Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation
Abstract
1. Introduction
2. Classification of Neurosteroids
3. Clinically Approved and Investigational Derivatives of P4 and DHEA in Neuroprotection
4. DHEA as a Neuroprotector
| Nerve Growth Factor | PREG | DHEA | P4 | ALLO |
|---|---|---|---|---|
| BDNF | normalizes the BDNF level in dopamine-depleted striatum [71] | increases the expression of BDNF in rats [72] | markedly mitigates the increased level of mature BDNF [73] | mitigates the decreases in truncated BDNF [74] |
| GDNF | no specific data on the direct effect on GDNF are available | no specific data on the direct effect on GDNF are available | the intracellular content of GDNF is not affected by progesterone treatment [75] | promotes neurogenesis and neuroprotection and modulates neurotrophic pathways indirectly through its influence on neuronal and glial cell activity [24] |
| CNTF | no specific data on the direct effect on GDNF are available | DHEA’s ability to enhance neural stem cell proliferation and neurogenesis may create cellular environments where CNTF is upregulated, as CNTF supports neuronal survival and differentiation [76,77] | P4 inhibits CNTF expression in cultured C6 astroglioma cells. Progesterone treatment also reduces CNTF expression in the amygdala and decreases immobility time in female CNTF+/+ but not in CNTF−/− mice [78] | no specific data on the direct effect on CNTF are available |
5. Progesterone as a Neuroprotector
6. Pregnenolone as a Neuroprotector
7. Allopregnanolone as a Neuroprotector
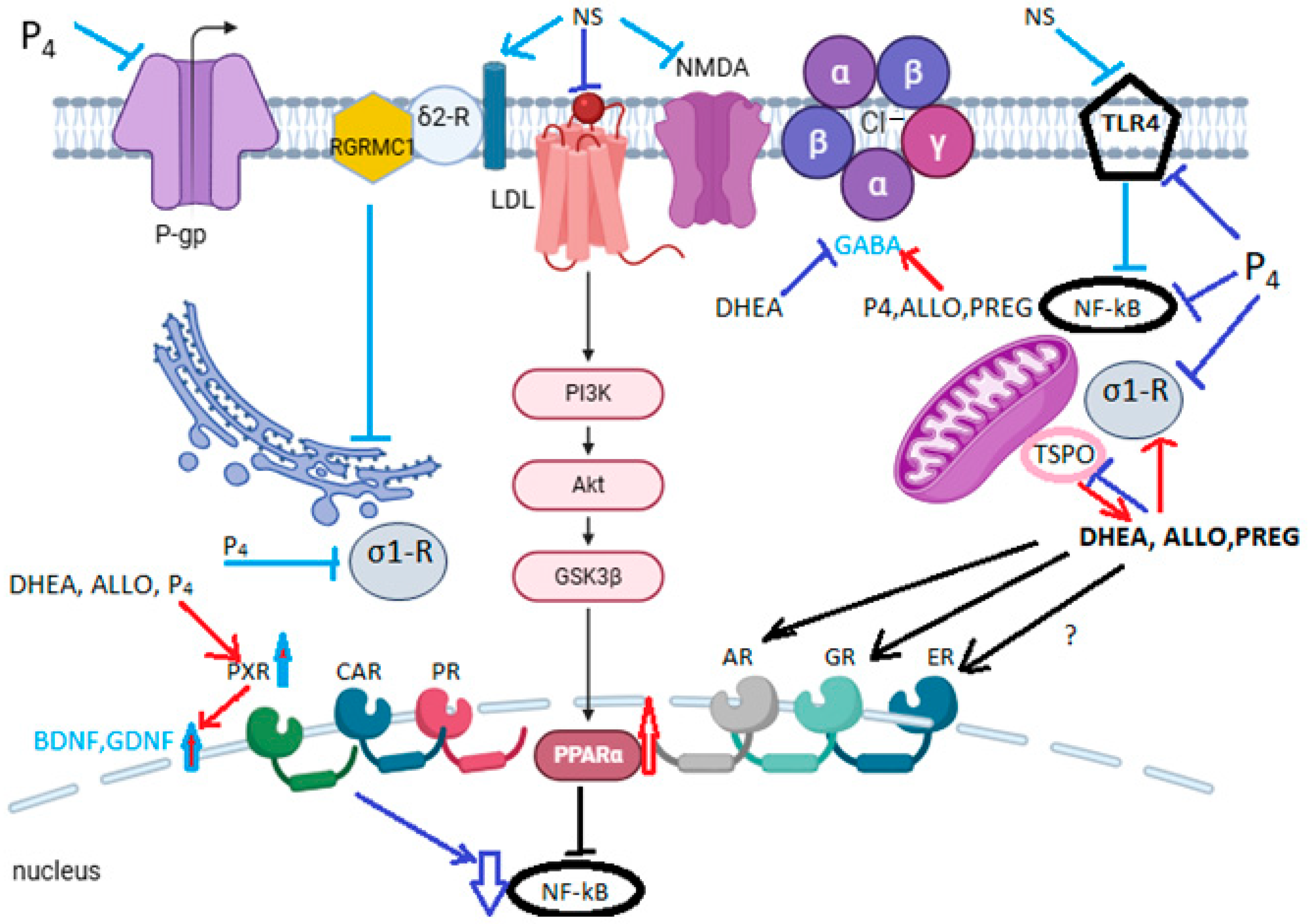
8. New Molecular Mechanisms of the Neuroprotective Action of Neurosteroids
8.1. The Mechanism of Anti-Inflammatory Action
8.2. Sigma-1 Receptor (σ1-R) and Sigma-2 Receptor (σ2-R) Signaling
8.2.1. Sigma-1 Receptor (σ1-R)
8.2.2. Sigma-2 Receptor (σ2-R) or TMEM97
8.3. PXR-Mediated Cytoprotection and BDNF Synthesis
8.4. TSPO-Mediated Neuroprotection
8.5. mPTP-Mediated Neuroprotection
9. Limitations and Prospects of DHEA and P4 Usage in Neuroprotection
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Buccilli, B.; Alan, A.; Aljeradat, B.G.; Shahzad, A.; Almealawy, Y.F.; Chisvo, N.S.; Ennabe, M.; Weinand, M. Neuroprotection: Surgical Approaches in Traumatic Brain Injury. Surg. Neurol. Int. 2024, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Yin, Y.; Wang, L.; Li, L.; Lan, C.; Shi, J.; Jiang, Z.; Ge, H.; Li, X.; et al. Clot removAl with or without decompRessive Craniectomy under ICP Monitoring for Supratentorial IntraCerebral Hemorrhage (CARICH): A Randomized Controlled Trial. Int. J. Surg. 2024, 110, 4804–4809. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, M.; Ibrahim, A.; Hamide, A.; Tran, T.; Candreva, E.; Baltaji, J. Exercise-Induced Neuroplasticity: Adaptive Mechanisms and Preventive Potential in Neurodegenerative Disorders. Physiologia 2025, 5, 13. [Google Scholar] [CrossRef]
- Fedotchev, A.I.; Zemlyanaya, A.A. Brain State-Dependent Non-Invasive Neurostimulation with EEG Feedback: Achievements and Prospects. Sovrem. Tehnol. Med. 2023, 15, 33. [Google Scholar] [CrossRef]
- Lan, Z.; Tan, F.; He, J.; Liu, J.; Lu, M.; Hu, Z.; Zhuo, Y.; Liu, J.; Tang, X.; Jiang, Z.; et al. Curcumin-Primed Olfactory Mucosa-Derived Mesenchymal Stem Cells Mitigate Cerebral Ischemia/Reperfusion Injury-Induced Neuronal PANoptosis by Modulating Microglial Polarization. Phytomedicine 2024, 129, 155635. [Google Scholar] [CrossRef]
- Hui, Z.; Lai-Fa, W.; Xue-Qin, W.; Ling, D.; Bin-Sheng, H.; Li, J.-M. Mechanisms and Therapeutic Potential of Chinonin in Nervous System Diseases. J. Asian Nat. Prod. Res. 2024, 26, 1405–1420. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Zhu, B.; Yang, Y.; Cai, C.; Wang, X.; Deng, L.; He, B.; Cui, Y.; Zhou, W. A Comparative Study of the Neuroprotective Effects of Dl-3-n-Butylphthalide and Edaravone Dexborneol on Cerebral Ischemic Stroke Rats. Eur. J. Pharmacol. 2023, 951, 175801. [Google Scholar] [CrossRef]
- Jhan, K.-Y.; Chen, K.-Y.; Chang, P.-K.; Chiu, C.-H.; Chou, C.-J.; Wang, L.-C. 7,8-Dihydroxyflavone Provides Neuroprotection and Rescues Behavioral Deficits in Angiostrongylus cantonensis-Infected Mice by Ameliorating Synaptic Loss. J. Microbiol. Immunol. Infect. 2025. [Google Scholar] [CrossRef]
- Cutler, A.J.; Mattingly, G.W.; Maletic, V. Understanding the Mechanism of Action and Clinical Effects of Neuroactive Steroids and GABAergic Compounds in Major Depressive Disorder. Transl. Psychiatry 2023, 13, 228. [Google Scholar] [CrossRef]
- Kolatorova, L.; Vitku, J.; Suchopar, J.; Hill, M.; Parizek, A. Progesterone: A Steroid with Wide Range of Effects in Physiology as Well as Human Medicine. Int. J. Mol. Sci. 2022, 23, 7989. [Google Scholar] [CrossRef]
- Skolnick, B.E.; Maas, A.I.; Narayan, R.K.; Van Der Hoop, R.G.; MacAllister, T.; Ward, J.D.; Nelson, N.R.; Stocchetti, N. A Clinical Trial of Progesterone for Severe Traumatic Brain Injury. N. Engl. J. Med. 2014, 371, 2467–2476. [Google Scholar] [CrossRef] [PubMed]
- Borowicz, K.K.; Piskorska, B.; Banach, M.; Czuczwar, S.J. Neuroprotective Actions of Neurosteroids. Front. Endocrin. 2011, 2, 50. [Google Scholar] [CrossRef] [PubMed]
- Guennoun, R. Progesterone in the Brain: Hormone, Neurosteroid and Neuroprotectant. Int. J. Mol. Sci. 2020, 21, 5271. [Google Scholar] [CrossRef]
- González, S.; Ferreyra, S. Neurosteroids and Neuropathic Pain: An up-to-Date Perspective. Curr. Opin. Endocr. Metab. Res. 2022, 22, 100314. [Google Scholar] [CrossRef]
- Angeloni, E.; Germelli, L.; Costa, B.; Martini, C.; Da Pozzo, E. Neurosteroids and Translocator Protein (TSPO) in Neuroinflammation. Neurochem. Int. 2025, 182, 105916. [Google Scholar] [CrossRef]
- Mallah, K.; Couch, C.; Borucki, D.M.; Toutonji, A.; Alshareef, M.; Tomlinson, S. Anti-Inflammatory and Neuroprotective Agents in Clinical Trials for CNS Disease and Injury: Where Do We Go from Here? Front. Immunol. 2020, 11, 2021. [Google Scholar] [CrossRef]
- Yilmaz, C.; Karali, K.; Fodelianaki, G.; Gravanis, A.; Chavakis, T.; Charalampopoulos, I.; Alexaki, V.I. Neurosteroids as Regulators of Neuroinflammation. Front. Neuroendocrinol. 2019, 55, 100788. [Google Scholar] [CrossRef]
- Guennoun, R.; Labombarda, F.; Gonzalez Deniselle, M.C.; Liere, P.; De Nicola, A.F.; Schumacher, M. Progesterone and Allopregnanolone in the Central Nervous System: Response to Injury and Implication for Neuroprotection. J. Steroid Biochem. Mol. Biol. 2015, 146, 48–61. [Google Scholar] [CrossRef]
- Sohan, M.S.R.; Hossain, M.F.; Hossain, S.; Omori, Y.; Amin, M.T.; Hasan, M.E.; Tokumoto, T. Biochemical Characterization of Zebrafish Paqr5b. Biochem. Biophys. Rep. 2025, 42, 101994. [Google Scholar] [CrossRef]
- Mathouchanh, M.; Lessman, C.A. Effects of Progestogen Neurosteroids on Locomotor Activity in Zebrafish Embryos and Larvae. Fish. Physiol. Biochem. 2025, 51, 105. [Google Scholar] [CrossRef]
- Parakala, M.L.; Zhang, Y.; Modgil, A.; Chadchankar, J.; Vien, T.N.; Ackley, M.A.; Doherty, J.J.; Davies, P.A.; Moss, S.J. Metabotropic, but Not Allosteric, Effects of Neurosteroids on GABAergic Inhibition Depend on the Phosphorylation of GABAA Receptors. J. Biol. Chem. 2019, 294, 12220–12230. [Google Scholar] [CrossRef] [PubMed]
- Raciti, L.; Formica, C.; Raciti, G.; Quartarone, A.; Calabrò, R.S. Gender and Neurosteroids: Implications for Brain Function, Neuroplasticity and Rehabilitation. Int. J. Mol. Sci. 2023, 24, 4758. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2010; Volume 186, pp. 113–137. ISBN 978-0-444-53630-3. [Google Scholar]
- Reddy, D.S.; Estes, W.A. Clinical Potential of Neurosteroids for CNS Disorders. Trends Pharmacol. Sci. 2016, 37, 543–561. [Google Scholar] [CrossRef] [PubMed]
- Tuem, K.B.; Atey, T.M. Neuroactive Steroids: Receptor Interactions and Responses. Front. Neurol. 2017, 8, 442. [Google Scholar] [CrossRef]
- Tateiwa, H.; Evers, A.S. Neurosteroids and Their Potential as a Safer Class of General Anesthetics. J. Anesth. 2024, 38, 261–274. [Google Scholar] [CrossRef]
- Rahmani, B.; Ghasemi, R.; Dargahi, L.; Ahmadiani, A.; Haeri, A. Neurosteroids; Potential Underpinning Roles in Maintaining Homeostasis. Gen. Comp. Endocrinol. 2016, 225, 242–250. [Google Scholar] [CrossRef]
- Mueller, J.W.; Gilligan, L.C.; Idkowiak, J.; Arlt, W.; Foster, P.A. The Regulation of Steroid Action by Sulfation and Desulfation. Endocr. Rev. 2015, 36, 526–563. [Google Scholar] [CrossRef]
- Maguire, J.L.; Mennerick, S. Neurosteroids: Mechanistic Considerations and Clinical Prospects. Neuropsychopharmacology 2024, 49, 73–82. [Google Scholar] [CrossRef]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid Hormone Metabolites Are Barbiturate-like Modulators of the GABA Receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef]
- Sripada, R.K.; Welsh, R.C.; Marx, C.E.; Liberzon, I. The Neurosteroids Allopregnanolone and Dehydroepiandrosterone Modulate Resting-State Amygdala Connectivity: Neurosteroids Modulate Amygdala Resting fcMRI. Hum. Brain Mapp. 2014, 35, 3249–3261. [Google Scholar] [CrossRef]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and Neuropsychiatric Effects of Dehydroepiandrosterone (DHEA) and DHEA Sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.-E.; Robel, P. Dehydroepiandrosterone (DHEA) and Dehydroepiandrosterone Sulfate (DHEAS) as Neuroactive Neurosteroids. Proc. Natl. Acad. Sci. USA 1998, 95, 4089–4091. [Google Scholar] [CrossRef] [PubMed]
- Reddy, D.S. Neurosteroids as Novel Anticonvulsants for Refractory Status Epilepticus and Medical Countermeasures for Nerve Agents: A 15-Year Journey to Bring Ganaxolone from Bench to Clinic. J. Pharmacol. Exp. Ther. 2024, 388, 273–300. [Google Scholar] [CrossRef]
- Xu, J.; Zhou, Y.; Yan, C.; Wang, X.; Lou, J.; Luo, Y.; Gao, S.; Wang, J.; Wu, L.; Gao, X.; et al. Neurosteroids: A Novel Promise for the Treatment of Stroke and Post-stroke Complications. J. Neurochem. 2022, 160, 113–127. [Google Scholar] [CrossRef]
- Doron, R.; Rozevich, L.; Bregman-Yemini, N.; Yadid, G. The Influence of Chronic or Acute DHEA Exposure on β-Endorphin Levels in the Nucleus Accumbens. Eur. J. Pharmacol. 2025, 996, 177446. [Google Scholar] [CrossRef]
- Richardson, E.; Patterson, R.; Meltzer-Brody, S.; McClure, R.; Tow, A. Transformative Therapies for Depression: Postpartum Depression, Major Depressive Disorder, and Treatment-Resistant Depression. Annu. Rev. Med. 2025, 76, 81–93. [Google Scholar] [CrossRef]
- Mayer, E.; Winkler, I.; Huber, E.; Urbanek, M.; Kiechl-Kohlendorfer, U.; Griesmaier, E.; Posod, A. Effects of DHEA and DHEAS in Neonatal Hypoxic–Ischemic Brain Injury. Antioxidants 2024, 13, 1542. [Google Scholar] [CrossRef]
- Stárka, L.; Hill, M.; Kolatorova, L.; Dušková, M. Androst-5-Ene-3β,7α/β,17β-Triols, Their Plasma Levels and Dependence on the Hypothalamic–Pituitary–Adrenal Axis. Steroids 2018, 134, 88–95. [Google Scholar] [CrossRef]
- Haroon, J.; Jordan, K.; Mahdavi, K.; Rindner, E.; Becerra, S.; Surya, J.R.; Zielinski, M.; Venkatraman, V.; Goodenowe, D.; Hofmeister, K.; et al. A Phase 2, Open-Label Study of Anti-Inflammatory NE3107 in Patients with Dementias. Medicine 2024, 103, e39027. [Google Scholar] [CrossRef]
- Georgelou, K.; Saridaki, E.-A.; Karali, K.; Papagiannaki, A.; Charalampopoulos, I.; Gravanis, A.; Tzeranis, D.S. Microneurotrophin BNN27 Reduces Astrogliosis and Increases Density of Neurons and Implanted Neural Stem Cell-Derived Cells after Spinal Cord Injury. Biomedicines 2023, 11, 1170. [Google Scholar] [CrossRef]
- Malik, A.S.; Narayan, R.K.; Wendling, W.W.; Cole, R.W.; Pashko, L.L.; Schwartz, A.G.; Strauss, K.I. A Novel Dehydroepiandrosterone Analog Improves Functional Recovery in a Rat Traumatic Brain Injury Model. J. Neurotrauma 2003, 20, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Sato, R.; Da Fonseca, G.W.P.; Das Neves, W.; Von Haehling, S. Mechanisms and Pharmacotherapy of Cancer Cachexia-associated Anorexia. Pharmacol. Res. Perspect. 2025, 13, e70031. [Google Scholar] [CrossRef] [PubMed]
- Ulchenko, D.; Miloykovich, L.; Zemlyanaya, O.; Shimanovsky, N.; Fedotcheva, T. Possible Participation of Adenine Nucleotide Translocase ANT1 in the Cytotoxic Action of Progestins, Glucocorticoids, and Diclofenac on Tumor Cells. Pharmaceutics 2023, 15, 2787. [Google Scholar] [CrossRef] [PubMed]
- Carl, P.; Høgskilde, S.; Lang–Jensen, T.; Bach, V.; Jagobsen, J.; Sørensen, M.B.; Grälls, M.; Widlund, L. Pharmacokinetics and Pharmacodynamics of Eltanolone (Pregnanolone), a New Steroid Intravenous Anaesthetic, in Humans. Acta Anaesthesiol. Scand. 1994, 38, 734–741. [Google Scholar] [CrossRef]
- Naylor, J.C.; Kilts, J.D.; Shampine, L.J.; Parke, G.J.; Wagner, H.R.; Szabo, S.T.; Smith, K.D.; Allen, T.B.; Telford-Marx, E.G.; Dunn, C.E.; et al. Effect of Pregnenolone vs Placebo on Self-Reported Chronic Low Back Pain Among US Military Veterans: A Randomized Clinical Trial. JAMA Netw. Open 2020, 3, e200287. [Google Scholar] [CrossRef]
- Meltzer-Brody, S.; Colquhoun, H.; Riesenberg, R.; Epperson, C.N.; Deligiannidis, K.M.; Rubinow, D.R.; Li, H.; Sankoh, A.J.; Clemson, C.; Schacterle, A.; et al. Brexanolone Injection in Post-Partum Depression: Two Multicentre, Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trials. Lancet 2018, 392, 1058–1070. [Google Scholar] [CrossRef]
- Wang, J.Y.; Trivedi, A.M.; Carrillo, N.R.; Yang, J.; Schneider, A.; Giulivi, C.; Adams, P.; Tassone, F.; Kim, K.; Rivera, S.M.; et al. Open-Label Allopregnanolone Treatment of Men with Fragile X-Associated Tremor/Ataxia Syndrome. Neurotherapeutics 2017, 14, 1073–1083. [Google Scholar] [CrossRef]
- De, S.K. Ganaxolone: First FDA-Approved Medicine for the Treatment ofSeizures Associated with Cyclin-Dependent Kinase-like 5 DeficiencyDisorder. Curr. Med. Chem. 2024, 31, 388–392. [Google Scholar] [CrossRef]
- Bixo, M.; Ekberg, K.; Poromaa, I.S.; Hirschberg, A.L.; Jonasson, A.F.; Andréen, L.; Timby, E.; Wulff, M.; Ehrenborg, A.; Bäckström, T. Treatment of Premenstrual Dysphoric Disorder with the GABA A Receptor Modulating Steroid Antagonist Sepranolone (UC1010)—A Randomized Controlled Trial. Psychoneuroendocrinology 2017, 80, 46–55. [Google Scholar] [CrossRef]
- Parikh, S.V.; Aaronson, S.T.; Mathew, S.J.; Alva, G.; DeBattista, C.; Kanes, S.; Lasser, R.; Bullock, A.; Kotecha, M.; Jung, J.; et al. Efficacy and Safety of Zuranolone Co-Initiated with an Antidepressant in Adults with Major Depressive Disorder: Results from the Phase 3 CORAL Study. Neuropsychopharmacology 2024, 49, 467–475. [Google Scholar] [CrossRef]
- Cutler, A.J.; Mattingly, G.W.; Kornstein, S.G.; Aaronson, S.T.; Lasser, R.; Zhang, H.; Rana, N.; Brown, C.; Levin, S.; Miller, C.; et al. Long-Term Safety and Efficacy of Initial and Repeat Treatment Courses with Zuranolone in Adult Patients with Major Depressive Disorder: Interim Results From the Open-Label, Phase 3 SHORELINE Study. J. Clin. Psychiatry 2023, 85, 23m14845. [Google Scholar] [CrossRef] [PubMed]
- Marecki, R.; Kałuska, J.; Kolanek, A.; Hakało, D.; Waszkiewicz, N. Zuranolone–Synthetic Neurosteroid in Treatment of Mental Disorders: Narrative Review. Front. Psychiatry 2023, 14, 1298359. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Tao, Y.; Duan, A.; Wei, X.; Wang, M.; Xie, M.; Chen, Z.; Shang, J.; Wang, Z. Efficacy and Tolerability of Zuranolone in Patients with Depression: A Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2024, 14, 1334694. [Google Scholar] [CrossRef]
- Singhal, M.; Modi, N.; Bansal, L.; Abraham, J.; Mehta, I.; Ravi, A. The Emerging Role of Neurosteroids: Novel Drugs Brexanalone, Sepranolone, Zuranolone, and Ganaxolone in Mood and Neurological Disorders. Cureus 2024, 16, e65866. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Uspenskaya, M.E.; Ulchenko, D.N.; Shimanovsky, N.L. Dehydroepiandrosterone and Its Metabolite 5-Androstenediol: New Therapeutic Targets and Possibilities for Clinical Application. Pharmaceuticals 2024, 17, 1186. [Google Scholar] [CrossRef]
- Tomczyk, K.; Chmaj-Wierzchowska, K.; Wszołek, K.; Wilczak, M. New Possibilities for Hormonal Vaginal Treatment in Menopausal Women. J. Clin. Med. 2023, 12, 4740. [Google Scholar] [CrossRef]
- Andguladze, M.; Tevdorashvili, G.; Tevdorashvili, M.; Tevdorashvili, D. (273) Influences of Oral Dehydroepiandrosterone (DHEA) Administration on Hormonal Profile, Menopausal Clinical Symptoms and Sexual Function in Early Postmenopausal Symptomatic Women. J. Sex. Med. 2024, 21 (Suppl. S2), qdae002.236. [Google Scholar] [CrossRef]
- Arlt, W. Dehydroepiandrosterone and Ageing. Best Pract. Res. Clin. Endocrinol. Metab. 2004, 18, 363–380. [Google Scholar] [CrossRef]
- Dutheil, F.; De Saint Vincent, S.; Pereira, B.; Schmidt, J.; Moustafa, F.; Charkhabi, M.; Bouillon-Minois, J.-B.; Clinchamps, M. DHEA as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Front. Psychiatry 2021, 12, 688367. [Google Scholar] [CrossRef]
- Liu, D.; Dillon, J.S. Dehydroepiandrosterone Activates Endothelial Cell Nitric-Oxide Synthase by a Specific Plasma Membrane Receptor Coupled to Gαi2,3. J. Biol. Chem. 2002, 277, 21379–21388. [Google Scholar] [CrossRef]
- Erceg, N.; Micic, M.; Forouzan, E.; Knezevic, N.N. The Role of Cortisol and Dehydroepiandrosterone in Obesity, Pain, and Aging. Diseases 2025, 13, 42. [Google Scholar] [CrossRef]
- Karakus, E.; Schmid, A.; Schäffler, A.; Wudy, S.A.; Geyer, J. Intracrine Formation of Steroid Hormones in Breast Cancer, Epidermal Keratinocyte, Dermal Fibroblast, and Adipocyte Cell Lines Measured by LC-MS/MS. Int. J. Mol. Sci. 2025, 26, 1188. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, Q.; Shen, X.; Yao, Y.; Li, L.; Ma, H. Dehydroepiandrosterone attenuates LPS-induced inflammatory responses via activation of Nrf2 in RAW264.7 macrophages. Mol. Immunol. 2021, 131, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, F.; Auci, D.L.; Mangano, K.; Flores-Riveros, J.; Villegas, S.; Frincke, J.M.; Reading, C.L.; Offner, H. 5-androstenediol ameliorates pleurisy, septic shock, and experimental autoimmune encephalomyelitis in mice. Autoimmune Dis. 2010, 2010, 757432. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.G. Dehydroepiandrosterone, Cancer, and Aging. Aging Dis. 2022, 13, 423–432. [Google Scholar] [CrossRef]
- Bairamova, S.P.; Petelin, D.S.; Akhapkin, R.V.; Kudryashov, N.; Sorokina, O.Y.; Semin, S.A.; Panfilova, V.; Volel, B.A. The endogenic neurosteroid system and its role in the pathogenesis and therapy of mental disorders. Res. Results Pharmacol. 2023, 9, 61–69. [Google Scholar] [CrossRef]
- Garcia, M.; Thirouard, L.; Sedès, L.; Monrose, M.; Holota, H.; Caira, F.; Volle, D.H.; Beaudoin, C. Nuclear Receptor Metabolism of Bile Acids and Xenobiotics: A Coordinated Detoxification System with Impact on Health and Diseases. Int. J. Mol. Sci. 2018, 19, 3630. [Google Scholar] [CrossRef]
- Kohalmy, K.; Tamási, V.; Kóbori, L.; Sárváry, E.; Pascussi, J.M.; Porrogi, P.; Rozman, D.; Prough, R.A.; Meyer, U.A.; Monostory, K. Dehydroepiandrosterone induces human CYP2B6 through the constitutive androstane receptor. Drug Metab. Dispos. 2007, 35, 1495–1501. [Google Scholar] [CrossRef]
- Labrie, C.; Flamand, M.; Bélanger, A.; Labrie, F. High Bioavailability of Dehydroepiandrosterone Administered Percutaneously in the Rat. J. Endocrinol. 1996, 150 (Suppl. S3), S107–S118. [Google Scholar]
- Corsi, S.; Scheggi, S.; Pardu, A.; Braccagni, G.; Caruso, D.; Cioffi, L.; Diviccaro, S.; Gentile, M.; Fanni, S.; Stancampiano, R.; et al. Pregnenolone for the Treatment of L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Exp. Neurol. 2023, 363, 114370. [Google Scholar] [CrossRef]
- Wu, S.; Ye, M.; Li, Z.; Bu, S.; Zhang, Y. Long-Term Supplementation of Dehydroepiandrosterone Improved Depressive-like Behaviors by Increasing BDNF Expression in the Hippocampus in Ovariectomized Rats. Heliyon 2020, 6, e05180. [Google Scholar] [CrossRef]
- Cao, T.; Tang, M.; Jiang, P.; Zhang, B.; Wu, X.; Chen, Q.; Zeng, C.; Li, N.; Zhang, S.; Cai, H. A Potential Mechanism Underlying the Therapeutic Effects of Progesterone and Allopregnanolone on Ketamine-Induced Cognitive Deficits. Front. Pharmacol. 2021, 12, 612083. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Shoae-Hassani, A.; Keyhanvar, P.; Kheradmand, D.; Darbandi-Azar, A. Dehydroepiandrosterone Stimulates Nerve Growth Factor and Brain Derived Neurotrophic Factor in Cortical Neurons. Adv. Pharmacol. Sci. 2013, 2013, 506191. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh, P.; Arbabi, E. The Effects of Progesterone on Glial Cell Line-derived Neurotrophic Factor Secretion from C6 Glioma Cells. Iran. J. Basic Med. Sci. 2012, 15, 1046–1052. [Google Scholar] [PubMed]
- Suzuki, M.; Wright, L.S.; Marwah, P.; Lardy, H.A.; Svendsen, C.N. Mitotic and Neurogenic Effects of Dehydroepiandrosterone (DHEA) on Human Neural Stem Cell Cultures Derived from the Fetal Cortex. Proc. Natl. Acad. Sci. USA 2004, 101, 3202–3207. [Google Scholar] [CrossRef]
- Perugini, J.; Di Mercurio, E.; Giuliani, A.; Sabbatinelli, J.; Bonfigli, A.R.; Tortato, E.; Severi, I.; Cinti, S.; Olivieri, F.; Le Roux, C.W.; et al. Ciliary Neurotrophic Factor Is Increased in the Plasma of Patients with Obesity and Its Levels Correlate with Diabetes and Inflammation Indices. Sci. Rep. 2022, 12, 8331. [Google Scholar] [CrossRef]
- Jia, C.; Brown, R.W.; Malone, H.M.; Burgess, K.C.; Gill, W.D.; Keasey, M.P.; Hagg, T. Ciliary Neurotrophic Factor Is a Key Sex-Specific Regulator of Depressive-like Behavior in Mice. Psychoneuroendocrinology 2019, 100, 96–105. [Google Scholar] [CrossRef]
- Fedotcheva, T.A. Clinical Use of Progestins and Their Mechanisms of Action: Present and Future (Review). Sovrem. Tekhnologii Med. 2021, 13, 93–106. [Google Scholar] [CrossRef]
- Reddy, D.S. The Role of Neurosteroids in the Pathophysiology and Treatment of Catamenial Epilepsy. Epilepsy Res. 2009, 85, 1–30. [Google Scholar] [CrossRef]
- Frye, C.A.; Cleveland, D.M.; Sadarangani, A.; Torgersen, J.K. Progesterone Promotes Anti-Anxiety/Depressant-like Behavior and Trophic Actions of BDNF in the Hippocampus of Female Nuclear Progesterone Receptor, but Not 5α-Reductase, Knockout Mice. Int. J. Mol. Sci. 2025, 26, 1173. [Google Scholar] [CrossRef]
- Lonsdale And, D.; Burnham, W.M. The Anticonvulsant Effects of Progesterone and 5α-dihydroprogesterone on Amygdala-kindled Seizures in Rats. Epilepsia 2003, 44, 1494–1499. [Google Scholar] [CrossRef]
- Söderpalm, A.H.V.; Lindsey, S.; Purdy, R.H.; Hauger, R.; De Wit, H. Administration of Progesterone Produces Mild Sedative-like Effects in Men and Women. Psychoneuroendocrinology 2004, 29, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G. Catamenial Epilepsy: Definition, Prevalence Pathophysiology and Treatment. Seizure 2008, 17, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Herzog, A.G. Catamenial Epilepsy: Update on Prevalence, Pathophysiology and Treatment from the Findings of the NIH Progesterone Treatment Trial. Seizure 2015, 28, 18–25. [Google Scholar] [CrossRef]
- Herzog, A.G.; Fowler, K.M.; Sperling, M.R.; Massaro, J.M.; Progesterone Trial Study Group. Distribution of Seizures across the Menstrual Cycle in Women with Epilepsy. Epilepsia 2015, 56, e58–e62. [Google Scholar] [CrossRef]
- Russu, M.C.; Antonescu, A.C. New Insights for Hormone Therapy in Perimenopausal Women Neuroprotection. In Sex Hormones in Neurodegenerative Processes and Diseases; Drevenšek, G., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Wendler, A.; Wehling, M. Many or Too Many Progesterone Membrane Receptors? Clinical Implications. Trends Endocrinol. Metab. 2022, 33, 850–868. [Google Scholar] [CrossRef]
- Miller, M.R.; Mannowetz, N.; Iavarone, A.T.; Safavi, R.; Gracheva, E.O.; Smith, J.F.; Hill, R.Z.; Bautista, D.M.; Kirichok, Y.; Lishko, P.V. Unconventional Endocannabinoid Signaling Governs Sperm Activation via the Sex Hormone Progesterone. Science 2016, 352, 555–559. [Google Scholar] [CrossRef]
- Luo, T.; Chen, H.; Zou, Q.; Wang, T.; Cheng, Y.; Wang, H.; Wang, F.; Jin, Z.; Chen, Y.; Weng, S.; et al. A Novel Copy Number Variation in CATSPER2 Causes Idiopathic Male Infertility with Normal Semen Parameters. Human. Reprod. 2019, 34, 414–423. [Google Scholar] [CrossRef]
- Castelnovo, L.F.; Thomas, P. Progesterone Exerts a Neuroprotective Action in a Parkinson’s Disease Human Cell Model through Membrane Progesterone Receptor α (mPRα/PAQR7). Front. Endocrinol. 2023, 14, 1125962. [Google Scholar] [CrossRef]
- Schumacher, M.; Mattern, C.; Ghoumari, A.; Oudinet, J.P.; Liere, P.; Labombarda, F.; Sitruk-Ware, R.; De Nicola, A.F.; Guennoun, R. Revisiting the Roles of Progesterone and Allopregnanolone in the Nervous System: Resurgence of the Progesterone Receptors. Progress Neurobiol. 2014, 113, 6–39. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progestins as Anticancer Drugs and Chemosensitizers, New Targets and Applications. Pharmaceutics 2021, 13, 1616. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of Sex Hormones on Immune Function and Multiple Sclerosis Development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Hall, O.J.; Klein, S.L. Progesterone-Based Compounds Affect Immune Responses and Susceptibility to Infections at Diverse Mucosal Sites. Mucosal Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Krishnamoorthy, V.R.; Kim, S.; Khurana, S.; LaPorte, H.M. Brain-Derived Neuerotrophic Factor and Related Mechanisms That Mediate and Influence Progesterone-Induced Neuroprotection. Front. Endocrinol. 2024, 15, 1286066. [Google Scholar] [CrossRef] [PubMed]
- Gruber, C.J.; Huber, J.C. Differential Effects of Progestins on the Brain. Maturitas 2003, 46, 71–75. [Google Scholar] [CrossRef]
- Baulieu, E.-E.; Schumacher, M. Progesterone as a Neuroactive Neurosteroid, with Special Reference to the Effect of Progesterone on Myelination. Steroids 2000, 65, 605–612. [Google Scholar] [CrossRef]
- Hsu, S.; Bove, R. Hormonal Therapies in Multiple Sclerosis: A Review of Clinical Data. Curr. Neurol. Neurosci. Rep. 2024, 24, 1–15. [Google Scholar] [CrossRef]
- Almeida, F.B.; Barros, H.M.T.; Pinna, G. Neurosteroids and Neurotrophic Factors: What Is Their Promise as Biomarkers for Major Depression and PTSD? Int. J. Mol. Sci. 2021, 22, 1758. [Google Scholar] [CrossRef]
- Liang, J.J.; Rasmusson, A.M. Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone. Chronic Stress. 2018, 2, 2470547018818555. [Google Scholar] [CrossRef]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef]
- Balan, I.; Boero, G.; Chéry, S.L.; McFarland, M.H.; Lopez, A.G.; Morrow, A.L. Neuroactive Steroids, Toll-like Receptors, and Neuroimmune Regulation: Insights into Their Impact on Neuropsychiatric Disorders. Life 2024, 14, 582. [Google Scholar] [CrossRef]
- Marx, C.E.; Keefe, R.S.E.; Buchanan, R.W.; Hamer, R.M.; Kilts, J.D.; Bradford, D.W.; Strauss, J.L.; Naylor, J.C.; Payne, V.M.; Lieberman, J.A.; et al. Proof-of-Concept Trial with the Neurosteroid Pregnenolone Targeting Cognitive and Negative Symptoms in Schizophrenia. Neuropsychopharmacology 2009, 34, 1885–1903. [Google Scholar] [CrossRef] [PubMed]
- Ritsner, M.S.; Bawakny, H.; Kreinin, A. Pregnenolone Treatment Reduces Severity of Negative Symptoms in Recent-onset Schizophrenia: An 8-week, Double-blind, Randomized Add-on Two-center Trial. Psychiatry Clin. Neurosci. 2014, 68, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.S.; Park, J.; Marx, C.E.; Hynan, L.S.; Gardner, C.; Davila, D.; Nakamura, A.; Sunderajan, P.; Lo, A.; Holmes, T. A Randomized, Double-Blind, Placebo-Controlled Trial of Pregnenolone for Bipolar Depression. Neuropsychopharmacology 2014, 39, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Fung, L.K.; Libove, R.A.; Phillips, J.; Haddad, F.; Hardan, A.Y. Brief Report: An Open-Label Study of the Neurosteroid Pregnenolone in Adults with Autism Spectrum Disorder. J. Autism Dev. Disord. 2014, 44, 2971–2977. [Google Scholar] [CrossRef]
- Gao, H.; Magin, Z.; Fogelman, N.; Sinha, R.; Angarita, G.A.; Milivojevic, V. Stability and Reliability of Repeated Plasma Pregnenolone Levels After Oral Pregnenolone Dosing in Individuals with Cocaine Use Disorder: Pilot Findings. Life 2024, 14, 1483. [Google Scholar] [CrossRef]
- Morrow, A.L.; Boero, G.; Balan, I. Emerging Evidence for Endogenous Neurosteroid Modulation of Pro-Inflammatory and Anti-Inflammatory Pathways That Impact Neuropsychiatric Disease. Neurosci. Biobehav. Rev. 2024, 158, 105558. [Google Scholar] [CrossRef]
- Rustichelli, C.; Bellei, E.; Bergamini, S.; Monari, E.; Lo Castro, F.; Baraldi, C.; Tomasi, A.; Ferrari, A. Comparison of Pregnenolone Sulfate, Pregnanolone and Estradiol Levels between Patients with Menstrually-Related Migraine and Controls: An Exploratory Study. J. Headache Pain. 2021, 22, 13. [Google Scholar] [CrossRef]
- Maddahi, A.; Warfvinge, K.; Holm, A.; Edvinsson, J.C.A.; Reducha, P.V.; Kazantzi, S.; Haanes, K.A.; Edvinsson, L. Progesterone Distribution in the Trigeminal System and Its Role to Modulate Sensory Neurotransmission: Influence of Sex. J. Headache Pain. 2023, 24, 154. [Google Scholar] [CrossRef]
- Pinna, G. Allopregnanolone, the Neuromodulator Turned Therapeutic Agent: Thank You, Next? Front. Endocrinol. 2020, 11, 236. [Google Scholar] [CrossRef]
- Available online: https://www.kegg.jp/entry/D11149 (accessed on 7 April 2025).
- Zhou, Y.; Zhang, Y.; Zhao, D.; Yu, X.; Shen, X.; Zhou, Y.; Wang, S.; Qiu, Y.; Chen, Y.; Zhu, F. TTD: Therapeutic Target Database Describing Target Druggability Information. Nucleic Acids Res. 2024, 52, D1465–D1477. [Google Scholar] [CrossRef]
- Lamb, Y.N. Ganaxolone: First Approval. Drugs 2022, 82, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Raikes, A.C.; Hernandez, G.D.; Matthews, D.C.; Lukic, A.S.; Law, M.; Shi, Y.; Schneider, L.S.; Brinton, R.D. Exploratory Imaging Outcomes of a Phase 1b/2a Clinical Trial of Allopregnanolone as a Regenerative Therapeutic for Alzheimer’s Disease: Structural Effects and Functional Connectivity Outcomes. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12258. [Google Scholar] [CrossRef] [PubMed]
- Sethi, R.; Gómez-Coronado, N.; Walker, A.J.; Robertson, O.D.; Agustini, B.; Berk, M.; Dodd, S. Neurobiology and Therapeutic Potential of Cyclooxygenase-2 (COX-2) Inhibitors for Inflammation in Neuropsychiatric Disorders. Front. Psychiatry 2019, 10, 605. [Google Scholar] [CrossRef]
- Pavlik, T.I.; Shimanovsky, N.L.; Zemlyanaya, O.A.; Fedotcheva, T.A. The Effect of Progestins on Cytokine Production in the Peripheral Blood Mononuclear Cells of Menopausal Women and Their Luminol-Dependent Chemiluminescence. Molecules 2023, 28, 4354. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, J.; Ding, H.; Wu, W.; Xiao, G. Progesterone Provides the Pleiotropic Neuroprotective Effect on Traumatic Brain Injury Through the Nrf2/ARE Signaling Pathway. Neurocrit. Care 2017, 26, 292–300. [Google Scholar] [CrossRef]
- Lucchi, C.; Codeluppi, A.; Filaferro, M.; Vitale, G.; Rustichelli, C.; Avallone, R.; Mandrioli, J.; Biagini, G. Human Microglia Synthesize Neurosteroids to Cope with Rotenone-Induced Oxidative Stress. Antioxidants 2023, 12, 963. [Google Scholar] [CrossRef]
- O’Connor, J.L.; Nissen, J.C. The Pathological Activation of Microglia Is Modulated by Sexually Dimorphic Pathways. Int. J. Mol. Sci. 2023, 24, 4739. [Google Scholar] [CrossRef]
- Fedotcheva, T.A.; Fedotcheva, N.I.; Shimanovsky, N.L. Progesterone as an Anti-Inflammatory Drug and Immunomodulator: New Aspects in Hormonal Regulation of the Inflammation. Biomolecules 2022, 12, 1299. [Google Scholar] [CrossRef]
- Taliani, S.; Da Settimo, F.; Da Pozzo, E.; Chelli, B.; Martini, C. Translocator Protein Ligands as Promising Therapeutic Tools for Anxiety Disorders. Curr. Med. Chem. 2009, 16, 3359–3380. [Google Scholar] [CrossRef]
- Germelli, L.; Angeloni, E.; Da Pozzo, E.; Tremolanti, C.; De Felice, M.; Giacomelli, C.; Marchetti, L.; Muscatello, B.; Barresi, E.; Taliani, S.; et al. 18 kDa TSPO Targeting Drives Polarized Human Microglia towards a Protective and Restorative Neurosteroidome Profile. Cell. Mol. Life Sci. 2025, 82, 34. [Google Scholar] [CrossRef]
- Lavender, E.; Hirasawa-Fujita, M.; Domino, E.F. Ketamine’s Dose Related Multiple Mechanisms of Actions: Dissociative Anesthetic to Rapid Antidepressant. Behav. Brain Res. 2020, 390, 112631. [Google Scholar] [CrossRef] [PubMed]
- Riad, A.; Lengyel-Zhand, Z.; Zeng, C.; Weng, C.-C.; Lee, V.M.-Y.; Trojanowski, J.Q.; Mach, R.H. The Sigma-2 Receptor/TMEM97, PGRMC1, and LDL Receptor Complex Are Responsible for the Cellular Uptake of Aβ42 and Its Protein Aggregates. Mol. Neurobiol. 2020, 57, 3803–3813. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.S.A.; Chamberlain, C.; Craven, R.J. S2RPgrmc1: The Cytochrome-Related Sigma-2 Receptor That Regulates Lipid and Drug Metabolism and Hormone Signaling. Expert Opin. Drug Metab. Toxicol. 2012, 8, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, E.; de Haan, W.; Scheijbeler, E.; Hamby, M.E.; Catalano, S.; Scheltens, P.; Grundman, M.; Caggiano, A.O. A Pilot Electroencephalography Study of the Effect of CT1812 Treatment on Synaptic Activity in Patients with Mild to Moderate Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2024, 11, 1809–1817. [Google Scholar] [CrossRef]
- Steinfield, S.R.; Stenn, D.F.; Chen, H.; Kalisch, B.E. A Review of the Clinical Progress of CT1812, a Novel Sigma-2 Receptor Antagonist for the Treatment of Alzheimer’s Disease. Pharmaceuticals 2025, 18, 659. [Google Scholar] [CrossRef]
- Kelp, N.C.; Pru, C.A.; Paudel, S.; Lydon, J.P.; Kim, J.J.; Peluso, J.J.; Pru, J.K. Uterine Pgrmc2 Deficiency Attenuates Endometrial Hyperplasia and Cancer and Prolongs Lifespan in a Pten Loss-of-Function-Induced Cancer Model. Cancers 2025, 17, 1178. [Google Scholar] [CrossRef]
- Willibald, M.; Wurster, I.; Meisner, C.; Vogel, U.; Seeger, H.; Mueck, A.; Fehm, T.; Neubauer, H. High Level of Progesteron Receptor Membrane Component 1 (PGRMC 1) in Tissue of Breast Cancer Patients Is Associated with Worse Response to Anthracycline-Based Neoadjuvant Therapy. Horm. Metab. Res. 2017, 49, 595–603. [Google Scholar] [CrossRef]
- Zhou, C.; Zhu, T.; Ni, W.; Zhou, H.; Song, J.; Wang, M.; Jin, G.; Zhou, Y.; Han, J.; Hua, F. Gain-of-function of Progesterone Receptor Membrane Component 2 Ameliorates Ischemic Brain Injury. CNS Neurosci. Ther. 2023, 29, 1585–1601. [Google Scholar] [CrossRef]
- Su, T.-P.; Su, T.-C.; Nakamura, Y.; Tsai, S.-Y. The Sigma-1 Receptor as a Pluripotent Modulator in Living Systems. Trends Pharmacol. Sci. 2016, 37, 262–278. [Google Scholar] [CrossRef]
- Fu, C.; Xiao, Y.; Zhou, X.; Sun, Z. Insight into Binding of Endogenous Neurosteroid Ligands to the Sigma-1 Receptor. Nat. Commun. 2024, 15, 5619. [Google Scholar] [CrossRef]
- Hashimoto, K. Sigma-1 receptor chaperone and brain-derived neurotrophic factor: Emerging links between cardiovascular disease and depression. Prog. Neurobiol. 2013, 100, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. Activation of Sigma-1 Receptor Chaperone in the Treatment of Neuropsychiatric Diseases and Its Clinical Implication. J. Pharmacol. Sci. 2015, 127, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hornung, R.S.; Raut, N.G.; Cantu, D.J.; Lockhart, L.M.; Averitt, D.L. Sigma-1 Receptors and Progesterone Metabolizing Enzymes in Nociceptive Sensory Neurons of the Female Rat Trigeminal Ganglia: A Neural Substrate for the Antinociceptive Actions of Progesterone. Mol. Pain. 2022, 18, 17448069211069255. [Google Scholar] [CrossRef] [PubMed]
- Niitsu, T.; Iyo, M.; Hashimoto, K. Sigma-1 Receptor Agonists as Therapeutic Drugs for Cognitive Impairment in Neuropsychiatric Diseases. Curr. Pharm. Des. 2012, 18, 875–883. [Google Scholar] [CrossRef]
- Ryskamp, D.A.; Korban, S.; Zhemkov, V.; Kraskovskaya, N.; Bezprozvanny, I. Neuronal Sigma-1 Receptors: Signaling Functions and Protective Roles in Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 862. [Google Scholar] [CrossRef]
- Moriguchi, S.; Shinoda, Y.; Yamamoto, Y.; Sasaki, Y.; Miyajima, K.; Tagashira, H.; Fukunaga, K. Stimulation of the Sigma-1 Receptor by DHEA Enhances Synaptic Efficacy and Neurogenesis in the Hippocampal Dentate Gyrus of Olfactory Bulbectomized Mice. PLoS ONE 2013, 8, e60863. [Google Scholar] [CrossRef]
- Wu, N.; Ye, Y.; Wan, B.; Yu, Y.; Liu, C.; Chen, Q. Emerging Benefits: Pathophysiological Functions and Target Drugs of the Sigma-1 Receptor in Neurodegenerative Diseases. Mol. Neurobiol. 2021, 58, 5649–5666. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, C.; Wang, C.; Sun, M.; Yin, D.; Sun, T. Sigma-2 Receptor—A Potential Target for Cancer/Alzheimer’s Disease Treatment via Its Regulation of Cholesterol Homeostasis. Molecules 2020, 25, 5439. [Google Scholar] [CrossRef]
- Vázquez-Rosa, E.; Watson, M.R.; Sahn, J.J.; Hodges, T.R.; Schroeder, R.E.; Cintrón-Pérez, C.J.; Shin, M.-K.; Yin, T.C.; Emery, J.L.; Martin, S.F.; et al. Neuroprotective Efficacy of a Sigma 2 Receptor/TMEM97 Modulator (DKR-1677) after Traumatic Brain Injury. ACS Chem. Neurosci. 2019, 10, 1595–1602. [Google Scholar] [CrossRef]
- Wang, H.; Peng, Z.; Li, Y.; Sahn, J.J.; Hodges, T.R.; Chou, T.-H.; Liu, Q.; Zhou, X.; Jiao, S.; Porciatti, V.; et al. σ2R/TMEM97 in Retinal Ganglion Cell Degeneration. Sci. Rep. 2022, 12, 20753. [Google Scholar] [CrossRef]
- Garrett, M.; Curry, S.; Feris, S.; Lu, Y.; Gu, Q.; Clark, A.; Martin, S.F.; Kastellorizios, M. Delivery of a Novel Neuroprotective Compound to the Retina in Rat and Rabbit Animal Models. J. Control Release 2025, 382, 113659. [Google Scholar] [CrossRef] [PubMed]
- Thitilertdecha, P.; Brimson, J.M. CT1812, a Small Molecule Sigma-2 Receptor Antagonist for Alzheimer’s Disease Treatment: A Systematic Review of Available Clinical Data. J. Alzheimer’s Dis. 2024, 101, S115–S128. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Mecca, A.P.; O’Dell, R.S.; Bartlett, H.H.; Diepenbrock, N.G.; Huang, Y.; Hamby, M.E.; Grundman, M.; Catalano, S.M.; Caggiano, A.O.; et al. A pilot study to evaluate the effect of CT1812 treatment on synaptic density and other biomarkers in Alzheimer’s disease. Alzheimers Res. Ther. 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- LaBarbera, K.M.; Sheline, Y.I.; Izzo, N.J.; Yuede, C.M.; Waybright, L.; Yurko, R.; Edwards, H.M.; Gardiner, W.D.; Blennow, K.; Zetterberg, H.; et al. A Phase 1b Randomized Clinical Trial of CT1812 to Measure Aβ Oligomer Displacement in Alzheimer’s Disease Using an Indwelling CSF Catheter. Transl. Neurodegener. 2023, 12, 24. [Google Scholar] [CrossRef]
- Riad, A.; Xu, J.; Mach, R.H. Sigma-2 Receptors: An Emerging Target for CNS PET Imaging Studies. In PET and SPECT of Neurobiological Systems; Dierckx, R.A.J.O., Otte, A., De Vries, E.F.J., Van Waarde, A., Lammertsma, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 973–991. [Google Scholar] [CrossRef]
- Lizama, B.N.; Kahle, J.; Catalano, S.M.; Caggiano, A.O.; Grundman, M.; Hamby, M.E. Sigma-2 Receptors—From Basic Biology to Therapeutic Target: A Focus on Age-Related Degenerative Diseases. Int. J. Mol. Sci. 2023, 24, 6251. [Google Scholar] [CrossRef]
- Santacruz, C.A.; Vincent, J.-L.; Imbault, V.; Bruneau, M.; Creteur, J.; Brimioulle, S.; Vincent, R.; Communi, D.; Taccone, F.S. Cerebral Apolipoprotein E and Amyloid Precursor-like Protein 1 as Risk Factors for Chronic Neurodegeneration after Non-Traumatic Acute Brain Injury (ABI). Crit. Care 2023, 27, 249. [Google Scholar] [CrossRef]
- Izzo, N.J.; Xu, J.; Zeng, C.; Kirk, M.J.; Mozzoni, K.; Silky, C.; Rehak, C.; Yurko, R.; Look, G.; Rishton, G.; et al. Alzheimer’s Therapeutics Targeting Amyloid Beta 1–42 Oligomers II: Sigma-2/PGRMC1 Receptors Mediate Abeta 42 Oligomer Binding and Synaptotoxicity. PLoS ONE 2014, 9, e111899. [Google Scholar] [CrossRef]
- Peluso, J.J.; Romak, J.; Liu, X. Progesterone Receptor Membrane Component-1 (PGRMC1) Is the Mediator of Progesterone’s Antiapoptotic Action in Spontaneously Immortalized Granulosa Cells as Revealed by PGRMC1 Small Interfering Ribonucleic Acid Treatment and Functional Analysis of PGRMC1 Mutations. Endocrinology 2008, 149, 534–543. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Zhao, L.; Nilsen, J.; McClure, K.; Wong, K.; Brinton, R.D. Progesterone Increases Rat Neural Progenitor Cell Cycle Gene Expression and Proliferation Via Extracellularly Regulated Kinase and Progesterone Receptor Membrane Components 1 and 2. Endocrinology 2009, 150, 3186–3196. [Google Scholar] [CrossRef]
- Willson, T.M.; Kliewer, S.A. Pxr, Car and Drug Metabolism. Nat. Rev. Drug Discov. 2002, 1, 259–266. [Google Scholar] [CrossRef]
- Harmsen, S.; Meijerman, I.; Febus, C.L.; Maas-Bakker, R.F.; Beijnen, J.H.; Schellens, J.H.M. PXR-Mediated Induction of P-Glycoprotein by Anticancer Drugs in a Human Colon Adenocarcinoma-Derived Cell Line. Cancer Chemother. Pharmacol. 2010, 66, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Koonce, C.J.; Walf, A.A. Novel Receptor Targets for Production and Action of Allopregnanolone in the Central Nervous System: A Focus on Pregnane Xenobiotic Receptor. Front. Cell. Neurosci. 2014, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Langmade, S.J.; Gale, S.E.; Frolov, A.; Mohri, I.; Suzuki, K.; Mellon, S.H.; Walkley, S.U.; Covey, D.F.; Schaffer, J.E.; Ory, D.S. Pregnane X Receptor (PXR) Activation: A Mechanism for Neuroprotection in a Mouse Model of Niemann–Pick C Disease. Proc. Natl. Acad. Sci. USA 2006, 103, 13807–13812. [Google Scholar] [CrossRef]
- Thejer, B.M.; Infantino, V.; Santarsiero, A.; Pappalardo, I.; Abatematteo, F.S.; Teakel, S.; Van Oosterum, A.; Mach, R.H.; Denora, N.; Lee, B.C.; et al. Sigma-2 Receptor Ligand Binding Modulates Association between TSPO and TMEM97. Int. J. Mol. Sci. 2023, 24, 6381. [Google Scholar] [CrossRef]
- Rupprecht, R.; Pradhan, A.K.; Kufner, M.; Brunner, L.M.; Nothdurfter, C.; Wein, S.; Schwarzbach, J.; Puig, X.; Rupprecht, C.; Rammes, G. Neurosteroids and Translocator Protein 18 kDa (TSPO) in Depression: Implications for Synaptic Plasticity, Cognition, and Treatment Options. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1477–1487. [Google Scholar] [CrossRef]
- Rupprecht, R.; Rupprecht, C.; Di Benedetto, B.; Rammes, G. Neuroinflammation and Psychiatric Disorders: Relevance of C1q, Translocator Protein (18 kDa) (TSPO), and Neurosteroids. World J. Biol. Psychiatry 2022, 23, 257–263. [Google Scholar] [CrossRef]
- Jung, M.E. A Protective Role of Translocator Protein in Alzheimer’s Disease Brain. Curr. Alzheimer Res. 2020, 17, 3–15. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Hanson, B.J.; Thai, P.N.; Schaefer, S.; Bers, D.M.; Dedkova, E.N. PK11195 Protects from Cell Death Only When Applied During Reperfusion: Succinate-Mediated Mechanism of Action. Front. Physiol. 2021, 12, 628508. [Google Scholar] [CrossRef]
- Wang, J.; Beecher, K. TSPO: An Emerging Role in Appetite for a Therapeutically Promising Biomarker. Open Biol. 2021, 11, 210173. [Google Scholar] [CrossRef]
- Corsi, F.; Baglini, E.; Barresi, E.; Salerno, S.; Cerri, C.; Martini, C.; Da Settimo Passetti, F.; Taliani, S.; Gargini, C.; Piano, I. Targeting TSPO Reduces Inflammation and Apoptosis in an In Vitro Photoreceptor-Like Model of Retinal Degeneration. ACS Chem. Neurosci. 2022, 13, 3188–3197. [Google Scholar] [CrossRef]
- Powrie, Y.S.L.; Smith, C. Central Intracrine DHEA Synthesis in Ageing-Related Neuroinflammation and Neurodegeneration: Therapeutic Potential? J. Neuroinflamm. 2018, 15, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, J.; Wu, R.; Bai, J.; Hou, Y.; Zeng, Y.; Zhang, Y.; Wang, X.; Wang, Z.; Meng, X. Mitochondrial MPTP: A Novel Target of Ethnomedicine for Stroke Treatment by Apoptosis Inhibition. Front. Pharmacol. 2020, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Naryzhnaya, N.V.; Maslov, L.N.; Oeltgen, P.R. Pharmacology of Mitochondrial Permeability Transition Pore Inhibitors. Drug Dev. Res. 2019, 80, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Andrabi, S.S.; Parvez, S.; Tabassum, H. Progesterone Induced Neuroprotection in Reperfusion Promoted Mitochondrial Dysfunction Following Focal Cerebral Ischemia in Rats. Dis. Models Mech. 2017, 10, 787–796. [Google Scholar] [CrossRef]
- Bernardi, P.; Gerle, C.; Halestrap, A.P.; Jonas, E.A.; Karch, J.; Mnatsakanyan, N.; Pavlov, E.; Sheu, S.-S.; Soukas, A.A. Identity, Structure, and Function of the Mitochondrial Permeability Transition Pore: Controversies, Consensus, Recent Advances, and Future Directions. Cell Death Differ. 2023, 30, 1869–1885. [Google Scholar] [CrossRef]
- Fedotcheva, N.I.; Teplova, V.V.; Fedotcheva, T.A.; Rzheznikov, V.M.; Shimanovskii, N.L. Effect of Progesterone and Its Synthetic Analogues on the Activity of Mitochondrial Permeability Transition Pore in Isolated Rat Liver Mitochondria. Biochem. Pharmacol. 2009, 78, 1060–1068. [Google Scholar] [CrossRef]
- Andrianova, N.V.; Brezgunova, A.A.; Buyan, M.I.; Makievskaya, C.I.; Buyan, A.I.; Cherkesova, K.S.; Pevzner, I.B.; Zorova, L.D.; Zorov, D.B.; Plotnikov, E.Y.; et al. Sex-Specific Effects of Estradiol and Progesterone in Ischemic Kidney Injury. Int. J. Mol. Sci. 2024, 25, 3155. [Google Scholar] [CrossRef]
- Karlsson, M.; Pukenas, B.; Chawla, S.; Ehinger, J.K.; Plyler, R.; Stolow, M.; Gabello, M.; Hugerth, M.; Elmér, E.; Hansson, M.J.; et al. Neuroprotective Effects of Cyclosporine in a Porcine Pre-Clinical Trial of Focal Traumatic Brain Injury. J. Neurotrauma 2019, 36, 14–24. [Google Scholar] [CrossRef]
- Hansson, M.J.; Elmér, E. Cyclosporine as Therapy for Traumatic Brain Injury. Neurotherapeutics 2023, 20, 1482–1495. [Google Scholar] [CrossRef]
- Akhtar, A. The Flaws and Human Harms of Animal Experimentation. Camb. Q. Healthc. Ethics 2015, 24, 407–419. [Google Scholar] [CrossRef]
- Coyoy-Salgado, A.; Segura-Uribe, J.; Salgado-Ceballos, H.; Castillo-Mendieta, T.; Sánchez-Torres, S.; Freyermuth-Trujillo, X.; Orozco-Barrios, C.; Orozco-Suarez, S.; Feria-Romero, I.; Pinto-Almazán, R.; et al. Evaluating Sex Steroid Hormone Neuroprotection in Spinal Cord Injury in Animal Models: Is It Promising in the Clinic? Biomedicines 2024, 12, 1478. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; Reed, S.C.; Brooks, D.J.; Levin, F.R.; Evans, S.M. Safety and Tolerability of Progesterone Treatment for Women with Cocaine Use Disorder: A Pilot Treatment Trial. Am. J. Drug Alcohol Abus. 2022, 48, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Nucera, B.; Rinaldi, F.; Dono, F.; Lanzone, J.; Evangelista, G.; Consoli, S.; Tappatà, M.; Narducci, F.; Troisi, S.; Trinka, E.; et al. Progesterone and Its Derivatives for the Treatment of Catamenial Epilepsy: A Systematic Review. Seizure Eur. J. Epilepsy 2023, 109, 52–59. [Google Scholar] [CrossRef]
- Hernandez, G.D.; Brinton, R.D. Allopregnanolone: Regenerative Therapeutic to Restore Neurological Health. Neurobiol. Stress 2022, 21, 100502. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Sussman, H.; Brinton, R.; Schumacher, M.; Singer, P.; Kumar, N.; De Nicola, A.F.; El-Etr, M.; Guennoun, R.V.; Borlongan, C. Nestorone (segesterone acetate) effects on neuroregeneration. Front. Neuroendocrinol. 2024, 73, 101136. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Bonsack, B.; Brinton, R.; Schumacher, M.; Kumar, N.; Lee, J.-Y.; Castelli, V.; Corey, S.; Coats, A.; Sadanandan, N.; et al. Progress in Progestin-Based Therapies for Neurological Disorders. Neurosci. Biobehav. Rev. 2021, 122, 38–65. [Google Scholar] [CrossRef]
- Alhaj, H.A.; Massey, A.E.; McAllister-Williams, R.H. Effects of DHEA Administration on Episodic Memory, Cortisol and Mood in Healthy Young Men: A Double-Blind, Placebo-Controlled Study. Psychopharmacology 2006, 188, 541–551. [Google Scholar] [CrossRef]
- Wolkowitz, O.M.; Kramer, J.H.; Reus, V.I.; Costa, M.M.; Yaffe, K.; Walton, P.; Raskind, M.; Peskind, E.; Newhouse, P.; Sack, D.; et al. DHEA Treatment of Alzheimer’s Disease: A Randomized, Double-Blind, Placebo-Controlled Study. Neurology 2003, 60, 1071–1076. [Google Scholar] [CrossRef]
- Minghetti, P.; Cilurzo, F.; Casiraghi, A.; Montanari, L.; Santoro, A. Development of Patches for the Controlled Release of Dehydroepiandrosterone. Drug Dev. Ind. Pharm. 2001, 27, 711–717. [Google Scholar] [CrossRef]
- Kusama, S.; Sato, K.; Matsui, Y.; Kimura, N.; Abe, H.; Yoshida, S.; Nishizawa, M. Transdermal Electroosmotic Flow Generated by a Porous Microneedle Array Patch. Nat. Commun. 2021, 12, 658. [Google Scholar] [CrossRef]
- Alex, M.; Alsawaftah, N.M.; Husseini, G.A. State-of-All-the-Art and Prospective Hydrogel-Based Transdermal Drug Delivery Systems. Appl. Sci. 2024, 14, 2926. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Özcan Bülbül, E.; Okur, M.E.; Karantas, I.D.; Üstündağ Okur, N. The Application of Nanogels as Efficient Drug Delivery Platforms for Dermal/Transdermal Delivery. Gels 2023, 9, 753. [Google Scholar] [CrossRef] [PubMed]
- Lunter, D.; Klang, V.; Eichner, A.; Savic, S.M.; Savic, S.; Lian, G.; Erdő, F. Progress in Topical and Transdermal Drug Delivery Research—Focus on Nanoformulations. Pharmaceutics 2024, 16, 817. [Google Scholar] [CrossRef] [PubMed]
- Walther, A.; Seuffert, J. Testosterone and Dehydroepiandrosterone Treatment in Ageing Men: Are We All Set? World J. Mens. Health 2020, 38, 178. [Google Scholar] [CrossRef]
| Name | Chemical Structure | Condition/Disease | Ref. |
|---|---|---|---|
| DHEA and its derivatives | |||
| DHEA | 3beta-hydroxyandrost-5-en-17-one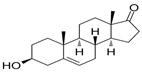 | neonatal hypoxic–ischemic brain injury | [38] |
| Bezisterim (ne3107) | 3α-ethynyl-androst-5-ene-3β,7β,17β-triol | neuroinflammation and dementia | [39,40] |
| BNN27 | 17α,20R-epoxypregn-5-ene-3β,21-diol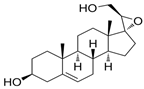 | small-molecule mimetics of endogenous neurotrophin/Spinal Cord Injury | [41] |
| Fluasterone | 3β-dehydroxy-16α-fluoro-DHEA | traumatic brain injury | [42] |
| P4 derivatives | |||
| Megestrole acetate | 17-Hydroxy-6-methylpregna-3,6-diene-3,20-dione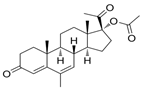 | anorexia and cachexia or serious unexplained weight loss | [43] |
| Gestobutanoyl | 17α-Acetoxy-3β-butanoyloxy-6-methyl-pregna-4,6-dien-20-one  | inflammation and MPTP opening inhibitor | [44] |
| PREG (eltanolone) | |||
| Eltanolone (stereoisomer of 5α-pregnan-3α-ol-20-one (allopregnanolone) | 3alpha-Hydroxy-5beta-pregnan-20-one | anesthetic/low back pain | [45,46] |
| ALLO and its derivatives | |||
| Brexanolone (Zulresso™) or ALLO | 5alpha-Pregnan-3alpha-ol-20-one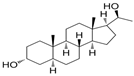 | postpartum depression and Fragile X-Associated Tremor/Ataxia Syndrome | [47,48] |
| Ganaxolone (Ztalmy™) | 3α-hydroxy-3β-methyl-5α-pregnan-20-one | inhibition of epileptic seizures associated with cyclin-dependent kinase-like 5 deficiency disorder | [49] |
| Sepranolone | Isoallopregnanolone, 3-hydroxy-5alpha-pregnan-20-one 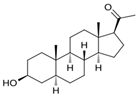 | premenstrual dysphoric disorder | [50] |
| Zuranolone (Zurzuvae™, SAGE-217). | 3beta-methyl-21-(4-cyano-1H-pyrazol-1′-yl)-19-norpregnanolone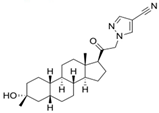 | postpartum depression/major depressive disorder | [51,52,53,54] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedotcheva, T.A.; Shimanovsky, N.L. Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation. Pharmaceuticals 2025, 18, 945. https://doi.org/10.3390/ph18070945
Fedotcheva TA, Shimanovsky NL. Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation. Pharmaceuticals. 2025; 18(7):945. https://doi.org/10.3390/ph18070945
Chicago/Turabian StyleFedotcheva, Tatiana A., and Nikolay L. Shimanovsky. 2025. "Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation" Pharmaceuticals 18, no. 7: 945. https://doi.org/10.3390/ph18070945
APA StyleFedotcheva, T. A., & Shimanovsky, N. L. (2025). Neurosteroids Progesterone and Dehydroepiandrosterone: Molecular Mechanisms of Action in Neuroprotection and Neuroinflammation. Pharmaceuticals, 18(7), 945. https://doi.org/10.3390/ph18070945





