Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution
Abstract
1. Introduction
2. Clinical Evidence
3. Basic Evidence
3.1. IAH’s Effectiveness Depending on the Organ(s) Investigated
3.2. Agents’ Effectiveness Depending on the IAH’s Level(s) Investigated
3.3. Agents’ Effectiveness Depending on the Organ(s) Investigated
3.4. Agents’ Effectiveness Considering Application Time
3.5. Agents’ Effectiveness Considering the Animal Model
4. BPC 157 Evidence
4.1. BPC 157 Primary Abdominal Compartment Syndrome
4.2. BPC 157 and Reperfusion After Decompression
4.3. Final Remarks for BPC 157 and Other Agents Used in ACS/IAH Studies
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Vukojević, J.; Siroglavić, M.; Kašnik, K.; Kralj, T.; Stanćić, D.; Kokot, A.; Kolarić, D.; Drmić, D.; Sever, A.Z.; Barišić, I.; et al. Rat inferior caval vein (ICV) ligature and particular new insights with the stable gastric pentadecapeptide BPC 157. Vasc. Pharmacol. 2018, 106, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Horvat Pavlov, K.; Drmic, D.; et al. Complex syndrome of complete occlusion of the end of the superior mesenteric vein, opposed with the stable gastric pentadecapeptide BPC 157 in rats. Biomedicines 2021, 9, 1029. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Knezevic, T.; Vranes, H.; Drmic, D.; Staroveski, M.; et al. Occluded superior mesenteric artery and vein. Therapy with the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 792. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Malekinusic, D.; Vrdoljak, B.; Vranes, H.; Knezevic, T.; Barisic, I.; Horvat Pavlov, K.; et al. Occlusion of the superior mesenteric artery in rats reversed by collateral pathways activation: Gastric pentadecapeptide BPC 157 therapy counteracts multiple organ dysfunction syndrome, intracranial, portal and caval hypertension, and aortal hypotension. Biomedicines 2021, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Kralj, T.; Kokot, A.; Zlatar, M.; Masnec, S.; Kasnik Kovac, K.; Milkovic Perisa, M.; Batelja Vuletic, L.; Giljanovic, A.; Strbe, S.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy of rat glaucoma. Biomedicines 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Horvat Pavlov, K.; Petrovic, A.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; et al. BPC 157 therapy and permanent occlusion of the superior sagittal sinus in rats: Vascular recruitment. Biomedicines 2021, 9, 744. [Google Scholar] [CrossRef] [PubMed]
- Tepes, M.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Madzar, Z.; Santak, G.; Batelja, L.; Milavic, M.; Sikiric, S.; et al. Stable gastric pentadecapeptide BPC 157 therapy for primary abdominal compartment syndrome. Front. Pharmacol. 2021, 12, 718147. [Google Scholar] [CrossRef] [PubMed]
- Tepes, M.; Krezic, I.; Vranes, H.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Vukovic, V.; Oroz, K.; Kovac, K.K.; Madzar, Z.; et al. Stable gastric pentadecapeptide BPC 157: Effect on reperfusion following maintained intra-abdominal hypertension (grade III and grade IV) in rats. Pharmaceuticals 2023, 16, 1554. [Google Scholar] [CrossRef] [PubMed]
- Smoday, I.M.; Petrovic, I.; Kalogjera, L.; Vranes, H.; Zizek, H.; Krezic, I.; Gojkovic, S.; Skorak, I.; Hriberski, K.; Brizic, I.; et al. Therapy effect of the stable gastric pentadecapeptide BPC 157 on acute pancreatitis as vascular failure-induced severe peripheral and central syndrome in rats. Biomedicines 2022, 10, 1299. [Google Scholar] [CrossRef] [PubMed]
- Kalogjera, L.; Krezic, I.; Smoday, I.M.; Vranes, H.; Zizek, H.; Yago, H.; Oroz, K.; Vukovic, V.; Kavelj, I.; Novosel, L.; et al. Stomach perforation-induced general occlusion/occlusion-like syndrome and stable gastric pentadecapeptide BPC 157 therapy effect. World J. Gastroenterol. 2023, 29, 4289–4316. [Google Scholar] [CrossRef] [PubMed]
- Vukojević, J.; Vrdoljak, B.; Malekinušić, D.; Siroglavić, M.; Milavić, M.; Kolenc, D.; Boban Blagaić, A.; Batelja, L.; Drmić, D.; Seiverth, S.; et al. The effect of pentadecapeptide BPC 157 on hippocampal ischemia/reperfusion injuries in rats. Brain Behav. 2020, 10, e01726. [Google Scholar] [CrossRef] [PubMed]
- Barisic, I.; Balenovic, D.; Udovicic, M.; Bardak, D.; Strinic, D.; Vlainić, J.; Vranes, H.; Smoday, I.M.; Krezic, I.; Milavic, M.; et al. Stable gastric pentadecapeptide BPC 157 may counteract myocardial infarction induced by isoprenaline in rats. Biomedicines 2022, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Strbe, S.; Gojkovic, S.; Krezic, I.; Zizek, H.; Vranes, H.; Barisic, I.; Strinic, D.; Orct, T.; Vukojevic, J.; Ilic, S.; et al. Over-dose lithium toxicity as an occlusive-like syndrome in rats and gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1506. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vranes, H.; Zizek, H.; Drmic, D.; Batelja Vuletic, L.; Milavic, M.; Sikiric, S.; Stilinovic, I.; Simeon, P.; et al. Robert’s intragastric alcohol-induced gastric lesion model as an escalated general peripheral and central syndrome, counteracted by the stable gastric pentadecapeptide BPC 157. Biomedicines 2021, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Skoro, M.; Kovac, K.K.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Pentadecapeptide BPC 157 as therapy for inferior caval vein embolization: Recovery of sodium laurate-post-embolization syndrome in rats. Pharmaceuticals 2023, 16, 1507. [Google Scholar] [CrossRef] [PubMed]
- Premuzic Mestrovic, I.; Smoday, I.M.; Kalogjera, L.; Krezic, I.; Zizek, H.; Vranes, H.; Vukovic, V.; Oroz, K.; Skorak, I.; Brizic, I.; et al. Antiarrhythmic sotalol, occlusion/occlusion-like syndrome in rats, and stable gastric pentadecapeptide BPC 157 therapy. Pharmaceuticals 2023, 16, 977. [Google Scholar] [CrossRef] [PubMed]
- Strbe, S.; Smoday, I.M.; Krezic, I.; Kalogjera, L.; Vukovic, V.; Zizek, H.; Gojkovic, S.; Vranes, H.; Barisic, I.; Sikiric, S.; et al. Innate vascular failure by application of neuroleptics, amphetamine, and domperidone rapidly induced severe occlusion/occlusion-like syndromes in rats and stable gastric pentadecapeptide BPC 157 as therapy. Pharmaceuticals 2023, 16, 788. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Roberts, D.J.; Sugrue, M.; De Keulenaer, B.L.; Ivatury, R.; Pelosi, P.; Verbrugge, F.; Wise, R.; Mullens, W. The polycompartment syndrome: A concise state-of-the-art review. Anaesthesiol. Intensive Ther. 2014, 46, 433–450. [Google Scholar] [CrossRef] [PubMed]
- De Laet, I.E.; Ravyts, M.; Vidts, W.; Valk, J.; De Waele, J.J.; Malbrain, M.L. Current insights in intra-abdominal hypertension and abdominal compartment syndrome: Open the abdomen and keep it open! Langenbecks Arch. Surg. 2008, 393, 833–847. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, R.; Wise, R.D.; Myatchin, I.; Vanhonacker, D.; Minini, A.; Mekeirele, M.; Kirkpatrick, A.W.; Pereira, B.M.; Sugrue, M.; De Keulenaer, B.; et al. Fluid managenent, intra-abdominal hypertension and the abdominal compartment syndrome: A narrrative review. Life 2022, 12, 1390. [Google Scholar] [CrossRef] [PubMed]
- De Laet, I.E.; Malbrain, M.L.N.G.; De Waele, J.J. A clinician’s guide to managemet of intra-abdominal hypertensio and abdominal compartment syndrome in critically ill patients. Crit. Care 2020, 24, 97. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I.E.; De Waele, J.J.; Kirkpatrick, A.W. Intra-abdominal hypertension: Definitions, monitoring, interpretation and management. Best Pract. Res. Clin. Anaesthesiol. 2013, 27, 249–270. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I.E.; De Waele, J.J. IAH/ACS: The rationale for surveillance. World J. Surg. 2009, 33, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I.E. Intra-abdominal hypertension: Evolving concepts. Clin. Chest Med. 2009, 30, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I.E. Intra-abdominal hypertension: Evolving concepts. Crit. Care Nurs. Clin. N. Am. 2012, 24, 275–309. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.J., Jr.; Cullinane, D.C.; Dutton, W.D.; Jerome, R.; Bagdonas, R.; Bilaniuk, J.W.; Collier, B.R.; Como, J.J.; Cumming, J.; Griffen, M.; et al. The management of the open abdomen in trauma and emergency general surgery: Part 1—damage control. J. Trauma 2010, 68, 1425–1438. [Google Scholar] [CrossRef] [PubMed]
- Diaz, J.J., Jr.; Dutton, W.D.; Ott, M.M.; Cullinane, D.C.; Alouidor, R.; Armen, S.B.; Bilanuik, J.W.; Collier, B.R.; Gunter, O.L.; Jawa, R.; et al. Eastern Association for the Surgery of Trauma: A review of the management of the open abdomen—Part 2 “Management of the open abdomen”. J. Trauma 2011, 71, 502–512. [Google Scholar] [CrossRef] [PubMed]
- Ylimartimo, A.T.; Lahtinen, S.; Nurkkala, J.; Koskela, M.; Kaakinen, T.; Vakkala, M.; Hietanen, S.; Liisanantti, J. Long-term outcomes after emergency laparatomy: A retrospective study. J. Gastrointest. Surg. 2022, 26, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Ylimartimo, A.T.; Nurkkala, J.; Koskela, M.; Lahtinen, S.; Kaakinen, M.; Vakkala, M.; Hietanene, S.; Liisanantti, J. Postoperative complications and outcome after emergency laparatomy: A retrospective study. J. Surg. 2023, 47, 119–129. [Google Scholar] [CrossRef]
- Li, F.; Jiang, L.; Pan, S.; Jiang, S.; Fan, Y.; Jiang, C.; Gao, C.; Leng, Y. Multi-omic profiling reveals that intra-abdominal-hypertension-induced intestinal damage can be prevented by microbiome and metabolic modulations with 5-hydroxyindoleacetic acid as a diagnostic marker. mSystems 2022, 7, e0120421. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Jia, L.; Yan, Q.Q.; Deng, Q.; Wei, B. Effect of Clostridium butyricum and butyrate on intestinal barrier functions: Study of a rat model of severe acute pancreatitis with intra-abdominal hypertension. Front. Physiol. 2020, 11, 561061. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Tang, H.; Zhang, Q.; Xu, L.; Zhou, W.; Hu, X.; Deng, Y.; Zhang, L. Basic fibroblast growth factor (bFGF) protects the blood-brain barrier by binding of FGFR1 and activating the ERK signaling pathway after intra-abdominal hypertension and traumatic brain injury. Med. Sci. Monit. 2020, 26, e922009. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Jiang, L.; Li, Y.; Bai, G.; Zhao, J.; Zhang, M.; Zhang, J. Hydrogen protects against liver injury during CO(2)pneumoperitoneum in rats. Oncotarget 2017, 9, 2631–2645. [Google Scholar] [CrossRef] [PubMed]
- Al-Saeedi, M.; Nickkholgh, A.; Schultze, D.; Flechtenmacher, C.; Zorn, M.; Liang, R.; Gutt, C.N.; Schemmer, P. Glycine protects liver from reperfusion injury following pneumoperitoneum. Eur. Surg. Res. 2018, 59, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.B.; Bihari, A.; Parry, N.G.; Ball, I.; Leslie, K.; Vogt, K.; Lawendy, A.R. Carbon monoxide and hydrogen sulphide reduce reperfusion injury in abdominal compartment syndrome. J. Surg. Res. 2018, 222, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Tang, H.; Liu, D.; Li, Y.; Zhang, L. Comparison of melatonin, hypertonic saline and hydroxethyl starch for resuscitation of secondary intra-abdominal hypertension in an animal model. PLoS ONE 2016, 11, e0161688. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Li, Y.; Liu, D.; Zhang, L.; Zhang, H.; Tang, H.; Zhang, H. Melatonin. Free Radic. Biol. Med. 2016, 97, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Oh, Y.J.; Kim, O.S.; Na, S. The effects of arginase inhibitor on lung oxidative stress and inflammation caused by pneumoperitoneum in rats. BMC Anesthesiol. 2015, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, S.A.; Ceylan, C.; Serel, T.A.; Doluoglu, O.G.; Soyupek, A.S.; Guzel, A.; Özorak, A.; Uz, E.; Savas, H.B.; Baspinar, S. Protective effect of theophylline on renal functions in experimental pneumoperiotoneum model. Ren. Fail. 2015, 37, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.G.; Chang, M.T.; Li, Y.; Zhang, L.Y. Melanocortin-4 receptor agonists alleviate intestinal dysfunction in secondary intra-abdominal hypertension in rat model. J. Surg. Res. 2015, 195, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Zhang, H.G.; Zhao, Z.A.; Chang, M.T.; Li, Y.; Yu, J.; Zhang, Y.; Zhang, L.Y. Melanocortin MC4 receptor agonists alleviate brain damage in abdominal compartment syndrome in the rat. Neuropeptides 2015, 49, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Davarci, I.; Alp, H.; Ozgur, T.; Karcioglu, M.; Tuzcu, K.; Evliyaoglu, O.; Motor, S.; Durgun Yetim, T. Ameliorating effects of CAPE on oxidative damage caused by pneumoperitoneum in rat lung tissue. Int. J. Clin. Exp. Med. 2014, 7, 1698–16705. [Google Scholar] [PubMed]
- Baltatzis, M.; Pavlidis, T.E.; Ouroumidis, O.; Koliakos, G.; Nikolaidou, C.; Venizelos, I.; Michopoulou, A.; Sakantamis, A.J. Aprotinin reduces oxidative stress induced by pneumoperitoneum in rats. Surg. Res. 2014, 189, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Xingwei, X.; Xin, G.; Peng, Z.; Tao, F.; Bowen, D.; Xiaoming, K.; Wu, J.; Ning, L.; Jieshou, L. Low-dose ketamine pretreatment reduces oxidative damage and inflammatory response following CO2 pneumoperitoneum in rats. Clin. Investig. Med. 2014, 37, E124. [Google Scholar] [CrossRef] [PubMed]
- Rifaioglu, M.M.; Davarci, M.; Nacar, A.; Alp, H.; Celik, M.; Sefil, N.K.; Inci, M. Caffeic acid phenethyl ester (CAPE) protects against acute urogenital injury following pneumoperitoneum in the rat. Ren. Fail. 2014, 36, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Cekic, B.; Geze, S.; Ozkan, G.; Besir, A.; Sonmez, M.; Karahan, S.C.; Mentese, A. The effect of dexmedetomidine on oxidative stress during pneumoperitoneum. BioMed Res. Int. 2014, 2014, 760323. [Google Scholar] [CrossRef] [PubMed]
- Saracoglu, K.T.; Saracoglu, A.; Umuroglu, T.; Ugurlu, M.U.; Deniz, M.; Gogus, F.Y. The preventive effect of dopamine infusion in rats with abdominal compartment syndrome. J. Investig. Surg. 2013, 26, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Tsai, P.S.; Huang, C.J. Minocycline ameliorates lung and liver dysfunction in a rodent model of hemorrhagic shock/resuscitation plus abdominal compartment syndrome. J. Surg. Res. 2013, 180, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Geze, S.; Cekic, B.; Imamoğlu, M.; Yörük, M.F.; Yuluğ, E.; Alver, A.; Mentese, A.; Ertürk, E.; Tusat, M. Use of dexmedetomidine to prevent pulmonary injury after pneumoperitoneum in ventilated rats. Surg. Laparosc. Endosc. Percutaneous Tech. 2012, 22, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Bishara, B.; Abu-Saleh, N.; Awad, H.; Ghrayeb, N.; Goltsman, I.; Aronson, D.; Khamaysi, I.; Assady, S.; Armaly, Z.; Haddad, S.; et al. Phosphodiesterase 5 inhibition protects against increased intra-abdominal pressure-induced renal dysfunction in experimental congestive heart failure. Eur. J. Heart Fail. 2012, 14, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Wan, M.H.; Li, J.; Tang, W.F.; Gong, H.L.; Chen, G.Y.; Xue, P.; Zhao, X.L.; Xia, Q. The influence of dachengqi tang on acute lung injury and intraabdominal hypertension in rats with acute pancreatitis. J. Sichuan Univ. Med. Sci. Ed. 2011, 42, 707–711. [Google Scholar] [PubMed]
- Saracoglu, A.; Saracoglu, K.T.; Deniz, M.; Ercna, F.; Yavuz, Y.; Gogus, Y. Dopamine—A preventive agent for mesenteric ischemia and reperfusion injury in abdominal compartment syndrome. Adv. Clin. Exp. Med. 2011, 20, 613–621. [Google Scholar]
- Ihtiyar, E.; Yaşar, N.F.; Erkasap, N.; Köken, T.; Tosun, M.; Oner, S.; Erkasap, S. Effects of doxycycline on renal ischemia reperfusion injury induced by abdominal compartment syndrome. J. Surg. Res. 2011, 167, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Tihan, D.N.; Erbil, Y.; Seven, R.; Arkaya, S.; Türkoğlu, U.; Hepgül, G.; Borucu, I. The effect of glutamine on oxidative damage in an experimental abdominal compartment syndrome model in rats. Ulus Travma Acil Cerrahi Derg. 2011, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fatih Yaşar, N.; Ozdemir, R.; Ihtiyar, E.; Erkasap, N.; Köken, T.; Tosun, M.; Oner, S.; Erkasap, S. Effects of doxycycline on intestinal ischemia reperfusion injury induced by abdominal compartment syndrome in a rat model. Curr. Ther. Res. Clin. Exp. 2010, 71, 186–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dinckan, A.; Sahin, E.; Ogus, M.; Emek, K.; Gumuslu, S. The effect of pentoxifylline on oxidative stress in CO2 pneumoperitoneum. Surg. Endosc. 2009, 23, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Bishara, B.; Karram, T.; Khatib, S.; Ramadan, R.; Schwartz, H.; Hoffman, A.; Abassi, Z. Impact of pneumoperitoneum on renal perfusion and excretory function: Beneficial effects of nitroglycerine. Surg. Endosc. 2009, 23, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Kaçmaz, A.; Polat, A.; User, Y.; Tilki, M.; Ozkan, S.; Sener, G. Octreotide improves reperfusion-induced oxidative injury in acute abdominal hypertension in rats. J. Gastrointest. Surg. 2004, 8, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Sener, G.; Kaçmaz, A.; User, Y.; Ozkan, S.; Tilki, M.; Yeğen, B.C. Melatonin ameliorates oxidative organ damage induced by acute intra-abdominal compartment syndrome in rats. J. Pineal Res. 2003, 35, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Kaçmaz, A.; Polat, A.; User, Y.; Tilki, M.; Ozkan, S.; Sener, G. Octreotide: A new approach to the management of acute abdominal hypertension. Peptides 2003, 24, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Kim, Z.G.; Sanli, E.; Brinkmann, L.; Lorenz, M.; Gutt, C.N. Impact of dopamine and endothelin-1 antagonism on portal venous blood flow during laparoscopic surgery. Surg. Endosc. 2002, 16, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Dolgor, B.; Kitano, S.; Yoshida, T.; Bandoh, T.; Ninomiya, K.; Matsumoto, T. Vasopressin. J. Surg. Res. 1998, 79, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Bajramagic, S.; Sever, M.; Rasic, F.; Staresinic, M.; Skrtic, A.; Beketic Oreskovic, L.; Oreskovic, I.; Strbe, S.; Loga Zec, S.; Hrabar, J.; et al. Stable gastric pentadecapeptide BPC 157 and intestinal anastomoses therapy in rats. A review. Pharmaceuticals 2024, 17, 1081. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Sever, M.; Krezic, I.; Vranes, H.; Kalogjera, L.; Smoday, I.M.; Vukovic, V.; Oroz, K.; Coric, L.; Skoro, M.; et al. New studies with stable gastric pentadecapeptide protecting gastrointestinal tract. Significance of counteraction of vascular and multiorgan failure of occlusion/occlusion-like syndrome in cytoprotection/organoprotection. Inflammopharmacology 2024, 32, 3119–3161. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Gojkovic, S.; Knezevic, M.; Tepes, M.; Strbe, S.; Vukojevic, J.; Duzel, A.; Kralj, T.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC 157: Prompt particular activation of collateral pathways. Curr. Med. Chem. 2023, 30, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. Novel cytoprotective mediator, stable gastric pentadecapeptide BPC 157. Vascular recruitment and gastrointestinal tract healing. Curr. Pharm. Des. 2018, 24, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Robert, A. Cytoprotection by prostaglandins. Gastroenterology 1979, 77, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Robert, A. Current history of cytoprotection. Prostaglandins 1981, 21 (Suppl. 1), 89–96. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Nezamis, J.E.; Lancaster, C.; Davis, J.P.; Field, S.O.; Hanchar, A.J. Mild irritants prevent gastric necrosis through “adaptive cytoprotection” mediated by prostaglandins. Am. J. Physiol. 1983, 245, G113–G121. [Google Scholar] [CrossRef] [PubMed]
- Szabó, S. Role of sulfhydryls and early vascular lesions in gastric mucosal injury. Acta Physiol. Hung. 1984, 64, 203–214. [Google Scholar] [PubMed]
- Trier, J.S.; Szabo, S.; Allan, C.H. Ethanol-induced damage to mucosal capillaries of rat stomach. Ultrastructural features and effects of prostaglandin F2 beta and cysteamine. Gastroenterology 1987, 92, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Pihan, G.; Majzoubi, D.; Haudenschild, C.; Trier, J.S.; Szabo, S. Early microcirculatory stasis in acute gastric mucosal injury in the rat and prevention by 16;16-dimethyl prostaglandin E2 or sodium thiosulfate. Gastroenterology 1986, 91, 1415–2146. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S.; Trier, J.S.; Brown, A.; Schnoor, J. Early vascular injury and increased vascular permeability in gastric mucosal injury caused by ethanol in the rat. Gastroenterology 1985, 88, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Szabo, S. Experimental basis for a role for sulfhydryls and dopamine in ulcerogenesis: A primer for cytoprotection—Organoprotection. Klin. Wochenschr. 1986, 64 (Suppl. 7), 16–122. [Google Scholar] [PubMed]
- Szabo, S.; Usadel, K.H. Cytoprotection-organoprotection by somatostatin: Gastric and hepatic lesions. Experientia 1982, 38, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Lum, J.T.; Lancaster, C.; Olafsson, A.S.; Kolbasa, K.P.; Nezamis, J.E. Prevention by prostaglandins of caerulein-induced pancreatitis in rats. Lab. Investig. 1989, 60, 677–691. [Google Scholar] [PubMed]
- Fietsam, R., Jr.; Villalba, M.; Glover, J.L.; Clark, K. Intra-abdominal compartment syndrome as a complication of ruptured abdominal aortic aneurysm repair. Am. Surg. 1989, 55, 396–402. [Google Scholar] [PubMed]
- Wendt, E. Ueber den einflussdes intraabdominalen Druckes auf die absonderungs gechwindigkeit des harnes. Arch. Physiol. Heilk. 1867, 52, 7–575. [Google Scholar]
- Malbrain, M.L.; Cheatham, M.L.; Kirkpatrick, A.; Sugrue, M.; Parr, M.; De Waele, J.; Balogh, Z.; Leppäniemi, A.; Olvera, C.; Ivatury, R.; et al. Results from the International Conference of Experts on Intra-abdominal Hypertension and Abdominal Compartment Syndrome. I. Definitions. Intensive Care Med. 2006, 32, 1722–1732. [Google Scholar] [CrossRef]
- Łagosz, P.; Sokolski, M.; Biegus, J.; Tycinska, A.; Zymlinski, R. Elevated intra-abdominal pressure: A review of current knowledge. World J. Clin. Cases 2022, 10, 3005–3013. [Google Scholar] [CrossRef] [PubMed]
- Ertel, W.; Oberholzer, A.; Platz, A.; Stocker, R.; Trentz, O. Incidence and clinical pattern of the abdominal compartment syndrome after “damage-control” laparotomy in 311 patients with severe abdominal and/or pelvic trauma. Crit. Care Med. 2000, 28, 1747–1753. [Google Scholar] [CrossRef] [PubMed]
- Tengberg, L.T.; Cihoric, M.; Foss, N.B.; Bay-Nielsen, M.; Gögenur, I.; Henriksen, R.; Jensen, T.K.; Tolstrup, M.B.; Nielsen, L.B. Complications after emergency laparotomy beyond the immediate postoperative period—A retrospective, observational cohort study of 1139 patients. Anaesthesia 2017, 72, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Havens, J.M.; Peetz, A.B.; Do, W.S.; Cooper, Z.; Kelly, E.; Askari, R.; Reznor, G.; Salim, A. The excess morbidity and mortality of emergency general surgery. J. Trauma Acute Care Surg. 2015, 78, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Tolstrup, M.B.; Watt, S.K.; Gögenur, I. Morbidity and mortality rates after emergency abdominal surgery: An analysis of 4346 patients scheduled for emergency laparotomy or laparoscopy. Langenbecks Arch. Surg. 2017, 402, 615–623. [Google Scholar] [CrossRef] [PubMed]
- Sharoky, C.E.; Bailey, E.A.; Sellers, M.M.; Kaufman, E.J.; Sinnamon, A.J.; Wirtalla, C.J.; Holena, D.N.; Kelz, R.R. Outcomes of hospitalized patients undergoing emergency general surgery remote from admission. Surgery 2017, 162, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Jeppesen, M.M.; Thygesen, L.C.; Ekeloef, S.; Gögenur, I. A nationwide cohort study of short- and long-term outcomes following emergency laparotomy. Dan. Med. J. 2019, 66, A5523. [Google Scholar] [PubMed]
- Awad, S.; Herrod, P.J.; Palmer, R.; Carty, H.M.; Abercrombie, J.F.; Brooks, A.; de Beer, T.; Mole, J.; Lobo, D.N. One- and two-year outcomes and predictors of mortality following emergency laparotomy: A consecutive series from a United Kingdom teaching hospital. World J. Surg. 2012, 36, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Peden, C.; Scott, M.J. Anesthesia for emergency abdominal surgery. Anesthesiol. Clin. 2015, 33, 209–221. [Google Scholar] [CrossRef] [PubMed]
- Ogola, G.O.; Gale, S.C.; Haider, A.; Shafi, S. The financial burden of emergency general surgery: National estimates 2010 to 2060. J. Trauma Acute Care Surg. 2015, 79, 444–448. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.W.; Olufajo, O.A.; Brat, G.A.; Rose, J.A.; Zogg, C.K.; Haider, A.H.; Salim, A.; Havens, J.M. Use of national burden to define operative emergency general surgery. JAMA Surg. 2016, 151, e160480. [Google Scholar] [CrossRef] [PubMed]
- Ingraham, A.M.; Cohen, M.E.; Raval, M.V.; Ko, C.Y.; Nathens, A.B. Comparison of hospital performance in emergency versus elective general surgery operations at 198 hospitals. J. Am. Coll. Surg. 2011, 212, 20–28.e1. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Chiumello, D.; Pelosi, P.; Bihari, D.; Innes, R.; Ranieri, V.M.; Del Turco, M.; Wilmer, A.; Brienza, N.; Malcangi, V.; et al. Incidence and prognosis of intraabdominal hypertension in a mixed population of critically ill patients: A multiple-center epidemiological study. Crit. Care Med. 2005, 33, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Chiumello, D.; Pelosi, P.; Wilmer, A.; Brienza, N.; Malcangi, V.; Bihari, D.; Innes, R.; Cohen, J.; Singer, P.; et al. Prevalence of intra-abdominal hypertension in critically ill patients: A multicentre epidemiological study. Intensive Care Med. 2004, 30, 822–829. [Google Scholar] [CrossRef] [PubMed]
- Sugrue, M.; Buhkari, Y. Intra-abdominal pressure and abdominal compartment syndrome in acute general surgery. World J. Surg. 2009, 33, 1123–1127. [Google Scholar] [CrossRef] [PubMed]
- Gutt, C.N.; Schmandra, T.C. Portal venous flow during CO2 pneumoperitoneum in the rat. Surg. Endosc. 1999, 13, 902–905. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Wise, R.D.; Smit, M.; Acosta, S.; D’Amours, S.; Beaubien-Souligny, W.; Bodnar, Z.; Coccolini, F.; Dangayach, N.S.; Dabrowski, W.; et al. International cross-sectional survey on current and updated definitions of intra-abdominal hypertension and abdominal compartment syndrome. World J. Emerg. Surg. 2024, 19, 39. [Google Scholar] [CrossRef] [PubMed]
- Scalea, T.M.; Bochicchio, G.V.; Habashi, N.; McCunn, M.; Shih, D.; McQuillan, K.; Aarabi, B. Increased intra-abdominal, intrathoracic, and intracranial pressure after severe brain injury: Multiple compartment syndrome. J. Trauma 2007, 62, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Kavanagh, B.P.; Meyer, L.J. Normalizing physiological variables in acute illness: Five reasons for caution. Intensive Care Med. 2005, 31, 1161–1167. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.; Rodseth, R.; Blaser, A.; Roberts, D.; De Waele, J.; Kirkpatrick, A.; De Keulenaer, B.; Malbrain, M. The Abdominal Compartment Society FTW. Awareness and knowledge of intra-abdominal hypertension and abdominal compartment syndrome: Results of a repeat, international, cross-sectional survey. Anaesthesiol. Intensive Ther. 2019, 51, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Wilmer, A. The polycompartment syndrome: Towards an understanding of the interactions between different compartments! Intensive Care Med. 2007, 33, 1869–1872. [Google Scholar] [CrossRef] [PubMed]
- Balogh, Z.J.; Butcher, N.E. Compartment syndromes from head to toe. Crit. Care Med. 2010, 38 (Suppl. 9), S445–S451. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Roberts, D.J.; De Waele, J.; Jaeschke, R.; Malbrain, M.L.; De Keulenaer, B.; Duchesne, J.; Bjorck, M.; Leppaniemi, A.; Ejike, J.C.; et al. Intra-abdominal hypertension and the abdominal compartment syndrome: Updated consensus definitions and clinical practice guidelines from the World Society of the Abdominal Compartment Syndrome. Intensive Care Med. 2013, 39, 1190–1206. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; De Laet, I. AIDS is coming to your ICU: Be prepared for acute bowel injury and acute intestinal distress syndrome. Intensive Care Med. 2008, 34, 1565–1569. [Google Scholar] [CrossRef] [PubMed]
- De Laet, I.E.; De Waele, J.J.; Malbrain, M.L.N.G. Fluid resuscitation and intra-abdominal hypertension. In Yearbook of Intensive Care and Emergency Medicine; Vincent, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 536–548. [Google Scholar]
- Malbrain, M.L.; De Laet, I. It’s all in the gut: Introducing the concept of acute bowel injury and acute intestinal distress syndrome. Crit Care Med. 2009, 37, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Malbrain, M.L.; Vidts, W.; Ravyts, M.; De Laet, I.; De Waele, J. Acute intestinal distress syndrome: The importance of intra-abdominal pressure. Minerva Anestesiol. 2008, 74, 657–673. [Google Scholar] [PubMed]
- Andrews, P.J.; Citerio, G. Intracranial pressure. Part one: Historical overview and basic concepts. Intensive Care Med. 2004, 30, 1730–1733. [Google Scholar] [CrossRef] [PubMed]
- Citerio, G.; Andrews, P.J. Intracranial pressure. Part two: Clinical applications and technology. Intensive Care Med. 2004, 30, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.; Zilkoski, M.; Mullins, R.; Mayberry, J.; Deveney, C.; Trunkey, D. Delayed celiotomy for the treatment of bile leak, compartment syndrome, and other hazards of nonoperative management of blunt liver injury. Am. J. Surg. 2003, 185, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Pearl, L.B.; Trunkey, D.D. Compartment syndrome of the liver. J. Trauma 1999, 47, 796–798. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, A.W.; Colistro, R.; Laupland, K.B.; Fox, D.L.; Konkin, D.E.; Kock, V.; Mayo, J.R.; Nicolaou, S. Renal arterial resistive index response to intraabdominal hypertension in a porcine model. Crit. Care Med. 2007, 35, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Wauters, J.; Claus, P.; Brosens, N.; McLaughlin, M.; Malbrain, M.; Wilmer, A. Pathophysiology of renal hemodynamics and renal cortical microcirculation in a porcine model of elevated intra-abdominal pressure. J. Trauma 2009, 66, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Bradley, S.E.; Mudge, G.H.; Blake, W.D.; Alphonse, P. The effect of increased intra-abdominal pressure on the renal excretion of water and electrolytes in normal human subjects and in patients with diabetes insipidus. Acta Clin. Belg. 1955, 10, 209–223. [Google Scholar] [CrossRef] [PubMed]
- De Laet, I.; Malbrain, M.L.; Jadoul, J.L.; Rogiers, P.; Sugrue, M. Renal implications of increased intra-abdominal pressure: Are the kidneys the canary for abdominal hypertension? Acta Clin. Belg. 2007, 62 (Suppl. 1), 119–130. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.H.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Hartleb, M.; Gutkowski, K. Kidneys in chronic liver diseases. World J. Gastroenterol. 2012, 18, 3035–3049. [Google Scholar] [CrossRef] [PubMed]
- Kramer, L.; Hörl, W.H. Hepatorenal syndrome. Semin. Nephrol. 2002, 22, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, G.L.; Malbrain, M.L.; Coremans, P.; Verbist, L.; Verhaegen, H. Orthodeoxia and platypnea in liver cirrhosis: Effects of propranolol. Acta Clin. Belg. 1994, 49, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Krowka, M.J.; Swanson, K.L.; Frantz, R.P.; McGoon, M.D.; Wiesner, R.H. Portopulmonary hypertension: Results from a 10-year screening algorithm. Hepatology 2006, 44, 1502–1510. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.A. The search for a magic bullet to fight multiple organ failure secondary to ischemia/reperfusion injury and abdominal compartment syndrome. J. Surg. Res. 2013, 184, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Skoog, P.; Seilitz, J.; Oikonomakis, I.; Hörer, T.M.; Nilsson, K.F. NO-donation increases visceral circulation in a porcine model of abdominal hypertension. J. Cardiovasc. Transl. Res. 2023, 16, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, S.H.; Yu, J.B.; Zhang, Y.F.; He, S.M.; Dong, S.A.; Zhang, Y.; Wu, L.L.; Li, C.; Li, H.B. Hydromorphone protects against CO(2) pneumoperitoneum induced lung injury via heme oxygenase-1-regulated mitochondrial dynamics. Oxidative Med. Cell. Longev. 2021, 2021, 9034376. [Google Scholar] [CrossRef] [PubMed]
- Sapegin, V.I.; Sapegin, I.D.; Il’chenko, F.N. Influence of mydocalm on the degree of intra-abdominal hypertension and local blood circulation in the intestinal wall in experiment. Eksp. Klin. Farmakol. 2014, 77, 15–19. [Google Scholar] [PubMed]
- Shimazutsu, K.; Uemura, K.; Auten, K.M.; Baldwin, M.F.; Belknap, S.W.; La Banca, F.; Jones, M.C.; McClaine, D.J.; McClaine, R.J.; Eubanks, W.S.; et al. Inclusion of a nitric oxide congener in the insufflation gas repletes S-nitrosohemoglobin and stabilizes physiologic status during prolonged carbon dioxide pneumoperitoneum. Clin. Transl. Sci. 2009, 2, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Hamidian Jahromi, A.; Youssef, A.M. Potential cerebroprotective role of pentoxifyllin in preventing the detrimental cerebral consequences of intra-abdominal hypertension. Am. Surg. 2014, 80, 218. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Cai, L.; Zhang, S.; Chen, Y.; Liu, G.; Zhang, C. Oxygen inhalation improves survival time of mice with acute intra-abdominal hypertension and protects liver cells. Transplant. Proc. 2012, 44, 1201–1205. [Google Scholar] [CrossRef] [PubMed]
- Dörfelt, R.; Ambrisko, T.D.; Moens, Y. Influence of fentanyl on intra-abdominal pressure during laparoscopy in dogs. Vet. Anaesth. Analg. 2012, 39, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.A.; Eubanks, W.S.; Stamler, J.S.; Gow, A.J.; Lagoo-Deenadayalan, S.A.; Villegas, L.; El-Moalem, H.E.; Reynolds, J.D. A method to attenuate pneumoperitoneum-induced reductions in splanchnic blood flow. Ann. Surg. 2005, 241, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Borba, M.R.; Lopes, R.I.; Carmona, M.; Neto, B.M.; Nahas, S.C.; Pereira, P.R. Effects of enalaprilat on the renin-angiotensin-aldosterone system and on renal function during CO2 pneumoperitoneum. J. Endourol. 2005, 19, 1026–1031. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Elizalde, J.I.; Adàlia, R.; Martínez-Pallí, G.; García-Valdecasas, J.C.; Piqué, J.M.; Taurà, P. The effects of vasoactive drugs on hepatic blood flow changes induced by CO2 laparoscopy: An animal study. Anesth. Analg. 2001, 93, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Agustí, M.; Elizalde, J.I.; Adàlia, R.; Cifuentes, A.; Fontanals, J.; Taurà, P. Dobutamine restores intestinal mucosal blood flow in a porcine model of intra-abdominal hyperpressure. Crit. Care Med. 2000, 28, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Chassard, D.; Berrada, K.; Tournadre, J.; Boulétreau, P. The effects of neuromuscular block on peak airway pressure and abdominal elastance during pneumoperitoneum. Anesth. Analg. 1996, 82, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Ruenzi, M.; Stolte, M.; Veljaca, M.; Oreskovic, K.; Peterson, J.; Ulcerative Colitis Study Group. A multicenter, randomized, double blind, placebo-controlled phase II study of PL 14736 enema in the treatment of mild-to-moderate ulcerative colitis. Gastroenterology 2005, 128, A584. [Google Scholar] [CrossRef]
- Veljaca, M.; Pavic Sladoljev, D.; Mildner, B.; Brajsa, K.; Bubenik, M.; Stipanicic, S.; Parnham, M. Safety, tolerability and pharmacokinetics of PL14736, a novel agent for treatment of ulcerative colitis, in healthy male volunteers. Gut 2003, 51 (Suppl. III), A309. [Google Scholar]
- Lee, E.; Padgett, B. Intra-articular injection of BPC 157 for multiple types of knee pain. Altern. Ther. Health Med. 2021, 27, 8–13. [Google Scholar] [PubMed]
- Lee, E.; Walker, C.; Ayadi, B. Effect of BPC-157 on symptoms in patients with interstitial cystitis: A pilot study. Altern. Ther. Health Med. 2024, 30, 12–17. [Google Scholar] [PubMed]
- Csiszko, A.; Nemeth, N.; Peto, K.; Deak, A.; Balog, K.; Bodnar, Z.; Szentkereszty, Z. Experimental models on abdominal compartment syndrome. Emerg. Med. Investig. 2019, 4, 1094. [Google Scholar]
- Schachtrupp, A.; Wauters, J.; Wilmer, A. What is the best animal model for acs? Acta Clin. Belg. 2007, 62 (Suppl. 1), 225–232. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, B.; Dabrowski, W.; Marciniak, A.; Wacinski, P.; Rzecki, Z.; Kotlinska, E.; Pilat, J. Increase in intra-abdominal pressure raises brain venous pressure; leads to brain ischaemia and decreases brain magnesium content. Magnes. Res. 2012, 25, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Roque, S.; Pêgo, J.M.; Correia-Pinto, J. Neurodevelopment impact of CO(2)-pneumoperitoneum in neonates: Experimental study in a rat model. J. Surg. Res. 2018, 221, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Sare, M.; Yürekli, M.; Kurukahvecioğlu, O.; Tekin, E.H.; Taneri, F.; Yusif-Zade, K.; Onuk, E. The effects of carbon dioxide pneumoperitoneum on tyrosine hydroxylase activity. Surg. Laparosc. Endosc. Percutaneous Tech. 2006, 16, 25–28. [Google Scholar] [CrossRef] [PubMed]
- Balci, C.; Akbulut, G.; Karabekir, H.S.; Karagoz, F.; Sungurtekin, H. Comparison of SPO2; hearth rate and body temperature values in abdominal compartment syndrome in a rat model with intraabdominal sepsis and intraabdominal hypertension. Saudi Med. J. 2006, 27, 1254–1257. [Google Scholar] [PubMed]
- Bishara, B.; Abu-Saleh, N.; Awad, H.; Goltsman, I.; Ramadan, R.; Khamaysi, I.; Abassi, Z. Pneumoperitoneum aggravates renal function in cases of decompensated but not compensated experimental congestive heart failure: Role of nitric oxide. J. Urol. 2011, 186, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Hazebroek, E.J.; Haitsma, J.J.; Lachmann, B.; Steyerberg, E.W.; de Bruin, R.W.; Bouvy, N.D.; Bonjer, H.J. Impact of carbon dioxide and helium insufflation on cardiorespiratory function during prolonged pneumoperitoneum in an experimental rat model. Surg. Endosc. 2002, 16, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Santos, R.S.; Moraes, L.; Samary, C.S.; Felix, N.S.; Silva, J.D.; Morales, M.M.; Huhle, R.; Abreu, M.G.; Schanaider, A.; et al. Effects of pressure support and pressure-controlled ventilation on lung damage in a model of mild extrapulmonary acute lung injury with intra-abdominal hypertension. PLoS ONE 2017, 12, e0178207. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.; Silva, P.L.; Capelozzi, V.L.; Oliveira, M.G.; Santana, M.C.E.; Cruz, F.F.; Pelosi, P.; Schanaider, A.; Malbrain, M.L.N.G.; Rocco, P.R.M. Early impact of abdominal compartment syndrome on liver, kidney and lung damage in a rodent model. Anaesthesiol. Intensive Ther. 2017, 49, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Papakrivou, E.; Makris, D.; Manoulakas, E.; Mitroudi, M.; Tepetes, K.; Papazoglou, K.; Zakynthinos, E. Intra-Abdominal hypertension causes bacterial growth in lungs: An animal study. BioMed Res. Int. 2017, 2017, 4601348. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.L.; Moraes, L.; Santos, R.S.; dos Santos Samary, C.; Silva, J.D.; Morales, M.M.; Capelozzi, V.L.; de Abreu, M.G.; Schanaider, A.; Silva, P.L.; et al. The biological effects of higher and lower positive end-expiratory pressure in pulmonary and extrapulmonary acute lung injury with intra-abdominal hypertension. Crit. Care 2014, 18, R121. [Google Scholar] [CrossRef] [PubMed]
- Runck, H.; Schumann, S.; Tacke, S.; Haberstroh, J.; Guttmann, J. Effects of intra-abdominal pressure on respiratory system mechanics in mechanically ventilated rats. Respir. Physiol. Neurobiol. 2012, 180, 204–210. [Google Scholar] [CrossRef]
- Akbulut, G.; Yazicioglu, M.B.; Şahin, Ö.; Tosun, M.; Dilek, O.N. Lung tissue apoptosis in abdominal hypertension: Apoptosis and necrosis of lung tissue in abdominal hypertension. Eur. J. Trauma Emerg. Surg. 2011, 37, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Chadi, S.A.; Abdo, H.; Bihari, A.; Parry, N.; Lawendy, A.R. Hepatic microvascular changes in rat abdominal compartment syndrome. J. Surg. Res. 2015, 197, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, J.A.; Zhang, W.W.; Gu, G.S.; Fan, C.G.; Wang, X.B.; Li, J.S. Experimental study of liver injury in rats of abdominal infection with abdominal compartment syndrome. Chin. J. Gastrointest. Surg. 2011, 14, 511–515. [Google Scholar] [PubMed]
- Chen, J.; Ren, J.A.; Zhang, W.W.; Gu, G.S.; Fan, C.G.; Wang, X.B.; Li, J.S. Open abdomen management treatment of liver injury in rats with abdominal compartment syndrome and sepsis. Chin. J. Gastrointest. Surg. 2011, 49, 335–340. [Google Scholar] [PubMed]
- Zabelin, M.V.; Zubritskiĭ, V.F.; Maĭorov, A.V.; Baranov, M.A. Pathomorphological alterations in the liver and small intestine in experimental modelling of abdominal compartment syndrome. Eksp. Klin. Gastroenterol. 2010, 6, 44–47. [Google Scholar] [PubMed]
- Mogilner, J.G.; Bitterman, H.; Hayari, L.; Brod, V.; Coran, A.G.; Shaoul, R.; Lurie, M.; Eldar, S.; Sukhotnik, I. Effect of elevated intra-abdominal pressure and hyperoxia on portal vein blood flow; hepatocyte proliferation and apoptosis in a rat model. Eur. J. Pediatr. Surg. 2008, 18, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Muftuoglu, M.A.; Aktekin, A.; Ozdemir, N.C.; Saglam, A. Liver injury in sepsis and abdominal compartment syndrome in rats. Surg. Today 2006, 36, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.P.; Chen, R.J.; Fang, J.F.; Lin, B.C.; Huang, T.L.; Cheng, M.L.; Chiu, D.T.; Tsay, P.K. Increased susceptibility to oxidant injury in hepatocytes from rats with intra-abdominal hypertension. J. Trauma 2004, 57, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Morozov, D.; Morozova, O.; Pervouchine, D.; Severgina, L.; Tsyplakov, A.; Zakharova, N.; Sushentsev, N.; Maltseva, L.; Budnik, I. Hypoxic renal injury in newborns with abdominal compartment syndrome (clinical and experimental study). Pediatr. Res. 2018, 83, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Köşüm, A.; Borazan, E.; Maralcan, G.; Aytekin, A. Biochemical and histopathological changes of intra-abdominal hypertension on the kidneys: Experimental study in rats. Ulus Cerrahi Derg. 2013, 29, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Strang, S.G.; van der Hoven, B.; Monkhorst, K.; Ali, S.; van Lieshout, E.M.M.; van Waes, O.J.F.; Verhofstad, M.H.J. Relation between intra-abdominal pressure and early intestinal ischemia in rats. Trauma Surg. Acute Care Open 2020, 5, e000595. [Google Scholar] [CrossRef]
- Strier, A.; Kravarusic, D.; Coran, A.G.; Srugo, I.; Bitterman, N.; Dorfman, T.; Pollak, Y.; Matter, I.; Sukhotnik, I. The effect of elevated intra-abdominal pressure on TLR4 signaling in intestinal mucosa and intestinal bacterial translocation in a rat. J. Laparoendosc. Adv. Surg. Tech. A 2017, 27, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Yi, M.; Fan, J.; Bai, Y.; Ge, Q.; Yao, G. Effects of acute intra-abdominal hypertension on multiple intestinal barrier functions in rats. Sci. Rep. 2016, 6, 22814. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Zhang, K.; Fan, J.; Yi, M.; Ge, Q.; Chen, L.; Zhang, L.; Yao, G. Effect of acute, slightly increased intra-abdominal pressure on intestinal permeability and oxidative stress in a rat model. PLoS ONE 2014, 9, e109350. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.T.; Wei, Z.Y.; Liu, D.J.; Zhang, Y.; Li, X.Y.; Chen, Z.H.; Xiao, G.X. Influence of haemoxygenase 1 (HO-1) gene expression on intestinal mucosa injury induced by intra-abdominal hypertension in rats. Chin. J. Burns 2013, 29, 239–244. [Google Scholar] [PubMed]
- Deenichin, G.; Dimov, R. Intraabdominal hypertention—Predisposing factor for visceral alterations and abdominal compartment syndrome. Khirurgiia (Sofiia) 2013, 4, 19–24. [Google Scholar] [PubMed]
- Gong, G.; Wang, P.; Ding, W.; Zhao, Y.; Li, J. Microscopic and ultrastructural changes of the intestine in abdominal compartment syndrome. J. Investig. Surg. 2009, 22, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Lou, Z.; Li, J.S. Interstitial cells of Cajal in abdominal sepsis and hypertension-induced ileus in rats. Eur. Surg. Res. 2009, 43, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Sukhotnik, I.; Mogilner, J.; Hayari, L.; Brod, V.; Shaoul, R.; Slijper, N.; Bejar, Y.; Coran, A.G.; Bitterman, H. Effect of elevated intra-abdominal pressure and 100% oxygen on superior mesenteric artery blood flow and enterocyte turnover in a rat. Pediatr. Surg. Int. 2008, 24, 1347–1353. [Google Scholar] [CrossRef] [PubMed]
- Turgunov, Y.; Matyushko, D.; Nurbekov, A.; Kaliyeva, D.; Alibekov, A. Influence of the intra-abdominal hypertension on the blood coagulation system (experimental study). Georgian Med. News. 2016, 256–257, 97–106. [Google Scholar] [PubMed]
- Nemeth, N.; Peto, K.; Deak, A.; Sogor, V.; Varga, G.; Tanczos, B.; Balog, K.; Csiszko, A.; Godo, Z.; Szentkereszty, Z. Hemorheological factors can be informative in comparing treatment possibilities of abdominal compartment syndrome. Clin. Hemorheol. Microcirc. 2016, 64, 765–775. [Google Scholar] [CrossRef] [PubMed]
- Stupnisek, M.; Franjic, S.; Drmic, D.; Hrelec, M.; Kolenc, D.; Radic, B.; Bojic, D.; Vcev, A.; Seiwerth, S.; Sikiric, P. Pentadecapeptide BPC 157 reduces bleeding time and thrombocytopenia after amputation in rats treated with heparin; warfarin or aspirin. Thromb. Res. 2012, 129, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Konosic, S.; Petricevic, M.; Ivancan, V.; Konosic, L.; Goluza, E.; Krtalic, B.; Drmic, D.; Stupnisek, M.; Seiwerth, S.; Sikiric, P. Intragastric application of aspirin, clopidogrel, cilostazol, and BPC 157 in rats: Platelet aggregation and blood clot. Oxidative Med. Cell. Longev. 2019, 2019, 9084643. [Google Scholar] [CrossRef] [PubMed]
- Gojkovic, S.; Krezic, I.; Vrdoljak, B.; Malekinusic, D.; Barisic, I.; Petrovic, A.; Horvat Pavlov, K.; Kolovrat, M.; Duzel, A.; Knezevic, M.; et al. Pentadecapeptide BPC 157 resolves suprahepatic occlusion of the inferior caval vein, Budd-Chiari syndrome model in rats. World J. Gastrointest. Pathophysiol. 2020, 11, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Drmic, D.; Boban Blagaic, A.; Tvrdeic, A.; Krezic, I.; Gojkovic, S.; Zizek, H.; Sikiric, S.; Strbe, S.; Smoday, I.M.; et al. Stable gastric pentadecapeptide BPC 157 and NO-system. In Nitric Oxide: From Research to Therapeutics; Advances in Biochemistry in Health and Disease 22; Ray, A., Gulati, K., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 349–375. [Google Scholar] [CrossRef]
- Sikiric, P.; Seiwerth, S.; Rucman, R.; Turkovic, B.; Rokotov, D.S.; Brcic, L.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; et al. Stable gastric pentadecapeptide BPC 157-NO-system relation. Curr. Pharm. Des. 2014, 20, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Sikirić, P.; Seiwerth, S.; Grabarević, Z.; Rucman, R.; Petek, M.; Jagić, V.; Turković, B.; Rotkvić, I.; Mise, S.; Zoricić, I.; et al. The influence of a novel pentadecapeptide, BPC 157, on N(G)-nitro-L-arginine methylester and L-arginine effects on stomach mucosa integrity and blood pressure. Eur. J. Pharmacol. 1997, 332, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Turkovic, B.; Sikiric, P.; Seiwerth, S.; Mise, S.; Anic, T.; Petek, M. Stable gastric pentadecapeptide BPC 157 studied for inflammatory bowel disease (PLD-116, PL14736, Pliva) induces nitric oxide synthesis. Gastroenterology 2004, 126, 287. [Google Scholar] [CrossRef]
- Stupnisek, M.; Kokot, A.; Drmic, D.; Hrelec Patrlj, M.; Zenko Sever, A.; Kolenc, D.; Radic, B.; Suran, J.; Bojic, D.; Vcev, A.; et al. Pentadecapeptide BPC 157 reduces bleeding and thrombocytopenia after amputation in rats treated with heparin, warfarin, L-NAME and L-arginine. PLoS ONE 2015, 10, e0123454. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wei, M.; Li, N.; Lu, Q.; Shrestha, S.M.; Tan, J.; Zhang, Z.; Wu, G.; Shi, R. Clopidogrel-induced gastric injury in rats is attenuated by stable gastric pentadecapeptide BPC 157. Drug Des. Dev. Ther. 2020, 14, 5599–5610. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Lee, C.H.; Chueh, H.Y.; Chang, G.J.; Huang, H.Y.; Lin, Y.; Pang, J.S. Modulatory effects of BPC 157 on vasomotor tone and the activation of Src-Caveolin-1-endothelial nitric oxide synthase pathway. Sci. Rep. 2020, 10, 17078. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.J.; Liu, H.T.; Wang, C.N.; Huang, H.Y.; Lin, Y.; Ko, Y.S.; Wang, J.S.; Chang, V.H.; Pang, J.S. Therapeutic potential of pro-angiogenic BPC157 is associated with VEGFR2 activation and up-regulation. J. Mol. Med. 2017, 95, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Lee, H.J.; Sikiric, P.; Hahm, K.B. BPC 157 rescued NSAID-cytotoxicity via stabilizing intestinal permeability and enhancing cytoprotection. Curr. Pharm. Des. 2020, 26, 2971–2981. [Google Scholar] [CrossRef] [PubMed]
- Tkalcević, V.I.; Cuzić, S.; Brajsa, K.; Mildner, B.; Bokulić, A.; Situm, K.; Perović, D.; Glojnarić, I.; Parnham, M.J. Enhancement by PL 14736 of granulation and collagen organization in healing wounds and the potential role of egr-1 expression. Eur. J. Pharmacol. 2007, 570, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.A.; Han, Y.M.; An, J.M.; Park, Y.J.; Sikiric, P.; Kim, D.H.; Kwon, K.A.; Kim, Y.J.; Yang, D.; Tchah, H.; et al. BPC157 as potential agent rescuing from cancer cachexia. Curr. Pharm. Des. 2018, 24, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Radeljak, S.; Seiwerth, S.; Sikiric, P. BPC 157 inhibits cell growth and VEGF signalling via the MAPK kinase pathway in the human melanoma cell line. Melanoma Res. 2004, 14, A14–A15. [Google Scholar] [CrossRef]
- Huang, T.; Zhang, K.; Sun, L.; Xue, X.; Zhang, C.; Shu, Z.; Mu, N.; Gu, J.; Zhang, W.; Wang, Y.; et al. Body protective compound-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Des. Dev. Ther. 2015, 9, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.S.; Huang, S.C.; Chen, F.H.; Chang, Y.; Mei, H.F.; Huang, H.Y.; Chen, W.Y.; Pang, J.S. Pentadecapeptide BPC 157 efficiently reduces radiation-induced liver injury and lipid accumulation through Kruppel-like factor 4 upregulation both in vivo and in vitro. Life Sci. 2022, 310, 121072. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Qu, M.; Duan, R.; Shi, D.; Jin, L.; Gao, J.; Wood, J.D.; Li, J.; Wang, G.D. Cytoprotective mechanism of the novel gastric peptide in gastrointestinal tract and cultured enteric neurons and glial cells. Neurosci. Bull. 2019, 35, 167–170. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Hsu, Y.H.; Pang, J.H. Pentadecapeptide BPC 157 enhances the growth hormone receptor expression in tendon fibroblasts. Molecules 2014, 19, 19066–19077. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Tsai, W.C.; Lin, M.S.; Hsu, Y.H.; Pang, J.H. The promoting effect of pentadecapeptide BPC 157 on tendon healing involves tendon outgrowth, cell survival, and cell migration. J. Appl. Physiol. 2011, 110, 74–780. [Google Scholar] [CrossRef] [PubMed]
- Depauw, P.R.A.M.; Groen, R.J.M.; Van Loon, J.; Peul, W.C.; Malbrain, M.L.N.G.; De Waele, J.J. The significance of intra-abdominal pressure in neurosurgery and neurological diseases: A narrative review and a conceptual proposal. Acta Neurochir. 2019, 161, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Youssef, A.M.; Hamidian Jahromi, A.; Vijay, C.G.; Granger, D.N.; Alexander, J.S. Intra-abdominal hypertension causes reversible blood-brain barrier disruption. J. Trauma Acute Care Surg. 2012, 72, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.I. From neurogenic pulmonary edema to fat embolism syndrome: A brief review of experimental and clinical investigations of acute lung injury and acute respiratory distress syndrome. Chin. J. Physiol. 2009, 52 (Suppl. 5), 339–344. [Google Scholar] [CrossRef] [PubMed]
- Seiwerth, S.; Rucman, R.; Turkovic, B.; Sever, M.; Klicek, R.; Radic, B.; Drmic, D.; Stupnisek, M.; Misic, M.; Vuletic, L.B.; et al. BPC 157 and standard angiogenic growth factors. Gastrointestinal tract healing, lessons from tendon, ligament, muscle and bone healing. Curr. Pharm. Des. 2018, 24, 1972–1989. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Gojkovic, S.; Krezic, I.; Smoday, I.M.; Kalogjera, L.; Zizek, H.; Oroz, K.; Vranes, H.; Vukovic, V.; Labidi, M.; et al. Stable gastric pentadecapeptide BPC 157 may recover brain-gut axis and gut-brain axis function. Pharmaceuticals 2023, 16, 676. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Boban Blagaic, A.; Strbe, S.; Beketic Oreskovic, L.; Oreskovic, I.; Sikiric, S.; Staresinic, M.; Sever, M.; Kokot, A.; Jurjevic, I.; et al. The stable gastric pentadecapeptide BPC 157 pleiotropic beneficial activity and its possible relations with neurotransmitter activity. Pharmaceuticals 2024, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Staresinic, M.; Japjec, M.; Vranes, H.; Prtoric, A.; Zizek, H.; Krezic, I.; Gojkovic, S.; Smoday, I.M.; Oroz, K.; Staresinic, E.; et al. Stable gastric pentadecapeptide BPC 157 and striated, smooth, and heart muscle. Biomedicines 2022, 10, 3221. [Google Scholar] [CrossRef] [PubMed]
- Sikiric, P.; Udovicic, M.; Barisic, I.; Balenovic, D.; Zivanovic Posilovic, G.; Strinic, D.; Uzun, S.; Sikiric, S.; Krezic, I.; Zizek, H.; et al. Stable gastric pentadecapeptide BPC157 as useful cytoprotective peptide therapy in the heart disturbances, myocardial infarction, pulmonary hypertension, arrhythmias, and thrombosis presentation. Biomedicines 2022, 10, 2696. [Google Scholar] [CrossRef] [PubMed]
- Grabarevic, Z.; Tisljar, M.; Artukovic, B.; Bratulic, M.; Dzaja, P.; Seiwerth, S.; Sikiric, P.; Peric, J.; Geres, D.; Kos, J. The influence of BPC 157 on nitric oxide agonist and antagonist induced lesions in broiler chicken. J. Physiol. Paris 1997, 91, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Smodiš Škerl, M.I.; Šoštarić, P.; Šuran, J.; Sikirić, P.; Vlainić, J. Physiological and immunological status of adult honeybees (Apis mellifera) fed sugar syrup supplemented with pentadecapeptide BPC 157. Biology 2021, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Tlak Gajger, I.; Ribarić, J.; Smodiš Škerl, M.; Vlainić, J.; Sikirić, P. Stable gastric pentadecapeptide BPC 157 in honeybee (Apis mellifera) therapy, to control Nosema ceranae invasions in apiary conditions. J. Vet. Pharmacol. Ther. 2018, 41, 614–621. [Google Scholar] [CrossRef] [PubMed]
- Filosevic, A.; Andretic Waldowski, R.; Sikiric, P.; Drmic, D. Stable gastric pentadecapeptide BPC 157 antagonizes hydrogen peroxide induced oxidative stress in Drosophila melanogaster. FASEB J. 2018, 31 (Suppl. S1), 667.14. [Google Scholar] [CrossRef]
- Gardner, A.K.; Schroeder, E.L. Pathophysiology of intraabdominal hypertension and abdominal compartment syndrome and relevance to veterinary critical care. J. Vet. Emerg. Crit. Care 2022, 32, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.C.; Teerenstra, S.; Meijboom, F.L.B.; Bleich, A. Methodical advances in reproducibility research: A proof of concept qualitative comparative analysis of reproducing animal data in humans. J. Neurosci. Methods 2023, 397, 109931. [Google Scholar] [CrossRef] [PubMed]
- Dirven, H.; Vist, G.E.; Bandhakavi, S.; Mehta, J.; Fitch, S.E.; Pound, P.; Ram, R.; Kincaid, B.; Leenaars, C.H.C.; Chen, M.; et al. Performance of preclinical models in predicting drug-induced liver injury in humans: A systematic review. Sci. Rep. 2021, 11, 6403. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.-M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [PubMed]
- Litchfield, J.T., Jr. Symposium on clinical drug evaluation and human pharmacology. XVI. Evaluation of the safety of new drugs by means of tests in animals. Clin. Pharmacol. Ther. 1962, 3, 665–672. [Google Scholar] [CrossRef] [PubMed]


| Ref. | Agent | IAH Procedure | Target | Outcome |
|---|---|---|---|---|
| [8] | Stable gastric pentadecapeptide BPC 157 (10 µg/kg, 10 ng/kg sc) given at 3 min reperfusion times | Reperfusion following maintained intra-abdominal hypertension (i) grade III: 25 mmHg/60 min or (ii), (iii) grade IV: (ii) 30 mmHg/30 min; (iii) 40 mmHg/30 min. Reperfusion for 60 min (i) or for 30 min (ii/iii) | Decompression/reperfusion-induced occlusion/occlusion-like syndrome: lesions and malondialdehyde (MDA) in the brain, heart, lung, liver, kidney, and gastrointestinal tract, thrombosis, hemorrhage, intracranial, portal, caval hypertension, aortal hypotension | Counteracted decompression/reperfusion-induced occlusion/occlusion-like syndrome as a whole. Counteracted: lesions and malondialdehyde (MDA) in the brain, heart, lung, liver, kidney, and gastrointestinal tract, thrombosis, hemorrhage, intracranial, portal, caval hypertension, aortal hypotension |
| [30] | L92, containing the single species L. acidophilus, 2.1 × 109 CFU/kg/day, calculated as 200 × 108 CFU for 7 days. Amino acid (AA) mixture treatment (trade name Elental) 25 g/kg/day for 7 days | 90-min nitrogen pneumoperitoneum: IAP was 12 mm Hg | Colon histology, colonic reduced glutathione (GSH) and malondialdehyde (MDA) were used to evaluate the changes in oxidative responses. Colonic interleukin-1β (IL-1β) was measured to assess the inflammatory responses, 5-HT and 5-HIAA in plasma. | Orally gavaged Lactobacillus acidophilus L-92 (L92) and a mixture of AA in rats with induced IAH. The results showed that both L92 and AA pretreatments effectively mitigated IAH-induced intestinal damage. Interestingly, L92 but not AA prevented metagenomic changes induced by IAH. |
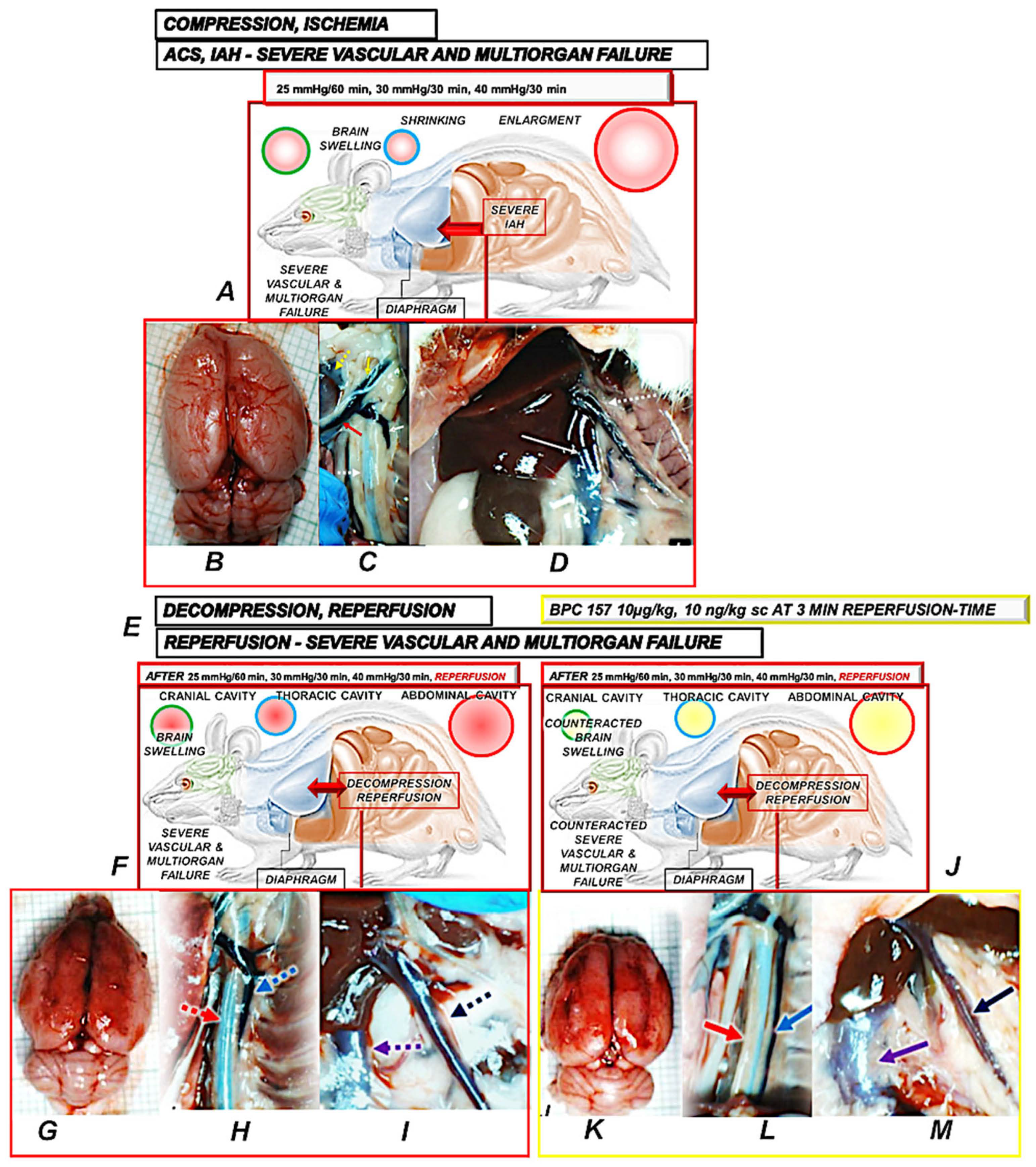
| [7] | Rats with intra-abdominal hypertension (grade III, grade IV) received BPC 157 (10 µg or 10 ng/kg sc) or saline (5 mL) after 10 min. | Intra-abdominal pressure in thiopental-anesthetized rats at 25 mmHg (60 min), 30 mmHg (30 min), 40 mmHg (30 min), 50 mmHg (15 min), and in esketamine-anesthetized rats at 25 mmHg for 120 min | Lesions in the brain, heart, lung, liver, kidney, and gastrointestinal tract, thrombosis, hemorrhage, intracranial, portal, caval hypertension, aortal hypotension | Counteracted occlusion/occlusion-like syndrome as a whole. Counteracted: lesions and malondialdehyde (MDA) in the brain, heart, lung, liver, kidney, and gastrointestinal tract, thrombosis, hemorrhage, intracranial, portal, caval hypertension, aortal hypotension |
| [31] | C. butyricum 1 × 109 colony-forming units (CFUs) of C. butyricum, Butyrate 100 mg/kg body weight of sodium butyrate in 1.0 mL of normal saline; for 10 days | Severe acute pancreatitis retrograde infusion, 4.5% sodium taurocholate (0.1 mL/100 g) 24 h after the operation, the IAP of each rat was determined | The plasma levels of several markers (amylase, diamine oxidase (DAO), fluorescein isothiocyanate (FITC)-dextran, tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, IL-1β, IL-12, lipopolysaccharide (LPS)) and fecal butyric acid level were determined. The pancreas and intestine were examined using histology, and RT-PCR and Western blotting of intestinal tissues were used to measure the expression of six markers (tight junction proteins (zonula occludens protein-1 (ZO-1), claudin-1, claudin-2, occluding) matrix metalloproteinase 9 (MMP9), and TNF-α). The gut flora of the rats was examined by 16S rRNA sequencing | Rats that received oral C. butyricum or butyrate had reduced intestinal injury and plasma levels of DAO, LPS, and inflammatory cytokines. |
| [32] | Before blood was drawn, rats in the combined + bFGF group were treated with bFGF (10 μg/kg; intraperitoneal (IP) injection). FGFR1 antagonist, PD173074 (10 mg/kg; IP injection) 2 min before the administration of bFGF. PD98059 (ERK antagonist; 20 mg/kg; IP injection) 2 min before bFGF administration. | For moderate TBI, the impact depth was set as 2.0 mm, the dwell time was set as 100 ms, and the velocity was set as 3.5 m/s. Blood samples were taken (0.5 mL/min) within 10 min after the surgery, blood pressure was maintained at 30–40 mmHg for 1.5 h, then the reperfusion was induced by Ringer’s solution (30 mL/h, using an infusion pump). After 5 min, nitrogen was peritoneally injected until the IAP reached 12 mmHg. | Intracranial pressure (ICP) monitoring, brain water content, Evans blue permeability detection, immunofluorescence staining, real-time PCR, and Western blot analysis | The effects of bFGF on alleviating the rat BBB injuries were determined, indicating that bFGF regulated the expression levels of the tight junction (TJ), adhesion junction (AJ), matrix metalloproteinase (MMP), and IL-1β, as well as reduced BBB permeability, brain edema, and intracranial pressure. Moreover, the FGFR1 antagonist PD 173074 and the ERK antagonist PD 98059 decreased the protective effects of bFGF. |
| [33] | Hypodermic injection of hydrogen gas (0.2 mL/kg), and after 10 min they received an abdominal insufflation of CO2 for 90 min at an intra-abdominal pressure of 15 mmHg. | Abdominal insufflation of CO2 for 90 min at an intra-abdominal pressure of 15 mmHg. | Alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were measured to evaluate liver function. Malondialdehyde (MDA), superoxide dismutase (SOD) and glutathione (GSH) content were measured to evaluate oxidative stress. Nuclear factor E2-related factor 2 (Nrf2) and Nrf2 downstream target genes, apoptosis-related genes and inflammatory cytokine mRNA and protein expression were detected. Liver injury was detected under the microscope. | Liver function, antioxidants content, inflammation and liver injury were improved after hydrogen preconditioning |
| [34] | i.v. glycine (1.5 mL, 300 mM) 10 min before pneumoperitoneum. | CO2 pneumoperitoneum (12 mmHg) for 90 min. Assessment at 1, 2, and 8 h after pneumoperitoneum | Transaminases, hepatic microcirculation, and phagocytosis of latex beads indexing both liver injury and KC activation were examined following pneumoperitoneum. | Glycine significantly decreased lactate dehydrogenase at 1 h and both aspartate aminotransferase and alanine aminotransferase at 2 h after pneumoperitoneum. In parallel, glycine significantly decreased both the rate of permanent adherence of leukocytes to the endothelium by up to 35% and the rate of phagocytosis by >50% compared to the control group. |
| [35] | CORM-3, CO donor, and GYY4137, H2S donor, were administered at the dose of 10 mg/kg and 50 mg/kg, respectively, via the carotid artery, just before decompression of the abdomen. Decompression 20–30 min. | 2 h, an abdominal plaster cast and intraperitoneal CO2 insufflation at 20 mmHg. Decompression 20–30 min | Sinusoidal perfusion, inflammatory response and cell death were quantified in exteriorized livers. Respiratory, liver, and renal dysfunction was assessed biochemically. | Improved hepatic microvasculature, counteracted hepatic cell death, and inflammatory, metabolic, and renal dysfunction in a rat model of ACS |
| [36] | In the melatonin group (MT, n = 8), after blood reinfusion, rats were infused with melatonin (50 mg/kg), then resuscitated with Ringer solution (LR) (30 mL/h × 6 h). In the hypertonic saline group (HS, n = 8), after blood reinfusion, rats were infused with 7.5% hypertonic saline (HS) (6 ml/kg), then resuscitated with LR (30 mL/h × 6 h). In the hydroxyethyl starch group (HES, n = 8), after blood reinfusion, rats were infused with hydroxyethyl starch 130/0.4 (HES) (30 mL/kg), then resuscitated with LR (30 mL/h × 6 h). | Portal hypertension 1 h, abdominal restraint device, and hemorrhaging to mean arterial pressure (MAP) of 40 mmHg for 2 h. After blood reinfusion, the rats were treated with lactated Ringer solution (LR) (30 mL/h), for 6 h. The secondary IAH was determined by an elevation of 12.5 mmHg (170 mmH2O) of IVCP from the starting point. | The intestinal permeability, immunofluorescence of tight junction proteins, transmission electron microscopy, level of inflammatory mediators (TNF-a, IL-1β, IL-6) and of biochemical markers of oxidative stress (malondialdehyde, myeloperoxidase activity, and glutathione peroxidase) were assessed. Expressions of the protein kinase B (Akt) and of tight junction proteins were detected by Western blot. | Compared with LR, HS, and HES, melatonin was associated with less inflammatory and oxidative injury, less intestinal permeability and injury, and lower incidence of secondary IAH in this model. The salutary effect of melatonin in this model was associated with the upregulation of intestinal Akt phosphorylation. |
| [37] | Melatonin (50 mg/kg), and SB-203580 (10 μmol/kg) immediately after blood reinfusion | Portal hypertension 1 h, abdominal restraint device, and hemorrhaging to mean arterial pressure (MAP) of 40 mmHg for 2 h. After blood reinfusion, the rats were treated with lactated Ringer solution (LR) (30 mL/h), for 6 h. The secondary IAH was determined by an elevation of 12.5 mmHg (170 mmH2O) of IVCP from the starting point. | MAP, the inferior vena cava pressure and urine output were monitored. Intestine histopathological examination, immunofluorescence of tight junction proteins, and transmission electron microscopy were administered. Intestinal permeability, myeloperoxidase activity, malondialdehyde, glutathione peroxidase, and levels of TNF-a, IL-2, and IL-6. The expression of extracellular signal-regulated kinase, p38, c-Jun NH2-terminal kinase, translocation of nuclear factor kappa B subunit, signal transducers and activators of transcription and tight junction proteins were detected by Western blot. | Melatonin inhibited the inflammatory responses, decreased expression of p38 MAPK, attenuated intestinal injury, and prevented secondary IAH. Moreover, administration of SB203580 abolished the increase in p38 MAPK and also attenuated intestinal injury. |
| [38] | An arginase inhibitor 2(S)-amino-6-boronohexanoic acid (ABH) subcutaneous injection (5 mg/kg) 1 h before induction of pneumoperitoneum (insufflation to intraperitoneal pressure of 15 mmHg for 60 min) | Pneumoperitoneum (insufflation to intraperitoneal pressure of 15 mmHg for 60 min) | After desufflation, blood was collected to determine levels of plasma nitrite, NOS, inflammatory cytokines, and malondialdehyde, a marker of oxidative stress. Lung tissue was obtained for histological evaluation. | Pretreatment with an arginase inhibitor may protect against lung injury caused by pneumoperitoneum. |
| [39] | 5 mg/kg of theophylline intraperitoneally before setting pneumoperitoneum model. | The pneumoperitoneum was generated by insufflating inside the abdomen by CO2 at 14 mmHg fixed pressure for 1 h, and desufflation was waited for 30 min | Urea, creatinine, cystatin-C, tissue and serum total antioxidant capacity, total oxidant capacity and oxidative stress index in two groups were measured and compared with each other. Apoptosis and histopathological conditions in the renal tissues were examined. | Lower cystatin-C levels in the group, where Theophylline was given, are suggestive of lower renal injury in this group. However, this opinion is interrogated as there is no difference in terms of tissue and serum TAS, TOS, OSI and urea values between the groups. |
| [40] | Selective melanocortin 4 receptor agonist RO27-3225 (180 μg/kg ip) 2 min before blood was drawn. The selective melanocortin 4 receptor antagonist HS024 (130 μg/kg) 2 min before the RO27 3225 administration. The nicotinic acetylcholine receptor antagonist chlorisondamine (250 μg/kg) 2 min before the RO27 3225 administration | IAH rat models were induced by hemorrhagic shock/resuscitation with the mean arterial pressure (MAP) maintained at 30 mm Hg for 90 min followed by the reinfusion of the withdrawn blood with lactated Ringer’s solution. Then, air was injected into the peritoneal cavity of the rats to maintain an intra-abdominal pressure of 20 mm Hg for 4 h. | Mean arterial pressure, reduced tumor necrosis factor-a, and interleukin-1b messenger RNA expression increased by IAH, histologic damage, and superoxide dismutase activity in the intestine, the levels of intestinal fatty acid-binding protein, intestinal edema and intestinal permeability, the expression of Rho-associated coiledecoil-containing protein kinase 1 and phosphorylated myosin light chain. | RO27-3225 restored mean arterial pressure, reduced tumor necrosis factor-a, and interleukin-1b messenger RNA expression increased by IAH, alleviated histologic damage, and improved superoxide dismutase activity in the intestine. Compared with the IAH group, the levels of intestinal fatty acid-binding protein, intestinal edema and intestinal permeability were lower in the RO group. Furthermore, the RO27-3225 treatment increased the expression of Rho-associated coiledecoil-containing protein kinase 1 and phosphorylated myosin light chain. Chl and HS024 abrogated the protective effects of RO27-3225. |
| [41] | Selective melanocortin 4 receptor agonist RO27-3225 (180 μg/kg ip) 2 min before blood was drawn. Selective melanocortin 4 receptor antagonist HS024 (130 μg/kg) 2 min before the RO27 3225 administration. The nicotinic acetylcholine receptor antagonist chlorisondamine (250 μg/kg) 2 min before the RO27 3225 administration | IAH rat models were induced by hemorrhagic shock/resuscitation (with the mean arterial pressure (MAP) maintained at 30 mm Hg for 90 min followed by the reinfusion of the withdrawn blood with lactated Ringer’s solution). Then, air was injected into the peritoneal cavity of the rats to maintain an intra-abdominal pressure of 20 mmHg for 4 h. | The permeability of the BBB, brain water content. The left brain hippocampus AQP4, MMP9, IL-1β and TNF-α concentrations were detected using an ELISA kit | The effects of the melanocortin 4 receptor agonist RO27-3225 in alleviating the rats’ IAH brain injuries were observed, which indicated that RO27-3225 could reduce brain edema, the expressions of the IL-1β and TNF-α inflammatory cytokines, the blood–brain barrier’s permeability and the aquaporin4 (AQP4) and matrix metalloproteinase 9 (MMP9) levels. Moreover, the nicotinic acetylcholine receptor antagonist chlorisondamine and the selective melanocortin 4 receptor antagonist HS024 can negate the protective effects of the RO27-3225. |
| [42] | Caffeic acid phenethyl ester (CAPE) at 10 μmol/kg was administered as a single intraperitoneal injection 1 h before the desufflation period | CO2 pneumoperitoneum 15 mmHg for 60 min. | The bronchoalveolar lavage was obtained twice with 3 mL of saline to investigate biochemical parameters, including paraoxonase (PON1) activity, total antioxidant status (TAS), total oxidative status (TOS) levels, and cytokine concentration. | CAPE to prevent CO2 pneumoperitoneum-induced oxidative stress and inflammatory reactions in lung tissue |
| [43] | Loading aprotinin dose of 28,000 KIU/kg ip straight after the onset of pneumoperitoneum, followed by lower maintenance doses (7500 KIU/kg), which were administered per hour until the termination of insufflation | Constant 12 mmHg pneumoperitoneum was maintained for 4 h. The duration of the reperfusion period was 60 or 180 min. | Several cytokines and markers of oxidative stress were measured in liver, small intestine, and lungs to compare the aprotinin group with the control group. Tissue inflammation was also evaluated and compared between groups using a five-scaled histopathologic score. | In the aprotinin group values of biochemical markers (tumor necrosis factor-a, interleukin 6, endothelin 1, C-reactive protein, pro-oxidant-antioxidant balance, and carbonyl proteins) were lower in all tissues studied. Statistical significance was greater in liver and lungs. Histopathologic examination revealed a significant difference between the control and aprotinin groups in all tissues examined. Aprotinin groups showed mild to moderate lesions, while in control groups, severe to very severe inflammation was present. The aprotinin subgroup with prolonged reperfusion period (180 min) showed milder lesions in all tissues than the rest of the groups. |
| [44] | Low-dose ketamine (KP1, 5 mg/kg; KP2, 10 mg/kg) | CO2 pneumoperitoneum of 15 mmHg. | Three hours after pneumoperitoneum, serum concentrations of interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), malondialdehyde (MDA), superoxide dismutase (SOD) and intestinal fatty acid binding protein (iFABP) were measured and liver, kidney, lung, and intestine were evaluated for tissue damage. | Pretreatment with low-dose ketamine before general anesthesia protects against potential oxidative damage and inflammatory response caused by CO2 pneumoperitoneum. |
| [45] | Intraperitoneal injection of caffeic acid phenethyl ester (CAPE) 10 μmol/kg one hour before the desufflation period | 60 min of pneumoperitoneum with 15 mmHg IAP. | Kidneys, testicles, and prostate: Histology and the levels of the total oxidant status (TOS) and total antioxidant status (TAS) | In rat model, increased IAP had an oxidative effect on kidney and testis but not on prostate. Moreover, it could affect the testicular Johnsen score. According to kidney and testis tissues’ histologic evaluation, no significant alteration was obtained in 15 mmHg pressure groups for 1-h insufflation period. All these adverse effects of IAP on both kidney and testis could be prevented by CAPE administration. Further studies are needed to show oxidative effect of IAP against the tissues with more detailed morphological and biochemical analysis. |
| [46] | 100 µg intraperitoneal dexmedetomidine 30 min before establishment of pneumoperitoneum. | 60 min pneumoperitoneum was established under 12 mmHg pressure; intraperitoneal dexmedetomidine (100 µg) was administered 30 min before abdominal insufflation to establish 60 min pneumoperitoneum under 12 mmHg pressure. | Plasma total oxidant status (TOS), total antioxidant status (TAS), and oxidative stress index (OSI) activity were measured 30 min after the conclusion of pneumoperitoneum. | Dexmedetomidine decreases oxidative stress caused by pneumoperitoneum and strengthens the antioxidant defense system. |
| [47] | Dopamine infusion (3 μg/kg/min) before increasing IAP, a 60-min infusion of dopamine was performed; following this, IAP was raised, and the dopamine infusion (3 μg/kg/min) was continued for another 60 min. | IAP of 20 mmHg was maintained for 60 min by air insufflation | Renal artery perfusion was measured continuously for 30 min with a Doppler probe. Mean arterial pressure, myeloperoxidase (MPO) activity, lipid peroxidation and glutathione (GSH) levels were measured in tissue samples, and histopathological scoring was carried out. | Dopamine infusion before and during ACS, increases renal perfusion and decreases free oxygen radicals. Degenerations in the kidney tissues of the rats were clearly improved when the animals were treated by dopamine during and before ACS |
| [48] | Minocycline (20 mg/kg) was intravenously administered immediately after resuscitation. | Hemorrhagic shock/resuscitation was induced by blood drawing (mean arterial pressure: 40–45 mm Hg for 60 min) followed by shed blood/saline mixture reinfusion. Subsequently, intra-abdominal pressure (IAP) was increased to 25 mm Hg by injecting air into the preplaced intraperitoneal latex balloon to induce ACS. IAP was maintained at 25 mmHg for 6 h. | The levels of polymorphonuclear leukocyte infiltration, the wet/dry weight ratio, and the concentrations of inflammatory molecules (e.g., chemokine, cytokine, and prostaglandin E2) in lung and liver tissues | Minocycline ameliorates inflammatory response and organ dysfunction in the lungs and liver induced by hemorrhagic shock/resuscitation plus abdominal compartment syndrome, and ameliorated lung and liver injuries. |
| [49] | Dexmedetomidine administration (intraperitoneal injection of 100 mg/kg) 30 min before pneumoperitoneum. | Intra-abdominal pressure of 12 mmHg for 60 min. The rats were rested for 30 min after abdominal deflation. | Blood samples were obtained for plasma malondialdehyde and ischemia-modified albumin (IMA) analyses. Lung tissue samples were taken for histopathologic examination and malondialdehyde analysis. | Dexmedetomidine prophylaxis resulted in significantly less IMA production and significantly less neutrophil infiltration, thereby helping to protect the lungs from injury after pneumoperitoneum. |
| [50] | Tadalafil (10 mg/kg/day) for 4 days before the experiment | Rats with compensated and decompensated chronic heart failure (CHF) induced by the placement of an aorto-caval fistula (ACF), Rats with myocardial infarction induced by the left anterior descending (LAD) artery ligation and sham controls subjected to IAPs: 7, 10, 14 mmHg. | Urine flow rate (V), Na+ excretion (UNaV), glomerular filtration rate (GFR), renal plasma flow (RPF) | Amelioration of the adverse effects of high IAP |
| [51] | Two hours after operation, 10 mL/kg dachengqi tang (DCQT) was administered orally | Acute necrotic pancreatitis was induced by retrograde infusion of 5% taurocholic acid into the pancreatic duct. | Aterial blood, pancreas and lung tissues were collected for biomarkers and histopathology 24 h after operations. Intra-abdominal pressure and intestinal propulsion rate were also measured. | DCQT treatment reduced intra-abdominal pressure and improved intestinal propulsion rate compared with those treated with saline. The ANP rats treated with DCQT had a lower wet to dry weight ratio, and milder myeloperoxidase activity and histopathology changes in the pancreas and lung than those treated with saline. Higher pressure of oxygen (PO2) was found in the rats treated with DCQT. |
| [52] | Dopamine infusion (3 μg/kg/min) before increasing IAP, a 60-min infusion of dopamine was performed; following this, IAP was raised, and the dopamine infusion (3 μg/kg/min) was continued for another 60 min. | IAP of 20 mm Hg was maintained for 60 min by air insufflation. | Superior mesenteric artery (SMA) perfusion was measured continuously for 30 min with a Doppler probe. Mean arterial pressure, myeloperoxidase (MPO) activity, lipid peroxidation, and glutathione (GSH) levels were measured in tissue samples, and histopathological scoring was carried out. | Improved intestinal epithelium, improved glandular structure, SMA perfusion, counteracted hypotension, increased MPO activity, and decreased GSH. |
| [53] | Doxycycline (10 mg/kg i.p.) during the induction of ACS. Doxycycline (10 mg/kg i.p.) at 1 h after decompression | Intra-abdominal pressure at 20 mmHg by insufflating CO2 gas for 60 min. Decompression 1 h and 24 h. | Creatinine, kidney MDA, IL-1b, IL-6, TNF-a, MMP-2, and TIMP-1 were studied, and the apoptotic cells were enumerated histopathologically, and apoptosis and bcl-2 expression were immunohistochemically assessed. | Doxycycline had protective effects on I/R injury by decreasing apoptosis via reducing the level of pro-inflammatory cytokines, increasing the level of TIMP-1, and inhibiting the activity of MMP-2. |
| [54] | Glutamine through gastric gavage for 10 days at a dose of 1 mL per day (1 g/kg/day) | 20 mmHg pressure was applied for 2 h using CO2. | Intestine, lung, and liver samples were removed for determination of tissue malondialdehyde (MDA) and glutathione (GSH) levels as oxidative injury parameters and of myeloperoxidase (MPO) activity as an inflammatory parameter. the alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels. | Glutamine decreased MDA levels and MPO activities and increased GSH levels. |
| [55] | Doxycycline (10 mg/kg IP) was injected during induction of ACS, and, similarly, intestinal samples were removed at 1 and 24 h after decompression. | IAP of 20 mmHg was maintained by insufflating with carbon dioxide gas for 60 min. Decompression 1 h and 24 h. | Intestine malondialdehyde (MDA), interleukin (IL)-1β, IL-6, tumor necrosis factor (TNF)-α, matrix metalloproteinase-2 (MMP-2), and tissue inhibitor of metalloproteinase-1 were studied and the apoptotic cells were enumerated histopathologically. Apoptosis and β-cell lymphoma 2 (βcl-2) expression were assessed immunohistochemically. | Doxycycline was associated with protective effects against I/R injury through decreasing apoptosis via attenuating the response of proinflammatory cytokines and inhibiting the activity of MMP-2 in this rat model. |
| [56] | Pentoxifylline (50 mg/kg ip) immediately before pneumoperitoneum. | CO2 pneumoperitoneum of 13 mmHg was established. At the first hour of insufflation, blood samples were taken to study the same parameters as in the control group. One hour following desufflation of CO2, blood samples were drawn again to study the same parameters. | The arterial pH, partial arterial oxygen pressure (PaO(2)), venous PO(2), arterial and venous PO(2) difference (P((a-v))O(2)), serum aspartate aminotransferase (AST), serum alanine aminotransferase (ALT), and thiobarbituric acid-reactive substances (TBARS) were studied at the end of the first and second hours | Pentoxifylline may reduce the oxidative injury following laparoscopic procedures. |
| [57] | Nitroglycerine (NTG) (i.v., prime 1.5 mg/kg and sustained infusion of 15 mg/kg/h) beginning 60 min before the application of 14 mmHg insufflation pressure for 1 h, followed by desufflation to 0 mmHg (recovery). L-arginine methylester (L-NAME) 100 mg/l added to drinking water for 4 days before the experiment. | IAP of 14 mmHg, over 1 h, followed by a deflation period of 1 h (recovery). | Urine flow rate (V), Na+ excretion (UNaV), glomerular filtration rate (GFR), renal plasma flow (RPF), and blood pressure | Counteraction of pneumoperitoneum-induced renal hypoperfusion and dysfunction (nitroglycerine). Aggravation of pneumoperitoneum-induced renal hypoperfusion and dysfunction (L-NAME) |
| [58] | Octreotide (50 µg/kg intraperitoneally) immediately before the decompression. | IAH 20 mmHg for 1 h, decompression 1 h. | ALT and AST levels, liver and intestinal tissues, malondialdehyde (an index of lipid peroxidation) and glutathione (a key to antioxidant) levels and myeloperoxidase (an index of tissue neutrophil infiltration) activity | Octreotide treatment reversed these oxidant responses, and reduced the elevations in both ALT and AST levels. |
| [59] | Melatonin (10 mg/kg, i.p.) immediately before the decompression of IAP. | IAH 20 mmHg for 1 h, decompression 1 h. | ALT and AST levels, liver and intestinal tissue, malondialdehyde (an index of lipid peroxidation) and glutathione (a key to antioxidant) levels and myeloperoxidase (an index of tissue neutrophil infiltration) activity, urea nitrogen (BUN), creatinine | Octreotide treatment reversed these oxidant responses, and reduced the elevations in ALT and AST, BUN and creatinine levels. |
| [60] | Octreotide (50 µg/kg intraperitoneally) immediately before the decompression | IAH 20 mmHg for 1 h, decompression 1 h. | Lung and kidney tissue, malondialdehyde (an index of lipid peroxidation) and glutathione (a key to antioxidant) levels and myeloperoxidase (an index of tissue neutrophil infiltration) activity urea nitrogen (BUN), creatinine | Octreotide treatment reversed these oxidant responses, and reduced the elevations In ALT and AST, BUN and creatinine levels. |
| [61] | Dopamine was dissolved in saline and applied as a continuous intravenous infusion at a rate of 3 µg/kg/min using a microperfusion pump with a volume of 2 mL/h. The selective endothelin-1 (ET-I) antagonist, JKC-30 l, was dissolved in saline and administered at 200 µg/kg intravenously as a single-shot injection. | Intraabdominal insufflation pressures were elevated in a stepwise manner from 2 to 12 mmHg (2, 4, 6, 8, 10, and 12 mmHg) every 10 min. At the end of the experimental procedure, the abdomen was desufltated and the portal venous blood flow recovery was measured for another 30 mm. | Portal blood flow was measured during intraabdominal pressures 2 to 12 mmHg. | Dopamine and ET-1 antagonism restore portal blood flow during laparoscopic surgery independently of the insufflation gas. |
| [62] | AVP V2 receptor antagonist, OPC-31260 (5 mg/kg) before pneumoperitoneum | 1 h to carbon dioxide pneumoperitoneum (PP) with an intra-abdominal pressure of 8 mmHg. | Glomerular filtration rate (GFR). Urine output, excretion of water, and urea nitrogen, serum osmolality and serum sodium levels, blood urea nitrogen levels | OPC-31260 pretreatment did not affect GFR. Results suggest that plasma AVP contributes to the oliguria due to PP. OPC-31260 may be useful in treating the water retention associated with PP. |
| Ref. | Agent/Species | IAH Procedure | Target | Outcome |
|---|---|---|---|---|
| [121] | Pigs. After 2 h of IAH, infusion of NO-donor PDNO (in a dose of 30 nmol kg−1 min−1) and the placebo drug was initiated and continued for 4 h until the experiment was ended. | IAH was induced by CO2 insufflation to 30 mmHg, after 2 h, decompression 4 h until the experiment was ended | Blood gases, invasive venous and arterial blood pressure, intestinal microcirculation and superior mesenteric blood flow were measured | NO-donor PDNO decreased systemic and pulmonary vascular resistances, elevated the cardiac index score, and, most importantly, counteracted decreased microcirculatory blood flow in the intestinal mucosa during IAH in a porcine model. |
| [122] | Mice. 120 μg (10 μL) hydromorphone 15 min before the establishment of pneumoperitoneum | Abdominal pressure to 15 mmHg for insufflation (for 1 h) and deflation with CO2 (for 3 h). | Lung injury myeloperoxidase (MPO), total oxidant status (TOS), and oxidative stress index (OSI), total antioxidant status (TAS). HO-1-regulated mitochondrial dynamics. | Hydromorphone alleviated lung injury in mice that underwent CO2 insufflation, decreased the levels of myeloperoxidase (MPO), total oxidant status (TOS), and oxidative stress index (OSI), and increased total antioxidant status (TAS). Hydromorphone protects against CO2 pneumoperitoneum-induced lung injury via HO-1-regulated mitochondrial dynamics. |
| [123] | Rabbits. Mydocalm (tolperison, 5 mg/kg single dose) at initiation of 3 h of IAH. | IAH level was modeled at 200 mmH2O with the subsequent stopping of further receipt of liquid during 3 h in an elastic container in the abdominal cavity. | Tone of muscles of the frontal abdominal wall, local blood flow, dilatation, constriction oxygen tension | Mydocalm reduces the tone of muscles of the frontal abdominal wall, which leads to a decrease in IAH (maximum effect after 1.5 h) and prevents decrease in the local blood flow, suppression of dilation and constriction, reactivity of vessels, and reduction in oxygen tension at the end of experiment. |
| [124] | Pigs. Ethyl nitrite (ENO) at a rate of 1.0 L/min in pigs using a high-flow clinical insufflator | The peritoneal cavity was next insufflated to a final intraperitoneal pressure of 15 mmHg with CO2 in the presence or absence of ENO at a rate of 1.0 L/min using a high-flow clinical insufflator. Pneumoperitoneum was maintained for 4 h during which gas was constantly vented from the peritoneal cavity to ensure continual CO2 and ENO exposure. After 4 h, insufflation was discontinued and the abdomen manually deflated. Monitoring was continued under anesthesia for an additional 2 h. | Regional tissue blood flow brain, heart, liver, kidney, stomach, small intestine, colon, spleen, pancreas, venous red blood cell SNO-Hb concentration | The data indicate ethyl nitrite can effectively attenuate insufflation-induced decreases in organ blood flow and nitric oxide bioactivity leading to reductions in markers of acute tissue injury. |
| [125] | Mice. Pentoxifyllin before IAH induction. | IAH was induced in mice by intraperitoneal infusion of mineral oil to a pressure of 20 mm Hg. 4 h of IAH followed by 1 h of decompression. | Brain blood barrier (BBB) integrity was determined by extravasation of 2% Evans blue (EB) | Pentoxifyllin improved BBB integrity |
| [126] | Mice. 50%, 80%, or 100% oxygen inhalation after establishing acute IAH | 15, 20, 30, or 40 cmH2O IAP. The 40 cmH2O group seemed to be appropriate for follow-up experiments. | Liver and blood samples were used to compare the rates of apoptosis using the TUNEL assay as well as alanine aminotransferase (ALT), aspartate aminotransferase (AST); Caspase-3, 9, MDA, and SOD concentrations. | As the oxygen concentration increased, the survival time was prolonged among the 40 cmH2O IAP group, and the number of apoptotic hepatic cells decreased (P 0.01), with a concomittent decrease in caspase 3 and 9 as well as malondialdehyde, although superoxide dismutase showed the opposite results. |
| [127] | Dogs. Following preparation, fentanyl (1 μg kg(−1)) was injected over 30 s IV. | The abdomen was insufflated with CO(2) (11–16 cmH(2) O). Data were recorded 5 min before, during and 5 min after treatment. The following time points were selected for analysis: −160, −140, −120, −100, −80, −60, −40, −20, 0, 30, 50, 70, 90, 110, 130 and 150 s after the start of fentanyl injection. | Peak inspiratory and end-expiratory intra-abdominal pressures continuously decreased over time during the whole experiment and fentanyl exaggerated the decrease in inspiratory pressures but did not affect the rate of decrease in expiratory pressures. | Fentanyl did not increase intra-abdominal pressures in dogs. |
| [128] | Pigs. CO2 enriched with 100 ppm ethyl nitrite (ENO) in pigs. | Final intraperitoneal pressure of 15 mmHg. After 35 min, ENO was introduced into the peritoneal space by passing the CO2 gas insufflated for 60 min and then monitored for an additional 60 min after termination of insufflation | Liver and kidney blood flows. | Inclusion of ethyl nitrite (a nitric oxide-containing compound) within the insufflating gas significantly attenuated the decreases in liver blood flow produced during and after a 60-min period of carbon dioxide pneumoperitoneum. |
| [129] | Dogs. Enalaprilat (5 mg) 15 min before | Abdominal pressure was maintained automatically at 15 mmHg over a 60-min period. 15 and 30 min utes after deflation of the abdominal cavity | Body weight, hematologic values, hemodynamic parameters, and renal function (plasma renin activity, urinary debt, creatinine clearance, and sodium-excretory fraction) | The decline in urinary debt and in creatinine clearance observed during pneumoperitoneum was less accentuated with the administration of enalaprilat. |
| [130] | Pigs. CO2 was insufflated into the peritoneal cavity to reach an intraabdominal pressure of 15 mmHg. After 60 min, animals received dopamine (5 microg × kg(−1) × min(−1); n = 8), dobutamine (5 microg × kg(−1) × min(−1)). | CO2 was insufflated into the peritoneal cavity to reach an intraabdominal pressure of 15 mmHg. | Heart rate, mean arterial pressure, and systemic vascular resistance, with decreases in cardiac output and hepatic artery and portal vein blood flows | Dobutamine infusion, in contrast to dopamine, corrected, at least in part, cardiac output, systemic vascular resistance, and hepatic artery blood flow alterations, but neither drug restored total hepatic blood flow. |
| [131] | Pigs. CO2 was insufflated into the peritoneal cavity to reach an intra-abdominal pressure of 15 mmHg, and 60 min later, animals received dopamine (5 microg/kg/min; n = 10), dobutamine (5 microg/kg/min; n = 10), or saline (n = 5) for 30 min. | CO2 was insufflated into the peritoneal cavity to reach an intra-abdominal pressure of 15 mmHg, and 60 min. | A laser Doppler probe was positioned in the lumen of the ileum to measure arterial and intestinal mucosal blood flows. | Dobutamine infusion reversed the decrease in cardiac output, it failed to restore superior mesenteric artery blood flow; however, intestinal mucosal blood flow returned to baseline levels. Dopamine also attenuated the decrease in cardiac output, but it had no beneficial effect on splanchnic hemodynamic variables. |
| [132] | Pigs. The pneumoperitoneum was carefully evacuated, and after a l0-min rest, similar airway and abdominal pressure measurements were repeated after the pigs received a 4 mg/kg intravenous injection of atracurium. | Insufflation was discontinued when IAP was more than 15 mmHg. | Elastic properties of the abdomen (elastance). | High peak inspiratory airway pressures and intraabdominal pressures during laparoscopy are not affected by neuromuscular block. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sikiric, P.; Seiwerth, S.; Skrtic, A.; Staresinic, M.; Strbe, S.; Vuksic, A.; Sikiric, S.; Bekic, D.; Penovic, T.; Drazenovic, D.; et al. Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution. Pharmaceuticals 2025, 18, 866. https://doi.org/10.3390/ph18060866
Sikiric P, Seiwerth S, Skrtic A, Staresinic M, Strbe S, Vuksic A, Sikiric S, Bekic D, Penovic T, Drazenovic D, et al. Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution. Pharmaceuticals. 2025; 18(6):866. https://doi.org/10.3390/ph18060866
Chicago/Turabian StyleSikiric, Predrag, Sven Seiwerth, Anita Skrtic, Mario Staresinic, Sanja Strbe, Antonia Vuksic, Suncana Sikiric, Dinko Bekic, Toni Penovic, Dominik Drazenovic, and et al. 2025. "Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution" Pharmaceuticals 18, no. 6: 866. https://doi.org/10.3390/ph18060866
APA StyleSikiric, P., Seiwerth, S., Skrtic, A., Staresinic, M., Strbe, S., Vuksic, A., Sikiric, S., Bekic, D., Penovic, T., Drazenovic, D., Becejac, T., Tepes, M., Madzar, Z., Novosel, L., Beketic Oreskovic, L., Oreskovic, I., Stupnisek, M., Boban Blagaic, A., & Dobric, I. (2025). Acute Compartment Syndrome and Intra-Abdominal Hypertension, Decompression, Current Pharmacotherapy, and Stable Gastric Pentadecapeptide BPC 157 Solution. Pharmaceuticals, 18(6), 866. https://doi.org/10.3390/ph18060866






