Microbiological Contamination of Medicinal Products —Is It a Significant Problem?
Abstract
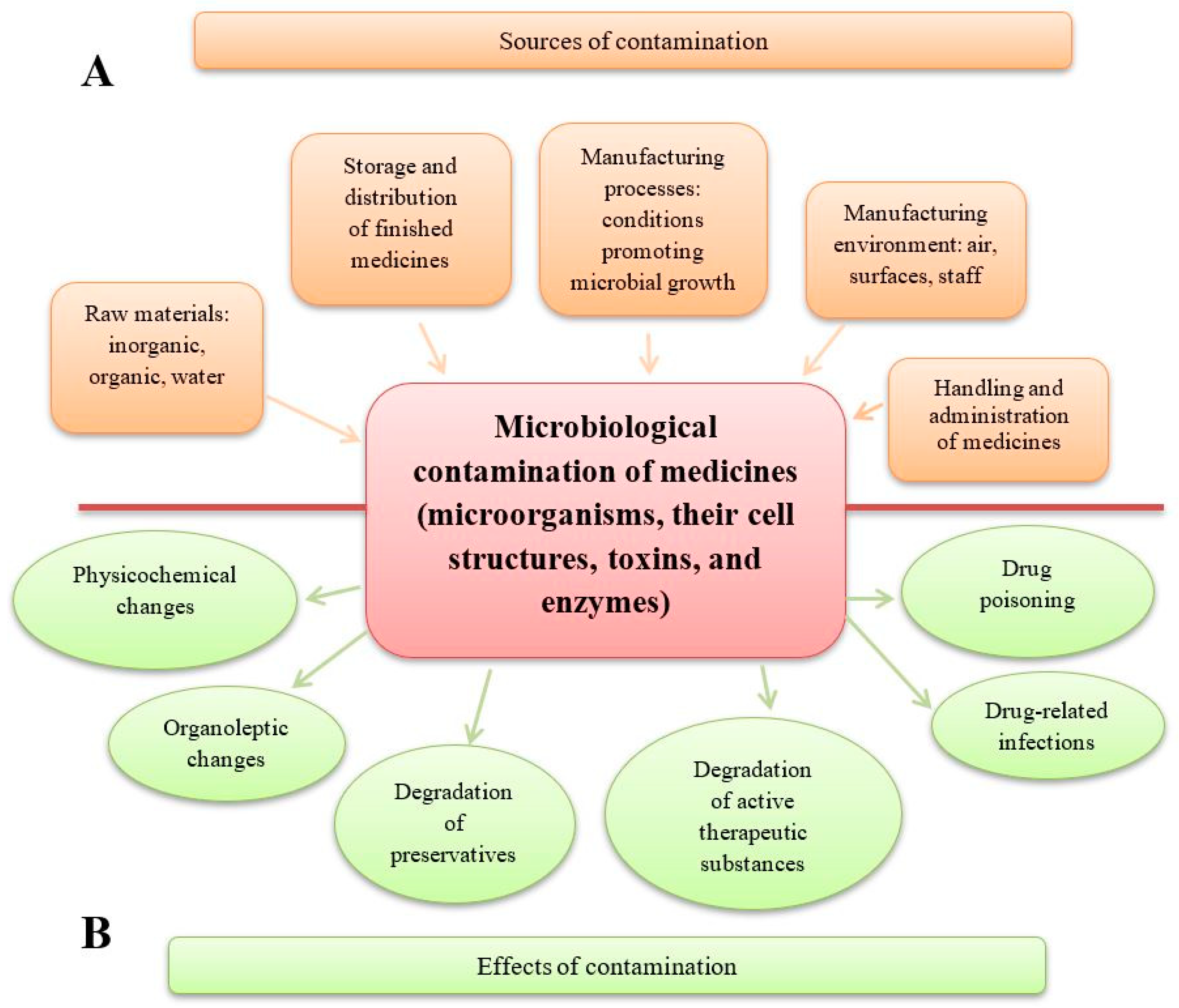
1. Introduction
- * A reduction in drug quality. This includes alterations in the pharmaceutical form (e.g., disintegration of tablets, agglomeration of powders and granules), organoleptic changes (e.g., deterioration in the appearance, smell, and taste of syrups, colour changes or stains on tablets), and breakdown of preservatives, which may enable the growth of pathogenic microorganisms (e.g., Pseudomonas aeruginosa). Additionally, microbial activity can lead to the degradation of active substances, such as the inactivation of penicillins by bacterial β-lactamases, the hydrolysis of aspirin in suspension by bacterial esterases, or the degradation of atropine in eye drops and steroids in ointments due to fungal contamination. The degradation of preservatives and the degradation of active substances are two distinct and harmful processes. A medicinal product may lose its preservatives due to microbial enzymes while retaining its therapeutic properties—for example, in the case of eye drops. Conversely, microorganisms may degrade the active substances in a drug without affecting the preservatives; this can happen in preparations such as antibiotic gels and ointments [1,2,3,4,5,6].
- * A reduction in patient safety. This includes drug-related infections caused by the presence of live pathogenic microorganisms in medicinal products and medical devices used for drug administration. When a contaminated drug is administered, microorganisms can grow, multiply, and release toxic substances in the patient’s body. Drug poisoning, on the other hand, is caused by cellular and extracellular factors—primarily toxins and enzymes—produced by microorganisms that were present in the raw materials and the finished product. In such cases, live microbial cells may no longer be present, so an infection does not occur [3,7,8,9,10,11].
2. Pharmacopoeial Requirements Concerning the Microbial Status of Drugs and Methods of Testing Their Microbiological Quality
3. Contamination of the Manufacturing Environment, Equipment, and Raw Materials
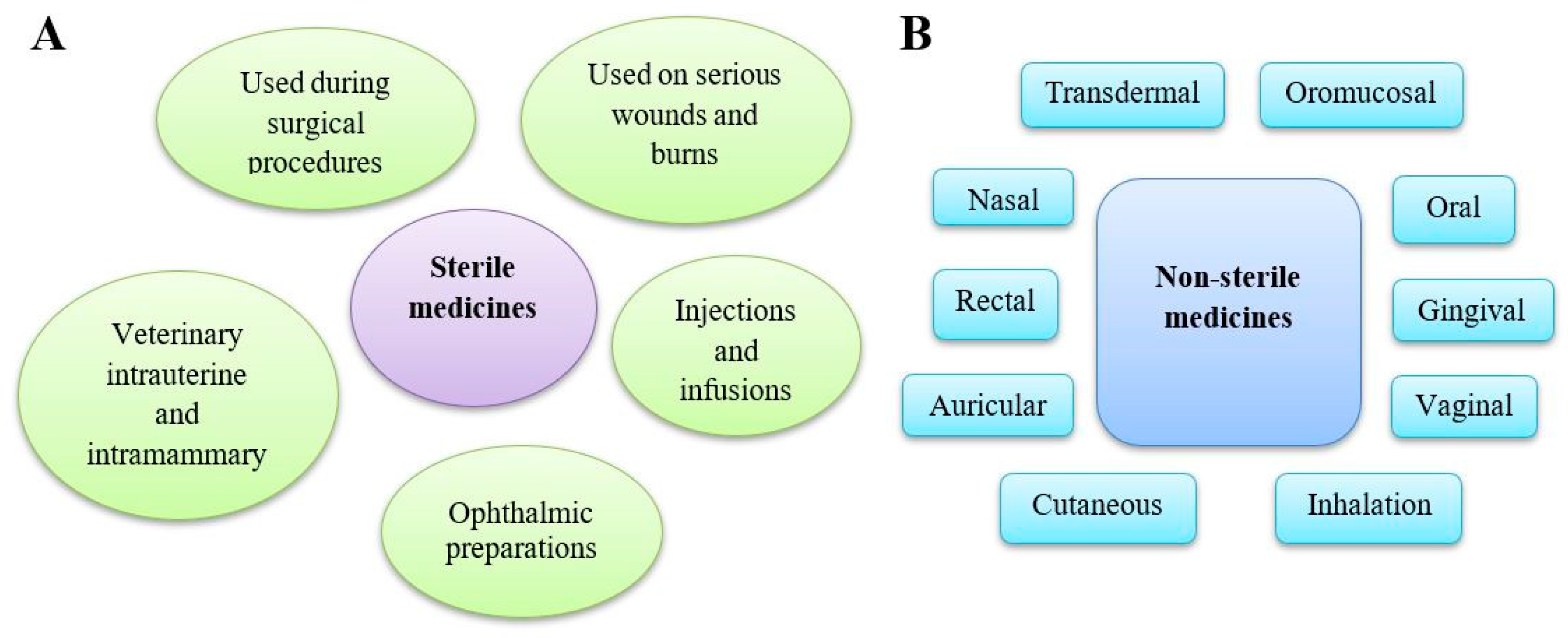
4. Contamination of Sterile Drugs
5. Contamination of Non-Sterile Drugs
6. Natural Pharmaceutical Products
7. Other Aspects of Medicinal Product Contamination
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dao, H.; Lakhani, P.; Police, A.; Kallakunta, V.; Ajjarapu, S.S.; Wu, K.W.; Ponkshe, P.; Repka, M.A.; Narasimha Murthy, S. Microbial stability of pharmaceutical and cosmetic products. AAPS PharmSciTech 2018, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Obuekwe, I.F.; Eichie, F. The presence of microorganisms in some common excipients used in tablet formulation. Acta Pol. Pharm. 2006, 63, 121–125. [Google Scholar] [PubMed]
- Eissa, M.E. Distribution of bacterial contamination in non-sterile pharmaceutical materials and assessment of its risk to the health of the final consumers quantitatively. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 217–230. [Google Scholar] [CrossRef][Green Version]
- Yilmaz, O.F.; Sarmis, A.; Mutlu, M.A.; Büsra Sahin, Z.; Pelin Kaya, S.; Oguz, H. Bacterial contamination of multi-use tear drops, gels, and ointments. Contact Lens Anterior Eye 2023, 46, 102064. [Google Scholar] [CrossRef]
- Hutchinson, J.; Runge, W.; Mulvey, M.; Norris, G.; Yetman, M.; Valkova, N.; Villemur, R.; Lepine, F. Burkholderia cepacia infections associated with intrinsically contaminated ultrasound gel: The role of microbial degradation of parabens. Infect. Control Hosp. Epidemiol. 2004, 25, 291–296. [Google Scholar] [CrossRef]
- Ratajczak, M.; Kubicka, M.M.; Kamińska, D.; Sawicka, P.; Długaszewska, J. Microbiological quality of non-sterile pharmaceutical products. Saudi Pharm. J. 2015, 23, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.E.E.; Abbas, H.S.; Kotakonda, M. Fungal diseases caused by serious contamination of pharmaceuticals and medical devices, and rapid fungal detection using nano-diagnostic tools: A Critical Review. Curr. Microbiol. 2024, 81, 10. [Google Scholar] [CrossRef]
- Vijayakumar, R.; Sandle, T.; Manoharan, C. A review of fungal contamination in pharmaceutical products and phenotypic identification of contaminants by conventional methods. Eur. J. Parent. Pharm. Sci. 2012, 17, 4–19. [Google Scholar]
- Taşli, H.; Coşar, G. Microbial contamination of eye drops. Cent. Eur. J. Public Health 2001, 9, 162–164. [Google Scholar]
- Tresoldi, A.T.; Padoveze, M.C.; Trabasso, P.; Veiga, J.F.; Marba, S.T.; von Nowakonski, A.; Branchini, M.L. Enterobacter cloacae sepsis outbreak in a newborn unit caused by contaminated total parenteral nutrition solution. Am. J. Infect. Control. 2000, 28, 258–261. [Google Scholar] [CrossRef]
- Murtaza, G.; Khan, M.A.; Nisa, Z.U.; Shafiq, S. A review on the microbial contamination in the non-sterile pharmaceutical products. Pharm. Sci. Technol. 2021, 5, 68–75. [Google Scholar] [CrossRef]
- Gunter, J.; Pandey, V. BBC News: Waldemar Haffkine: The Vaccine Pioneer the World Forgot. 2020. Available online: https://www.bbc.com/news/world-asia-india-55050012 (accessed on 24 February 2025).
- Ross, R. The inoculation accident at Mulkowal. Nature 1907, 75, 486–487. [Google Scholar] [CrossRef][Green Version]
- Prince, H.N.; Prince, D.L.; Contamination control. The life and Death of Bacteria and Other Germs. Contract Pharma. Microbiol. 2005, 1–7. Available online: https://www.nelsonlabs.com/wp-content/uploads/2019/08/Contamination-Control-The-life-and-death-of-bacteria-and-other-germs.pdf (accessed on 24 February 2025).
- Centers for Disease Control and Prevention (CDC). Epidemiologic notes and reports. Nosocomial bacteremias associated with intravenous fluid therapy-USA. 1971. MMWR Morb. Mortal. Wkly. Rep. 1997, 46, 1227–1233. Available online: https://pubmed.ncbi.nlm.nih.gov/9427215/ (accessed on 24 February 2025).
- Maki, D.G.; Anderson, R.L.; Shulman, J.A. In-use contamination of intravenous infusion fluid. Appl. Microbiol. 1974, 28, 778–784. [Google Scholar] [CrossRef]
- Mackel, D.C.; Maki, D.G.; Anderson, R.L.; Rhame, F.S.; Bennett, J.V. Nationwide epidemic of septicemia caused by contaminated intravenous products: Mechanisms of intrinsic contamination. J. Clin. Microbiol. 1975, 2, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Maki, D.G.; Rhame, F.S.; Mackel, D.C.; Bennett, J.V. Nationwide epidemic of septicemia caused by contaminated intravenous products. I. Epidemiologic and clinical features. Am. J. Med. 1976, 60, 471–485. [Google Scholar] [CrossRef]
- Abbas, K.M.; Dorratoltaj, N.; O’Dell, M.L.; Bordwine, P.; Kerkering, T.M.; Redican, K.J. Clinical Response, outbreak investigation, and epidemiology of the fungal meningitis epidemic in the United States: Systematic review. Disaster Med. Public Health Prep. 2016, 10, 145–151. [Google Scholar] [CrossRef]
- Ritter, J.M.; Muehlenbachs, A.; Blau, D.M.; Paddock, C.D.; Shieh, W.J.; Drew, C.P.; Batten, B.C.; Bartlett, J.H.; Metcalfe, M.G.; Pham, C.D.; et al. Exserohilum infections associated with contaminated steroid injections: A clinicopathologic review of 40 cases. Am. J. Pathol. 2013, 183, 881–892. [Google Scholar] [CrossRef]
- Renfrow, J.J.; Frenkel, M.B.; Hsu, W. Fungal contamination of methylprednisolone causing recurrent lumbosacral intradural abscess. Emerg. Infect. Dis. 2017, 23, 552–553. [Google Scholar] [CrossRef]
- Kabbara, A.; Rosenberg, S.K.; Untal, C. Methicillin-resistant Staphylococcus aureus epidural abscess after transforaminal epidural steroid injection. Pain Physician 2004, 7, 269–272. [Google Scholar] [CrossRef]
- Motamedifar, M.; Askarian, M. The prevalence of multidose vial contamination by aerobic bacteria in a major teaching hospital, Shiraz, Iran, 2006. Am. J. Infect. Control 2009, 37, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Conde Giner, S.; Bosó Ribelles, V.; Bellés Medall, M.D.; Raga Jiménez, C.; Ferrando Piqueres, R.; Bravo José, P. Catheter-related bloodstream infections in patients receiving central parenteral nutrition: Prevalence, associated factors, and treatment. Nutr. Hosp. 2020, 37, 890–894. [Google Scholar] [CrossRef]
- Miglani, A.; Saini, C.; Musyuni, P.; Aggarwal, G. A review and analysis of product recall for pharmaceutical drug product. J. Generic Med. 2022, 18, 72–81. [Google Scholar] [CrossRef]
- European Medicines Agency Defective Product Report. Available online: https://www.ema.europa.eu/en/search?search_api_fulltext=quality%20defects (accessed on 24 February 2025).
- Jimenez, L. Microbial diversity in pharmaceutical product recalls and environments. PDA J. Pharm. Sci. Technol. 2007, 61, 383–399. [Google Scholar] [PubMed]
- Sutton, S.; Jimenez, L. Review of Reported Recalls Involving Microbiological Control 2004–2011 with Emphasis on FDA Considerations of ‘Objectionable Organisms. Am. Pharm. Rev. 2012, 15, 42–57. [Google Scholar]
- Jimenez, L. Analysis of FDA Enforcement Reports (2012–2019) to Determine the Microbial Diversity in Contaminated Non-Sterile and Sterile Drugs. Am. Pharm. Rev. 2019, 21, 48–73. [Google Scholar]
- Patel, R.; Vhora, A.; Jain, D.; Patel, R.; Khunt, D.; Patel, R.; Dyawanapelly, S.; Junnuthula, V. A retrospective regulatory analysis of FDA recalls carried out by pharmaceutical companies from 2012 to 2023. Drug Discov. Today 2024, 29, 103993. [Google Scholar] [CrossRef]
- Tidswell, E.; Azab, W. The Cost of Microbial Control. PDA Letter. 2017. Available online: https://www.pda.org/pda-letter-portal/home/full-article/the-cost-of-microbial-control (accessed on 24 February 2025).
- European Pharmacopoeia. Microbiological quality of non-sterile pharmaceutical preparations and substances for pharmaceutical use. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.4. [Google Scholar]
- United States Pharmacopeia. Microbiological examination of non-sterile products: Acceptance criteria for pharmaceutical preparations and substances for pharmaceutical use. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 1111. [Google Scholar]
- European Pharmacopoeia. Sterility. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.1. [Google Scholar]
- United States Pharmacopeia. Sterility tests. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 71. [Google Scholar]
- Japanese Pharmacopoeia. Sterility test. In JP XVIII; The Ministry of Health, Labour And Welfare: Tokyo, Japan, 2021; pp. 144–147. [Google Scholar]
- Japanese Pharmacopoeia. Microbial attributes of non-sterile pharmaceutical products. In JP XVIII; The Ministry of Health, Labour And Welfare: Tokyo, Japan, 2021; pp. 2684–2686. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of non-sterile products: Microbial enumeration tests. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.12. [Google Scholar]
- United States Pharmacopeia. Microbiological examination of nonsterile products microbial enumeration tests. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 61. [Google Scholar]
- European Pharmacopoeia. Microbiological quality of herbal medicinal products for oral use and extracts used in their preparation. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.8. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of non-sterile products: Test for specified micro-organisms. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.13. [Google Scholar]
- United States Pharmacopeia. Microbiological examination of nonsterile products tests for specified microorganisms. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 62. [Google Scholar]
- United States Pharmacopeia. Microbiological examination of nonsterile products tests for Burkholderia cepacia complex. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 60. [Google Scholar]
- European Pharmacopoeia. Live Biotherapeutic Products. Ph. Eur. 2023, 11, 3053. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of live biotherapeutic products: Tests for enumeration of microbial contaminants. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.36. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of live biotherapeutic products: Tests for specified micro-organisms. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.38. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of cell-based preparations. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.27. [Google Scholar]
- European Pharmacopoeia. Viral safety. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.7. [Google Scholar]
- European Pharmacopoeia. Minimising the risk of transmitting animal spongiform encephalopathy agents via human and veterinary medicinal products. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.1. [Google Scholar]
- European Pharmacopoeia. Pyrogens. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.8. [Google Scholar]
- United States Pharmacopeia. Pyrogen Test. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 151. [Google Scholar]
- Japanese Pharmacopoeia. Pyrogen Test. In JP XVIII; The Ministry of Health, Labour And Welfare: Tokyo, Japan, 2021; pp. 133–134. [Google Scholar]
- Franco, E.; Garcia-Recio, V.; Jiménez, P.; Garrosa, M.; Girbés, T.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Endotoxins from a Pharmacopoeial Point of View. Toxins 2018, 10, 331. [Google Scholar] [CrossRef]
- Levin, J.; Bang, F.B. A description of cellular coacgulation in the limulus. Bull. Johns. Hopkins Hosp. 1964, 115, 337–345. [Google Scholar]
- Levin, J.; Bang, F.B. The Role of endotoxin in the extracellular coagulation of limulus blood. Bull. Johns. Hopkins Hosp. 1964, 115, 265–274. [Google Scholar] [PubMed]
- Tamura, H.; Reich, J.; Nagaoka, I. Outstanding Contributions of LAL Technology to Pharmaceutical and Medical Science: Review of Methods, Progress, Challenges, and Future Perspectives in Early Detection and Management of Bacterial Infections and Invasive Fungal Diseases. Biomedicines 2021, 11, 536. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia. Bacterial Endotoxins. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.14. [Google Scholar]
- United States Pharmacopeia. Bacterial Endotoxins Test. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 85. [Google Scholar]
- Japanese Pharmacopoeia. Bacterial Endotoxins Test. In JP XVIII; The Ministry of Health, Labour And Welfare: Tokyo, Japan, 2021; pp. 124–127. [Google Scholar]
- European Pharmacopoeia. Monocyte Activation Test. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.30. [Google Scholar]
- European Pharmacopoeia. Test for Bacterial Endotoxins Using Recombinant Factor C. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.32. [Google Scholar]
- United States Pharmacopeia. Use of Recombinant Reagents in the Bacterial Endotoxins Test. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 1085.1. [Google Scholar]
- European Pharmacopoeia. Depyrogenation of Items Used in the Production of Parenteral Preparations. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.12. [Google Scholar]
- Gudeman, J.; Jozwiakowski, M.; Chollet, J.; Randell, M. Potential risks of pharmacy compounding. Drugs RD 2013, 13, 1–8. [Google Scholar] [CrossRef]
- United States Pharmacopeia. Pharmaceutical Compounding—Sterile Preparations. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 797. [Google Scholar]
- European Pharmacopoeia. Microbiological examination of human tissues. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.39. [Google Scholar]
- Laudy, A.E.; Tyski, S. Infections connected with organ and tissue transplantation. Adv. Microbiol. 2024, 63, 65–80. [Google Scholar] [CrossRef]
- European Commission. Good Manufacturing Practice (GMP) Guidelines, EudraLex—Volume 4, Brussels. Available online: https://health.ec.europa.eu/medicinal-products/eudralex/eudralex-volume-4_en (accessed on 24 February 2025).
- The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use. Annex 1 Manufacture of Sterile Medicinal Products. Brussels. EudraLex—Volume 4—Good Manufacturing Practice (GMP). Available online: https://health.ec.europa.eu/document/download/e05af55b-38e9-42bf-8495-194bbf0b9262_en?filename=20220825_gmp-an1_en_0.pdf (accessed on 24 February 2025).
- Ashour, M.; Mansy, M.; Eissa, M. Microbiological environmental monitoring in pharmaceutical facility. Egypt. Acad. J. Biolog. Sci. G Microbiol. 2011, 3, 63–74. [Google Scholar] [CrossRef]
- Cundell, T. Mold Monitoring and control in pharmaceutical manufacturing areas. Am. Pharm. Rev. 2016, 19. Available online: https://www.americanpharmaceuticalreview.com/Featured-Articles/190686-Mold-Monitoring-and-Control-in-Pharmaceutical-Manufacturing-Areas/ (accessed on 24 February 2025).
- United States Pharmacopeia. Microbiological control and monitoring of aseptic processing environments. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 1116. [Google Scholar]
- Rubenfire, A. Baxter to Pay $18.2 Million in Settlement Over Mold at Manufacturing Facility. Modern Healthcare. 2017. Available online: https://www.modernhealthcare.com/article/20170112/NEWS/170119945/baxter-to-pay-18-2-million-in-settlement-over-mold-at-manufacturing-facility (accessed on 24 February 2025).
- Larmené-Beld, K.H.M.; Frijlink, H.W.; Taxis, K. A systematic review and meta-analysis of microbial contamination of parenteral medication prepared in a clinical versus pharmacy environment. Eur. J. Clin. Pharmacol. 2019, 75, 609–617. [Google Scholar] [CrossRef]
- Sandle, T. A review of cleanroom microflora: Types, trends, and patterns. PDA J. Pharm. Sci. Technol. 2011, 65, 392–403. [Google Scholar] [CrossRef]
- Wilson, S.C.; Palmatier, R.N.; Andriychuk, L.A.; Martin, J.M.; Jumper, C.A.; Holder, H.W.; Straus, D.C. Mold contamination and air handling units. J. Occup. Environ. Hyg. 2007, 4, 483–491. [Google Scholar] [CrossRef]
- Röder, F.; Sandle, T. Microbial contamination in water systems. PDA J. Pharm. Sci. Technol. 2022, 76, 434–443. [Google Scholar] [CrossRef]
- Guidance on Quality of Water for Pharmaceutical Use. EMA/CHMP/CVMP/QWP/496873/2018. 2020. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-quality-water-pharmaceutical-use_en.pdf (accessed on 24 February 2025).
- European Pharmacopoeia. Water purified. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 0008. [Google Scholar]
- European Pharmacopoeia. Water for injections. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 0169. [Google Scholar]
- European Pharmacopoeia. Water for preparation of extracts. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2249. [Google Scholar]
- Khaleque, A.; Sarkar, C.K.; Islam, A.; Kobid, A.; Nabi, A.H.M.; Islam, N.; Al Reza, S.; Uddin, A. Microbiological analysis of water used for pharmaceutical product preparation. IOSR J. Pharm. Biol. Sci. 2020, 15, 7–10. [Google Scholar]
- Cundell, A.M. Review of the media selection and incubation conditions for the compendial sterility and microbial limit tests. In Pharmacopeial Forum; USP: North Bethesda, MD, USA, 2002; Volume 28, pp. 2034–2041. [Google Scholar]
- Eissa, M.; Mahmoud, A. Evaluation of microbial recovery from raw materials for pharmaceutical use. J. Food Pharm. Sci. 2016, 4, 6–11. [Google Scholar]
- Robinson, L.B.; Wichelhausen, R.H.; Roizman, B. Contamination of human cell cultures by pleuropneumonialike organisms. Science 1956, 124, 1147–1148. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Mycoplasmas. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.6.7. [Google Scholar]
- Armstrong, S.E.; Mariano, J.A.; Lundin, D.J. The scope of mycoplasma contamination within the biopharmaceutical industry. Biologicals 2010, 38, 211–213. [Google Scholar] [CrossRef]
- Drexler, H.G.; Uphoff, C.C. Mycoplasma contamination of cell cultures: Incidence, sources, effects, detection, elimination, prevention. Cytotechnology 2002, 39, 75–90. [Google Scholar] [CrossRef]
- Schneier, M.; Razdan, S.; Miller, A.M.; Briceno, M.E.; Barua, S. Current technologies to endotoxin detection and removal for biopharmaceutical purification. Biotechnol. Bioeng. 2020, 117, 2588–2609. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, G.; Kan, S.; Hoshikawa, K.; Sato, K.; Fujita, Y.; Inada, K.; Inoue, Y. Endotoxin contamination of single-use sterile surgical gloves. Future Microbiol. 2020, 15, 1425–1430. [Google Scholar] [CrossRef]
- Bühlmann, X. Microbiological control in the manufacture of sterile pharmaceutical products. Pharm. Acta Helv. 1971, 46, 385–409. [Google Scholar]
- Jokl, D.H.; Wormser, G.P.; Nichols, N.S.; Montecalvo, M.A.; Karmen, C.L. Bacterial contamination of ophthalmic solutions used in an extended care facility. Br. J. Ophthalmol. 2007, 91, 1308–1310. [Google Scholar] [CrossRef]
- Tsegaw, A.; Abula, T.; Assefa, Y. Bacterial contamination of multi-dose eye drops at ophthalmology department, University of Gondar, Northwest Ethiopia. Middle East. Afr. J. Ophthalmol. 2017, 24, 81–86. [Google Scholar]
- Kim, M.S.; Choi, C.Y.; Kim, J.M.; Chang, H.R.; Woo, H.Y. Microbial contamination of multiply used preservative-free artificial tears packed in reclosable containers. Br. J. Ophthalmol. 2008, 92, 1518–1521. [Google Scholar] [CrossRef] [PubMed]
- Fazeli, M.R.; Nejad, H.B.; Mehrgan, H.; Elahian, L. Microbial contamination of preserved ophthalmic drops in outpatient departments: Possibility of an extended period of use. DARU 2004, 12, 151–155. [Google Scholar]
- Engemann, J.; Kaye, K.; Cox, G.; Perfect, J.; Schell, W.; McGarry, S.A.; Patterson, K.; Edupuganti, S.; Cook, P.; Rutala, W.A.; et al. Exophiala infection from contaminated injectable steroids prepared by a compounding pharmacy—United States, July-November 2002. Morb. Mortal. Wkly. Rep. 2002, 51, 1109–1112. [Google Scholar] [CrossRef]
- Oli, A.N.; Ibeabuchi, M.U.; Enweani, I.B.; Emencheta, S.C. Pharmaceutical quality of selected metronidazole and ciprofloxacin infusions marketed in south eastern Nigeria. Drug Healthc. Patient Saf. 2020, 12, 103–112. [Google Scholar] [CrossRef]
- Shah, H.; Honeybul, S.; Tang, S.; Arthur, I.; McLaren, S.; Boan, P. Mould meningitis associated with intravenous drug use. Med. Mycol. Case Rep. 2018, 20, 18–20. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; Hanly, A.M.; Francis, E.; Keane, N.; McNamara, D.A. Catheter associated blood stream infections in patients receiving parenteral nutrition: A prospective study of 850 patients. J. Clin. Med. Res. 2013, 5, 18–21. [Google Scholar] [CrossRef]
- Marra, A.R.; Opilla, M.; Edmond, M.B.; Kirby, D.F. Epidemiology of blood-stream infections in patients receiving long-term total parenteral nutrition. J. Clin. Gastroenterol. 2007, 41, 19–28. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Methods of Preparation of Sterile Products. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.1. [Google Scholar]
- Daehn, T.; Schneider, A.; Knobloch, J.; Hellwinkel, O.J.C.; Spitzer, M.S.; Kromer, R. Contamination of multi dose eyedrops in the intra and perioperative context. Sci. Rep. 2021, 11, 20364. [Google Scholar] [CrossRef]
- Chua, S.W.; Mustapha, M.; Wong, K.K.; Ami, M.; Mohd Zahidin, A.Z.; Nasaruddin, R.A. Microbial contamination of extended use ophthalmic drops in ophthalmology clinic. Clin. Ophthalmol. 2021, 15, 3147–3152. [Google Scholar] [CrossRef]
- Somner, J.E.; Cavanagh, D.J.; Wong, K.K.; Whitelaw, M.; Thomson, T.; Mansfield, D. The precautionary principle: What is the risk of reusing disposable drops in routine ophthalmology consultations and what are the costs of reducing this risk to zero? Eye 2010, 24, 361–363. [Google Scholar] [CrossRef]
- Yilmaz, O.F.; Sarmis, A.; Mutlu, M.A.; Ersoy, E.E.; Askarova, U.; Oguz, H. Bacterial contamination of multi-use antibiotic steroid eye ointments and drops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2023, 261, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kang, M.J.; Sim, H.E.; Hwang, J.H. Microbial contamination of preservative-free artificial tears based on instillationtechniques. Pathogens 2022, 11, 592. [Google Scholar] [CrossRef]
- Da Costa, A.X.; Yu, M.C.Z.; de Freitas, D.; Cristovam, P.C.; LaMonica, L.C.; Dos Santos, V.R.; Gomes, J.A.P. Microbial cross-contamination in multidose eyedrops: The impact of instillation angle and bottle geometry. Transl. Vis. Sci. Technol. 2020, 9, 7. [Google Scholar] [CrossRef]
- Costa, C.M.; Espósito, K.P.; Florêncio, V.M.B.; Aquino, P.R.L.; Firmo, E.F.; Costa, I.M.A.; Rocha, C.; Andrade, C.G.; Oliveira-Júnior, J.B.; Ventura, C.V. Evaluation of microbial contamination in multi-dose fluorescein eyedrops in a reference eye center. Arq. Bras. Oftalmol. 2021, 84, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Kyei, S.; France, D.; Asiedu, K. Microbial contamination of multiple-use bottles of fluorescein ophthalmic solution. Clin. Exp. Optom. 2019, 102, 30–34. [Google Scholar] [CrossRef]
- Watson, J.T.; Jones, R.C.; Siston, A.M.; Fernandez, J.R.; Martin, K.; Beck, E.; Sokalski, S.; Jensen, B.J.; Arduino, M.J.; Srinivasan, A.; et al. Outbreak of catheter-associated Klebsiella oxytoca and Enterobacter cloacae bloodstream infections in an oncology chemotherapy center. Arch. Intern. Med. 2005, 165, 2639–2643. [Google Scholar] [CrossRef] [PubMed]
- Blossom, D.; Noble-Wang, J.; Su, J.; Pur, S.R.N.; Chemaly, R.; Shams, A.; Jensen, B.; Pascoe, N.; Gullion, J.; Casey, E.; et al. Multistate outbreak of Serratia marcescens bloodstream infections caused by contamination of prefilled heparin and isotonic sodium chloride solution syringes. Arch. Intern. Med. 2009, 169, 1705–1711. [Google Scholar] [CrossRef]
- Su, J.R.; Blossom, D.B.; Chung, W.; Smartt Gullion, J.; Pascoe, N.; Heseltine, G.; Srinivasan, A. Epidemiologic investigation of a 2007 outbreak of Serratia marcescens bloodstream infection in Texas caused by contamination of syringes prefilled with heparin and saline. Infect. Control Hosp. Epidemiol. 2009, 30, 593–595. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Multistate outbreak of fungal infection associated with injection of methylprednisolone acetate solution from a single compounding pharmacy—United States, 2012. Morb. Mortal. Wkly. Rep. 2012, 61, 839–842. [Google Scholar]
- Casadevall, A.; Pirofski, L. Exserohilum rostratum fungal meningitis associated with methylprednisolone injections. Future Microbiol. 2013, 8, 135–137. [Google Scholar] [CrossRef]
- Chiller, T.M.; Roy, M.; Nguyen, D.; Guh, A.; Malani, A.N.; Latham, R.; Peglow, S.; Kerkering, T.; Kaufman, D.; McFadden, J.; et al. Multistate fungal infection clinical investigation team. clinical findings for fungal infections caused by methylprednisolone injections. N. Engl. J. Med. 2013, 369, 1610–1619. [Google Scholar] [CrossRef] [PubMed]
- Opilla, M. Epidemiology of bloodstream infection associated with parenteral nutrition. Am. J. Infect. Control 2008, 36, 173.e5–173.e8. [Google Scholar] [CrossRef] [PubMed]
- Beghetto, M.G.; Victorino, J.; Teixeira, L.; de Azevedo, M.J. Parenteral nutrition as a risk factor for central venous catheter-related infection. J. Parent. Ent. Nutr. 2005, 29, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Fennrich, S.; Hennig, U.; Toliashvili, L.; Schlensak, C.; Wendel, H.P.; Stoppelkamp, S. More than 70 Years of Pyrogen Detection: Current State and Future Perspectives. Altern. Lab. Anim. 2016, 44, 239–253. [Google Scholar] [CrossRef]
- Janů, M. New approaches to the issue of pyrogens in parenteral preparations. Ceska Slov. Farm. 2025, 73, 169–174. [Google Scholar] [CrossRef]
- Zhang, C.; Tian, F.; Zhang, M.; Zhang, Z.; Bai, M.; Guo, G.; Zheng, W.; Wang, Q.; Shi, Y.; Wang, L. Endotoxin contamination, a potentially important inflammation factor in water and wastewater: A Review. Sci. Total Environ. 2019, 681, 365–378. [Google Scholar] [CrossRef]
- Kawakami, H.; Fuchino, H.; Kawahara, N. Endotoxin Contamination and Reaction Interfering Substances in the Plant Extract Library. Biol. Pharm. Bull. 2020, 43, 1767–1775. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Endotoxin-like reactions associated with intravenous gentamicin—California, 1998. Morb. Mortal. Wkly. Rep. 1998, 47, 877–880. [Google Scholar]
- Krieger, J.A.; Duncan, L. Gentamicin Contaminated with Endotoxin. N. Engl. J. Med. 1999, 340, 1122. [Google Scholar] [CrossRef]
- Tidswell, E.C. A Nontrivial Analysis of Patient Safety Risk from Parenteral Drug- and Medical Device-Borne Endotoxin. Drugs RD 2023, 23, 65–76. [Google Scholar] [CrossRef]
- Yang, K.H.; Lee, M.G. Effects of endotoxin derived from Escherichia coli lipopolysaccharide on the pharmacokinetics of drugs. Arch. Pharm. Res. 2008, 31, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Notice to readers: Manufacturer’s recall of nasal spray contaminated with Burkholderia cepacia complex. MMWR 2004, 53, 246. [Google Scholar]
- Glowicz, J.; Crist, M.; Gould, C.; Moulton-Meissner, H.; Noble-Wang, J.; de Man, T.J.B.; Perry, K.A.; Miller, Z.; Yang, W.C.; Langille, S.; et al. B. cepacia investigation workgroup. A multistate investigation of health care-associated Burkholderia cepacia complex infections related to liquid docusate sodium contamination, January–October 2016. Am. J. Infect. Control 2018, 46, 649–655. [Google Scholar] [CrossRef]
- Charnock, C. The microbial content of non-sterile pharmaceuticals distributed in Norway. J. Hosp. Infect. 2004, 57, 233–240. [Google Scholar] [CrossRef]
- Myemba, D.T.; Bwire, G.M.; Sangeda, R.Z. Microbiological quality of selected local and imported non-sterile pharmaceutical products in Dar es Salaam, Tanzania. Infect. Drug Resist. 2022, 15, 2021–2034. [Google Scholar] [CrossRef]
- Ekeleme, U.G.; Ikwuagwu, V.O.; Chukwuocha, U.M.; Nwakanma, J.C.; Adiruo, S.A.; Ogini, I.O.; Ude, I.U. Detection and characterization of micro-organisms linked to unsealed drugs sold in Ihiagwa community, Owerri, Imo State, Nigeria. Access Microbiol. 2024, 6, 000752.v3. [Google Scholar] [CrossRef] [PubMed]
- Mugoyela, V.; Mwambete, K.D. Microbial contamination of nonsterile pharmaceuticals in public hospital settings. Ther. Clin. Risk Manag. 2010, 6, 443–448. [Google Scholar] [CrossRef] [PubMed]
- United States Pharmacopeia. Application of water activity determination to nonsterile pharmaceutical products. In USP 46—NF 41; United States Pharmacopeia: Rockville, MD, USA, 2023; Chapter 1112. [Google Scholar]
- European Pharmacopoeia. Water-solid interactions; determination of sorption-desorption isotherms and of water activity. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 2.9.39. [Google Scholar]
- Ali, M.E. Burkholderia cepacia in pharmaceutical industries. Int. J. Vaccines Vaccin. 2016, 3, 00064. [Google Scholar] [CrossRef]
- Kumar, S.P.; Uthra, K.T.; Chitra, V.; Damodharan, N.; Pazhani, G.P. Challenges and mitigation strategies associated with Burkholderia cepacia complex contamination in pharmaceutical manufacturing. Arch. Microbiol. 2024, 206, 159. [Google Scholar] [CrossRef]
- Tavares, M.; Kozak, M.; Balola, A.; Sá-Correia, I. Burkholderia cepacia complex bacteria: A feared contamination risk in water-based pharmaceutical products. Clin. Microbiol. Rev. 2020, 33, 10. [Google Scholar] [CrossRef]
- Contaminated Povidone-Iodine Solution–Texas. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001358.htm (accessed on 24 February 2025).
- Song, J.E.; Kwak, Y.G.; Um, T.H.; Cho, C.R.; Kim, S.; Park, I.S.; Hwang, J.H.; Kim, N.; Oh, G.-B. Outbreak of Burkholderia cepacia pseudobacteraemia caused by intrinsically contaminated commercial 0.5% chlorhexidine solution in neonatal intensive care units. J. Hosp. Infect. 2018, 98, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Weber DJRutala, W.A.; Sickbert-Bennett, E.E. Outbreaks associated with contaminated antiseptics and disinfectants. Antimicrob. Agents Chemother. 2007, 51, 4217–4224. [Google Scholar] [CrossRef]
- Zou, Q.; Li, N.; Liu, J.; Li, X.; Wang, Z.; Ai, X.; Tao, F.; Qu, M.; Cai, M.; Hu, Y. Investigation of an outbreak of Burkholderia cepacia infection caused by drug contamination in a tertiary hospital in China. Am. J. Infect. Control 2020, 48, 199–203. [Google Scholar] [CrossRef]
- Nannini, E.C.; Ponessa, A.; Muratori, R.; Marchiaro, P.; Ballerini, V.; Flynn, L.; Limansky, A.S. Polyclonal outbreak of bacteremia caused by Burkholderia cepacia complex and the presumptive role of ultrasound gel. Braz. J. Infect. Dis. 2015, 19, 543–545. [Google Scholar] [CrossRef]
- Solaimalai, D.; Devanga Ragupathi, N.K.; Ranjini, K.; Paul, H.; Verghese, V.P.; Michael, J.S.; Veeraraghavan, B.; James, E.J. Ultrasound gel as a source of hospital outbreaks: Indian experience and literature review. Indian J. Med. Microbiol. 2019, 37, 263–267. [Google Scholar] [CrossRef] [PubMed]
- FDA Updates on Multistate Outbreak of Burkholderia cepacia Infections. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-updates-multistate-outbreak-burkholderia-cepacia-infections (accessed on 24 February 2025).
- Torbeck, L.; Raccasi, D.; Guilfoyle, D.E.; Friedman, R.L.; Hussong, D. Burkholderia cepacia: This decision is overdue. PDA J. Pharm. Sci. Tech. 2011, 65, 535–543. [Google Scholar] [CrossRef] [PubMed]
- FDA Advises Drug Manufacturers that Burkholderia cepacia Complex Poses a Contamination Risk in Non-Sterile, Water-Based Drug Products. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-advises-drug-manufacturers-burkholderia-cepacia-complex-poses-contamination-risk-non-sterile?source=govdelivery&utm_medium=email&utm_source=govdelivery (accessed on 24 February 2025).
- Duong, H.; Minogue, E.; Fullbrook, S.; Barry, T.; Reddington, K. A culture-independent nucleic acid diagnostics method for use in the detection and quantification of Burkholderia cepacia complex contamination in aqueous finished pharmaceutical products. PLoS ONE 2024, 16, 19. [Google Scholar] [CrossRef]
- Sandle, T. Fungal contamination of pharmaceutical products: The growing menace. Eur. Pharm. Rev. 2014, 19, 68–71. [Google Scholar]
- Cheng, V.C.C.; Chan, J.F.W.; Ngan, A.H.Y.; To, K.K.W.; Leung, S.Y.; Tsoi, H.W.; Yam, W.C.; Tai, J.W.M.; Wong, S.S.Y.; Tse, H.; et al. Outbreak of intestinal infection due to Rhizopus microsporus. J. Clin. Microbiol. 2009, 47, 2834–2843. [Google Scholar] [CrossRef]
- Opoku, S.; Nyanor, I. Qualitative and quantitative microbiological studies of paediatric artemether-lumefantrine dry powders and paracetamol syrups obtained from selected drug stores in Accra, Ghana. J. Trop. Med. 2019, 2019, 7062016. [Google Scholar] [CrossRef]
- Khana, M.; Teotia, U.V.S.; Singh, Y. Effect of primary packaging on microbiological status of oral solid dosage form. J. Appl. Pharm. Res. 2018, 6, 1–6. [Google Scholar]
- Abba, D.; Inabo, H.I.; Yakubu, S.E.; Olonitola, O.S. Contamination of herbal medicinal products marketed in Kaduna metropolis with selected pathogenic bacteria. Afr. J. Tradit. Complement. Altern. Med. 2008, 25, 70–77. [Google Scholar] [CrossRef]
- Igbeneghu, O.A.; Lamikanra, A. Assessment of the microbial quality of some oral liquid herbal medicines marketed in Ile-Ife, South-Western Nigeria. AJMR 2016, 10, 1618–1624. [Google Scholar] [CrossRef]
- Ahiabor, W.K.; Darkwah, S.; Donkor, E.S. Microbial contamination of herbal medicines in Africa 2000–2024: A systematic review. Environ. Health Insights 2024, 30, 18. [Google Scholar] [CrossRef]
- Alharbi, S.F.; Althbah, A.I.; Mohammed, A.H.; Alrasheed, M.A.; Ismail, M.; Allemailem, K.S.; Alnuqaydan, A.M.; Baabdullah, A.M.; Alkhalifah, A. Microbial and heavy metal contamination in herbal medicine: A prospective study in the central region of Saudi Arabia. BMC Complement. Med. Ther. 2024, 24, 2. [Google Scholar] [CrossRef] [PubMed]
- De Sousa Lima, C.M.; Fujishima, M.A.T.; de Paula Lima, B.; Mastroianni, P.C.; de Sousa, F.F.O.; da Silva, J.O. Microbial contamination in herbal medicines: A serious health hazard to elderly consumers. BMC Complement. Med. Ther. 2020, 20, 17. [Google Scholar] [CrossRef]
- Wang, G.; Jiao, M.; Hu, J.; Xun, Y.; Chen, L.; Qiu, J.; Ji, F.; Lee, Y.-W.; Shi, J.; Xu, J. Quantitative analysis of fungal contamination of different herbal medicines in China. Toxins 2024, 16, 229. [Google Scholar] [CrossRef] [PubMed]
- Kneifel, W.; Czech, E.; Kopp, B. Microbial contamination of medicinal plants—A review. Planta Med. 2002, 68, 5–15. [Google Scholar] [CrossRef]
- Yu, J.; Yang, M.; Han, J.; Pang, X. Fungal and mycotoxin occurrence, affecting factors, and prevention in herbal medicines: A review. Toxin Rev. 2021, 41, 976–994. [Google Scholar] [CrossRef]
- Qin, X.; Guo, S. Mould and mycotoxin contamination of medicinal materials. Zhongguo Zhong Yao Za Zhi 2011, 6, 3397–3401. [Google Scholar]
- Chen, L.; Guo, W.; Zheng, Y.; Zhou, J.; Liu, T.; Chen, W.; Liang, D.; Zhao, M.; Zhu, Y.; Wu, Q.; et al. Occurrence and characterization of fungi and mycotoxins in contaminated medicinal herbs. Toxins 2020, 12, 30. [Google Scholar] [CrossRef]
- Govender, S.; Plessis-Stoman, D.; Downing, T.G.; Venter, M. Traditional herbal medicines: Microbial contamination, consumer safety and the need for standards. South Afr. J. Sci. 2006, 102, 253–255. [Google Scholar]
- Walusansa, A.; Asiimwe, S.; Kafeero, H.M.; Stanley, I.J.; Ssenku, J.E.; Nakavuma, J.L.; Kakudidi, E.K. Prevalence and dynamics of clinically significant bacterial contaminants in herbal medicines sold in East Africa from 2000 to 2020: A systematic review and meta-analysis. Trop. Med. Health 2021, 49, 10. [Google Scholar] [CrossRef]
- Jameel Mahdi Alsammarraie, H. Evaluation of the pharmaceutical and microbial safety, as well as the reliability, of some natural pharmaceutical products available in Iraq. Arch. Razi Inst. 2022, 77, 1465–1471. [Google Scholar] [CrossRef] [PubMed]
- Al Akeel, M.M.; Al Ghamdi, W.M.; Al Habib, S.A.; Koshm, M.; Al Otaibi, F. Herbal medicines: Saudi population knowledge, attitude, and practice at a glance. J. Fam. Med. Prim. Care 2018, 7, 865–875. [Google Scholar] [CrossRef]
- European Pharmacopoeia. Alternative methods for control of microbiological quality. In Ph. Eur., 11th ed.; European Directorate for the Quality of Medicines and HealthCare (EDQM) Council of Europe: Strasbourg, France, 2023; Chapter 5.1.6. [Google Scholar]
- De Brito, N.M.R.; Lourenço, F.R. Rapid identification of microbial contaminants in pharmaceutical products using a PCA/LDA-based FTIR-ATR method. Braz. J. Pharm. Sci. 2021, 57, e18899. [Google Scholar] [CrossRef]
- Wu, S.M.; Chen, J.; Ai, X.X.; Yan, Z.Y. Detection of Escherichia coli in drugs with antibody conjugated quantum dots as immunofluorescence probes. J. Pharm. Biomed. Anal. 2013, 78–79, 9–13. [Google Scholar] [CrossRef]
- Pei, L.; Hu, C.Q.; Ma, S.H.; Dai, H.; Hang, T.J. Correlation of bacteria in the contaminated drug and the environmental microbes in the clean room for pharmaceutical microbial test investigated by FTIR. Yao Xue Xue Bao 2007, 42, 1189–1194. [Google Scholar]
- Civen, R.; Vugia, D.J.; Alexander, R.; Brunner, W.; Taylor, S.; Parris, N.; Wasserman, R.; Abbott, S.; Werner, S.B.; Rosenberg, J. Outbreak of Serratia marcescens infections following injection of betamethasone compounded at a community pharmacy. Clin. Infect. Dis. 2006, 43, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Hocevar, S.N.; Moulton-Meissner, H.A.; Stevens, K.M.; McIntyre, M.G.; Jensen, B.; Kuhar, D.T.; Noble-Wang, J.A.; Schnatz, R.G.; Becker, S.C.; et al. Outbreak of Serratia marcescens bloodstream infections in patients receiving parenteral nutrition prepared by a compounding pharmacy. Clin. Infect. Dis. 2014, 59, 1–8. [Google Scholar] [CrossRef]
- Held, M.R.; Begier, E.M.; Beardsley, D.S.; Browne, F.A.; Martinello, R.A.; Baltimore, R.S.; McDonald, L.C.; Jensen, B.; Hadler, J.L.; Dembry, L.M. Life-threatening sepsis caused by Burkholderia cepacia from contaminated intravenous flush solutions prepared by a compounding pharmacy in another state. Pediatrics 2006, 118, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Gershman, M.D.; Kennedy, D.J.; Noble-Wang, J.; Kim, C.; Gullion, J.; Kacica, M.; Jensen, B.; Pascoe, N.; Saiman, L.; McHale, J.; et al. Pseudomonas fluorescens investigation team: Multistate outbreak of Pseudomonas fluorescens bloodstream infection after exposure to contaminated heparinized saline flush prepared by a compounding pharmacy. Clin. Infect. Dis. 2008, 47, 1372–1379. [Google Scholar] [CrossRef] [PubMed]
- Pullirsch, D.; Bellemare, J.; Hackl, A.; Trottier, Y.L.; Mayrhofer, A.; Schindl, H.; Taillon, C.; Gartner, C.; Hottowy, B.; Beck, G.; et al. Microbiological contamination in counterfeit and unapproved drugs. BMC Pharmacol. Toxicol. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.; Chauhan, S.; Dare, M.; Bansal, A.K. Degradation of parabens by Pseudomonas beteli and Burkholderia latens. Eur. J. Pharm. Biopharm. 2010, 75, 206–212. [Google Scholar] [CrossRef]
- Pfizer Inc. Issues a Voluntary Nationwide Recall for 2 Lots of RELPAX® (Eletriptan Hydrobromide) 40 mg Tablets Due to Potential Microbiological Contamination of Non-Sterile Products. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pfizer-inc-issues-voluntary-nationwide-recall-2-lots-relpaxr-eletriptan-hydrobromide-40-mg-tablets (accessed on 24 February 2025).
- AvKARE, LLC. Issues Voluntary Nationwide Recall of Atovaquone Oral Suspension, USP 750 mg/5 mL Due to Potential Bacillus cereus Contamination. Available online: https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/avkare-llc-issues-voluntary-nationwide-recall-atovaquone-oral-suspension-usp-750-mg5-ml-due (accessed on 24 February 2025).
| Years of FDA Analysis | Number of Microbiologically Related Recalls | Reason for Recalls | References |
|---|---|---|---|
| 1998–2006 | Total = 327; 134—non-sterile products; 193—sterile products. | Main contaminated microorganisms: Burkholderia cepacia (35 cases), Pseudomonas spp. (20 cases), Salmonella spp. (6 cases), Ralstonia pickettii (3 cases); 78% lack of sterility assurance. | [27] |
| 2004–2011 | Total = 642; 22% non-sterile; 78% sterile. | 103 cases of objectionable organisms, including: B. cepacia (35 cases), Pseudomonas spp. (15 cases), yeast/mould (23 cases); 79% of sterile recalls—lack of sterility assurance. | [28] |
| 2012–2019 | 713 non-sterile preparations; 1056—lack of sterility assurance. | Main contaminated microorganisms: B. cepacia (102 cases), R. picketti (45 cases), Salmonella spp. (28 cases), Clostridioides difficile (13 cases). | [29] |
| 2012–2023 | Total recalls = 15,710. | Sterility recalls (5766 cases), including lack of sterility assurance (2785 cases) and non-sterility (2621 cases); contamination (1712), including microbial contamination (686 cases). Main contaminated microorganisms: B. cepacia Bacillus spp., Klebsiella spp., Candida albicans, Aspergillus spp. | [30] |
| Raw Materials | Bacteria | Fungi | Endotoxins | References |
|---|---|---|---|---|
| Substances for pharmaceutical use | TAMC 103 CFU/g or CFU/mL | TYMC 102 CFU/g or CFU/mL | nt | [32] |
| Water purified | TAMC 102 CFU/mL | nt | nt | [79] |
| Water for injections in bulk | TAMC 10 CFU/mL | nt | <0.25 IU/mL | [80] |
| Water for injections sterile | TAMC 0 CFU/mL | TYMC 0 CFU/mL | <0.25 IU/mL | [80] |
| Water for preparation of extracts | TAMC 102 CFU/mL | nt | nt | [81] |
| Medicine Types | Other Information | Isolated Microorganisms | References |
|---|---|---|---|
| Ophtalmic preparations | A total of 271 multi-use tear containers used by 168 patients were tested. Microbial contamination was detected in 33 (12.2%) of all containers, including ointments (32.0%), gels (11.7%), and drops (7.9%). Notably, collapsible tubes without preservatives had a significantly higher contamination rate (32%). | Contamination mostly by opportunistic bacterial and fungal strains, including Pseudomonas stutzeri, P. aeruginosa, Bacillus licheniformis, Paenibacillus pabuli, Proteus mirabilis, P. agglomerans, Morganella morganii, Serratia marcescens, and Serratia liquefaciens. | [4] |
| A total of 92 eye drop bottles were examined: 43 bottles were opened and used for two weeks, and 49 bottles were unopened and sealed. The contamination rate was 34.8% in opened bottles and 10.2% in unopened bottles. | Six samples from opened bottles contain coagulase-negative staphylococci, and nine samples yielded one or two different microorganisms. Among unopened eye drop bottles, two samples contained S. aureus, two coagulase-negative staphylococci, and one Bacillus spp. | [9] | |
| Out of 123 multi-dose eye solutions, 10 were contaminated. | P. mirabilis was detected in 8 of 10 contaminated solutions. | [92] | |
| Out of 100 in-use eye drop vials, 11 were contaminated. | Staphylococci (7 strains), Bacillus spp. (2 strains), and single strains of E. coli and Enterobacter spp. | [93] | |
| In total, 242 eye drop vials were analysed, and bacterial contamination was detected in 5 vials. | 3.9% coagulase-negative staphylococci and 1.0% Acinetobacter spp. | [94] | |
| A total of 200 eye drop bottles were tested after 1, 2, 4, and 7 days of use, and the contamination rates were as follows: 34%, 52%, 56%, and 58%. | Dominant contamination: Staphylococcus epidermidis (in 29.5% of tested containers), Bacillus spp. (16%), Micrococcus spp. (13.5%), S. aureus (8.5%), Penicillium spp. (15%), and Aspergillus flavus (8%). | [95] | |
| Injection and infusion preparations | Two cases of meningitis infection due to contaminated injectable steroid (methylprednisolone) prepared by a compounding pharmacy. | Exophiala spp. | [96] |
| In total, 8 metronidazole and 8 ciprofloxacin infusions preparations were tested; 2 of the metronidazole and 1 of the ciprofloxacin preparations were contaminated. | Microorganism identification was not performed. | [97] | |
| Fungal meningitis was caused by contaminated injections. | Pithomyces chartarum | [98] | |
| Parenteral nutrition preparations | Eleven neonates were infected after parenteral nutrition | E. cloacae | [11] |
| Multi-dose vials of medicinal salts, including potassium chloride, sodium chloride, and sodium bicarbonate, were examined. Bacterial contamination was identified in 36 of 637 (5.6%) vials. | Contamination by aerobic normal commensal microbiota. In Gram-positive bacteria (88.9%), S. epidermidis was the most common contaminant (44.4%). Gram-negative bacteria (11.1%). | [23] | |
| Catheter-related bloodstream infections in 850 patients who underwent central venous catheterisation for total parenteral nutrition. In total, 11.2% of patients in intensive care units and 12.1% of patients in non-intensive care units were infected. | Microorganism identification was not performed | [99] | |
| Bloodstream infections occurred in 37 of 47 long-term parenteral nutrition patients, and 23.8% of infection episodes were polymicrobial. | The most prevalent pathogen was coagulase-negative staphylococci (33.5%). Moreover, the following strains were also isolated: Corynebacterium spp., S. aureus, Streptococcus spp., Leuconostoc spp., Lactobacillus spp., Bacillus spp., Propionibacterium spp., E. coli, Proteus spp., Acinetobacter baumannii, Serratia spp., P. aeruginosa, E. cloacae, Agrobacterium radiobacter, P. agglomerans, Citrobacter freundii, Acinetobacter lwoffi, Bacteriodes fragilis, Fusobacterium nucleatum, Ewingella americana, Kluyvera ascorbata, Candida tropicalis, Candida lusitaniae, Candida krusei, Rhodotorula rubra, Malassezia furfur, and Aureobasidium spp. | [100] |
| Medicine Types | Other Information | Isolated Microorganisms | References |
|---|---|---|---|
| Nasal spray | CDC reported a manufacturer’s recall of over-the-counter oxymetazoline HCl 0.05% nasal spray due to bacterial contamination. | Burkholderia cepacia complex | [126] |
| Molecular analysis confirmed a close genetic relationship between bacterial isolates from the manufacturer’s purified water, the liquid docusate sodium product, and patient clinical samples. | B. cepacia complex | [127] | |
| Oral and topical preparations | Most of the 77 tested products met quantitative microbiological requirements of Ph. Eur.; 29 samples contained objectionable bacterial strains. | B. circulans (8 isolates) and single isolates of Micrococcus luteus, Enterococcus faecium, P. agglomerans, R. pickettii, S. maltophilia, and Bordetella bronchiseptica. | [128] |
| All investigated products (mainly from India) were contaminated with microorganisms, with most exceeding the maximum acceptable counts. Syrups and suspensions were more contaminated than tablets and capsules. | P. aeruginosa, S. epidermidis, and Klebsiella pneumoniae were the most frequently isolated pathogens. | [129] | |
| Tablets | Sealed containers of paracetamol, chloroquine, and metronidazole tablets were opened by personnel and distributed to people. These opened containers were stored at room temperature, and microbial contamination was detected in 42 of 50 (84%) samples tested. | S. aureus, E. coli, P. aeruginosa, C. albicans, and A. niger. The most common isolates were S. aureus (51.8% of bacterial isolates) and C. albicans (73.3% of fungal isolates). | [130] |
| Tablets, capsules, ointments, and syrups | In total, 1285 non-sterile pharmaceutical products manufactured by various pharmaceutical plants, before they were marketed, were tested, and 1.87% of the tested drugs were non-compliant with Ph. Eur. because of excessive microbial counts and the presence of pathogens excluded by Ph. Eur. | Bacillus spp., Microccocus spp., Enterococcus spp., Aspergillus spp., Rhizopus spp., Alternaria spp., and Mucor spp. were isolated. | [6] |
| Among 10 non-sterile drugs, 5 contained between 10 and more than 1000 CFU/mL of pathogenic bacteria. | Bacillus spp. and Klebsiella spp., Candida spp. and Aspergillus spp. | [131] | |
| Mouthwashes, syrups, skin creams, and other products. | Fungal contamination by moulds and yeasts was detected. | Aspergillus spp., Fusarium spp., Rhizopus spp., Penicillium spp., and Candida spp. were recorded most frequently. | [8] |
| Medicine Types | Other Information | Isolated Microorganisms | References |
|---|---|---|---|
| Herbal remedies | Widespread microbial contamination of 150 samples. The TAMC exceeded 5 × 107 CFU/g in 59.33% of the samples, while 28% of the preparations had microbial counts below this threshold. | S. aureus in 65.33% samples, E. coli in 58.67% samples, Salmonella typhi in 46.67% samples, and Shigella spp. in 19.33% samples. | [151] |
| Herbal oral liquid preparations | The mean bacterial load of 50 analysed herbal medicine samples ranged from 0.0 CFU/mL to 2.9 × 1012 CFU/mL, while the mean fungal load ranged from 0.0 CFU/mL to 3.5 × 1012 CFU/mL. A total of 52% samples contained one bacterial contaminant each, 26% of the samples had two, while 20% had three contaminants. Four contaminants were recovered from one sample. | A total of 85 bacteria were recovered from 49 of the 50 samples, including 36 Gram-positive and 49 Gram-negative bacteria. Most often, it was Bacillus spp. (40%) and Klebsiella spp. (31.8%). Other contaminants: E. coli, Staphylococcus spp., Salmonella spp., and P. aeruginosa. | [152] |
| Herbal medicines | Data from 50 publications (2000–2024) on microbial contamination of herbal medicines in different African regions. | The most frequently isolated bacteria: E. coli (62%), S. aureus (60%), Bacillus spp. (54%), and Pseudomonas spp. (46%). The most frequently isolated fungi: Aspergillus spp. (40%), Penicillium spp. (28%), and Candida spp. (24%). | [153] |
| From 47 products, including 18 creams, 15 liquids, and 14 powders, 58 bacterial strains were isolated, and all but 3 samples were contaminated with at least one microorganism. Most Gram-positive bacterial isolates were found to be multidrug-resistant. | Most commonly, Klebsiella spp., Pseudomonas spp., and E. coli were isolated. | [154] | |
| In total, 132 oral and topical products were tested. Bacterial and fungal contamination was detected in 51.5% and 35.6% of samples, respectively. A total of 31.8% of the herbal medicine samples exceeded the safety limits (≤105 CFU/g), with 16.7% of homemade and 15.1% of commercial herbal medicines surpassing this threshold. Moreover, the tested water samples contained coliforms, rendering the water unfit for consumption. | The most commonly isolated were S. aureus (49.2%), Salmonella spp. (34.8%), E. coli (25.8%), and P. aeruginosa (14.4%). | [155] | |
| Commonly used herbal medicines | A total of 173 fungal strains were isolated from 138 samples. Mycotoxin analysis revealed that Fusarium spp. primarily produced acetylated forms of deoxynivalenol, while Alternaria spp. mainly produced altertoxins. | The most frequently isolated were Fusarium spp. (28%) and Alternaria spp. (21%). | [156] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyski, S.; Burza, M.; Laudy, A.E. Microbiological Contamination of Medicinal Products —Is It a Significant Problem? Pharmaceuticals 2025, 18, 946. https://doi.org/10.3390/ph18070946
Tyski S, Burza M, Laudy AE. Microbiological Contamination of Medicinal Products —Is It a Significant Problem? Pharmaceuticals. 2025; 18(7):946. https://doi.org/10.3390/ph18070946
Chicago/Turabian StyleTyski, Stefan, Magdalena Burza, and Agnieszka Ewa Laudy. 2025. "Microbiological Contamination of Medicinal Products —Is It a Significant Problem?" Pharmaceuticals 18, no. 7: 946. https://doi.org/10.3390/ph18070946
APA StyleTyski, S., Burza, M., & Laudy, A. E. (2025). Microbiological Contamination of Medicinal Products —Is It a Significant Problem? Pharmaceuticals, 18(7), 946. https://doi.org/10.3390/ph18070946






