Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities
Abstract
1. Introduction
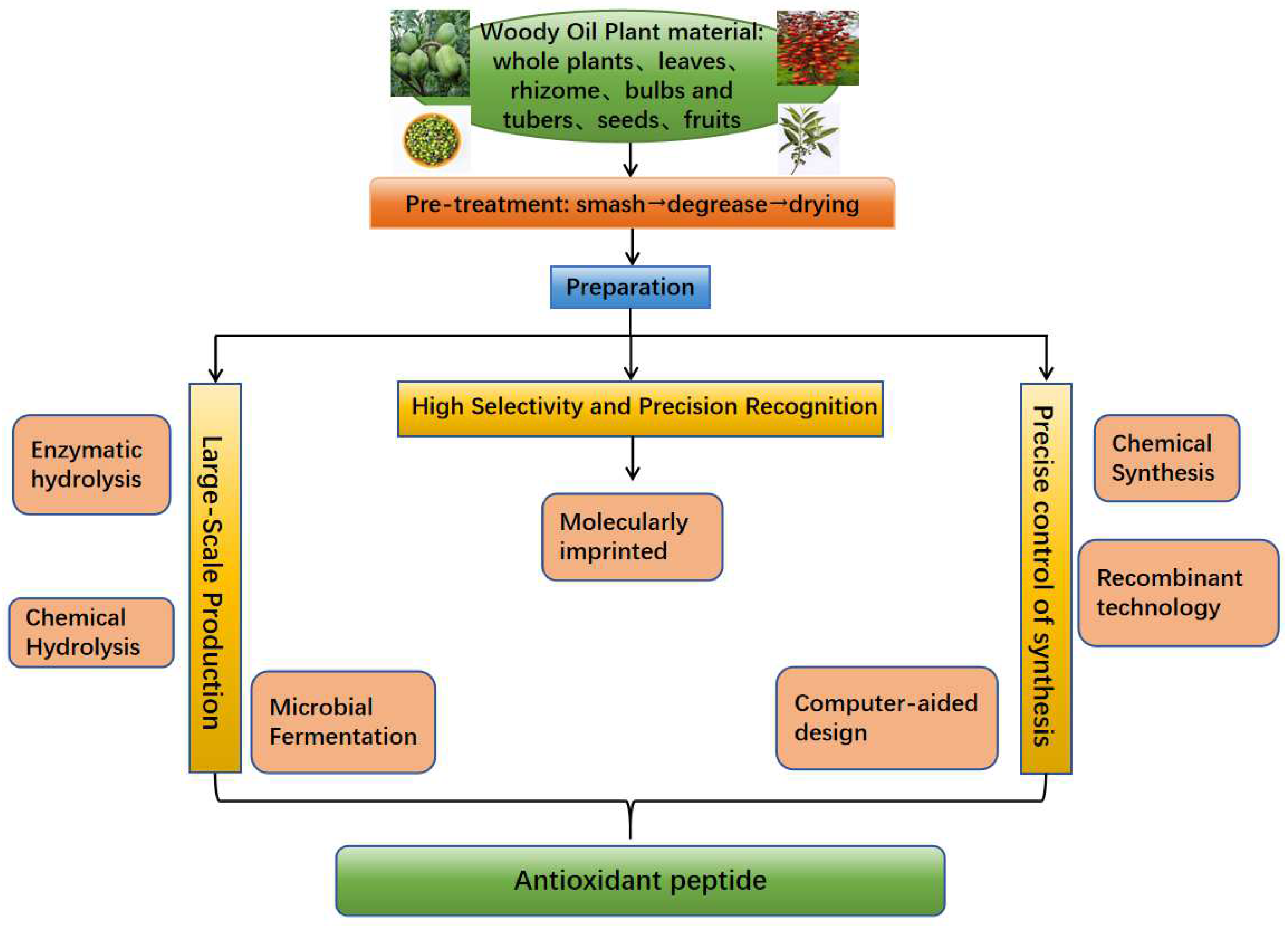
2. Advances in the Preparation of and Functional Insights into Antioxidant Peptides Derived from Woody Oil Resources
2.1. Large-Scale Production with Low Purity Requirements
2.2. Synthesis of Functional Peptides with Precise Control
2.3. Peptide Preparation with High Selectivity and Precision Recognition
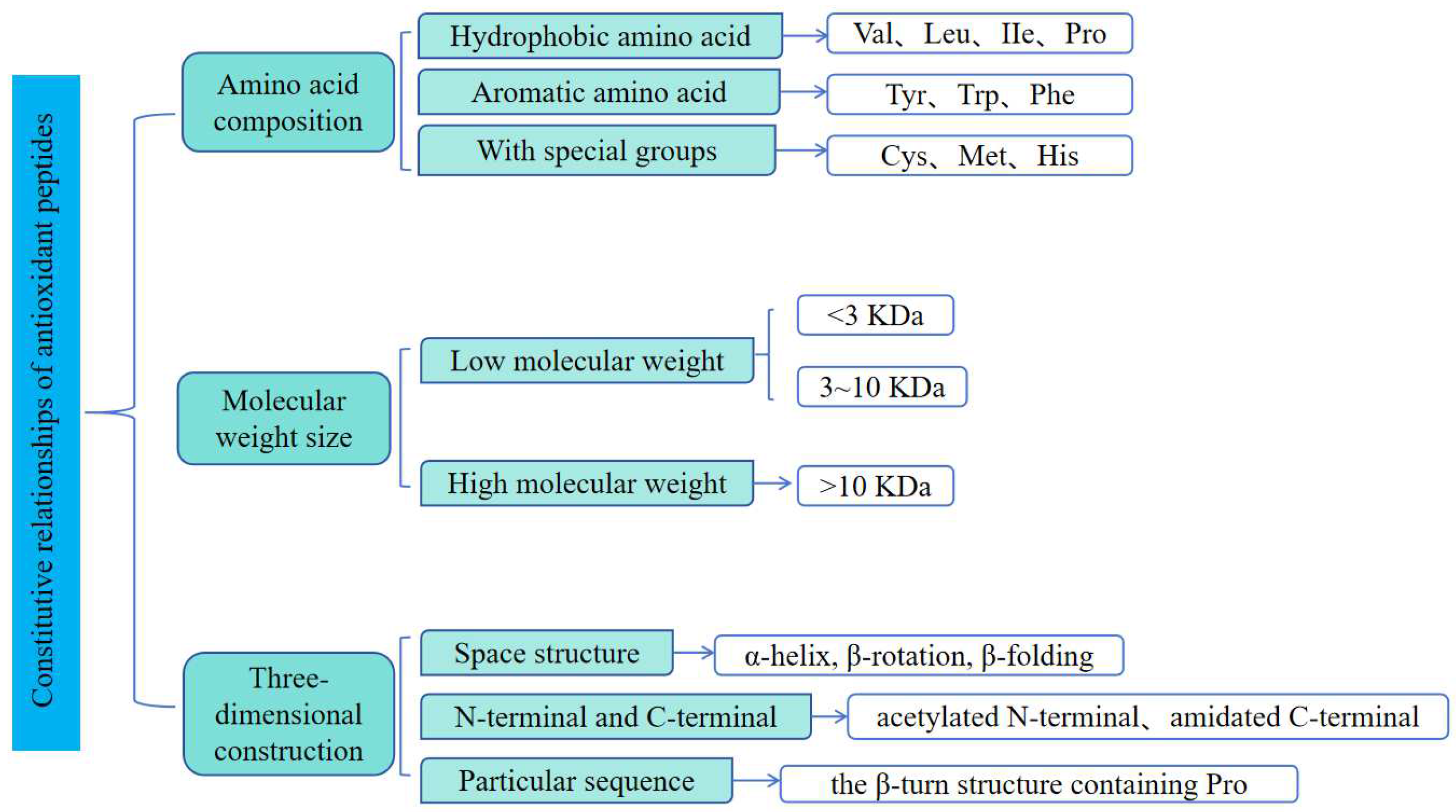
3. Effects of Structure–Activity Relationship of Antioxidant Peptides
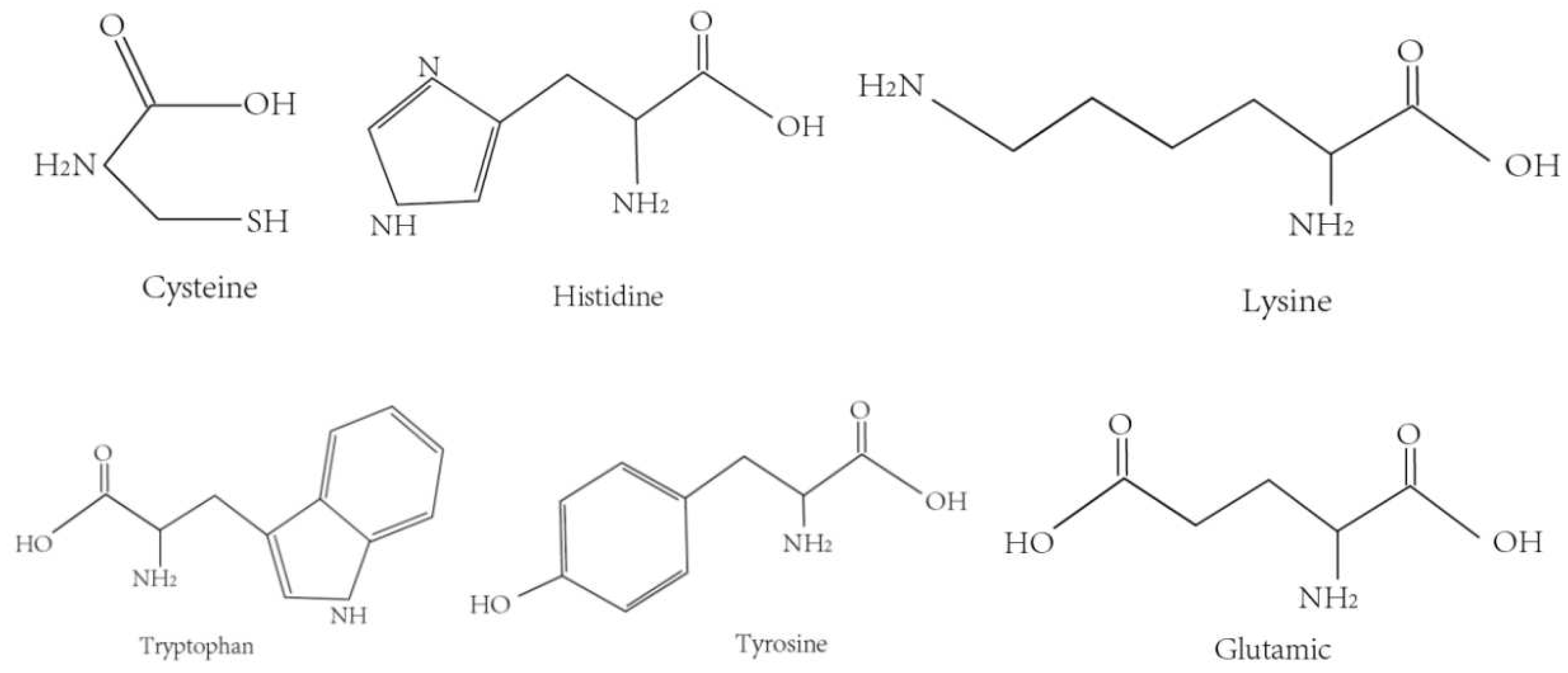
3.1. Effects of Amino Acid Sequence on Activity
3.2. Effects of Molecular Weight on Activity
3.3. Effects of Three-Dimensional Structure on Activity
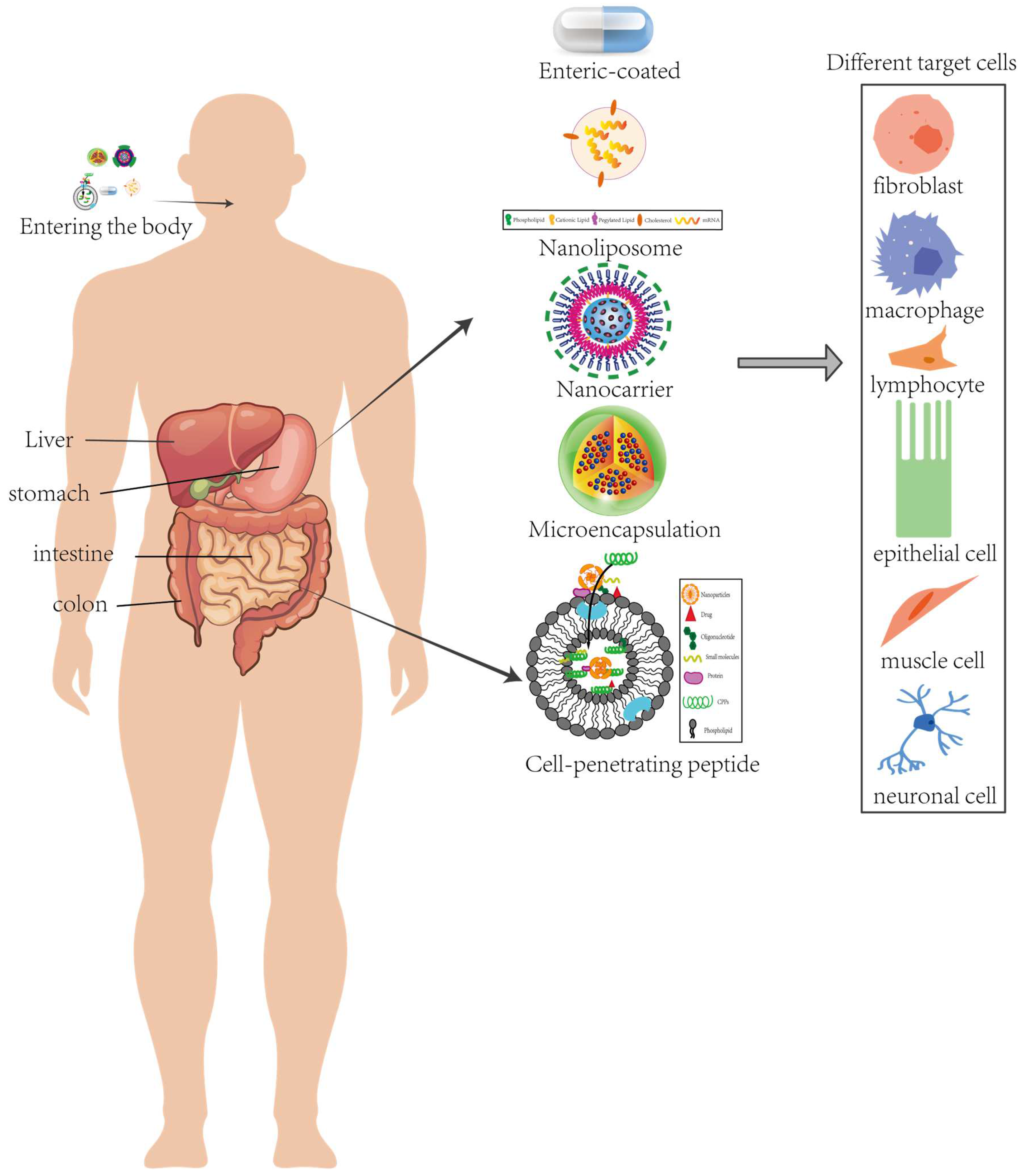
4. Delivery Systems with Antioxidant Peptides
4.1. Nanotechnology Applications
4.2. Biological Polymer Carriers: Natural Polymers and Synthetic Polymers
4.3. Targeted Delivery Systems
4.4. Encapsulated Delivery Systems for Microcapsules and Nano-Emulsions
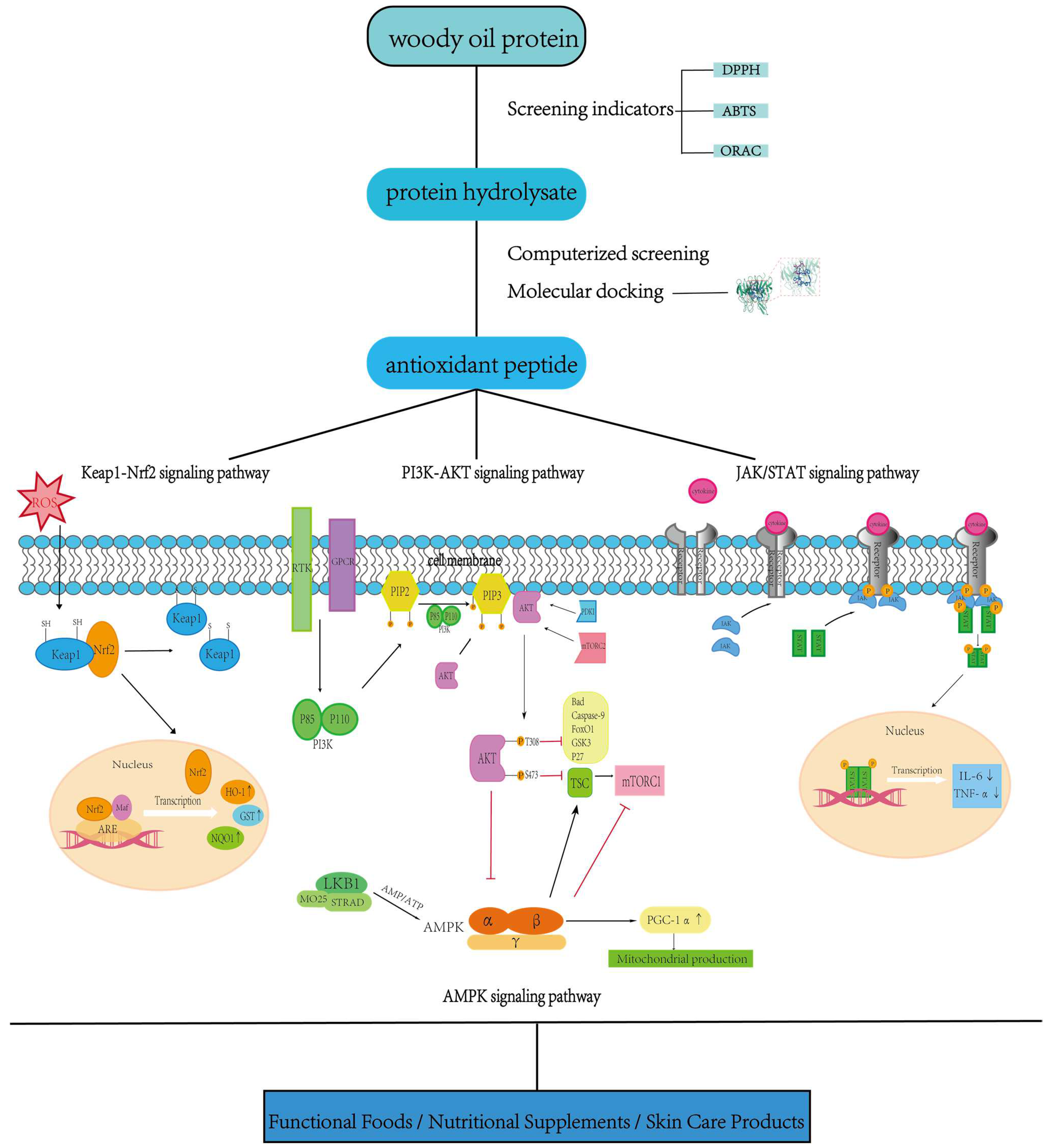
5. Studies on the Signaling Pathway Regulation Mechanism of Antioxidant Peptides from Woody Oil Resources
5.1. Integrated Regulatory Mechanisms of Core Antioxidant Signaling Pathways
5.1.1. Keap1/Nrf2/ARE Pathway: A Redox-Sensing Hub for Peptide Intervention
5.1.2. PI3K/Akt Pathway: Enhancing Redox Signaling and Cytoprotection
5.1.3. JAK/STAT Pathway: Coordinating Anti-Inflammatory and Redox Responses
5.2. Exploration of Auxiliary Pathways and Systemic Crossover Mechanisms
5.2.1. AMPK Pathway: Energy–Redox Coupling and Mitochondrial Repair
5.2.2. Emerging Mechanisms: Non-Coding RNAs and Microecological Interactions for Regulation
6. Safety, Immunogenicity, and Toxicity of Antioxidant Peptides Derived from Woody Oil Resources
6.1. Acute and Sub-Chronic Toxicity
6.2. Immunogenicity
6.3. Pro-Oxidant and Metabolic Liabilities
7. Clinical Translation and Ongoing Human Studies
8. Application Prospects and Industrialization Potential
- (1)
- Precision Nutrition for Aging and Metabolic Syndromes
- (2)
- Functional Foods via Multi-Pathway Synergy
- (3)
- Cosmeceutical Applications: Anti-UV and Skin Barrier Repair
- (4)
- Mechanism-Guided Peptide Innovation for Industrial Translation
9. Future Perspectives
- (1)
- Structure–function elucidation
- (2)
- Improved bioavailability
- (3)
- Mechanistic integration via multi-omics
- (4)
- Human validation
- (5)
- Sustainable industrialization
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chiou, A.; Kalogeropoulos, N. Virgin Olive Oil as Frying Oil. Compr. Rev. Food Sci. Food Saf. 2017, 16, 632–646. [Google Scholar] [CrossRef] [PubMed]
- Mat Yasin, M.H.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F.; Ali, M.H. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef]
- Deen, A.; Visvanathan, R.; Wickramarachchi, D.; Marikkar, N.; Nammi, S.; Jayawardana, B.C.; Liyanage, R. Chemical composition and health benefits of coconut oil: An overview. J. Sci. Food Agric. 2020, 101, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, A.; Du, Q.; Zhu, W.; Liu, H.; Naeem, A.; Guan, Y.; Chen, L.; Ming, L. Bioactive substances and therapeutic potential of camellia oil: An overview. Food Biosci. 2022, 49, 101855. [Google Scholar] [CrossRef]
- Bartoszek, A.; Makaro, A.; Bartoszek, A.; Kordek, R.; Fichna, J.; Salaga, M. Walnut Oil Alleviates Intestinal Inflammation and Restores Intestinal Barrier Function in Mice. Nutrients 2020, 12, 1302. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.L.; Xiao, X.Z.; Xu, X.Q. Chemical composition analysis of seed oil from five wild almond species in China as potential edible oil resource for the future. S. Afr. J. Bot. 2019, 121, 274–281. [Google Scholar] [CrossRef]
- Deng, R.; Gao, J.; Yi, J.; Liu, P. Could peony seeds oil become a high-quality edible vegetable oil? The nutritional and phytochemistry profiles, extraction, health benefits, safety and value-added-products. Food Res. Int. 2022, 156, 111200. [Google Scholar] [CrossRef]
- Khalid, N.; Khan, R.S.; Hussain, M.I.; Farooq, M.; Ahmad, A.; Ahmed, I. A comprehensive characterisation of safflower oil for its potential applications as a bioactive food ingredient—A review. Trends Food Sci. Technol. 2017, 66, 176–186. [Google Scholar] [CrossRef]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Borotová, P.; Galovičová, L.; Vukovic, N.L.; Vukic, M.; Kunová, S.; Hanus, P.; Kowalczewski, P.Ł.; Bakay, L.; Kačániová, M. Role of Litsea cubeba Essential Oil in Agricultural Products Safety: Antioxidant and Antimicrobial Applications. Plants 2022, 11, 1504. [Google Scholar] [CrossRef]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crops Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Mohanty, A.; Rout, P.R.; Dubey, B.; Meena, S.S.; Pal, P.; Goel, M. A critical review on biogas production from edible and non-edible oil cakes. Biomass Convers. Biorefin. 2021, 12, 949–966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ma, H.; Quaisie, J.; Gu, C.; Guo, L.; Liu, D.; Chen, Y.; Zhang, T. Tea saponin extracted from seed pomace of Camellia oleifera Abel ameliorates DNCB-induced atopic dermatitis-like symptoms in BALB/c mice. J. Funct. Foods 2022, 91, 105001. [Google Scholar] [CrossRef]
- Teli, M.D.; Valia, S.P.; Mifta, J. Application of functionalized coir fibre as eco-friendly oil sorbent. J. Text. Inst. 2016, 108, 1106–1111. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Chi, J.; Ma, J. Bioactive Peptides from Walnut Residue Protein. Molecules 2020, 25, 1285. [Google Scholar] [CrossRef]
- Sheng, J.; Yang, X.; Chen, J.; Peng, T.; Yin, X.; Liu, W.; Liang, M.; Wan, J.; Yang, X. Antioxidative Effects and Mechanism Study of Bioactive Peptides from Defatted Walnut (Juglans regia L.) Meal Hydrolysate. J. Agric. Food Chem. 2019, 67, 3305–3312. [Google Scholar] [CrossRef]
- Wang, M.; Wu, W.; Xiao, J.; Li, C.; Chen, B.; Shen, Y. Recent Development in Antioxidant Peptides of Woody Oil Plant By-Products. Food Rev. Int. 2022, 39, 5479–5500. [Google Scholar] [CrossRef]
- Qusa, M.H.; Siddique, A.B.; Nazzal, S.; El Sayed, K.A. Novel olive oil phenolic (−)-oleocanthal (+)-xylitol-based solid dispersion formulations with potent oral anti-breast cancer activities. Int. J. Pharm. 2019, 569, 118596. [Google Scholar] [CrossRef]
- Lin, C.-H.; Chen, Y.-K.; Wang, W.-C. The production of bio-jet fuel from palm oil derived alkanes. Fuel 2020, 260, 116345. [Google Scholar] [CrossRef]
- Acevedo-Juárez, S.; Guajardo-Flores, D.; Heredia-Olea, E.; Antunes-Ricardo, M. Bioactive peptides from nuts: A review. Int. J. Food Sci. Technol. 2022, 57, 2226–2234. [Google Scholar] [CrossRef]
- Boruzi, A.I.; Nour, V. Walnut (Juglans regia L.) leaf powder as a natural antioxidant in cooked pork patties. CyTA-J. Food 2019, 17, 431–438. [Google Scholar] [CrossRef]
- Elisha, C.; Bhagwat, P.; Pillai, S. Emerging production techniques and potential health promoting properties of plant and animal protein-derived bioactive peptides. Crit. Rev. Food Sci. Nutr. 2024, 1–30. [Google Scholar] [CrossRef]
- Chan, B.A. Peptidomimetic Polymers: Advances in Monomer Design and Polymerization Methods. Ph.D. Thesis, Louisiana State University and Agricultural and Mechanical College, Baton Rouge, LA, USA, 2016. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, X.; Pei, H.; Chen, Y.; Meng, H.; Yuan, J.; Xing, H.; Wu, Y. An overview of walnuts application as a plant-based. Front. Endocrinol. 2022, 13, 1083707. [Google Scholar] [CrossRef]
- Zhang, L.; Bai, Y.-Y.; Hong, Z.-S.; Xie, J.; Tian, Y. Isolation, Identification, Activity Evaluation, and Mechanism of Action of Neuroprotective Peptides from Walnuts: A Review. Nutrients 2023, 15, 4085. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. Mol. Sci. 2021, 3, 100047. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Hong, J.; Aihaiti, A.; Mu, Y.; Yin, X.; Zhang, M.; Liu, X.; Wang, L. Preparation of sea buckthorn (Hippophae rhamnoides L.) seed meal peptide by mixed fermentation and its effect on volatile compounds and hypoglycemia. Front. Nutr. 2024, 11, 1355116. [Google Scholar] [CrossRef]
- Rodríguez, V.; Asenjo, J.A.; Andrews, B.A. Design and implementation of a high yield production system for recombi-nant expression of peptides. Microb. Cell Factories 2014, 13, 65. [Google Scholar] [CrossRef]
- Lu, H.; Xie, T.; Wu, Q.; Hu, Z.; Luo, Y.; Luo, F. Alpha-Glucosidase Inhibitory Peptides: Sources, Preparations, Identifications, and Action Mechanisms. Nutrients 2023, 15, 4267. [Google Scholar] [CrossRef]
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51. [Google Scholar] [CrossRef]
- Hassan, A.; Ali, S.; Farooq, M.A.; Tahir, H.M.; Awan, M.U.; Mughal, T.A. Optimization of enhanced microbial production of zinc bacitracin by submerged fermentation technology. J. Basic Microbiol. 2020, 60, 585–599. [Google Scholar] [CrossRef]
- Saubenova, M.; Oleinikova, Y.; Rapoport, A.; Maksimovich, S.; Yermekbay, Z.; Khamedova, E. Bioactive Peptides Derived from Whey Proteins for Health and Functional Beverages. Fermentation 2024, 10, 359. [Google Scholar] [CrossRef]
- Khiari, Z.; Mason, B. Comparative dynamics of fish by-catch hydrolysis through chemical and microbial methods. Lwt 2018, 97, 135–143. [Google Scholar] [CrossRef]
- Wang, S.; Zheng, L.; Zhao, T.; Zhang, Q.; Liu, Y.; Sun, B.; Su, G.; Zhao, M. Inhibitory Effects of Walnut (Juglans regia) Peptides on Neuroinflammation and Oxidative Stress in Lipopolysaccharide-Induced Cognitive Impairment Mice. J. Agric. Food Chem. 2020, 68, 2381–2392. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Du, M.; Zhang, Y.; Xu, W.; Wang, C.; Wang, K.; Zhang, L. Purification and Identification of an ACE Inhibitory Peptide from Walnut Protein. J. Agric. Food Chem. 2013, 61, 4097–4100. [Google Scholar] [CrossRef]
- Bader, M.; Xu, F.; Wang, K.Y.; Wang, N.; Li, G.; Liu, D. Bioactivity of a modified human Glucagon-like peptide-1. PLoS ONE 2017, 12, e0171601. [Google Scholar]
- Zhong, H.; Li, B.; Fan, Z.; Fang, Z.; Li, Y.; Lu, S.; Shen, Y.; Wang, W.; Zhang, R. Isolation, purification, characterization, and analysis of the antioxidant activity of antioxidant peptides from walnut milk fermented with Lactobacillus paracasei SMN-LBK. Food Biosci. 2024, 61, 104547. [Google Scholar] [CrossRef]
- Nazarian-Firouzabadi, F.; Torres, M.D.T.; de la Fuente-Nunez, C. Recombinant production of antimicrobial peptides in plants. Biotechnol. Adv. 2024, 71, 108296. [Google Scholar] [CrossRef]
- Hudon, A.; Gaudreau-Ménard, C.; Bouchard-Boivin, M.; Godin, F.; Cailhol, L. The Use of Computer-Driven Technologies in the Treatment of Borderline Personality Disorder: A Systematic Review. J. Clin. Med. 2022, 11, 3685. [Google Scholar] [CrossRef]
- Malibari, A.A.; Alzahrani, J.S.; Eltahir, M.M.; Malik, V.; Obayya, M.; Duhayyim, M.A.; Lira Neto, A.V.; de Albuquerque, V.H.C. Optimal deep neural network-driven computer aided diagnosis model for skin cancer. Comput. Electr. Eng. 2022, 103, 108318. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y. Quantitative Structure-activity Relationship Study on Antioxidant Dipeptides. In Proceedings of the 2022 AOCS Annual Meeting & Expo, Atlanta, GA, USA, 1–4 May 2022. [Google Scholar]
- Medeiros, I.; Gomes, A.F.T.; Oliveira e Silva, E.G.; Bezerra, I.W.L.; da Silva Maia, J.K.; Piuvezam, G.; Morais, A.H.D.A. Proteins and Peptides Studied In Silico and In Vivo for the Treatment of Diabetes Mellitus: A Systematic Review. Nutrients 2024, 16, 2395. [Google Scholar] [CrossRef]
- Ali, M.M.; Liu, X.; Amin, F.R.; Zhou, J.; Hu, L. Recent advances in molecularly imprinted polymers for glycosylated molecule analysis. TrAC Trends Anal. Chem. 2025, 185, 118168. [Google Scholar] [CrossRef]
- Yang, X.; Dong, X.; Zhang, K.; Yang, F.; Guo, Z. A molecularly imprinted polymer as an antibody mimic with affinity for lysine acetylated peptides. J. Mater. Chem. B 2016, 4, 920–928. [Google Scholar] [CrossRef]
- Balcer, E.; Sobiech, M.; Luliński, P. Molecularly Imprinted Carriers for Diagnostics and Therapy—A Critical Appraisal. Pharmaceutics 2023, 15, 1647. [Google Scholar] [CrossRef]
- Incel, A.; Arribas Díez, I.; Wierzbicka, C.; Gajoch, K.; Jensen, O.N.; Sellergren, B. Selective Enrichment of Histidine Phosphorylated Peptides Using Molecularly Imprinted Polymers. Anal. Chem. 2021, 93, 3857–3866. [Google Scholar] [CrossRef] [PubMed]
- Zangiabadi, M.; Zhao, Y. Molecularly Imprinted Polymeric Receptors with Interfacial Hydrogen Bonds for Peptide Recognition in Water. ACS Appl. Polym. Mater. 2020, 2, 3171–3180. [Google Scholar] [CrossRef]
- Zou, T.-B.; He, T.-P.; Li, H.-B.; Tang, H.-W.; Xia, E.-Q. The Structure-Activity Relationship of the Antioxidant Peptides from Natural Proteins. Molecules 2016, 21, 72. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.-R.; Zhang, L.; Ding, D.-G.; Chi, C.-F.; Wang, B.; Huo, J.-C. Preparation, Identification, and Activity Evaluation of Eight Antioxidant Peptides from Protein Hydrolysate of Hairtail (Trichiurus japonicas) Muscle. Mar. Drugs 2019, 17, 23. [Google Scholar] [CrossRef]
- Lopez-Huertas, E.; Alcaide-Hidalgo, J.M. Characterisation of Endogenous Peptides Present in Virgin Olive Oil. Int. J. Mol. Sci. 2022, 23, 1712. [Google Scholar] [CrossRef]
- Shi, C.; Liu, M.; Zhao, H.; Lv, Z.; Liang, L.; Zhang, B. A Novel Insight into Screening for Antioxidant Peptides from Hazelnut Protein: Based on the Properties of Amino Acid Residues. Antioxidants 2022, 11, 127. [Google Scholar] [CrossRef]
- Matsui, R.; Honda, R.; Kanome, M.; Hagiwara, A.; Matsuda, Y.; Togitani, T.; Ikemoto, N.; Terashima, M. Designing antioxidant peptides based on the antioxidant properties of the amino acid side-chains. Food Chem. 2018, 245, 750–755. [Google Scholar] [CrossRef]
- Ishak, N.H.; Sarbon, N.M. A Review of Protein Hydrolysates and Bioactive Peptides Deriving from Wastes Generated by Fish Processing. Food Bioprocess. Technol. 2017, 11, 2–16. [Google Scholar] [CrossRef]
- Kumar, A.; Elavarasan, K.; Hanjabam, M.D.; Binsi, P.K.; Mohan, C.O.; Zynudheen, A.A.; Kumar, K.A. Marine collagen peptide as a fortificant for biscuit: Effects on biscuit attributes. Lwt 2019, 109, 450–456. [Google Scholar] [CrossRef]
- Wu, R.; Huang, J.; Huan, R.; Chen, L.; Yi, C.; Liu, D.; Wang, M.; Liu, C.; He, H. New insights into the structure-activity relationships of antioxidative peptide PMRGGGGYHY. Food Chem. 2021, 337, 127678. [Google Scholar] [CrossRef] [PubMed]
- Landi, N.; Clemente, A.; Pedone, P.V.; Ragucci, S.; Di Maro, A. An Updated Review of Bioactive Peptides from Mushrooms in a Well-Defined Molecular Weight Range. Toxins 2022, 14, 84. [Google Scholar] [CrossRef]
- Wang, J.; Wang, K.; Lin, S.; Zhao, P.; Jones, G.; Trang, H.; Liu, J.; Ye, H. Improvement of antioxidant activity of peptides with molecular weights ranging from 1 to 10kDa by PEF technology. Int. J. Biol. Macromol. 2012, 51, 244–249. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Liu, R.; Mats, L.; Zhu, H.; Pauls, K.P.; Deng, Z.; Tsao, R. Antioxidant and anti-inflammatory polyphenols and peptides of common bean (Phaseolus vulga L.) milk and yogurt in Caco-2 and HT-29 cell models. J. Funct. Foods 2019, 53, 125–135. [Google Scholar] [CrossRef]
- Zhuang, Y.; Hou, H.; Zhao, X.; Zhang, Z.; Li, B. Effects of Collagen and Collagen Hydrolysate from Jellyfish (Rhopilema esculentum) on Mice Skin Photoaging Induced by UV Irradiation. J. Food Sci. 2009, 74, H183–H188. [Google Scholar] [CrossRef]
- Esteve, C.; Marina, M.L.; García, M.C. Novel strategy for the revalorization of olive (Olea europaea) residues based on the extraction of bioactive peptides. Food Chem. 2015, 167, 272–280. [Google Scholar] [CrossRef]
- Akbarbaglu, Z.; Mohammadi, M.; Arefi, A.; Laein, S.S.; Sarabandi, K.; Peighambardoust, S.H.; Hesarinejad, M.A. Biological properties of LMW-peptide fractions from apricot kernel protein: Nutritional, antibacterial and ACE-inhibitory activities. J. Agric. Food Res. 2024, 16, 101176. [Google Scholar] [CrossRef]
- Martí-Quijal, F.J.; Castagnini, J.M.; Ruiz, M.-J.; Barba, F.J. Sea Bass Side Streams Extracts Obtained by Pulsed Electric Fields: Nutritional Characterization and Effect on SH-SY5Y Cells. Foods 2023, 12, 2717. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Zhang, G.; Tian, Y.; Kong, F.; Xiong, S.; Zhao, S.; Jia, D.; Manyande, A.; Du, H. Identification of novel antioxidant peptides from snakehead (Channa argus) soup generated during gastrointestinal digestion and insights into the anti-oxidation mechanisms. Food Chem. 2021, 337, 127921. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Qi, J.; Lu, Y.; He, H.; Wu, W. PLGA-based implants for sustained delivery of peptides/proteins: Current status, challenge and perspectives. Chin. Chem. Lett. 2023, 34, 108250. [Google Scholar] [CrossRef]
- Balkrishna, A.; Kumar, A.; Arya, V.; Rohela, A.; Verma, R.; Nepovimova, E.; Krejcar, O.; Kumar, D.; Thakur, N.; Kuca, K.; et al. Phytoantioxidant Functionalized Nanoparticles: A Green Approach to Combat Nanoparticle-Induced Oxidative Stress. Oxid. Med. Cell. Longev. 2021, 2021, 3155962. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.-M.; Wu, L.-J.; Lin, M.-T.; Lu, Y.-Y.; Wang, T.-T.; Han, M.; Zhang, B.; Xu, D.-H. Construction and Evaluation of Chitosan-Based Nanoparticles for Oral Administration of Exenatide in Type 2 Diabetic Rats. Polymers 2022, 14, 2181. [Google Scholar] [CrossRef]
- Jeong, W.-j.; Bu, J.; Kubiatowicz, L.J.; Chen, S.S.; Kim, Y.; Hong, S. Peptide–nanoparticle conjugates: A next generation of diagnostic and therapeutic platforms? Nano Converg. 2018, 5, 1–18. [Google Scholar] [CrossRef]
- Shrestha, H.; Bala, R.; Arora, S. Lipid-Based Drug Delivery Systems. J. Pharm. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Wang, F.; Pu, C.; Liu, M.; Li, R.; Sun, Y.; Tang, W.; Sun, Q.; Tian, Q. Fabrication and characterization of walnut peptides-loaded proliposomes with three lyoprotectants: Environmental stabilities and antioxidant/antibacterial activities. Food Chem. 2022, 366, 130643. [Google Scholar] [CrossRef]
- Zhang, G.; Fang, S.; Regenstein, J.M.; Wang, F. Preparation, characterization and stability of nanoliposomes loaded with peptides from defatted walnut (Juglans regia L.) meal. J. Food Sci. Technol. 2022, 59, 3180–3191. [Google Scholar] [CrossRef] [PubMed]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377–1397. [Google Scholar] [CrossRef]
- Ismail, R.; Bocsik, A.; Katona, G.; Gróf, I.; Deli, M.A.; Csóka, I. Encapsulation in Polymeric Nanoparticles Enhances the Enzymatic Stability and the Permeability of the GLP-1 Analog, Liraglutide, Across a Culture Model of Intestinal Permeability. Pharmaceutics 2019, 11, 599. [Google Scholar] [CrossRef]
- Reddy, K.T.K.; Reddy, A.S. Recent breakthroughs in drug delivery systems for targeted cancer therapy: An overview. Cell. Mol. Biomed. Rep. 2025, 5, 13–27. [Google Scholar] [CrossRef]
- Wang, T.-Y.; Sun, Y.; Muthukrishnan, N.; Erazo-Oliveras, A.; Najjar, K.; Pellois, J.-P. Membrane Oxidation Enables the Cytosolic Entry of Polyarginine Cell-penetrating Peptides. J. Biol. Chem. 2016, 291, 7902–7914. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Ejaz, W.; Dutta, K.; Thayumanavan, S. Antibody Delivery for Intracellular Targets: Emergent Therapeutic Potential. Bioconjug. Chem. 2019, 30, 1028–1041. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, P.G.; Wen, J.; Pan, X.; Koley, A.; Ren, J.-G.; Sahni, A.; Basu, R.; Salim, H.; Appiah Kubi, G.; Qian, Z.; et al. Enhancing the Cell Permeability of Stapled Peptides with a Cyclic Cell-Penetrating Peptide. J. Med. Chem. 2019, 62, 10098–10107. [Google Scholar] [CrossRef] [PubMed]
- Ildefonso, C.J.; Jaime, H.; Brown, E.E.; Iwata, R.L.; Ahmed, C.M.; Massengill, M.T.; Biswal, M.R.; Boye, S.E.; Hauswirth, W.W.; Ash, J.D.; et al. Targeting the Nrf2 Signaling Pathway in the Retina with a Gene-Delivered Secretable and Cell-Penetrating Peptide. Investig. Ophthalmol. Vis. Sci. 2016, 57, 372–386. [Google Scholar] [CrossRef]
- Aytar Çelik, P.; Erdogan-Gover, K.; Barut, D.; Enuh, B.M.; Amasya, G.; Sengel-Türk, C.T.; Derkus, B.; Çabuk, A. Bacterial Membrane Vesicles as Smart Drug Delivery and Carrier Systems: A New Nanosystems Tool for Current Anticancer and Antimicrobial Therapy. Pharmaceutics 2023, 15, 1052. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Wang, Y.; Chen, H.; Zhang, X.; Luo, C.; Zhou, W.; Li, L.; Teng, L.; Yu, H.; et al. Smart drug delivery systems for precise cancer therapy. Acta Pharm. Sin. B 2022, 12, 4098–4121. [Google Scholar] [CrossRef]
- Ma, B.; Li, Q.; Mi, Y.; Zhang, J.; Tan, W.; Guo, Z. pH-responsive nanogels with enhanced antioxidant and antitumor activities on drug delivery and smart drug release. Int. J. Biol. Macromol. 2024, 257, 128590. [Google Scholar] [CrossRef]
- Xun, X.-M.; Zhang, Z.-A.; Yuan, Z.-X.; Tuhong, K.; Yan, C.-H.; Zhan, Y.-F.; Kang, G.-P.; Wu, Q.-Y.; Wang, J. Novel caffeic acid grafted chitosan-sodium alginate microcapsules generated by microfluidic technique for the encapsulation of bioactive peptides from silkworm pupae. Sustain. Chem. Pharm. 2023, 32, 100974. [Google Scholar] [CrossRef]
- Chaurasia, M.; Singh, R.; Sur, S.; Flora, S.J.S. A review of FDA approved drugs and their formulations for the treatment of breast cancer. Front. Pharmacol. 2023, 14, 1184472. [Google Scholar] [CrossRef]
- Fardous, J.; Omoso, Y.; Joshi, A.; Yoshida, K.; Patwary, M.K.A.; Ono, F.; Ijima, H. Development and characterization of gel-in-water nanoemulsion as a novel drug delivery system. Mater. Sci. Eng. C 2021, 124, 112076. [Google Scholar] [CrossRef]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Keum, Y.-S.; Choi, B. Molecular and Chemical Regulation of the Keap1-Nrf2 Signaling Pathway. Molecules 2014, 19, 10074–10089. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Srivastav, S.; Saha, S.; Das, P.K.; Ukil, A. Leishmania donovani inhibits macrophage apoptosis and pro-inflammatory response through AKT-mediated regulation of β-catenin and FOXO-1. Cell Death Differ. 2016, 23, 1815–1826. [Google Scholar] [CrossRef]
- Rawlings, J.S.; Rosler, K.M.; Harrison, D.A. The JAK/STAT signaling pathway. J. Cell Sci. 2004, 117, 1281–1283. [Google Scholar] [CrossRef]
- Qi, Y.; Wu, D.; Fang, L.; Leng, Y.; Wang, X.; Liu, C.; Liu, X.; Wang, J.; Min, W. Anti-inflammatory effect of walnut-derived peptide via the activation of Nrf2/Keap1 pathway against oxidative stress. J. Funct. Foods 2023, 110, 105839. [Google Scholar] [CrossRef]
- Zhang, B.; Zhao, Z.; Meng, X.; Chen, H.; Fu, G.; Xie, K. Hydrogen ameliorates oxidative stress via PI3K-Akt signaling pathway in UVB-induced HaCaT cells. Int. J. Mol. Med. 2018, 41, 3653–3661. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Sternberg, P.; Cai, J. Essential Roles of the PI3 Kinase/Akt Pathway in Regulating Nrf2-Dependent Antioxidant Functions in the RPE. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1671–1678. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, L.; Luo, D.; Huang, M.; Feng, Y.; Zhao, M. Peptide WCPFSRSF alleviates sleep deprivation-induced memory impairment by inhibiting neuroinflammation and modulating IL-6/JAK/STAT signaling pathway. Food Biosci. 2022, 50, 102176. [Google Scholar] [CrossRef]
- Wu, H.; Qu, L.; Bai, X.; Zhu, C.; Liu, Y.; Duan, Z.; Liu, H.; Fu, R.; Fan, D. Ginsenoside Rk1 induces autophagy-dependent apoptosis in hepatocellular carcinoma by AMPK/mTOR signaling pathway. Food Chem. Toxicol. 2024, 186, 114587. [Google Scholar] [CrossRef]
- Peng, X.; Ma, L.; Cheng, Y.; Wu, G.; Xiao, S.; Zeng, X.; Zhang, S.; Zhou, J. Protective properties of milk-derived peptide QEPV in attenuating oxidative stress through AMPK/PPARα signaling pathways. J. Funct. Foods 2024, 122, 106503. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Farag, M.A.; Cai, X.; Wang, S. Identification of novel antioxidant peptides from Lateolabrax japonicus and the underlying molecular mechanisms against oxidative stress injury in Caco-2 cells. Food Biosci. 2024, 62, 105538. [Google Scholar] [CrossRef]
- Yan, Z.; Wang, D.; An, C.; Xu, H.; Zhao, Q.; Shi, Y.; Song, N.; Deng, B.; Guo, X.; Rao, J.; et al. The antimicrobial peptide YD attenuates inflammation via miR-155 targeting CASP12 during liver fibrosis. Acta Pharm. Sin. B 2021, 11, 100–111. [Google Scholar] [CrossRef]
- Bayati, P.; Kalantari, M.; Assarehzadegan, M.-A.; Poormoghim, H.; Mojtabavi, N. MiR-27a as a diagnostic biomarker and potential therapeutic target in systemic sclerosis. Sci. Rep. 2022, 12, 18932. [Google Scholar] [CrossRef]
- Mei, F.; Duan, Z.; Chen, M.; Lu, J.; Zhao, M.; Li, L.; Shen, X.; Xia, G.; Chen, S. Effect of a high-collagen peptide diet on the gut microbiota and short-chain fatty acid metabolism. J. Funct. Foods 2020, 75, 104278. [Google Scholar] [CrossRef]
- Li, W.; Deng, M.; Gong, J.; Zhang, X.; Ge, S.; Zhao, L. Sodium Acetate Inhibit TGF-β1-Induced Activation of Hepatic Stellate Cells by Restoring AMPK or c-Jun Signaling. Front. Nutr. 2021, 8, 729583. [Google Scholar] [CrossRef]
- Yao, G.; Tang, X.; Ye, Z.; Yan, W.; Yu, J.; Wu, Y.; Zhang, J.; Yang, D. Protective effect of Camellia vietnamensis active peptide on alcohol-induced hepatocyte injury. Food Agric. Immunol. 2021, 32, 425–449. [Google Scholar] [CrossRef]
- Li, D.; Ren, J.; Wang, T.; Wu, L.; Liu, P.; Li, Y. Anti-hypoxia effects of walnut oligopeptides (Juglans regia L.) in mice. Am. J. Transl. Res. 2021, 13, 4581. [Google Scholar]
- Yeh, A.-L.; Chao, C.-L.; Huang, W.-F.; Lin, H.-C.; Wang, C.-J. Walnut (Juglans regia L.) Oligopeptide Effects on Enhancing Memory, Cognition and Improving Sleep Quality in Teenagers and Elderly People in a Randomized Double-Blind Controlled Trial. Nat. Prod. Commun. 2022, 17, 1934578X221089065. [Google Scholar] [CrossRef]
- Liu, R.; Wu, L.; Du, Q.; Ren, J.-W.; Chen, Q.-H.; Li, D.; Mao, R.-X.; Liu, X.-R.; Li, Y. Small Molecule Oligopeptides Isolated from Walnut (Juglans regia L.) and Their Anti-Fatigue Effects in Mice. Molecules 2018, 24, 45. [Google Scholar] [CrossRef]
- Liu, R.; Zhu, N.; Hao, Y.; Liu, X.; Kang, J.; Mao, R.; Yu, X.; Li, Y. The Protective Effect of Walnut Oligopeptides against Indomethacin-Induced Gastric Ulcer in Rats. Nutrients 2023, 15, 1675. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, J.; Yan, W.; Ye, Z.; Yu, J.; Yao, G.; Wu, Y.; Zhang, J.; Yang, D. Preparation of Active Peptides from Camellia vietnamensis and Their Metabolic Effects in Alcohol-Induced Liver Injury Cells. Molecules 2022, 27, 1790. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Lin, L.; Zhang, Y.; Li, C.; Chen, B.; Shen, Y. Effects of walnut kernel pellicle on the composition and properties of enzymatic hydrolysates of walnut meal by peptidomics and bioinformatics. J. Food Sci. 2025, 90, e17604. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, I.; Bangov, I.; Flower, D.R.; Doytchinova, I. AllerTOP v.2—A server for in silico prediction of allergens. J. Mol. Model. 2014, 20, 2278. [Google Scholar] [CrossRef] [PubMed]
- Naegeli, H.; Birch, A.N.; Casacuberta, J.; De Schrijver, A.; Gralak, M.A.; Guerche, P.; Jones, H.; Manachini, B.; Messéan, A.; Nielsen, E.E.; et al. Guidance on allergenicity assessment of genetically modified plants. EFSA J. 2017, 15, e04862. [Google Scholar]
- Yin, J.-J.; Fu, P.P.; Lutterodt, H.; Zhou, Y.-T.; Antholine, W.E.; Wamer, W. Dual Role of Selected Antioxidants Found in Dietary Supplements: Crossover between Anti- and Pro-Oxidant Activities in the Presence of Copper. J. Agric. Food Chem. 2012, 60, 2554–2561. [Google Scholar] [CrossRef]
- Di, L. Strategic Approaches to Optimizing Peptide ADME Properties. AAPS J. 2014, 17, 134–143. [Google Scholar] [CrossRef]
- Smith, D.E.; Clémençon, B.; Hediger, M.A. Proton-coupled oligopeptide transporter family SLC15: Physiological, pharmacological and pathological implications. Mol. Asp. Med. 2013, 34, 323–336. [Google Scholar] [CrossRef]
- Hasegawa, T.; Oka, T.; Son, H.G.; Oliver-García, V.S.; Azin, M.; Eisenhaure, T.M.; Lieb, D.J.; Hacohen, N.; Demehri, S. Cytotoxic CD4+ T cells eliminate senescent cells by targeting cytomegalovirus antigen. Cell 2023, 186, 1417–1431.e20. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Zhang, N.; Li, Y.; Cai, Z.; Li, G.; Liu, Z.; Liu, Z.; Wang, Y.; Shao, X.; et al. Anti-aging activity and their mechanisms of natural food-derived peptides: Current advancements. Food Innov. Adv. 2023, 2, 272–290. [Google Scholar] [CrossRef]
- Tonolo, F.; Folda, A.; Cesaro, L.; Scalcon, V.; Marin, O.; Ferro, S.; Bindoli, A.; Rigobello, M.P. Milk-derived bioactive peptides exhibit antioxidant activity through the Keap1-Nrf2 signaling pathway. J. Funct. Foods 2020, 64, 103696. [Google Scholar] [CrossRef]
- Yang, H.; Wang, F.; Zhao, P.; Ullah, S.; Ma, Y.; Zhao, G.; Cheng, Y.; Li, Q.; Li, T.; Qiao, M.; et al. Black soybean peptide mediates the AMPK/SIRT1/NF-κB signaling pathway to alleviate Alzheimer’s-related neuroinflammation in lead-exposed HT22 cells. Int. J. Biol. Macromol. 2025, 286, 138404. [Google Scholar] [CrossRef]
- Das, D.; Kabir, M.E.; Sarkar, S.; Wann, S.B.; Kalita, J.; Manna, P. Antidiabetic potential of soy protein/peptide: A therapeutic insight. Int. J. Biol. Macromol. 2022, 194, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Feng, J.; Du, Z.; Zhen, H.; Lin, M.; Jia, S.; Li, T.; Huang, X.; Ostenson, C.-G.; Chen, Z. Oral administration of soybean peptide Vglycin normalizes fasting glucose and restores impaired pancreatic function in Type 2 diabetic Wistar rats. J. Nutr. Biochem. 2014, 25, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Han, S.; Lee, S.; Kim, J.; Kim, J.; Kang, D.-K. Synergistic Antioxidant and Anti-Inflammatory Effects of Phenolic Acid-Conjugated Glutamine–Histidine–Glycine–Valine (QHGV) Peptides Derived from Oysters (Crassostrea talienwhanensis). Antioxidants 2024, 13, 447. [Google Scholar] [CrossRef]
- Cheng, W.; Shi, X.; Zhang, J.; Li, L.; Di, F.; Li, M.; Wang, C.; An, Q.; Zhao, D. Role of PI3K-AKT Pathway in Ultraviolet Ray and Hydrogen Peroxide-Induced Oxidative Damage and Its Repair by Grain Ferments. Foods 2023, 12, 806. [Google Scholar] [CrossRef]
- Song, Z.; Geng, J.; Wang, D.; Fang, J.; Wang, Z.; Wang, C.; Li, M. Reparative effects of Schizophyllum commune oat bran fermentation broth on UVB-induced skin inflammation via the JAK/STAT pathway. Bioresour. Bioprocess. 2024, 11, 73. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food Protein-Derived Bioactive Peptides: Production, Processing, and Potential Health Benefits. J. Food Sci. 2011, 77, R11–R24. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, T.; Li, H.; Xu, W.; Maas, K.; Singh, V.; Chen, M.-H.; Dorsey, S.G.; Starkweather, A.R.; Cong, X.S. Multi-Omics Analysis of Gut Microbiota and Host Transcriptomics Reveal Dysregulated Immune Response and Metabolism in Young Adults with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2024, 25, 3514. [Google Scholar] [CrossRef]
- Qiu, Y.; Ozturk, S.; Cui, X.; Qin, W.; Wu, Q.; Liu, S. Increased heat tolerance and transcriptome analysis of Salmonella enterica Enteritidis PT 30 heat-shocked at 42 °C. Food Res. Int. 2023, 167, 112636. [Google Scholar] [CrossRef]
- Czyżewski, B.; Matuszczak, A.; Polcyn, J.; Smędzik-Ambroży, K.; Staniszewski, J. Deadweight loss in environmental policy: The case of the European Union member states. J. Clean. Prod. 2020, 260, 121064. [Google Scholar] [CrossRef]
| Woody Oil Resources | Main Products | Major Distribution Regions | Application Industries |
|---|---|---|---|
| Olive [1] | Olive Oil | Mediterranean region, Americas, Asia | Food and Cosmetics |
| Oil Palm [2] | Palm Oil and Palm Kernel Oil | Asia, Africa, Americas | Food, Cosmetics, and Biofuel |
| Coconut [3] | Coconut Oil | Africa, Latin America, Asia | Food, Cosmetics, and Pharmaceutical Industry |
| Camellia oleifera [4] | Camellia Oil | Guangdong, Hong Kong, Guangxi, Hunan, Jiangxi | Edible and Industrial Uses |
| Walnut [5] | Walnut Oil | China, USA, Turkey, Mexico, Iran | Edible and Medicinal |
| Almond [6] | Almond Oil | USA, Turkey, Australia, EU, China | Food and Cosmetics |
| Peony Seed [7] | Peony Seed Oil | Japan, France, UK, USA, China | Food and Cosmetics |
| Safflower Seed [8] | Safflower Seed Oil | India, Mexico, China | Food and Industrial Applications |
| Grape Seed [9] | Grape Seed Oil | Europe, Asia, Americas | Food and Cosmetics |
| Litsea cubeba [10] | Litsea cubeba Essential Oil | Eastern Asia, Oceania, Pacific Islands | Edible and Medicinal |
| Cornus wilsoniana Wangerin [11] | Cornus wilsoniana Wangerin Essential Oil | China | Food and Biofuel |
| Method | Key Advantages | Key Limitations |
|---|---|---|
| Chemical Synthesis [23] | High accuracy Good controllability Diversity | Many side reactions High cost Difficult to synthesize long-chain peptides |
| Chemical Hydrolysis | Rapid reaction Low cost [24] Simple operation | Poor specificity Environmental pollution [25] Amino acid damage |
| Enzymatic Hydrolysis [24] | Gentle conditions Controlled reaction Safety | Low yield Purification difficulties |
| Microbial Fermentation [26] | Gentle conditions Eco-friendly Low cost | Limited yield Time-consuming [27] Fermentation conditions require precise control |
| Recombinant technology [28] | Strong expression orientation Safety Low cost | Purification difficulties Not suitable for small-molecule peptides Long cycle times |
| Computer-aided [29] | Low cost Efficiency Flexibility | High computer resource requirements [30] Experimental verification needed |
| Evidence Tier | Model/Protocol | Dose and Duration | Key Observations |
|---|---|---|---|
| In vitro | Human L-02 hepatocytes, alcohol–injury model | Camellia vietnamensis peptide A1–2, ≤200 µg mL−1, 24 h | No cytotoxicity to normal cells; restored viability after EtOH insult [99] |
| Sub-acute in vivo | BALB/c mice, 30-day oral study | Walnut oligopeptides (WOPs) 110–440 mg kg−1 day−1 | No mortality, normal serum liver–renal panels; behavioral indices unchanged [100] |
| Human (Phase I/II) | RCT, teenagers and elderly (n = 36) | WOPs 170 mg or 340 mg day−1, 90 days | No adverse events recorded; hematology and biochemistry within reference ranges [101] |
| Evidence Tier | Woody Oil Source and Study ID | Population/Design | Principal Findings |
|---|---|---|---|
| Development stage | Walnut oligopeptides (ChiCTR1900028160)—90-day, randomized, double-blind, placebo-controlled trial | 18 teenagers + 18 elderly volunteers; 170 mg and 340 mg day−1 | ↑ Wechsler Adult Intelligence Scale, ↓ Pittsburgh Sleep Quality Index; no adverse events or abnormal hematology/biochemistry [101] |
| Phase I/II completed | Fermented Camellia seed peptide-rich extract (Shiseido internal study) | Healthy adults, topical application (open-label) | ↑ epidermal CXCL9 expression, proposed to boost immune clearance of senescent fibroblasts [111] |
| Exploratory cosmetic study | Camellia, Tung, and Eucommia peptides | ClinicalTrials.gov and ChiCTR | No interventional trials yet registered |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tu, J.; Peng, J.; Wen, L.; Li, C.; Xiao, Z.; Wu, Y.; Xu, Z.; Hu, Y.; Zhong, Y.; Miao, Y.; et al. Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities. Pharmaceuticals 2025, 18, 842. https://doi.org/10.3390/ph18060842
Tu J, Peng J, Wen L, Li C, Xiao Z, Wu Y, Xu Z, Hu Y, Zhong Y, Miao Y, et al. Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities. Pharmaceuticals. 2025; 18(6):842. https://doi.org/10.3390/ph18060842
Chicago/Turabian StyleTu, Jia, Jie Peng, Li Wen, Changzhu Li, Zhihong Xiao, Ying Wu, Zhou Xu, Yuxi Hu, Yan Zhong, Yongjun Miao, and et al. 2025. "Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities" Pharmaceuticals 18, no. 6: 842. https://doi.org/10.3390/ph18060842
APA StyleTu, J., Peng, J., Wen, L., Li, C., Xiao, Z., Wu, Y., Xu, Z., Hu, Y., Zhong, Y., Miao, Y., Xiao, J., & Liu, S. (2025). Antioxidant Peptides Derived from Woody Oil Resources: Mechanisms of Redox Protection and Emerging Therapeutic Opportunities. Pharmaceuticals, 18(6), 842. https://doi.org/10.3390/ph18060842








