Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing
Abstract
1. Introduction
2. Results
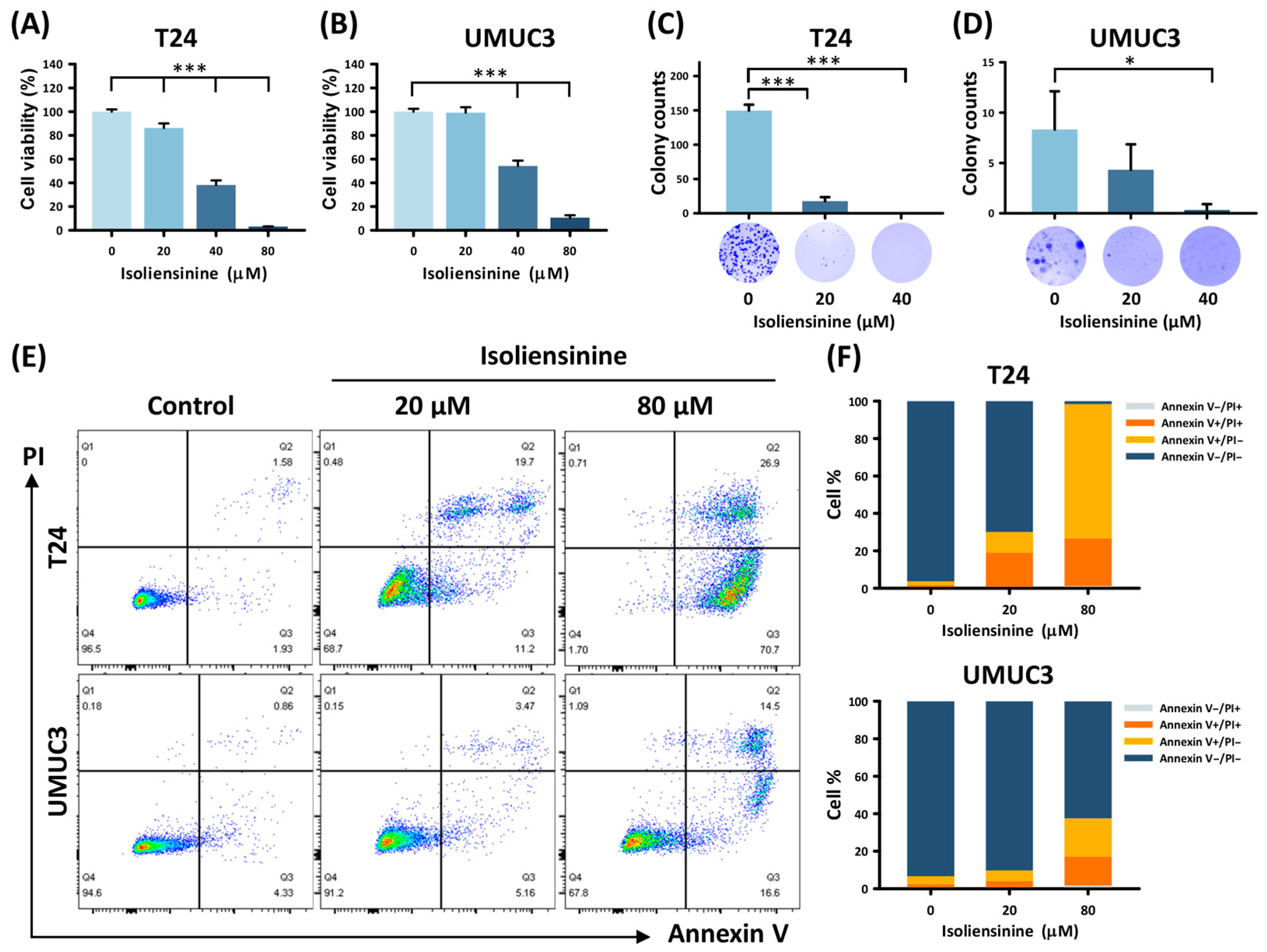
2.1. Isoliensinine Suppresses UC Cell Growth and Induces Tumor Apoptosis In Vitro
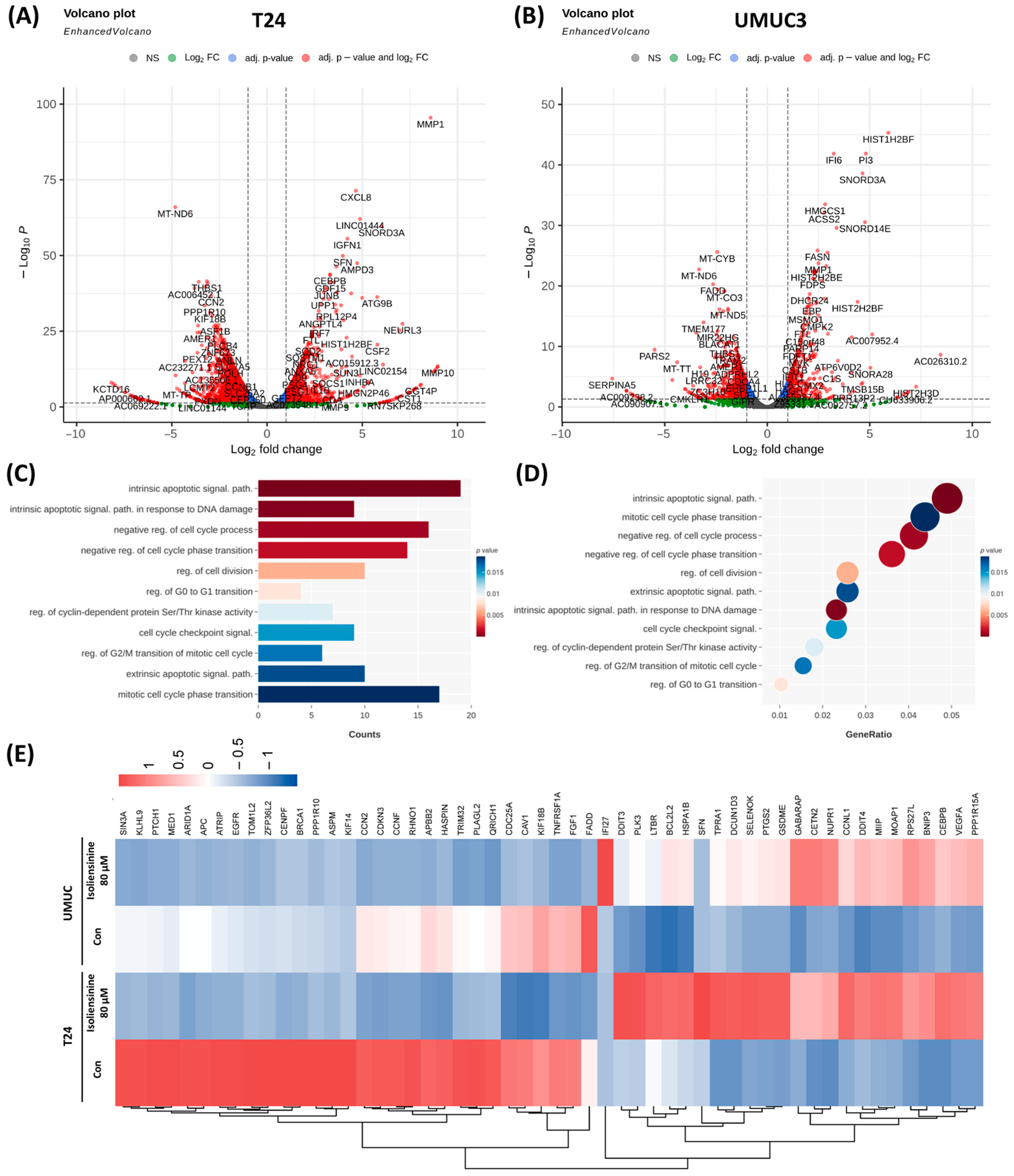
2.2. Isoliensinine Inhibits UC Cell Growth by Affecting Intracellular RNA Expression
2.3. Isoliensinine Inhibits UC Cell Growth Through Several Signaling Pathways
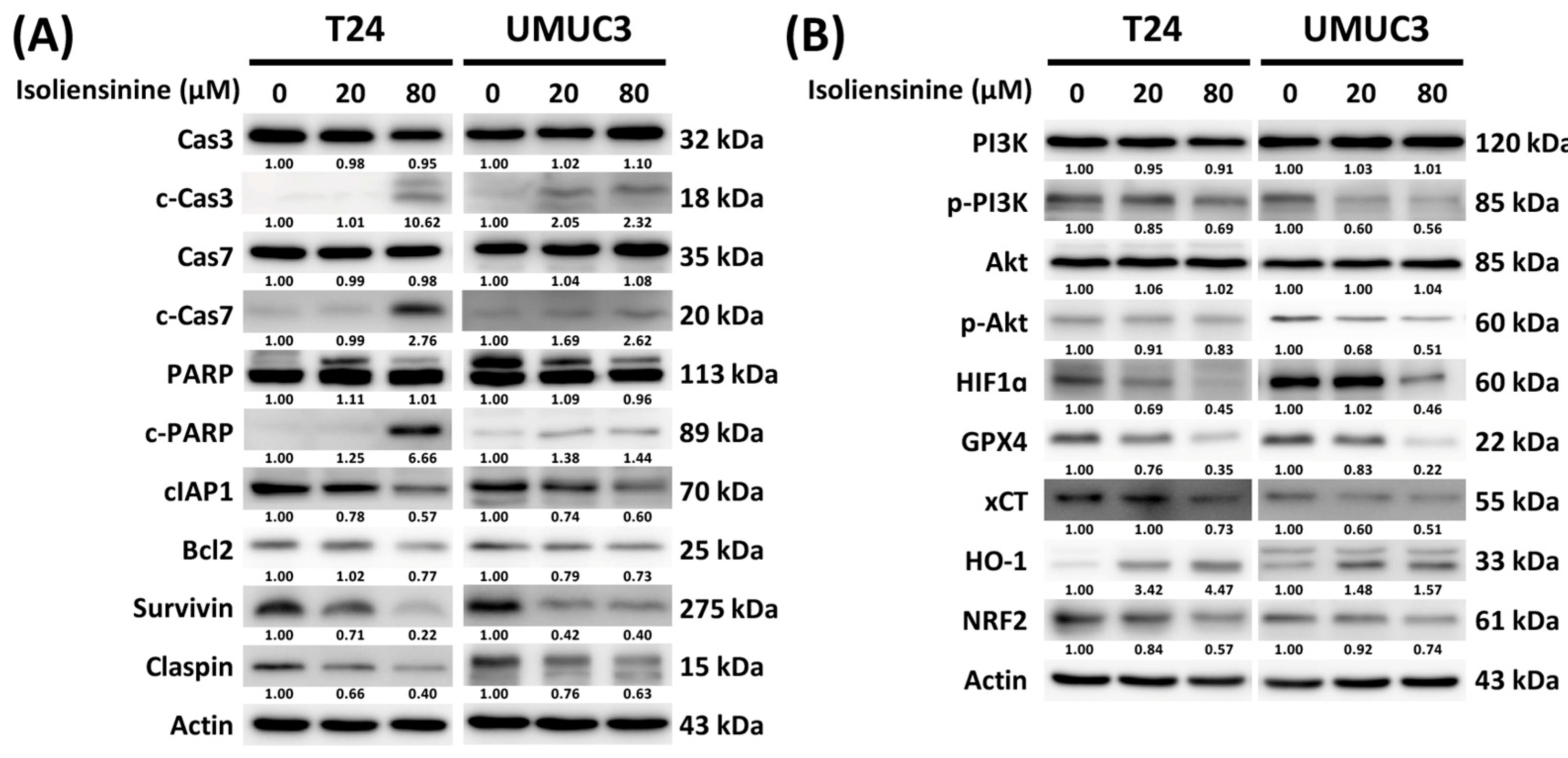
2.4. Isoliensinine Induces Ferroptosis in UC Cell Lines
2.5. Isoliensinine Prohibits UC Cell Growth by Affecting the Expression of Protein Targets in Apoptosis, Ferroptosis, the PI3K/AKT Pathway, and the Hypoxia-Inducible Factor 1 (HIF-1) Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. MTT Assay
4.3. Colony Formation Assay
4.4. Flow Cytometry Assay
4.5. Western Blotting
4.6. RNA Sequence Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCG | Bacillus Calmette–Guerin |
| DEGs | Differentially expressed genes |
| ddMVAC | Dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin |
| GC | Gemcitabine and cisplatin |
| GO | Gene ontology |
| HIF-1 | Hypoxia-inducible factor 1 |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| NMIBC | Non-muscle-invasive bladder cancer |
| NGS | Next-generation sequencing |
| PBS | Phosphate-buffered saline |
| RIPA | Radio-immunoprecipitation assay |
| TSC2 | Tuberous sclerosis complex 2 |
| TNF | Tumor necrosis factor |
| UC | Urothelial carcinoma |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Miyazaki, J.; Nishiyama, H. Epidemiology of urothelial carcinoma. Int. J. Urol. 2017, 24, 730–734. [Google Scholar] [CrossRef]
- Vlaming, M.; Kiemeney, L.; van der Heijden, A.G. Survival after radical cystectomy: Progressive versus De novo muscle invasive bladder cancer. Cancer Treat. Res. Commun. 2020, 25, 100264. [Google Scholar] [CrossRef]
- Babjuk, M.; Burger, M.; Capoun, O.; Cohen, D.; Compérat, E.M.; Dominguez Escrig, J.L.; Gontero, P.; Liedberg, F.; Masson-Lecomte, A.; Mostafid, A.H.; et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur. Urol. 2022, 81, 75–94. [Google Scholar] [CrossRef] [PubMed]
- Alfred Witjes, J.; Max Bruins, H.; Carrión, A.; Cathomas, R.; Compérat, E.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Lorch, A.; Martini, A.; et al. European Association of Urology Guidelines on Muscle-invasive and Metastatic Bladder Cancer: Summary of the 2023 Guidelines. Eur. Urol. 2024, 85, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ye, Z.W.; Tew, K.D.; Townsend, D.M. Cisplatin chemotherapy and renal function. Adv. Cancer Res. 2021, 152, 305–327. [Google Scholar] [CrossRef] [PubMed]
- Galsky, M.D.; Chen, G.J.; Oh, W.K.; Bellmunt, J.; Roth, B.J.; Petrioli, R.; Dogliotti, L.; Dreicer, R.; Sonpavde, G. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann. Oncol. 2012, 23, 406–410. [Google Scholar] [CrossRef]
- Khaki, A.R.; Shan, Y.; Nelson, R.E.; Kaul, S.; Gore, J.L.; Grivas, P.; Williams, S.B. Cost-effectiveness analysis of neoadjuvant immune checkpoint inhibition vs. cisplatin-based chemotherapy in muscle invasive bladder cancer. Urol. Oncol. 2021, 39, e732.e9–e732.e16. [Google Scholar] [CrossRef]
- Alameddine, R.; Mallea, P.; Shahab, F.; Zakharia, Y. Antibody Drug Conjugates in Bladder Cancer: Current Milestones and Future Perspectives. Curr. Treat. Options Oncol. 2023, 24, 1167–1182. [Google Scholar] [CrossRef]
- Wang, S.C.; Sung, W.W.; Kao, Y.L.; Hsieh, T.Y.; Chen, W.J.; Chen, S.L.; Chang, H.R. The gender difference and mortality-to-incidence ratio relate to health care disparities in bladder cancer: National estimates from 33 countries. Sci. Rep. 2017, 7, 4360. [Google Scholar] [CrossRef]
- Huang, C.Y.; Wang, S.C.; Chan, L.; Hsieh, T.Y.; Sung, W.W.; Chen, S.L. Gender differences in trends of bladder cancer mortality-to-incidence ratios according to health expenditure in 55 countries. PLoS ONE 2021, 16, e0244510. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, B.; Li, X.Z.; Qi, L.F.; Liang, Y.Z. Preparative separation and purification of liensinine, isoliensinine and neferine from seed embryo of Nelumbo nucifera GAERTN using high-speed counter-current chromatography. J. Sep. Sci. 2009, 32, 2476–2481. [Google Scholar] [CrossRef] [PubMed]
- Ren, H.L.; Zhang, J.H.; Xiao, J.H. Benzylisoquinoline alkaloids inhibit lung fibroblast activation mainly via inhibiting TGF-β1/Smads and ERK1/2 pathway proteins. Heliyon 2023, 9, e16849. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, L.; Zhang, Z.; Song, L.; Zhang, P.; Cao, Z.; Ma, J. Isoliensinine Eliminates Afterdepolarizations Through Inhibiting Late Sodium Current and L-Type Calcium Current. Cardiovasc. Toxicol. 2021, 21, 67–78. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.L.; Zhou, Z.W.; Long, H.Z.; Luo, H.Y.; Wen, D.D.; Cheng, L.; Gao, L.C. Isoliensinine: A Natural Compound with “Drug-Like” Potential. Front. Pharmacol. 2021, 12, 630385. [Google Scholar] [CrossRef]
- Manogaran, P.; Beeraka, N.M.; Huang, C.-Y.; Vijaya Padma, V. Neferine and isoliensinine enhance ‘intracellular uptake of cisplatin’ and induce ‘ROS-mediated apoptosis’ in colorectal cancer cells—A comparative study. Food Chem. Toxicol. 2019, 132, 110652. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, X.; Wu, T.; Li, B.; Liu, T.; Wang, R.; Liu, Q.; Liu, Z.; Gong, Y.; Shao, C. Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci. Rep. 2015, 5, 12579. [Google Scholar] [CrossRef]
- Wang, T.; Li, Z.; Xia, S.; Xu, Z.; Chen, X.; Sun, H. Dexmedetomidine promotes cell proliferation and inhibits cell apoptosis by regulating LINC00982 and activating the phosphoinositide-3-kinase (PI3K)/protein kinase B (AKT) signaling in hypoxia/reoxygenation-induced H9c2 cells. Bioengineered 2022, 13, 10159–10167. [Google Scholar] [CrossRef]
- Shu, G.; Zhang, L.; Jiang, S.; Cheng, Z.; Wang, G.; Huang, X.; Yang, X. Isoliensinine induces dephosphorylation of NF-kB p65 subunit at Ser536 via a PP2A-dependent mechanism in hepatocellular carcinoma cells: Roles of impairing PP2A/I2PP2A interaction. Oncotarget 2016, 7, 40285–40296. [Google Scholar] [CrossRef]
- Shu, G.; Yue, L.; Zhao, W.; Xu, C.; Yang, J.; Wang, S.; Yang, X. Isoliensinine, a Bioactive Alkaloid Derived from Embryos of Nelumbo nucifera, Induces Hepatocellular Carcinoma Cell Apoptosis through Suppression of NF-κB Signaling. J. Agric. Food Chem. 2015, 63, 8793–8803. [Google Scholar] [CrossRef]
- Song, Y.; Li, M.; Li, Y.; Zhang, T.; Zhang, J.; Han, D.; Lian, F.; Liu, X.; Fang, X. Identification of Isoliensinine as a Ferroptosis Suppressor with Iron-Chelating Activity. J. Nat. Prod. 2025, 88, 245–254. [Google Scholar] [CrossRef]
- Long, H.-Z.; Li, F.-J.; Gao, L.-C.; Zhou, Z.-W.; Luo, H.-Y.; Xu, S.-G.; Dai, S.-M.; Hu, J.-D. Isoliensinine activated the Nrf2/GPX4 pathway to inhibit glutamate-induced ferroptosis in HT-22 cells. J. Biochem. Mol. Toxicol. 2024, 38, e23794. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef] [PubMed]
- Sendoel, A.; Kohler, I.; Fellmann, C.; Lowe, S.W.; Hengartner, M.O. HIF-1 antagonizes p53-mediated apoptosis through a secreted neuronal tyrosinase. Nature 2010, 465, 577–583. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Zhao, T.; Chen, J.; Kang, L.; Wei, Y.; Han, L.; Shen, L.; Long, C.; Wu, S.; et al. Di-(2-ethylhexyl) phthalate exposure leads to ferroptosis via the HIF-1α/HO-1 signaling pathway in mouse testes. J. Hazard. Mater. 2022, 426, 127807. [Google Scholar] [CrossRef] [PubMed]
- Ho, G.Y.; Woodward, N.; Coward, J.I. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol. Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef]
- Cathomas, R.; Lorch, A.; Bruins, H.M.; Compérat, E.M.; Cowan, N.C.; Efstathiou, J.A.; Fietkau, R.; Gakis, G.; Hernández, V.; Espinós, E.L.; et al. The 2021 Updated European Association of Urology Guidelines on Metastatic Urothelial Carcinoma. Eur. Urol. 2022, 81, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Boormans, J.L.; van der Heijden, M.S.; van der Heijden, A.G.; Meijer, R.P.; Mehra, N.; Kiemeney, L.; Aben, K.K.H. Overall Survival of Patients Receiving Cisplatin or Carboplatin for Primary Metastatic Urothelial Carcinoma of the Bladder: A Contemporary Dutch Nationwide Cohort Study. Eur. Urol. Focus 2022, 8, 995–1002. [Google Scholar] [CrossRef]
- Manogaran, P.; Somasundaram, B.; Viswanadha, V.P. Reversal of cisplatin resistance by neferine/isoliensinine and their combinatorial regimens with cisplatin-induced apoptosis in cisplatin-resistant colon cancer stem cells (CSCs). J. Biochem. Mol. Toxicol. 2022, 36, e22967. [Google Scholar] [CrossRef]
- Muema, J.M.; Mutunga, J.M.; Obonyo, M.A.; Getahun, M.N.; Mwakubambanya, R.S.; Akala, H.M.; Cheruiyot, A.C.; Yeda, R.A.; Juma, D.W.; Andagalu, B.; et al. Isoliensinine from Cissampelos pariera rhizomes exhibits potential gametocytocidal and anti-malarial activities against Plasmodium falciparum clinical isolates. Malar. J. 2023, 22, 161. [Google Scholar] [CrossRef]
- Hu, G.; Xu, R.A.; Dong, Y.Y.; Wang, Y.Y.; Yao, W.W.; Chen, Z.C.; Chen, D.; Bu, T.; Ge, R.S. Simultaneous determination of liensinine, isoliensinine and neferine in rat plasma by UPLC-MS/MS and application of the technique to pharmacokinetic studies. J. Ethnopharmacol. 2015, 163, 94–98. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, J.H.; Gao, L.; Xiao, J.H. Correlation between the dynamic changes of gammadeltaT cells, Th17 cells, CD4(+)CD25(+) regulatory T cells in peripheral blood and pharmacological interventions against bleomycin-induced pulmonary fibrosis progression in mice. Exp. Cell Res. 2024, 439, 114098. [Google Scholar] [CrossRef]
- Yao, M.; Wu, M.; Yuan, M.; Wu, M.; Shen, A.; Chen, Y.; Lian, D.; Liu, X.; Peng, J. Enhancing the therapeutic potential of isoliensinine for hypertension through PEG-PLGA nanoparticle delivery: A comprehensive in vivo and in vitro study. Biomed. Pharmacother. 2024, 174, 116541. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Li, H.; Zhang, Y.; Lin, Y.; Chen, C.; Chen, J.; Huang, Y.; Zhou, Y.; Tang, Y.; Ding, J.; et al. Isoliensinine suppresses bone loss by targeted inhibition of RANKL-RANK binding. Biochem. Pharmacol. 2023, 210, 115463. [Google Scholar] [CrossRef] [PubMed]
- Dabrell, S.N.; Li, Y.C.; Yamaguchi, H.; Chen, H.F.; Hung, M.C. Herbal Compounds Dauricine and Isoliensinine Impede SARS-CoV-2 Viral Entry. Biomedicines 2023, 11, 2914. [Google Scholar] [CrossRef] [PubMed]
- Hadaschik, B.A.; ter Borg, M.G.; Jackson, J.; Sowery, R.D.; So, A.I.; Burt, H.M.; Gleave, M.E. Paclitaxel and cisplatin as intravesical agents against non-muscle-invasive bladder cancer. BJU Int. 2008, 101, 1347–1355. [Google Scholar] [CrossRef]
- Maruyama, T.; Yamamoto, S.; Qiu, J.; Ueda, Y.; Suzuki, T.; Nojima, M.; Shima, H. Apoptosis of bladder cancer by sodium butyrate and cisplatin. J. Infect. Chemother. 2012, 18, 288–295. [Google Scholar] [CrossRef]
- Muramaki, M.; So, A.; Hayashi, N.; Sowery, R.; Miyake, H.; Fujisawa, M.; Gleave, M.E. Chemosensitization of gemcitabine-resistant human bladder cancer cell line both in vitro and in vivo using antisense oligonucleotide targeting the anti-apoptotic gene, clusterin. BJU Int. 2009, 103, 384–390. [Google Scholar] [CrossRef]
- Papadopoulos, E.I.; Yousef, G.M.; Scorilas, A. Gemcitabine impacts differentially on bladder and kidney cancer cells: Distinct modulations in the expression patterns of apoptosis-related microRNAs and BCL2 family genes. Tumour Biol. 2015, 36, 3197–3207. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef]
- Zhao, L.; Zhou, X.; Xie, F.; Zhang, L.; Yan, H.; Huang, J.; Zhang, C.; Zhou, F.; Chen, J.; Zhang, L. Ferroptosis in cancer and cancer immunotherapy. Cancer Commun. 2022, 42, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Lu, Y.; Zhang, J.; Ren, Y.; Mo, Y.; Liu, D.; Duan, L.; Yuan, Z.; Wang, C.; Wang, Q. Levistilide a Induces Ferroptosis by Activating the Nrf2/HO-1 Signaling Pathway in Breast Cancer Cells. Drug Des. Dev. Ther. 2022, 16, 2981–2993. [Google Scholar] [CrossRef]
- Koppula, P.; Zhuang, L.; Gan, B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell 2021, 12, 599–620. [Google Scholar] [CrossRef]
- Smits, V.A.J.; Cabrera, E.; Freire, R.; Gillespie, D.A. Claspin-checkpoint adaptor and DNA replication factor. FEBS J. 2019, 286, 441–455. [Google Scholar] [CrossRef]
- Jiang, Y.; Cui, J.; Cui, M.; Jing, R. SLC7A11 promotes the progression of gastric cancer and regulates ferroptosis through PI3K/AKT pathway. Pathol. Res. Pract. 2023, 248, 154646. [Google Scholar] [CrossRef]
- Uranga, R.M.; Katz, S.; Salvador, G.A. Enhanced Phosphatidylinositol 3-kinase (PI3K)/Akt Signaling Has Pleiotropic Targets in Hippocampal Neurons Exposed to Iron-induced Oxidative Stress. J. Biol. Chem. 2013, 288, 19773–19784. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Ye, Z.; Hu, Y.; Ye, L.; Gao, L.; Wang, Y.; Sun, Q.; Tong, S.; Zhang, S.; Wu, L.; et al. Fatostatin induces ferroptosis through inhibition of the AKT/mTORC1/GPX4 signaling pathway in glioblastoma. Cell Death Dis. 2023, 14, 211. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, W.; Huang, Z. Bupivacaine modulates the apoptosis and ferroptosis in bladder cancer via phosphatidylinositol 3-kinase (PI3K)/AKT pathway. Bioengineered 2022, 13, 6794–6806. [Google Scholar] [CrossRef] [PubMed]
- Wheatley, S.P.; Altieri, D.C. Survivin at a glance. J. Cell Sci. 2019, 132, jcs223826. [Google Scholar] [CrossRef]
- Rashid, M.; Zadeh, L.R.; Baradaran, B.; Molavi, O.; Ghesmati, Z.; Sabzichi, M.; Ramezani, F. Up-down regulation of HIF-1α in cancer progression. Gene 2021, 798, 145796. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Zhu, W.; Han, J.; Yang, X.; Zhou, R.; Lu, H.C.; Yu, H.; Yuan, W.B.; Li, P.C.; Tao, J.; et al. The role of the HIF-1α/ALYREF/PKM2 axis in glycolysis and tumorigenesis of bladder cancer. Cancer Commun. 2021, 41, 560–575. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, J.; Zheng, Z.; Yang, F.; Liu, S.; Wu, Y.; Chen, Y.; Xu, T.; Mao, S.; Yan, Y.; et al. PHGDH Inhibits Ferroptosis and Promotes Malignant Progression by Upregulating SLC7A11 in Bladder Cancer. Int. J. Biol. Sci. 2022, 18, 5459–5474. [Google Scholar] [CrossRef] [PubMed]
- Sathe, A.; Nawroth, R. Targeting the PI3K/AKT/mTOR Pathway in Bladder Cancer. Methods Mol. Biol. 2018, 1655, 335–350. [Google Scholar] [CrossRef]
- Lee, Y.-S.; Lee, D.-H.; Choudry, H.A.; Bartlett, D.L.; Lee, Y.J. Ferroptosis-induced endoplasmic reticulum stress: Cross-talk between ferroptosis and apoptosis. Mol. Cancer Res. 2018, 16, 1073–1076. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, X.; Duan, D.; Zhao, L. Organelle-specific mechanisms in crosstalk between apoptosis and ferroptosis. Oxid. Med. Cell. Longev. 2023, 2023, 3400147. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-C.; Huang, H.-E.; Yu, C.-Y.; Chang, Y.-C.; Lin, S.-Y.; Wang, S.-C.; Sung, W.-W. Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing. Pharmaceuticals 2025, 18, 1008. https://doi.org/10.3390/ph18071008
Li Y-C, Huang H-E, Yu C-Y, Chang Y-C, Lin S-Y, Wang S-C, Sung W-W. Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing. Pharmaceuticals. 2025; 18(7):1008. https://doi.org/10.3390/ph18071008
Chicago/Turabian StyleLi, Yun-Chen, Hsuan-En Huang, Chia-Ying Yu, Ya-Chuan Chang, Shu-Yu Lin, Shao-Chuan Wang, and Wen-Wei Sung. 2025. "Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing" Pharmaceuticals 18, no. 7: 1008. https://doi.org/10.3390/ph18071008
APA StyleLi, Y.-C., Huang, H.-E., Yu, C.-Y., Chang, Y.-C., Lin, S.-Y., Wang, S.-C., & Sung, W.-W. (2025). Isoliensinine Induces Ferroptosis in Urothelial Carcinoma Cells via the PI3K/AKT/HIF-1α Axis: Molecular Evidence from Next-Generation Sequencing. Pharmaceuticals, 18(7), 1008. https://doi.org/10.3390/ph18071008









