Oxidative Stress Score as a Simplified Surrogate for Prognostic Stratification and Therapeutic Decision-Making in Multiple Myeloma
Abstract
1. Introduction
2. Results
2.1. Model Construction and Analysis
2.2. Relationship Between OSS and Clinical Characteristics
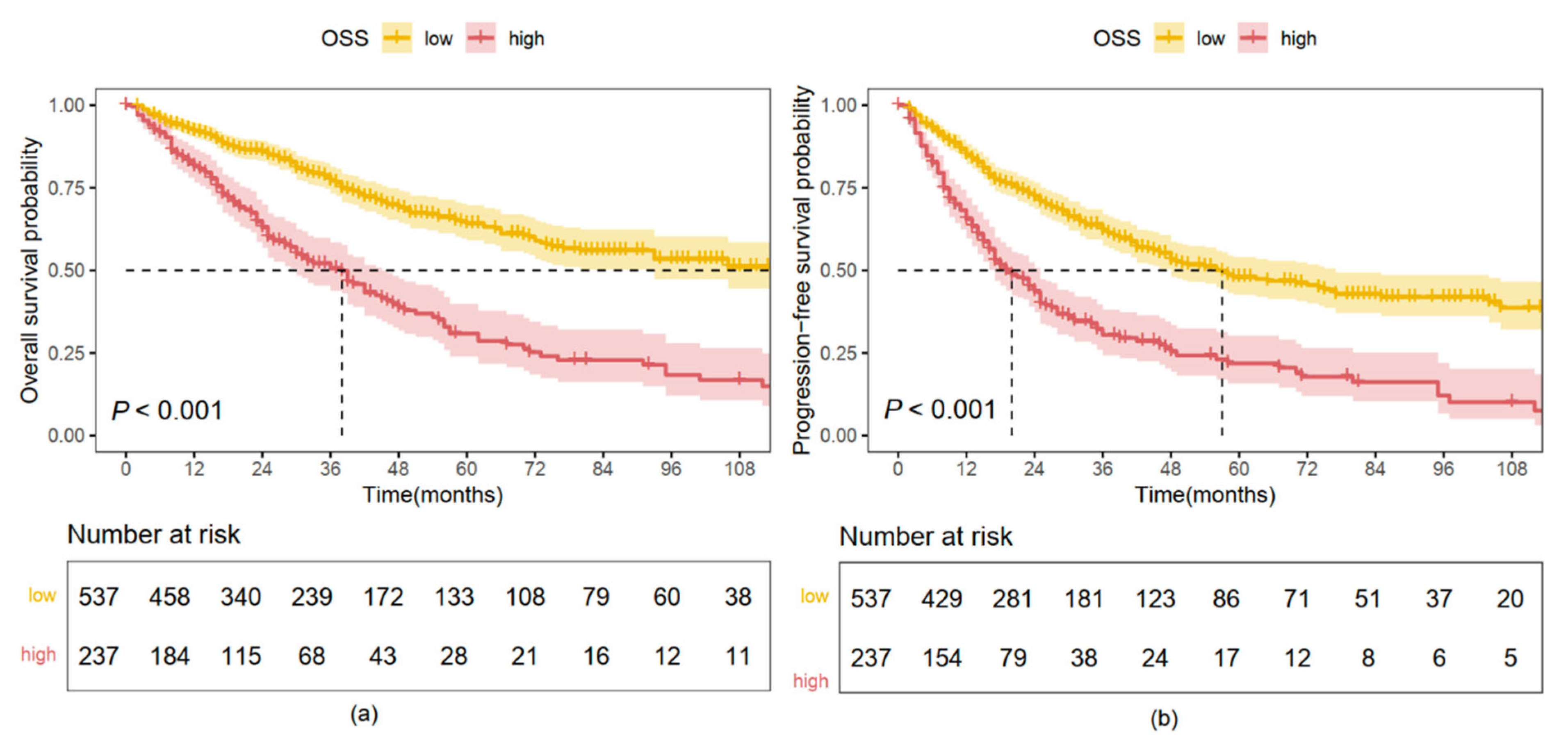
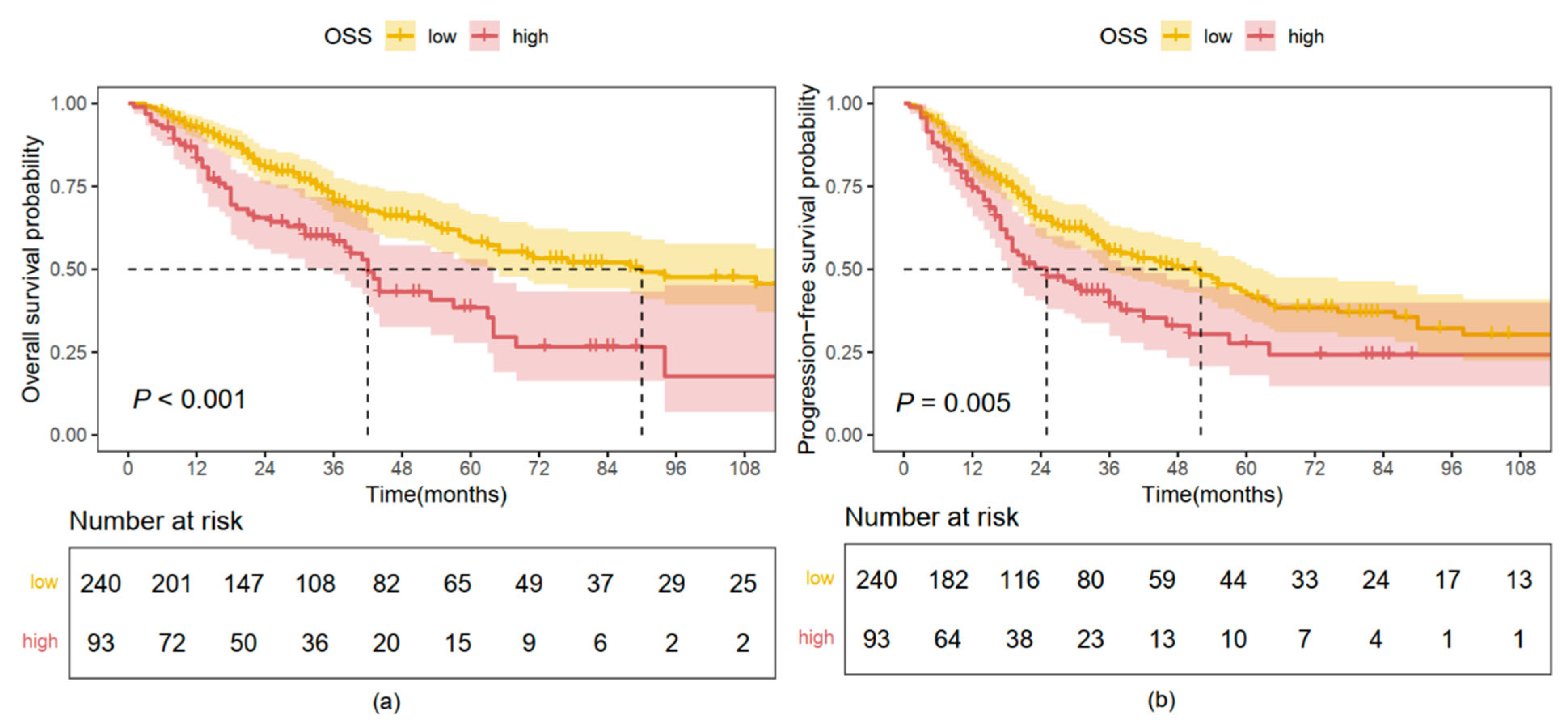
2.3. Kaplan–Meier Analysis Based on OS and PFS
2.4. Independent Prognostic Significance of OSS
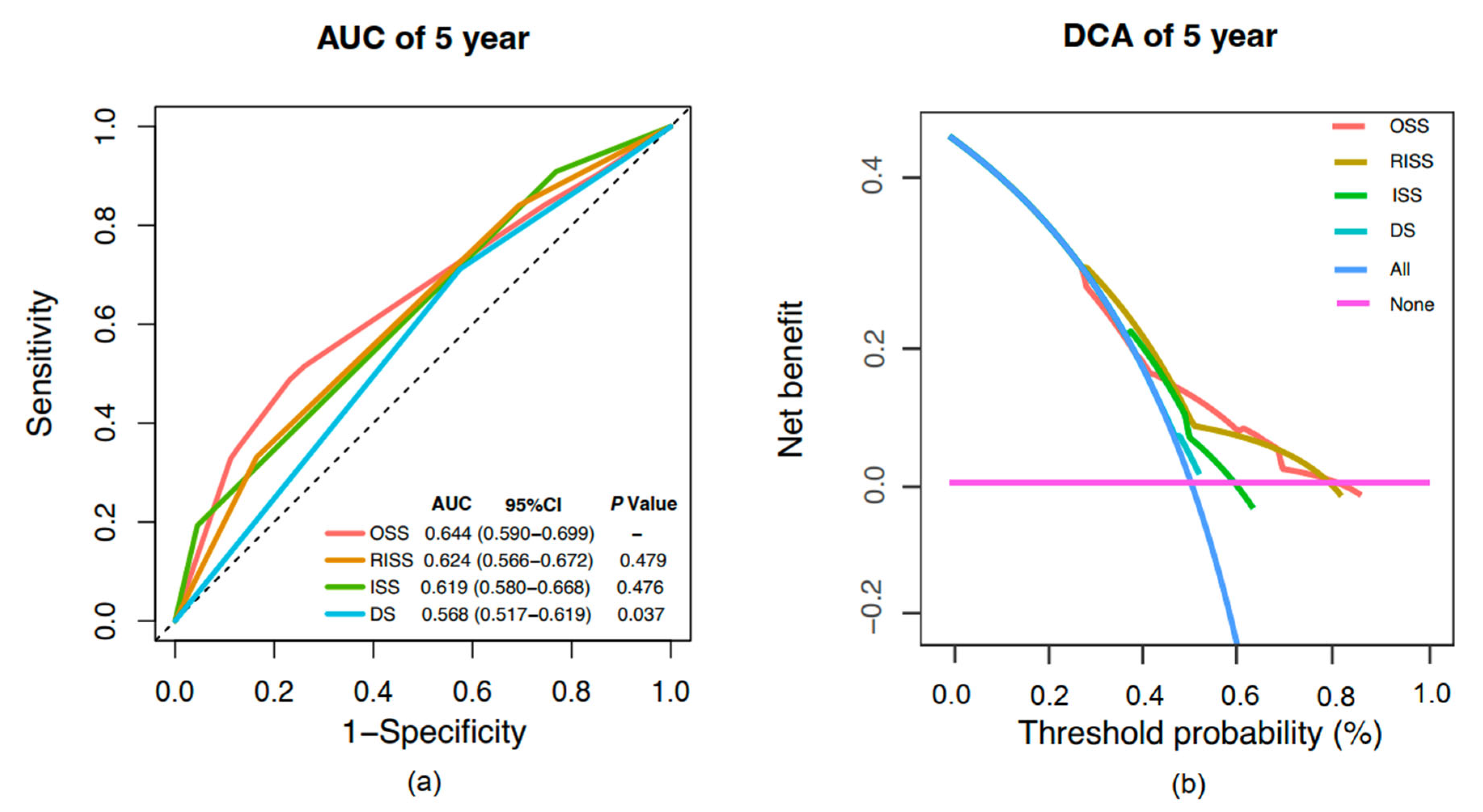
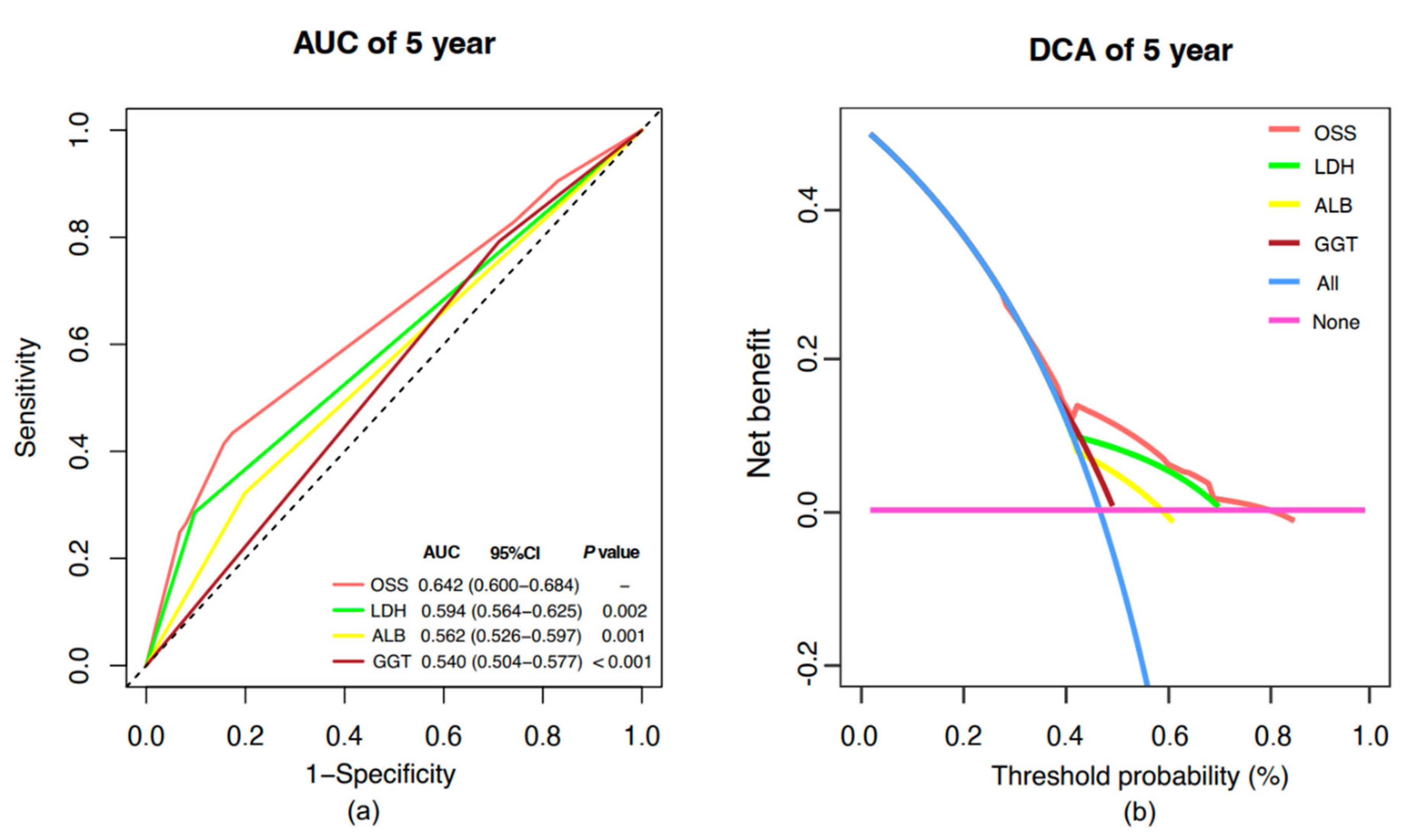
2.5. Model Comparative Assessment and Clinical Benefit Evaluation
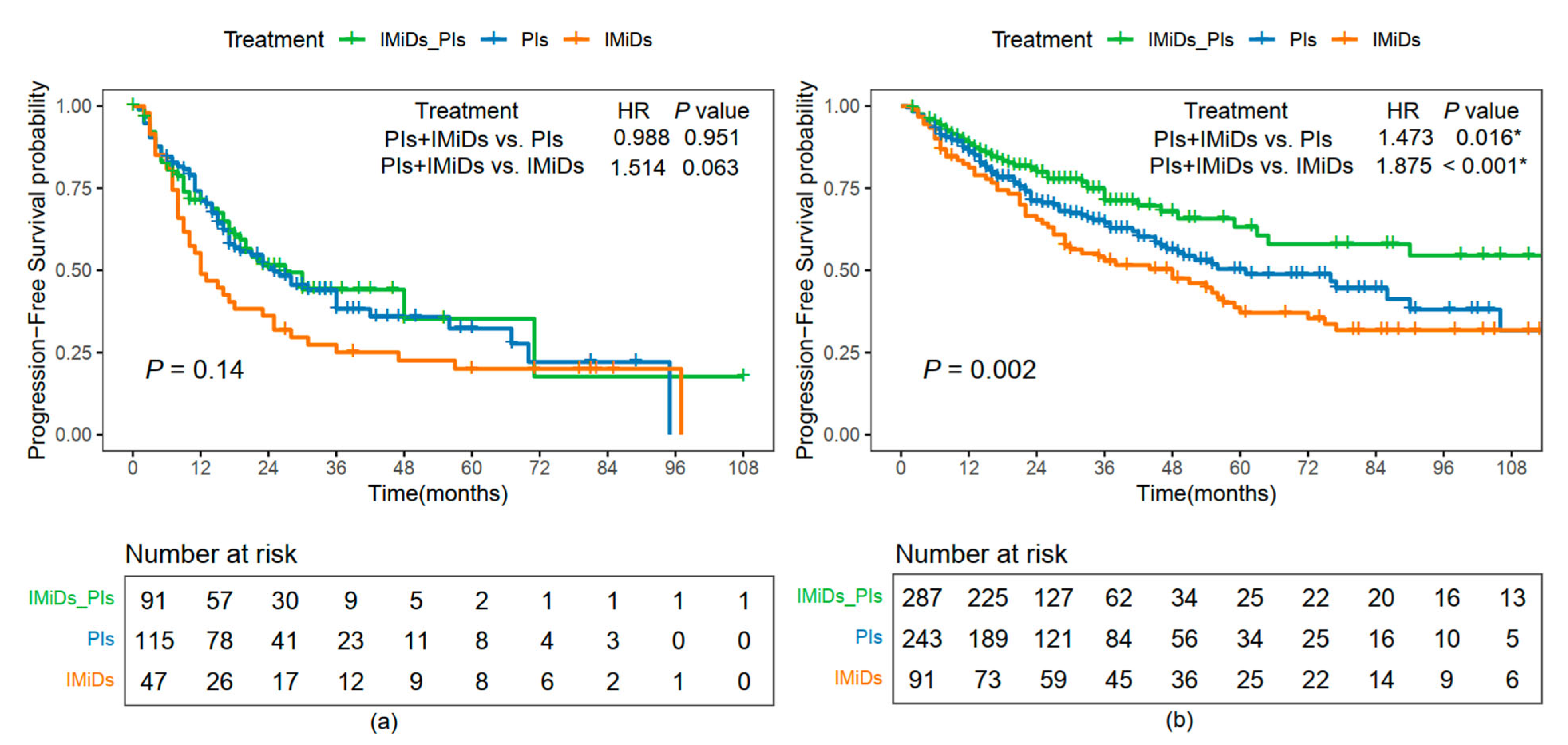
2.6. PFS Comparison Among Different Treatment Options
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Data Collection
4.3. Outcomes
4.4. Model Construction and OSS Calculation
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lipchick, B.C.; Fink, E.E.; Nikiforov, M.A. Oxidative stress and proteasome inhibitors in multiple myeloma. Pharmacol. Res. 2016, 105, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Chan, S.C.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E., III; Xu, W.; Zheng, Z.J.; Elcarte, E.; Withers, M.; Wong, M.C.S.; et al. The epidemiological landscape of multiple myeloma: A global cancer registry estimate of disease burden, risk factors, and temporal trends. Lancet Haematol. 2022, 9, e670–e677. [Google Scholar] [CrossRef] [PubMed]
- Cowan, A.J.; Allen, C.; Barac, A.; Basaleem, H.; Bensenor, I.; Curado, M.P.; Foreman, K.; Gupta, R.; Harvey, J.; Hosgood, H.D.; et al. Global Burden of Multiple Myeloma: A Systematic Analysis for the Global Burden of Disease Study 2016. JAMA Oncol. 2018, 4, 1221–1227. [Google Scholar] [CrossRef]
- Leleu, X.; Hulin, C.; Lambert, J.; Bobin, A.; Perrot, A.; Karlin, L.; Roussel, M.; Montes, L.; Cherel, B.; Chalopin, T.; et al. Isatuximab, lenalidomide, dexamethasone and bortezomib in transplant-ineligible multiple myeloma: The randomized phase 3 BENEFIT trial. Nat. Med. 2024, 30, 2235–2241. [Google Scholar] [CrossRef]
- Yu, B.; Jiang, T.; Liu, D. BCMA-targeted immunotherapy for multiple myeloma. J. Hematol. Oncol. 2020, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Puertas, B.; González-Calle, V.; Sobejano-Fuertes, E.; Escalante, F.; Queizán, J.A.; Bárez, A.; Labrador, J.; Alonso-Alonso, J.M. Novel Agents as Main Drivers for Continued Improvement in Survival in Multiple Myeloma. Cancers 2023, 15, 1558. [Google Scholar] [CrossRef]
- Liu, J.; Chu, B.; Wang, Q.; Dong, J.; Lu, Q.; Shi, L.; Gao, S.; Wang, T.; Wang, F.; Chen, M.; et al. Front-line treatment efficacy and clinical outcomes of elderly patients with multiple myeloma in a real-world setting: A multicenter retrospective study in China. Cancer Med. 2023, 12, 3101–3111. [Google Scholar]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Xiong, S.; Chng, W.-J.; Zhou, J. Crosstalk between endoplasmic reticulum stress and oxidative stress: A dynamic duo in multiple myeloma. Cell. Mol. Life Sci. 2021, 78, 3883–3906. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Y.; Ying, M.; Jin, C.; Li, J.; Hu, X. Lactate dehydrogenases amplify reactive oxygen species in cancer cells in response to oxidative stimuli. Signal Transduct. Target. Ther. 2021, 6, 242. [Google Scholar] [CrossRef]
- Guan, C.; Wang, Y. LncRNA CASC9 attenuates lactate dehydrogenase-mediated oxidative stress and inflammation in spinal cord injury via sponging miR-383-5p. Inflammation 2021, 44, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yao, C.; Zhou, X.; Liu, S.; Qi, L.; Zhu, S.; Zhao, C.; Hu, D.; Shen, W. Glutathione-degrading enzymes in the complex landscape of tumors (review). Int. J. Oncol. 2024, 65, 5660. [Google Scholar] [CrossRef] [PubMed]
- Mitrić, A.; Castellano, I. Targeting gamma-glutamyl transpeptidase: A pleiotropic enzyme involved in glutathione metabolism and in the control of redox homeostasis. Free Radic. Biol. Med. 2023, 208, 672–683. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Zahoor, A.; Ibrahim, M.; Younus, M.; Nawaz, S.; Naseer, R.; Akram, Q.; Deng, C.-L.; Ojha, S.C. Enhanced Efficacy of Direct-Acting Antivirals in Hepatitis C Patients by Coadministration of Black Cumin and Ascorbate as Antioxidant Adjuvants. Oxid. Med. Cell. Longev. 2020, 2020, 7087921. [Google Scholar] [CrossRef]
- Sitar, M.E.; Aydin, S.; Çakatay, U. Human Serum Albumin and Its Relation with Oxidative Stress. Clin. Lab 2013, 59, 945–952. [Google Scholar] [CrossRef]
- Liu, L.; Xie, K.; Yin, M.; Chen, X.; Chen, B.; Ke, J.; Wang, C. Serum potassium, albumin and vitamin B12 as potential oxidative stress markers of fungal peritonitis. Ann. Med. 2021, 53, 2132–2141. [Google Scholar] [CrossRef]
- Sezer, M.T.; Akin, H.; Demir, M.; Erturk, J.; Aydin, Z.D.; Savik, E.; Tunc, N. The Effect of serum albumin level on iron-induced oxidative stress in chronic renal failure patients. J. Nephrol. 2007, 20, 196–203. [Google Scholar]
- Periasamy, S.; Hsu, D.-Z.; Fu, Y.-H.; Liu, M.-Y. Sleep deprivation-induced multi-organ injury: Role of oxidative stress and inflammation. EXCLI J. 2015, 14, 672–683. [Google Scholar]
- Sandesc, M.; Rogobete, A.F.; Bedreag, O.H.; Dinu, A.; Papurica, M.; Cradigati, C.A.; Sarandan, M.; Popovici, S.E.; Bratu, L.M.; Bratu, T.; et al. Analysis of oxidative stress-related markers in critically ill polytrauma patients: An observational prospective single-center study. Bosn. J. Basic Med. Sci. 2018, 18, 191–197. [Google Scholar] [CrossRef]
- Sánchez de Medina, F.; Martínez-Augustin, O.; González, R.; Ballester, I.; Nieto, A.; Gálvez, J.; Zarzuelo, A. Induction of alkaline phosphatase in the inflamed intestine: A novel pharmacological target for inflammatory bowel disease. Biochem. Pharmacol. 2004, 68, 2317–2326. [Google Scholar] [CrossRef]
- Cavo, M.; Capra, A.; Wester, R.; Bonello, F.; Creixenti, J.B.; van de Donk, N.W.; Boccadoro, M.; Larocca, A.; Gay, F.; Mateos, M.-V.; et al. Second Revision of the International Staging System (R2-ISS) for Overall Survival in Multiple Myeloma: A European Myeloma Network (EMN) Report Within the HARMONY Project. J. Clin. Oncol. 2022, 40, 3406–3418. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report from International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef] [PubMed]
- Plano, F.; Gigliotta, E.; Corsale, A.M.; Azgomi, M.S.; Santonocito, C.; Ingrascì, M.; Di Carlo, L.; Augello, A.E.; Speciale, M.; Vullo, C.; et al. Ferritin Metabolism Reflects Multiple Myeloma Microenvironment and Predicts Patient Outcome. Int. J. Mol. Sci. 2023, 24, 8852. [Google Scholar] [CrossRef] [PubMed]
- Misra, U.K.; Kalita, J.; Singh, S.K.; Rahi, S.K. Oxidative Stress Markers in Vitamin B12 Deficiency. Mol. Neurobiol. 2017, 54, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Li, L.; Chen, Q.; Li, M.; Feng, J.; Sun, Y.; Zhao, R.; Zhu, Y.; Lv, Y.; Zhu, Z.; et al. PGC1β regulates multiple myeloma tumor growth through LDHA-mediated glycolytic metabolism. Mol. Oncol. 2018, 12, 1579–1595. [Google Scholar] [CrossRef]
- Ndrepepa, G.; Kastrati, A. Gamma-glutamyl transferase and cardiovascular disease. Ann. Transl. Med. 2016, 4, 481. [Google Scholar] [CrossRef]
- Blomhoff, R.; Jacobs, D.R.; Lee, D.-H. Is serum gamma glutamyltransferase a marker of oxidative stress? Free Radic. Res. 2004, 38, 535–539. [Google Scholar] [CrossRef]
- Simone, G.D.; di Masi, A.; Ascenzi, P. Serum Albumin: A Multifaced Enzyme. Int. J. Mol. Sci. 2021, 22, 10086. [Google Scholar] [CrossRef]
- Ria, R.; Vacca, A. Bone Marrow Stromal Cells-Induced Drug Resistance in Multiple Myeloma. Int. J. Mol. Sci. 2020, 21, 613. [Google Scholar] [CrossRef]
- Lang, E.; Lang, F. Mechanisms and pathophysiological significance of eryptosis, the suicidal erythrocyte death. Semin. Cell Dev. Biol. 2015, 39, 35–42. [Google Scholar] [CrossRef]
- Bissinger, R.; Bhuyan, A.A.M.; Qadri, S.M.; Lang, F. Oxidative stress, eryptosis and anemia: A pivotal mechanistic nexus in systemic diseases. FEBS J. 2019, 286, 826–854. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, S. Oxidative stress in the regulation of normal and neoplastic hematopoiesis. Antioxid. Redox Signal 2008, 10, 1923–1940. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Generating survival times to simulate cox proportional hazards models with time-varying covariates. Stat. Med. 2012, 31, 3946–3958. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | OSS-Low (n = 537, %) | OSS-High (n = 237, %) | p Value |
|---|---|---|---|
| Sex | 0.132 | ||
| Female | 239 (44.5) | 91 (38.4) | |
| Male | 298 (55.5) | 146 (61.6) | |
| Age (years) | 0.017 | ||
| Median (IQR) | 59 (52–65) | 62 (55–68) | |
| <65 | 379 (70.6) | 146 (61.6) | |
| ≥65 | 158 (29.4) | 91 (38.4) | |
| ECOG * | 0.729 | ||
| <2 | 521 (97.7) | 228 (97.0) | |
| ≥2 | 12 (2.3) | 7 (3.0) | |
| DS | <0.001 | ||
| I | 71 (13.2) | 14 (5.9) | |
| II | 113 (21.0) | 27 (11.4) | |
| III | 353 (65.7) | 196 (82.7) | |
| ISS | <0.001 | ||
| I | 203 (37.8) | 29 (12.2) | |
| II | 147 (27.4) | 82 (34.6) | |
| III | 187 (34.8) | 126 (53.2) | |
| RISS * | <0.001 | ||
| I | 88 (24.9) | 8 (3.9) | |
| II | 246 (69.5) | 131 (63.3) | |
| III | 20 (5.6) | 68 (32.9) | |
| R2ISS * | <0.001 | ||
| I | 77 (28.1) | 4 (3.0) | |
| II | 76 (27.7) | 29 (21.8) | |
| III | 113 (41.2) | 80 (60.2) | |
| IV | 8 (2.9) | 20 (15.0) | |
| Extramedullary disease | 0.226 | ||
| No | 410 (76.4) | 191 (80.6) | |
| Yes | 127 (23.6) | 46 (19.4) | |
| Cytogenetics * | 0.543 | ||
| Standard Risk | 203 (74.1) | 94 (70.7) | |
| High Risk | 71 (25.9) | 39 (29.3) | |
| Transplant | 0.009 | ||
| No | 444 (82.7) | 214 (90.3) | |
| Yes | 93 (17.3) | 23 (9.7) | |
| Treatment | 0.377 | ||
| PIs | 172 (32.0) | 78 (32.9) | |
| IMiDs | 61 (11.4) | 33 (13.9) | |
| IMiDs–PIs | 198 (36.9) | 72 (30.4) | |
| Other | 106 (19.7) | 54 (22.8) | |
| β2-MG (mg/L) | <0.001 | ||
| Mean (SD) | 5.2 (±7.1) | 7.6 (±7.7) | |
| <3.5 | 287 (53.4) | 55 (23.2) | |
| ≥3.5 | 250 (46.6) | 182 (76.8) | |
| HGB (g/L) | <0.001 | ||
| Mean (SD) | 106.2 (±26.7) | 90.2 (±27.1) | |
| <120 | 353 (65.7) | 193 (81.4) | |
| ≥120 | 184 (34.3) | 44 (18.6) |
| Variables | Univariate Cox Analysis | Multivariate Cox Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Female | Reference | |||
| Male | 1.158 (0.916–1.465) | 0.220 | ||
| Age (years) | ||||
| <65 | Reference | Reference | ||
| ≥65 | 1.480 (1.165–1.880) | 0.001 | 1.077 (0.841–1.379) | 0.555 |
| ECOG | ||||
| <2 | Reference | Reference | ||
| ≥2 | 4.797 (2.735–8.411) | <0.001 | 3.834 (2.151–6.832) | <0.001 |
| DS | ||||
| I | Reference | Reference | ||
| II | 1.198 (0.773–1.856) | 0.420 | 1.034 (0.653–1.637) | 0.887 |
| III | 1.675 (1.149–2.442) | 0.007 | 1.043 (0.680–1.600) | 0.847 |
| ISS | ||||
| I | Reference | |||
| II | 2.042 (1.493–2.791) | <0.001 | ||
| III | 2.481 (1.830–3.365) | <0.001 | ||
| RISS | ||||
| I | Reference | Reference | ||
| II | 2.515 (1.823–4.813) | <0.001 | 1.401 (0.802–2.449) | 0.236 |
| III | 5.711 (3.339–9.768) | <0.001 | 1.529 (0.781–2.995) | 0.216 |
| R2ISS | ||||
| I | Reference | |||
| II | 1.880 (1.038–3.408) | 0.037 | ||
| III | 2.493 (1.438–4.322) | 0.001 | ||
| IV | 4.280 (2.044–8.964) | <0.001 | ||
| Cytogenetic | ||||
| Standard Risk | Reference | 0.896 | ||
| High Risk | 1.028 (0.677–1.562) | |||
| Extramedullary Disease | ||||
| No | Reference | Reference | ||
| Yes | 0.717 (0.527–0.976) | 0.035 | 0.808 (0.584–1.118) | 0.198 |
| Transplant | ||||
| No | Reference | Reference | ||
| Yes | 0.263 (0.157–0.443) | <0.001 | 0.319 (0.188–0.540) | <0.001 |
| β2-MG (mg/L) | ||||
| <3.5 | Reference | Reference | ||
| ≥3.5 | 2.198 (1.720–2.809) | <0.001 | 1.427 (1.056–1.926) | 0.020 |
| HGB (g/L) | ||||
| <120 | Reference | Reference | ||
| ≥120 | 0.532 (0.402–0.704) | <0.001 | 0.783 (0.565–1.084) | 0.141 |
| OSS | ||||
| Low | Reference | Reference | ||
| High | 2.680 (2.125–3.380) | <0.001 | 2.038 (1.552–2.677) | <0.001 |
| Variables | Univariate Cox Analysis | Multivariate Cox Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | |
| Sex | ||||
| Female | Reference | |||
| Male | 1.070 (0.871–1.314) | 0.519 | ||
| Age (year) | ||||
| <65 | Reference | Reference | ||
| ≥65 | 1.306 (1.045–1.613) | 0.013 | 0.980 (0.786–1.221) | 0.855 |
| ECOG | ||||
| <2 | Reference | Reference | ||
| ≥2 | 3.068 (1.759–5.352) | <0.001 | 2.608 (1.486–4.575) | 0.001 |
| DS | ||||
| I | Reference | |||
| II | 1.411 (0.949–2.098) | 0.089 | ||
| III | 1.839 (1.302–2.598) | 0.001 | ||
| ISS | ||||
| I | Reference | |||
| II | 1.672 (1.275–2.192) | <0.001 | ||
| III | 2.027 (1.563–2.2629) | <0.001 | ||
| RISS | ||||
| I | Reference | Reference | ||
| II | 1.788 (1.214–2.632) | 0.003 | 1.140 (0.738–1.761) | 0.554 |
| III | 4.181 (2.690–6.498) | <0.001 | 1.708 (0.999–2.920) | 0.051 |
| R2ISS | ||||
| I | Reference | |||
| II | 1.517 (0.949–2.424) | 0.082 | ||
| III | 1.797 (1.169–2.764) | 0.008 | ||
| IV | 4.020 (2.233–7.237) | <0.001 | ||
| Extramedullary Disease | ||||
| No | Reference | |||
| Yes | 0.874 (0.678–1.126) | 0.296 | ||
| Cytogenetic | ||||
| Standard Risk | Reference | |||
| High Risk | 1.092 (0.777–1.536) | 0.611 | ||
| Transplant | ||||
| No | Reference | Reference | ||
| Yes | 0.466 (0.326–0.667) | <0.001 | 0.537 (0.372–0.775) | 0.001 |
| β2-MG (mg/L) | ||||
| <3.5 | Reference | Reference | ||
| ≥3.5 | 1.797 (1.455–2.219) | <0.001 | 1.231 (0.956–1.568) | 0.107 |
| HGB (g/L) | ||||
| <120 | Reference | Reference | ||
| ≥120 | 0.550 (0.432–0.699) | <0.001 | 0.680 (0.522–0.887) | 0.004 |
| OSS | ||||
| Low | Reference | Reference | ||
| High | 2.441 (1.986–3.000) | <0.001 | 1.793 (1.413–2.274) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, Q.; Zhang, L.; Huang, Q.; Lv, W.; Liang, Z.; Liu, S.; Nie, R.; Xia, Z.; Liang, Y.; Wang, Y. Oxidative Stress Score as a Simplified Surrogate for Prognostic Stratification and Therapeutic Decision-Making in Multiple Myeloma. Pharmaceuticals 2025, 18, 878. https://doi.org/10.3390/ph18060878
Liang Q, Zhang L, Huang Q, Lv W, Liang Z, Liu S, Nie R, Xia Z, Liang Y, Wang Y. Oxidative Stress Score as a Simplified Surrogate for Prognostic Stratification and Therapeutic Decision-Making in Multiple Myeloma. Pharmaceuticals. 2025; 18(6):878. https://doi.org/10.3390/ph18060878
Chicago/Turabian StyleLiang, Qi, Limei Zhang, Qianqian Huang, Weiran Lv, Zhijian Liang, Shutong Liu, Runcong Nie, Zhongjun Xia, Yang Liang, and Yun Wang. 2025. "Oxidative Stress Score as a Simplified Surrogate for Prognostic Stratification and Therapeutic Decision-Making in Multiple Myeloma" Pharmaceuticals 18, no. 6: 878. https://doi.org/10.3390/ph18060878
APA StyleLiang, Q., Zhang, L., Huang, Q., Lv, W., Liang, Z., Liu, S., Nie, R., Xia, Z., Liang, Y., & Wang, Y. (2025). Oxidative Stress Score as a Simplified Surrogate for Prognostic Stratification and Therapeutic Decision-Making in Multiple Myeloma. Pharmaceuticals, 18(6), 878. https://doi.org/10.3390/ph18060878








