Expanding the Therapeutic Profile of Topical Cannabidiol in Temporomandibular Disorders: Effects on Sleep Quality and Migraine Disability in Patients with Bruxism-Associated Muscle Pain
Abstract
1. Introduction
1.1. Rationale
1.2. Objectives
2. Results
2.1. Baseline Demographic and Clinical Characteristics of Participants
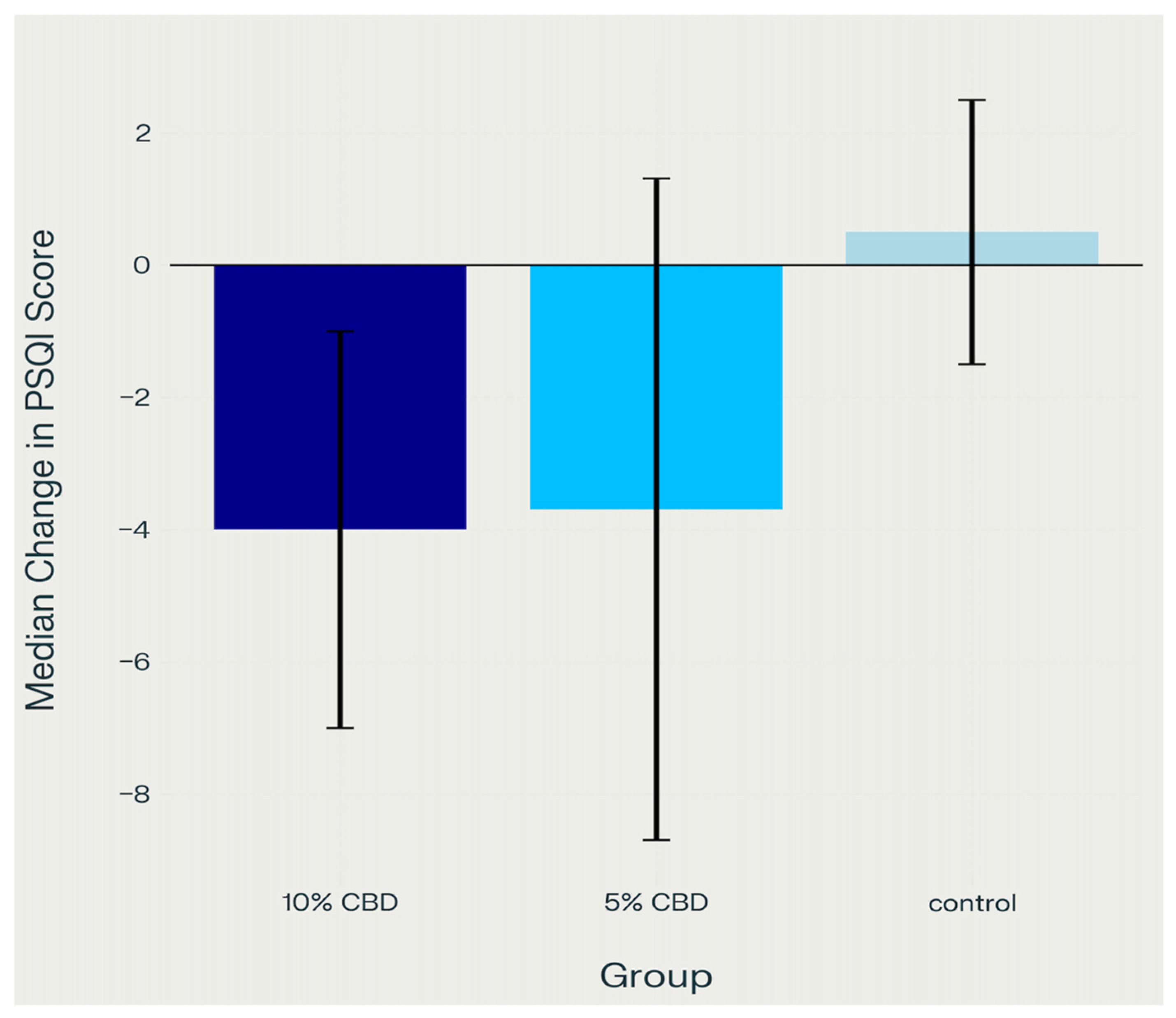
2.2. Sleep Quality (PSQI)
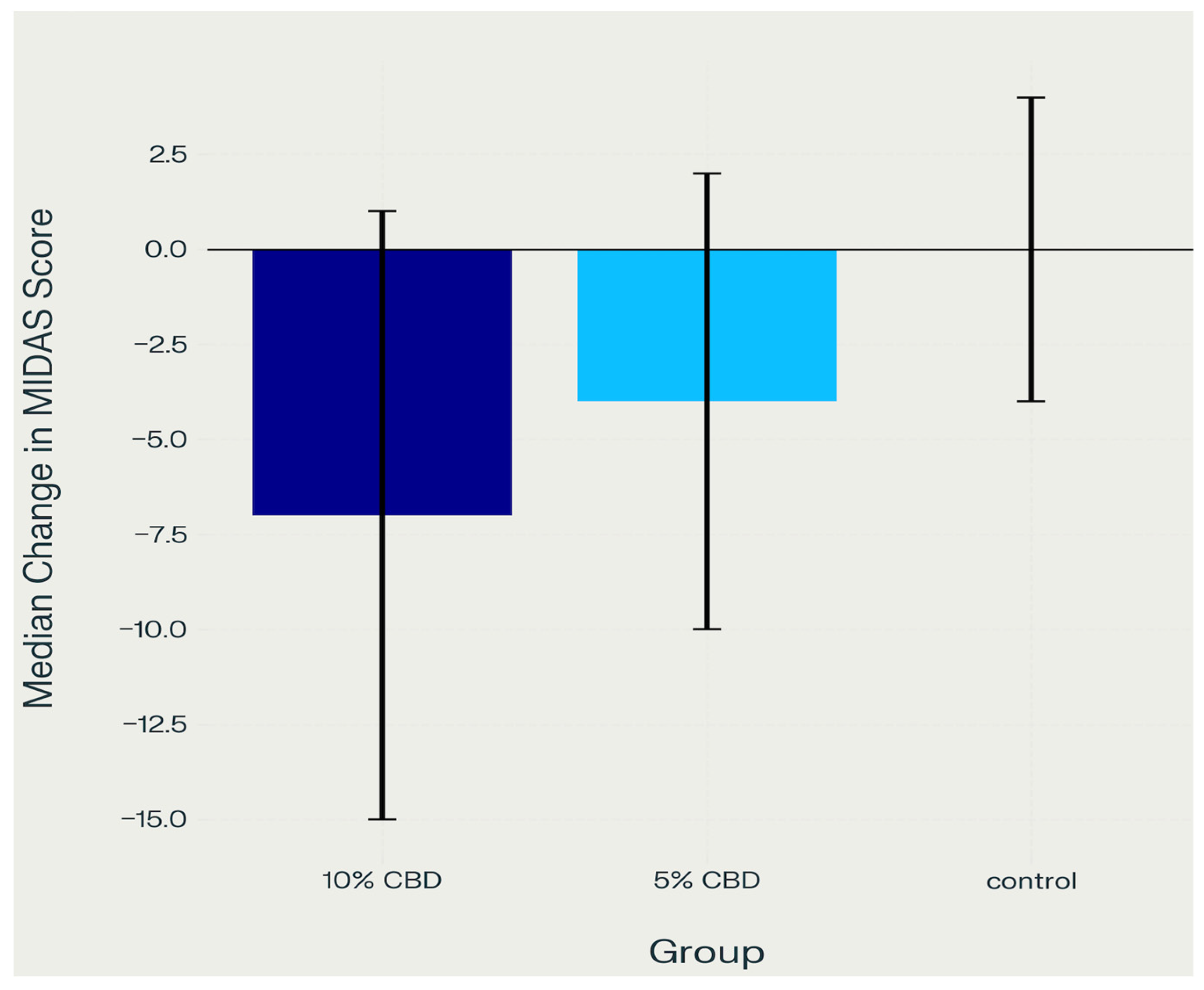
2.3. Migraine Disability (MIDAS)
2.4. Summary of Key Findings
2.4.1. Sleep Quality (PSQI)
- Control group vs. 5% CBD: p = 0.002
- Control group vs. 10% CBD: p < 0.001.
2.4.2. Migraine Disability (MIDAS)
- Control group vs. 5% CBD: p = 0.010
- Control group vs. 10% CBD: p < 0.001.
2.5. Safety and Adverse Events
2.6. VAS and sEMG Results
3. Discussion
4. Materials and Methods
4.1. Study Design
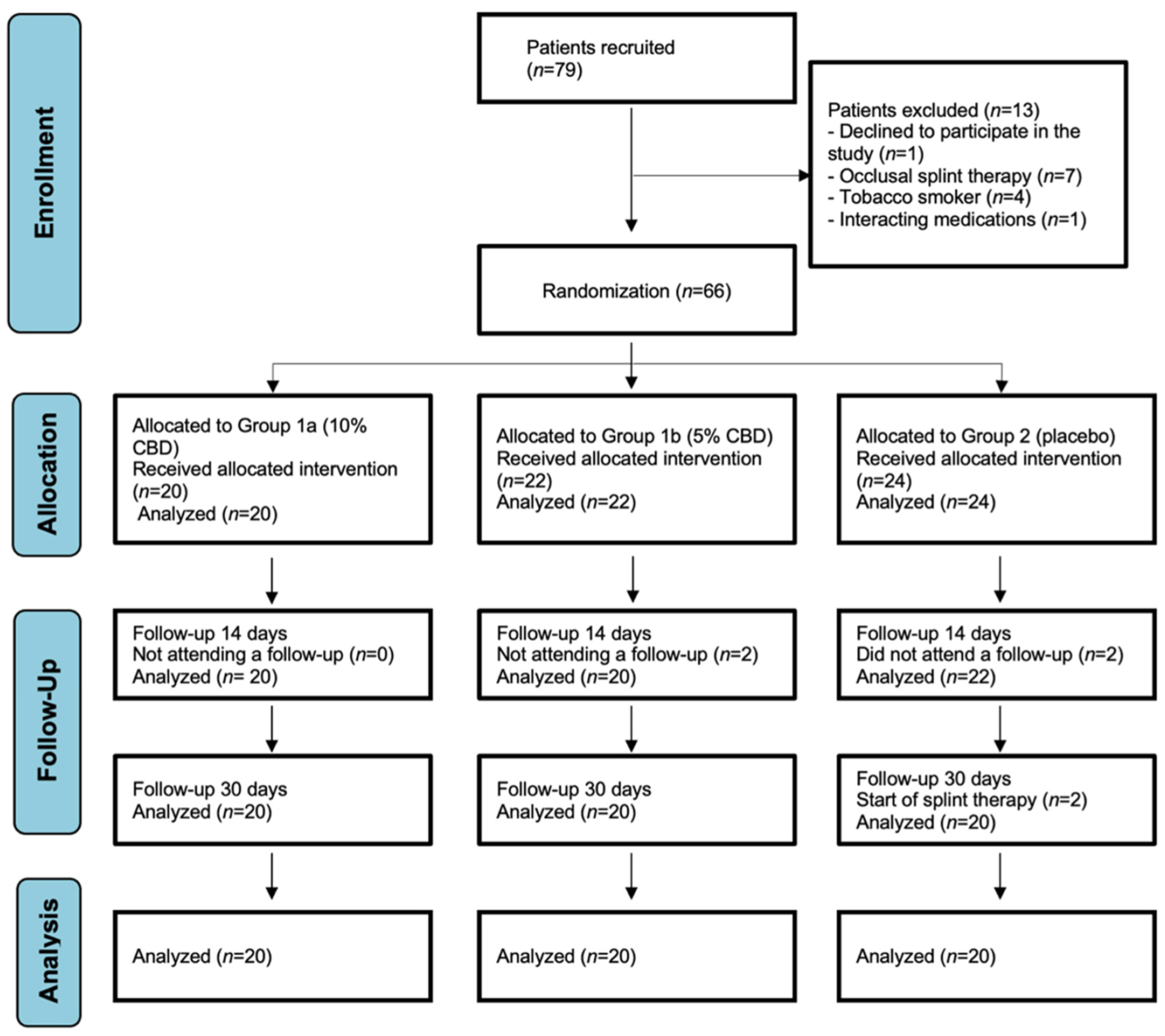
4.2. Participant Recruitment and Sample Size
4.3. Study Groups
- Group 1 (control group) received a placebo gel without cannabidiol.
- Group 2 (CBD 5% group) received a 5% CBD hydrogel.
- Group 3 (CBD 10% group) received a 10% CBD hydrogel.
4.4. Ethical Considerations and Registration
4.5. Study Protocol
Randomization and Blinding
4.6. Experimental Gel Preparation and Application
4.7. Assessment Methods
4.7.1. Bruxism Assessment
4.7.2. Muscle Tension (sEMG)
4.7.3. Pain Levels (VAS)
4.7.4. Sleep Quality (PSQI)
- 0–5 points: Good sleep quality (no significant problems)
- 6–10 points: Mild sleep disturbances (may stem from lifestyle, stress, or poor sleep habits)
- 11–15 points: Moderate sleep disturbances (suggesting possible chronic issues, e.g., insomnia or excessive daytime sleepiness)
- 16–21 points: Severe sleep disturbances (indicating serious problems requiring medical intervention, e.g., sleep apnea, chronic insomnia, circadian rhythm disorders).
4.7.5. Migraine Disability (MIDAS)
- 0–5 points (Grade I)—No or minimal disability;
- ○
- Migraine has negligible impact on daily life;
- 6–10 points (Grade II)—Mild disability;
- ○
- Migraine occasionally interferes with daily functioning;
- 11–20 points (Grade III)—Moderate disability;
- ○
- Migraine significantly affects work and daily life;
- ≥21 points (Grade IV)—Severe disability;
- ○
- Severe restriction of daily activities; requires advanced treatment.
4.8. Statistical Analysis
4.9. Safety Monitoring
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cásedas, G.; Yarza-Sancho, M.d.; López, V. Cannabidiol (CBD): A Systematic Review of Clinical and Preclinical Evidence in the Treatment of Pain. Pharmaceuticals 2024, 17, 1438. [Google Scholar] [CrossRef]
- Russo, E.B.; Marcu, J. Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. Adv. Pharmacol. 2017, 80, 67–134. [Google Scholar]
- Etemad, L.; Karimi, G.; Alavi, M.S.; Roohbakhsh, A. Pharmacological Effects of Cannabidiol by Transient Receptor Potential Channels. Life Sci. 2022, 300, 120582. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fan, M.; An, C.; Ni, F.; Huang, W.; Luo, J. A Narrative Review of Molecular Mechanism and Therapeutic Effect of Cannabidiol (CBD). Basic Clin. Pharmacol. Toxicol. 2022, 130, 439–456. [Google Scholar] [CrossRef] [PubMed]
- Walczyńska-Dragon, K.; Kurek-Górecka, A.; Fiegler-Rudol, J.; Nitecka-Buchta, A.; Baron, S. The Therapeutic Potential of Cannabidiol in the Management of Temporomandibular Disorders and Orofacial Pain. Pharmaceutics 2025, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Fine, P.G.; Rosenfeld, M.J. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med. J. 2013, 4, e0022. [Google Scholar] [CrossRef]
- Matei, D.; Trofin, D.; Iordan, D.A.; Onu, I.; Condurache, I.; Ionite, C.; Buculei, I. The Endocannabinoid System and Physical Exercise. Int. J. Mol. Sci. 2023, 24, 1989. [Google Scholar] [CrossRef]
- Tanganeli, J.P.; Haddad, D.S.; Rode, S.M.; Tambeli, C.H.; Grossmann, E. The endocannabinoid system and orofacial pains: Updates and perspectives. BrJP 2023, 6 (Suppl. S2), S131–S138. [Google Scholar] [CrossRef]
- Grinspoon, P. The Endocannabinoid System: Essential and Mysterious. Harvard Health Blog. 11 August 2021. Available online: https://www.health.harvard.edu/blog/the-endocannabinoid-system-essential-and-mysterious-202108112569 (accessed on 11 August 2021).
- Shetty, S.; Pitti, V.; Satish Babu, C.L.; Surendra Kumar, G.P.; Deepthi, B.C. Bruxism: A literature review. J. Indian. Prosthodont. Soc. 2010, 10, 141–148. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martínez-Hernández, M.M.; González-Alamilla, R.; Gutiérrez-Sánchez, J.E.; Cuevas-Suárez, C.E.; Monjarás-Ávila, C.U.; Monjarás-Ávila, A.J. Self-Report of the Perception of Stress and Signs of Bruxism Generated during the Pandemic in Students of the Health Area. Odovtos Int. J. Dent. Sci. 2022, 24, 200–212. [Google Scholar] [CrossRef]
- Nowińska, M.; Kozyra, M.; Zimnicki, P.; Kaczerska, J.; Śmiech, N.; Milanowska, J. The influence of stress on the occurrence of bruxism. J. Educ. Health Sport. 2020, 10, 200–211. [Google Scholar] [CrossRef]
- Lal, S.J.; Sankari, A.; Kurt, K.; Weber, D.D.S. Bruxism Management. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482466/ (accessed on 1 May 2024).
- Kapos, F.P.; Exposto, F.G.; Oyarzo, J.F.; Durham, J. Temporomandibular disorders: A review of current concepts in aetiology, diagnosis and management. Oral Surg. 2020, 13, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Réus, J.C.; Duarte, J.; Pauletto, P.; Polmann, H.; de Queiroz, L.P.; Maia, I.S.; De Luca Canto, G. Associations between sleep bruxism and primary headaches: A descriptive study. J. Oral Facial Pain Headache 2024, 38, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Xu, Q.; Shi, Y.; Zhao, R.; Li, H.; Zheng, J.; Liu, F.; Wan, Y.; Wei, B. Pathology of pain and its implications for therapeutic interventions. Signal Transduct. Target. Ther. 2024, 9, 155. [Google Scholar] [CrossRef]
- De La Rosa, J.S.; Brady, B.R.; Ibrahim, M.M.; Herder, K.E.; Wallace, J.S.; Padilla, A.R.; Vanderah, T.W. Co-occurrence of chronic pain and anxiety/depression symptoms in U.S. adults: Prevalence, functional impacts, and opportunities. Pain 2024, 165, 666–673. [Google Scholar] [CrossRef]
- Manzanares, J.; Julian, M.; Carrascosa, A. Role of the cannabinoid system in pain control and therapeutic implications for the management of acute and chronic pain episodes. Curr. Neuropharmacol. 2006, 4, 239–257. [Google Scholar] [CrossRef]
- Leinen, Z.J.; Mohan, R.; Premadasa, L.S.; Acharya, A.; Mohan, M.; Byrareddy, S.N. Therapeutic Potential of Cannabis: A Comprehensive Review of Current and Future Applications. Biomedicines 2023, 11, 2630. [Google Scholar] [CrossRef]
- Matos, C.; Pereira, A.T.; Dias, M.J.; Sousa, C.; Vinha, A.F.; Moutinho, C.; Carvalho, M. Cannabis for Chronic Pain: Mechanistic Insights and Therapeutic Challenges. Stresses 2025, 5, 7. [Google Scholar] [CrossRef]
- Fillingim, R.B.; Loeser, J.D.; Baron, R.; Edwards, R.R. Assessment of Chronic Pain: Domains, Methods, and Mechanisms. J. Pain. 2016, 17 (Suppl. S9), T10–T20. [Google Scholar] [CrossRef]
- Silberstein, S.D. Outcomes, efficacy, and complications of headache management. In Raj’s Practical Management of Pain, 4th ed.; Benzon, H.T., Rathmell, J.P., Wu, C.L., Turk, D.C., Argoff, C.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2008; pp. 1261–1279. [Google Scholar] [CrossRef]
- Edmeads, J.; Láinez, J.M.; Brandes, J.L.; Schoenen, J.; Freitag, F. Potential of the Migraine Disability Assessment (MIDAS) Questionnaire as a public health initiative and in clinical practice. Neurology 2001, 56 (Suppl. S1), S29–S34. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F., III; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Doi, Y.; Minowa, M.; Uchiyama, M.; Okawa, M.; Kim, K.; Shibui, K.; Kamei, Y. Psychometric assessment of subjective sleep quality using the Japanese version of the Pittsburgh Sleep Quality Index (PSQI-J) in psychiatric disordered and control subjects. Psychiatry Res. 2000, 97, 165–172. [Google Scholar] [CrossRef]
- Esmaeli, M.; Dehabadi, M.D.; Khaleghi, A.A. Cannabidiol as a novel therapeutic agent in breast cancer: Evidence from literature. BMC Cancer 2025, 25, 772. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.I.; Nguyen, L.C.; Oumeslakht, L.; Bensussan, A.; Ben Mkaddem, S. Cannabinoids as Immune System Modulators: Cannabidiol Potential Therapeutic Approaches and Limitations. Cannabis Cannabinoid Res. 2023, 8, 254–269. [Google Scholar] [CrossRef] [PubMed]
- Navarro, G.; Varani, K.; Lillo, A.; Vincenzi, F.; Rivas-Santisteban, R.; Raïch, I.; Reyes-Resina, I.; Ferreiro-Vera, C.; Borea, P.A.; Sánchez de Medina, V.; et al. Pharmacological data of cannabidiol- and cannabigerol-type phytocannabinoids acting on cannabinoid CB1, CB2 and CB1/CB2 heteromer receptors. Pharmacol Res. 2020, 159, 104940. [Google Scholar] [CrossRef] [PubMed]
- Khalid, S.; Almalki, F.A.; Hadda, T.B.; Bader, A.; Abu-Izneid, T.; Berredjem, M.; Elsharkawy, E.R.; Alqahtani, A.M. Medicinal Applications of Cannabinoids Extracted from Cannabis sativa (L.): A New Route in the Fight Against COVID-19? Curr. Pharm. Des. 2021, 27, 1564–1578. [Google Scholar] [CrossRef]
- Müller, C.E. Fortschritte in der Cannabis-Forschung aus pharmazeutisch-chemischer Sicht [Progress in cannabis research from a pharmaceutical chemist’s point of view]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2019, 62, 818–824. (In German) [Google Scholar] [CrossRef]
- Hameed, M.; Prasad, S.; Jain, E.; Dogrul, B.N.; Al-Oleimat, A.; Pokhrel, B.; Chowdhury, S.; Co, E.L.; Mitra, S.; Quinonez, J.; et al. Medical Cannabis for Chronic Nonmalignant Pain Management. Curr. Pain Headache Rep. 2023, 27, 57–63. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the Art and New Challenges for Therapeutic Applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- de Souza, R.S.; Takahashi, R.N.; Corrêa, F.M.A. Cannabinoids in Migraine: A Review of Clinical Evidence. Front. Neurol. 2022, 13, 871187. [Google Scholar] [CrossRef]
- Martynowicz, H.; Smardz, J.; Michalek-Zrabkowska, M.; Mazur, G.; Wojakowska, A. Correlation Between Sleep Bruxism and Headache Impact Test (HIT-6) Scores Among Adults. Front. Neurol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Walczyńska-Dragon, K.; Fiegler-Rudol, J.; Nitecka-Buchta, A.; Baron, S. Cannabidiol for Orofacial and Upper-Quarter Pain: A Systematic Evaluation of Therapeutic Potential. J. Clin Med. 2025, 14, 4186. [Google Scholar] [CrossRef]
- Arantes, A.L.F.; Carvalho, M.C.; Brandão, M.L.; Prado, W.A.; Crippa, J.A.; de Souza Crippa, J.A.; Lovick, T.A.; Genaro, K. Antinociceptive Action of Cannabidiol on Thermal Sensitivity and Post-Operative Pain in Male and Female Rats. Behav. Brain Res. 2024, 459, 114793. [Google Scholar] [CrossRef]
- Sepulveda, D.E.; Morris, D.P.; Raup-Konsavage, W.M.; Sun, D.; Vrana, K.E.; Graziane, N.M. Evaluating the Antinociceptive Efficacy of Cannabidiol Alone or in Combination with Morphine Using the Formalin Test in Male and Female Mice. Cannabis Cannabinoid Res. 2022, 7, 648–657. [Google Scholar] [CrossRef]
- Vivanco-Estela, A.N.; dos-Santos-Pereira, M.; Guimaraes, F.S.; Del-Bel, E.; Nascimento, G.C. do Cannabidiol Has Therapeutic Potential for Myofascial Pain in Female and Male Parkinsonian Rats. Neuropharmacology 2021, 196, 108700. [Google Scholar] [CrossRef]
- ISO 15378:2018; Primary Packaging Materials for Medicinal Products. International Organization for Standardization: Geneva, Switzerland, 2018.
| Characteristic | Group 1a (10% CBD) (n = 20) | Group 1b (5% CBD) (n = 20) | Group 2 (Placebo) (n = 20) | Total (n = 60) |
|---|---|---|---|---|
| Age, years | 24.2 | 24.0 | 23.4 | 23.87 |
| Sex (%) | ||||
| Female | 13 (65%) | 11 (55%) | 12 (60%) | 36 (60%) |
| Male | 7 (35%) | 9 (45%) | 8 (40%) | 24 (40%) |
| VAS score (mean, SD) | ||||
| Day 0 | 6.0 (2.0) | 6.0 (2.0) | 6.0 (3.5) | 6.0 (3.0) |
| Day 14 | 4.0 (2.0) | 4.5 (3.0) | 5.0 (2.5) | 4.0 (2.0) |
| Day 30 | 2.0 (3.0) | 3.5 (2.0) | 5.5 (3.0) | 4.0 (3.0) |
| Group |
Baseline (Median [IQR]) |
Day 14 (Median [IQR]) |
Day 30 (Median [IQR]) |
|---|---|---|---|
| 10% CBD | 12.0 [9.0–14.0] | 10.0 [8.0–12.0] | 7.0 [6.0–9.0] |
| 5% CBD | 12.0 [9.0–14.0] | 11.0 [9.0–13.0] | 8.0 [6.0–10.0] |
| Control | 9.0 [6.0–11.0] | 9.0 [6.0–12.0] | 9.0 [6.0–11.0] |
| Comparison | Mean Rank Difference | Standard Error | Standardized Test Statistic | p-Value |
|---|---|---|---|---|
| Control Group vs. 5% CBD | −12.829 | 4.972 | −2.58 | 0.01 |
| Control Group vs. 10% CBD | −19.842 | 4.767 | −4.162 | <0.001 |
| 5% CBD vs. 10% CBD | −7.013 | 5.289 | −1.326 | 0.185 |
| Group | Baseline (Median [IQR]) | Day 30 (Median [IQR]) |
|---|---|---|
| 10% CBD | 17.0 [12.0–22.0] | 8.0 [6.0–10.0] |
| 5% CBD | 15.0 [10.0–19.0] | 10.0 [7.0–13.0] |
| Control | 12.0 [8.0–15.0] | 12.0 [8.0–15.0] |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walczyńska-Dragon, K.; Fiegler-Rudol, J.; Baron, S.; Nitecka-Buchta, A. Expanding the Therapeutic Profile of Topical Cannabidiol in Temporomandibular Disorders: Effects on Sleep Quality and Migraine Disability in Patients with Bruxism-Associated Muscle Pain. Pharmaceuticals 2025, 18, 1064. https://doi.org/10.3390/ph18071064
Walczyńska-Dragon K, Fiegler-Rudol J, Baron S, Nitecka-Buchta A. Expanding the Therapeutic Profile of Topical Cannabidiol in Temporomandibular Disorders: Effects on Sleep Quality and Migraine Disability in Patients with Bruxism-Associated Muscle Pain. Pharmaceuticals. 2025; 18(7):1064. https://doi.org/10.3390/ph18071064
Chicago/Turabian StyleWalczyńska-Dragon, Karolina, Jakub Fiegler-Rudol, Stefan Baron, and Aleksandra Nitecka-Buchta. 2025. "Expanding the Therapeutic Profile of Topical Cannabidiol in Temporomandibular Disorders: Effects on Sleep Quality and Migraine Disability in Patients with Bruxism-Associated Muscle Pain" Pharmaceuticals 18, no. 7: 1064. https://doi.org/10.3390/ph18071064
APA StyleWalczyńska-Dragon, K., Fiegler-Rudol, J., Baron, S., & Nitecka-Buchta, A. (2025). Expanding the Therapeutic Profile of Topical Cannabidiol in Temporomandibular Disorders: Effects on Sleep Quality and Migraine Disability in Patients with Bruxism-Associated Muscle Pain. Pharmaceuticals, 18(7), 1064. https://doi.org/10.3390/ph18071064







