2.1. Phytochemical Mapping of Common Vervain
The yields of extracts, calculated as percentage of dry weight, were as follows: B1—9.38%; BE7.5—7.56%; BE5—11.23%; and BE2.5—6.83%. The content of total phenolics and flavonoids in the aerial parts of four
Verbena officinalis extracts is summarized in
Table 1. Generally, the highest amount of total phenolics and total flavonoids was noted in the BE5 extract, while the lowest content was discovered in the BE7.5 extract. Butanol fractions of
V. officinalis have been previously described as rich in secondary metabolites, especially phenolic compounds [
17]. The results presented in
Table 1 indicate that the combination of two solvents in a 50:50 ratio (BE5) is the most efficient method for the isolation of phenolic compounds.
Chemical analysis of bioactive compounds extracted from
Verbena officinalis, conducted using the high-resolution LC/MS technique, led to the identification of 75 compounds. Metabolite identification was achieved through the study of the exact mass of the compounds in a full-scan experiment, as well as MS
2 fragmentation at high resolution. The structures of the compounds were proposed based on a comprehensive review of the literature on
Verbena metabolites, where available. Base peak chromatograms of all four different extracts are depicted in
Figure S1, and there it can be seen that the most abundant peaks are hastatoside and verbenalin, whose molecular ions in our experiment appear as formic acid adducts.
Table S1 lists the identified compounds, together with retention times and key mass spectrometry attributes (exact mass and MS
2 fragmentation), divided into several groups according to their basic chemical structures: benzoic acid derivatives (12 compounds), cinnamic acid derivatives (10 compounds), iridoid glycosides (8 compounds), phenylethanoids (8 compounds), flavonoid C-glycosides (3 compounds), flavonoid O-glycosides (14 compounds), flavonoid aglycones (14 compounds), and xanthones (6 compounds). Notably, the majority of detected metabolites belong to the groups of iridoid glycosides, followed by flavonoid glycosides and aglycones. These findings are in line with previous reports but also provide additional insight into the chemical complexity of this species.
Among the most relevant identified compounds were rosmarinic acid, verbascoside, verbenalin, and hastatoside, which are consistently reported as key constituents of
V. officinalis in earlier studies [
13,
17,
18]. The presence of rosmarinic acid, a major antioxidant phenolic compound, supports findings by Rehecho et al. (2011) [
13], who associated this molecule with the high antioxidant capacity of the plant. Likewise, verbenalin and hastatoside, two bioactive iridoid glycosides with reported anti-inflammatory, sedative, and neuroprotective properties [
6,
19], were also confirmed in our profile.
In addition to known compounds, this analysis identified a wide range of flavonoid derivatives, including apigenin, luteolin, quercetin, and isorhamnetin, both in aglycone and glycosylated forms. These flavonoids have previously been associated with anti-inflammatory and neuroprotective activity [
6]. The detection of flavonoid C-glycosides (e.g., apigenin 6,8-di-C-hexoside) and acetylated/rhamnosylated forms (e.g., myricetin 3-O-(acetyl)-rhamnoside, quercetin 3-O-(acetyl)-rhamnoside) suggests a complex and diverse flavonoid metabolism that has not been equally emphasized in earlier studies.
Interestingly, several xanthone derivatives, including trihydroxy- and dihydroxy-methoxyxanthones, were identified. These compounds are less frequently reported in
V. officinalis, and their presence may represent chemotaxonomic markers or be indicative of environmental influences on secondary metabolite biosynthesis. Some compounds reported by El-Wakil et al. (2022) [
17], such as specific sesquiterpenes or non-polar components, were not detected in our LC-MS profile, likely due to methodological differences in detection sensitivity or polarity of the extraction solvents.
A set of heatmaps (
Figure 1,
Figure 2,
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8) was created to show the distribution of individual compounds across four solvent systems of increasing polarity, namely B1 (100% butanol), BE7.5 (75:25 butanol:ethanol), BE5 (50:50), and BE2.5 (25:75), in order to thoroughly assess the phytochemical diversity and extraction efficiency of
V. officinalis.
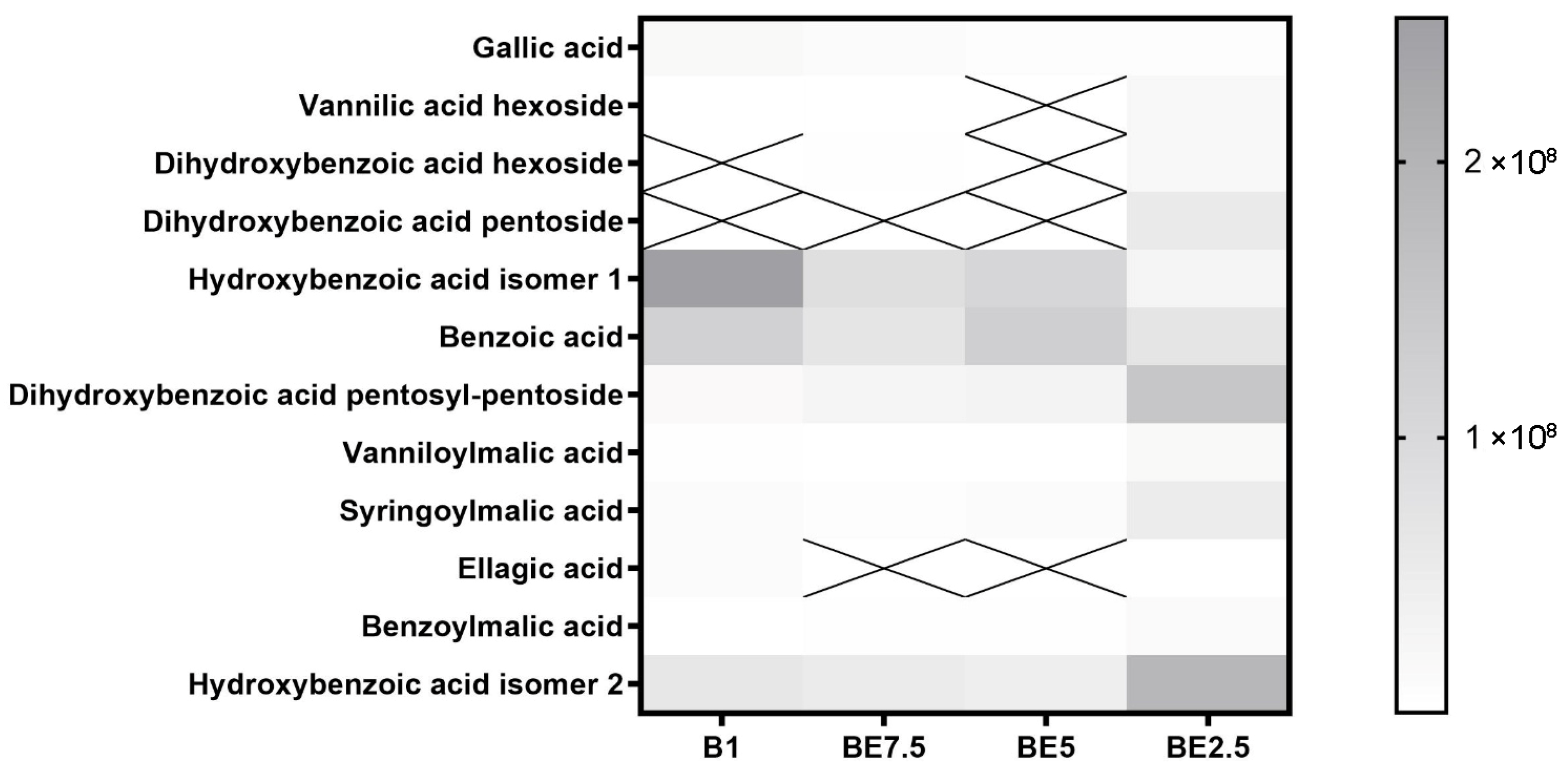
Different extraction patterns influenced by solvent polarity are shown in the heatmap of benzoic acid derivatives across four
V. officinalis extracts (
Figure 1). The most abundant and consistent compounds among those found are benzoic acid and hydroxybenzoic acid isomer 1, dihydroxybenzoic acid pentosyl-pentoside, especially in B1 and BE5 samples. This suggests that these relatively simple phenolic acids are easily extracted across a range of polarities, with a slight preference for mid-polar systems. Good solubility in ethanol-rich solutions is indicated by the moderate and uniform distribution of hydroxybenzoic acid isomer 2, and dihydroxybenzoic acid pentoside, vanillic acid hexoside, dihydroxybenzoic acid hexoside. Their increased polarity and affinity for ethanol-enriched solvents are reflected in their more pronounced extraction in BE2.5. All extracts include very low intensities of compounds, such as gallic acid, benzoylmalic acid, syringoylmalic acid, and vanilloylmalic acid, with no clear solvent preference. BE2.5 demonstrates the broadest and most balanced extraction capacity for benzoic acid derivatives, recovering both free acids and more polar glycosylated forms. These findings highlight the importance of tuning solvent polarity to target specific subgroups within the phenolic acid class and underscore the potential of mixed alcohol systems for comprehensive phytochemical recovery.
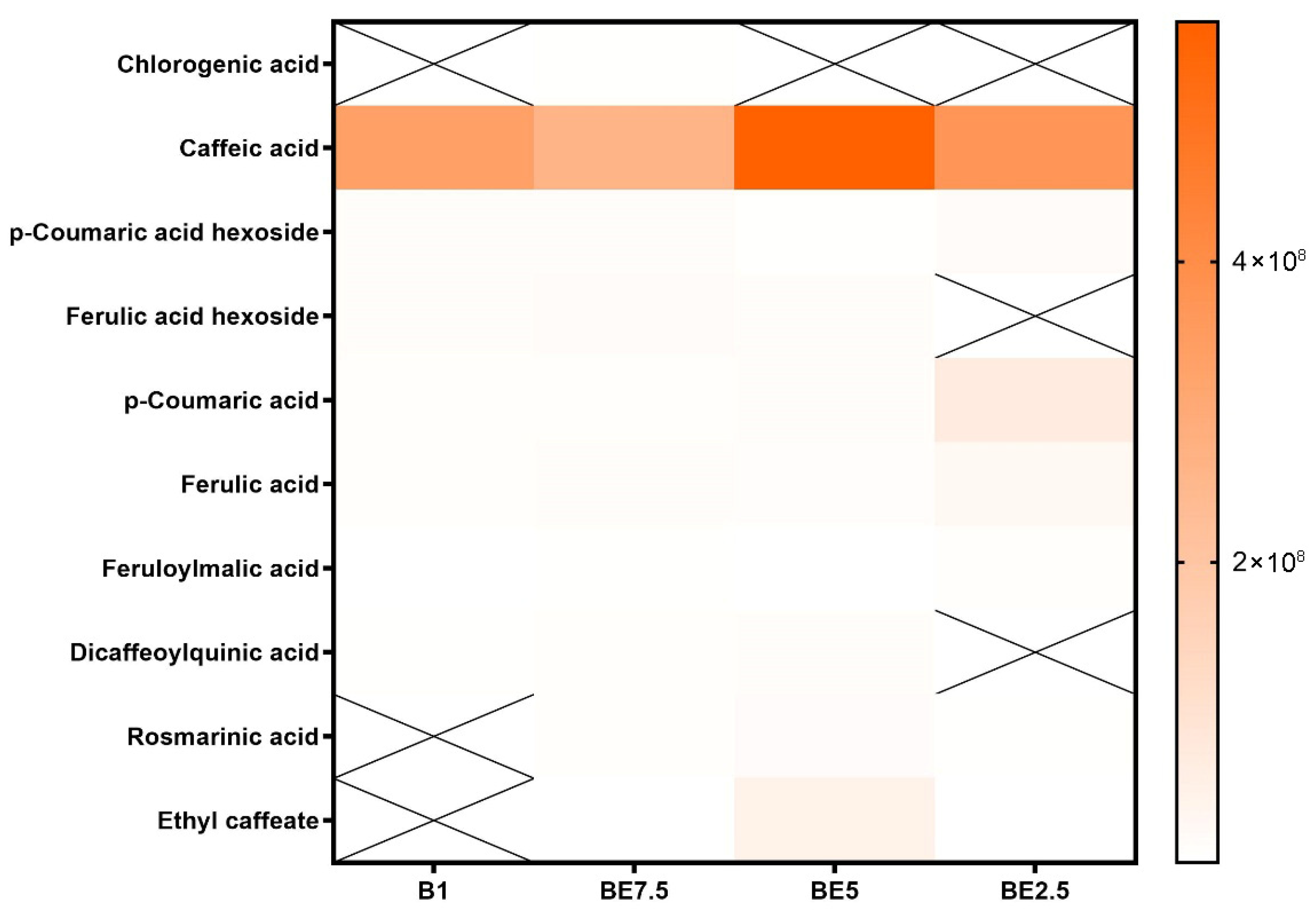
Caffeic acid is found in all four extracts and has the maximum intensity in BE5, followed by BE2.5 and B1, with the lowest signal in BE7.5, according to the heatmap of cinnamic acid derivatives (
Figure 2). This distinct polarity-dependent pattern shows that mid-ethanol solvent systems, especially the 50:50 butanol:ethanol mixture (BE5), which offers the highest extraction yield, are the most effective for extracting caffeic acid, a moderately polar hydroxycinnamic acid. The relevance of caffeic acid as a major phenolic element in extracts is shown by its widespread occurrence across samples. The majority of other derivatives of cinnamic acid, on the other hand, show very little or no detection, indicating either low natural abundance or poor solubility under the extraction conditions used. Once more demonstrating the significance of polar solvents, p-coumaric acid and ferulic acid exhibit very low intensity, with modest signals in BE2.5 and BE5. The results provide compelling evidence that the best solvent for obtaining hydroxycinnamic acids from
V. officinalis is BE5 (50:50 butanol:ethanol), especially caffeic acid, which seems to be the most prevalent component.
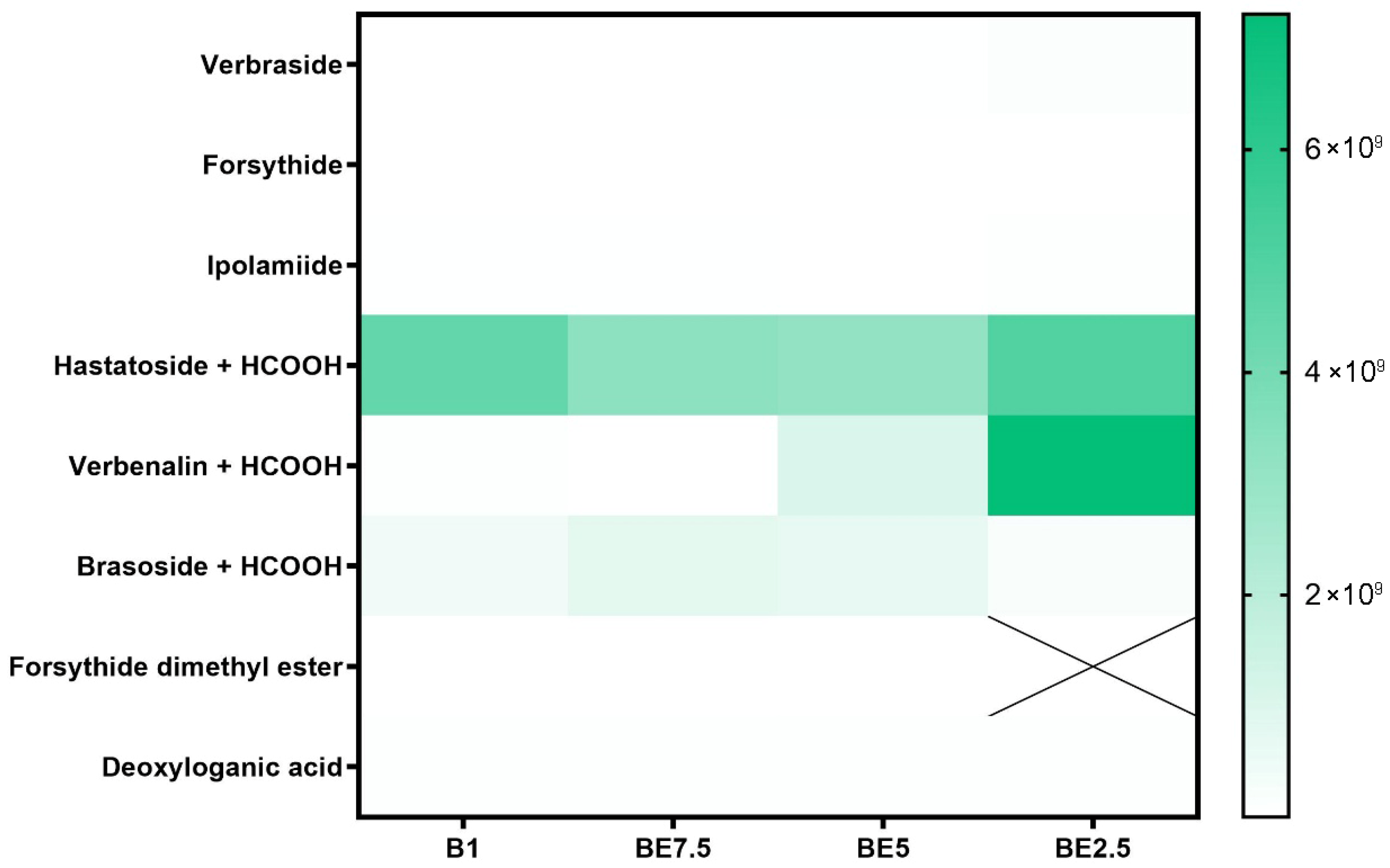
Iridoid glycosides’ heatmap (
Figure 3) shows a distinct polarity-dependent extraction pattern, with BE2.5 and, to a lesser extent, BE5 showing the most effective recovery. Out of all the chemicals listed, hastatoside + HCOOH has the largest and most stable abundance in all four extracts, peaking in BE2.5. Its high natural content and generally advantageous solubility in a range of polarities are highlighted by its extensive presence across extraction techniques. The second most common compound is verbenalin + HCOOH, whose extraction gets better over time from B1 to BE2.5. Its polarity and the beneficial effects of ethanol on solubilization and matrix permeability are evident in this trend. The appropriateness of mid-ethanol concentrations for the extraction of glycosidic iridoids is further supported by the low to moderate quantities of brasoside + HCOOH that are present in all extracts, with a minor enrichment in BE7.5 and BE5. The examined sample also contains other iridoid glycosides, including verbraside, forsythide, and ipolamiide. The fact that forsythide dimethyl ester is completely undetectable as well (designated with a “X” in BE2.5) highlights the solvents selectivity. The findings show that the best extracts for obtaining the main iridoid glycosides from
V. officinalis are those that are ethanol-rich, especially BE2.5. This is especially true for extracts that contain formate derivatives, such as verbenalin and hastatoside.
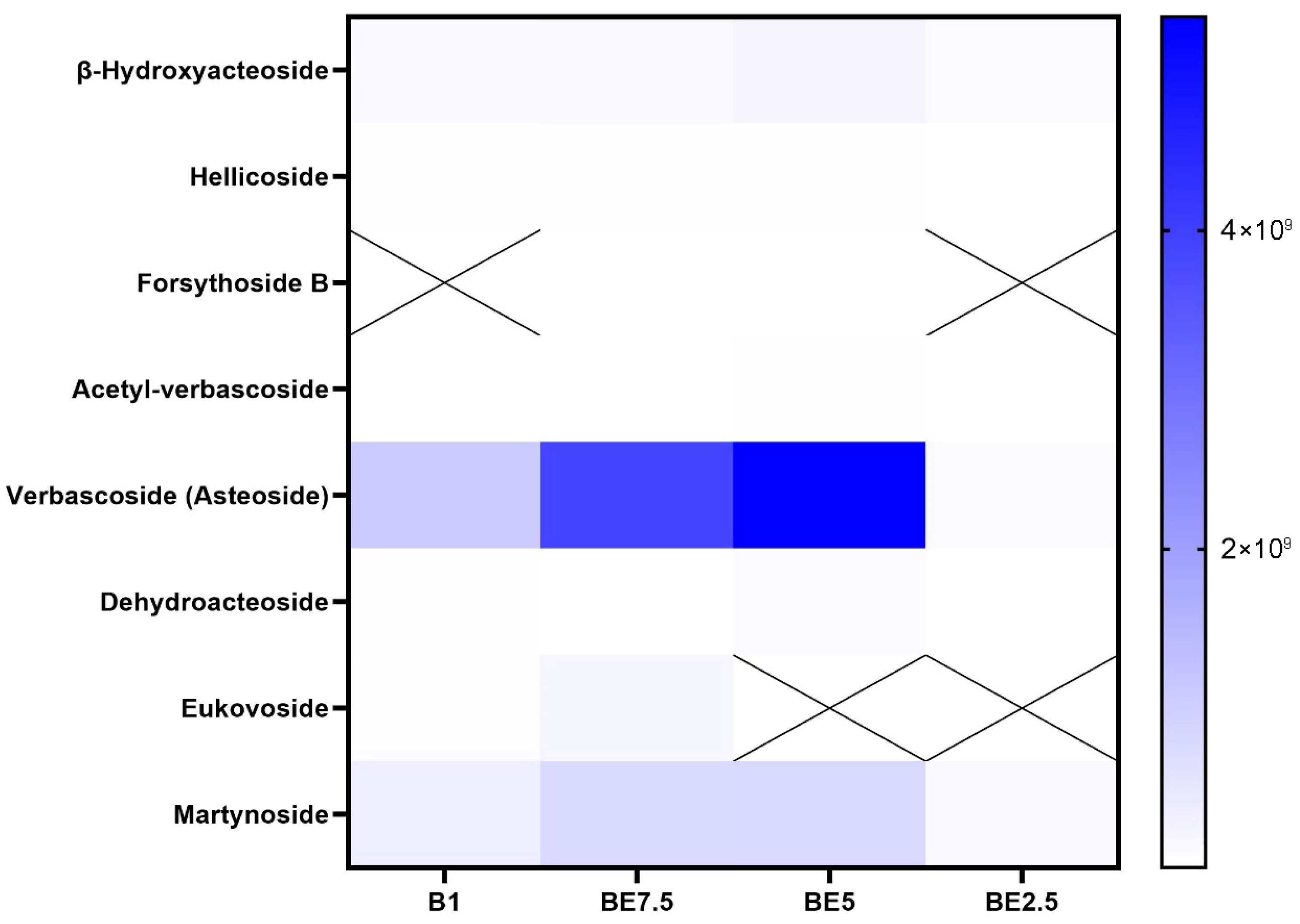
Verbascoside, often referred to as acteoside, is clearly dominant in all examined extracts, according to the phenylethanoids heatmap (
Figure 4), with BE5 showing the highest intensity, followed by BE7.5 and B1. This pattern makes it abundantly evident that a 50:50 butanol:ethanol mixture (BE5) provides the best polarity for verbascoside extraction. Verbascoside is a molecule that requires a moderately polar environment for effective solubilization due to its numerous hydroxyl groups and glycosidic linkages. Verbascoside’s constant high concentration highlights both its significance as a chemotaxonomic and pharmacologically active marker and its richness in the plant material. Martynoside appears in all extracts, suggesting moderate solubility. Other phenylethanoids, including dehydroacteoside, acetyl-verbascoside, hellicoside, and
β-hydroxyacteoside, are found at much lower concentrations, with weaker signals in extracts. Despite having structural similarities to verbascoside, some derivatives might be less abundant in nature or might be harder to extract under the tested circumstances. They may need specific extraction techniques or be minor ingredients, as indicated by their low intensities in all extracts. Forsythoside B and eukovoside exhibit intermittent detection. Because of their low native abundance or poor solubility in the chosen solvent systems, eukovoside and forsythoside B are only found in a few extracts or not at all. Verbascoside is the most abundant and extractable phenylethanoid in vervain extracts, according to the data, and the BE5 extract performs noticeably better than the others in terms of recovery efficiency.
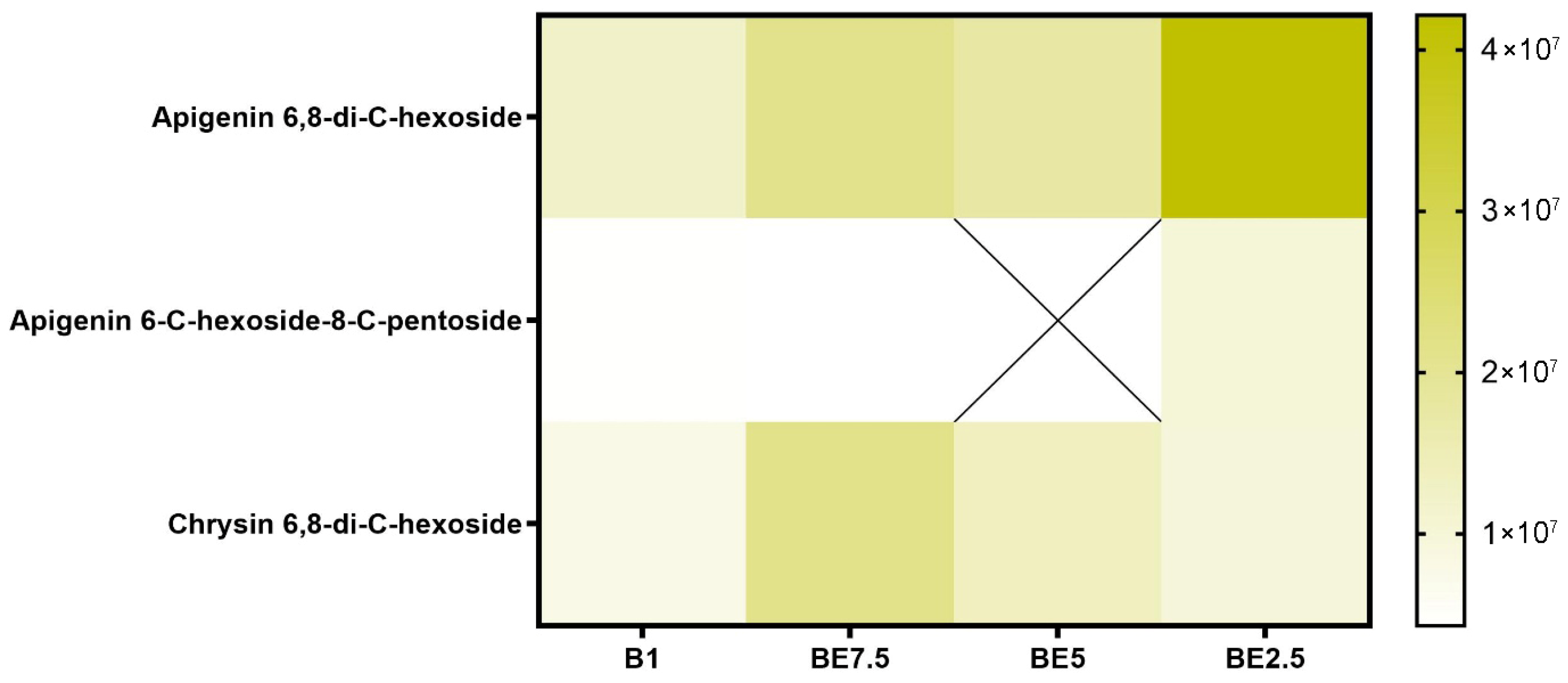
The most prevalent identified molecule, according to the flavonoid
C-glycoside heatmap (
Figure 5), is apigenin 6,8-di-
C-hexoside, which is present in all four extracts. The BE2.5 extract has the largest abundance, suggesting a strong preference for extraction under conditions that are high in ethanol. This molecule is relatively polar due to its double glycosylation via C-C bonds, and its extraction is improved with increasing solvent polarity. This compound’s great natural abundance in the plant matrix is further supported by the fact that it is present in all extracts, although to differing degrees. Additionally, chrysin 6,8-di-
C-hexoside is consistently found in all extracts. The uneven distribution of apigenin 6-
C-hexoside-8-
C-pentoside may indicate reduced abundance, variations in stability, or selective extraction based on the delicate relationships between solvent polarity and glycoside composition. All things considered, this heatmap shows that the most effective extracts for apigenin
C-glycosides are those that are high in ethanol, especially BE2.5.
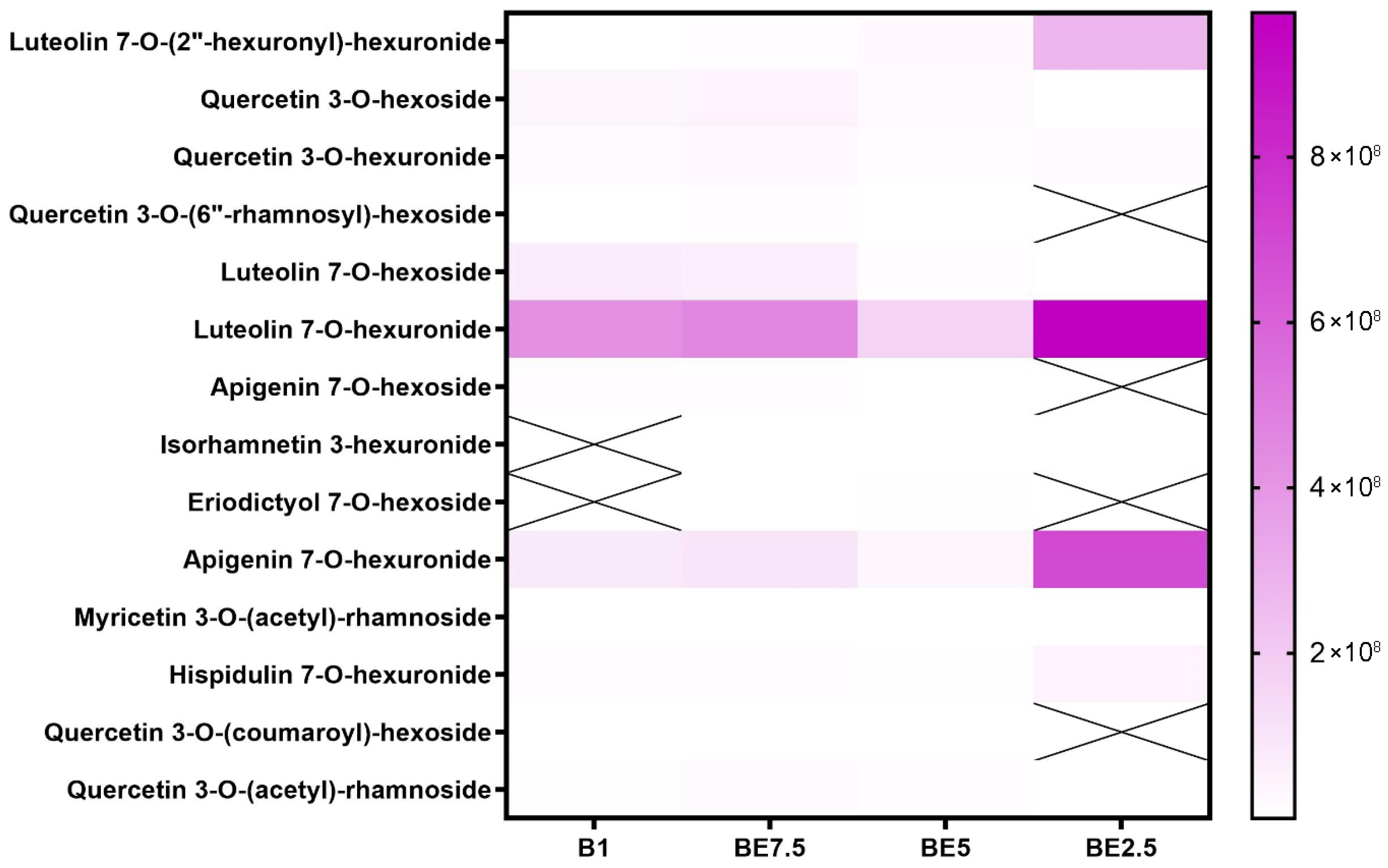
Luteolin 7-
O-hexuronide is the most prevalent and reliably extracted flavonoid of the components listed; it is especially prominent in BE2.5, where it reaches its maximum intensity (
Figure 6). Additionally, it is found in BE5 and is well-represented in BE7.5 and B1, suggesting that it is relatively soluble in a variety of polarities, although obviously preferring more polar ones. The strong extraction of luteolin 7-
O-hexoside and apigenin 7-
O-hexuronide in BE2.5 further supports the idea that ethanol-rich environments are the best conditions for the recovery of these flavonoids. However, certain extracts do not contain compounds such as isorhamnetin 3-hexuronide, eriodictyol 7-
O-hexoside, and quercetin 3-
O-(6″-rhamnosyl)-hexoside. Furthermore, only at low intensities are more complicated acylated forms found, such as myricetin 3-
O-(acetyl)-rhamnoside, quercetin 3-
O-(coumaroyl)-hexoside, and quercetin 3-
O-(acetyl)-rhamnoside. This heatmap provides compelling evidence that ethanol-dominant solvents are the most effective for extracting flavonoid
O-glycosides, especially glucuronidated versions of luteolin and apigenin. The best extraction method for these substances turns out to be BE2.5.
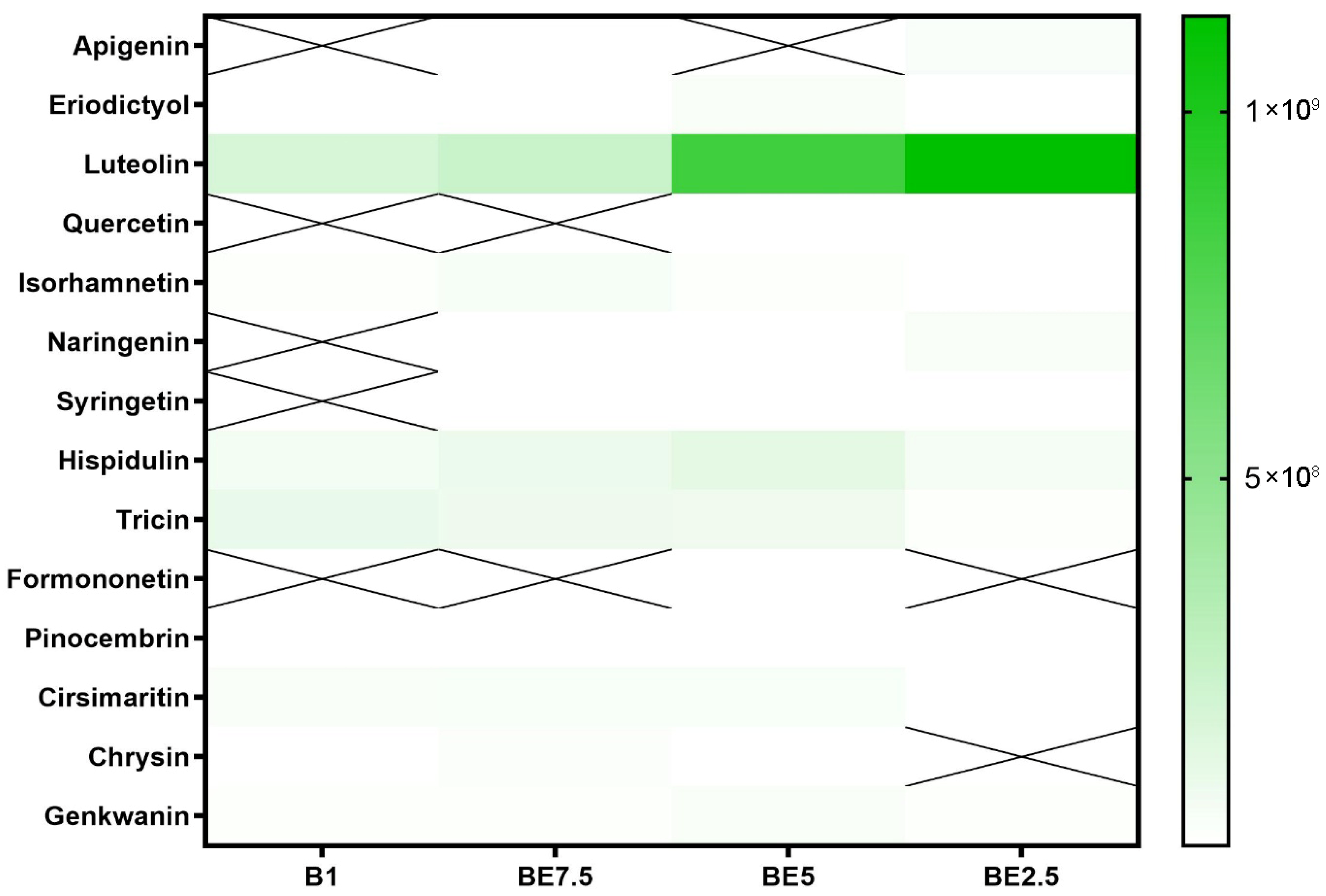
Luteolin is the most prevalent compound in the class of flavonoid aglycones, according to the heatmap depicting them (
Figure 7). It can be found in smaller quantities in BE7.5 and B1, but it is most prominent in BE5, with much greater intensity in BE2.5. Given the comparatively low polarity of flavonoid aglycones, this pattern is a little surprising, but it implies that luteolin may still prefer ethanol-rich solvents even though it is an aglycone because it contains several hydroxyl groups, which provide intermediate polarity. Given its great abundance, it is evident that
V. officinalis contains a significant amount of this bioactive flavone, which has cytoprotective and anti-inflammatory properties. The moderate representation of other aglycones, such as hispidulin, tricin, and cirsimaritin, which are mostly detectable in BE5 and BE7.5, suggests that ethanol-containing solvents are also useful for their extraction. These substances are structurally similar to luteolin, but their substitution patterns are different, which could explain why they are not as soluble.
Apigenin, quercetin, naringenin, syringetin, formononetin, and chrysin are among the other aglycones that are either nonexistent or just occasionally seen in extracts. Low natural concentrations in the plant material or specific solubility profiles that the studied extraction techniques are unable to adequately handle could be the cause of their limited presence. It is interesting to note that formononetin was found in BE5, indicating that this molecule may be extractable in conditions of balanced polarity, but it may only be found in trace amounts in this plant. When creating bioactive extracts from this species, the extraction pattern demonstrates the importance of using hydroalcoholic solvents to maximize aglycone recovery.
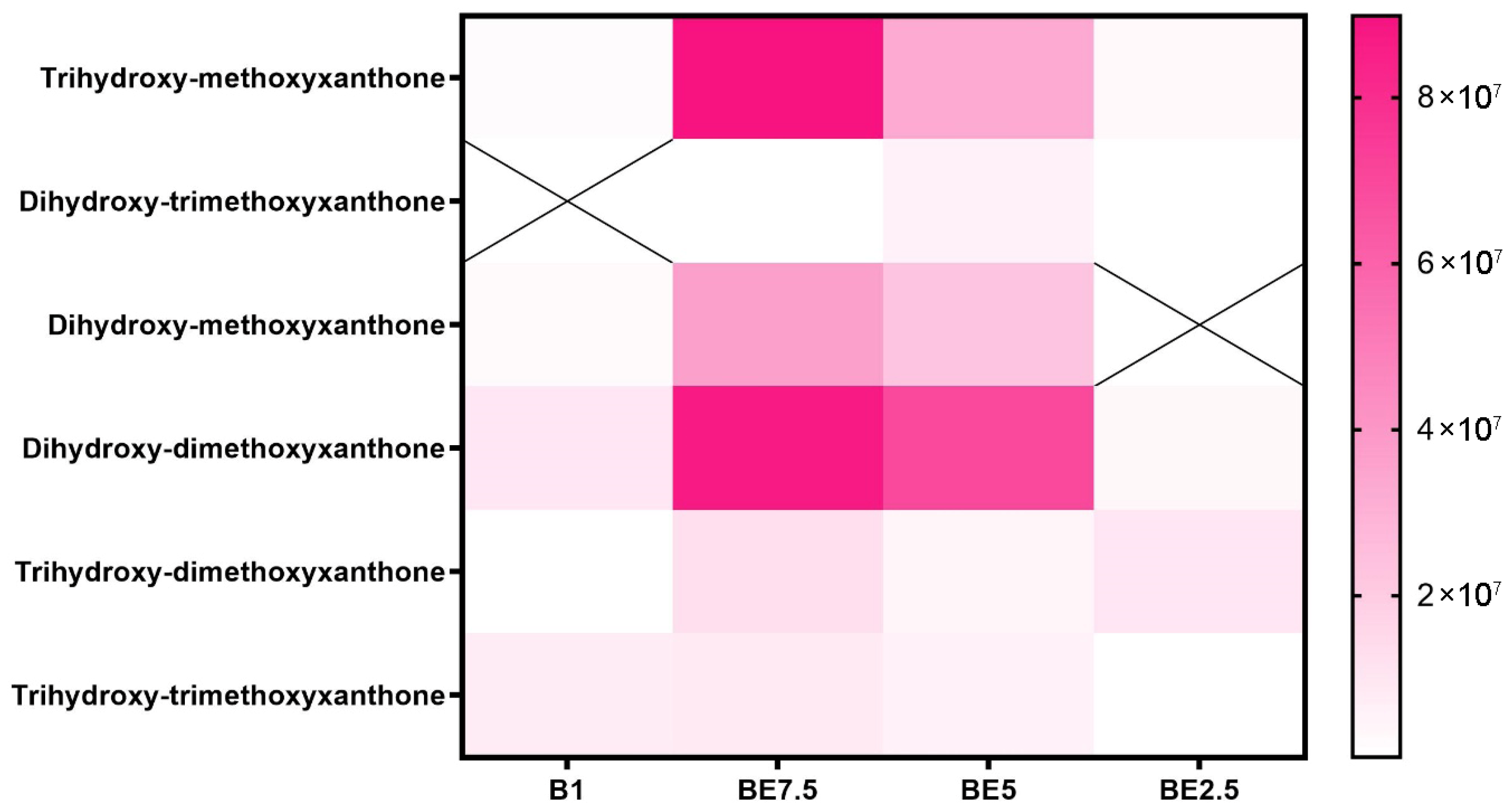
Across the four examined solvent systems, the xanthone distribution heatmap (
Figure 8) shows a profile of these phenolic chemicals that is extract-specific but generally balanced. The most prevalent molecules are trihydroxy-methoxyxanthone and dihydroxy-dimethoxyxanthone, which are especially abundant in the BE7.5 extract. BE5 has a considerable presence of these compounds, and B1 and BE2.5 have modest detection of them. This pattern suggests that these xanthones are best extracted using a moderately polar solvent composition (75:25 butanol:ethanol), most likely because of their amphipathic nature brought on by both hydroxyl and methoxy substitutions.
According to these results, BE7.5 provides the most effective and varied xanthone extraction, especially for those with several methoxy and hydroxyl groups that provide intermediate polarity. The information highlights the significance of adjusting solvent polarity to maximize recovery and reflects the structurally dependent solubility of xanthones. The antioxidant, anti-inflammatory, and antibacterial qualities of these chemicals make them noteworthy, which emphasizes the importance of maximizing their extraction from V. officinalis.
2.2. Biological Activities of the Extracts
Table 2 presents the antioxidant activity of four different extracts (B1, BE7.5, BE5, and BE2.5), as assessed using six standard assays: DPPH, ABTS, CUPRAC, FRAP, MCA, and PMA. Among all samples, BE5 exhibited the highest antioxidant activity in the DPPH (181.03 mg TE/g), ABTS (178.50 mg TE/g), CUPRAC (287.86 mg TE/g), and FRAP (170.99 mg TE/g) assays. These results are consistent with its presumed high phenolic content and suggest a strong capacity for radical scavenging and reducing power. Notably, BE5 also recorded the highest activity in the PMA assay (3.62 mmol TE/g), reflecting significant phosphomolybdenum-reducing potential. This may be attributed to the presence of verbascoside, a compound well-documented for its antioxidative properties [
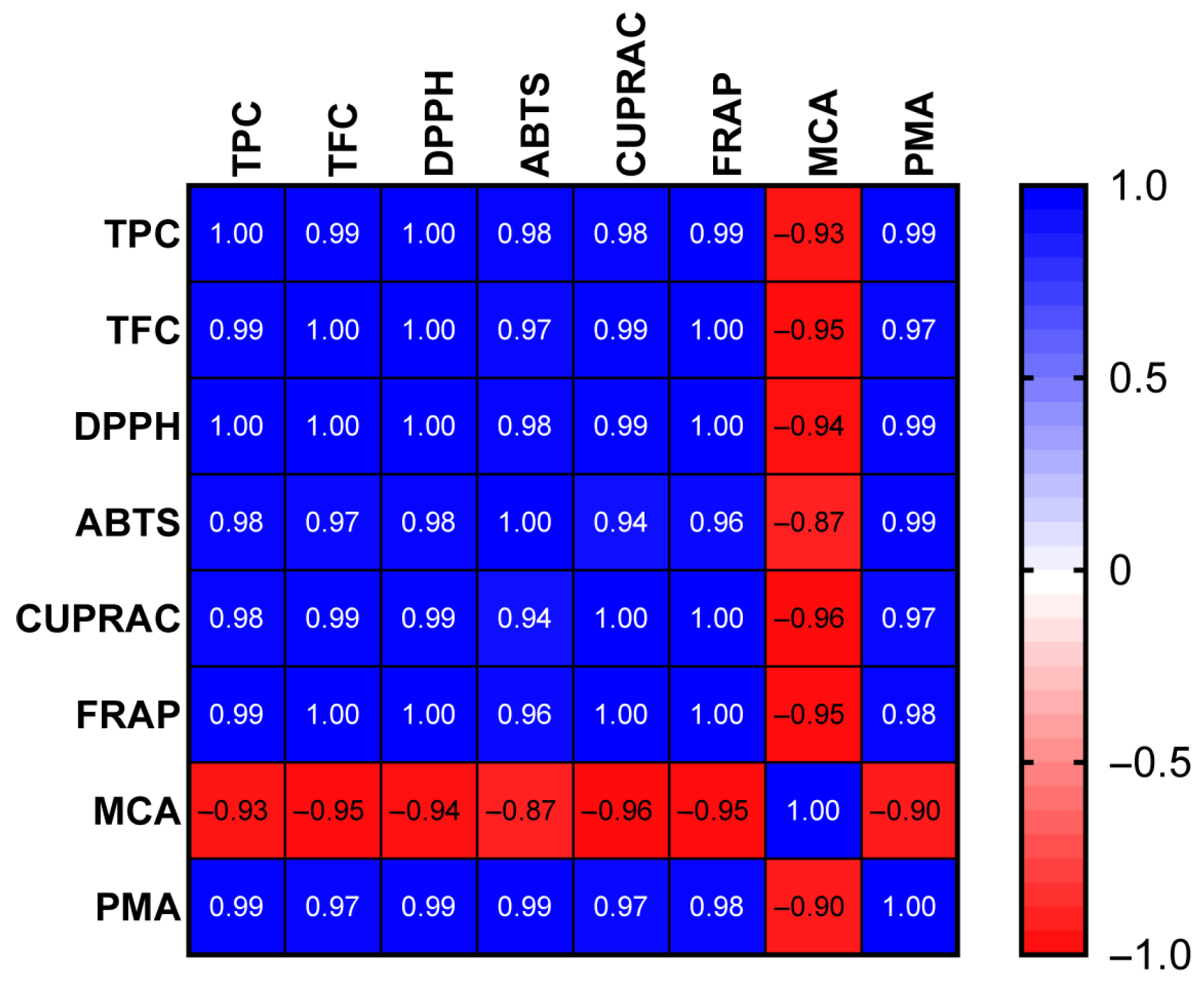
20]. In contrast, the BE2.5 and B1 extracts exhibited the best performance in the MCA assay. Iridoid and flavonoid glucosides—particularly verbenalin, hastatoside, and apigenin glucoside—are most abundant in the BE2.5 extract, which is characterized by pronounced metal-chelating activity. To assess the correlation between bioactive compounds and antioxidant properties, we conducted a Pearson correlation analysis. The findings, depicted in
Figure 9, indicate that, except for metal-chelating capacity, all antioxidant properties exhibited a strong correlation with the total phenolics/flavonoids content (R
2 > 0.8). This observation highlighted that phenolic compounds were the primary contributors to the antioxidant properties of
V. officinalis. Among the samples tested, all extracts demonstrated comparable AChE inhibitory activity, with values ranging narrowly from 5.10 to 5.17 mg GALAE/g (
Table 3), suggesting similar capacities to inhibit enzymes involved in neurodegenerative processes, such as Alzheimer’s disease [
6]. BE5 demonstrated the strongest tyrosinase inhibitory activity (102.33 mg KAE/g), surpassing all other extracts. This highlights its potential for applications in managing hyperpigmentation and oxidative-stress-related dermatological conditions. In contrast, BE2.5 exhibited the lowest α-amylase inhibitory activity (0.29 mg ACAE/g), while BE5 again showed the highest (0.59 mg ACAE/g), suggesting a broader spectrum of bioactivity that may be linked to its phytochemical richness.
Taken together, the results demonstrate a relation between the phytochemical composition of the extracts, their antioxidant potential, and their enzyme inhibitory capacities, with BE5 standing out as the most promising candidate for further pharmacological exploration.
Table 4 displays the minimum bactericidal concentrations (MBC) and minimum inhibitory concentrations (MIC) of four extracts (B1, BE7.5, BE5, and BE2.5) that were evaluated against
Pseudomonas aeruginosa. With different extraction solvent compositions, a distinct trend in antibacterial potency can be seen. Among the extracts, the BE2.5 extract (25% butanol: 75% ethanol) exhibited the lowest MIC (0.25 mg/mL) and MBC (0.50 mg/mL) values, indicating the strongest antibacterial activity. This implies that compounds active against
P. aeruginosa are better extracted from solvent mixtures with a higher ethanol content. The antimicrobial activity followed the order: BE2.5 > BE5 > BE7.5 = B1 Nevertheless, ampicillin had lower MIC and MBC values (0.003 and 0.005 mg/mL, respectively) and was far more potent than any of the extracts. According to these findings, the extract’s antibacterial activity is highly influenced by the solvent’s polarity, and BE2.5 stands out as the most promising option for more research against
P. aeruginosa. A previous study reported the antimicrobial activity of various fractions of
Verbena carolina against
Staphylococcus aureus,
Enterococcus faecalis,
Escherichia coli,
Salmonella typhi,
Candida albicans,
Trichophyton mentagrophytes, and
T. rubrum. The major compounds identified in these fractions include hispidulin, verbenaline, hastatoside, and verbascoside. Our findings are consistent with these previously reported results on
V. carolina [
21].
2.3. Molecular Modeling
Since the most intense peaks were for the compounds hastatoside, luteolin 7-
O-(2″-glucuronyl)-glucuronide, luteolin-7-
O-glucuronide, martynoside, verbascoside, and verbenalin, we have carried out further investigation of these compounds and their interactions with enzymes included in anti-enzymatic assays and selected enzymes representing virulence factors in
P. aeruginosa.
Table 5,
Table 6,
Table 7,
Table 8,
Table 9 and
Table 10 show the docking scores (binding energy) values of the chosen prevalent compounds against each of the six enzymes and the co-crystalized ligands in the active site of enzyme from PDB for each enzyme used as the control molecule. Additionally, the interactions of specific protein–ligand complexes were thoroughly investigated as shown in
Figure 10,
Figure 11,
Figure 12,
Figure 13,
Figure 14 and
Figure 15.
Among the six tested compounds, luteolin 7-O-(2″-glucuronyl)-glucuronide shows best binding affinity to the active site of acetylcholinesterase with the binding energy of −10.1 kcal/mol, forming ten hydrogen bonds with the amino acids in the active site. Compared to the control molecule, 1-benzyl-4-[(5,6-dimethoxy-1-indanon-2-yl)methyl]piperidine with the binding energy of −11.4 kcal/mol, luteolin 7-O-(2″-glucuronyl)-glucuronide shows less effectiveness towards binding to the active site of acetylcholinesterase.
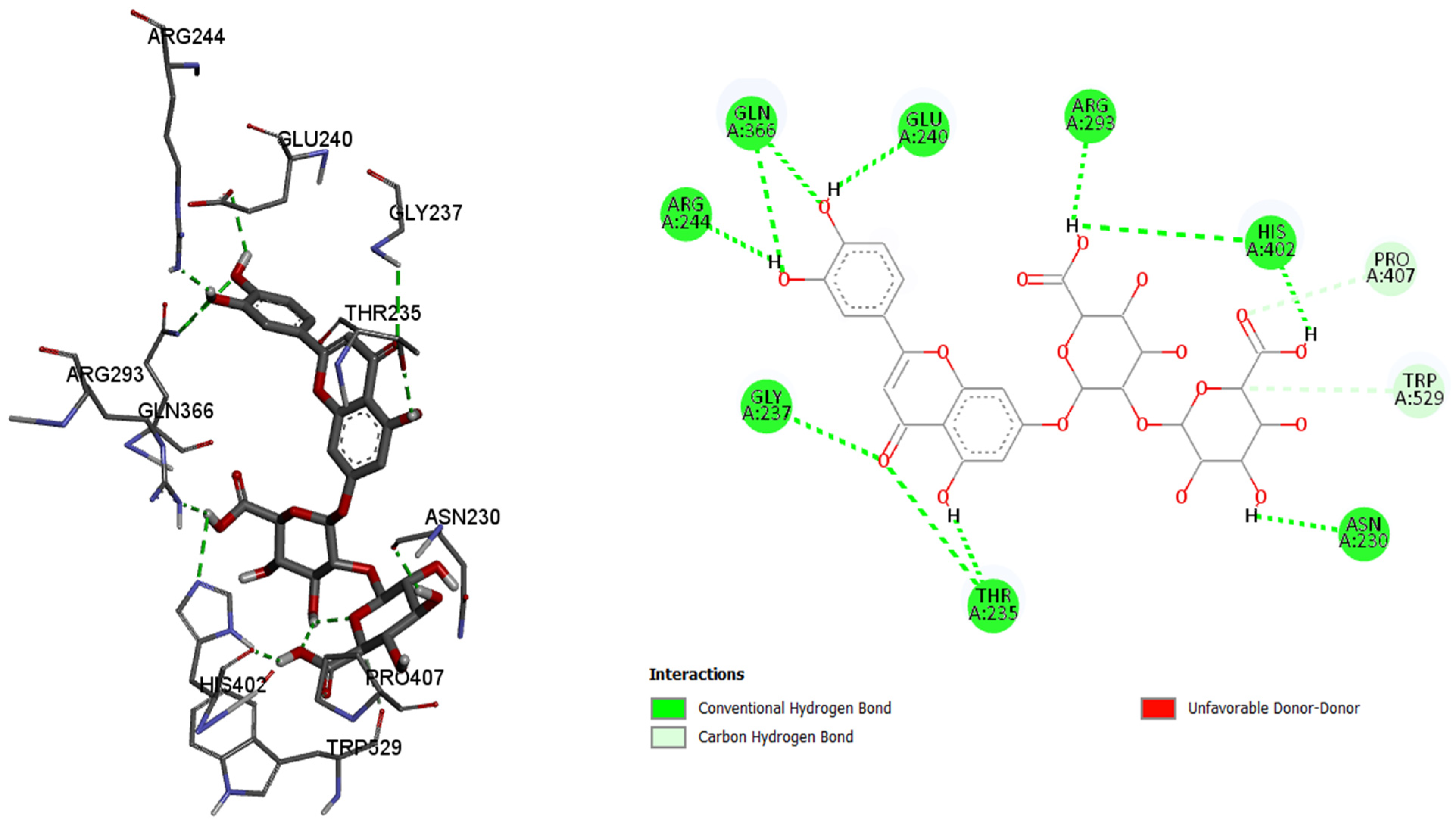
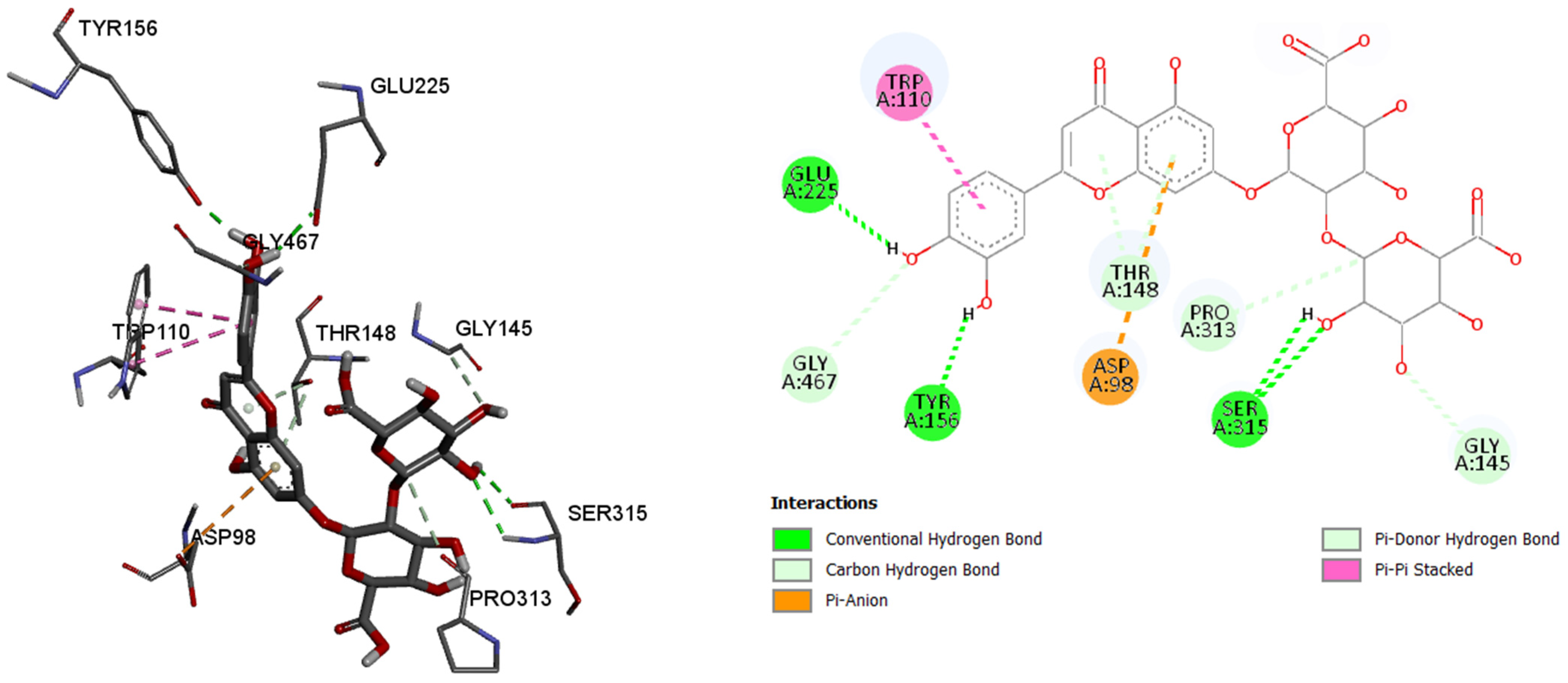
By analyzing the binding affinity to the active site of the butyrylcholinesterase, the same compound, luteolin 7-O-(2″-glucuronyl)-glucuronide, showed the best binding affinity (−10.3 kcal/mol) as in the case of the acetylcholinesterase. Conventional hydrogen bonds are formed between the 7-O-(2″-glucuronyl)-glucuronide and the amino acids, namely 225Glu, 156Tyr, and 315Ser, while the π–anion, π–π stacked, π–donor hydrogen, and carbon hydrogen bonds are formed between the rest of the six binding amino acids, and their residues. By comparing the binding affinity of the co-crystalized control molecule (2-{1-[4-(12-Amino-3-chloro-6,7,10,11-tetrahydro-7,11-methanocycloocta[b]quinolin-9-yl)butyl]-1H-1,2,3-triazol-4-yl}-N-[4-hydroxy-3-methoxybenzyl]acetamide), and the molecule with the best binding affinity among six tested molecules, the control molecule showed slightly better binding affinity (−10.7 kcal/mol).
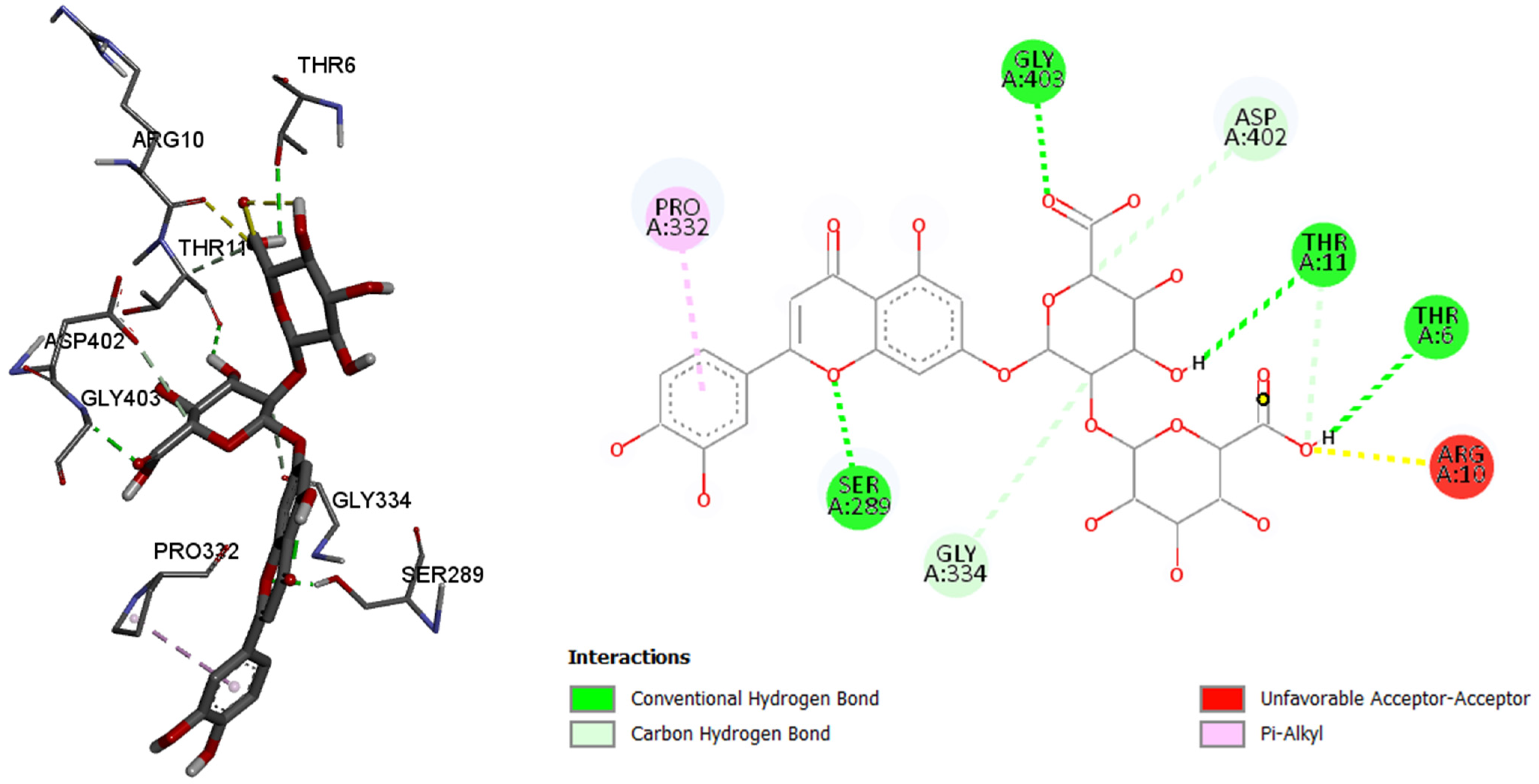
Like in the other two previously tested enzymes, in this case, luteolin 7-O-(2″-glucuronyl)-glucuronide showed the best binding affinity among the six tested compounds. By comparing with the control molecule, 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)-4H-chromen-4-one, 7-O-(2″-glucuronyl)-glucuronide showed better binding affinity to the pancreatic alpha amylase, with the binding energy of −9.5 kcal/mol (comparing to the control molecule binding energy of −7.8 kacl/mol), binding with the six amino acids and their residues in the active site of enzyme via conventional and carbon hydrogen, as well as other bonds.
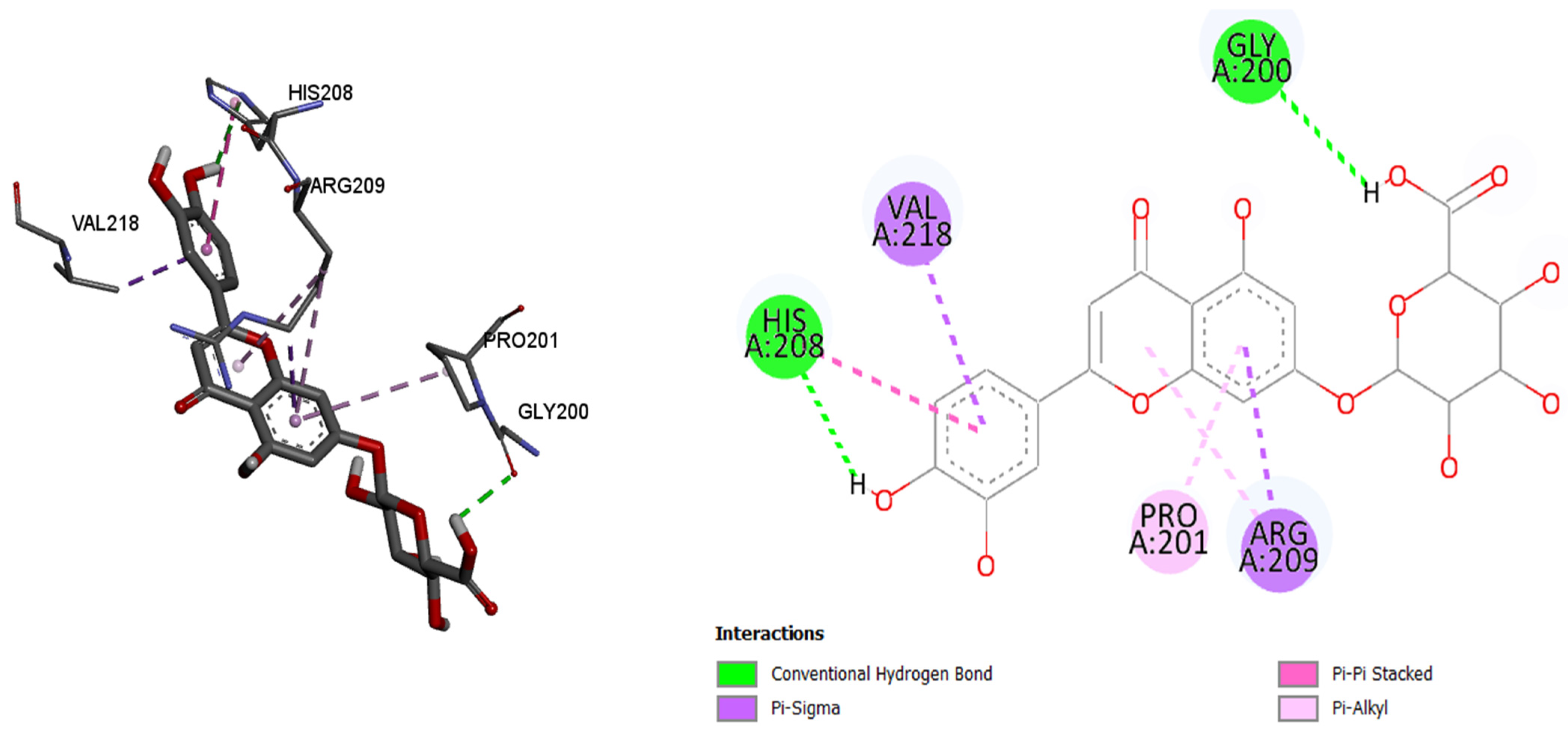
As in the case of tyrosinase, luteolin-7-O-glucuronide showed the best binding affinity (binding energy of −7.9 kcal/mol) by comparing it with the other tested molecules. Luteolin-7-O-glucuronide bind to the active site of tyrosinase via conventional hydrogen bonds (208His and 200His), π–sigma, π–π stacked, and π–alkyl bonds (218Val, 201Pro, and 209Arg). Luteolin-7-O-glucuronide showed better bind affinity compared to the control compound kojic acid (binding energy −5.4 kcal/mol).
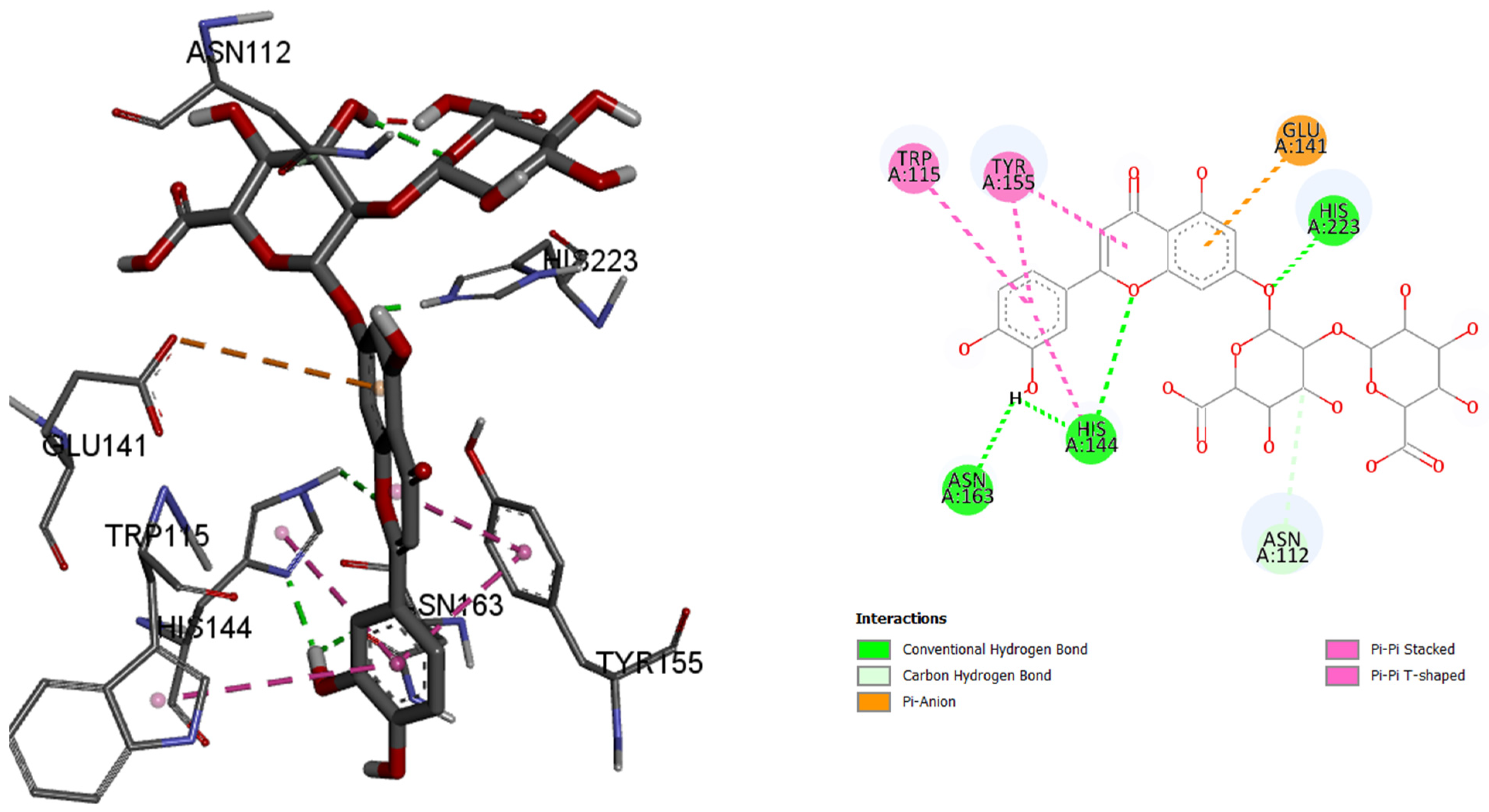
In the case of enzyme elastase B from P. aeruginosa, luteolin 7-O-(2″-glucuronyl)-glucuronide had best binding affinity with the binding energy of −9.6 kcal/mol, making this ligand a better ‘binder’ to the active site of the enzyme instead of the co-crystalized control molecule. Luteolin 7-O-(2″-glucuronyl)-glucuronide binds to the active site via hydrogen bonds and π–type bonds (115Trp, 155Tyr, 163Asn, 144His, 112Asn, 141Glu, and 223His).
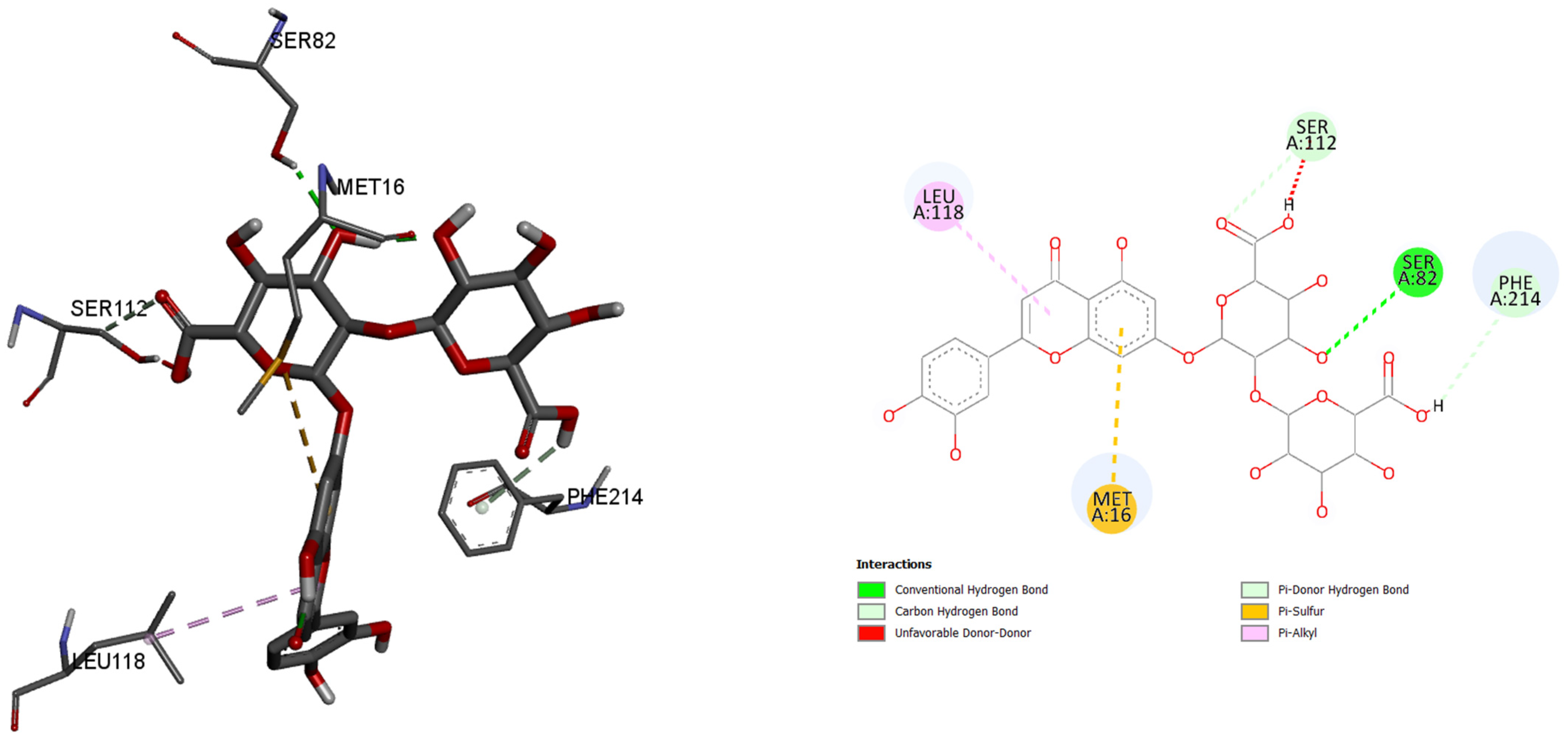
Furthermore, in the case of lipase A from P. aeruginosa, best binding affinity to the active site of the enzyme had luteolin 7-O-(2″-glucuronyl)-glucuronide, with the binding energy of −8.5 kcal/mol. This molecule also had better binding energy compared to the control molecule binding energy of −6.1 kacl/mol. Only five amino acids and their residues play roles in binding this molecule: 118Leu, 112Ser, 82Ser, 214Phe, and 16Met.
These molecular modeling studies show the high inhibitory potential of two luteolin glycosides, 7-O-(2″-glucuronyl)-glucuronide and luteolin-7-O-glucuronide, regarding their relatively high abundance in analyzed extracts compared to other flavonoid-O-glycosides. In the cases of in vitro testing of these extracts against the four enzymes, all tested extracts exhibited similar inhibitory potential. These results should suggest that these two compounds could be the carriers of biological activity among all compounds in the extracts.
Further studies should focus on isolating these compounds from the V. officinalis that showed great inhibitory potential in in silico studies and testing them as the potential carriers of biological activity, regarding their high relative abundance, and testing these compounds for the inhibitory activity of presented enzymes in vitro.