Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress
Abstract
1. Introduction
2. Results
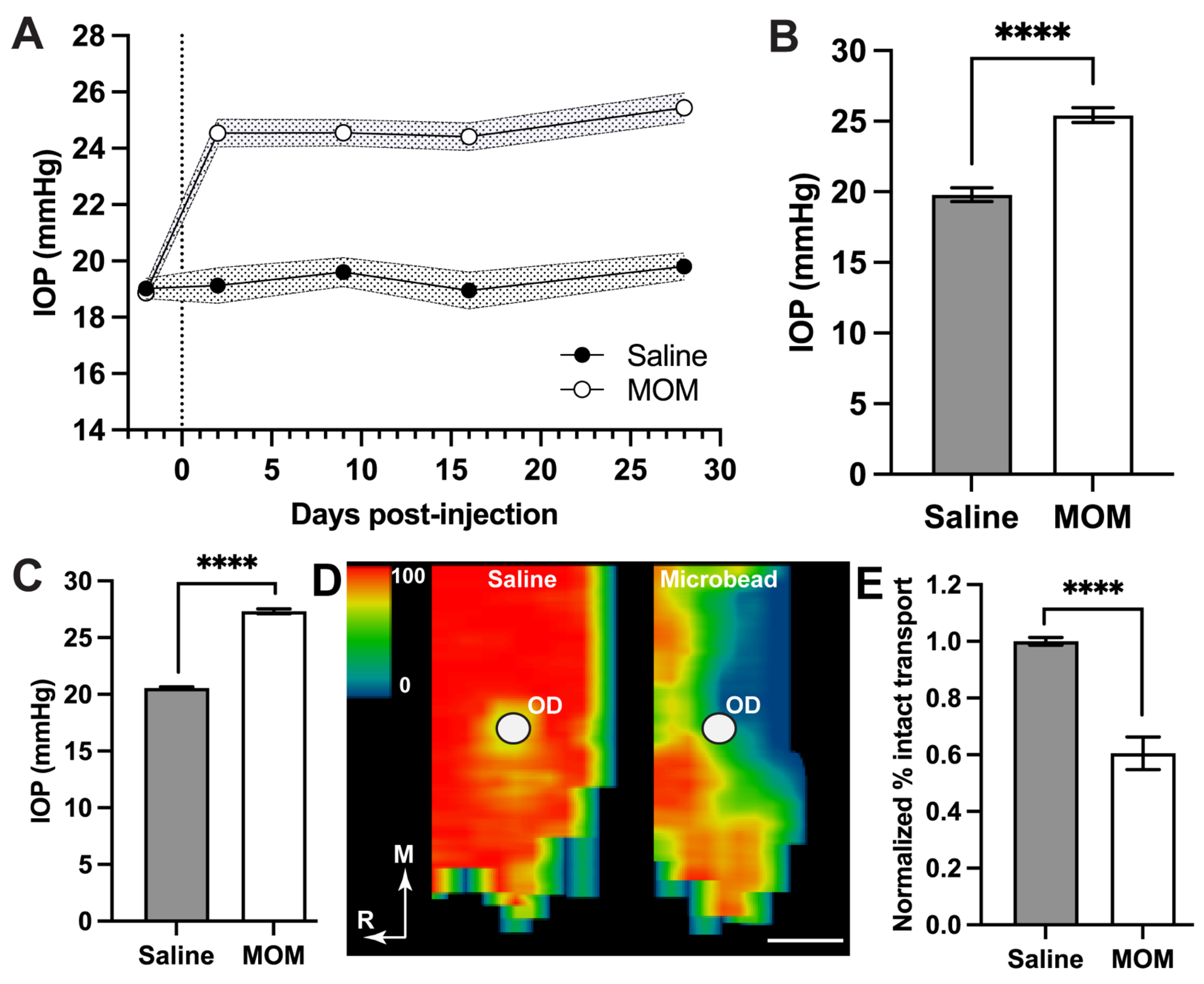
2.1. IOP Elevation Decreases Young’s Modulus for Peripapillary Sclera and Glial Lamina
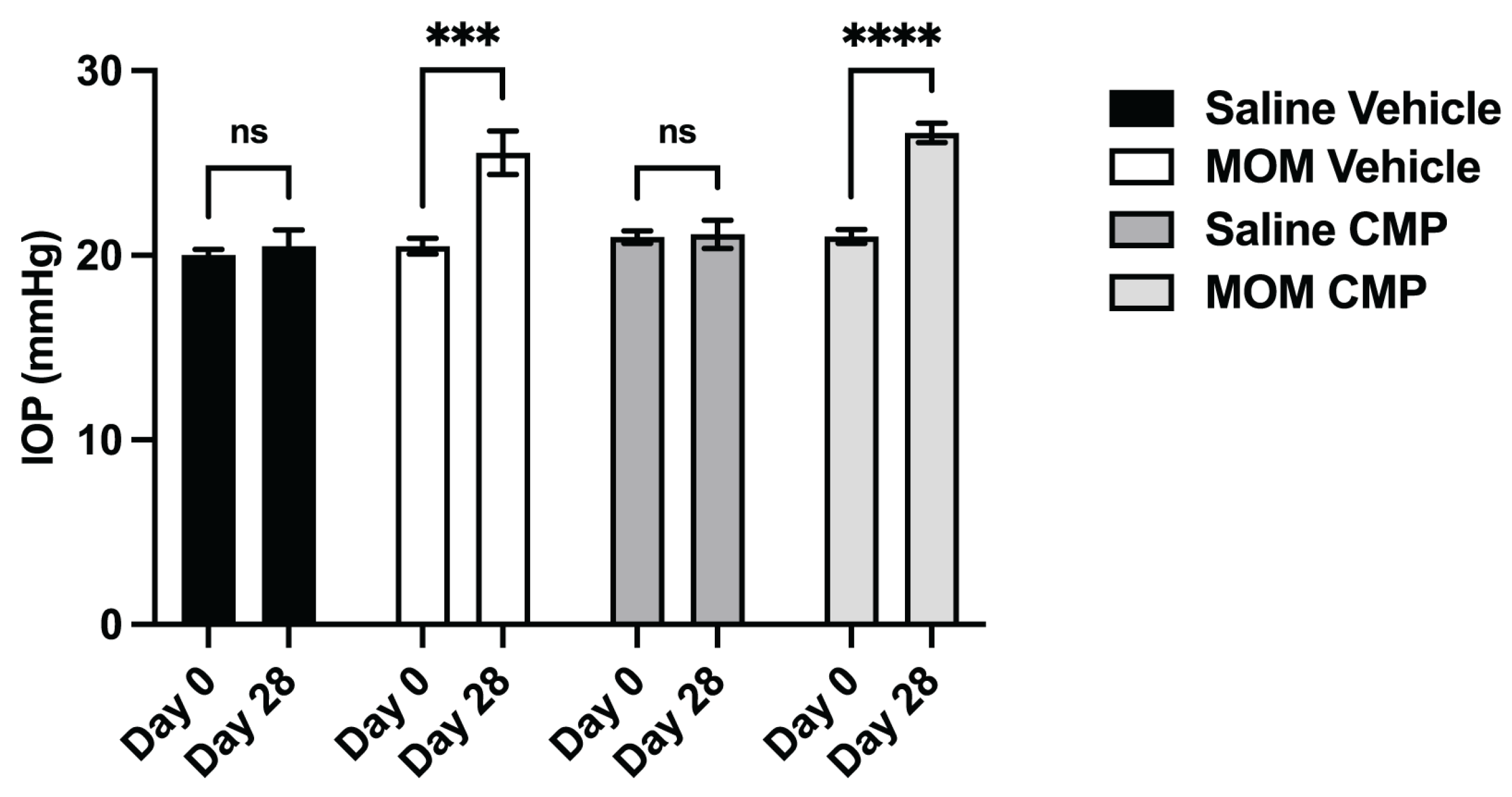
2.2. Collagen Mimetic Peptides Counter the Influence of IOP Elevation on Tissue Stiffness
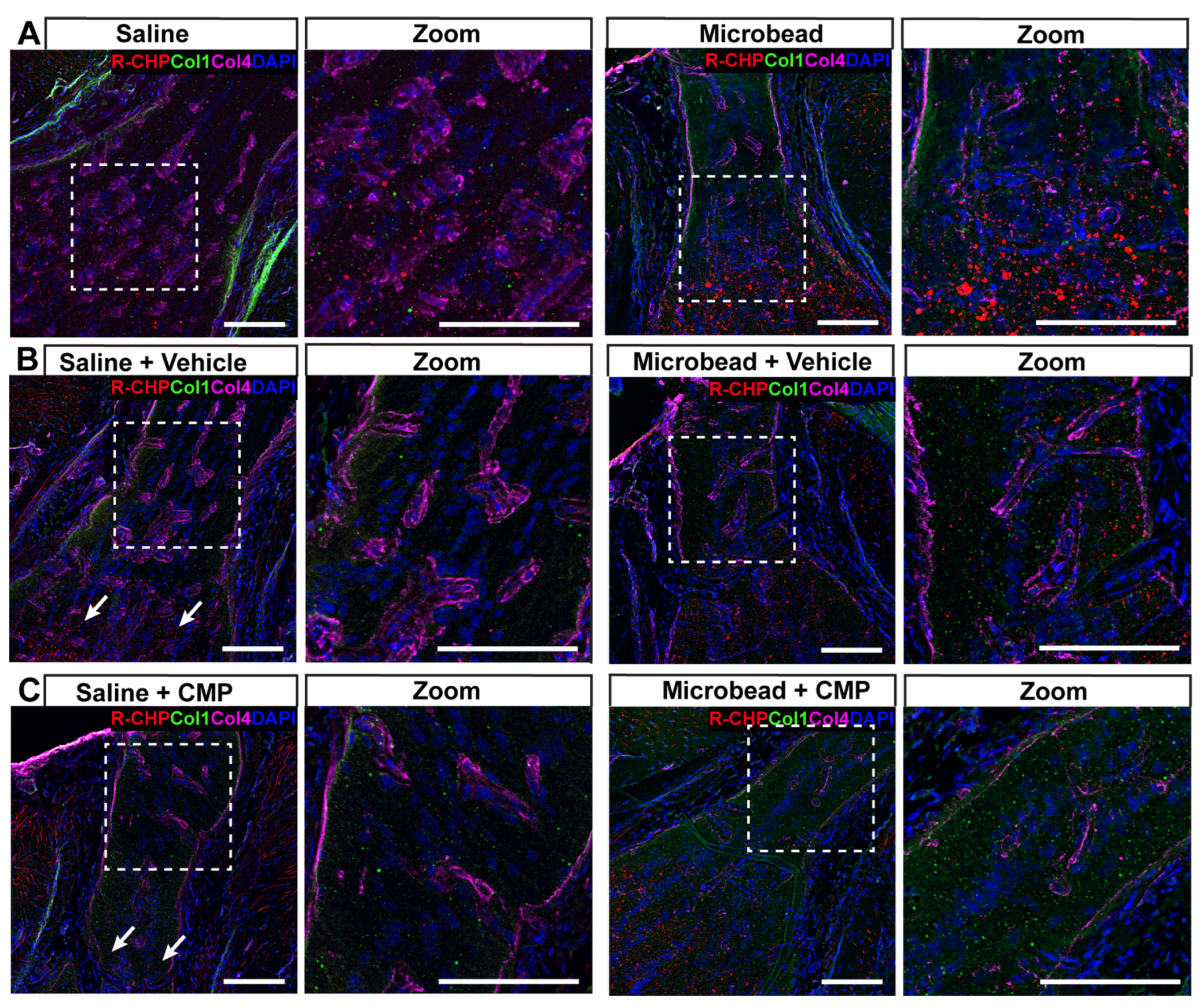
2.3. Collagen Mimetic Peptides Reduce Levels of Fragmented Collagen
3. Discussion
4. Materials and Methods
4.1. Animal Model
4.2. Collagen Mimetic Peptide
4.3. Cholera Toxin B Assessment of RGC Axon Transport in Brain
4.4. Tissue Preparation
4.5. Atomic Force Microscopy (AFM)
4.6. Immunohistochemistry
4.7. Confocal Microscopy
4.8. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFM | Atomic force microscopy |
| CTB | Cholera toxin B |
| CMP | Collagen mimetic peptide |
| ECM | Extracellular matrix |
| IOP | Intraocular pressure |
| GL | Glial lamina |
| MMP | Matrix metalloprotease |
| NDS | Normal donkey serum |
| ONH | Optic nerve head |
| PBS | Phosphate-buffered saline |
| PDL | Poly-D-Lysine |
| PPS | Peripapillary sclera |
| RGC | Retinal ganglion cell |
References
- Fazio, M.A.; Grytz, R.; Morris, J.S.; Bruno, L.; Girkin, C.A.; Downs, J.C. Human scleral structural stiffness increases more rapidly with age in donors of African descent compared to donors of European descent. Investig. Ophthalmol. Vis. Sci. 2014, 55, 7189–7198. [Google Scholar] [CrossRef] [PubMed]
- Yuhas, P.T.; Roberts, C.J. Clinical Ocular Biomechanics: Where Are We after 20 Years of Progress? Curr. Eye Res. 2023, 48, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hu, G.; Yan, Y.; Wang, Z.; Liu, X.; Shi, H. Biomechanical analysis of ocular diseases and its in vitro study methods. Biomed. Eng. Online 2022, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Sigal, I.A.; Flanagan, J.G.; Ethier, C.R. Factors influencing optic nerve head biomechanics. Investig. Ophthalmol. Vis. Sci. 2005, 46, 4189–4199. [Google Scholar] [CrossRef]
- Pallikaris, I.G.; Kymionis, G.D.; Ginis, H.S.; Kounis, G.A.; Tsilimbaris, M.K. Ocular rigidity in living human eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 409–414. [Google Scholar] [CrossRef]
- Wang, W.; Du, S.; Zhang, X. Corneal Deformation Response in Patients with Primary Open-Angle Glaucoma and in Healthy Subjects Analyzed by Corvis ST. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5557–5565. [Google Scholar] [CrossRef]
- Catania, F.; Morenghi, E.; Rosetta, P.; Paolo, V.; Vinciguerra, R. Corneal Biomechanics Assessment with Ultra High Speed Scheimpflug Camera in Primary Open Angle Glaucoma Compared with Healthy Subjects: A meta-analysis of the Literature. Curr. Eye Res. 2023, 48, 161–171. [Google Scholar] [CrossRef]
- Ferrara, M.; Lugano, G.; Sandinha, M.T.; Kearns, V.R.; Geraghty, B.; Steel, D.H.W. Biomechanical properties of retina and choroid: A comprehensive review of techniques and translational relevance. Eye 2021, 35, 1818–1832. [Google Scholar] [CrossRef]
- McMonnies, C.W. An examination of the relation between intraocular pressure, fundal stretching and myopic pathology. Clin. Exp. Optom. 2016, 99, 113–119. [Google Scholar] [CrossRef]
- Park, J.; Shin, A.; Jafari, S.; Demer, J.L. Material properties and effect of preconditioning of human sclera, optic nerve, and optic nerve sheath. Biomech. Model. Mechanobiol. 2021, 20, 1353–1363. [Google Scholar] [CrossRef]
- Coudrillier, B.; Campbell, I.C.; Read, A.T.; Geraldes, D.M.; Vo, N.T.; Feola, A.; Mulvihill, J.; Albon, J.; Abel, R.L.; Ethier, C.R. Effects of Peripapillary Scleral Stiffening on the Deformation of the Lamina Cribrosa. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2666–2677. [Google Scholar] [CrossRef] [PubMed]
- Schultz, D.S.; Lotz, J.C.; Lee, S.M.; Trinidad, M.L.; Stewart, J.M. Structural factors that mediate scleral stiffness. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4232–4236. [Google Scholar] [CrossRef]
- McBrien, N.A.; Gentle, A. Role of the sclera in the development and pathological complications of myopia. Prog. Retin. Eye Res. 2003, 22, 307–338. [Google Scholar] [CrossRef]
- Wallman, J.; Gottlieb, M.D.; Rajaram, V.; Fugate-Wentzek, L.A. Local retinal regions control local eye growth and myopia. Science 1987, 237, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Moeendarbary, E.; Weber, I.P.; Sheridan, G.K.; Koser, D.E.; Soleman, S.; Haenzi, B.; Bradbury, E.J.; Fawcett, J.; Franze, K. The soft mechanical signature of glial scars in the central nervous system. Nat. Commun. 2017, 8, 14787. [Google Scholar] [CrossRef]
- Keeley, F.W.; Morin, J.D.; Vesely, S. Characterization of collagen from normal human sclera. Exp. Eye Res. 1984, 39, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Schwaner, S.A.; Feola, A.J.; Ethier, C.R. Factors affecting optic nerve head biomechanics in a rat model of glaucoma. J. R. Soc. Interface 2020, 17, 20190695. [Google Scholar] [CrossRef]
- Zhang, L.; Albon, J.; Jones, H.; Gouget, C.L.; Ethier, C.R.; Goh, J.C.; Girard, M.J. Collagen microstructural factors influencing optic nerve head biomechanics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 2031–2042. [Google Scholar] [CrossRef]
- Sigal, I.A. Interactions between Geometry and Mechanical Properties on the Optic Nerve Head. Investig. Ophthalmol. Vis. Sci. 2009, 50, 2785–2795. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Luo, X.X.; Igoe, F.; Neufeld, A.H. Extracellular Matrix of the Human Lamina Cribrosa. Am. J. Ophthalmol. 1987, 104, 567–576. [Google Scholar] [CrossRef]
- Morrison, J.; Farrell, S.; Johnson, E.; Deppmeier, L.; Moore, C.; Grossmann, E. Structure and composition of the rodent lamina cribrosa. Exp. Eye Res. 1995, 60, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Lye-Barthel, M.; Masland, R.H.; Jakobs, T.C. The morphology and spatial arrangement of astrocytes in the optic nerve head of the mouse. J. Comp. Neurol. 2009, 516, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, A.P.; Jan, N.J.; Hua, Y.; Yang, B.; Sigal, I.A. Peripapillary sclera architecture revisited: A tangential fiber model and its biomechanical implications. Acta Biomater. 2018, 79, 113–122. [Google Scholar] [CrossRef]
- Agapova, O.A.; Ricard, C.S.; Salvador-Silva, M.; Hernandez, M.R. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia 2001, 33, 205–216. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Agapova, O.A.; Yang, P.; Salvador-Silva, M.; Ricard, C.S.; Aoi, S. Differential gene expression in astrocytes from human normal and glaucomatous optic nerve head analyzed by cDNA microarray. Glia 2002, 38, 45–64. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Lucarelli, M.J.; Gabelt, B.T.; Hernandez, M.R. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003, 967, 132–143. [Google Scholar] [CrossRef]
- Quigley, H.A.; Dorman-Pease, M.E.; Brown, A.E. Quantitative study of collagen and elastin of the optic nerve head and sclera in human and experimental monkey glaucoma. Curr. Eye Res. 1991, 10, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Morrison, J.C.; Farrell, S.; Deppmeier, L.; Moore, C.G.; McGinty, M.R. The Effect of Chronically Elevated Intraocular Pressure on the Rat Optic Nerve Head Extracellular Matrix. Exp. Eye Res. 1996, 62, 663–674. [Google Scholar] [CrossRef]
- Morrison, J.C.; Dorman-Pease, M.E.; Dunkelberger, G.R.; Quigley, H.A. Optic Nerve Head Extracellular Matrix in Primary Optic Atrophy and Experimental Glaucoma. Arch. Ophthalmol. 1990, 108, 1020–1024. [Google Scholar] [CrossRef]
- Hernandez, M.R.; Andrzejewska, W.M.; Neufeld, A.H. Changes in the Extracellular Matrix of the Human Optic Nerve Head in Primary Open-Angle Glaucoma. Am. J. Ophthalmol. 1990, 109, 180–188. [Google Scholar] [CrossRef]
- Burgoyne, C.F.; Crawford Downs, J.; Bellezza, A.J.; Francis Suh, J.K.; Hart, R.T. The optic nerve head as a biomechanical structure: A new paradigm for understanding the role of IOP-related stress and strain in the pathophysiology of glaucomatous optic nerve head damage. Prog. Retin. Eye Res. 2005, 24, 39–73. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, A.; Fox, M.A.; Pihlajaniemi, T. Collagens as New Players in Nervous System Diseases. In The Collagen Superfamily and Collagenopathies; Ruggiero, F., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 289–338. [Google Scholar]
- Wareham, L.K.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Collagen in the central nervous system: Contributions to neurodegeneration and promise as a therapeutic target. Mol. Neurodegener. 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liu, L.; Liu, Y.; Ren, J.; Qian, X. Mechanical property changes of glial LC and RGC axons in response to high intraocular pressure. Front. Bioeng. Biotechnol. 2025, 13, 1574231. [Google Scholar] [CrossRef]
- Lambert, W.S.; Carlson, B.J.; Formichella, C.R.; Sappington, R.M.; Ahlem, C.; Calkins, D.J. Oral Delivery of a Synthetic Sterol Reduces Axonopathy and Inflammation in a Rodent Model of Glaucoma. Front. Neurosci. 2017, 11, 45. [Google Scholar] [CrossRef]
- Lambert, W.S.; Pasini, S.; Collyer, J.W.; Formichella, C.R.; Ghose, P.; Carlson, B.J.; Calkins, D.J. Of Mice and Monkeys: Neuroprotective Efficacy of the p38 Inhibitor BIRB 796 Depends on Model Duration in Experimental Glaucoma. Sci. Rep. 2020, 10, 8535. [Google Scholar] [CrossRef]
- Calkins, D.J. Adaptive responses to neurodegenerative stress in glaucoma. Prog. Retin. Eye Res. 2021, 84, 100953. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Guthrie, K.M.; Teixeira, L.; Murphy, C.J.; Dubielzig, R.R.; McAnulty, J.F.; Raines, R.T. Anchoring a cytoactive factor in a wound bed promotes healing. J. Tissue Eng. Regen. Med. 2016, 10, 1012–1020. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Murphy, C.J.; McAnulty, J.F.; Raines, R.T. Peptides that anneal to natural collagen in vitro and ex vivo. Org. Biomol. Chem. 2012, 10, 5892–5897. [Google Scholar] [CrossRef] [PubMed]
- Bou Ghanem, G.O.; Koktysh, D.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Wareham, L.K.; Calkins, D.J. Collagen Mimetic Peptides Promote Repair of MMP-1-Damaged Collagen in the Rodent Sclera and Optic Nerve Head. Int. J. Mol. Sci. 2023, 24, 17031. [Google Scholar] [CrossRef]
- Calkins, D.J. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef]
- McGrady, N.R.; Pasini, S.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Restoring the Extracellular Matrix: A Neuroprotective Role for Collagen Mimetic Peptides in Experimental Glaucoma. Front. Pharmacol. 2021, 12, 764709. [Google Scholar] [CrossRef]
- Ribeiro, M.; McGrady, N.R.; Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Calkins, D.J. Intraocular Delivery of a Collagen Mimetic Peptide Repairs Retinal Ganglion Cell Axons in Chronic and Acute Injury Models. Int. J. Mol. Sci. 2022, 23, 2911. [Google Scholar] [CrossRef]
- Hwang, J.; Huang, Y.; Burwell, T.J.; Peterson, N.C.; Connor, J.; Weiss, S.J.; Yu, S.M.; Li, Y. In situ imaging of tissue remodeling with collagen hybridizing peptides. ACS Nano 2017, 11, 9825–9835. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Yu, S.M.; Li, Y. The chemistry and biology of collagen hybridization. J. Am. Chem. Soc. 2023, 145, 10901–10916. [Google Scholar] [CrossRef] [PubMed]
- Ratnatilaka Na Bhuket, P.; Li, Y.; Yu, S.M. From Collagen Mimetics to Collagen Hybridization and Back. Acc. Chem. Res. 2024, 57, 14767–14772. [Google Scholar] [CrossRef]
- Leon, S.; Yin, Y.; Nguyen, J.; Irwin, N.; Benowitz, L.I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 2000, 20, 4615–4626. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ho, D.; Meng, H.; Chan, T.R.; An, B.; Yu, H.; Brodsky, B.; Jun, A.S.; Michael Yu, S. Direct detection of collagenous proteins by fluorescently labeled collagen mimetic peptides. Bioconjug. Chem. 2013, 24, 9–16. [Google Scholar] [CrossRef]
- Sigal, I.A.; Grimm, J.L. A Few Good Responses: Which Mechanical Effects of IOP on the ONH to Study? Investig. Ophthalmol. Vis. Sci. 2012, 53, 4270–4278. [Google Scholar] [CrossRef] [PubMed]
- Sigal, I.A.; Ethier, C.R. Biomechanics of the optic nerve head. Exp. Eye Res. 2009, 88, 799–807. [Google Scholar] [CrossRef]
- Girard, M.J.A.; Suh, J.-K.F.; Bottlang, M.; Burgoyne, C.F.; Downs, J.C. Biomechanical Changes in the Sclera of Monkey Eyes Exposed to Chronic IOP Elevations. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5656–5669. [Google Scholar] [CrossRef]
- Downs, J.C.; Suh, J.-K.F.; Thomas, K.A.; Bellezza, A.J.; Hart, R.T.; Burgoyne, C.F. Viscoelastic Material Properties of the Peripapillary Sclera in Normal and Early-Glaucoma Monkey Eyes. Investig. Ophthalmol. Vis. Sci. 2005, 46, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Pijanka, J.K.; Coudrillier, B.; Ziegler, K.; Sorensen, T.; Meek, K.M.; Nguyen, T.D.; Quigley, H.A.; Boote, C. Quantitative Mapping of Collagen Fiber Orientation in Non-glaucoma and Glaucoma Posterior Human Sclerae. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5258–5270. [Google Scholar] [CrossRef]
- Coudrillier, B.; Pijanka, J.K.; Jefferys, J.L.; Goel, A.; Quigley, H.A.; Boote, C.; Nguyen, T.D. Glaucoma-related Changes in the Mechanical Properties and Collagen Micro-architecture of the Human Sclera. PLoS ONE 2015, 10, e0131396. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Jiang, J.; Lin, Z.; Zhang, C.; Moonasar, N.; Qian, S. Identification of key genes and pathways in scleral extracellular matrix remodeling in glaucoma: Potential therapeutic agents discovered using bioinformatics analysis. Int. J. Med. Sci. 2021, 18, 1554–1565. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Lim, S.H. Matrix Metalloproteinases and Glaucoma. Biomolecules 2022, 12, 1368. [Google Scholar] [CrossRef]
- Wang, M.; Corpuz, C.C.C.; Zhang, F. Shaping Eyeballs by Scleral Collagen Cross-Linking: A Hypothesis for Myopia Treatment. Front. Med. 2021, 8, 655822. [Google Scholar] [CrossRef]
- Eilaghi, A.; Flanagan, J.G.; Simmons, C.A.; Ethier, C.R. Effects of scleral stiffness properties on optic nerve head biomechanics. Ann. Biomed. Eng. 2010, 38, 1586–1592. [Google Scholar] [CrossRef]
- Cone-Kimball, E.; Nguyen, C.; Oglesby, E.N.; Pease, M.E.; Steinhart, M.R.; Quigley, H.A. Scleral structural alterations associated with chronic experimental intraocular pressure elevation in mice. Mol. Vis. 2013, 19, 2023–2039. [Google Scholar]
- Quigley, H.A.; Pitha, I.F.; Welsbie, D.S.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Berlinicke, C.A.; Mitchell, K.L.; et al. Losartan Treatment Protects Retinal Ganglion Cells and Alters Scleral Remodeling in Experimental Glaucoma. PLoS ONE 2015, 10, e0141137. [Google Scholar] [CrossRef]
- Kimball, E.C.; Nguyen, C.; Steinhart, M.R.; Nguyen, T.D.; Pease, M.E.; Oglesby, E.N.; Oveson, B.C.; Quigley, H.A. Experimental scleral cross-linking increases glaucoma damage in a mouse model. Exp. Eye Res. 2014, 128, 129–140. [Google Scholar] [CrossRef]
- Gerberich, B.G.; Hannon, B.G.; Brown, D.M.; Read, A.T.; Ritch, M.D.; Schrader Echeverri, E.; Nichols, L.; Potnis, C.; Sridhar, S.; Toothman, M.G.; et al. Evaluation of Spatially Targeted Scleral Stiffening on Neuroprotection in a Rat Model of Glaucoma. Transl. Vis. Sci. Technol. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Wareham, L.K.; Kuchtey, J.; Wu, H.J.; Krystofiak, E.; Wu, Y.; Reinhart-King, C.A.; Kuchtey, R.W. Lysyl oxidase-like 1 deficiency alters ultrastructural and biomechanical properties of the peripapillary sclera in mice. Matrix Biol. Plus 2022, 16, 100120. [Google Scholar] [CrossRef] [PubMed]
- Baratta, R.O.; Del Buono, B.J.; Schlumpf, E.; Ceresa, B.P.; Calkins, D.J. Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration. Front. Pharmacol. 2021, 12, 705623. [Google Scholar] [CrossRef]
- Panwar, P.; Butler, G.S.; Jamroz, A.; Azizi, P.; Overall, C.M.; Brömme, D. Aging-associated modifications of collagen affect its degradation by matrix metalloproteinases. Matrix Biol. 2018, 65, 30–44. [Google Scholar] [CrossRef]
- Kazaili, A.; Abdul-Amir Al-Hindy, H.; Madine, J.; Akhtar, R. Nano-Scale Stiffness and Collagen Fibril Deterioration: Probing the Cornea Following Enzymatic Degradation Using Peakforce-QNM AFM. Sensors 2021, 21, 1629. [Google Scholar] [CrossRef]
- Abass, A.; Eliasy, A.; Geraghty, B.; Elabd, M.; Hassan, A.; Elsheikh, A. Effect of freezing and thawing on the biomechanical characteristics of porcine ocular tissues. J. Biomech. 2019, 87, 93–99. [Google Scholar] [CrossRef]
- Clavert, P.; Kempf, J.F.; Bonnomet, F.; Boutemy, P.; Marcelin, L.; Kahn, J.L. Effects of freezing/thawing on the biomechanical properties of human tendons. Surg. Radiol. Anat. 2001, 23, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Weeber, H.A.; Eckert, G.; Soergel, F.; Meyer, C.H.; Pechhold, W.; van der Heijde, R.G. Dynamic mechanical properties of human lenses. Exp. Eye Res. 2005, 80, 425–434. [Google Scholar] [CrossRef]
- Wang, K.; Read, A.T.; Sulchek, T.; Ethier, C.R. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res. 2017, 158, 3–12. [Google Scholar] [CrossRef]
- Pyka-Fościak, G.; Fościak, M.; Pabijan, J.; Lis, G.J.; Litwin, J.A.; Lekka, M. Changes in stiffness of the optic nerve and involvement of neurofilament light chains in the course of experimental autoimmune encephalomyelitis, an animal model of multiple sclerosis. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2023, 1869, 166796. [Google Scholar] [CrossRef]
- Seifert, J.; Hammer, C.M.; Rheinlaender, J.; Sel, S.; Scholz, M.; Paulsen, F.; Schäffer, T.E. Distribution of Young’s Modulus in Porcine Corneas after Riboflavin/UVA-Induced Collagen Cross-Linking as Measured by Atomic Force Microscopy. PLoS ONE 2014, 9, e88186. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Li, T.; Li, L.; Qian, X.; Liu, Z. A feasible method for independently evaluating the mechanical properties of glial LC and RGC axons by combining atomic force microscopy measurement with image segmentation. J. Mech. Behav. Biomed. Mater. 2022, 126, 105041. [Google Scholar] [CrossRef] [PubMed]
- Braunsmann, C.; Hammer, C.M.; Rheinlaender, J.; Kruse, F.E.; Schaffer, T.E.; Schlotzer-Schrehardt, U. Evalution of lamina cribrosa and peripapillary sclera stiffness in pseudoexfoliation and normal eyes by atomic force microscopy. Investig. Ophthalmol. Vis. Sci. 2012, 53, 2960–2967. [Google Scholar] [CrossRef]
- Baratta, R.O.; Schlumpf, E.; Del Buono, B.J.; DeLorey, S.; Ousler, G.; Calkins, D.J. A Phase 2 Trial to Test Safety and Efficacy of ST-100, a Unique Collagen Mimetic Peptide Ophthalmic Solution for Dry Eye Disease. Opthalmol. Sci. 2023, 4, 100451. [Google Scholar] [CrossRef]
- Dapper, J.D.; Crish, S.D.; Pang, I.H.; Calkins, D.J. Proximal inhibition of p38 MAPK stress signaling prevents distal axonopathy. Neurobiol. Dis. 2013, 59, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Sappington, R.M.; Carlson, B.J.; Crish, S.D.; Calkins, D.J. The microbead occlusion model: A paradigm for induced ocular hypertension in rats and mice. Investig. Ophthalmol. Vis. Sci. 2010, 51, 207–216. [Google Scholar] [CrossRef]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wareham, L.K.; Ghanem, G.O.B.; Clark, K.L.; Schlumpf, E.; Del Buono, B.J.; Calkins, D.J. Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress. Pharmaceuticals 2025, 18, 875. https://doi.org/10.3390/ph18060875
Wareham LK, Ghanem GOB, Clark KL, Schlumpf E, Del Buono BJ, Calkins DJ. Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress. Pharmaceuticals. 2025; 18(6):875. https://doi.org/10.3390/ph18060875
Chicago/Turabian StyleWareham, Lauren K., Ghazi O. Bou Ghanem, Kristin L. Clark, Eric Schlumpf, Brian J. Del Buono, and David J. Calkins. 2025. "Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress" Pharmaceuticals 18, no. 6: 875. https://doi.org/10.3390/ph18060875
APA StyleWareham, L. K., Ghanem, G. O. B., Clark, K. L., Schlumpf, E., Del Buono, B. J., & Calkins, D. J. (2025). Topical Application of a Collagen Mimetic Peptide Restores Peripapillary Scleral Stiffness Reduced by Ocular Stress. Pharmaceuticals, 18(6), 875. https://doi.org/10.3390/ph18060875





