Validation of a Traditional Medicine, Achyrocline satureioides Infusion, for the Improvement of Mild Respiratory Infection Symptoms: A Randomized, Placebo-Controlled and Open-Label Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Participants and Baseline Characteristics
2.2. Overall Analysis
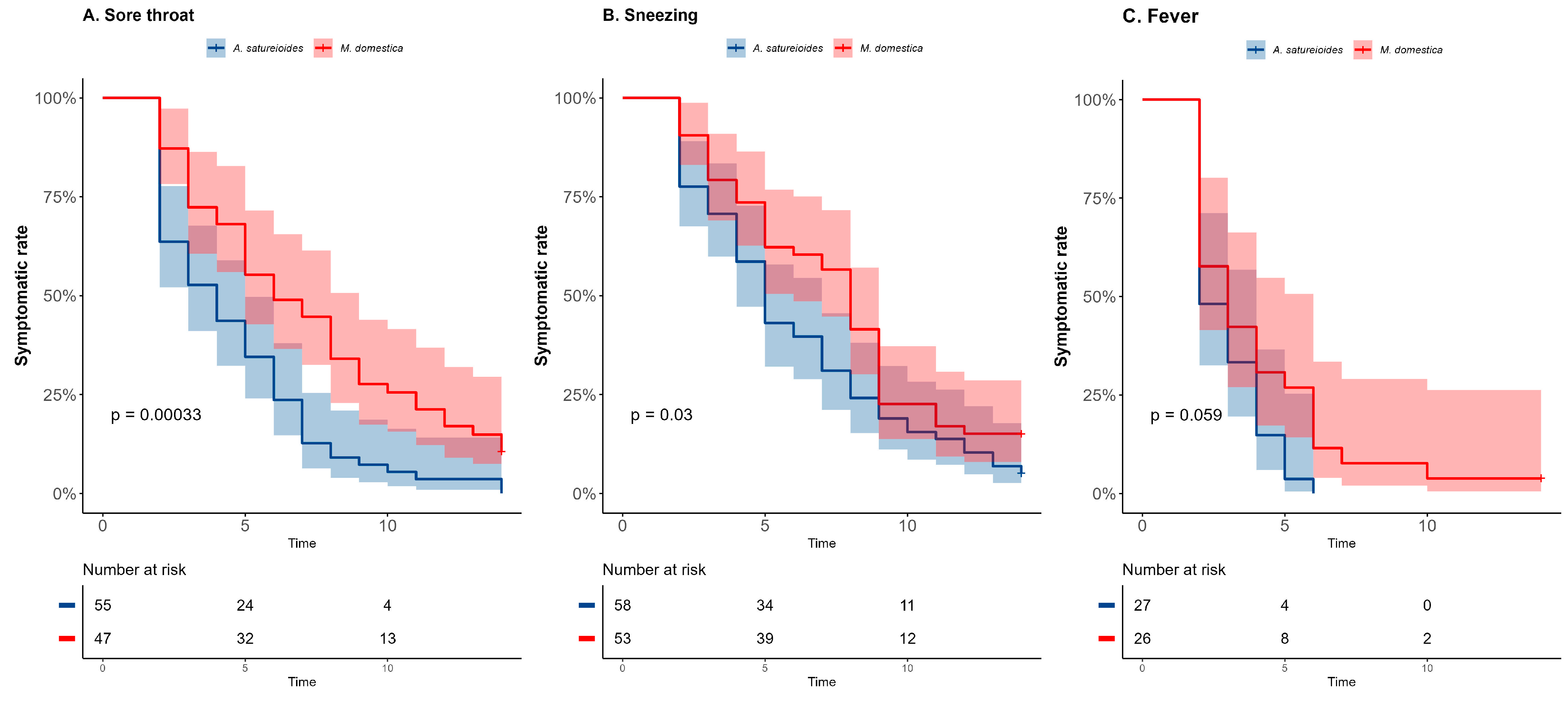
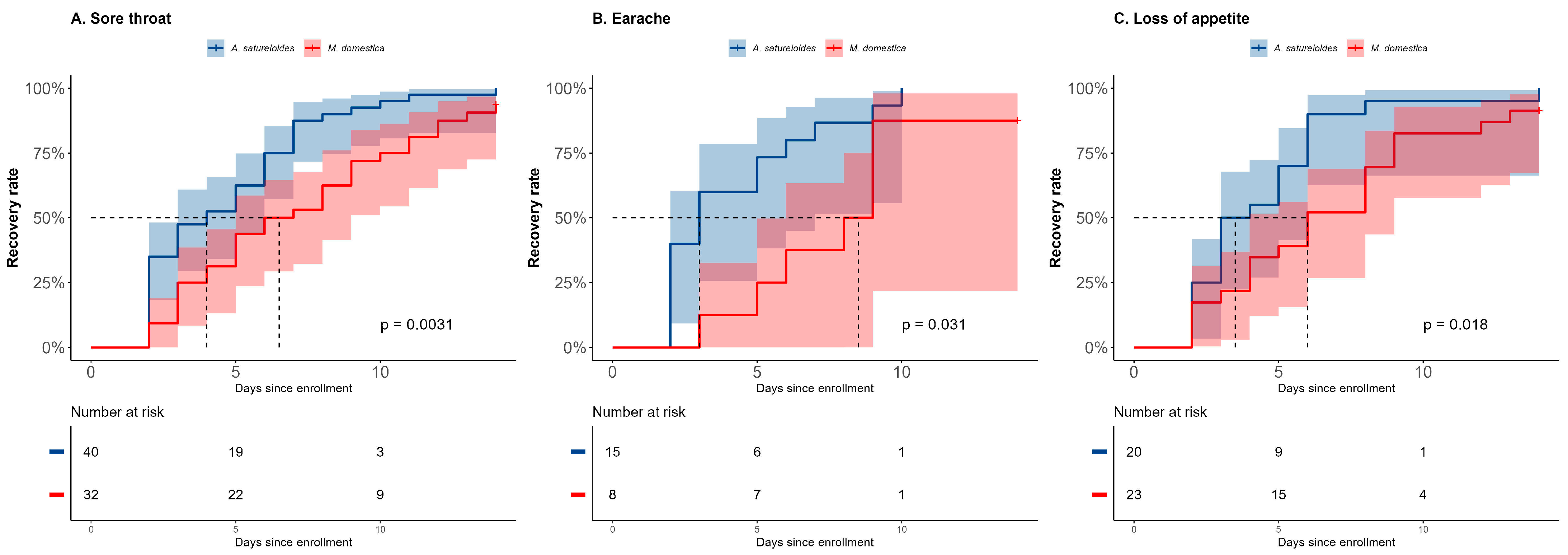
2.3. Subgroup Analysis
2.4. Safety
3. Discussion
4. Materials and Methods
4.1. Ethical Considerations
4.2. Trial Design and Randomization
4.3. Plant Material and Intervention
4.4. Assessment
4.5. Endpoints
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COVID-19 | Coronavirus disease 2019 |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| A. satureioides | Achyrocline satureioides |
| M. domestica | Malus domestica |
| NE | Not possible to estimate |
| ReBEC | Brazilian Registry of Clinical Trial |
| RT-PCR | Reverse transcription polymerase chain reaction |
| UMT | Municipal Screening Unit |
| SISGEN | Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado |
| PP | “per-protocol” principle |
| ACE2 | Angiotensin-converting enzyme 2 |
| TMPRSS2 | Transmembrane serine protease 2 |
| 3CLpro | 3-chymotrypsin-like protease |
References
- Braga, F.C. Brazilian Traditional Medicine: Historical Basis, Features and Potentialities for Pharmaceutical Development. J. Tradit. Chin. Med. Sci. 2021, 8, S44–S50. [Google Scholar] [CrossRef]
- Dutra, R.C.; Campos, M.M.; Santos, A.R.S.; Calixto, J.B. Medicinal Plants in Brazil: Pharmacological Studies, Drug Discovery, Challenges and Perspectives. Pharmacol. Res. 2016, 112, 4–29. [Google Scholar] [CrossRef]
- Ballabh, B.; Chaurasia, O.P. Traditional Medicinal Plants of Cold Desert Ladakh—Used in Treatment of Cold, Cough and Fever. J. Ethnopharmacol. 2007, 112, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Retta, D.; Dellacassa, E.; Villamil, J.; Suárez, S.A.; Bandoni, A.L. Marcela, a Promising Medicinal and Aromatic Plant from Latin America: A Review. Ind. Crops Prod. 2012, 38, 27–38. [Google Scholar] [CrossRef]
- Siqueira, I.R.; Simões, C.M.O.; Bassani, V.L. Achyrocline satureioides (Lam.) D.C. as a Potential Approach for Management of Viral Respiratory Infections. Phytother. Res. 2021, 35, 3–5. [Google Scholar] [CrossRef]
- Bastos, C.I.M.; Dani, C.; Cechinel, L.R.; da Silva Neves, A.H.; Rasia, F.B.; Bianchi, S.E.; da Silveira Loss, E.; Lamers, M.L.; Meirelles, G.; Bassani, V.L.; et al. Achyrocline satureioides as an Adjuvant Therapy for the Management of Mild Viral Respiratory Infections in the Context of COVID-19: Preliminary Results of a Randomized, Placebo-Controlled, and Open-Label Clinical Trial. Phytother. Res. 2023, 37, 5354–5365. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Esquivel, J.; Galvan-Salazar, H.R.; Guzman-Solorzano, H.P.; Cuevas-Velazquez, A.C.; Guzman-Solorzano, J.A.; Mokay-Ramirez, K.A.; Paz-Michel, B.A.; Murillo-Zamora, E.; Delgado-Enciso, J.; Melnikov, V.; et al. Efficacy of the Use of Mefenamic Acid Combined with Standard Medical Care vs. Standard Medical Care Alone for the Treatment of COVID-19: A Randomized Double-Blind Placebo-Controlled Trial. Int. J. Mol. Med. 2022, 49, 29. [Google Scholar] [CrossRef]
- Boschiero, M.N.; Duarte, A.; Palamim, C.V.C.; Alvarez, A.E.; Mauch, R.M.; Marson, F.A.L. Frequency of Respiratory Pathogens Other than SARS-CoV-2 Detected during COVID-19 Testing. Diagn. Microbiol. Infect. Dis. 2022, 102, 115576. [Google Scholar] [CrossRef] [PubMed]
- Kenealy, T. Sore Throat. BMJ Clin. Evid. 2014, 2014, 1509. [Google Scholar]
- Barioni, E.D.; Santin, J.R.; Machado, I.D.; Rodrigues, S.F.D.P.; Ferraz-de-Paula, V.; Wagner, T.M.; Cogliati, B.; Corrêa dos Santos, M.; Machado, M.D.S.; Andrade, S.F.D.; et al. Achyrocline satureioides (Lam.) D.C. Hydroalcoholic Extract Inhibits Neutrophil Functions Related to Innate Host Defense. Evid.-Based Complement. Altern. Med. 2013, 2013, 787916. [Google Scholar] [CrossRef]
- De Souza, K.C.B.; Bassani, V.L.; Schapoval, E.E.S. Influence of Excipients and Technological Process on Anti-Inflammatory Activity of Quercetin and Achyrocline satureioides (Lam.) D.C. Extracts by Oral Route. Phytomedicine 2007, 14, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Di Pierro, F.; Khan, A.; Iqtadar, S.; Mumtaz, S.U.; Chaudhry, M.N.A.; Bertuccioli, A.; Derosa, G.; Maffioli, P.; Togni, S.; Riva, A.; et al. Quercetin as a Possible Complementary Agent for Early-Stage COVID-19: Concluding Results of a Randomized Clinical Trial. Front. Pharmacol. 2023, 13, 1096853. [Google Scholar] [CrossRef]
- da Silva Araújo, N.P.; de Matos, N.A.; Leticia Antunes Mota, S.; Farias de Souza, A.B.; Dantas Cangussú, S.; Cunha Alvim de Menezes, R.; Silva Bezerra, F. Quercetin Attenuates Acute Lung Injury Caused by Cigarette Smoke Both In Vitro and In Vivo. COPD J. Chronic Obstr. Pulm. Dis. 2020, 17, 205–214. [Google Scholar] [CrossRef]
- Townsend, E.A.; Emala, C.W. Quercetin Acutely Relaxes Airway Smooth Muscle and Potentiates β-Agonist-Induced Relaxation via Dual Phosphodiesterase Inhibition of PLCβ and PDE4. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 305, L396–L403. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an Antiviral Agent Inhibits Influenza a Virus (IAV) Entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Gasmi, A.; Mujawdiya, P.K.; Lysiuk, R.; Shanaida, M.; Peana, M.; Gasmi Benahmed, A.; Beley, N.; Kovalska, N.; Bjørklund, G. Quercetin in the Prevention and Treatment of Coronavirus Infections: A Focus on SARS-CoV-2. Pharmaceuticals 2022, 15, 1049. [Google Scholar] [CrossRef]
- Roy, A.V.; Chan, M.; Banadyga, L.; He, S.; Zhu, W.; Chrétien, M.; Mbikay, M. Quercetin Inhibits SARS-CoV-2 Infection and Prevents Syncytium Formation by Cells Co-Expressing the Viral Spike Protein and Human ACE2. Virol. J. 2024, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Manjunathan, R.; Periyaswami, V.; Mitra, K.; Rosita, A.S.; Pandya, M.; Selvaraj, J.; Ravi, L.; Devarajan, N.; Doble, M. Molecular Docking Analysis Reveals the Functional Inhibitory Effect of Genistein and Quercetin on TMPRSS2: SARS-CoV-2 Cell Entry Facilitator Spike Protein. BMC Bioinform. 2022, 23, 180. [Google Scholar] [CrossRef]
- Chen, L.; Li, J.; Luo, C.; Liu, H.; Xu, W.; Chen, G.; Liew, O.W.; Zhu, W.; Puah, C.M.; Shen, X.; et al. Binding Interaction of Quercetin-3-β-Galactoside and Its Synthetic Derivatives with SARS-CoV 3CLpro: Structure–Activity Relationship Studies Reveal Salient Pharmacophore Features. Bioorganic Med. Chem. 2006, 14, 8295–8306. [Google Scholar] [CrossRef]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir–Ritonavir in Adults Hospitalized with Severe COVID-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef]
- Agência Nacional de Vigilância Sanitária (ANVISA). Formulário de Fitoterápicos da Farmacopeia Brasileira, 1st ed.; ANVISA: Brasília, Brazil, 2011; p. 20.
- Yakoot, M. Nonsignificant Trends in COVID-19 Trials: Is There a Significance? J. Med. Virol. 2022, 94, 1757–1760. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.Y.; Teo, S.P.; Abdullah, M.S.; Chong, P.L.; Asli, R.; Mani, B.I.; Momin, N.R.; Lim, A.C.A.; Rahman, N.A.; Chong, C.F.; et al. COVID-19 Symptom Duration: Associations with Age, Severity and Vaccination Status in Brunei Darussalam, 2021. West. Pac. Surveill. Response 2022, 13, 55–63. [Google Scholar] [CrossRef]
- Kumar, A.; Sharma, A.; Tirpude, N.V.; Padwad, Y.; Hallan, V.; Kumar, S. Plant-Derived Immuno-Adjuvants in Vaccines Formulation: A Promising Avenue for Improving Vaccines Efficacy against SARS-CoV-2 Virus. Pharmacol. Rep. 2022, 74, 1238–1254. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, R.; Wang, M.; Wei, G.-W. Mutations Strengthened SARS-CoV-2 Infectivity. J. Mol. Biol. 2020, 432, 5212–5226. [Google Scholar] [CrossRef]
- Tao, K.; Tzou, P.L.; Nouhin, J.; Gupta, R.K.; De Oliveira, T.; Kosakovsky Pond, S.L.; Fera, D.; Shafer, R.W. The Biological and Clinical Significance of Emerging SARS-CoV-2 Variants. Nat. Rev. Genet. 2021, 22, 757–773. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Barbosa, A.N.; Cimerman, S. Editorial: New Therapeutic Approaches for SARS-CoV-2/COVID-19. Front. Immunol. 2023, 14, 1276279. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Bhardwaj, K.; Klimova, B.; Nepovimova, E.; Wu, Q.; Landi, M.; Kuca, K.; Valis, M.; Wu, W. Malus domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants 2020, 9, 1408. [Google Scholar] [CrossRef] [PubMed]
- Scheim, D.E.; Aldous, C.; Osimani, B.; Fordham, E.J.; Hoy, W.E. When Characteristics of Clinical Trials Require Per-Protocol as Well as Intention-to-Treat Outcomes to Draw Reliable Conclusions: Three Examples. J. Clin. Med. 2023, 12, 3625. [Google Scholar] [CrossRef]
- Lima, V.; Melo, E.; Lima, D. Teor de Compostos Fenólicos Totais Em Chás Brasileiros. Braz. J. Food Technol. 2004, 7, 187–190. [Google Scholar]
- Balsan, G.; Pellanda, L.C.; Sausen, G.; Galarraga, T.; Zaffari, D.; Pontin, B.; Portal, V.L. Effect of Yerba Mate and Green Tea on Paraoxonase and Leptin Levels in Patients Affected by Overweight or Obesity and Dyslipidemia: A Randomized Clinical Trial. Nutr. J. 2019, 18, 5. [Google Scholar] [CrossRef]
- Davis, J.M.; Murphy, E.A.; McClellan, J.L.; Carmichael, M.D.; Gangemi, J.D. Quercetin Reduces Susceptibility to Influenza Infection Following Stressful Exercise. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 295, R505–R509. [Google Scholar] [CrossRef]
- Farazuddin, M.; Mishra, R.; Jing, Y.; Srivastava, V.; Comstock, A.T.; Sajjan, U.S. Quercetin Prevents Rhinovirus-Induced Progression of Lung Disease in Mice with COPD Phenotype. PLoS ONE 2018, 13, e0199612. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Faris, A.N.; Comstock, A.T.; Wang, Q.; Nanua, S.; Hershenson, M.B.; Sajjan, U.S. Quercetin Inhibits Rhinovirus Replication In Vitro and In Vivo. Antivir. Res. 2012, 94, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Uchide, N.; Toyoda, H. Antioxidant Therapy as a Potential Approach to Severe Influenza-Associated Complications. Molecules 2011, 16, 2032–2052. [Google Scholar] [CrossRef] [PubMed]
- Therneau, T.M. A Package for Survival Analysis in R.; R Package Version 3.5-5; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=survival (accessed on 1 April 2025).
- Kassambara, A.; Kosinski, M. Survminer: Drawing Survival Curves Using ‘ggplot2’; R Package Version 0.4.9; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=survminer (accessed on 1 April 2025).


| All Participants (n = 240) | Achyrocline satureioides Infusion (n = 120) | Malus domestica Infusion (n = 120) | |
|---|---|---|---|
| Age (years, mean ± SD) | 40.17 ± 14.41 | 41.45 ± 15.07 | 38.88 ± 13.71 |
| Male n (%) | 104 (43.51%) | 50 (41.67%) | 54 (45%) |
| Female | 136 (56.67%) | 70 (58.33%) | 66 (55%) |
| Ethnicity, n (%) | |||
| White | 177 (73.75%) | 90 (75%) | 87 (72.50%) |
| Pardo | 46 (19.17%) | 22 (18.33%) | 24 (20%) |
| Black | 13 (5.42%) | 6 (5.00%) | 7 (5.83%) |
| Not provided | 4 (1.67%) | 2 (1.67%) | 2 (1.67%) |
| Education Level, n (%) | |||
| Elementary school (incomplete) | 59 (24.58%) | 32 (26.67%) | 27 (22.50%) |
| Elementary school | 41 (17.08%) | 17 (14.17%) | 24 (20.00%) |
| High school (incomplete) | 14 (5.83%) | 6 (5.00%) | 12 (10.00%) |
| High school | 74 (30.83%) | 42 (35.00%) | 32 (26.67%) |
| Graduation (incomplete) | 15 (6.25%) | 6 (5.00%) | 9 (7.50%) |
| Graduated | 32 (13.33%) | 15 (12.50%) | 13 (10.83%) |
| Not provided | 5 (2.08%) | 2 (1.67%) | 3 (2.50%) |
| Comorbidities, n (%) | |||
| Diabetes | 27 (11.30%) | 17 (14.17%) | 10 (8.33%) |
| Hypertension | 69 (28.75%) | 34 (28.33%) | 34 (28.33%) |
| Obesity (BMI > 30 kg/m2) | 68 (28.33%) | 33 (27.50%) | 34 (28.33%) |
| Smoking | 27 (11.30%) | 14 (11.67%) | 13 (10.83%) |
| Heart diseases | 21 (8.79%) | 15 (12.50%) | 6 (5.00%) |
| Neoplasias | 12 (5.02%) | 8 (6.67%) | 4 (3.33%) |
| Respiratory diseases | 150 (62.50%) | 78 (65.00%) | 72 (60.00%) |
| User of Medicinal Plants, n (%) | |||
| Yes | 180 (75.00%) | 94 (78.33%) | 86 (71.67%) |
| No | 52 (21.67%) | 24 (20.00%) | 28 (23.33%) |
| Not informed | 8 (3.33%) | 2 (1.67%) | 6 (5.00%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bastos, C.I.M.; Dani, C.; Cechinel, L.R.; Neves, A.H.d.S.; Rasia, F.B.; Lamers, M.L.; Bianchi, S.E.; Meirelles, G.; Worm, P.V.; Bassani, V.L.; et al. Validation of a Traditional Medicine, Achyrocline satureioides Infusion, for the Improvement of Mild Respiratory Infection Symptoms: A Randomized, Placebo-Controlled and Open-Label Clinical Trial. Pharmaceuticals 2025, 18, 861. https://doi.org/10.3390/ph18060861
Bastos CIM, Dani C, Cechinel LR, Neves AHdS, Rasia FB, Lamers ML, Bianchi SE, Meirelles G, Worm PV, Bassani VL, et al. Validation of a Traditional Medicine, Achyrocline satureioides Infusion, for the Improvement of Mild Respiratory Infection Symptoms: A Randomized, Placebo-Controlled and Open-Label Clinical Trial. Pharmaceuticals. 2025; 18(6):861. https://doi.org/10.3390/ph18060861
Chicago/Turabian StyleBastos, Catherina Isdra Moszkowicz, Caroline Dani, Laura Reck Cechinel, Arthur Hipolito da Silva Neves, Fabiana Briato Rasia, Marcelo Lazzaron Lamers, Sara Elis Bianchi, Gabriela Meirelles, Paulo Valdeci Worm, Valquiria Linck Bassani, and et al. 2025. "Validation of a Traditional Medicine, Achyrocline satureioides Infusion, for the Improvement of Mild Respiratory Infection Symptoms: A Randomized, Placebo-Controlled and Open-Label Clinical Trial" Pharmaceuticals 18, no. 6: 861. https://doi.org/10.3390/ph18060861
APA StyleBastos, C. I. M., Dani, C., Cechinel, L. R., Neves, A. H. d. S., Rasia, F. B., Lamers, M. L., Bianchi, S. E., Meirelles, G., Worm, P. V., Bassani, V. L., & Siqueira, I. R. (2025). Validation of a Traditional Medicine, Achyrocline satureioides Infusion, for the Improvement of Mild Respiratory Infection Symptoms: A Randomized, Placebo-Controlled and Open-Label Clinical Trial. Pharmaceuticals, 18(6), 861. https://doi.org/10.3390/ph18060861






