Navigating the Dry Eye Therapeutic Puzzle: A Mechanism-Based Overview of Current Treatments
Abstract
1. Introduction
1.1. What Is Dry Eye Disease (DED)?
1.2. How Does DED Present?
1.3. How Is DED Profiled?
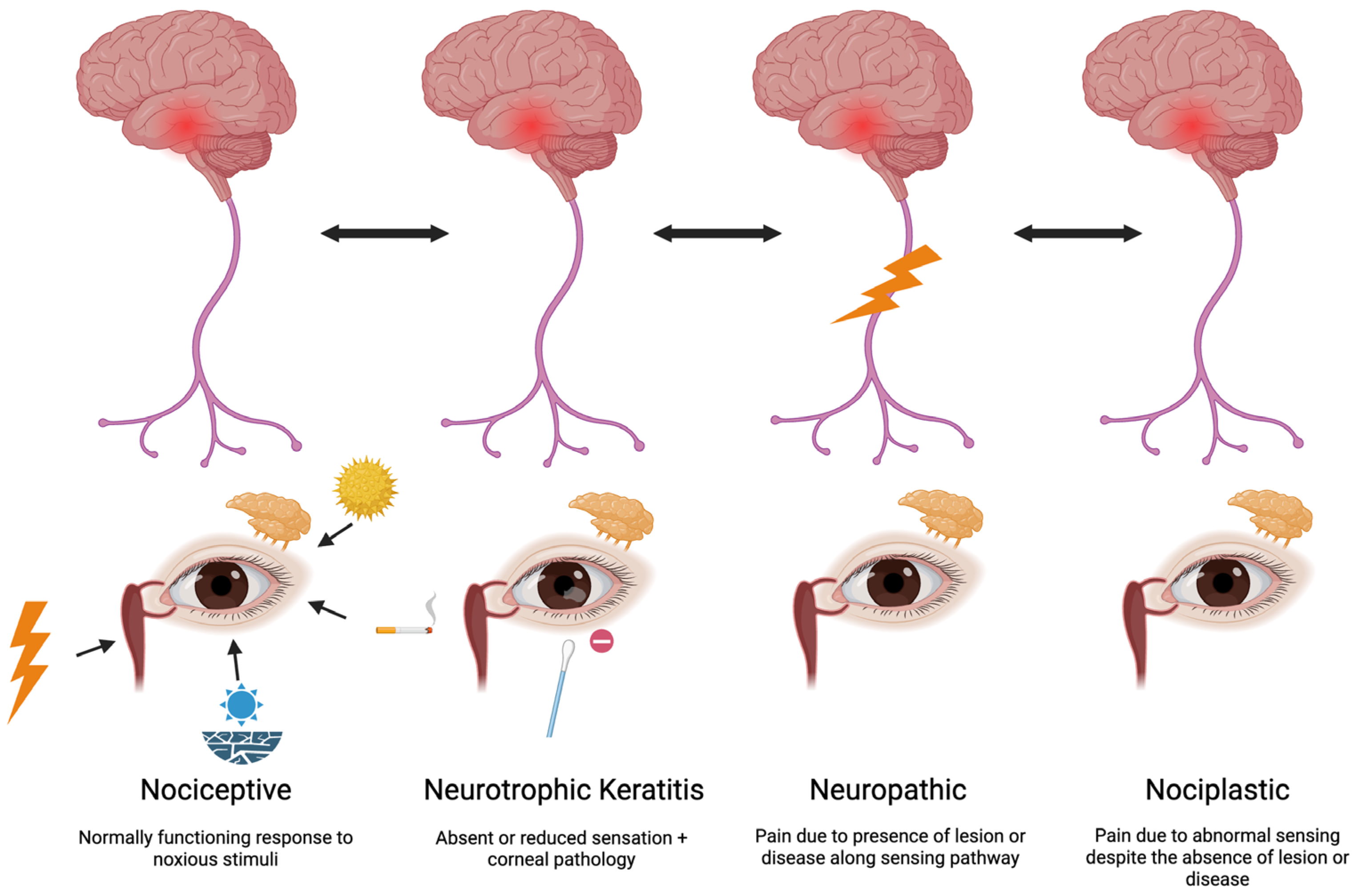
1.4. The Role of Nerve Dysfunction in DED
1.5. How Is Nerve Status Profiled?
1.6. Review Objective
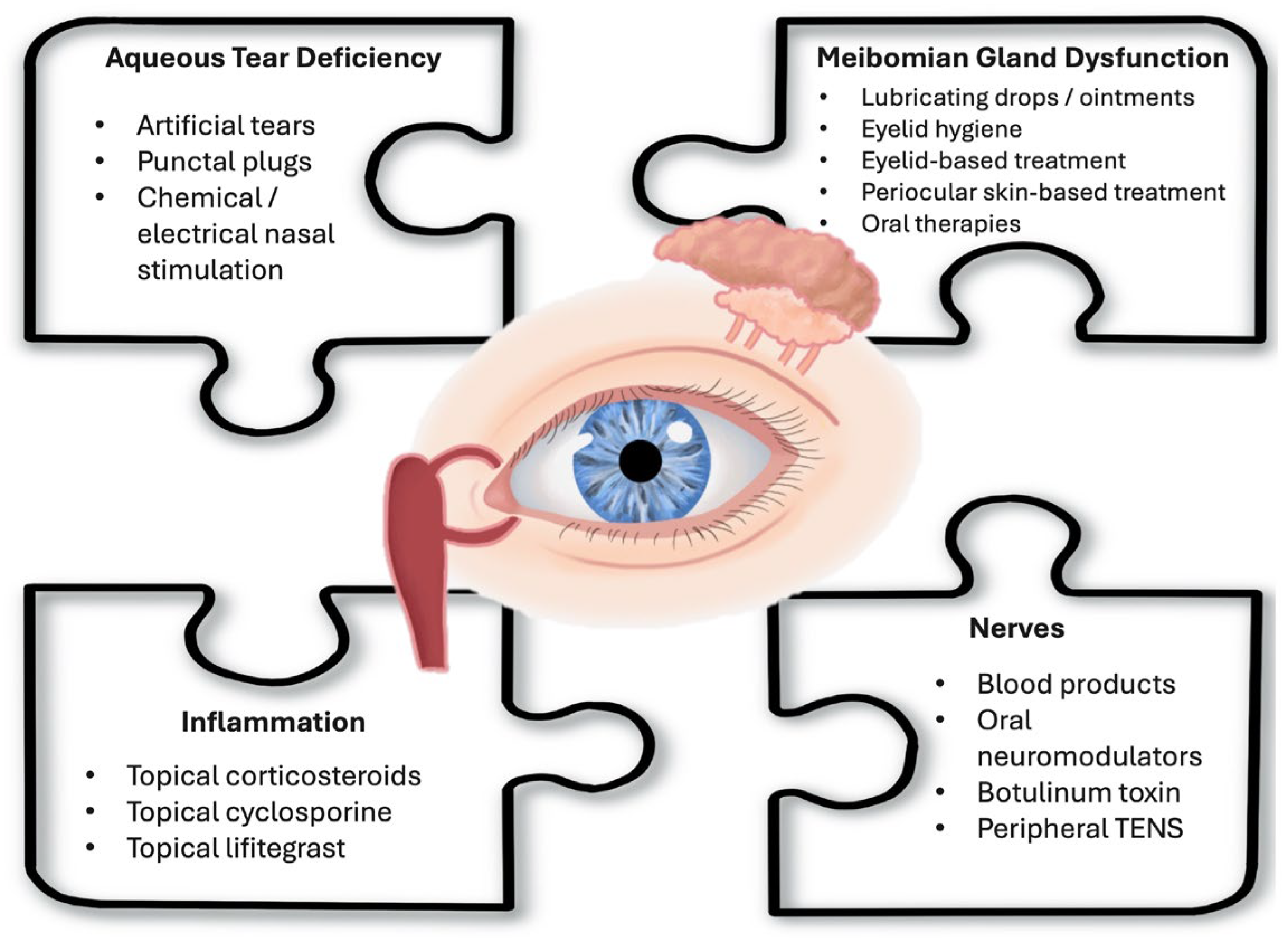
2. Review of Current Therapies
2.1. Therapies for Aqueous Tear Deficiency
2.1.1. Artificial Tears
2.1.2. Punctal Plugs
2.1.3. Tyrvaya (Varenicline Solution)
2.1.4. Nasal Stimulation—External Nasal Stimulator
2.2. Therapies for Meibomian Gland Dysfunction (MGD)
2.2.1. Lubricating Drops and Ointments
2.2.2. Perfluorohexyloctane Ophthalmic Solution (MIEBO)
2.2.3. Eyelid Hygiene
2.2.4. Eyelid-Based Treatments
LipiFlow Thermal Pulsation System
iLux
BlephEx
2.2.5. Periocular Skin-Based Treatments
Intense Pulsed Light (IPL) Therapy
2.2.6. Oral Therapies
Omega-3 Fatty Acid Supplementation
Oral Antibiotics
2.3. Therapies Targeting Inflammation
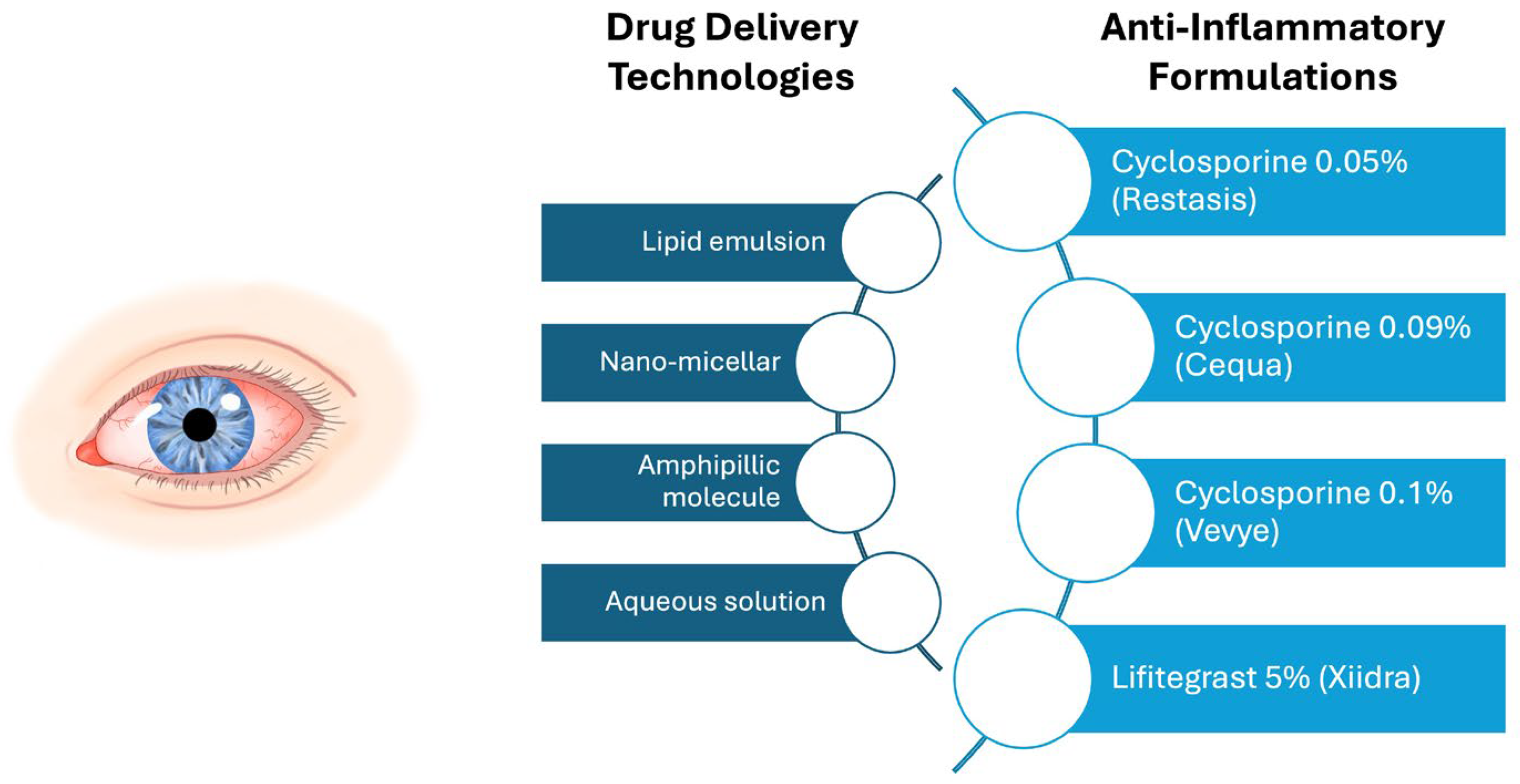
2.3.1. Topical Anti-Inflammatories
2.3.2. Topical Corticosteroids
2.4. Therapies Targeting Nerves
2.4.1. Blood Products
2.4.2. Oral Neuromodulators
2.4.3. Adjuvant Therapies
Botulinum Toxin
Peripheral Trigeminal Transcutaneous Electrical Nerve Stimulation (TENS)
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DED | Dry eye disease |
| ATD | Aqueous tear deficiency |
| MGD | Meibomian gland dysfunction |
| DEQ5 | 5-Item Dry Eye Questionnaire 5 |
| OSDI | Ocular Surface Disease Index |
| TBUT | Tear break-up time |
| NK | Neurotrophic keratitis |
| ATs | Artificial tears |
| tCFS | Total corneal fluorescein staining |
| MGS | Meibomian gland secretion |
| IVCM | In vivo confocal microscopy |
| CsA | Cyclosporine |
| ASTs | Autologous serum tears |
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.-K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef] [PubMed]
- Mandell, J.T.; Idarraga, M.; Kumar, N.; Galor, A. Impact of Air Pollution and Weather on Dry Eye. J. Clin. Med. 2020, 9, 3740. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Kim, M.; Galor, A. Beyond dry eye: How co-morbidities influence disease phenotype in dry eye disease. Clin. Exp. Optom. 2022, 105, 177–185. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Aggarwal, S.; Galor, A. What’s new in dry eye disease diagnosis? Current advances and challenges. F1000Res 2018, 7, 1952. [Google Scholar] [CrossRef]
- Chhadva, P.; McClellan, A.L.; Alabiad, C.R.; Feuer, W.J.; Batawi, H.; Galor, A. Impact of Eyelid Laxity on Symptoms and Signs of Dry Eye Disease. Cornea 2016, 35, 531–535. [Google Scholar] [CrossRef]
- Chalmers, R.L.; Begley, C.G.; Caffery, B. Validation of the 5-Item Dry Eye Questionnaire (DEQ-5): Discrimination across self-assessed severity and aqueous tear deficient dry eye diagnoses. Cont. Lens Anterior Eye 2010, 33, 55–60. [Google Scholar] [CrossRef]
- Schiffman, R.M.; Christianson, M.D.; Jacobsen, G.; Hirsch, J.D.; Reis, B.L. Reliability and validity of the Ocular Surface Disease Index. Arch. Ophthalmol. 2000, 118, 615–621. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Chhadva, P.; Alexander, A.; McClellan, A.L.; McManus, K.T.; Seiden, B.; Galor, A. The impact of conjunctivochalasis on dry eye symptoms and signs. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2867–2871. [Google Scholar] [CrossRef] [PubMed]
- Mehra, D.; Cohen, N.K.; Galor, A. Ocular Surface Pain: A Narrative Review. Ophthalmol. Ther. 2020, 9, 1–21. [Google Scholar] [CrossRef] [PubMed]
- NaPier, E.; Camacho, M.; McDevitt, T.F.; Sweeney, A.R. Neurotrophic keratopathy: Current challenges and future prospects. Ann. Med. 2022, 54, 666–673. [Google Scholar] [CrossRef] [PubMed]
- De Lott, L.B.; Kaplan, C.; Harte, S.; Clauw, D.J.; Galor, A.; Vehof, J.; Shtein, R.M. Nociplastic pain among individuals with chronic ocular surface pain: One cause for “pain without stain”? Surv. Ophthalmol. 2025, 70, 536–543. [Google Scholar] [CrossRef]
- Lacorzana, J.; Neo, Y.N.; Maubon, L.; Sibley, D.; Ahmad, S. Current and Emerging Therapeutic Strategies for the Management of Neurotrophic Keratitis. Drugs 2025, 85, 283–291. [Google Scholar] [CrossRef]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef]
- IASP Terminology. Available online: http://www.iasp-pain.org/Education/Content.aspx?ItemNumber=1698. (accessed on 18 February 2025).
- Ong, E.S.; Felix, E.R.; Levitt, R.C.; Feuer, W.J.; Sarantopoulos, C.D.; Galor, A. Epidemiology of discordance between symptoms and signs of dry eye. Br. J. Ophthalmol. 2018, 102, 674–679. [Google Scholar] [CrossRef]
- Xiong, F.; Arnold, B.F.; Lietman, T.M.; Gonzales, J.A. Predictors of Discordance Between Dry Eye Symptoms and Signs: Insights From the Sjögren’s International Collaborative Clinical Alliance. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3. [Google Scholar] [CrossRef]
- Galor, A.; Hamrah, P.; Haque, S.; Attal, N.; Labetoulle, M. Understanding chronic ocular surface pain: An unmet need for targeted drug therapy. Ocul. Surf. 2022, 26, 148–156. [Google Scholar] [CrossRef]
- Galor, A.; Lighthizer, N. Corneal Sensitivity Testing Procedure for Ophthalmologic and Optometric Patients. J. Vis. Exp. 2024, 210, e66597. [Google Scholar] [CrossRef]
- Chao, C.; Stapleton, F.; Badarudin, E.; Golebiowski, B. Ocular surface sensitivity repeatability with Cochet-Bonnet esthesiometer. Optom. Vis. Sci. 2015, 92, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Tesón, M.; Calonge, M.; Fernández, I.; Stern, M.E.; González-García, M.J. Characterization by Belmonte’s gas esthesiometer of mechanical, chemical, and thermal corneal sensitivity thresholds in a normal population. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3154–3160. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.Y.; Chow, J.; Liu, J. Corneal Innervation and Sensation: The Eye and Beyond. Yale J. Biol. Med. 2018, 91, 13–21. [Google Scholar]
- Merayo-Lloves, J.; Gomez Martin, C.; Lozano-Sanroma, J.; Renedo Laguna, C. Assessment and safety of the new esthesiometer BRILL: Comparison with the Cochet-Bonnet Esthesiometer. Eur. J. Ophthalmol. 2024, 34, 1036–1045. [Google Scholar] [CrossRef]
- Dieckmann, G.; Goyal, S.; Hamrah, P. Neuropathic Corneal Pain: Approaches for Management. Ophthalmology 2017, 124, S34–S47. [Google Scholar] [CrossRef]
- Crane, A.M.; Feuer, W.; Felix, E.R.; Levitt, R.C.; McClellan, A.L.; Sarantopoulos, K.D.; Galor, A. Evidence of central sensitisation in those with dry eye symptoms and neuropathic-like ocular pain complaints: Incomplete response to topical anaesthesia and generalised heightened sensitivity to evoked pain. Br. J. Ophthalmol. 2017, 101, 1238–1243. [Google Scholar] [CrossRef]
- Aggarwal, S.; Kheirkhah, A.; Cavalcanti, B.M.; Cruzat, A.; Jamali, A.; Hamrah, P. Correlation of corneal immune cell changes with clinical severity in dry eye disease: An in vivo confocal microscopy study. Ocul. Surf. 2021, 19, 183–189. [Google Scholar] [CrossRef]
- Moein, H.R.; Akhlaq, A.; Dieckmann, G.; Abbouda, A.; Pondelis, N.; Salem, Z.; Muller, R.T.; Cruzat, A.; Cavalcanti, B.M.; Jamali, A.; et al. Visualization of microneuromas by using in vivo confocal microscopy: An objective biomarker for the diagnosis of neuropathic corneal pain? Ocul. Surf. 2020, 18, 651–656. [Google Scholar] [CrossRef]
- Dartt, D.A. Dysfunctional Neural Regulation of Lacrimal Gland Secretion and its Role in the Pathogenesis of Dry Eye Syndromes. Ocul. Surf. 2004, 2, 76–91. [Google Scholar] [CrossRef]
- Nassar, A.; Tabbara, K.F.; Aljurf, M. Ocular manifestations of graft-versus-host disease. Saudi J. Ophthalmol. 2013, 27, 215–222. [Google Scholar] [CrossRef]
- Donthineni, P.R.; Doctor, M.B.; Shanbhag, S.; Kate, A.; Galor, A.; Djalilian, A.R.; Singh, S.; Basu, S. Aqueous-deficient dry eye disease: Preferred practice pattern guidelines on clinical approach, diagnosis, and management. Indian. J. Ophthalmol. 2023, 71, 1332–1347. [Google Scholar] [CrossRef] [PubMed]
- Semp, D.A.; Beeson, D.; Sheppard, A.L.; Dutta, D.; Wolffsohn, J.S. Artificial Tears: A Systematic Review. Clin. Optom. 2023, 15, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Pucker, A.D.; Ng, S.M.; Nichols, J.J. Over the counter (OTC) artificial tear drops for dry eye syndrome. Cochrane Database Syst. Rev. 2016, 2, Cd009729. [Google Scholar] [CrossRef] [PubMed]
- Lievens, C.; Berdy, G.; Douglass, D.; Montaquila, S.; Lin, H.; Simmons, P.; Carlisle-Wilcox, C.; Vehige, J.; Haque, S. Evaluation of an enhanced viscosity artificial tear for moderate to severe dry eye disease: A multicenter, double-masked, randomized 30-day study. Contact Lens Anterior Eye 2019, 42, 443–449. [Google Scholar] [CrossRef]
- Baxter, S.A.; Laibson, P.R. Punctal plugs in the management of dry eyes. Ocul. Surf. 2004, 2, 255–265. [Google Scholar] [CrossRef]
- Ervin, A.M.; Law, A.; Pucker, A.D. Punctal occlusion for dry eye syndrome. Cochrane Database Syst. Rev. 2017, 6, Cd006775. [Google Scholar] [CrossRef]
- Hirai, K.; Takano, Y.; Uchio, E.; Kadonosono, K. Clinical evaluation of the therapeutic effects of atelocollagen absorbable punctal plugs. Clin. Ophthalmol. 2012, 6, 133–138. [Google Scholar] [CrossRef]
- Dieckmann, G.; Fregni, F.; Hamrah, P. Neurostimulation in dry eye disease—Past, present, and future. Ocul. Surf. 2019, 17, 20–27. [Google Scholar] [CrossRef]
- Frampton, J.E. Varenicline Solution Nasal Spray: A Review in Dry Eye Disease. Drugs 2022, 82, 1481–1488. [Google Scholar] [CrossRef]
- Wirta, D.; Vollmer, P.; Paauw, J.; Chiu, K.-H.; Henry, E.; Striffler, K.; Nau, J.; Wirta, D.; Rubin, J.; Reilly, C.; et al. Efficacy and Safety of OC-01 (Varenicline Solution) Nasal Spray on Signs and Symptoms of Dry Eye Disease: The ONSET-2 Phase 3 Randomized Trial. Ophthalmology 2022, 129, 379–387. [Google Scholar] [CrossRef]
- Ji, M.H.; Moshfeghi, D.M.; Periman, L.; Kading, D.; Matossian, C.; Walman, G.; Markham, S.; Mu, A.; Jayaram, A.; Gertner, M.; et al. Novel Extranasal Tear Stimulation: Pivotal Study Results. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, X.; Li, X. Effectiveness of Intranasal Tear Neurostimulation for Treatment of Dry Eye Disease: A Meta-Analysis. Ophthalmol. Ther. 2023, 12, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.J.; Butron, K.; Robledo, N.; Loudin, J.; Baba, S.N.; Chayet, A. A nonrandomized, open-label study to evaluate the effect of nasal stimulation on tear production in subjects with dry eye disease. Clin. Ophthalmol. 2016, 10, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Adil, M.Y.; Chen, X.; Utheim Ø, A.; Ræder, S.; Tønseth, K.A.; Lagali, N.S.; Dartt, D.A.; Utheim, T.P. Functional and Morphological Evaluation of Meibomian Glands in the Assessment of Meibomian Gland Dysfunction Subtype and Severity. Am. J. Ophthalmol. 2020, 209, 160–167. [Google Scholar] [CrossRef]
- Garrigue, J.S.; Amrane, M.; Faure, M.O.; Holopainen, J.M.; Tong, L. Relevance of Lipid-Based Products in the Management of Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 647–661. [Google Scholar] [CrossRef]
- Baudouin, C.; Galarreta, D.J.; Mrukwa-Kominek, E.; Böhringer, D.; Maurino, V.; Guillon, M.; Rossi, G.C.; Van der Meulen, I.J.; Ogundele, A.; Labetoulle, M. Clinical evaluation of an oil-based lubricant eyedrop in dry eye patients with lipid deficiency. Eur. J. Ophthalmol. 2017, 27, 122–128. [Google Scholar] [CrossRef]
- Yang, L.; Li, J.; Zhou, B.; Wang, Y. An Injectable Copolymer for in Situ Lubrication Effectively Relieves Dry Eye Disease. ACS Mater. Lett. 2025, 7, 884–890. [Google Scholar] [CrossRef]
- Sheppard, J.D.; Kurata, F.; Epitropoulos, A.T.; Krösser, S.; Vittitow, J.L. NOV03 for Signs and Symptoms of Dry Eye Disease Associated With Meibomian Gland Dysfunction: The Randomized Phase 3 MOJAVE Study. Am. J. Ophthalmol. 2023, 252, 265–274. [Google Scholar] [CrossRef]
- Tian, L.; Gao, Z.; Zhu, L.; Shi, X.; Zhao, S.; Gu, H.; Xu, G.; Wang, L.; Dai, H.; Zhang, H.; et al. Perfluorohexyloctane Eye Drops for Dry Eye Disease Associated With Meibomian Gland Dysfunction in Chinese Patients: A Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 385–392. [Google Scholar] [CrossRef]
- Guillon, M.; Maissa, C.; Wong, S. Symptomatic relief associated with eyelid hygiene in anterior blepharitis and MGD. Eye Contact Lens 2012, 38, 306–312. [Google Scholar] [CrossRef]
- Ahn, H.; Kim, B.Y.; Kim, J.; Ji, Y.W.; Jun, I.; Kim, T.I.; Lee, H.K.; Seo, K.Y. How Long to Continue Eyelid Hygiene to Treat Meibomian Gland Dysfunction. J. Clin. Med. 2022, 11, 529. [Google Scholar] [CrossRef] [PubMed]
- Nichols, K.K.; Foulks, G.N.; Bron, A.J.; Glasgow, B.J.; Dogru, M.; Tsubota, K.; Lemp, M.A.; Sullivan, D.A. The international workshop on meibomian gland dysfunction: Executive summary. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1922–1929. [Google Scholar] [CrossRef] [PubMed]
- Aryasit, O.; Uthairat, Y.; Singha, P.; Horatanaruang, O. Efficacy of baby shampoo and commercial eyelid cleanser in patients with meibomian gland dysfunction: A randomized controlled trial. Medicine 2020, 99, e20155. [Google Scholar] [CrossRef]
- Beining, M.W.; Magnø, M.S.; Moschowits, E.; Olafsson, J.; Vehof, J.; Dartt, D.A.; Utheim, T.P. In-office thermal systems for the treatment of dry eye disease. Surv. Ophthalmol. 2022, 67, 1405–1418. [Google Scholar] [CrossRef]
- Lam, P.Y.; Shih, K.C.; Fong, P.Y.; Chan, T.C.Y.; Ng, A.L.; Jhanji, V.; Tong, L. A Review on Evidence-Based Treatments for Meibomian Gland Dysfunction. Eye Contact Lens 2020, 46, 3–16. [Google Scholar] [CrossRef]
- Pucker, A.D.; Yim, T.W.; Rueff, E.; Ngo, W.; Tichenor, A.A.; Conto, J.E. LipiFlow for the treatment of dry eye disease. Cochrane Database Syst. Rev. 2024, 2, Cd015448. [Google Scholar] [CrossRef]
- Hu, J.; Zhu, S.; Liu, X. Efficacy and safety of a vectored thermal pulsation system (Lipiflow®) in the treatment of meibomian gland dysfunction: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 25–39. [Google Scholar] [CrossRef]
- Meng, Z.; Chu, X.; Zhang, C.; Liu, H.; Yang, R.; Huang, Y.; Zhao, S. Efficacy and Safety evaluation of a single thermal pulsation system treatment (Lipiflow(®)) on meibomian gland dysfunction: A randomized controlled clinical trial. Int. Ophthalmol. 2023, 43, 1175–1184. [Google Scholar] [CrossRef]
- Chen, K.Y.; Chan, H.C.; Chan, C.M. Is a thermal pulsation system (LipiFlow) effective as a standalone treatment for meibomian gland dysfunction and dry eye? A systematic review and meta-analysis. Ther. Adv. Ophthalmol. 2025, 17, 25158414251338775. [Google Scholar] [CrossRef]
- Tauber, J.; Owen, J.; Bloomenstein, M.; Hovanesian, J.; Bullimore, M.A. Comparison of the iLUX and the LipiFlow for the Treatment of Meibomian Gland Dysfunction and Symptoms: A Randomized Clinical Trial. Clin. Ophthalmol. 2020, 14, 405–418. [Google Scholar] [CrossRef]
- Núñez, M.X.; Acosta-Ortega, A.; Vera-Duarte, G.R.; Gómez-Duarte, C. The ILux(®) compared to the mechanical meibomian gland expression for the treatment of moderate and severe meibomian gland dysfunction. Int. J. Ophthalmol. 2024, 17, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Siegel, H.; Merz, A.; Gross, N.; Bründer, M.C.; Böhringer, D.; Reinhard, T.; Maier, P. BlephEx-treatment for blepharitis: A prospective randomized placebo-controlled trial. BMC Ophthalmol. 2024, 24, 503. [Google Scholar] [CrossRef]
- Mohammad-Rabei, H.; Arabi, A.; Shahraki, T.; Rezaee-Alam, Z.; Baradaran-Rafii, A. Role of Blepharoexfoliation in Demodex Blepharitis: A Randomized Comparative Study. Cornea 2023, 42, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benitez-del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Giannaccare, G.; Taroni, L.; Senni, C.; Scorcia, V. Intense Pulsed Light Therapy In The Treatment Of Meibomian Gland Dysfunction: Current Perspectives. Clin. Optom. 2019, 11, 113–126. [Google Scholar] [CrossRef]
- Demolin, L.; Es-Safi, M.; Soyfoo, M.S.; Motulsky, E. Intense Pulsed Light Therapy in the Treatment of Dry Eye Diseases: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 3039. [Google Scholar] [CrossRef]
- Yates, B.; Que, S.K.; D’Souza, L.; Suchecki, J.; Finch, J.J. Laser treatment of periocular skin conditions. Clin. Dermatol. 2015, 33, 197–206. [Google Scholar] [CrossRef]
- Martignago, C.C.S.; Bonifacio, M.; Ascimann, L.T.; Vassão, P.G.; Parisi, J.R.; Renno, A.P.; Garcia, L.A.; Ribeiro, D.A.; Renno, A.C.M. Efficacy and safety of intense pulsed light in rosacea: A systematic review. Indian. J. Dermatol. Venereol. Leprol. 2024, 90, 599–605. [Google Scholar] [CrossRef]
- Toyos, R.; Desai, N.R.; Toyos, M.; Dell, S.J. Intense pulsed light improves signs and symptoms of dry eye disease due to meibomian gland dysfunction: A randomized controlled study. PLoS ONE 2022, 17, e0270268. [Google Scholar] [CrossRef]
- Pellegrini, M.; Senni, C.; Bernabei, F.; Cicero, A.F.G.; Vagge, A.; Maestri, A.; Scorcia, V.; Giannaccare, G. The Role of Nutrition and Nutritional Supplements in Ocular Surface Diseases. Nutrients 2020, 12, 952. [Google Scholar] [CrossRef]
- Giannaccare, G.; Pellegrini, M.; Sebastiani, S.; Bernabei, F.; Roda, M.; Taroni, L.; Versura, P.; Campos, E.C. Efficacy of Omega-3 Fatty Acid Supplementation for Treatment of Dry Eye Disease: A Meta-Analysis of Randomized Clinical Trials. Cornea 2019, 38, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.X.; Ko, M.L. Efficacy of Omega-3 Intake in Managing Dry Eye Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2023, 12, 7026. [Google Scholar] [CrossRef]
- Jo, Y.J.; Lee, J.S. Effects of dietary high dose DHA omega-3 supplement in dry eye with meibomian gland dysfunction. Int. J. Ophthalmol. 2021, 14, 1700–1706. [Google Scholar] [CrossRef] [PubMed]
- Asbell, P.A.; Maguire, M.G.; Pistilli, M.; Ying, G.S.; Szczotka-Flynn, L.B.; Hardten, D.R.; Lin, M.C.; Shtein, R.M. n-3 Fatty Acid Supplementation for the Treatment of Dry Eye Disease. N. Engl. J. Med. 2018, 378, 1681–1690. [Google Scholar] [CrossRef] [PubMed]
- Vernhardsdottir, R.R.; Magno, M.S.; Hynnekleiv, L.; Lagali, N.; Dartt, D.A.; Vehof, J.; Jackson, C.J.; Utheim, T.P. Antibiotic treatment for dry eye disease related to meibomian gland dysfunction and blepharitis—A review. Ocul. Surf. 2022, 26, 211–221. [Google Scholar] [CrossRef]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The international workshop on meibomian gland dysfunction: Report of the subcommittee on management and treatment of meibomian gland dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef]
- Upaphong, P.; Tangmonkongvoragul, C.; Phinyo, P. Pulsed Oral Azithromycin vs 6-Week Oral Doxycycline for Moderate to Severe Meibomian Gland Dysfunction: A Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 423–429. [Google Scholar] [CrossRef]
- Yoo, S.E.; Lee, D.C.; Chang, M.H. The effect of low-dose doxycycline therapy in chronic meibomian gland dysfunction. Korean J. Ophthalmol. 2005, 19, 258–263. [Google Scholar] [CrossRef]
- Stern, M.E.; Pflugfelder, S.C. Inflammation in dry eye. Ocul. Surf. 2004, 2, 124–130. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; de Paiva, C.S. The Pathophysiology of Dry Eye Disease: What We Know and Future Directions for Research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef] [PubMed]
- Levine, H.; Hwang, J.; Dermer, H.; Mehra, D.; Feuer, W.; Galor, A. Relationships between activated dendritic cells and dry eye symptoms and signs. Ocul. Surf. 2021, 21, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Periman, L.M.; Perez, V.L.; Saban, D.R.; Lin, M.C.; Neri, P. The Immunological Basis of Dry Eye Disease and Current Topical Treatment Options. J. Ocul. Pharmacol. Ther. 2020, 36, 137–146. [Google Scholar] [CrossRef]
- Anitua, E.; de la Sen-Corcuera, B.; Orive, G.; Sánchez-Ávila, R.M.; Heredia, P.; Muruzabal, F.; Merayo-Lloves, J. Progress in the use of plasma rich in growth factors in ophthalmology: From ocular surface to ocular fundus. Expert. Opin. Biol. Ther. 2022, 22, 31–45. [Google Scholar] [CrossRef]
- Perez, V.L.; Mah, F.S.; Willcox, M.; Pflugfelder, S. Anti-Inflammatories in the Treatment of Dry Eye Disease: A Review. J. Ocul. Pharmacol. Ther. 2023, 39, 89–101. [Google Scholar] [CrossRef]
- Ames, P.; Galor, A. Cyclosporine ophthalmic emulsions for the treatment of dry eye: A review of the clinical evidence. Clin. Investig. 2015, 5, 267–285. [Google Scholar] [CrossRef]
- Sall, K.; Stevenson, O.D.; Mundorf, T.K.; Reis, B.L. Two multicenter, randomized studies of the efficacy and safety of cyclosporine ophthalmic emulsion in moderate to severe dry eye disease. CsA Phase 3 Study Group. Ophthalmology 2000, 107, 631–639. [Google Scholar] [CrossRef]
- Goldberg, D.F.; Malhotra, R.P.; Schechter, B.A.; Justice, A.; Weiss, S.L.; Sheppard, J.D. A Phase 3, Randomized, Double-Masked Study of OTX-101 Ophthalmic Solution 0.09% in the Treatment of Dry Eye Disease. Ophthalmology 2019, 126, 1230–1237. [Google Scholar] [CrossRef]
- Akpek, E.K.; Wirta, D.L.; Downing, J.E.; Tauber, J.; Sheppard, J.D.; Ciolino, J.B.; Meides, A.S.; Krösser, S. Efficacy and Safety of a Water-Free Topical Cyclosporine, 0.1%, Solution for the Treatment of Moderate to Severe Dry Eye Disease: The ESSENCE-2 Randomized Clinical Trial. JAMA Ophthalmol. 2023, 141, 459–466. [Google Scholar] [CrossRef]
- Haber, S.L.; Benson, V.; Buckway, C.J.; Gonzales, J.M.; Romanet, D.; Scholes, B. Lifitegrast: A novel drug for patients with dry eye disease. Ther. Adv. Ophthalmol. 2019, 11, 2515841419870366. [Google Scholar] [CrossRef]
- Semba, C.P.; Torkildsen, G.L.; Lonsdale, J.D.; McLaurin, E.B.; Geffin, J.A.; Mundorf, T.K.; Kennedy, K.S.; Ousler, G.W. A phase 2 randomized, double-masked, placebo-controlled study of a novel integrin antagonist (SAR 1118) for the treatment of dry eye. Am. J. Ophthalmol. 2012, 153, 1050–1060.e1051. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, J.D.; Torkildsen, G.L.; Lonsdale, J.D.; D’Ambrosio, F.A., Jr.; McLaurin, E.B.; Eiferman, R.A.; Kennedy, K.S.; Semba, C.P. Lifitegrast ophthalmic solution 5.0% for treatment of dry eye disease: Results of the OPUS-1 phase 3 study. Ophthalmology 2014, 121, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tauber, J.; Karpecki, P.; Latkany, R.; Luchs, J.; Martel, J.; Sall, K.; Raychaudhuri, A.; Smith, V.; Semba, C.P. Lifitegrast Ophthalmic Solution 5.0% versus Placebo for Treatment of Dry Eye Disease: Results of the Randomized Phase III OPUS-2 Study. Ophthalmology 2015, 122, 2423–2431. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Luchs, J.; Karpecki, P.M.; Nichols, K.K.; Jackson, M.A.; Sall, K.; Tauber, J.; Roy, M.; Raychaudhuri, A.; Shojaei, A. Lifitegrast for the Treatment of Dry Eye Disease: Results of a Phase III, Randomized, Double-Masked, Placebo-Controlled Trial (OPUS-3). Ophthalmology 2017, 124, 53–60. [Google Scholar] [CrossRef]
- Li, J.X.; Tsai, Y.Y.; Lai, C.T.; Li, Y.L.; Wu, Y.H.; Chiang, C.C. Lifitegrast Ophthalmic Solution 5% Is a Safe and Efficient Eyedrop for Dry Eye Disease: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 5014. [Google Scholar] [CrossRef]
- Locatelli, E.V.T.; Acuna, K.A.; Betz, J.; Tovar, A.A.; Galor, A. Comparison of Subjective Responses to Cyclosporine 0.05% Versus Lifitegrast 5.0% in Individuals With Dry Eye Disease. Cornea 2024, 43, 88–94. [Google Scholar] [CrossRef]
- Ryu, K.J.; Kim, S.; Kim, M.K.; Paik, H.J.; Kim, D.H. Short-Term Therapeutic Effects of Topical Corticosteroids on Refractory Dry Eye Disease: Clinical Usefulness of Matrix Metalloproteinase 9 Testing as a Response Prediction Marker. Clin. Ophthalmol. 2021, 15, 759–767. [Google Scholar] [CrossRef]
- Byun, Y.J.; Kim, T.I.; Kwon, S.M.; Seo, K.Y.; Kim, S.W.; Kim, E.K.; Park, W.C. Efficacy of combined 0.05% cyclosporine and 1% methylprednisolone treatment for chronic dry eye. Cornea 2012, 31, 509–513. [Google Scholar] [CrossRef]
- Prinz, J.; Maffulli, N.; Fuest, M.; Walter, P.; Bell, A.; Migliorini, F. Efficacy of Topical Administration of Corticosteroids for the Management of Dry Eye Disease: Systematic Review and Meta-Analysis. Life 2022, 12, 1932. [Google Scholar] [CrossRef]
- Pinto-Fraga, J.; López-Miguel, A.; González-García, M.J.; Fernández, I.; López-de-la-Rosa, A.; Enríquez-de-Salamanca, A.; Stern, M.E.; Calonge, M. Topical Fluorometholone Protects the Ocular Surface of Dry Eye Patients from Desiccating Stress: A Randomized Controlled Clinical Trial. Ophthalmology 2016, 123, 141–153. [Google Scholar] [CrossRef]
- Cutolo, C.A.; Barabino, S.; Bonzano, C.; Traverso, C.E. The Use of Topical Corticosteroids for Treatment of Dry Eye Syndrome. Ocul. Immunol. Inflamm. 2019, 27, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Soni, N.G.; Jeng, B.H. Blood-derived topical therapy for ocular surface diseases. Br. J. Ophthalmol. 2016, 100, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.C.; Fisher, J.; Mondy, P.; Segatchian, J.; Dennington, P.M. Serum eye drop preparation in Australia: Current manufacturing practice. Transfus. Apher. Sci. 2015, 53, 92–94. [Google Scholar] [CrossRef]
- Quan, N.G.; Leslie, L.; Li, T. Autologous Serum Eye Drops for Dry Eye: Systematic Review. Optom. Vis. Sci. 2023, 100, 564–571. [Google Scholar] [CrossRef]
- Shtein, R.M.; Shen, J.F.; Kuo, A.N.; Hammersmith, K.M.; Li, J.Y.; Weikert, M.P. Autologous Serum-Based Eye Drops for Treatment of Ocular Surface Disease: A Report by the American Academy of Ophthalmology. Ophthalmology 2020, 127, 128–133. [Google Scholar] [CrossRef]
- He, C.Z.; Zeng, Z.J.; Liu, J.Q.; Qiu, Q.; He, Y. Autologous serum eye drops for patients with dry eye disease: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2024, 11, 1430785. [Google Scholar] [CrossRef]
- Zheng, N.; Zhu, S.Q. Randomized controlled trial on the efficacy and safety of autologous serum eye drops in dry eye syndrome. World J. Clin. Cases 2023, 11, 6774–6781. [Google Scholar] [CrossRef]
- Soifer, M.; Tovar, A.; Wang, M.; Mousa, H.M.; Yennam, S.; Sabater, A.L.; Pflugfelder, S.C.; Perez, V.L. A multicenter report of the use of plasma rich in growth factors (PRGF) for the treatment of patients with ocular surface diseases in North America. Ocul. Surf. 2022, 25, 40–48. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Dogru, M.; Goto, E.; Ohashi, Y.; Kojima, T.; Ishida, R.; Tsubota, K. Autologous serum application in the treatment of neurotrophic keratopathy. Ophthalmology 2004, 111, 1115–1120. [Google Scholar] [CrossRef]
- Rao, K.; Leveque, C.; Pflugfelder, S.C. Corneal nerve regeneration in neurotrophic keratopathy following autologous plasma therapy. Br. J. Ophthalmol. 2010, 94, 584–591. [Google Scholar] [CrossRef]
- Shen, X.; Chen, X.; He, Y.; Xu, H.; Zhu, J. Efficacy and safety of pregabalin in eye pain: A systematic review. Medicine 2023, 102, e32875. [Google Scholar] [CrossRef] [PubMed]
- Tei, Y.; Mikami, Y.; Ito, M.; Tomida, T.; Ohshima, D.; Hori, Y.; Adachi-Akahane, S. Pathogenic Mechanism of Dry Eye-Induced Chronic Ocular Pain and a Mechanism-Based Therapeutic Approach. Investig. Ophthalmol. Vis. Sci. 2022, 63, 7. [Google Scholar] [CrossRef] [PubMed]
- Ozmen, M.C.; Dieckmann, G.; Cox, S.M.; Rashad, R.; Paracha, R.; Sanayei, N.; Morkin, M.I.; Hamrah, P. Efficacy and tolerability of nortriptyline in the management of neuropathic corneal pain. Ocul. Surf. 2020, 18, 814–820. [Google Scholar] [CrossRef] [PubMed]
- Small, L.R.; Galor, A.; Felix, E.R.; Horn, D.B.; Levitt, R.C.; Sarantopoulos, C.D. Oral Gabapentinoids and Nerve Blocks for the Treatment of Chronic Ocular Pain. Eye Contact Lens 2020, 46, 174–181. [Google Scholar] [CrossRef]
- Patel, S.; Mittal, R.; Sarantopoulos, K.D.; Galor, A. Neuropathic ocular surface pain: Emerging drug targets and therapeutic implications. Expert. Opin. Ther. Targets 2022, 26, 681–695. [Google Scholar] [CrossRef]
- Dieckmann, G.; Ozmen, M.C.; Cox, S.M.; Engert, R.C.; Hamrah, P. Low-dose naltrexone is effective and well-tolerated for modulating symptoms in patients with neuropathic corneal pain. Ocul. Surf. 2021, 20, 33–38. [Google Scholar] [CrossRef]
- Ongun, N.; Ongun, G.T. Is gabapentin effective in dry eye disease and neuropathic ocular pain? Acta Neurol. Belg. 2021, 121, 397–401. [Google Scholar] [CrossRef]
- Xu, X.M.; Liu, Y.; Dong, M.X.; Zou, D.Z.; Wei, Y.D. Tricyclic antidepressants for preventing migraine in adults. Medicine 2017, 96, e6989. [Google Scholar] [CrossRef]
- Belmonte, C.; Acosta, M.C.; Merayo-Lloves, J.; Gallar, J. What Causes Eye Pain? Curr. Ophthalmol. Rep. 2015, 3, 111–121. [Google Scholar] [CrossRef]
- Locatelli, E.V.T.; Huang, J.J.; Betz, J.D.; Huang, J.J.; Kantor, N.B.; Reyes, N.; Felix, E.R.; Lee, W.W.; Galor, A. Impact of botulinum toxin type A on ocular pain with neuropathic features. Ocul. Surf. 2025, 37, 24–32. [Google Scholar] [CrossRef]
- Dailey, D.L.; Rakel, B.A.; Vance, C.G.T.; Liebano, R.E.; Amrit, A.S.; Bush, H.M.; Lee, K.S.; Lee, J.E.; Sluka, K.A. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. J. Pain 2013, 154, 2554–2562. [Google Scholar] [CrossRef] [PubMed]
- Evans, A.G.; Horrar, A.N.; Ibrahim, M.M.; Burns, B.L.; Kalmar, C.L.; Assi, P.E.; Brooks-Horrar, K.N.; Kesayan, T.; Al Kassis, S. Outcomes of transcutaneous nerve stimulation for migraine headaches: A systematic review and meta-analysis. J. Neurol. 2022, 269, 4021–4029. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.M.; Zhang, J. Effectiveness of transcutaneous electrical stimulation combined with artificial tears for the treatment of dry eye: A randomized controlled trial. Exp. Ther. Med. 2020, 20, 175. [Google Scholar] [CrossRef] [PubMed]

| Therapy | Type of DED Targeted | Proposed Mechanism of Action | Key Clinical Outcomes |
|---|---|---|---|
| Artificial tears | ATD/MGD | Supplement aqueous layer of tear film and/or improve tear film stability using lipid-based components | Improved signs and symptoms across multiple formulations and trials in ATD and MGD-associated DED |
| Punctal plugs | ATD | Augment ocular surface tear retention | Improved tear retention in some studies and improved symptoms; evidence variable |
| Tyrvaya (varenicline Solution) | ATD | Stimulate tear production via trigeminal pathway | Increased tear production, variable improvement in symptoms versus placebo |
| External nasal stimulation | ATD | Stimulate external nasal nerves to promote tear production | Increased tear production after regular use |
| Perfluorohexyloctane ophthalmic solution (MEIBO) | MGD | Improve tear film function | Improved corneal staining and symptoms in patients with MGD; no significant effect on MGD score or TBUT versus placebo |
| Eyelid hygiene | MGD | Reduce debris and bacteria at lid margin | Limited change in gland function or signs; improved symptoms |
| LipiFlow | MGD | Heat and pulsation to evacuate Meibomian glands | Improved gland function, tear film stability, and symptoms versus warm compress; treatment durability varied across studies |
| iLux | MGD | Targeted heat and compression to clear gland obstructions | Improved gland function, tear film stability, and symptoms versus manual gland expression; comparable efficacy to LipiFlow; variable overall efficacy across studies |
| BlephEx | MGD | Mechanical cleaning of lid margin | Improved Demodex count and symptoms versus sham treatment; limited comparative data |
| IPL | MGD | Reduce inflammation and improve gland function | Improved tear stability and symptoms in MGD with skin inflammation versus manual gland expression alone |
| Omega-3 supplementation | MGD | Anti-inflammatory effect; may alter meibum composition | Mixed findings; some studies showed symptom and tear stability benefit, while others did not |
| Oral antibiotics | MGD/Inflammation | Reduce inflammation and bacterial load | Improved gland function and symptoms in MGD across several regimens |
| Topical anti-inflammatories | Inflammation | Suppress ocular surface inflammation (e.g., CsA, lifitegrast) | Improved corneal staining across all studies; variable improvement in other signs and symptoms versus vehicle |
| Topical corticosteroids | Inflammation | Suppress ocular surface inflammation | Short-term improvement in signs and symptoms in inflammation-driven DED versus control |
| Blood products | Nerves | Provide growth factors and anti-inflammatory cytokines | Improved signs and symptoms compared to artificial tears, strongest signal in moderate or greater DED and neurotrophic keratitis-related DED |
| Oral neuromodulators | Nerves | Modulate central pain signaling pathways | Reduced ocular pain symptoms in some patients with neuropathic features |
| Botulinum toxin | Nerves | Modulate nerve activity | Improved ocular pain in some patients with neuropathic features; limited data overall |
| Peripheral TENS | Nerves | Stimulate trigeminal nerve branches to modulate pain | Improved symptoms versus artificial tears alone; variability in tear parameter improvement between devices and studies |
| Drug | Mechanism | Dose Range Used in Individuals with Chronic Ocular Pain | Ocular Pain Studies |
|---|---|---|---|
| Gabapentin Pregabalin | α2δ voltage-gated calcium channel ligand | Gabapentin—300 mg–900 mg TID Pregabalin—75 mg–150 mg BID | Shen, X. et al. [112] Tei, Y. et al. [113] |
| Amitriptyline, Nortriptyline | Serotonin and norepinephrine reuptake inhibition, sodium channel modulation | Nortriptyline—10 mg–100 mg daily Amitriptyline—10 mg–100 mg daily | Ozmen, M.C. et al. [114] |
| Duloxetine | Selective norepinephrine reuptake inhibitor | 20–60 mg daily | Small, L.R. et al. [115] Patel, S. et al. [116] |
| Topiramate | Voltage-gated sodium channel modulation, enhances GABA-A receptor activity | 100 mg daily | Patel, S. et al. [116] |
| Low-Dose Naltrexone | Pure opioid antagonist with μ− and δ opioid receptor affinity | 1.5–4.5 mg daily | Dieckmann, G. et al. [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Betz, J.; Galor, A. Navigating the Dry Eye Therapeutic Puzzle: A Mechanism-Based Overview of Current Treatments. Pharmaceuticals 2025, 18, 994. https://doi.org/10.3390/ph18070994
Betz J, Galor A. Navigating the Dry Eye Therapeutic Puzzle: A Mechanism-Based Overview of Current Treatments. Pharmaceuticals. 2025; 18(7):994. https://doi.org/10.3390/ph18070994
Chicago/Turabian StyleBetz, Jason, and Anat Galor. 2025. "Navigating the Dry Eye Therapeutic Puzzle: A Mechanism-Based Overview of Current Treatments" Pharmaceuticals 18, no. 7: 994. https://doi.org/10.3390/ph18070994
APA StyleBetz, J., & Galor, A. (2025). Navigating the Dry Eye Therapeutic Puzzle: A Mechanism-Based Overview of Current Treatments. Pharmaceuticals, 18(7), 994. https://doi.org/10.3390/ph18070994






