Superhydrophobicity Effects on Spheroid Formation, Structure, and Viability on Co-Culture Conditions
Abstract
1. Introduction
2. Results
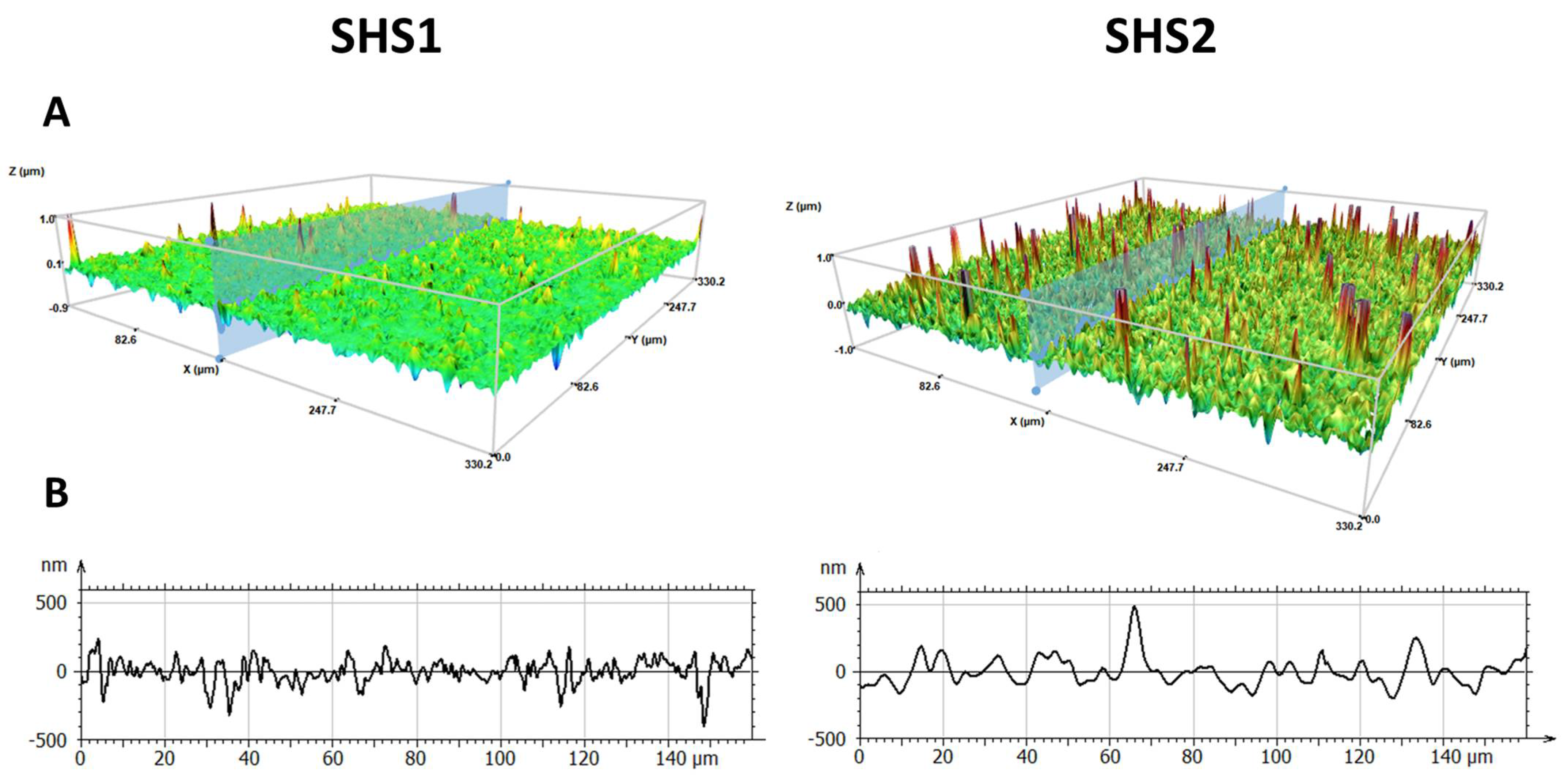
2.1. Surface Characterization of Superhydophobic Coatings
2.2. Cell Behavior in SHS Substrates
2.3. Cell Viability on SHS Spheroids
2.4. Morphological Parameters Determined by 3D Profilometry Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Methods
4.2.1. Surface Preparation and Characterization
4.2.2. Cell Cultures
4.2.3. Cell Culture in Superhydrophobic Substrates
4.2.4. Fluorescence Microscopy Studies
4.2.5. Profilometry Studies
4.2.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shao, C.; Chi, J.; Zhang, H.; Fan, Q.; Zhao, Y.; Ye, F.; Shao, C.M.; Zhao, Y.J.; Ye, F.F.; Chi, J.J.; et al. Development of Cell Spheroids by Advanced Technologies. Adv. Mater. Technol. 2020, 5, 2000183. [Google Scholar] [CrossRef]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-Dimensional Cell Culture Systems and Their Applications in Drug Discovery and Cell-Based Biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Itaka, K.; Ohba, S.; Nishiyama, N.; Chung, U.; Yamasaki, Y.; Kataoka, K. 3D Spheroid Culture System on Micropatterned Substrates for Improved Differentiation Efficiency of Multipotent Mesenchymal Stem Cells. Biomaterials 2009, 30, 2705–2715. [Google Scholar] [CrossRef] [PubMed]
- Bates, R.C.; Edwards, N.S.; Yates, J.D. Spheroids and Cell Survival. Crit. Rev. Oncol. Hematol. 2000, 36, 61–74. [Google Scholar] [CrossRef]
- Baldari, S.; Di Rocco, G.; Piccoli, M.; Pozzobon, M.; Muraca, M.; Toietta, G. Challenges and Strategies for Improving the Regenerative Effects of Mesenchymal Stromal Cell-Based Therapies. Int. J. Mol. Sci. 2017, 18, 2087. [Google Scholar] [CrossRef] [PubMed]
- Breslin, S.; O’Driscoll, L. Three-Dimensional Cell Culture: The Missing Link in Drug Discovery. Drug Discov. Today 2013, 18, 240–249. [Google Scholar] [CrossRef]
- Ivascu, A.; Kubbies, M. Rapid Generation of Single-Tumor Spheroids for High-Throughput Cell Function and Toxicity Analysis. SLAS Discov. 2006, 11, 922–932. [Google Scholar] [CrossRef]
- Yakavets, I.; Jenard, S.; Francois, A.; Maklygina, Y.; Loschenov, V.; Lassalle, H.P.; Dolivet, G.; Bezdetnaya, L. Stroma-Rich Co-Culture Multicellular Tumor Spheroids as a Tool for Photoactive Drugs Screening. J. Clin. Med. 2019, 8, 1686. [Google Scholar] [CrossRef]
- Azizipour, N.; Avazpour, R.; Sawan, M.; Rosenzweig, D.H.; Ajji, A. Uniformity of Spheroids-on-a-Chip by Surface Treatment of PDMS Microfluidic Platforms. Sens. Diagn. 2022, 1, 750–764. [Google Scholar] [CrossRef]
- Aung, A.; Davey, S.K.; Theprungsirikul, J.; Kumar, V.; Varghese, S.; Aung, A.; Davey, S.K.; Theprungsirikul, J.; Varghese, S.; Kumar, V. Deciphering the Mechanics of Cancer Spheroid Growth in 3D Environments through Microfluidics Driven Mechanical Actuation. Adv. Healthc. Mater. 2023, 12, 2201842. [Google Scholar] [CrossRef]
- Białkowska, K.; Komorowski, P.; Bryszewska, M.; Miłowska, K. Spheroids as a Type of Three-Dimensional Cell Cultures—Examples of Methods of Preparation and the Most Important Application. Int. J. Mol. Sci. 2020, 21, 6225. [Google Scholar] [CrossRef] [PubMed]
- Harnett, E.M.; Alderman, J.; Wood, T. The Surface Energy of Various Biomaterials Coated with Adhesion Molecules Used in Cell Culture. Colloids Surf. B Biointerfaces 2007, 55, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, Y.; Wu, W.; Zhao, Q.; Li, G. A Superhydrophobic Chip Integrated with an Array of Medium Reservoirs for Long-Term Hanging Drop Spheroid Culture. Acta Biomater. 2021, 135, 234–242. [Google Scholar] [CrossRef]
- Urzì, O.; Gasparro, R.; Costanzo, E.; De Luca, A.; Giavaresi, G.; Fontana, S.; Alessandro, R. Three-Dimensional Cell Cultures: The Bridge between In Vitro and In Vivo Models. Int. J. Mol. Sci. 2023, 24, 12046. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.D.C. Super Liquid-Repellent Surfaces and 3D Spheroids Growth. Front. Biosci.-Landmark 2022, 27, 144. [Google Scholar] [CrossRef]
- Morán, M.D.C.; Cirisano, F.; Ferrari, M. Spheroid Formation and Recovery Using Superhydrophobic Coating for Regenerative Purposes. Pharmaceutics 2023, 15, 2226. [Google Scholar] [CrossRef]
- Laschke, M.W.; Menger, M.D. Life Is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Cavestany, M.; Wright, O.A.; Reckhorn, N.T.; Carter, A.T.; Jayawardana, K.; Nguyen, T.; Briggs, D.P.; Koktysh, D.S.; Esteban Linares, A.; Li, D.; et al. Superhydrophobic Array Devices for the Enhanced Formation of 3D Cancer Models. ACS Nano 2024, 18, 23637–23654. [Google Scholar] [CrossRef]
- Mohapatra, O.; Gopu, M.; Ashraf, R.; Easo George, J.; Patil, S.; Mukherjee, R.; Kumar, S.; Mampallil, D. Spheroids Formation in Large Drops Suspended in Superhydrophobic Paper Cones. Biomicrofluidics 2024, 18, 24107. [Google Scholar] [CrossRef]
- Rolver, M.G.; Elingaard-Larsen, L.O.; Pedersen, S.F. Assessing Cell Viability and Death in 3D Spheroid Cultures of Cancer Cells. J. Vis. Exp. 2019, 2019, e59714. [Google Scholar] [CrossRef]
- Chatzinikolaidou, M. Cell Spheroids: The New Frontiers in in Vitro Models for Cancer Drug Validation. Drug Discov. Today 2016, 21, 1553–1560. [Google Scholar] [CrossRef]
- Yakavets, I.; Francois, A.; Benoit, A.; Merlin, J.L.; Bezdetnaya, L.; Vogin, G. Advanced Co-Culture 3D Breast Cancer Model for Investigation of Fibrosis Induced by External Stimuli: Optimization Study. Sci. Rep. 2020, 10, 21273. [Google Scholar] [CrossRef]
- Lindner, E.; Arias, E. Surface Free Energy Characteristics of Polyfluorinated Silane Films. Langmuir 1992, 8, 1195–1198. [Google Scholar] [CrossRef]
- Bhowmick, A.K.; Konar, J.; Kole, S.; Narayanan, S. Surface Properties of EPDM, Silicone Rubber, and Their Blend during Aging. J. Appl. Polym. Sci. 1995, 57, 631–637. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.D.C. Mammalian Cell Spheroids on Mixed Organic–Inorganic Superhydrophobic Coating. Molecules 2022, 27, 1247. [Google Scholar] [CrossRef]
- del Carmen Morán, M.; Cirisano, F.; Ferrari, M. Superhydrophobicity Effects on Spheroid Formation and Polarization of Macrophages. Pharmaceuticals 2024, 17, 1042. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Reis, R.L.; Pirraco, R.P. Modelling the Complex Nature of the Tumor Microenvironment: 3D Tumor Spheroids as an Evolving Tool. J. Biomed. Sci. 2024, 31, 13. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D Tumor Spheroids as in Vitro Models to Mimic in Vivo Human Solid Tumors Resistance to Therapeutic Drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef]
- Rodrigues, T.; Kundu, B.; Silva-Correia, J.; Kundu, S.C.; Oliveira, J.M.; Reis, R.L.; Correlo, V.M. Emerging Tumor Spheroids Technologies for 3D in Vitro Cancer Modeling. Pharmacol. Ther. 2018, 184, 201–211. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Majety, M.; Pradel, L.P.; Gies, M.; Ries, C.H. Fibroblasts Influence Survival and Therapeutic Response in a 3D Co-Culture Model. PLoS ONE 2015, 10, e0127948. [Google Scholar] [CrossRef] [PubMed]
- Morán, M.D.C.; Cirisano, F.; Ferrari, M. 3D Profilometry and Cell Viability Studies for Drug Response Screening. Mater. Sci. Eng. C 2020, 115, 111142. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Yin, L.; Yang, Y.; Guan, Y.; Wang, W.; Xu, H.; Tao, N. Quantification of Epidermal Growth Factor Receptor Expression Level and Binding Kinetics on Cell Surfaces by Surface Plasmon Resonance Imaging. Anal. Chem. 2015, 87, 9960–9965. [Google Scholar] [CrossRef] [PubMed]
- Diameter of NIH/3T3 Embryonic Fibroblast Cell. Available online: https://bionumbers.hms.harvard.edu/bionumber.aspx?&id=108905&ver=0 (accessed on 5 May 2025).
- Gehler, S.; Ponik, S.M.; Riching, K.M.; Keely, P.J. Bi-Directional Signaling: Extracellular Matrix and Integrin Regulation of Breast Tumor Progression. Crit. Rev. Trade Eukaryot. Gene Expr. 2013, 23, 139–157. [Google Scholar] [CrossRef]
- Janson, I.A.; Putnam, A.J. Extracellular Matrix Elasticity and Topography: Material-Based Cues That Affect Cell Function via Conserved Mechanisms. J. Biomed. Mater. Res. A 2015, 103, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-Based Drug Screen: Considerations and Practical Approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Denys, H.; Derycke, L.; Hendrix, A.; Westbroek, W.; Gheldof, A.; Narine, K.; Pauwels, P.; Gespach, C.; Bracke, M.; De Wever, O. Differential Impact of TGF-β and EGF on Fibroblast Differentiation and Invasion Reciprocally Promotes Colon Cancer Cell Invasion. Cancer Lett. 2008, 266, 263–274. [Google Scholar] [CrossRef]
- Kim, S.A.; Lee, E.K.; Kuh, H.J. Co-Culture of 3D Tumor Spheroids with Fibroblasts as a Model for Epithelial–Mesenchymal Transition in Vitro. Exp. Cell Res. 2015, 335, 187–196. [Google Scholar] [CrossRef]
- Agrawal, A.; Lasli, S.; Javanmardi, Y.; Coursier, D.; Micalet, A.; Watson, S.; Shahreza, S.; Serwinski, B.; Djordjevic, B.; Szita, N.; et al. Stromal Cells Regulate Mechanics of Tumour Spheroid. Mater. Today Bio 2023, 23, 100821. [Google Scholar] [CrossRef]
- Cirisano, F.; Benedetti, A.; Liggieri, L.; Ravera, F.; Santini, E.; Ferrari, M. Amphiphobic Coatings for Antifouling in Marine Environment. Colloids Surf. A Physicochem. Eng. Asp. 2016, 505, 158–164. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.D.C. Regenerable Superhydrophobic Coatings for Biomedical Fabrics. Coatings 2020, 10, 578. [Google Scholar] [CrossRef]
- Liggieri, L.; Passerone, A. An Automatic Technique for Measuring the Surface Tension of Liquid Metals. High. Temp. Technol. 1989, 7, 82–86. [Google Scholar] [CrossRef]
- ISO 25178-2:2021; Geometrical Product Specifications (GPS)—Surface Texture: Area—Part 2: Terms, Definitions and Surface Texture Parameters. International Organisation for Standardization: Geneva, Switzerland, 2021.
- McGahon, A.J.; Martin, S.J.; Bissonnette, R.P.; Mahboubi, A.; Shi, Y.; Mogil, R.J.; Nishioka, W.K.; Green, D.R. The End of the (Cell) Line: Methods for the Study of Apoptosis in Vitro. Methods Cell Biol. 1995, 46, 153–174. [Google Scholar] [CrossRef]





| Surface | 3T3/A431 | Circularity (Mean ± SEM) | Size (Mean ± SEM) | Cell Viability | ||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | 48 h | ||
| SHS1 | 20:80 | 0.896 ± 0.018 | 0.900 ± 0.017 | 184 ± 22 | 153 ± 15 | Green (viable) ●●○ Red (dead) ●●○ |
| 50:50 | 0.923 ± 0.018 | 0.945 ± 0.012 | 407 ± 39 | 354 ± 33 | Green (viable) ●●● Red (dead) ●○○ | |
| 80:20 | 0.939 ± 0.011 | 0.931 ± 0.013 | 302 ± 21 | 422 ± 90 | Green (viable) ●●● Red (dead) ●○○ | |
| SHS2 | 20:80 | 0.878 ± 0.019 | 0.899 ± 0.032 | 168 ± 40 | 230 ± 59 | Green (viable) ●●○ Red (dead) ●●○ |
| 50:50 | 0.841 ± 0.064 | 0.887 ± 0.027 | 498 ± 75 | 500 ± 46 | Green (viable) ●●● Red (dead) ●○○ | |
| 80:20 | 0.749 ± 0.068 | 0.953 ± 0.029 | 614 ± 63 | 520 ± 83 | Green (viable) ●●○ Red (dead) ●●○ | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morán, M.d.C.; Cirisano, F.; Ferrari, M. Superhydrophobicity Effects on Spheroid Formation, Structure, and Viability on Co-Culture Conditions. Pharmaceuticals 2025, 18, 953. https://doi.org/10.3390/ph18070953
Morán MdC, Cirisano F, Ferrari M. Superhydrophobicity Effects on Spheroid Formation, Structure, and Viability on Co-Culture Conditions. Pharmaceuticals. 2025; 18(7):953. https://doi.org/10.3390/ph18070953
Chicago/Turabian StyleMorán, María del Carmen, Francesca Cirisano, and Michele Ferrari. 2025. "Superhydrophobicity Effects on Spheroid Formation, Structure, and Viability on Co-Culture Conditions" Pharmaceuticals 18, no. 7: 953. https://doi.org/10.3390/ph18070953
APA StyleMorán, M. d. C., Cirisano, F., & Ferrari, M. (2025). Superhydrophobicity Effects on Spheroid Formation, Structure, and Viability on Co-Culture Conditions. Pharmaceuticals, 18(7), 953. https://doi.org/10.3390/ph18070953









