Integrating Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Inonotus obliquus Polysaccharide in the Treatment of Rheumatoid Arthritis
Abstract
1. Introduction
2. Results
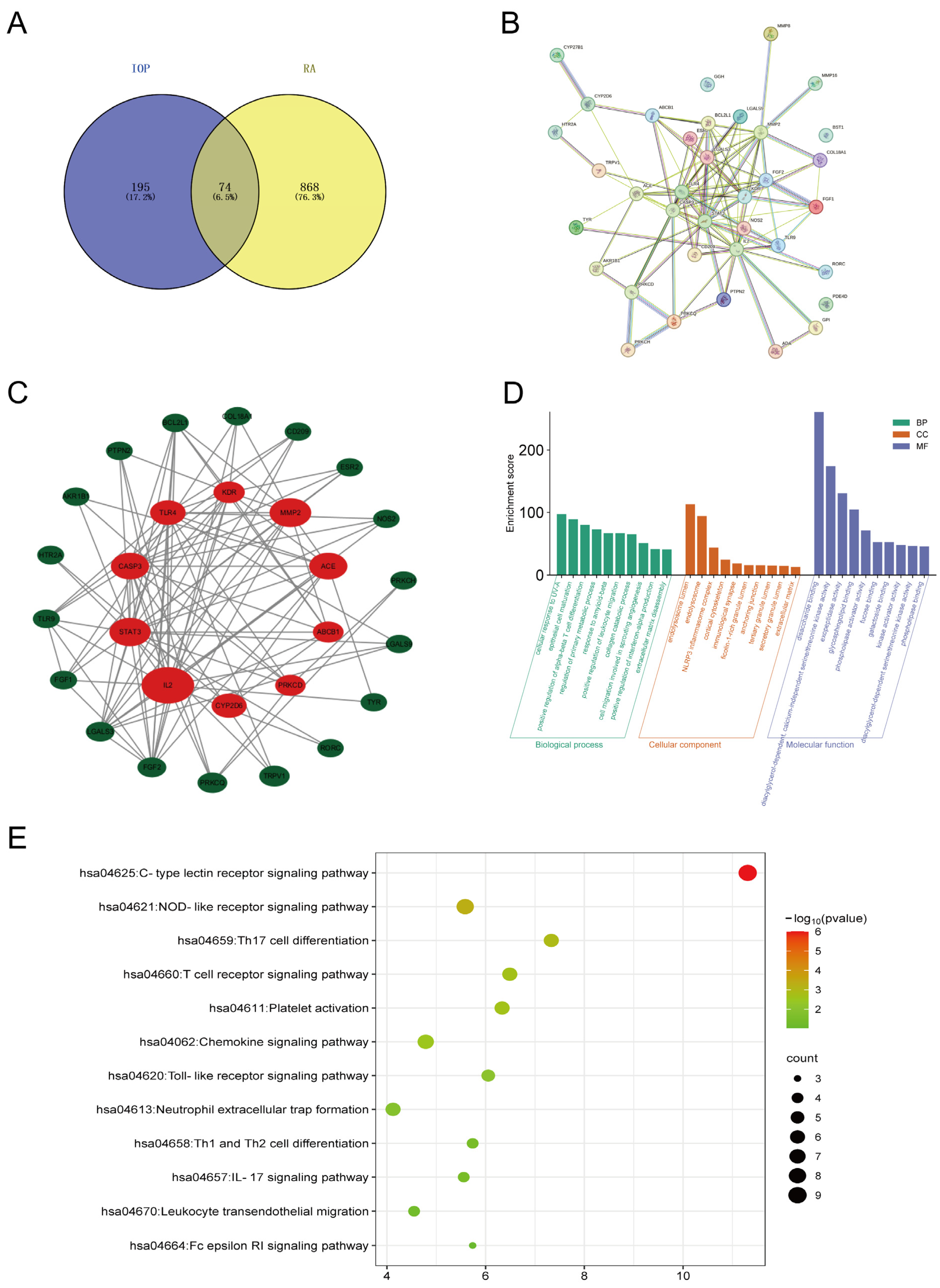
2.1. Key Targets and Pathways of IOP in RA Identified by Network Pharmacology
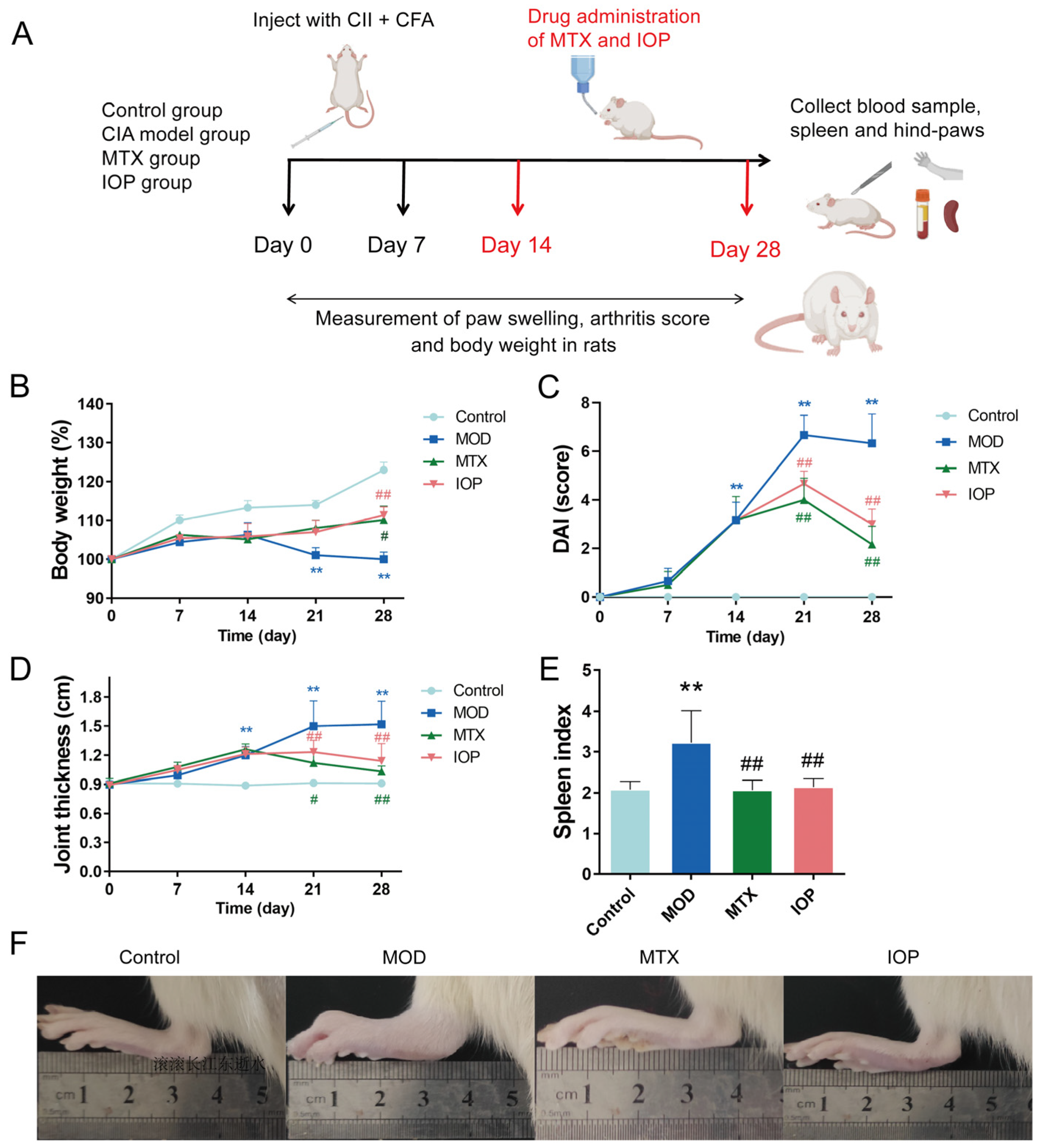
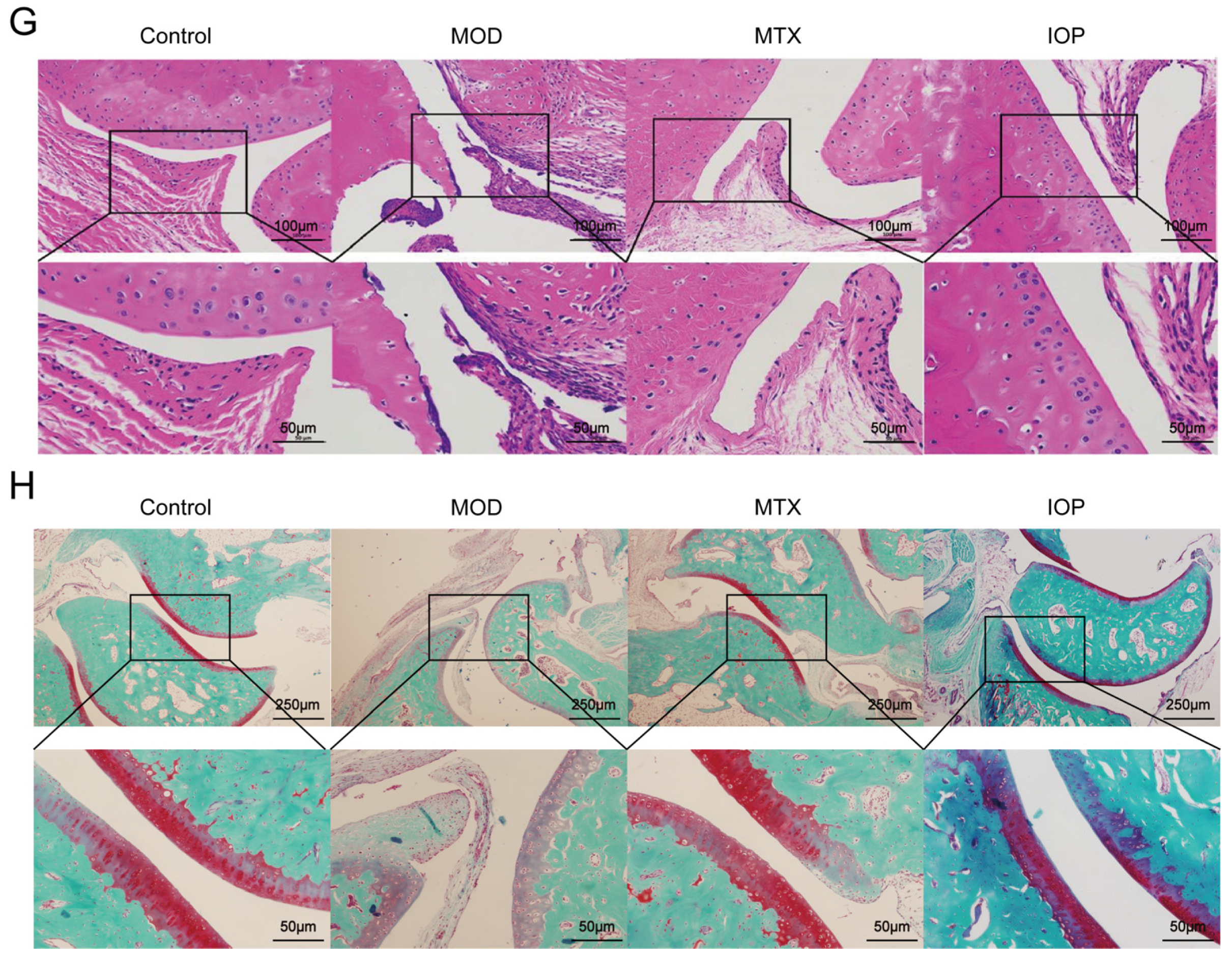
2.2. IOP Alleviates the Severity of Arthritis in Rats with CIA
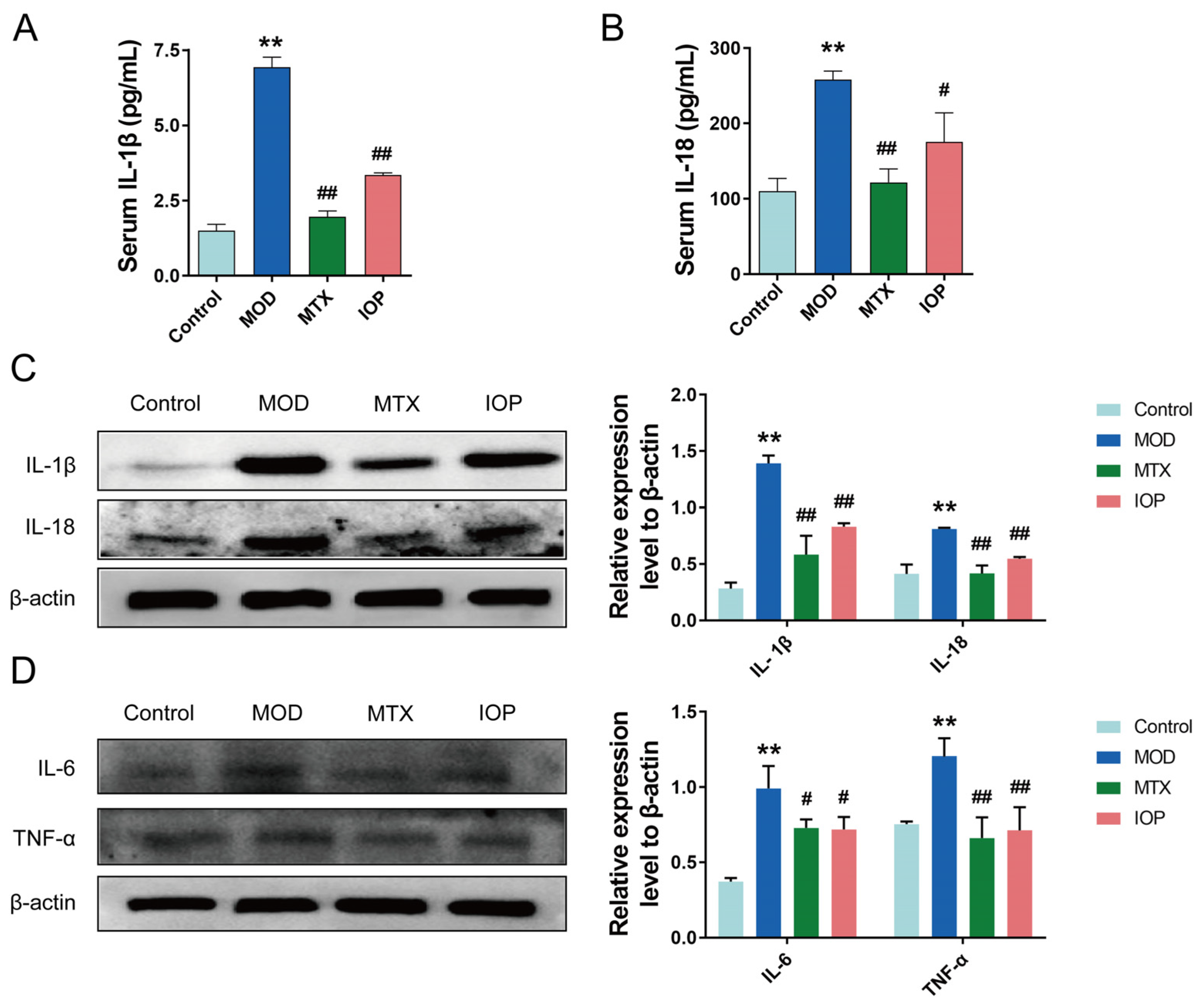
2.3. IOP Inhibits the Expression of Inflammatory Mediators in CIA Rats
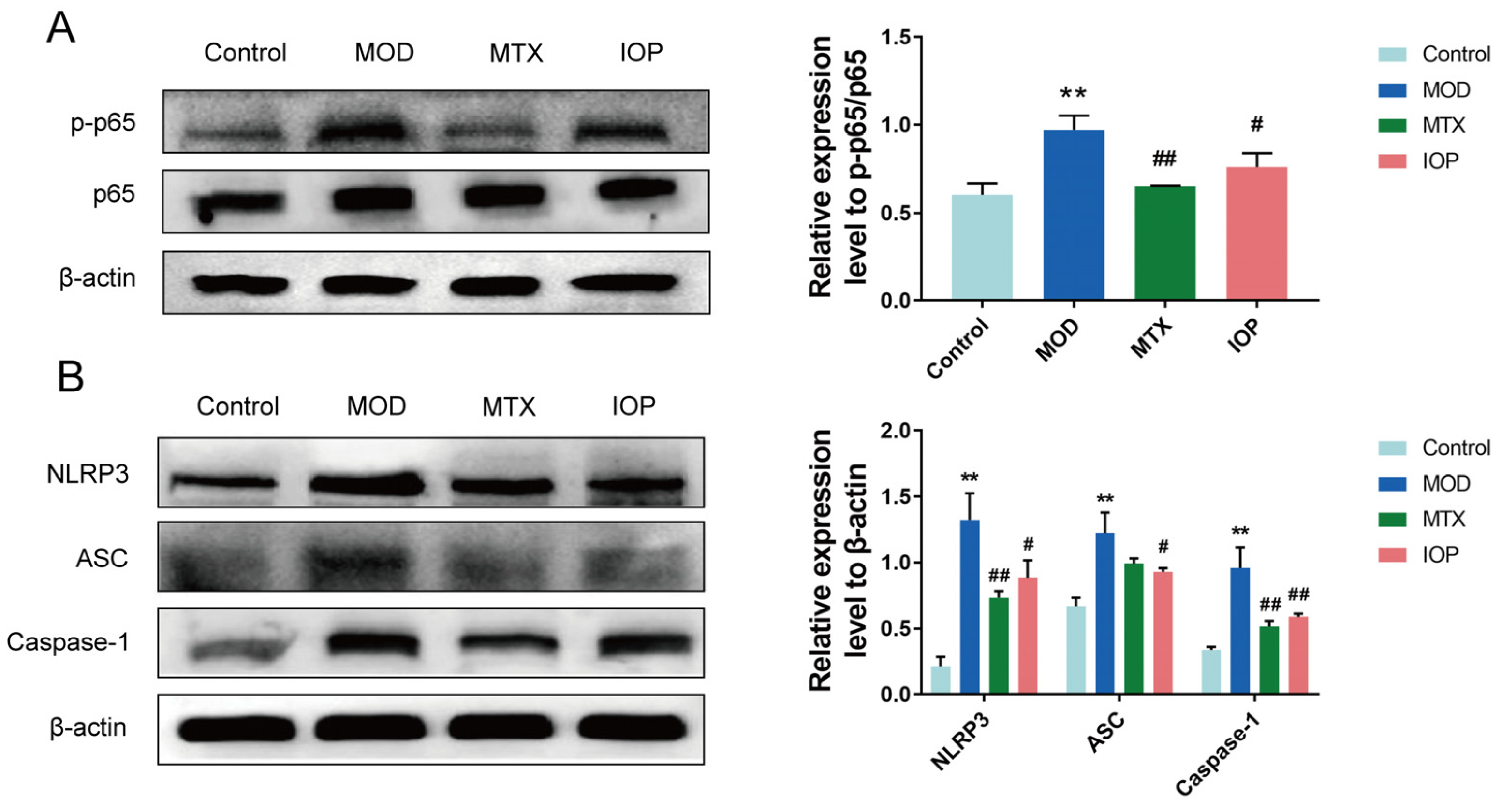
2.4. IOP Inhibits NF-κB and NLRP3 Inflammasome Signaling Pathways in CIA Rats
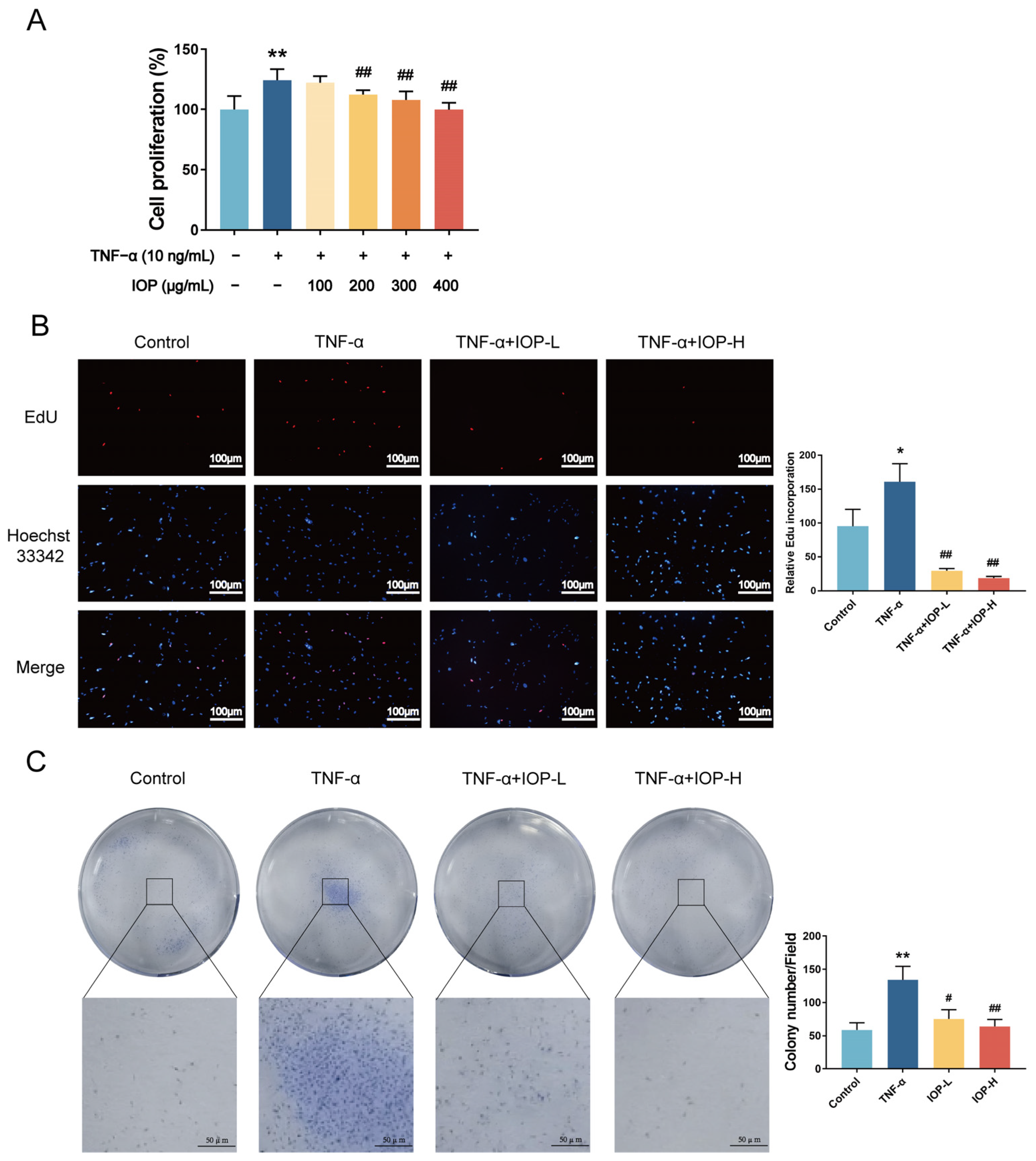
2.5. IOP Affects Cell Viability and Proliferation of MH7A Cells
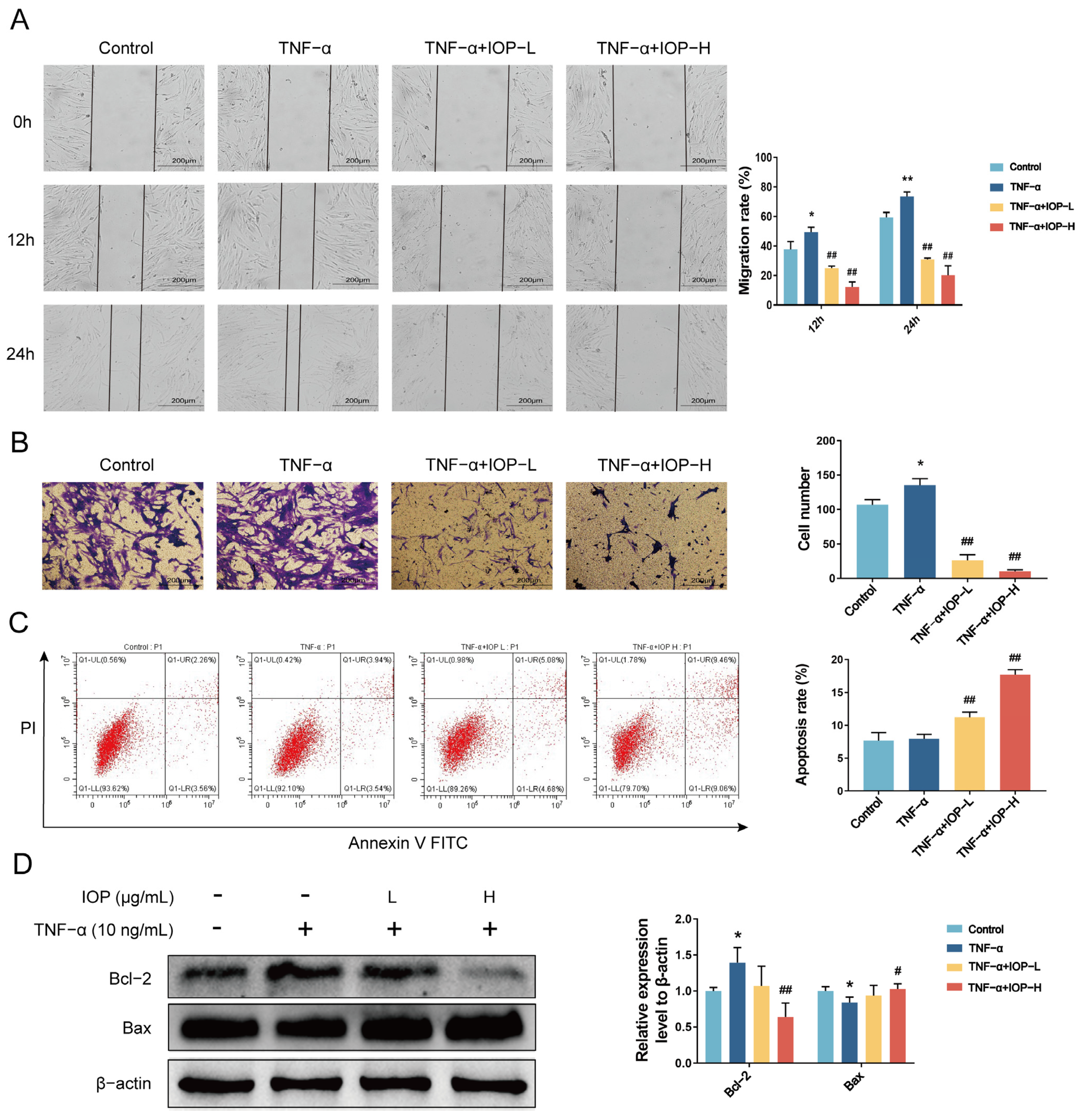
2.6. IOP Inhibits Migration, Invasion and Induces Apoptosis of MH7A Cells
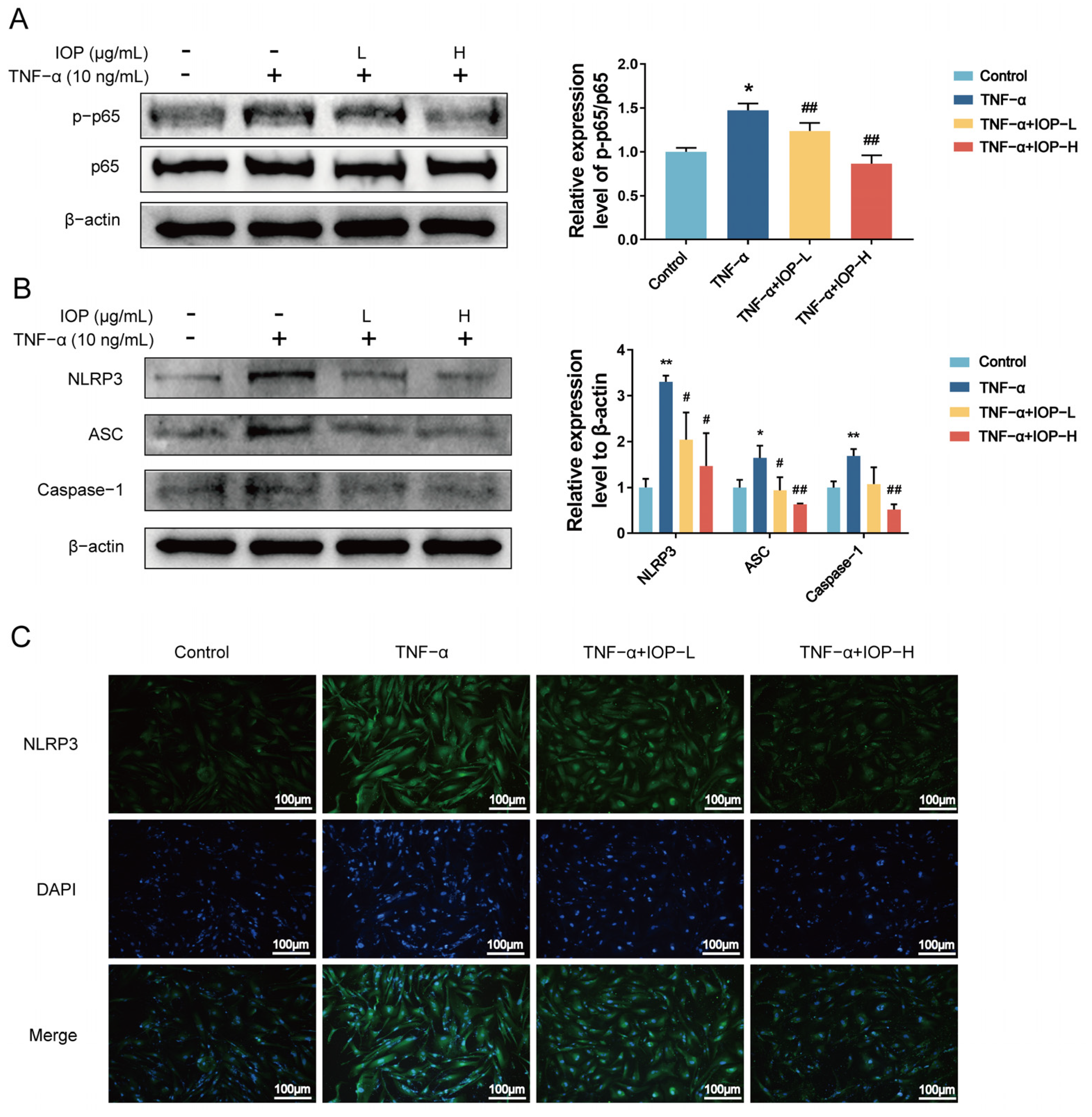
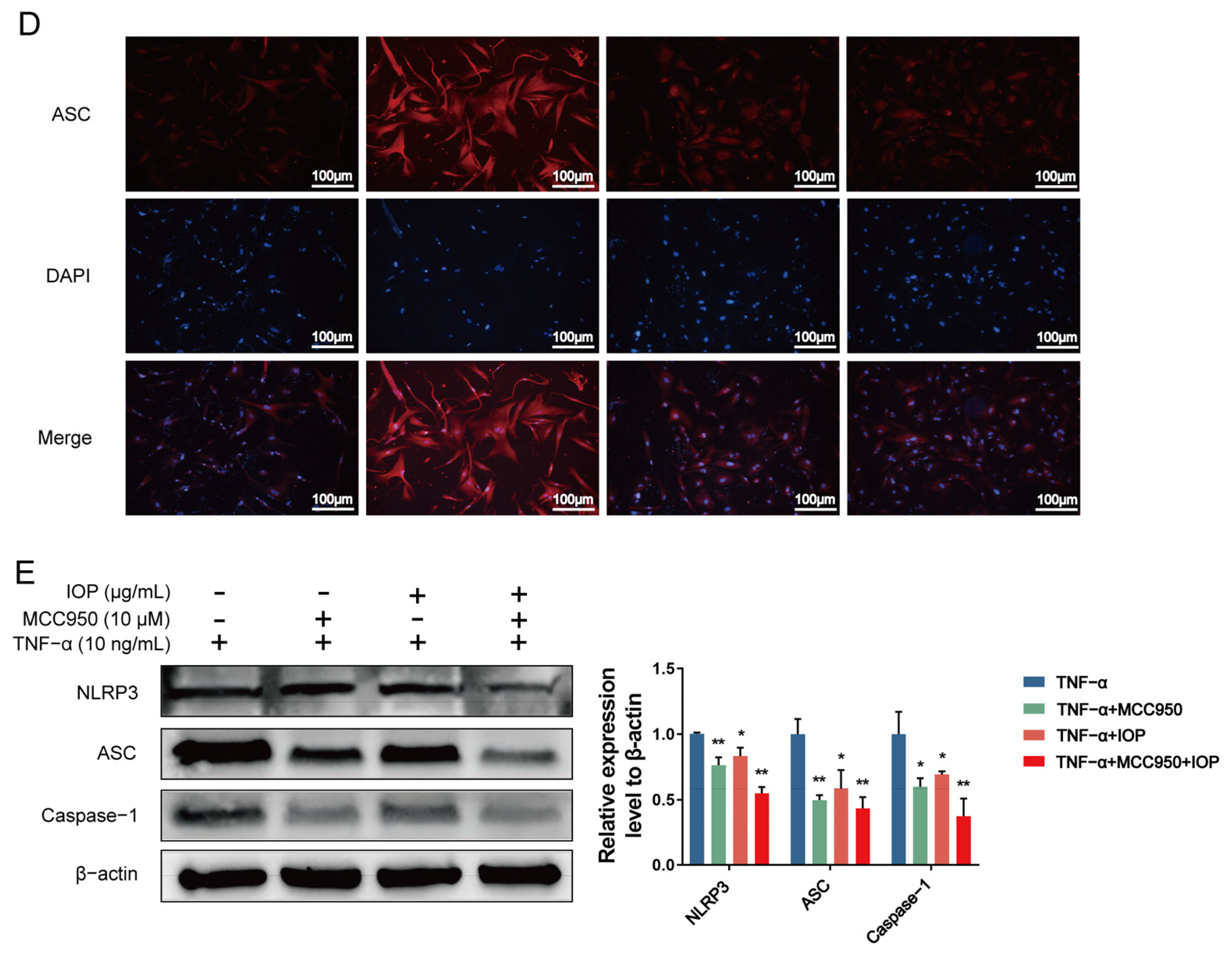
2.7. IOP Inhibits NF-κB and NLRP3 Inflammasome Signaling Pathways in MH7A Cells
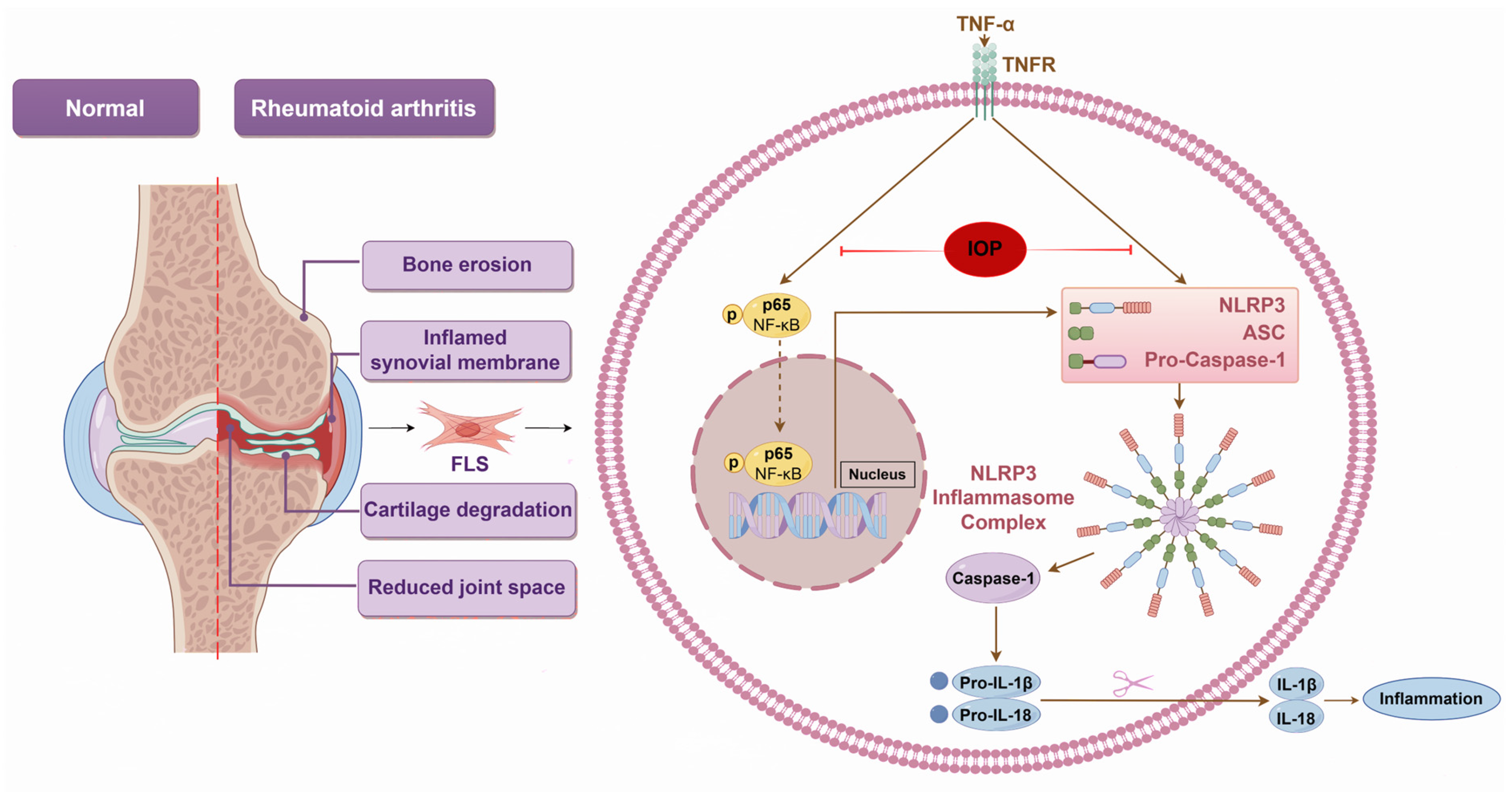
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of IOP
4.3. Target Prediction and Network Pharmacology Analysis
4.4. Cell Culture
4.5. Establishment of CIA Model and Treatment of Drugs
4.6. Histological Analysis
4.7. ELISA Assay
4.8. Cell Counting Kit-8 (CCK-8) Assay
4.9. 5-Ethynyl-2′-Deoxyuridine Incorporation Assay
4.10. Colony Formation Assay
4.11. Migration and Invasion Assay
4.12. Apoptosis Assay
4.13. Western Blot Analysis
4.14. Immunofluorescence
4.15. Statistical Analysis
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | biological processes |
| CC | cellular components |
| CIA | collagen-induced arthritis |
| DAVID | database for annotation, visualization, and integrated discovery |
| DL | drug-likeness |
| DMARDs | disease-modifying antirheumatic drugs |
| DMEM | Dulbecco’s Modified Eagle’s Medium |
| DSS | dextran sulfate sodium |
| EdU | 5-ethynyl-2′-deoxyuridine |
| FBS | fetal bovine serum |
| GC | glucocorticoids |
| GO | Gene ontology |
| H&E | hematoxylin–eosin |
| IL | interleukin |
| iNOS | inducible nitric oxide synthase |
| IOP | Inonotus obliquus polysaccharide |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| Man | mannose |
| MF | molecular functions |
| MOD | model |
| MTX | methotrexate |
| NF-κB | nuclear factor kappa B |
| NLRP3 | NOD-like receptor family pyrin domain containing 3 |
| NOD | nucleotide-binding oligonucleotide domain |
| OB | oral absorption |
| PPI | protein–protein interaction |
| RA | Rheumatoid arthritis |
| SD | standard deviation |
| Th1 | T helper cell 1 |
| TNF-α | tumor necrosis factor-α |
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef] [PubMed]
- Bergstra, S.A.; Sepriano, A.; Kerschbaumer, A.; van der Heijde, D.; Caporali, R.; Edwards, C.J.; Verschueren, P.; de Souza, S.; Pope, J.E.; Takeuchi, T.; et al. Efficacy, duration of use and safety of glucocorticoids: A systematic literature review informing the 2022 update of the EULAR recommendations for the management of rheumatoid arthritis. Ann. Rheum. Dis. 2023, 82, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine networks in the pathogenesis of rheumatoid arthritis. Int. J. Mol. Sci. 2021, 22, 10922. [Google Scholar] [CrossRef]
- Liu, S.; Ma, H.X.; Zhang, H.X.; Deng, C.J.; Xin, P. Recent advances on signaling pathways and their inhibitors in rheumatoid arthritis. Clin. Immunol. 2021, 230, 108793. [Google Scholar] [CrossRef]
- Malhotra, H.; Garg, V.; Singh, G. Biomarker approach towards rheumatoid arthritis treatment. Curr. Rheumatol. Rev. 2021, 17, 162–175. [Google Scholar] [CrossRef]
- Radu, A.; Bungau, S.G. Management of rheumatoid arthritis: An overview. Cells 2021, 10, 2857. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by ssteoclasts. Science. 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.Y.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Sun, S. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 2017, 17, 545–558. [Google Scholar] [CrossRef]
- Jimi, E.; Fei, H.; Nakatomi, C. NF-κB signaling regulates physiological and pathological chondrogenesis. Int. J. Mol. Sci. 2019, 20, 6275. [Google Scholar] [CrossRef]
- Zhang, J.L.; Wei, K.H. Necrosulfonamide reverses pyroptosis-induced inhibition of proliferation and differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD pathway. Exp. Cell Res. 2021, 405, 112648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, M.; Niu, W.T.; Mao, H.J.; Yang, P.; Xu, B.B.; Sun, Y.H. Tricalcium phosphate particles promote pyroptotic death of calvaria osteocytes through the ROS/NLRP3/Caspase-1 signaling axis in amouse osteolysis model. Int. Immunopharmacol. 2022, 107, 108699. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Huang, Y.R.; Cui, Z.M.; Liu, J.J. Purification, characterization and biological activity of a novel polysaccharide from Inonotus obliquus. Int. J. Biol. Macromol. 2015, 79, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.P.; Jia, Y.N.; Xue, Z.H.; Li, N.N.; Liu, J.Y.; Chen, H.X. Recent developments in Inonotus obliquus (Chaga mushroom) polysaccharides: Isolation, structural characteristics, biological activities and application. Polymers 2021, 13, 1441. [Google Scholar] [CrossRef]
- Jiang, S.P.; Shi, F.L.; Lin, H.; Ying, Y.; Luo, L.Y.; Huang, D.Q.; Luo, Z.J. Inonotus obliquus polysaccharides induces apoptosis of lung cancer cells and alters energy metabolism via the LKB1/AMPK axis. Int. J. Mol. Sci. 2020, 151, 1277–1286. [Google Scholar] [CrossRef]
- Li, J.W.; Qu, C.; Li, F.F.; Chen, Y.F.; Zheng, J.J.; Xiao, Y.; Jin, Q.X.; Jin, G.H.; Huang, X.Z.; Jin, D. Inonotus obliquus polysaccharide ameliorates azoxymethane/dextran sulfate sodium-induced colitis-associated cancer in mice via activation of the NLRP3 inflammasome. Front. Pharmacol. 2021, 11, 621835. [Google Scholar] [CrossRef]
- Mishra, S.K.; Kang, J.; Kim, D.; Oh, S.H.; Kim, M.K. Orally administered aqueous extract of Inonotus obliquus ameliorates acute inflammation in dextran sulfate sodium (DSS)-induced colitis in mice. J. Ethnopharmacol. 2012, 143, 524–532. [Google Scholar] [CrossRef]
- Hu, Y.; Sheng, Y.; Yu, M.; Li, K.K.; Ren, G.M.; Xu, X.H.; Qu, J.J. Antioxidant activity of Inonotus obliquus polysaccharide and its amelioration for chronic pancreatitis in mice. Int. J. Mol. Sci. 2016, 87, 348–356. [Google Scholar] [CrossRef]
- Han, Y.Q.; Nan, S.J.; Fan, J.; Chen, Q.H.; Zhang, Y.Z. Inonotus obliquus polysaccharides protect against Alzheimer’s disease by regulating Nrf2 signaling and exerting antioxidative and antiapoptotic effects. Int. J. Mol. Sci. 2019, 131, 769–778. [Google Scholar] [CrossRef]
- Park, J.; Ryu, J.H.; Kim, B.Y.; Chun, H.S.; Kim, M.S.; Shin, Y.I. Fermented lettuce extract containing nitric oxide metabolites attenuates inflammatory parameters in model mice and in human fibroblast-like synoviocytes. Nutrients 2023, 15, 1106. [Google Scholar] [CrossRef]
- Chen, Y.F.; Zheng, J.J.; Qu, C.; Xiao, Y.; Li, F.F.; Jin, Q.X.; Li, H.H.; Meng, F.P.; Jin, G.H.; Jin, D. Inonotus obliquus polysaccharide ameliorates dextran sulphate sodium induced colitis involving modulation of Th1/Th2 and Th17/Treg balance. Artif. Cells Nanomed. Biotechnol. 2019, 47, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.F.; Zheng, Y.; Li, H.B. NLRP3 inflammasome plays an important role in the pathogenesis of collagen-induced arthritis. Mediat. Inflamm. 2016, 2016, 9656270. [Google Scholar] [CrossRef] [PubMed]
- Greenhill, C.J.; Jones, G.W.; Nowell, M.A.; Newton, Z.; Harvey, A.K.; Moideen, A.N.; Collins, F.L.; Bloom, A.C.; Coll, R.C.; Robertson, A.A.; et al. Interleukin-10 regulates the inflammasome-driven augmentation of inflammatory arthritis and joint destruction. Arthritis Res. Ther. 2014, 16, 419–429. [Google Scholar] [CrossRef]
- Bartok, B.; Firestein, G.S. Fibroblast-like synoviocytes: Key effector cells in rheumatoid arthritis. Immunol. Rev. 2010, 233, 233–255. [Google Scholar] [CrossRef]
- Bottini, N.; Firestein, G.S. Duality of fibroblast-like synoviocytes in RA: Passive responders and imprinted aggressors. Nat. Rev. Rheumatol. 2013, 9, 24–33. [Google Scholar] [CrossRef]
- Bao, Y.G.; Sun, Y.W.; Ji, J.; Gan, L.; Zhang, C.F.; Wang, C.Z.; Yuan, C.S. Genkwanin ameliorates adjuvant-induced arthritis in rats through inhibiting JAK/STAT and NF-κB signaling pathways. Phytomedicine 2019, 63, 153036. [Google Scholar] [CrossRef]
- Goode, K.M.; Petrov, D.P.; Vickman, R.E.; Crist, S.A.; Pascuzzi, P.E.; Ratliff, T.L.; Davisson, V.J.; Hazbun, T.R. Targeting the Hsp90 C-terminal domain to induce allosteric inhibition and selective client downregulation. Biochim. Biophys. Acta (BBA) Gen. Subj. 2017, 1861, 1992–2006. [Google Scholar] [CrossRef]
- Zheng, C.J.; Zhao, X.X.; Ai, H.W.; Lin, B.; Han, T.; Jiang, Y.P.; Xing, X.; Qin, L.P. Therapeutic effects of standardized Vitex negundo seeds extract on complete Freund’s adjuvant induced arthritis in rats. Phytomedicine 2014, 21, 838–846. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bergstra, S.A.; Kerschbaumer, A.; Sepriano, A.; Aletaha, D.; Caporali, R.; Edwards, C.J.; Hyrich, K.L.; Pope, J.E.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann. Rheum. Dis. 2023, 82, 3–18. [Google Scholar] [CrossRef]
- Xu, L.; Yu, Y.F.; Sang, R.; Ge, B.J.; Wang, M.; Zhou, H.Y.; Zhang, X.M. Inonotus obliquus polysaccharide protects against adverse pregnancy caused by Toxoplasma gondii infection through regulating Th17/Treg balance via TLR4/NF-κB pathway. Int. J. Biol. Macromol. 2020, 146, 832–840. [Google Scholar] [CrossRef]
- Yan, K.X.; Zhou, H.Y.; Wang, M.; Li, H.T.; Sang, R.; Ge, B.J.; Zhao, X.; Li, C.T.; Wang, W.; Zhang, X.M. Inhibitory effects of Inonotus obliquus polysaccharide on inflammatory response in Toxoplasma gondii-infected RAW264.7 macrophages. Evid-Based Compl. Alt. 2021, 2021, 2245496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.Q.; Cheng, S.Y.; Liang, J.S.; Qu, J.J. Polysaccharide from fermented mycelium of Inonotus obliquus attenuates the ulcerative colitis and adjusts the gut microbiota in mice. Microb. Pathogenesis 2023, 177, 105990. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.Y.; Xie, S.Z.; Min, P.F.; Li, H.; Zhao, F.L.; Liu, M.; Jin, W.D.; Wang, L.S.; Zhao, J.H.; Jia, L.J. Protective effect of Inonotus obliquus polysaccharide on mice infected with Neospora caninum. Int. J. Biol. Macromol. 2024, 261, 129906. [Google Scholar] [CrossRef]
- Li, D.Y.; Wu, M.H. Pattern recognition receptors in health and diseases. Signal Transduct. Target. 2021, 6, 291–315. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, L.; Ma, R.; Wang, M.; He, J.; Wang, P.; Tao, Q.; Xu, Y. Jolkinolide B ameliorates rheumatoid arthritis by regulating the JAK2/STAT3 signaling pathway. Phytomedicine 2024, 124, 155311. [Google Scholar] [CrossRef]
- Ge, G.R.; Bai, J.X.; Wang, Q.; Liang, X.L.; Tao, H.Q.; Chen, H.; Wei, M.G.; Niu, J.J.; Yang, H.L.; Xu, Y.Z.; et al. Punicalagin ameliorates collagen-induced arthritis by downregulating M1 macrophage and pyroptosis via NF-κB signaling pathway. Sci. China Life Sci. 2022, 65, 588–603. [Google Scholar] [CrossRef]
- Cai, L.; Zong, P.; Zhou, M.Y.; Liu, F.Y.; Meng, B.; Liu, M.M.; Li, Z.; Li, R. 7-Hydroxycoumarin mitigates the severity of collagen-induced arthritis in rats by inhibiting proliferation and inducing apoptosis of fibroblast-like synoviocytes via suppression of Wnt/β-catenin signaling pathway. Phytomedicine 2022, 94, 153841. [Google Scholar] [CrossRef]
- Shen, Y.; Teng, L.; Qu, Y.H.; Liu, J.; Zhu, X.D.; Chen, S.; Yang, L.F.; Huang, Y.H.; Song, Q.; Fu, Q. Anti-proliferation and anti-inflammation effects of corilagin in rheumatoid arthritis by downregulating NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2022, 284, 114791. [Google Scholar] [CrossRef]
- Zhang, Q.; Peng, W.; Wei, S.J.; Wei, D.N.; Li, R.L.; Liu, J.; Peng, L.Y.; Yang, S.; Gao, Y.X.; Wu, C.J.; et al. Guizhi-Shaoyao-Zhimu decoction possesses anti-arthritic effects on type II collagen-induced arthritis in rats via suppression of inflammatory reactions, inhibition of invasion & migration and induction of apoptosis in synovial fibroblasts. Biomed. Pharmacother. 2019, 118, 109367. [Google Scholar] [CrossRef]
- Hong, R.; Sur, B.; Yeom, M.; Lee, B.; Kim, K.S.; Rodriguez, J.P.; Lee, S.; Kang, K.S.; Huh, C.K.; Lee, S.C.; et al. Anti-inflammatory and anti-arthritic effects of the ethanolic extract of Aralia continentalis Kitag. in IL-1β-stimulated human fibroblast-like synoviocytes and rodent models of polyarthritis and nociception. Phytomedicine 2018, 38, 45–56. [Google Scholar] [CrossRef]
- Haidar, O.; O’Neill, N.; Staunton, C.A.; Bavan, S.; O’Brien, F.; Zouggari, S.; Sharif, U.; Mobasheri, A.; Kumagai, K.; Barrett-Jolley, R. Pro-inflammatory cytokines drive deregulation of potassium channel expression in primary synovial fibroblasts. Front. Physiol. 2020, 11, 226. [Google Scholar] [CrossRef] [PubMed]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- McHugh, J. SUMOylation links metabolic and aggressive phenotype of RA FLS. Nat. Rev. Rheumatol. 2020, 16, 668. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xu, Q.; Liu, Q.P.; Pan, D.M.; Jiang, Y.B.; Liu, M.Y.; Liu, M.L.; Xu, H.S.; Lin, C.S. Leonurine attenuates fibroblast-like synoviocyte-mediated synovial inflammation and joint destruction in rheumatoid arthritis. Rheumatology 2017, 56, 1417–1427. [Google Scholar] [CrossRef]
- Shen, Y.; Fan, X.T.; Qu, Y.H.; Tang, M.; Huang, Y.H.; Peng, Y.; Fu, Q. Magnoflorine attenuates inflammatory responses in RA by regulating the PI3K/Akt/NF-κB and Keap1-Nrf2/HO-1 signalling pathways in vivo and in vitro. Phytomedicine 2022, 104, 154339. [Google Scholar] [CrossRef]
- Li, W.J.; Wang, K.X.; Liu, Y.D.; Wu, H.; He, Y.; Li, C.C.; Wang, Q.; Su, X.H.; Yan, S.K.; Su, W.W.; et al. A novel drug combination of mangiferin and cinnamic acid alleviates rheumatoid arthritis by inhibiting TLR4/NFκB/NLRP3 activation-induced pyroptosis. Front. Immunol. 2022, 13, 912933. [Google Scholar] [CrossRef]
- Fu, D.; O’Neill, R.A. Monosaccharide composition analysis of oligosaccharides and glycoproteins by high-performance liquid chromatography. Anal. Biochem. 1995, 227, 377–384. [Google Scholar] [CrossRef]
- Ru, J.L.; Li, P.; Wang, J.N.; Zhou, W.; Li, B.H.; Huang, C.; Li, P.D.; Guo, Z.H.; Tao, W.Y.; Yang, Y.F.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Yang, B.; Xu, Y.; Zhang, W.Y.; Zhu, D.D.; Huang, B.; Yang, Y.J.; Jia, X.B.; Feng, L. Oral absorption mechanisms of polysaccharides and potential as carriers for the construction of nano-delivery systems: A review. Int. J. Biol. Macromol. 2025, 310, 143184. [Google Scholar] [CrossRef]
- Wang, X.; Shen, Y.H.; Wang, S.W.; Li, S.L.; Zhang, W.L.; Liu, X.F.; Lai, L.H.; Pei, J.F.; Li, H.L. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.L.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W.Z. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.C.; Luo, H.T.; Huo, P.P.; Wang, Z.H.; Zhang, S.; He, Z.H.; Wu, Y.; Zhao, L.H.; Liu, J.J.; Guo, J.C.; et al. KOBAS-i: Intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 2021, 49, W317–W325. [Google Scholar] [CrossRef]
- Li, J.W.; Li, F.F.; Zhao, Y.Q.; Jin, D. Integrating network pharmacology and experimental validation to explore the effect and mechanism of AD-1 in the treatment of colorectal cancer. Front. Pharmacol. 2023, 14, 1159712. [Google Scholar] [CrossRef]
- Choudhary, N.; Bhatt, L.K.; Prabhavalkar, K.S. Experimental animal models for rheumatoid arthritis. Immunopharm. Immunot. 2018, 40, 193–200. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, Y.; Lao, M.; Wang, J.; Xu, S.; Li, R.; Xu, X.; Kuang, Y.; Shi, M.; Zou, Y.; et al. Increased SUMO-activating enzyme SAE1/UBA2 promotes glycolysis and pathogenic behavior of rheumatoid fibroblast-like synoviocytes. JCI Insight 2020, 5, e135935. [Google Scholar] [CrossRef]
- Joosten, L.A.; Lubberts, E.; Durez, P.; Helsen, M.M.; Jacobs, M.J.; Goldman, M.; van den Berg, W.B. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997, 40, 249–260. [Google Scholar] [CrossRef]
- Zhang, L.; Hao, M.; Yao, L.; Xing, C.; Wen, Q.; Zhang, Z.; Yu, J.; Wang, J.; Xing, D.; Zheng, T.; et al. Sericin “hairpin structure”-based multifunctional anthocyanin nanoencapsulation for remodeling ROS-dependent cutaneous wound healing. Chem. Eng. J. 2023, 475, 145863. [Google Scholar] [CrossRef]
- Kong, J.L.; Zou, R.Z.; Law, G.L.; Wang, Y. Biomimetic multifunctional persistent luminescence nanoprobes for long-term near-infrared imaging and therapy of cerebral and cerebellar gliomas. Sci. Adv. 2022, 8, m7077. [Google Scholar] [CrossRef]
- Fang, X.Z.; Lee, Y.H.; Jang, J.H.; Kim, S.J.; Kim, S.H.; Kim, D.H.; Na, H.K.; Kim, K.O.; Baek, J.H.; Surh, Y.J. ARD1 stabilizes NRF2 through direct interaction and promotes colon cancer progression. Life Sci. 2023, 313, 121217. [Google Scholar] [CrossRef]
| Number | Gene Symbol | Uniprot ID | Gene Full Name |
|---|---|---|---|
| 1 | IL-2 | P60568 | Interleukin-2 |
| 2 | STAT3 | P40763 | Signal transducer and activator of transcription 3 |
| 3 | CASP3 | P42574 | Caspase-3 |
| 4 | TLR4 | O00206 | Toll-like receptor 4 |
| 5 | KDR | P35968 | Kinase insert domain receptor |
| 6 | MMP2 | P08253 | Matrix metalloproteinase-2 |
| 7 | ACE | P12821 | Angiotensin-converting enzyme |
| 8 | ABCB1 | P08183 | ATP-binding cassette sub-family B member 1 |
| 9 | PRKCD | Q05655 | Protein kinase C delta type |
| 10 | CYP2D6 | P10635 | Cytochrome P450 2D6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, Y.; Jiang, T.; Fang, X.; Chen, Y.; Li, J.; Huang, S.; Li, F.; Jin, D. Integrating Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Inonotus obliquus Polysaccharide in the Treatment of Rheumatoid Arthritis. Pharmaceuticals 2025, 18, 1017. https://doi.org/10.3390/ph18071017
Fu Y, Jiang T, Fang X, Chen Y, Li J, Huang S, Li F, Jin D. Integrating Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Inonotus obliquus Polysaccharide in the Treatment of Rheumatoid Arthritis. Pharmaceuticals. 2025; 18(7):1017. https://doi.org/10.3390/ph18071017
Chicago/Turabian StyleFu, Yuan, Tianyi Jiang, Xizhu Fang, Yifang Chen, Jiawei Li, Shengnan Huang, Fangfang Li, and Dan Jin. 2025. "Integrating Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Inonotus obliquus Polysaccharide in the Treatment of Rheumatoid Arthritis" Pharmaceuticals 18, no. 7: 1017. https://doi.org/10.3390/ph18071017
APA StyleFu, Y., Jiang, T., Fang, X., Chen, Y., Li, J., Huang, S., Li, F., & Jin, D. (2025). Integrating Network Pharmacology and Experimental Validation to Explore the Effect and Mechanism of Inonotus obliquus Polysaccharide in the Treatment of Rheumatoid Arthritis. Pharmaceuticals, 18(7), 1017. https://doi.org/10.3390/ph18071017






