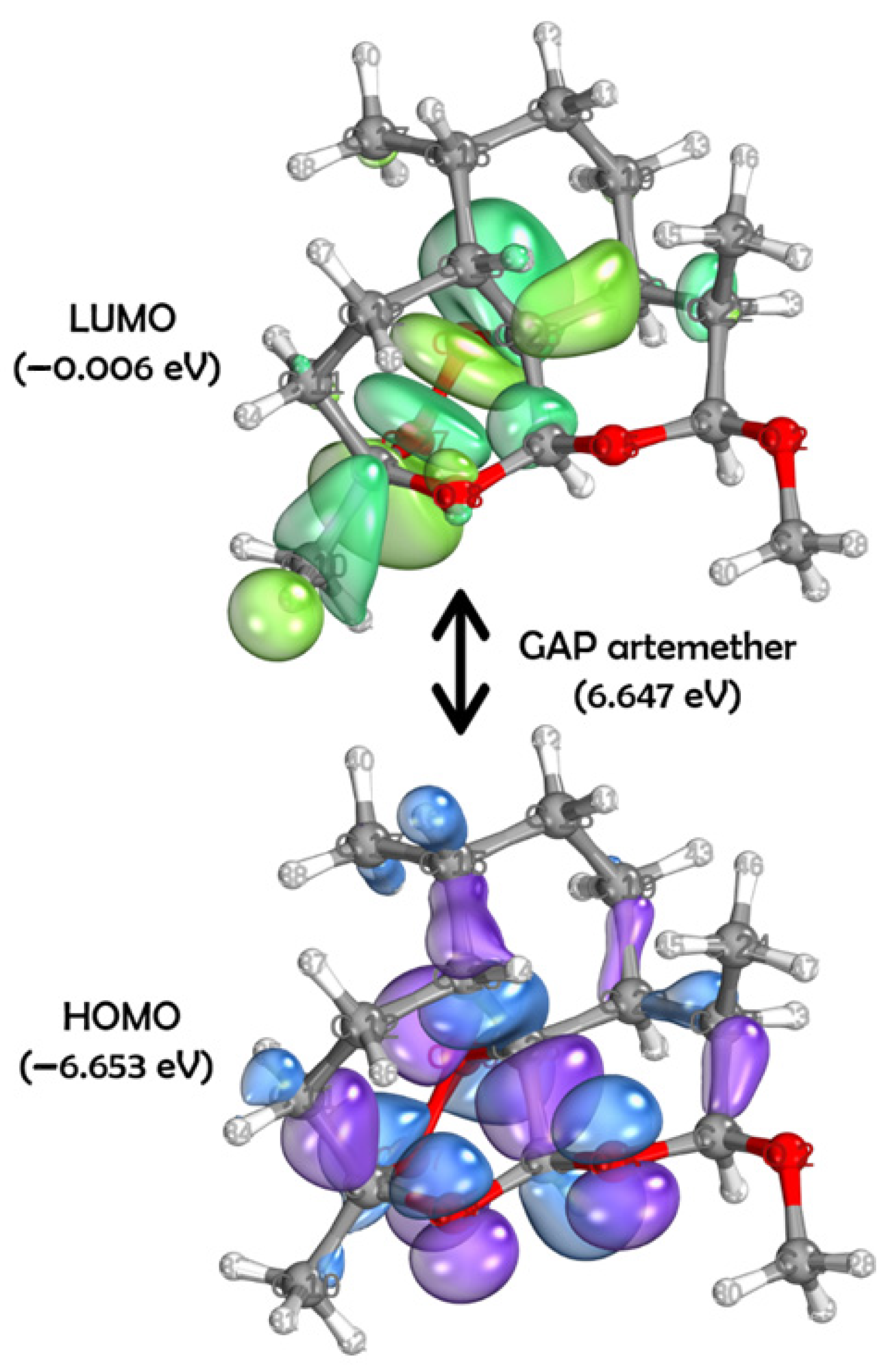
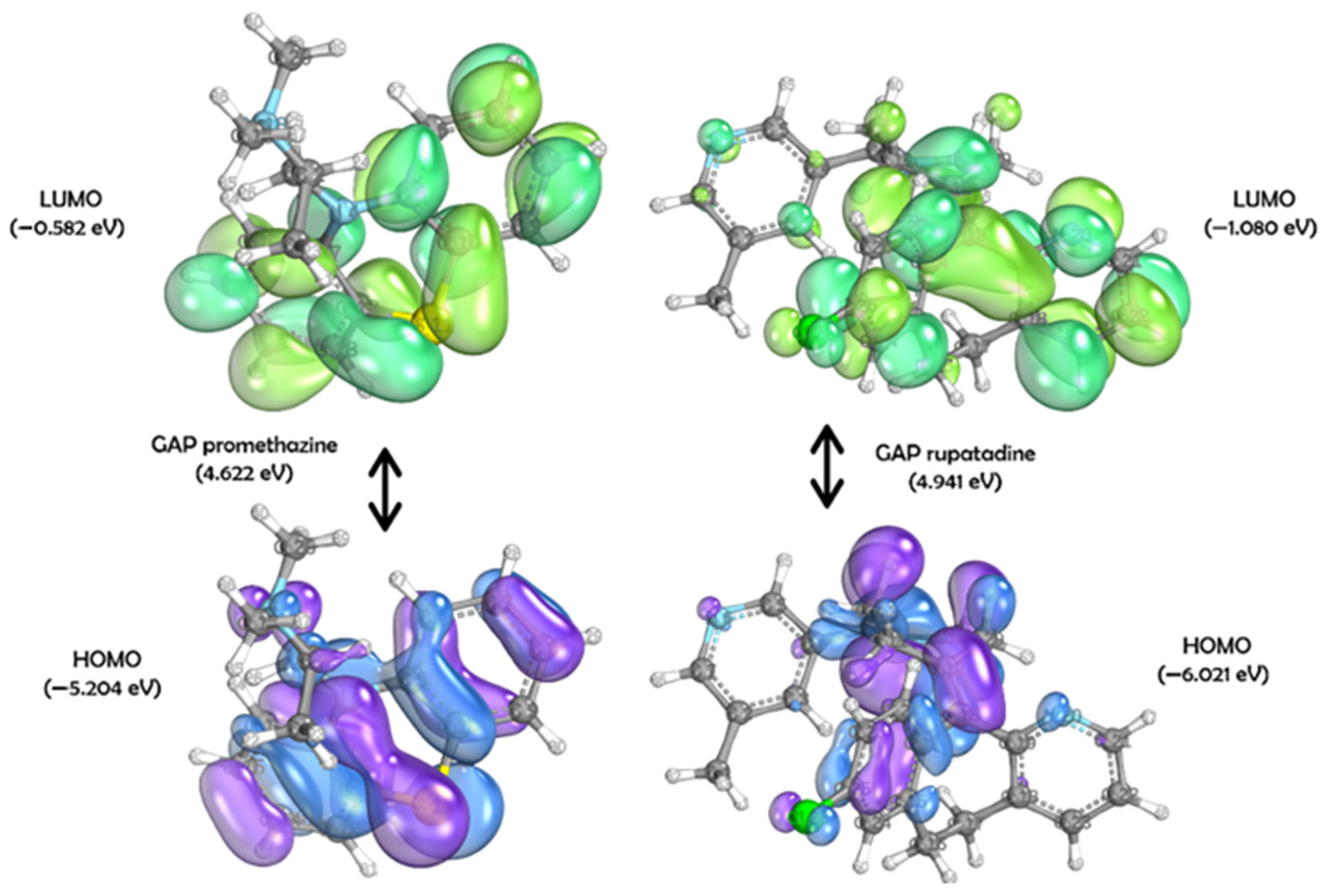
2. Results
3. Discussion
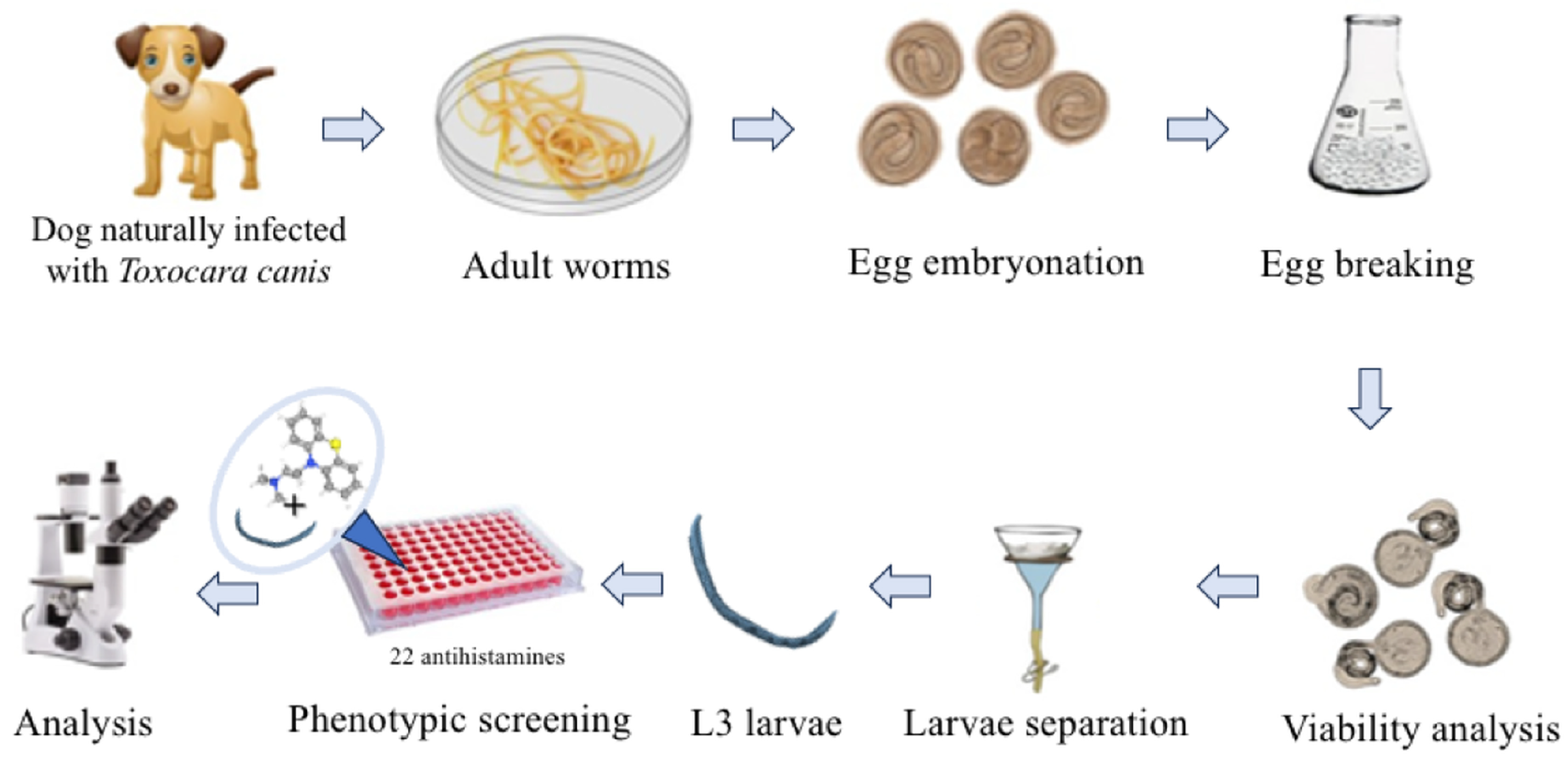
4. Materials and Methods
4.1. Drugs and Reagents
4.2. Parasite Collection and Preparation
4.3. In Vitro Assays with Toxocara canis L3 Larvae
4.4. Statistical Analyses
4.5. DFT Studies
4.6. Homology Modeling
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mengarda, A.C.; Silva, T.C.; Silva, A.S.; Roquini, D.B.; Fernandes, J.P.S.; De Moraes, J. Toward Anthelmintic Drug Candidates for Toxocariasis: Challenges and Recent Developments. Eur. J. Med. Chem. 2023, 251, 115268. [Google Scholar] [CrossRef]
- Cirino, M.E.; Teixeira, T.R.; Silva, A.M.H.; Borges, A.C.C.; Fukui-Silva, L.; Wagner, L.G.; Fernandes, C.; McCann, M.; Santos, A.L.S.; De Moraes, J. Anthelmintic Activity of 1,10-Phenanthroline-5,6-Dione-Based Metallodrugs. Sci. Rep. 2025, 15, 4699. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, Q.; Liu, G.-H.; Zheng, W.-B.; Hong, S.-J.; Sugiyama, H.; Zhu, X.-Q.; Elsheikha, H.M. Toxocariasis: A Silent Threat with a Progressive Public Health Impact. Infect. Dis. Poverty 2018, 7, 59. [Google Scholar] [CrossRef]
- Ma, G.; Holland, C.V.; Wang, T.; Hofmann, A.; Fan, C.-K.; Maizels, R.M.; Hotez, P.J.; Gasser, R.B. Human Toxocariasis. Lancet Infect. Dis. 2018, 18, e14–e24. [Google Scholar] [CrossRef]
- Auer, H.; Walochnik, J. Toxocariasis and the Clinical Spectrum. Adv. Parasitol. 2020, 109, 111–130. [Google Scholar] [CrossRef]
- Macpherson, C.N.L. The Epidemiology and Public Health Importance of Toxocariasis: A Zoonosis of Global Importance. Int. J. Parasitol. 2013, 43, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Rostami, A.; Ma, G.; Wang, T.; Koehler, A.V.; Hofmann, A.; Chang, B.C.H.; Macpherson, C.N.; Gasser, R.B. Human Toxocariasis—A Look at a Neglected Disease through an Epidemiological ‘Prism’. Infect. Genet. Evol. 2019, 74, 104002. [Google Scholar] [CrossRef]
- Alberich, M.; Garcia, M.; Petermann, J.; Blancfuney, C.; Jouffroy, S.; Jacquiet, P.; Lespine, A. Evaluation of Nematode Susceptibility and Resistance to Anthelmintic Drugs with a WMicrotracker Motility Assay. Sci. Rep. 2025, 15, 17968. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.L.G.; De Moraes, J.; Andricopulo, A.D. Approaches to Advance Drug Discovery for Neglected Tropical Diseases. Drug Discov. Today 2022, 27, 2278–2287. [Google Scholar] [CrossRef]
- Zhu, X.-Q.; Korhonen, P.K.; Cai, H.; Young, N.D.; Nejsum, P.; Von Samson-Himmelstjerna, G.; Boag, P.R.; Tan, P.; Li, Q.; Min, J.; et al. Genetic Blueprint of the Zoonotic Pathogen Toxocara canis. Nat. Commun. 2015, 6, 6145. [Google Scholar] [CrossRef]
- International Helminth Genomes Consortium. Comparative Genomics of the Major Parasitic Worms. Nat. Genet. 2019, 51, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Roquini, D.B.; Lemes, B.L.; Kreutz, A.L.B.; Spoladore, S.C.; Amaro, M.C.; Lopes, F.B.; Fernandes, J.P.S.; De Moraes, J. Antihistamines H1 as Potential Anthelmintic Agents against the Zoonotic Parasite Angiostrongylus cantonensis. ACS Omega 2024, 9, 31159–31165. [Google Scholar] [CrossRef]
- Lopes, F.B.; Aranha, C.M.S.Q.; Fernandes, J.P.S. Histamine H3 Receptor and Cholinesterases as Synergistic Targets for Cognitive Decline: Strategies to the Rational Design of Multitarget Ligands. Chem. Biol. Drug Des. 2021, 98, 212–225. [Google Scholar] [CrossRef] [PubMed]
- Rosikon, K.D.; Bone, M.C.; Lawal, H.O. Regulation and Modulation of Biogenic Amine Neurotransmission in Drosophila and Caenorhabditis elegans. Front. Physiol. 2023, 14, 970405. [Google Scholar] [CrossRef]
- Roquini, D.B.; Cogo, R.M.; Mengarda, A.C.; Mazloum, S.F.; Morais, C.S.; Xavier, R.P.; Salvadori, M.C.; Teixeira, F.S.; Ferreira, L.E.; Pinto, P.L.; et al. Promethazine Exhibits Antiparasitic Properties In Vitro and Reduces Worm Burden, Egg Production, Hepatomegaly, and Splenomegaly in a Schistosomiasis Animal Model. Antimicrob. Agents Chemother. 2019, 63, e01208-19. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.P.; Mengarda, A.C.; Silva, M.P.; Roquini, D.B.; Salvadori, M.C.; Teixeira, F.S.; Pinto, P.L.; Morais, T.R.; Ferreira, L.L.G.; Andricopulo, A.D.; et al. H1-Antihistamines as Antischistosomal Drugs: In Vitro and in Vivo Studies. Parasit. Vectors 2020, 13, 278. [Google Scholar] [CrossRef]
- Criado, P.R.; Criado, R.F.J.; Maruta, C.W.; Machado Filho, C.d. Histamine, histamine receptors and antihistamines: New concepts. An. Bras. Dermatol. 2010, 85, 195–210. [Google Scholar] [CrossRef]
- Alevizos, M.; Karagkouni, A.; Vasiadi, M.; Sismanopoulos, N.; Makris, M.; Kalogeromitros, D.; Theoharides, T.C. Rupatadine Inhibits Inflammatory Mediator Release from Human Laboratory of Allergic Diseases 2 Cultured Mast Cells Stimulated by Platelet-Activating Factor. Ann. Allergy Asthma Immunol. 2013, 111, 542–547. [Google Scholar] [CrossRef]
- Thilakarathne, S.S.; Taki, A.C.; Wang, T.; Nowell, C.; Chang, B.C.H.; Gasser, R.B. Evaluation of Serum Supplementation on the Development of Haemonchus contortus Larvae In Vitro and on Compound Screening Results. Int. J. Mol. Sci. 2025, 26, 1118. [Google Scholar] [CrossRef]
- Shanley, H.T.; Wang, T.; Taki, A.C.; Byrne, J.J.; Chang, B.C.H.; Sleebs, B.E.; Gasser, R.B. Advances in Anthelmintic Target Identification. Int. J. Mol. Sci. 2025, 26, 3738. [Google Scholar] [CrossRef]
- Sulpizi, M.; Folkers, G.; Rothlisberger, U.; Carloni, P.; Scapozza, L. Applications of Density Functional Theory-Based Methods in Medicinal Chemistry. Quant. Struct. Act.Relat. 2002, 21, 173–181. [Google Scholar] [CrossRef]
- LaPointe, S.; Weaver, D.A. Review of Density Functional Theory Quantum Mechanics as Applied to Pharmaceutically Relevant Systems. Curr. Comput Aided Drug. Des. 2007, 3, 290–296. [Google Scholar] [CrossRef]
- Dos Santos Nascimento, I.J.; De Aquino, T.M.; Da Silva-Júnior, E.F. Cruzain and Rhodesain Inhibitors: Last Decade of Advances in Seeking for New Compounds Against American and African Trypanosomiases. Curr. Top. Med. Chem. 2021, 21, 1871–1899. [Google Scholar] [CrossRef]
- Silva-Silva, J.V.; Feitosa, A.O.; Doring, T.H.; Watanabe, L.A.; Siqueira, J.E.; Andricopulo, A.D.; Marinho, P.S.; Marinho, A.M. Aflavinine Alkaloids from Aspergillus Sp.: Antibacterial Activity and Inosine 5’-Monophosphate Dehydrogenase (IMPDH) as a Potential Target in Bacillus subtilis. J. Braz. Chem. Soc. 2025, 36, 1–14. [Google Scholar] [CrossRef]
- Silva-Silva, J.V.; Moragas-Tellis, C.J.; Chagas, M.S.S.; Souza, P.V.R.; Moreira, D.L.; Hardoim, D.J.; Taniwaki, N.N.; Costa, V.F.A.; Bertho, A.L.; Brondani, D.; et al. Carajurin Induces Apoptosis in Leishmania amazonensis Promastigotes through Reactive Oxygen Species Production and Mitochondrial Dysfunction. Pharmaceuticals 2022, 15, 331. [Google Scholar] [CrossRef]
- Justino, G.C.; Vieira, A.J.S.C. Antioxidant Mechanisms of Quercetin and Myricetin in the Gas Phase and in Solution—A Comparison and Validation of Semi-Empirical Methods. J. Mol. Model 2010, 16, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Miar, M.; Shiroudi, A.; Pourshamsian, K.; Oliaey, A.R.; Hatamjafari, F. Theoretical Investigations on the HOMO–LUMO Gap and Global Reactivity Descriptor Studies, Natural Bond Orbital, and Nucleus-Independent Chemical Shifts Analyses of 3-Phenylbenzo[d]Thiazole-2(3H)-Imine and Its Para -Substituted Derivatives: Solvent and Substituent Effects. J. Chem. Res. 2021, 45, 147–158. [Google Scholar] [CrossRef]
- Yarsan, E.; Altinsaat, Ç.; Ayçiçek, H.; Şahindokuyucu, F.; Kalkan, F. Effects of Albendazole Treatment on Haematological and Biochemical Parameters in Healthy and Toxocara canis Infected Mice. Turk. J. Vet. Anim. Sci. 2003, 27, 1057–1063. [Google Scholar]
- Elgendy, D.I.; Elmahy, R.A.; Amer, A.I.M.; Ibrahim, H.A.; Eltantawy, A.F.; Mansour, F.R.; Salama, A.M. Efficacy of Artemether against Toxocariasis in Mice: Parasitological and Immunopathological Changes in Brain, Liver, and Lung. Pathog. Glob. Health. 2024, 118, 47–64. [Google Scholar] [CrossRef]
- Shu-Hua, X.; Ji-Qing, Y.; Hui-Fang, G.; Jin-Yan, M.; Pei-Ying, J.; Chollet, J.; Tanner, M.; Utzinger, J. Schistosoma japonicum: Effect of Artemether on Glutathione S-Transferase and Superoxide Dismutase. Exp. Parasitol. 2002, 102, 38–45. [Google Scholar] [CrossRef]
- El-Lakkany, N.M.; Seif el-Din, S.H. Haemin Enhances the in Vivo Efficacy of Artemether against Juvenile and Adult Schistosoma mansoni in Mice. Parasitol. Res. 2013, 112, 2005–2015. [Google Scholar] [CrossRef]
- Martínez-Espinosa, R.; Argüello-García, R.; Saavedra, E.; Ortega-Pierres, G. Albendazole Induces Oxidative Stress and DNA Damage in the Parasitic Protozoan Giardia duodenalis. Front. Microbiol. 2015, 6, 800. [Google Scholar] [CrossRef]
- Pons, V.; Beaumont, S.; Tran Huu Dau, M.E.; Iorga, B.I.; Dodd, R.H. Rigid Analogues of Antimitotic Indolobenzazepinones: New Insights into Tubulin Binding via Molecular Modeling. ACS Med. Chem. Lett. 2011, 2, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, R.P.V.J.; Vasanthathilake, V.W.S.M.; Lloyd, S.; Fernando, S.T. Collection of Eggs and Hatching and Culturing Second-Stage Larvae of Toxocara vitulorum In Vitro. J. Parasitol. 1992, 78, 1090. [Google Scholar] [CrossRef]
- Silva, T.C.; Mengarda, A.C.; Lemes, B.L.; Lescano, S.A.Z.; Souza, D.C.S.; Lago, J.H.G.; De Moraes, J. N-(4-Methoxyphenyl)Pentanamide, a Simplified Derivative of Albendazole, Displays Anthelmintic Properties against the Nematode Toxocara canis. Microbiol. Spectr. 2022, 10, e01807-22. [Google Scholar] [CrossRef] [PubMed]
- Sinott, F.A.; Sena-Lopes, Â.; Leal, K.S.; Thais De Oliveira Silva, M.; Cardoso De Freitas, M.; Quintana De Moura, M.; Aires Berne, M.E.; Borsuk, S. Essential Oil from Brazilian Red Propolis Exhibits Anthelmintic Activity against Larvae of Toxocara cati. Exp. Parasitol. 2019, 200, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Sessa, D.P.; Mengarda, A.C.; Simplicio, P.E.; Antar, G.M.; Lago, J.H.G.; De Moraes, J. 15β-Senecioyl-Oxy- Ent -Kaur-16-En-19-Oic Acid, a Diterpene Isolated from Baccharis lateralis, as Promising Oral Compound for the Treatment of Schistosomiasis. J. Nat. Prod. 2020, 83, 3744–3750. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Knizia, G.; Klein, J.E.M.N. Electron Flow in Reaction Mechanisms—Revealed from First Principles. Angew. Chem. Int. Ed. 2015, 54, 5518–5522. [Google Scholar] [CrossRef] [PubMed]
- Arnold, K.; Bordoli, L.; Kopp, J.; Schwede, T. The SWISS-MODEL Workspace: A Web-Based Environment for Protein Structure Homology Modelling. Bioinformatics 2006, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-Pdb Viewer: An Environment for Comparative Protein Modeling. J. Electrophor. 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]
- Schwede, T. SWISS-MODEL: An Automated Protein Homology-Modeling Server. Nucleic Acids Res. 2003, 31, 3381–3385. [Google Scholar] [CrossRef]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein Structure Homology Modeling Using SWISS-MODEL Workspace. Nat. Protoc. 2009, 4, 1–13. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-Atom Structure Validation for Macromolecular Crystallography. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Carrascoza, F.; Zaric, S.; Silaghi-Dumitrescu, R. Computational Study of Protein Secondary Structure Elements: Ramachandran Plots Revisited. J. Mol. Graph. Model. 2014, 50, 125–133. [Google Scholar] [CrossRef]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and Better Reference Data for Improved All-atom Structure Validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Benkert, P.; Tosatto, S.C.E.; Schomburg, D. QMEAN: A Comprehensive Scoring Function for Model Quality Assessment. Proteins 2008, 71, 261–277. [Google Scholar] [CrossRef]
- Wiederstein, M.; Sippl, M.J. ProSA-Web: Interactive Web Service for the Recognition of Errors in Three-Dimensional Structures of Proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef]
- Laisne, M.-C.; Michallet, S.; Lafanechère, L. Characterization of Microtubule Destabilizing Drugs: A Quantitative Cell-Based Assay That Bridges the Gap between Tubulin Based- and Cytotoxicity Assays. Cancers 2021, 13, 5226. [Google Scholar] [CrossRef] [PubMed]
- Dolinsky, T.J.; Czodrowski, P.; Li, H.; Nielsen, J.E.; Jensen, J.H.; Klebe, G.; Baker, N.A. PDB2PQR: Expanding and Upgrading Automated Preparation of Biomolecular Structures for Molecular Simulations. Nucleic Acids Res. 2007, 35, W522–W525. [Google Scholar] [CrossRef] [PubMed]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef] [PubMed]

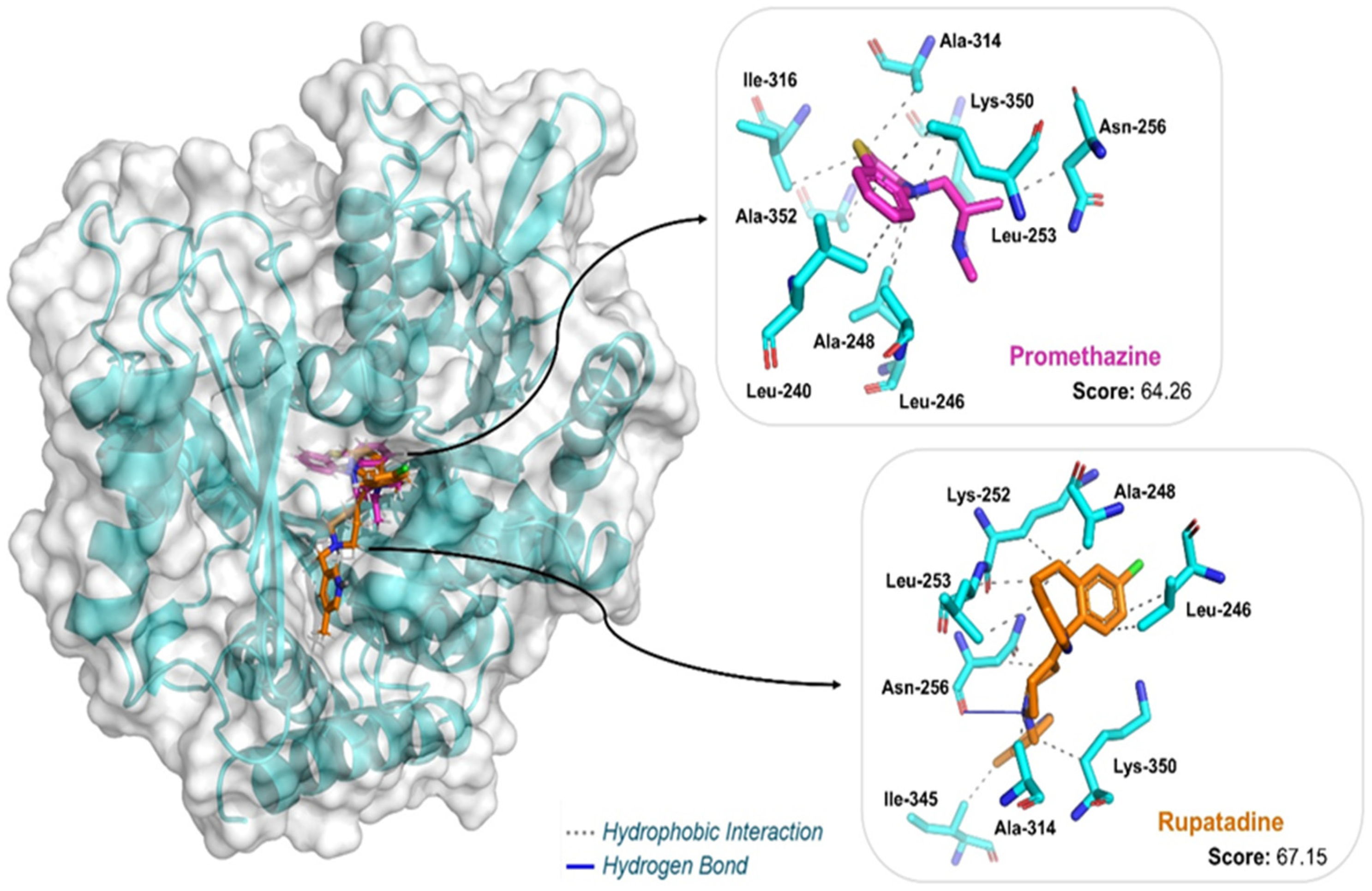
| Ligand | Docking Score | Interaction | Residues (Distance in Å) |
|---|---|---|---|
| Promethazine | 64.26 | Hydrophobic | Leu240 (3.44), Leu246 (3.43), Ala248 (3.10), Leu253 (3.52; 3.93), Asn256 (3.60), Ala314 (3.96), Ile316 (3.98), Lys350 (3.37), Ala352 (3.31) |
| Rupatadine | 67.15 | Hydrophobic | Leu246 (3.40; 3.48), Ala248 (3.07), Lys252 (3.53), Leu253 (3.43; 3.54), Asn256 (3.80), Ala314 (3.56), Ile345 (3.45), Lys350 (3.91) |
| Hydrogen Bond | Asn256 (3.23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, T.C.; Godoy-Silva, J.; Amaro, M.C.; Silva-Silva, J.V.; Doring, T.H.; Ferreira, L.L.G.; Andricopulo, A.D.; de Moraes, J. Phenotypic Screening of H1-Antihistamines Identifies Promethazine and Rupatadine as Active Compounds Against Toxocara canis Infective Larvae. Pharmaceuticals 2025, 18, 997. https://doi.org/10.3390/ph18070997
Silva TC, Godoy-Silva J, Amaro MC, Silva-Silva JV, Doring TH, Ferreira LLG, Andricopulo AD, de Moraes J. Phenotypic Screening of H1-Antihistamines Identifies Promethazine and Rupatadine as Active Compounds Against Toxocara canis Infective Larvae. Pharmaceuticals. 2025; 18(7):997. https://doi.org/10.3390/ph18070997
Chicago/Turabian StyleSilva, Taís C., Julia Godoy-Silva, Monique C. Amaro, João V. Silva-Silva, Thiago H. Doring, Leonardo L. G. Ferreira, Adriano D. Andricopulo, and Josué de Moraes. 2025. "Phenotypic Screening of H1-Antihistamines Identifies Promethazine and Rupatadine as Active Compounds Against Toxocara canis Infective Larvae" Pharmaceuticals 18, no. 7: 997. https://doi.org/10.3390/ph18070997
APA StyleSilva, T. C., Godoy-Silva, J., Amaro, M. C., Silva-Silva, J. V., Doring, T. H., Ferreira, L. L. G., Andricopulo, A. D., & de Moraes, J. (2025). Phenotypic Screening of H1-Antihistamines Identifies Promethazine and Rupatadine as Active Compounds Against Toxocara canis Infective Larvae. Pharmaceuticals, 18(7), 997. https://doi.org/10.3390/ph18070997







