Nerve Growth Factor in Pediatric Brain Injury: From Bench to Bedside
Abstract
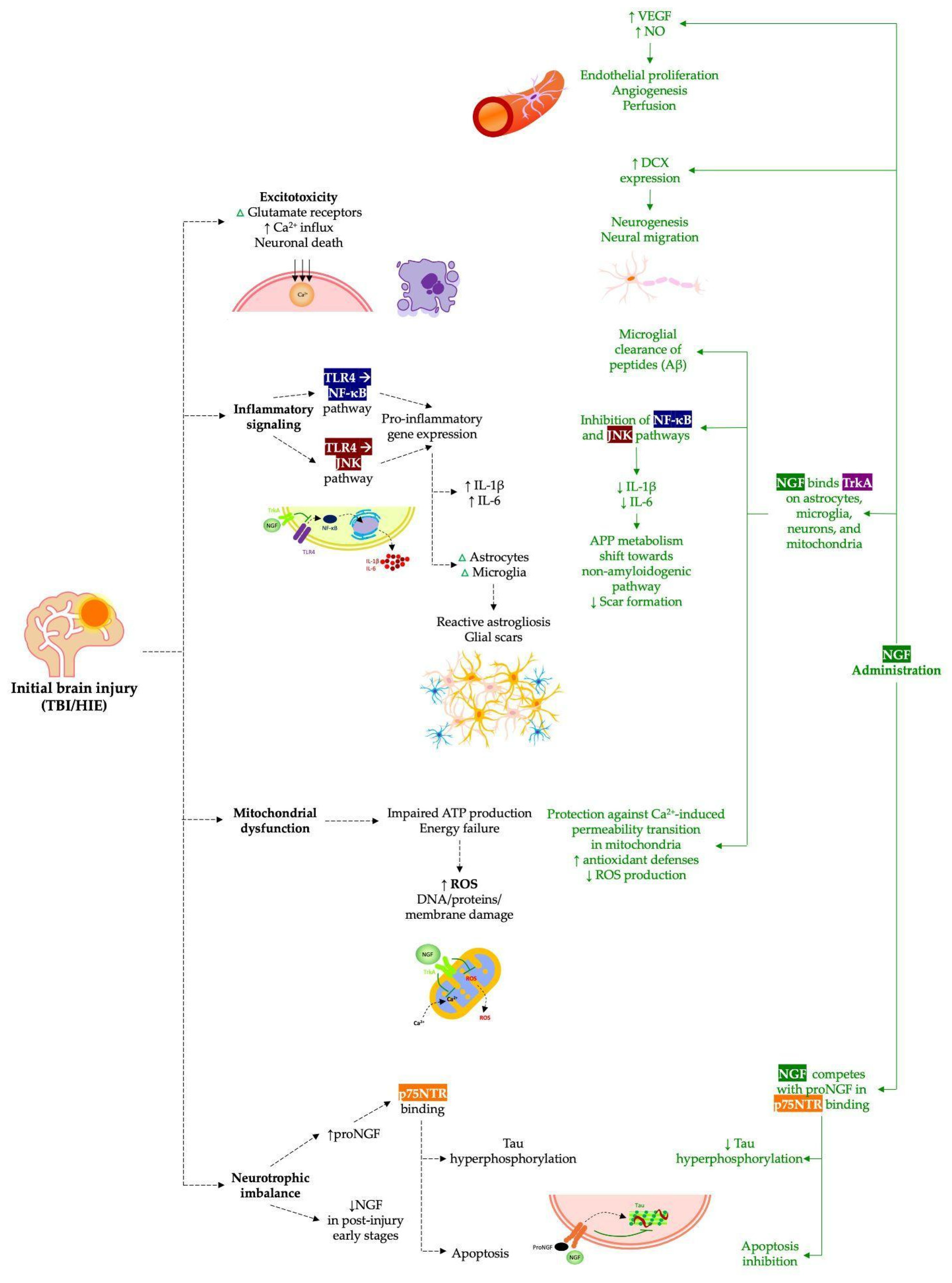
:1. Introduction
2. Materials and Methods
- Nerve growth factor;
- NGF;
- Neurotrophins;
- Neuroplasticity;
- Traumatic brain injury;
- TBI;
- HIE;
- Hypoxic–Ischemic Encephalopathy;
- Brain injury;
- NGF AND children.
3. Results
3.1. Nerve Growth Factor in Traumatic Brain Injury
| Study Type | Population | Intervention | Key Findings | Reference |
|---|---|---|---|---|
| Preclinical | 90 adult rats | 50 mg/day of NGF, delivered intranasally, for 14 days | NGF delivery promoted the decrease in TBI-induced Aβ deposits and improved TBI-induced functional impairment (p < 0.05) | Tian et al., 2012 [22] |
| Preclinical | 24 adult rats | 5 mg/day of NGF, delivered intranasally, for 12, 24, and 72 h | NGF treatment promoted the decrease in TBI-increased aquaporin-4 content and brain edema and the reduction in apoptosis by up-regulation of Bcl-2 and down-regulation of caspase-3 (p < 0.05) | Lv et al., 2013 [23] |
| Preclinical | 132 adult rats | 5 mg/day of NGF, delivered intranasally, for 3 days | NGF therapy promoted the attenuation of TBI-induced tau hyperphosphorylation and the decrease in IL-1β secretion (p < 0.05) | Lv et al., 2014 [24] |
| Preclinical | 48 adult rats | 5 mg/day of NGF, delivered intranasally, for 7 days | NGF treatment showed no effects on functional motor recovery after TBI (p < 0.05) | Young et al., 2015 [26] |
| Preclinical | 136 adult rats | 50 μg/kg of NGF, intranasally delivered in three rounds in a single day | NGF therapy prevented the onset of TBI-induced motor disabilities and reduced microglial activation, reactive astrogliosis, and IL-1β expression (p < 0.05) | Manni et al., 2023 [25] |
| Clinical | A 4-year-old boy | 0.1 mg/kg of murine NGF, delivered intranasally, twice a day for 10 consecutive days, for 4 cycles, at 1 month distance each | NGF treatment improved voluntary motor control, facial mimicry, phonation, attention and verbal comprehension, ability to cry, cough reflex, oral motor function, feeding ability, and bowel and bladder control | Chiaretti et al., 2017 [21] |
| Clinical | 3 children aged 3 to 10 years | 50 µg/kg of hr-NGF, administered intranasally, three times a day for 7 consecutive days for 4 cycles, at 1 month distance each. | NGF therapy reduced spasticity and improved the recovery of facial mimicry, voluntary movements, oral motor function, verbal comprehension, attention, cough reflex, crying ability, and feeding abilities | Gatto et al., 2023 [14] |
| Clinical | A 14-year-old boy | 50 µg/kg of hr-NGF, administered intranasally, three times a day for 7 consecutive days for 4 cycles, at 1 month distance each | NGF administration improved radiological functional assessment, cognitive processes, memory, communication strategy, execution skills, attention, and verbal expression | Capossela et al., 2024 [27] |
| Clinical | A 4-year-old boy | 50 µg/kg of hr-NGF, administered intranasally, three times a day for 7 consecutive days for 4 cycles, at 1 month distance each | NGF therapy improved motor function, verbal comprehension, executive functions, and EEG pattern | Di Sarno et al., 2025 [15] |
3.2. Nerve Growth Factor in Neonatal Hypoxic–Ischemic Brain Injury
| Study Type | Population | Intervention | Key Findings | Reference |
|---|---|---|---|---|
| Preclinical | 7 neonatal rats | Intraventricular administration of murine NGF | NGF reduced infarct size (~10% vs. 30–40% controls), enhanced TrkA phosphorylation, induced neuroprotection in cortex and striatum | Holtzman et al., 1996 [28] |
| Preclinical | 60 neonatal rats | Intramuscular injection of murine NGF at a dose of 20 ng/g/day once a day for 5 days | NGF promoted astrocyte activation (increased GFAP expression), supporting neuronal survival and synaptic formation after hypoxic–ischemic brain damage (p < 0.01) | Yin et al., 2013 [36] |
| Preclinical | 40 neonatal rats | Intraperitoneal injection of rhNGF at a dose of 0.5 µg administered for 3 days, with/without hyperbaric oxygen | NGF improved learning, memory, and sensory motor function post injury; combined NGF and hyperbaric oxygen had additive benefits (p < 0.01) | Wei et al., 2017 [37] |
| Preclinical | 110 neonatal rats | Single intranasal administration of CHF6467 (modified human NGF) at a dose of 20 μg/kg | CHF6467 reduced brain infarct volume, improved neurobehavioral outcomes; synergistic with therapeutic hypothermia (p < 0.05) | Landucci et al., 2025 [38] |
| Clinical | 2 infants (8 and 9 months old) | Intraventricular administration of murine NGF at a dose of 0.1 mg/day for 10 days | Improved neurological status (GCS increased from 4/5 to 8/9), enhanced EEG alpha/theta ratio, MRI showed reduction in malacic areas, and SPECT indicated improved cerebral perfusion in affected regions | Chiaretti et al., 2005 [39] |
| Clinical | 2 infants (8 and 13 months old) | Intraventricular administration of murine NGF at a dose of 0.1 mg/day for 10 days | Improved cerebral perfusion, elevated doublecortin expression (neurogenesis marker), enhanced EEG alpha/theta ratio, and neurological recovery | Chiaretti et al., 2008 [40] |
| Clinical | 4 patients: 2 children with hypoxic–ischemic brain damage, an adult patient with an optic glioma-induced visual loss, and a child with a severe crush syndrome of the lower left limb | Administration of murine NGF at a dose of 1 mg via external catheter into brain, eye drops, and subcutaneous skin injection | Amelioration of neurological and electrophysiological function in brain, improvement of visual function, and gradual healing of ischemic skin lesion | Chiaretti et al., 2011 [41] |
| Clinical | 2 children with HIE and coma | Intraventricular administration of murine NGF at a dose of 1 mg, once a day for 10 days | Significant improvement in EEG and SPECT after NGF; motor and cognitive improvement attributed to NGF’s neuroprotective effects on residual cholinergic neurons and network restoration | Fantacci et al., 2013 [34] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-beta |
| APP | Amyloid precursor protein |
| ATP | Adenosine triphosphate |
| CENTRAL | Cochrane Central Register of Controlled Trials |
| CNS | Central nervous system |
| CRS-R | Coma Recovery Scale-Revised |
| CSF | Cerebrospinal fluid |
| DCX | Doublecortin |
| DNA | Deoxyribonucleic acid |
| EEG | Electroencephalography |
| GCS | Glasgow Coma Scale |
| GFAP | Glial fibrillary acidic protein |
| HBO | Hyperbaric oxygen |
| HIE | Hypoxic–ischemic encephalopathy |
| hr-NGF | Human-recombinant NGF |
| IGF-1 | Insulin-like growth factor 1 |
| IL-1β | Interleukin-1β |
| JNK | c-Jun NH 2-terminal kinase |
| MRI | Magnetic Resonance Imaging |
| mNGF | Mouse nerve growth factor |
| NF-κB | Nuclear Factor-κB |
| NGF | Nerve growth factor |
| NO | Nitric oxide |
| p75NTR | p75 neurotrophin receptor |
| PET/CT | Positron Emission Tomography/Computed Tomography |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analysis |
| proNGF | Pro-form of nerve growth factor |
| ROS | Reactive oxygen species |
| SPECT | Single Photon Emission Computed Tomography |
| TBI | Traumatic brain injury |
| TLR4 | Toll-like receptor 4 |
| TrkA | Tropomyosin receptor kinase A |
| VEGF | Vascular Endothelial Growth Factor |
| VEP | Visual evoked potentials |
References
- Lingsma, H.F.; Roozenbeek, B.; Steyerberg, E.W.; Murray, G.D.; Maas, A.I. Early prognosis in traumatic brain injury: From prophecies to predictions. Lancet Neurol. 2010, 9, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Mohamadpour, M.; Whitney, K.; Bergold, P.J. The Importance of Therapeutic Time Window in the Treatment of Traumatic Brain Injury. Front. Neurosci. 2019, 13, 07. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ng, S.Y.; Lee, A.Y.W. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front. Cell. Neurosci. 2019, 13, 528. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiaretti, A.; Barone, G.; Riccardi, R.; Antonelli, A.; Pezzotti, P.; Genovese, O.; Tortorolo, L.; Conti, G. NGF, DCX, and NSE upregulation correlates with severity and outcome of head trauma in children. Neurology 2009, 72, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Loane, D.J. Neuroinflammation after traumatic brain injury: Opportunities for therapeutic intervention. Brain Behav. Immun. 2012, 26, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.H.; Kuo, L.T.; Luh, H.T. The Roles of Neurotrophins in Traumatic Brain Injury. Life 2021, 12, 26. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ristovska, S.; Stomnaroska, O.; Danilovski, D. Hypoxic Ischemic Encephalopathy (HIE) in Term and Preterm Infants. PRILOZI 2022, 43, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Nair, J.; Kumar, V.H.S. Current and Emerging Therapies in the Management of Hypoxic Ischemic Encephalopathy in Neonates. Children 2018, 5, 99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Sarno, L.; Curatola, A.; Cammisa, I.; Capossela, L.; Eftimiadi, G.; Gatto, A.; Chiaretti, A. Non-pharmacologic approaches to neurological stimulation in patients with severe brain injuries: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 6856–6870. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, L.F. Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1545–1564. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vink, R.; Van Den Heuvel, C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin. Investig. Drugs 2004, 13, 1263–1274. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Capossela, L.; Gatto, A.; Ferretti, S.; Di Sarno, L.; Graglia, B.; Massese, M.; Soligo, M.; Chiaretti, A. Multifaceted Roles of Nerve Growth Factor: A Comprehensive Review with a Special Insight into Pediatric Perspectives. Biology 2024, 13, 546. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gatto, A.; Capossela, L.; Conti, G.; Eftimiadi, G.; Ferretti, S.; Manni, L.; Curatola, A.; Graglia, B.; Di Sarno, L.; Calcagni, M.L.; et al. Intranasal human-recombinant NGF administration improves outcome in children with post-traumatic unresponsive wakefulness syndrome. Biol. Direct 2023, 18, 61. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Di Sarno, L.; Capossela, L.; Ferretti, S.; Manni, L.; Soligo, M.; Staccioli, S.; Napoli, E.; Burattini, R.; Gatto, A.; Chiaretti, A. Intranasal Human-Recombinant Nerve Growth Factor Enhances Motor and Cognitive Function Recovery in a Child with Severe Traumatic Brain Injury. Pharmaceuticals 2025, 18, 163. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiaretti, A.; Antonelli, A.; Mastrangelo, A.; Pezzotti, P.; Tortorolo, L.; Tosi, F.; Genovese, O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma 2008, 25, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Antonelli, A.; Genovese, O.; Pezzotti, P.; Rocco, C.D.; Viola, L.; Riccardi, R. Nerve growth factor and doublecortin expression correlates with improved outcome in children with severe traumatic brain injury. J. Trauma 2008, 65, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Antonelli, A.; Riccardi, R.; Genovese, O.; Pezzotti, P.; Di Rocco, C.; Tortorolo, L.; Piedimonte, G. Nerve growth factor expression correlates with severity and outcome of traumatic brain injury in children. Eur. J. Paediatr. Neurol. 2008, 12, 195–204. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Manni, L.; Conti, G.; Chiaretti, A.; Soligo, M. Intranasal nerve growth factor for prevention and recovery of the outcomes of traumatic brain injury. Neural Regen. Res. 2023, 18, 773–778. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chiaretti, A.; Conti, G.; Falsini, B.; Buonsenso, D.; Crasti, M.; Manni, L.; Soligo, M.; Fantacci, C.; Genovese, O.; Calcagni, M.L.; et al. Intranasal Nerve Growth Factor administration improves cerebral functions in a child with severe traumatic brain injury: A case report. Brain Inj. 2017, 31, 1538–1547. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Guo, R.; Yue, X.; Lv, Q.; Ye, X.; Wang, Z.; Chen, Z.; Wu, B.; Xu, G.; Liu, X. Intranasal administration of nerve growth factor ameliorate β-amyloid deposition after traumatic brain injury in rats. Brain Res. 2012, 1440, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Fan, X.; Xu, G.; Liu, Q.; Tian, L.; Cai, X.; Sun, W.; Wang, X.; Cai, Q.; Bao, Y.; et al. Intranasal delivery of nerve growth factor attenuates aquaporins-4-induced edema following traumatic brain injury in rats. Brain Res. 2013, 1493, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Lv, Q.; Lan, W.; Sun, W.; Ye, R.; Fan, X.; Ma, M.; Yin, Q.; Jiang, Y.; Xu, G.; Dai, J.; et al. Intranasal nerve growth factor attenuates tau phosphorylation in brain after traumatic brain injury in rats. J. Neurol. Sci. 2014, 345, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Manni, L.; Leotta, E.; Mollica, I.; Serafino, A.; Pignataro, A.; Salvatori, I.; Conti, G.; Chiaretti, A.; Soligo, M. Acute Intranasal Treatment with Nerve Growth Factor Limits the Onset of Traumatic Brain Injury in Young Rats. Br. J. Pharmacol. 2023, 180, 1949–1964. [Google Scholar] [CrossRef]
- Young, J.; Pionk, T.; Hiatt, I.; Geeck, K.; Smith, J.S. Environmental enrichment aides in functional recovery following unilateral controlled cortical impact of the forelimb sensorimotor area however intranasal administration of nerve growth factor does not. Brain Res. Bull. 2015, 115, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Capossela, L.; Graglia, B.; Ferretti, S.; Di Sarno, L.; Gatto, A.; Calcagni, M.L.; Di Giuda, D.; Cocciolillo, F.; Romeo, D.M.; Manni, L.; et al. Intranasal human-recombinant nerve growth factor administration improves cognitive functions in a child with severe traumatic brain injury. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 4302–4312. [Google Scholar] [CrossRef] [PubMed]
- Holtzman, D.M.; Sheldon, R.A.; Jaffe, W.; Cheng, Y.; Ferriero, D.M. Nerve growth factor protects the neonatal brain against hypoxic-ischemic injury. Ann. Neurol. 1996, 39, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Gillam-Krakauer, M.; Shah, M.; Gowen, C.W., Jr. Birth Asphyxia; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar] [PubMed]
- Gunn, A.J.; Thoresen, M. Neonatal encephalopathy and hypoxic-ischemic encephalopathy. Handb. Clin. Neurol. 2019, 162, 217–237. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.K.; Gulati, A. Advances in Therapies to Treat Neonatal Hypoxic-Ischemic Encephalopathy. J. Clin. Med. 2023, 12, 6653. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Conroy, J.N.; Coulson, E.J. High-affinity TrkA and p75 neurotrophin receptor complexes: A twisted affair. J. Biol. Chem. 2022, 298, 101568. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, T.H.; Kato, H.; Chen, S.T.; Kogure, K.; Itoyama, Y. Expression of nerve growth factor and trkA after transient focal cerebral ischemia in rats. Stroke 1998, 29, 1687–1696; discussion 1697. [Google Scholar] [CrossRef] [PubMed]
- Fantacci, C.; Capozzi, D.; Ferrara, P.; Chiaretti, A. Neuroprotective role of nerve growth factor in hypoxic-ischemic brain injury. Brain Sci. 2013, 3, 1013–1022. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhong, J.; Zhao, L.; Du, Y.; Wei, G.; Yao, W.G.; Lee, W.H. Delayed IGF-1 treatment reduced long-term hypoxia-ischemia-induced brain damage and improved behavior recovery of immature rats. Neurol. Res. 2009, 31, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Dong, L.; Wei, W.; Wang, Y.; Chai, Y.; Feng, Z. Effect of mouse nerve growth factor on the expression of glial fibrillary acidic protein in hippocampus of neonatal rats with hypoxic-ischemic brain damage. Exp. Ther. Med. 2013, 5, 419–422. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wei, L.; Ren, Q.; Zhang, Y.; Wang, J. Effects of hyperbaric oxygen and nerve growth factor on the long-term neural behavior of neonatal rats with hypoxic ischemic brain damage. Acta Cir. Bras. 2017, 32, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Mango, D.; Carloni, S.; Mazzantini, C.; Pellegrini-Giampietro, D.E.; Saidi, A.; Balduini, W.; Schiavi, E.; Tigli, L.; Pioselli, B.; et al. Beneficial effects of CHF6467, a modified human nerve growth factor, in experimental neonatal hypoxic-ischaemic encephalopathy. Br. J. Pharmacol. 2025, 182, 510–529. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Genovese, O.; Riccardi, R.; Di Rocco, C.; Di Giuda, D.; Mariotti, P.; Pulitanò, S.; Piastra, M.; Polidori, G.; Colafati, G.S.; et al. Intraventricular nerve growth factor infusion: A possible treatment for neurological deficits following hypoxic-ischemic brain injury in infants. Neurol. Res. 2005, 27, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Antonelli, A.; Genovese, O.; Fernandez, E.; Giuda, D.; Mariotti, P.; Riccardi, R. Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic-ischemic brain injury. Neurol. Res. 2008, 30, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Chiaretti, A.; Falsini, B.; Aloe, L.; Pierri, F.; Fantacci, C.; Riccardi, R. Neuroprotective role of nerve growth factor in hypoxicischemic injury. From brain to skin. Arch. Ital. Biol. 2011, 149, 275–282. [Google Scholar] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Sarno, L.; Ferretti, S.; Capossela, L.; Gatto, A.; Pansini, V.; Caroselli, A.; Manni, L.; Soligo, M.; Chiaretti, A. Nerve Growth Factor in Pediatric Brain Injury: From Bench to Bedside. Pharmaceuticals 2025, 18, 929. https://doi.org/10.3390/ph18060929
Di Sarno L, Ferretti S, Capossela L, Gatto A, Pansini V, Caroselli A, Manni L, Soligo M, Chiaretti A. Nerve Growth Factor in Pediatric Brain Injury: From Bench to Bedside. Pharmaceuticals. 2025; 18(6):929. https://doi.org/10.3390/ph18060929
Chicago/Turabian StyleDi Sarno, Lorenzo, Serena Ferretti, Lavinia Capossela, Antonio Gatto, Valeria Pansini, Anya Caroselli, Luigi Manni, Marzia Soligo, and Antonio Chiaretti. 2025. "Nerve Growth Factor in Pediatric Brain Injury: From Bench to Bedside" Pharmaceuticals 18, no. 6: 929. https://doi.org/10.3390/ph18060929
APA StyleDi Sarno, L., Ferretti, S., Capossela, L., Gatto, A., Pansini, V., Caroselli, A., Manni, L., Soligo, M., & Chiaretti, A. (2025). Nerve Growth Factor in Pediatric Brain Injury: From Bench to Bedside. Pharmaceuticals, 18(6), 929. https://doi.org/10.3390/ph18060929






