Rhodococcus rhodochrous IEGM 1362 Immobilized in Macroporous PVA Cryogel as an Effective Biocatalyst for the Production of Bioactive (–)-Isopulegol Compounds
Abstract
1. Introduction
2. Results and Discussion
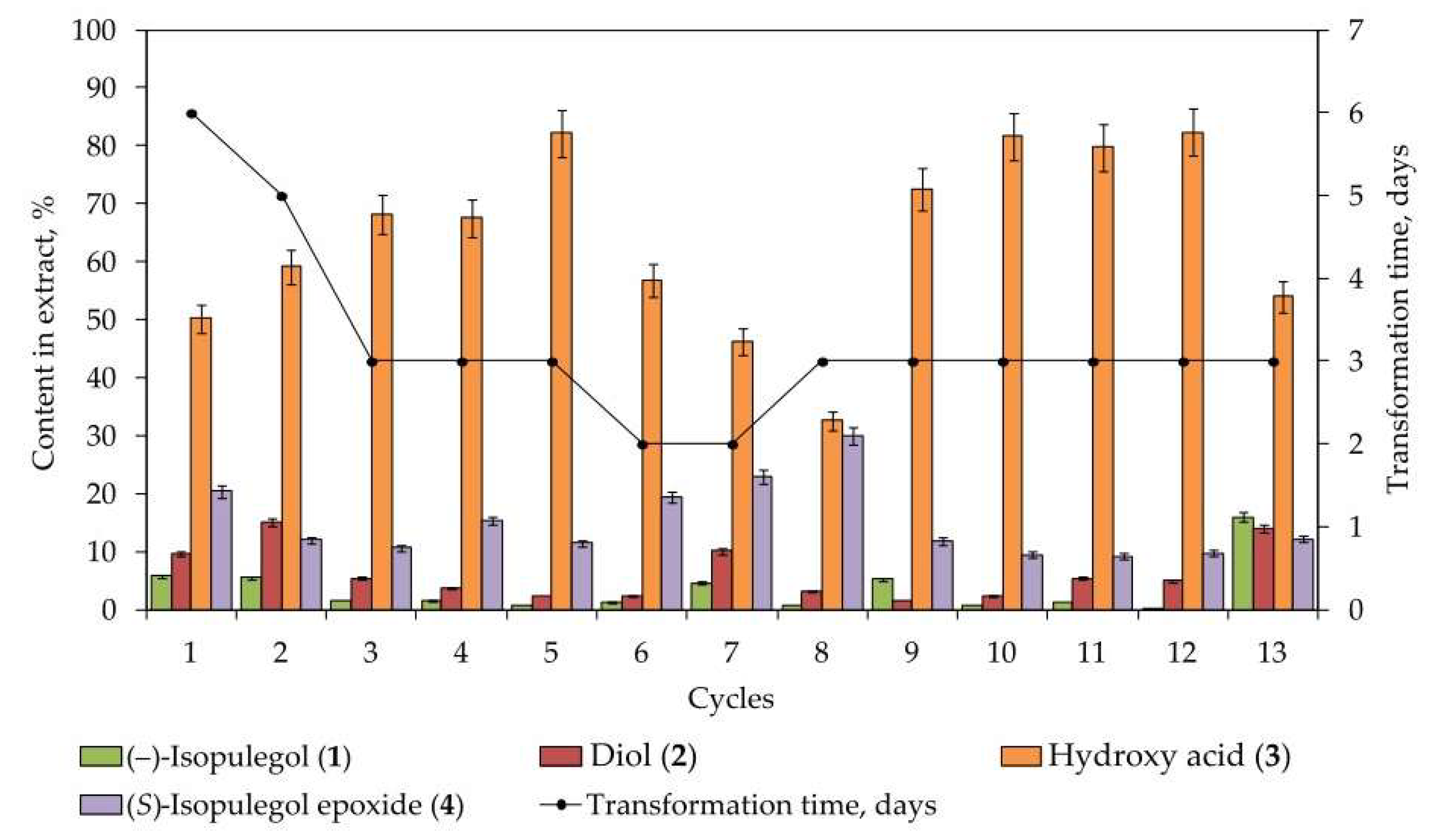
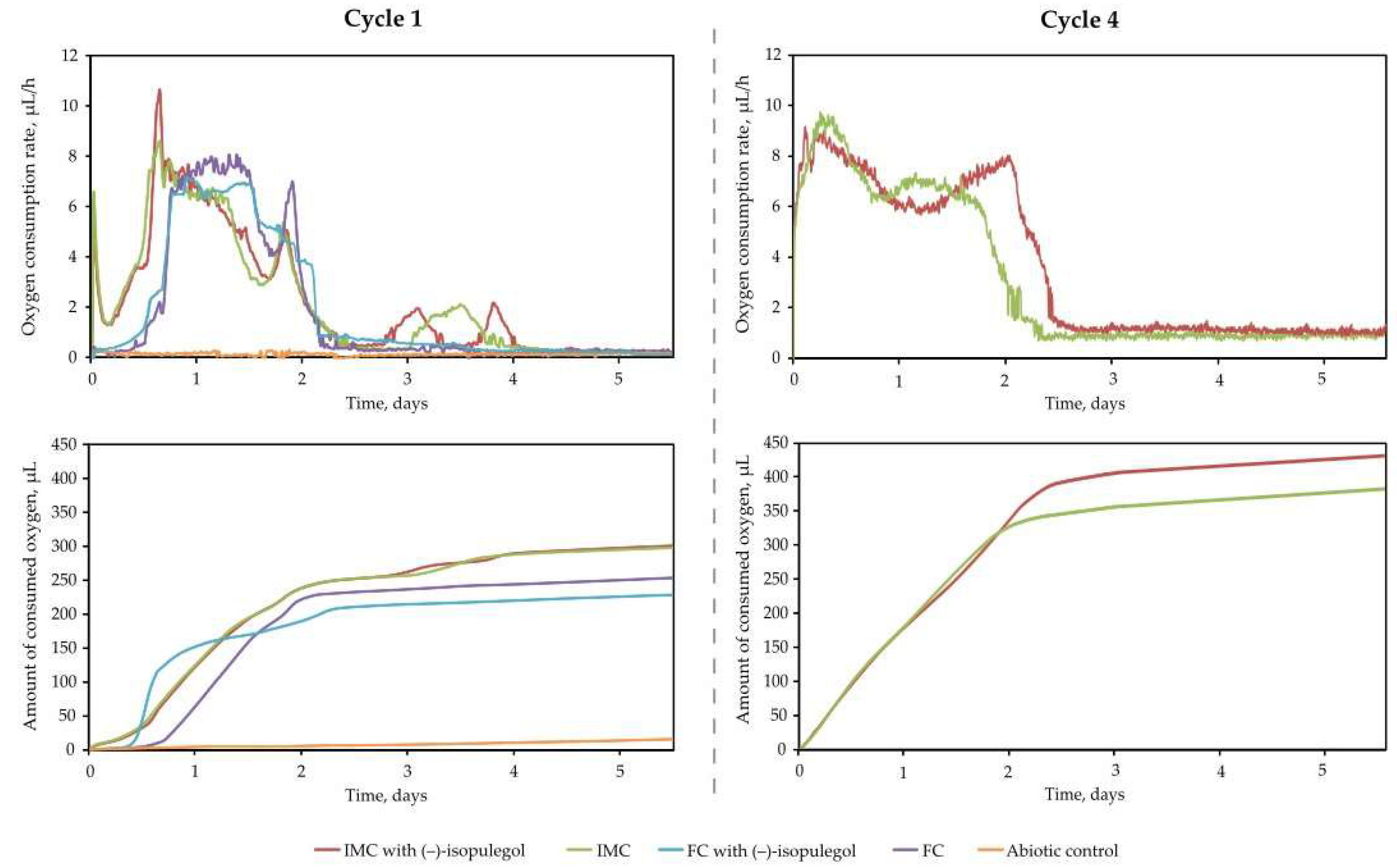
2.1. Biotransformation of (–)-Isopulegol Using Immobilized Cells
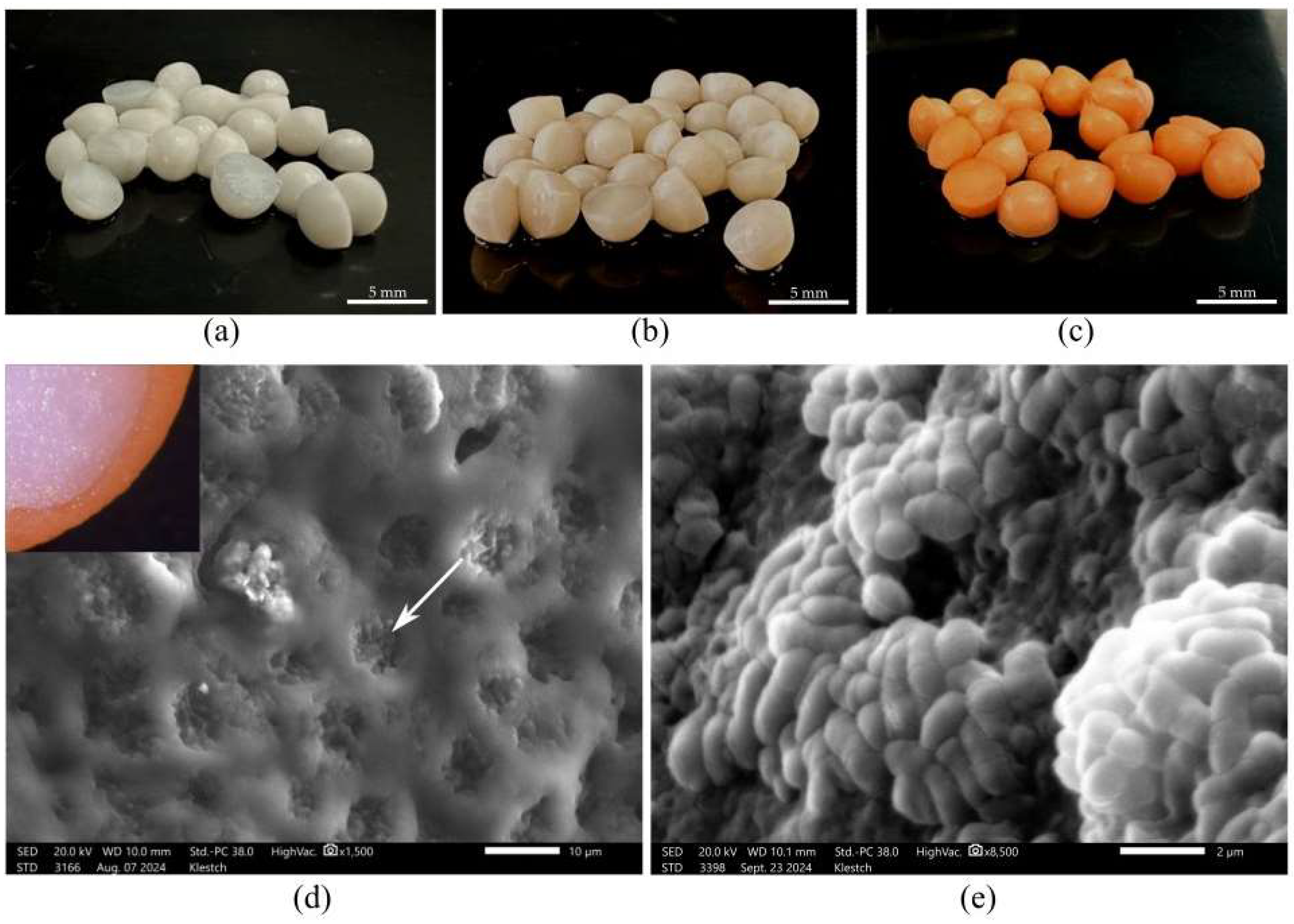
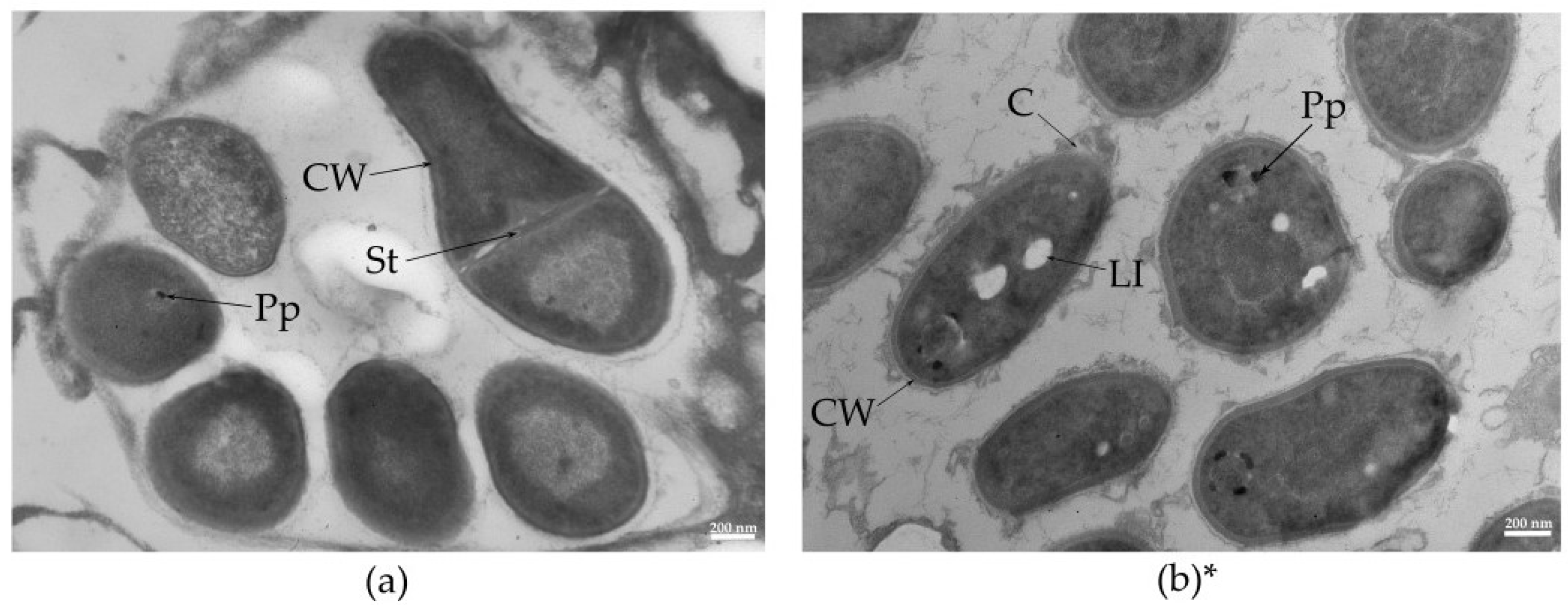
2.2. Morphology and Elemental Composition of R. rhodochrous IEGM 1362 Immobilized Cells
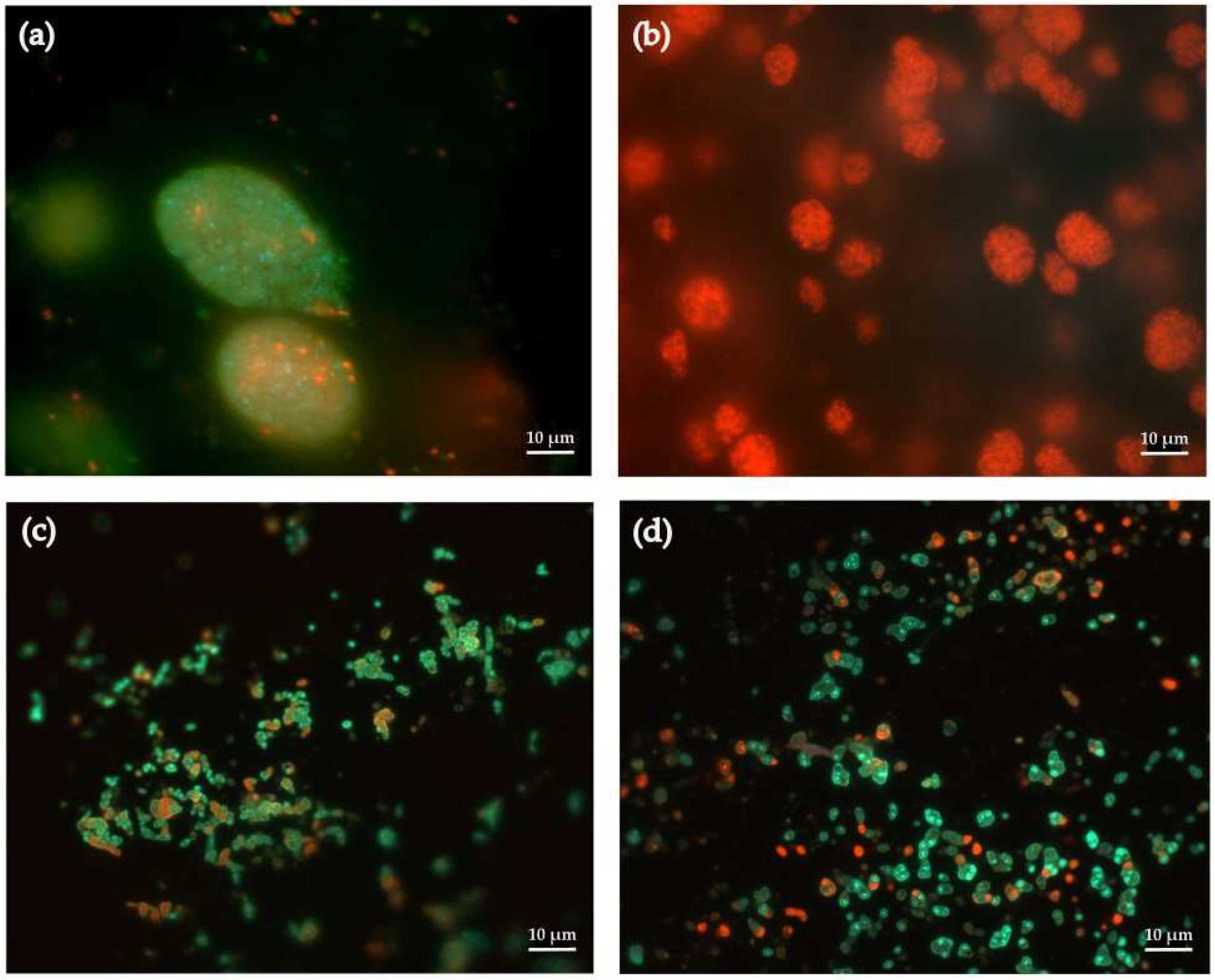
2.3. Viability of Immobilized R. rhodochrous IEGM 1362 Cells
3. Materials and Methods
3.1. Bacterial Strain
3.2. Chemicals
3.3. Immobilization of Bacterial Cells
3.4. Biotransformation of (–)-Isopulegol
3.5. Analytical Methods
3.6. Scanning Electron Microscopy (SEM), Transmission Electron Microscopy (TEM), and Energy-Dispersive X-Ray Spectroscopy (EDX) with Elemental Mapping
3.7. Respiratory Activity
3.8. Fluorescent Microscopy
3.9. In Silico Analysis of (–)-Isopulegol and Its Derivatives
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PVA | Polyvinyl alcohol |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EDX | Energy dispersive X-ray spectroscopy |
| GC-MS | Gas chromatography–mass spectrometry |
References
- Minh Le, T.; Szakonyi, Z. Enantiomeric Isopulegol as the Chiral Pool in the Total Synthesis of Bioactive Agents. Chem. Rec. 2022, 22, e202100194. [Google Scholar] [CrossRef] [PubMed]
- Ilyina, I.V.; Zarubaev, V.V.; Lavrentieva, I.N.; Shtro, A.A.; Esaulkova, I.L.; Korchagina, D.V.; Borisevich, S.S.; Volcho, K.P.; Salakhutdinov, N.F. Highly potent activity of isopulegol-derived substituted octahydro-2H-chromen-4-ols against influenza A and B viruses. Bioorg. Med. Chem. Lett. 2018, 28, 2061–2067. [Google Scholar] [CrossRef]
- Li-Zhulanov, N.S.; Pavlova, A.V.; Korchagina, D.V.; Gatilov, Y.V.; Volcho, K.P.; Tolstikova, T.G.; Salakhutdinov, N.F. Synthesis of 1,3-oxazine derivatives based on (−)-isopulegol using the Ritter reaction and study of their analgesic activity. Chem. Heterocycl. Compd. 2020, 56, 936–941. [Google Scholar] [CrossRef]
- Le, T.M.; Huynh, T.; Endre, G.; Szekeres, A.; Fülöp, F.; Szakonyi, Z. Stereoselective synthesis and application of isopulegol-based bi- and trifunctional chiral compounds. RSC Adv. 2020, 10, 38468–38477. [Google Scholar] [CrossRef]
- Le, T.M.; Bérdi, P.; Zupkó, I.; Fülöp, F.; Szakonyi, Z. Synthesis and transformation of (−)-isopulegol-based chiral β-aminolactones and β-aminoamides. Int. J. Mol. Sci. 2018, 19, 3522. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Kiokekli, S.; Naviwala, M.; Shirke, A.N.; Pavlidis, I.V.; Gross, R.A. Cutinases as stereoselective catalysts: Specific activity and enantioselectivity of cutinases and lipases for menthol and its analogs. Enzym. Microb. Technol. 2020, 133, 109467. [Google Scholar] [CrossRef] [PubMed]
- Shukla, O.P.; Bartholomus, R.C.; Gunsalus, I.C. Microbial Transformation of Menthol and Menthane-3,4-Diol. Can. J. Microbiol. 1987, 33, 489–497. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V. Hydrocarbon-Oxidizing Bacteria and Their Potential in Eco-Biotechnology and Bioremediation. In Microbial Resources: From Functional Existence in Nature to Industrial Applications; Kurtböke, I., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 121–148. [Google Scholar]
- Ivshina, I.B.; Luchnikova, N.A.; Maltseva, P.Y.; Ilyina, I.V.; Volcho, K.P.; Gatilov, Y.V.; Korchagina, D.V.; Kostrikina, N.A.; Sorokin, V.V.; Mulyukin, A.L.; et al. Biotransformation of (–)-Isopulegol by Rhodococcus rhodochrous. Pharmaceuticals 2022, 15, 964. [Google Scholar] [CrossRef]
- Najim, A.A.; Radeef, A.Y.; al-Doori, I.; Jabbar, Z.H. Immobilization: The Promising Technique to Protect and Increase the Efficiency of Microorganisms to Remove Contaminants. J. Chem. Technol. Biotechnol. 2024, 99, 1707–1733. [Google Scholar] [CrossRef]
- Lapponi, M.J.; Méndez, M.B.; Trelles, J.A.; Rivero, C.W. Cell Immobilization Strategies for Biotransformations. Curr. Opin. Green Sustain. Chem. 2022, 33, 100565. [Google Scholar] [CrossRef]
- Berillo, D.; Malika, T.; Baimakhanova, B.B.; Sadanov, A.K.; Berezin, V.E.; Trenozhnikova, L.P.; Baimakhanova, G.B.; Amangeldi, A.A.; Kerimzhanova, B. An Overview of Microorganisms Immobilized in a Gel Structure for the Production of Precursors, Antibiotics, and Valuable Products. Gels 2024, 10, 646. [Google Scholar] [CrossRef] [PubMed]
- Lozinsky, V.I.; Galaev, I.Y.; Plieva, F.M.; Savina, I.N.; Jungvid, H.; Mattiasson, B. Polymeric Cryogels as Promising Materials of Biotechnological Interest. Trends Biotechnol. 2003, 21, 445–451. [Google Scholar] [CrossRef]
- Kylosova, T.I.; Elkin, A.A.; Grishko, V.V.; Ivshina, I.B. Biotransformation of Prochiral Sulfides into (R)-Sulfoxides Using Immobilized Gordonia terrae IEGM 136 Cells. J. Mol. Catal. B Enzym. 2016, 123, 8–13. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Paulino, B.N.; da Silva, G.N.S.; Araújo, F.F.; Néri-Numa, I.A.; Pastore, G.M.; Bicas, J.L.; Molina, G. Beyond natural aromas: The bioactive and technological potential of monoterpenes. Trends Food Sci. Technol. 2022, 128, 188–201. [Google Scholar] [CrossRef]
- Bradley, J.M.; Svistunenko, D.A.; Wilson, M.T.; Hemmings, A.M.; Moore, G.R.; Le Brun, N.E. Bacterial Iron Detoxification at the Molecular Level. J. Biol. Chem. 2020, 295, 17602–17623. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Stok, J.E.; Soo, R.M.; De Voss, J.J. CYP108N12 Initiates p-Cymene Biodegradation in Rhodococcus globerulus. Arch. Biochem. Biophys. 2022, 730, 109410. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Buczynski, J.B.; Bell, S.G.; Stok, J.E.; De Voss, J.J. CYP108N14: A Monoterpene Monooxygenase from Rhodococcus globerulus. Arch. Biochem. Biophys. 2024, 752, 109852. [Google Scholar] [CrossRef]
- Giang, P.D.; Churchman, L.R.; Stok, J.E.; Bell, S.G.; De Voss, J.J. Cymredoxin, a [2Fe–2S] Ferredoxin, Supports Catalytic Activity of the p-Cymene Oxidising P450 Enzyme CYP108N12. Arch. Biochem. Biophys. 2023, 737, 109549. [Google Scholar] [CrossRef]
- Grogan, G.; Roberts, G.; Parsons, S.; Turner, N.; Flitsch, S. P450camr, a Cytochrome P450 Catalysing the Stereospecific 6-Endo-Hydroxylation of (1R)-(+)-Camphor. Appl. Microbiol. Biotechnol. 2002, 59, 449–454. [Google Scholar]
- Liu, S.H.; Zeng, G.M.; Niu, Q.Y.; Liu, Y.; Zhou, L.; Jiang, L.H.; Tan, X.F.; Xu, P.; Zhang, C.; Cheng, M. Bioremediation Mechanisms of Combined Pollution of PAHs and Heavy Metals by Bacteria and Fungi: A Mini Review. Bioresour. Technol. 2017, 224, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, A.N.; Liu, Y.J.; Liu, Z.; Liu, Y.; Wu, X.J. Analysis of the Mechanism for Enhanced Pyrene Biodegradation Based on the Interactions between Iron-Ions and Rhodococcus ruber Strain L9. Ecotoxicol. Environ. Saf. 2021, 225, 112789. [Google Scholar] [CrossRef] [PubMed]
- Bisht, M.; Thayallath, S.K.; Bharadwaj, P.; Franklin, G.; Mondal, D. Biomass-Derived Functional Materials as Carriers for Enzymes: Towards Sustainable and Robust Biocatalysts. Green Chem. 2023, 25, 4591–4624. [Google Scholar] [CrossRef]
- Benítez-Mateos, A.I.; Huber, C.; Nidetzky, B.; Bolivar, J.M.; López-Gallego, F. Design of the Enzyme-Carrier Interface to Overcome the O2 and NADH Mass Transfer Limitations of an Immobilized Flavin Oxidase. ACS Appl. Mater. Interfaces 2020, 12, 56027–56038. [Google Scholar] [CrossRef]
- Andryushina, V.; Balabanova, T.; Beklemishev, A.; Varfolomeev, S.; Vodyakova, M.; Demakov, V.; Ditchenko, T.; Dzhavahiya, V.; Drozdova, M.; Efremenko, E.; et al. Immobilized Cells: Biocatalysts and Processes; Publishing Center RIOR: Moscow, Russia, 2018; ISBN 978-5-369-02004-3. [Google Scholar]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An Emergent Form of Bacterial Life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Ivshina, I.B.; Lozinsky, V.I.; Podorozhko, E.A.; Jeffree, C.E.; Gavrin, A.Y.; Philp, J.C. Immobilization of Hydrocarbon-Oxidizing Bacteria in Poly(Vinyl) Alcohol Cryogels Hydrophobized Using a Biosurfactant. J. Microbiol. Methods 2005, 65, 596–603. [Google Scholar] [CrossRef]
- Bull, S.D.; Tibbetts, J.D.; Cunningham, W. Preparation of p-Menthane-3,8-diol Stereoisomers and Uses Thereof. WO Patent WO2024252135A1, 12 December 2024. [Google Scholar]
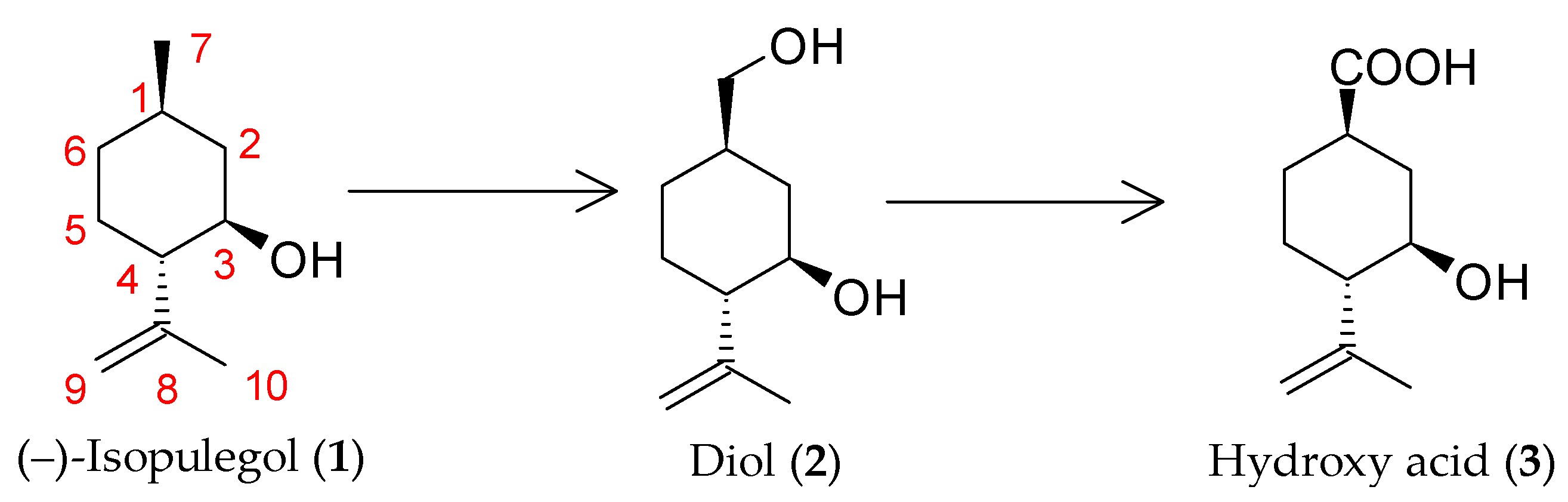



| Estimated Activity | (–)-Isopulegol (1) | Diol (2) | Hydroxy Acid (3) | (S)-Isopulegol Epoxide (4) |
|---|---|---|---|---|
| Carminative | 0.976 | 0.930 | 0.928 | 0.835 |
| Anti–eczematic | 0.929 | 0.918 | 0.908 | 0.844 |
| Neuromuscular acetyl choline blocker | 0.751 | 0.677 | 0.682 | – |
| Inhibitor of β-glucuronidase | – | 0.670 | 0.714 | 0.329 |
| Immunosuppressive | 0.755 | 0.720 | 0.731 | 0.631 |
| Stimulator of NFκB transcription factor | 0.747 | 0.730 | 0.730 | – |
| Inhibitor of retinol dehydrogenase | 0.738 | 0.739 | 0.693 | 0.277 |
| Respiratory analeptic | – | 0.568 | 0.686 | 0.588 |
| Anti-inflammatory | 0.690 | 0.645 | 0.692 | 0.413 |
| Antitumor | – | 0.550 | 0.482 | – |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maltseva, P.Y.; Plotnitskaya, N.A.; Chudinova, A.A.; Ilyina, I.V.; Volcho, K.P.; Salakhutdinov, N.F.; Ivshina, I.B. Rhodococcus rhodochrous IEGM 1362 Immobilized in Macroporous PVA Cryogel as an Effective Biocatalyst for the Production of Bioactive (–)-Isopulegol Compounds. Pharmaceuticals 2025, 18, 839. https://doi.org/10.3390/ph18060839
Maltseva PY, Plotnitskaya NA, Chudinova AA, Ilyina IV, Volcho KP, Salakhutdinov NF, Ivshina IB. Rhodococcus rhodochrous IEGM 1362 Immobilized in Macroporous PVA Cryogel as an Effective Biocatalyst for the Production of Bioactive (–)-Isopulegol Compounds. Pharmaceuticals. 2025; 18(6):839. https://doi.org/10.3390/ph18060839
Chicago/Turabian StyleMaltseva, Polina Y., Natalia A. Plotnitskaya, Alexandra A. Chudinova, Irina V. Ilyina, Konstantin P. Volcho, Nariman F. Salakhutdinov, and Irina B. Ivshina. 2025. "Rhodococcus rhodochrous IEGM 1362 Immobilized in Macroporous PVA Cryogel as an Effective Biocatalyst for the Production of Bioactive (–)-Isopulegol Compounds" Pharmaceuticals 18, no. 6: 839. https://doi.org/10.3390/ph18060839
APA StyleMaltseva, P. Y., Plotnitskaya, N. A., Chudinova, A. A., Ilyina, I. V., Volcho, K. P., Salakhutdinov, N. F., & Ivshina, I. B. (2025). Rhodococcus rhodochrous IEGM 1362 Immobilized in Macroporous PVA Cryogel as an Effective Biocatalyst for the Production of Bioactive (–)-Isopulegol Compounds. Pharmaceuticals, 18(6), 839. https://doi.org/10.3390/ph18060839









