Effect of Tegoprazan on Tacrolimus and Mycophenolate Levels in Kidney Transplant Recipients: A Randomized Controlled Study Using a Smart Trial Platform
Abstract
1. Introduction
2. Results
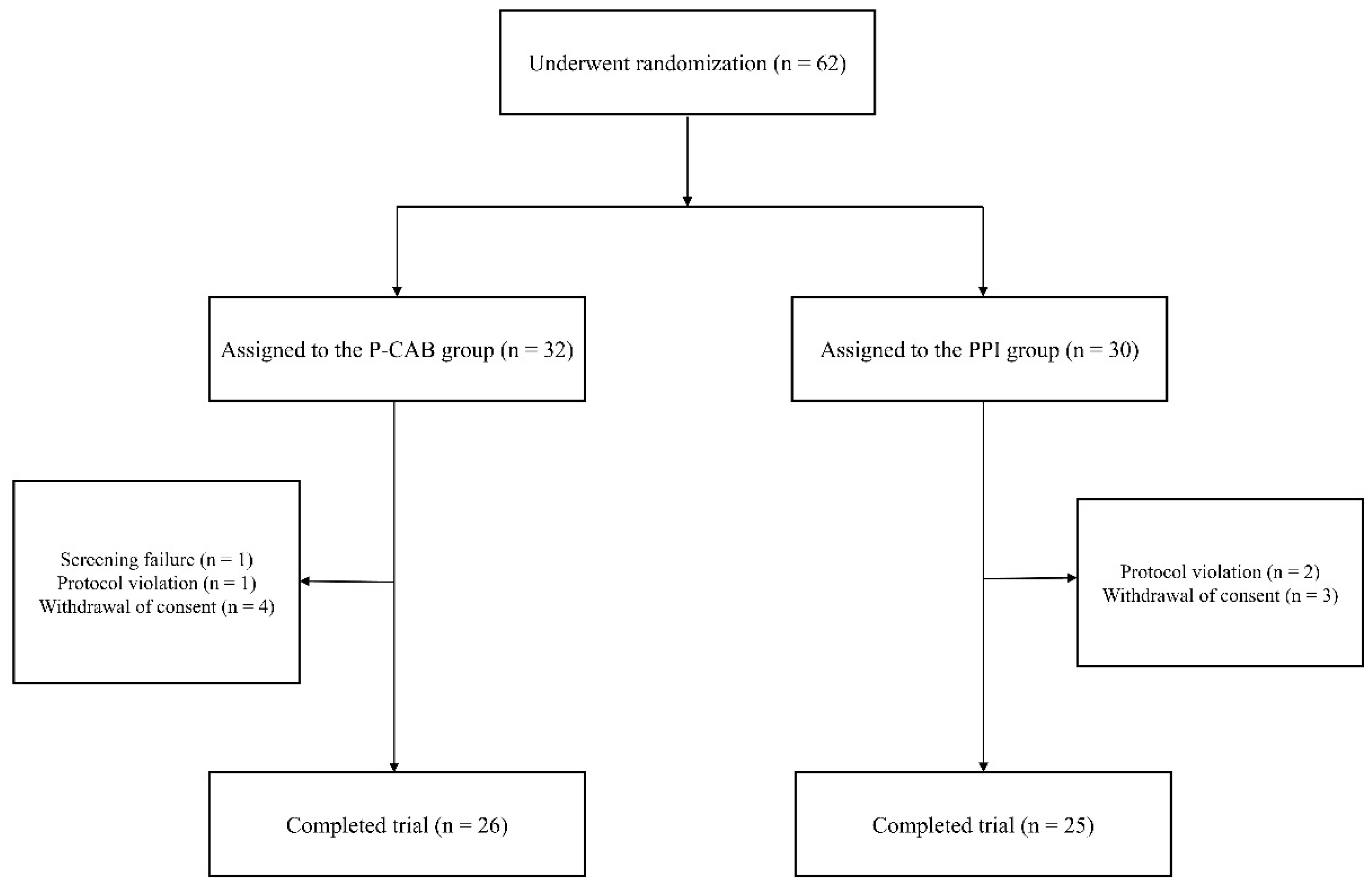
2.1. Participants and Baseline Characteristics
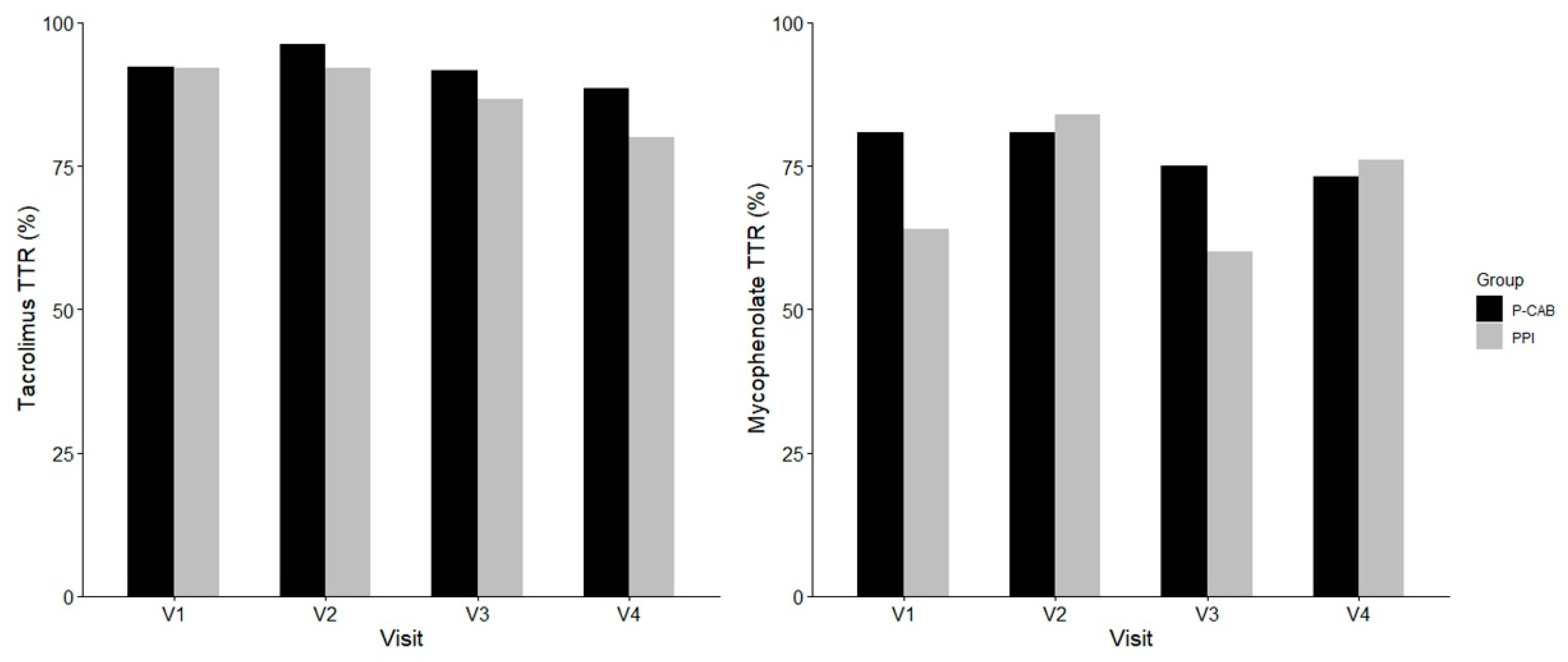
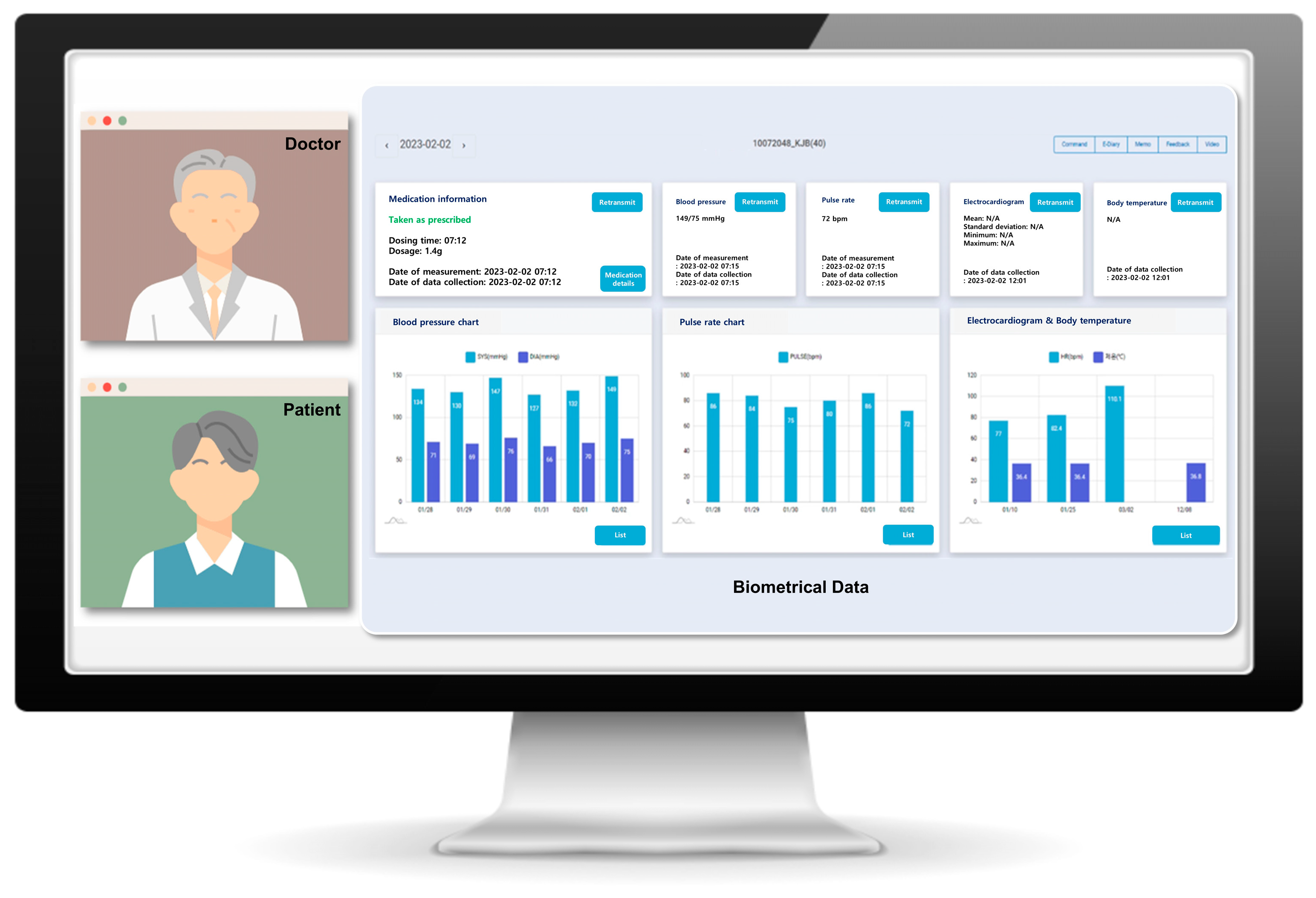
2.2. Collected Data from the Smart Clinical Trial Platform
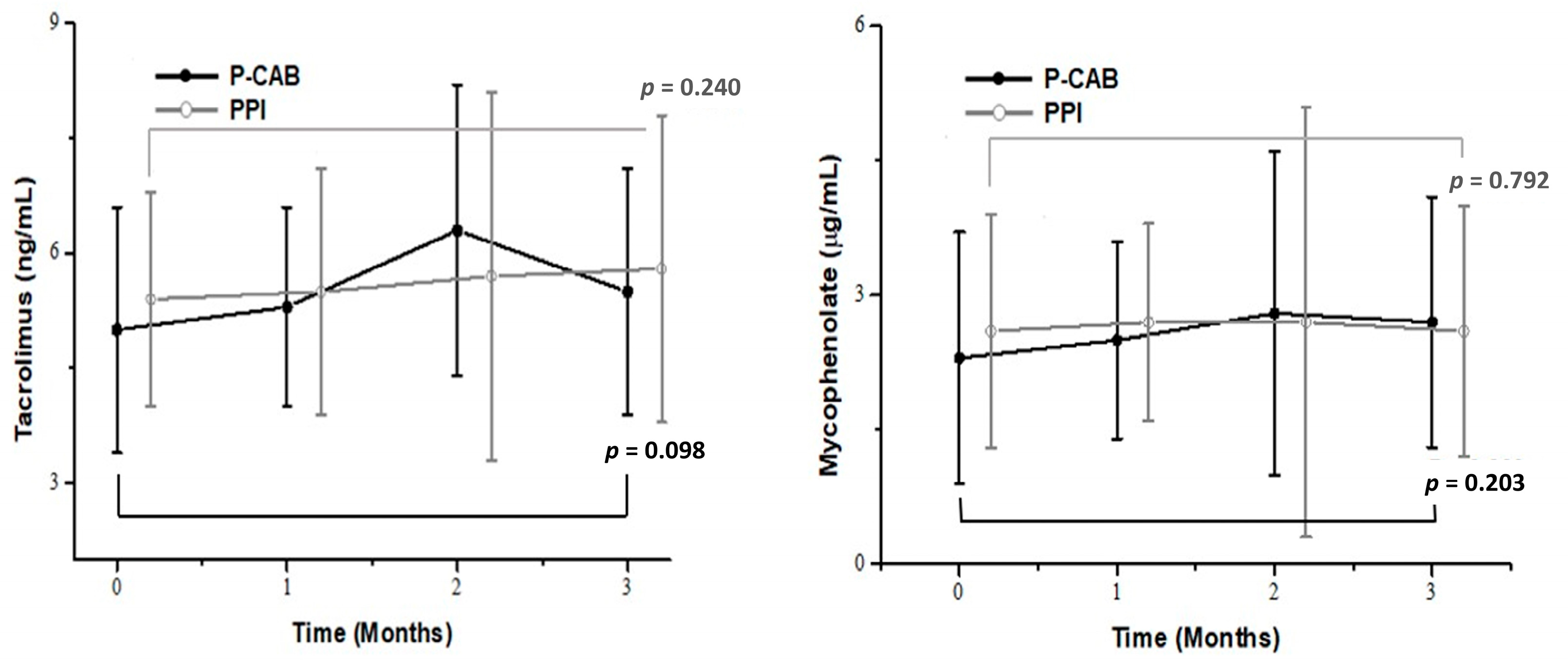
2.3. Effects of P-CABs and PPIs on Immunosuppressants
2.4. Transplant Outcomes and GI Symptoms
2.5. Adverse Events (AEs)
3. Discussion
4. Materials and Methods
4.1. Study Design
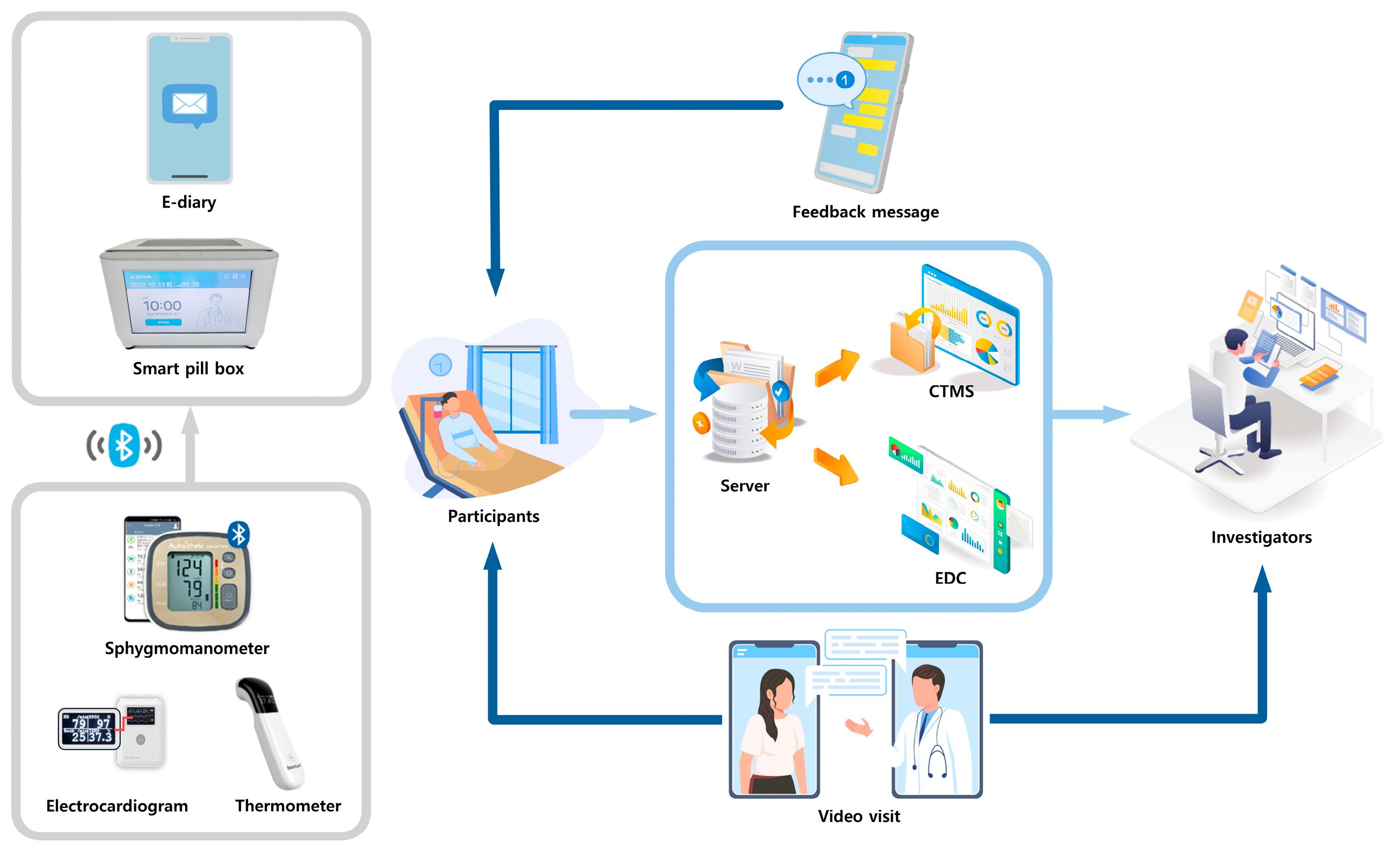
4.2. Smart Clinical Trial Platform: Components and Collected Information
4.3. Smart Pill Box
4.4. Remote Home Monitoring System
4.5. Electronic Diary App
4.6. Non-Face-to-Face Video Visit
4.7. Safety Management System
4.8. Data Collection
4.9. Study Outcomes
4.10. Measurements of the Immunosuppressant Trough Level
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AEs | Adverse events |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| BPAR | Biopsy-proven acute rejection |
| BMI | Body mass index |
| BUN | Blood urea nitrogen |
| CTMS | Clinical trial management system |
| eCRF | Electronic case report form |
| eGFR | Estimated glomerular filtration rate |
| GERD-HRQL | Gastroesophageal Reflux Disease–Health-Related Quality of Life |
| GI | Gastrointestinal |
| GTP | Gamma-glutamyl transferase |
| KTRs | Kidney transplant recipients |
| MPA | Mycophenolic acid |
| P-CABs | Potassium-competitive acid blockers |
| PPIs | Proton pump inhibitors |
| RDQ | Reflux Disease Questionnaire |
| SAEs | Severe adverse events |
References
- Starzl, T.E. The development of clinical renal transplantation. Am. J. Kidney Dis. 1990, 16, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic review: Kidney transplantation compared with dialysis in clinically relevant outcomes. Am. J. Transpl. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Wang, J.H.; Hart, A. Global Perspective on Kidney Transplantation: United States. Kidney360 2021, 2, 1836–1839. [Google Scholar] [CrossRef] [PubMed]
- Halloran, P.F. Immunosuppressive drugs for kidney transplantation. N. Engl. J. Med. 2004, 351, 2715–2729. [Google Scholar] [CrossRef]
- Jurewicz, W.A. Tacrolimus versus cyclosporin immunosuppression: Long-term outcome in renal transplantation. Nephrol. Dial. Transpl. 2003, 18 (Suppl. 1), i7–i11. [Google Scholar] [CrossRef] [PubMed]
- Webster, A.; Woodroffe, R.C.; Taylor, R.S.; Chapman, J.R.; Craig, J.C. Tacrolimus versus cyclosporin as primary immunosuppression for kidney transplant recipients. Cochrane Database Syst. Rev. 2005, Cd003961. [Google Scholar] [CrossRef]
- de Andrade, L.G.; Rodrigues, M.A.; Romeiro, F.G.; Garcia, P.D.; Contti, M.M.; de Carvalho, M.F. Clinicopathologic features and outcome of mycophenolate-induced colitis in renal transplant recipients. Clin. Transpl. 2014, 28, 1244–1248. [Google Scholar] [CrossRef]
- Gioco, R.; Corona, D.; Ekser, B.; Puzzo, L.; Inserra, G.; Pinto, F.; Schipa, C.; Privitera, F.; Veroux, P.; Veroux, M. Gastrointestinal complications after kidney transplantation. World J. Gastroenterol. 2020, 26, 5797–5811. [Google Scholar] [CrossRef]
- Saeki, T.; Ueda, K.; Tanigawara, Y.; Hori, R.; Komano, T. Human P-glycoprotein transports cyclosporin A and FK506. J. Biol. Chem. 1993, 268, 6077–6080. [Google Scholar] [CrossRef]
- Shiraga, T.; Niwa, T.; Teramura, Y.; Kagayama, A.; Tsutsui, M.; Ohno, Y.; Iwasaki, K. Oxidative metabolism of tacrolimus and its metabolite by human cytochrome P450 3A subfamily. Drug Metab. Pharmacokinet. 1999, 14, 277–285. [Google Scholar] [CrossRef][Green Version]
- Miura, M.; Inoue, K.; Kagaya, H.; Satoh, S.; Tada, H.; Sagae, Y.; Habuchi, T.; Suzuki, T. Influence of rabeprazole and lansoprazole on the pharmacokinetics of tacrolimus in relation to CYP2C19, CYP3A5 and MDR1 polymorphisms in renal transplant recipients. Biopharm. Drug Dispos. 2007, 28, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Yano, I.; Fukuhara, Y.; Katsura, T.; Takahashi, T.; Ito, N.; Yamamoto, S.; Ogawa, O.; Inui, K. Distinct effects of omeprazole and rabeprazole on the tacrolimus blood concentration in a kidney transplant recipient. Drug Metab. Pharmacokinet. 2007, 22, 441–444. [Google Scholar] [CrossRef]
- Kiberd, B.A.; Wrobel, M.; Dandavino, R.; Keown, P.; Gourishankar, S. The role of proton pump inhibitors on early mycophenolic acid exposure in kidney transplantation: Evidence from the CLEAR study. Ther. Drug Monit. 2011, 33, 120–123. [Google Scholar] [CrossRef]
- Gabardi, S.; Olyaei, A. Evaluation of potential interactions between mycophenolic acid derivatives and proton pump inhibitors. Ann. Pharmacother. 2012, 46, 1054–1064. [Google Scholar] [CrossRef]
- Oshima, T.; Miwa, H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J. Neurogastroenterol. Motil. 2018, 24, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Son, B.K.; Kim, G.H.; Jung, H.K.; Jung, H.Y.; Chung, I.K.; Sung, I.K.; Kim, J.I.; Kim, J.H.; Lee, J.S.; et al. Randomised phase 3 trial: Tegoprazan, a novel potassium-competitive acid blocker, vs. esomeprazole in patients with erosive oesophagitis. Aliment. Pharmacol. Ther. 2019, 49, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Choi, H.Y.; Kim, Y.H.; Nam, J.Y.; Kim, B.; Song, G.S.; Lim, H.S.; Bae, K.S. Effect of Food on the Pharmacokinetics and Pharmacodynamics of a Single Oral Dose of Tegoprazan. Clin. Ther. 2021, 43, 1371–1380. [Google Scholar] [CrossRef]
- Jung, H.Y.; Seong, S.J.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, C.D.; Yoon, Y.R.; Kim, H.K.; Huh, S.; Yoon, S.H.; et al. The efficacy and stability of an information and communication technology-based centralized monitoring system of adherence to immunosuppressive medication in kidney transplant recipients: Study protocol for a randomized controlled trial. Trials 2017, 18, 480. [Google Scholar] [CrossRef]
- Jung, H.Y.; Jeon, Y.; Seong, S.J.; Seo, J.J.; Choi, J.Y.; Cho, J.H.; Park, S.H.; Kim, C.D.; Yoon, Y.R.; Yoon, S.H.; et al. ICT-based adherence monitoring in kidney transplant recipients: A randomized controlled trial. BMC Med. Inf. Decis. Mak. 2020, 20, 105. [Google Scholar] [CrossRef]
- Wedemeyer, R.S.; Blume, H. Pharmacokinetic drug interaction profiles of proton pump inhibitors: An update. Drug Saf. 2014, 37, 201–211. [Google Scholar] [CrossRef]
- Birdwell, K.A.; Decker, B.; Barbarino, J.M.; Peterson, J.F.; Stein, C.M.; Sadee, W.; Wang, D.; Vinks, A.A.; He, Y.; Swen, J.J.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guidelines for CYP3A5 Genotype and Tacrolimus Dosing. Clin. Pharmacol. Ther. 2015, 98, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kofler, S.; Shvets, N.; Bigdeli, A.K.; König, M.A.; Kaczmarek, P.; Deutsch, M.A.; Vogeser, M.; Steinbeck, G.; Reichart, B.; Kaczmarek, I. Proton pump inhibitors reduce mycophenolate exposure in heart transplant recipients-a prospective case-controlled study. Am. J. Transpl. 2009, 9, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- Rouse, G.E.; Hardinger, K.; Tsapepas, D.; Tichy, E.M. A Comparison of Histamine Receptor Antagonists Versus Proton Pump Inhibitor Gastrointestinal Ulcer Prophylaxis in Kidney Transplant Recipients. Prog. Transpl. 2017, 27, 4–9. [Google Scholar] [CrossRef]
- Lorf, T.; Ramadori, G.; Ringe, B.; Schwörer, H. The effect of pantoprazole on tacrolimus and cyclosporin A blood concentration in transplant recipients. Eur. J. Clin. Pharmacol. 2000, 56, 439–440. [Google Scholar] [CrossRef] [PubMed]
- Bremer, S.C.B.; Reinhardt, L.; Sobotta, M.; Hasselluhn, M.C.; Lorf, T.; Ellenrieder, V.; Schwörer, H. Pantoprazole Does not Affect Serum Trough Levels of Tacrolimus and Everolimus in Liver Transplant Recipients. Front. Med. 2018, 5, 320. [Google Scholar] [CrossRef]
- Rissling, O.; Glander, P.; Hambach, P.; Mai, M.; Brakemeier, S.; Klonower, D.; Halleck, F.; Singer, E.; Schrezenmeier, E.V.; Dürr, M.; et al. No relevant pharmacokinetic interaction between pantoprazole and mycophenolate in renal transplant patients: A randomized crossover study. Br. J. Clin. Pharmacol. 2015, 80, 1086–1096. [Google Scholar] [CrossRef]
- Mei, T.; Noguchi, H.; Suetsugu, K.; Hisadome, Y.; Kaku, K.; Okabe, Y.; Masuda, S.; Nakamura, M. Effects of Concomitant Administration of Vonoprazan Fumarate on the Tacrolimus Blood Concentration in Kidney Transplant Recipients. Biol. Pharm. Bull. 2020, 43, 1600–1603. [Google Scholar] [CrossRef]
- Suzuki, Y.; Yoshihashi, T.; Takahashi, K.; Furuya, K.; Ohkohchi, N.; Oda, T.; Homma, M. Drug-Drug Interaction between Tacrolimus and Vonoprazan in Kidney Transplant Recipients. J. Clin. Med. 2021, 10, 3964. [Google Scholar] [CrossRef]
- Watari, S.; Araki, M.; Matsumoto, J.; Yoshinaga, K.; Sekito, T.; Maruyama, Y.; Mitsui, Y.; Sadahira, T.; Kubota, R.; Nishimura, S.; et al. Blood concentrations of tacrolimus upon conversion from rabeprazole to vonoprazan in renal transplant recipients: Correlation with cytochrome P450 gene polymorphisms. Drug Metab. Pharmacokinet. 2021, 40, 100407. [Google Scholar] [CrossRef]
- van Boekel, G.A.; Kerkhofs, C.H.; van de Logt, F.; Hilbrands, L.B. Proton pump inhibitors do not increase the risk of acute rejection. Neth. J. Med. 2014, 72, 86–90. [Google Scholar]
- Boonpheng, B.; Thongprayoon, C.; Bathini, T.; Sharma, K.; Mao, M.A.; Cheungpasitporn, W. Proton pump inhibitors and adverse effects in kidney transplant recipients: A meta-analysis. World J. Transpl. 2019, 9, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Flothow, D.J.G.; Suwelack, B.; Pavenstädt, H.; Schütte-Nütgen, K.; Reuter, S. The Effect of Proton Pump Inhibitor Use on Renal Function in Kidney Transplanted Patients. J. Clin. Med. 2020, 9, 258. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transpl. 2009, 9 (Suppl. 3), S1–S155. [Google Scholar] [CrossRef] [PubMed]
| Variables | P-CAB (n = 26) | PPI (n = 25) | p-Value |
|---|---|---|---|
| Age (year) | 53.9 ± 10.6 | 50.4 ± 8.3 | 0.204 |
| Height (cm) | 167.9 ± 7.1 | 165.5 ± 8.5 | 0.270 |
| Body weight (kg) | 60.1 ± 12.2 | 62.1 ± 12.2 | 0.127 |
| BMI (kg/m2) | 22.6 ± 3.2 | 22.5 ± 2.6 | 0.741 |
| Gender (male) | 18 (69.2) | 17 (68.0) | 0.925 |
| BUN (mg/dL) | 19.5 ± 6.3 | 20.0 ± 7.0 | 0.679 |
| Creatinine (mg/dL) | 1.1 ± 0.3 | 1.2 ± 0.4 | 0.178 |
| Glucose (mg/dL) | 115.7 ± 30.1 | 110.4 ± 22.6 | 0.940 |
| eGFR (mL/min/1.73 m2) | 62.8 ± 17.1 | 57.7 ± 14.9 | 0.397 |
| AST (U/L) | 17.6 ± 4.6 | 17.7 ± 4.3 | 0.934 |
| ALT (U/L) | 15.3 ± 6.1 | 13.9 ± 5.1 | 0.383 |
| GTP (U/L) | 32.3 ± 29.5 | 23.1 ± 19.4 | 0.117 |
| Immunosuppressants | P-CAB (n = 26) | PPI (n = 25) | p-Value | |
|---|---|---|---|---|
| Tacrolimus (ng/mL) | Baseline | 5.0 ± 1.6 | 5.4 ± 1.4 | 0.166 |
| Week 4 | 5.3 ± 1.3 | 5.5 ± 1.6 | 0.598 | |
| Week 8 | 6.3 ± 1.9 | 5.7 ± 2.4 | 0.479 | |
| Week 12 | 5.5 ± 1.6 | 5.8 ± 2.0 | 0.500 | |
| Mycophenolate (µg/mL) | Baseline | 2.3 ± 1.4 | 2.6 ± 1.3 | 0.350 |
| Week 4 | 2.5 ± 1.1 | 2.7 ± 1.1 | 0.515 | |
| Week 8 | 2.8 ± 1.8 | 2.7 ± 2.4 | 0.294 | |
| Week 12 | 2.7 ± 1.4 | 2.6 ± 1.4 | 0.565 |
| eGFR (mL/min/1.73 m2) | De Novo DSA | BPAR | Allograft Loss | ||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 4 | Week 8 | Week 12 | ||||
| P-CAB (n = 26) | 62.8 ± 17.1 | 61.8 ± 13.0 | 60.6 ± 12.8 | 63.5 ± 16.5 | 1 (4.2%) | 0 | 0 |
| PPI (n = 25) | 57.7 ± 14.9 | 60.1 ± 14.8 | 63.6 ± 14.1 | 60.6 ± 14.7 | 2 (8.3%) | 0 | 0 |
| p-value | 0.397 | 0.538 | 0.569 | 0.618 | 1.000 | N/A | N/A |
| Questionnaire for Quality of Life | P-CAB (n = 26) | PPI (n = 25) | p-Value | |
|---|---|---|---|---|
| GERD-HRQL | Baseline | 4.9 ± 4.3 | 2.8 ± 2.3 | 0.074 |
| Week 4 | 0.9 ± 1.4 | 0.7 ± 1.2 | 0.400 | |
| Week 8 | 0.7 ± 1.0 | 0.9 ± 1.8 | 0.583 | |
| Week 12 | 0.7 ± 1.6 | 0.5 ± 1.1 | 0.382 | |
| RDQ | Baseline | 4.7 ± 4.7 | 3.2 ± 3.3 | 0.426 |
| Week 4 | 1.0 ± 2.1 | 0.3 ± 0.9 | 0.322 | |
| Week 8 | 0.6 ± 1.2 | 0.4 ± 1.0 | 0.617 | |
| Week 12 | 0.7 ± 1.9 | 0.7 ± 2.0 | 0.822 |
| P-CAB (n = 26) | PPI (n = 25) | p-Value | |
|---|---|---|---|
| AEs | 15 (57.7) | 7 (28.0) | 0.032 |
| SAEs | 2 (7.7) | 0 (0.0) | NA |
| Withdrawal due to AEs | 0 (0.0) | 0 (0.0) | NA |
| AEs | |||
| Abdominal discomfort | 3 (11.5) | 2 (8.0) | 1.000 |
| Arthralgia | 1 (3.8) | 0 (0.0) | 1.000 |
| Constipation | 2 (7.7) | 0 (0.0) | 0.490 |
| COVID-19 | 0 (0.0) | 2 (8.0) | 0.235 |
| Diarrhea | 5 (19.2) | 0 (0.0) | 0.051 |
| Headache | 0 (0.0) | 1 (4.0) | 0.490 |
| Hypertension | 4 (15.4) | 0 (0.0) | 0.110 |
| Hypoglycemia | 1 (3.8) | 0 (0.0) | 1.000 |
| Myalgia | 0 (0.0) | 1 (4.0) | 0.490 |
| Nausea, vomiting | 3 (11.5) | 0 (0.0) | 0.235 |
| Productive cough, sputum | 4 (15.4) | 0 (0.0) | 0.110 |
| Pyrexia | 0 (0.0) | 1 (4.0) | 0.490 |
| Toothache | 0 (0.0) | 1 (4.0) | 0.490 |
| Upper respiratory tract infection | 2 (7.7) | 1 (4.0) | 1.000 |
| Urinary tract infection | 0 (0.0) | 1 (4.0) | 0.490 |
| Vertigo | 0 (0.0) | 1 (4.0) | 0.490 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-W.; Jeon, Y.H.; Lim, J.-H.; Seo, J.J.; Jung, H.-Y.; Choi, J.-Y.; Park, S.-H.; Kim, C.-D.; Kim, Y.-L.; Cho, J.-H. Effect of Tegoprazan on Tacrolimus and Mycophenolate Levels in Kidney Transplant Recipients: A Randomized Controlled Study Using a Smart Trial Platform. Pharmaceuticals 2025, 18, 830. https://doi.org/10.3390/ph18060830
Lee S-W, Jeon YH, Lim J-H, Seo JJ, Jung H-Y, Choi J-Y, Park S-H, Kim C-D, Kim Y-L, Cho J-H. Effect of Tegoprazan on Tacrolimus and Mycophenolate Levels in Kidney Transplant Recipients: A Randomized Controlled Study Using a Smart Trial Platform. Pharmaceuticals. 2025; 18(6):830. https://doi.org/10.3390/ph18060830
Chicago/Turabian StyleLee, Seong-Wook, You Hyun Jeon, Jeong-Hoon Lim, Jung Ju Seo, Hee-Yeon Jung, Ji-Young Choi, Sun-Hee Park, Chan-Duck Kim, Yong-Lim Kim, and Jang-Hee Cho. 2025. "Effect of Tegoprazan on Tacrolimus and Mycophenolate Levels in Kidney Transplant Recipients: A Randomized Controlled Study Using a Smart Trial Platform" Pharmaceuticals 18, no. 6: 830. https://doi.org/10.3390/ph18060830
APA StyleLee, S.-W., Jeon, Y. H., Lim, J.-H., Seo, J. J., Jung, H.-Y., Choi, J.-Y., Park, S.-H., Kim, C.-D., Kim, Y.-L., & Cho, J.-H. (2025). Effect of Tegoprazan on Tacrolimus and Mycophenolate Levels in Kidney Transplant Recipients: A Randomized Controlled Study Using a Smart Trial Platform. Pharmaceuticals, 18(6), 830. https://doi.org/10.3390/ph18060830










