Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets
Abstract
1. Introduction
2. Results
2.1. Screening for Active Ingredients of P. japonicus var. major and Gastric Cancer Targets
2.2. Main Components and Targets Associated with Gastric Cancer
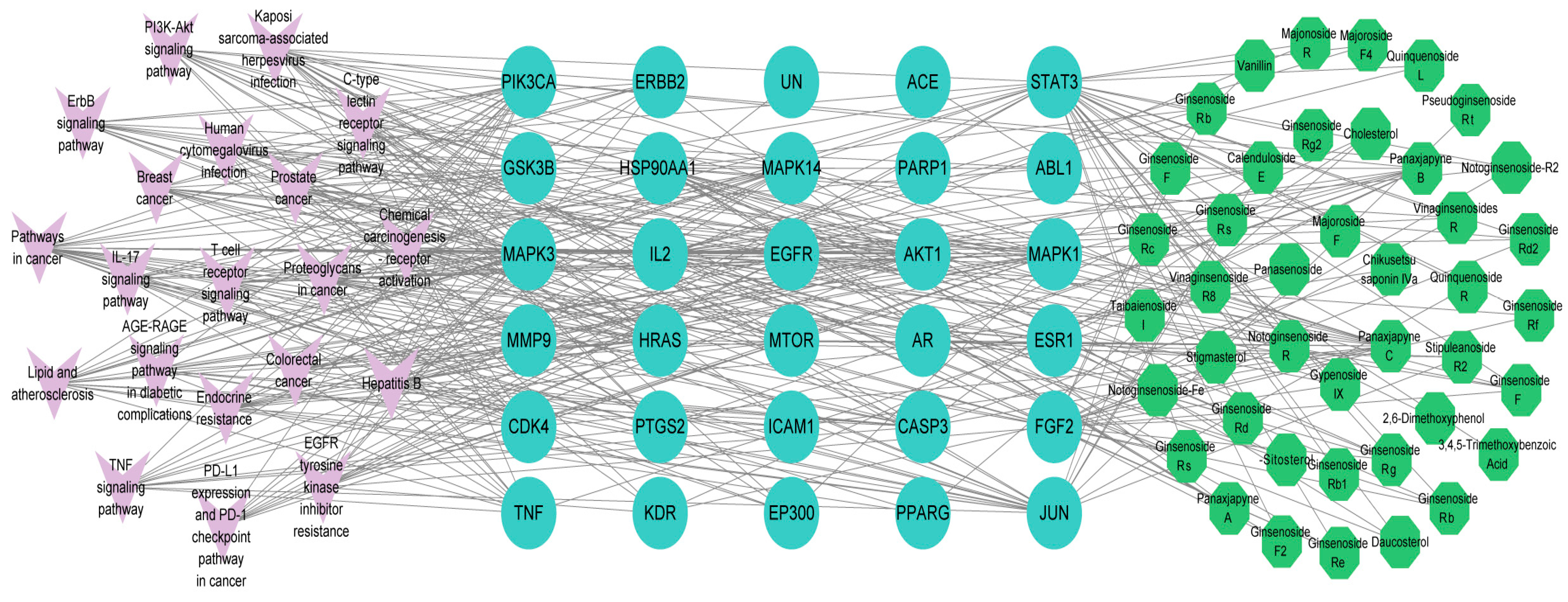
2.3. Potential Functions and Pathways Associated with Targets for GC Treatment
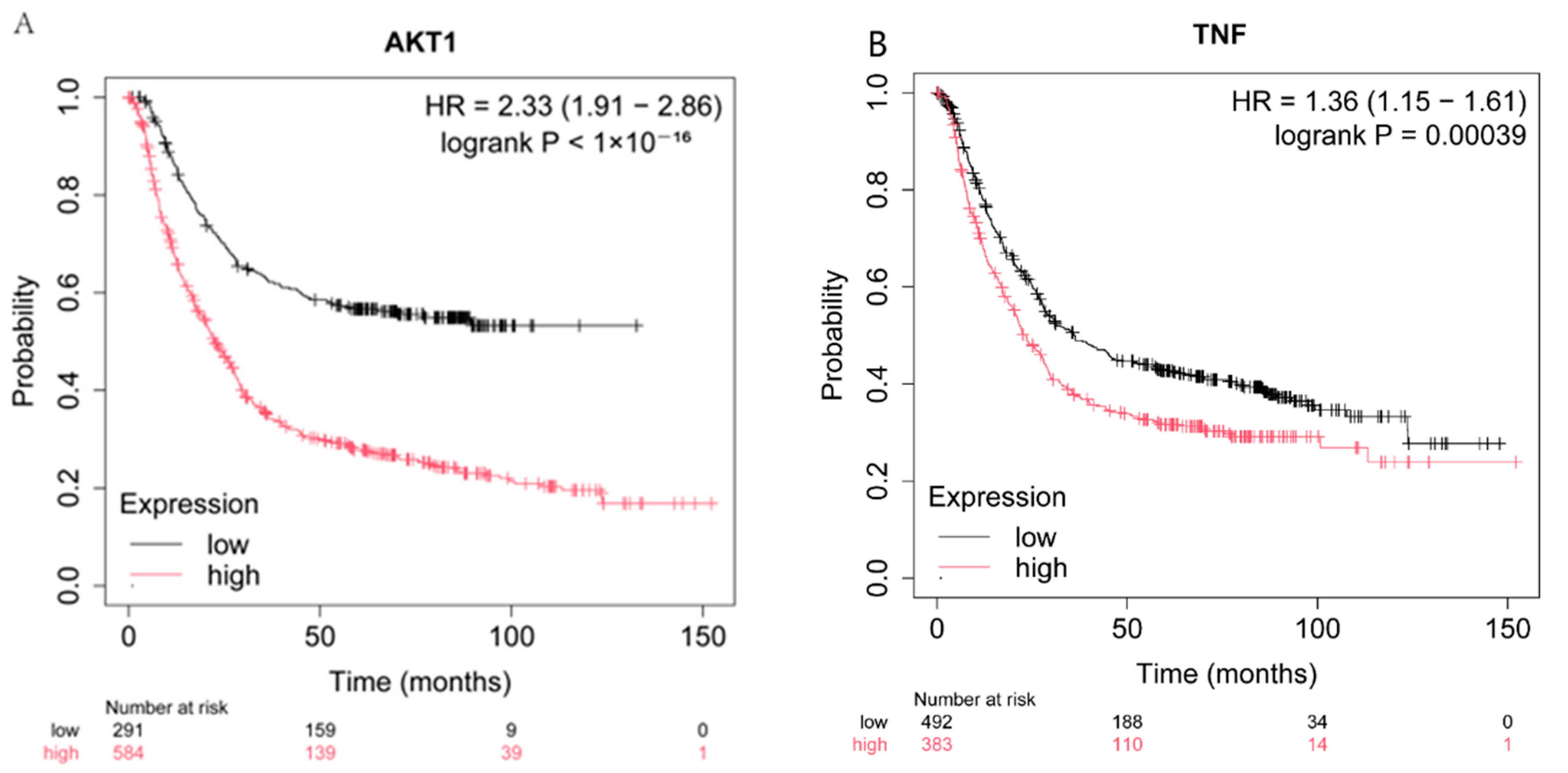
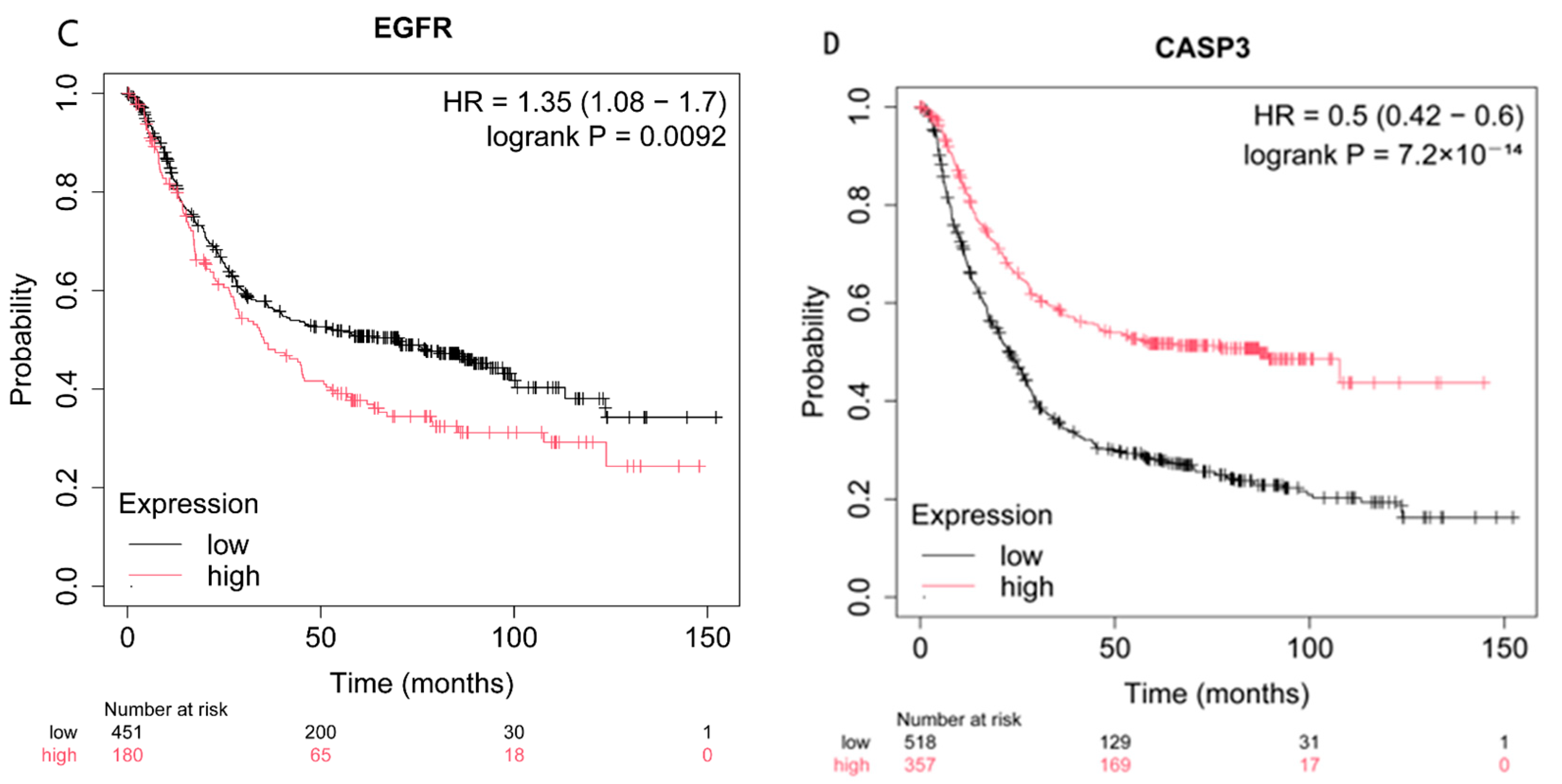
2.4. Effect of the Expression of CASP3, AKT1, TNF, and EGFR on Patient Survival
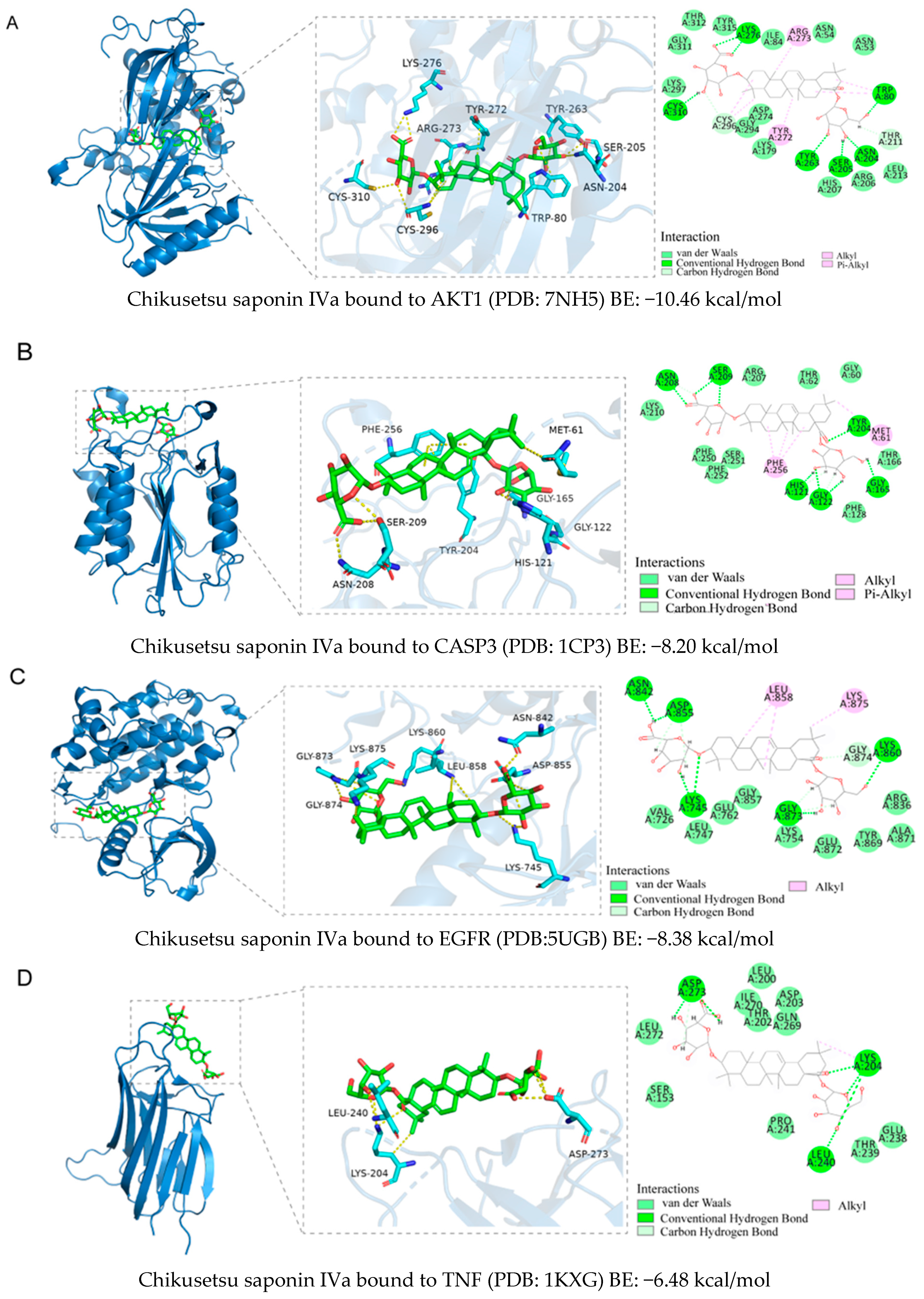
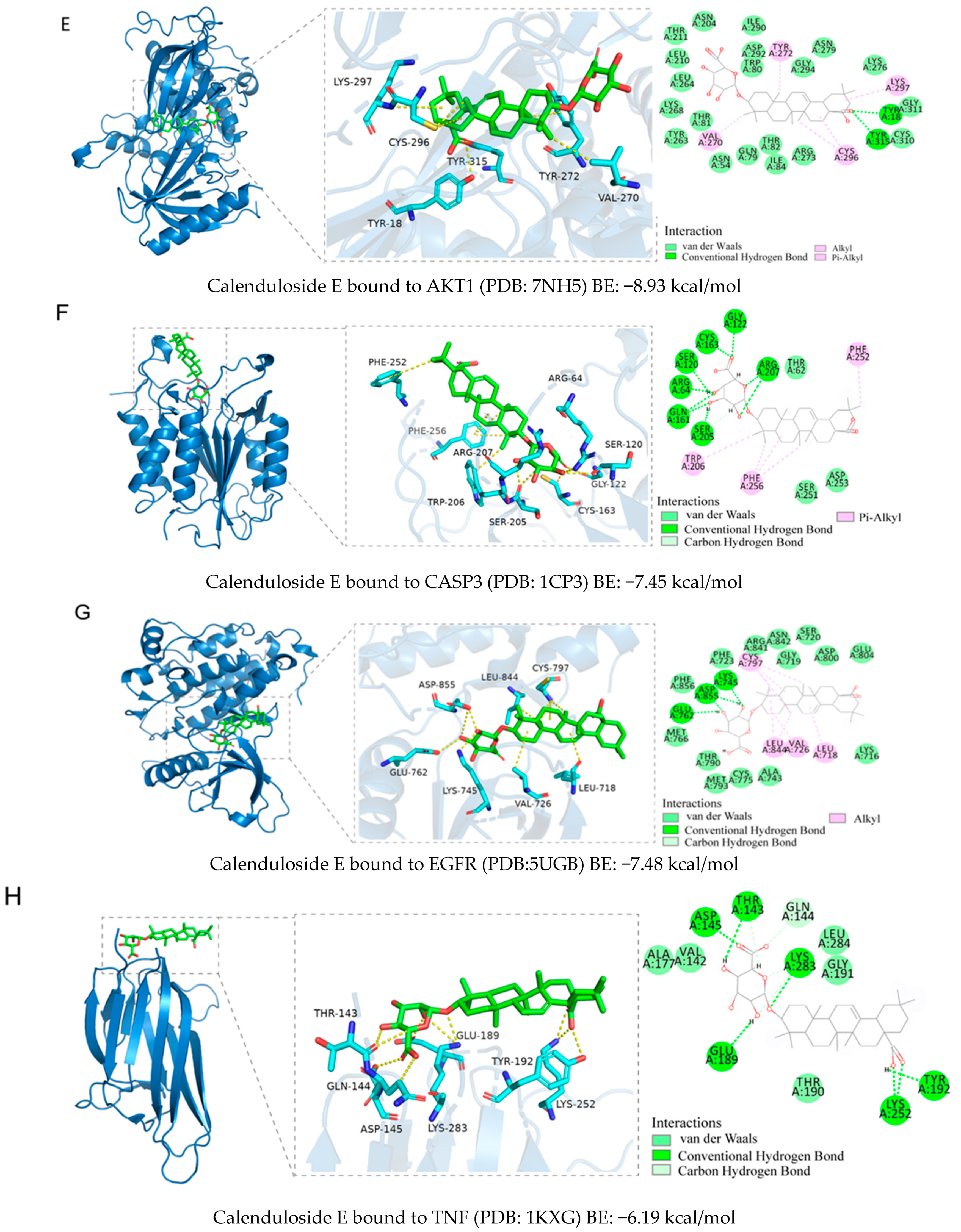
2.5. Binding Ability Between Main Active Compounds and Protein Targets
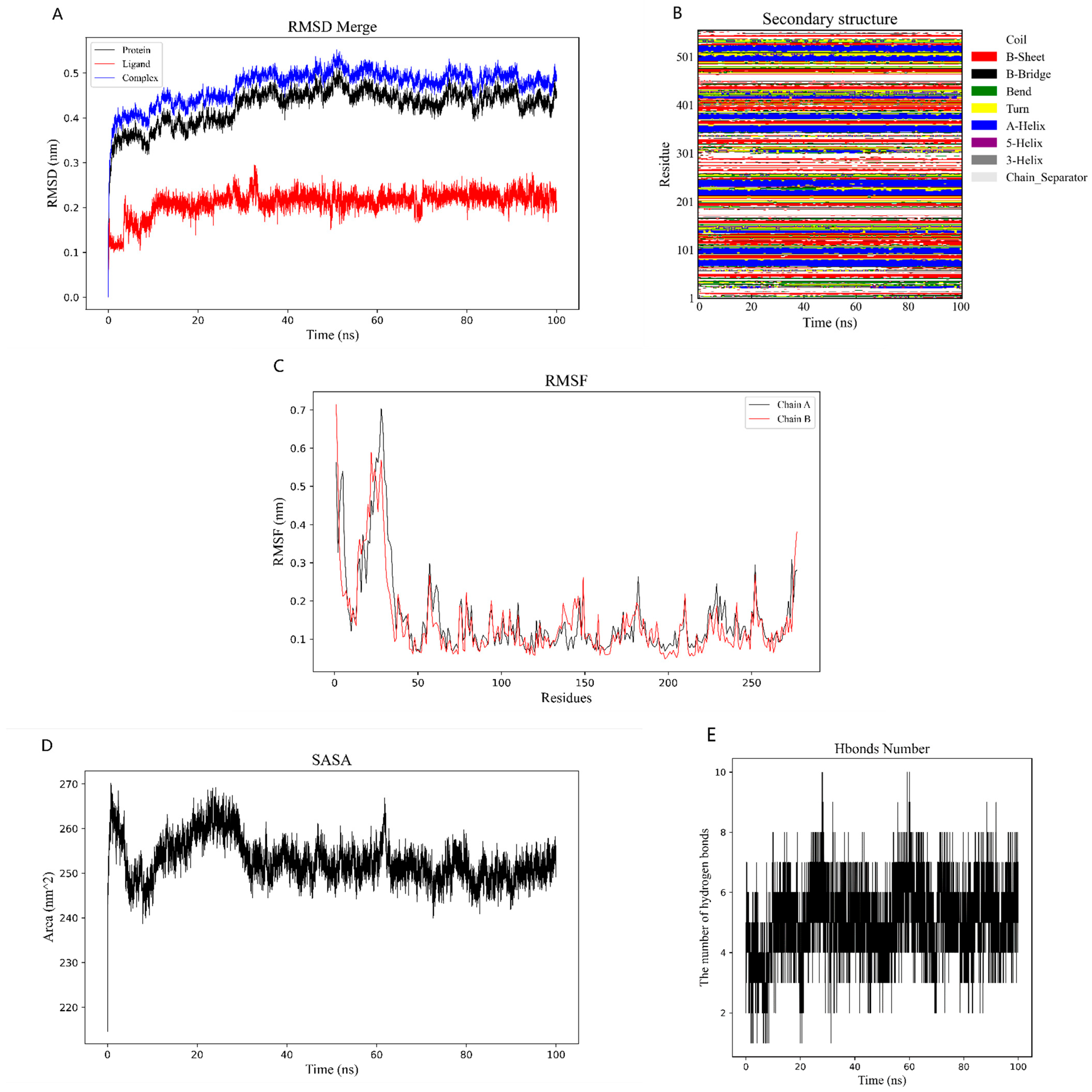
2.6. Binding Energy and Stability of Chikusetsu Saponin IVa–CASP3
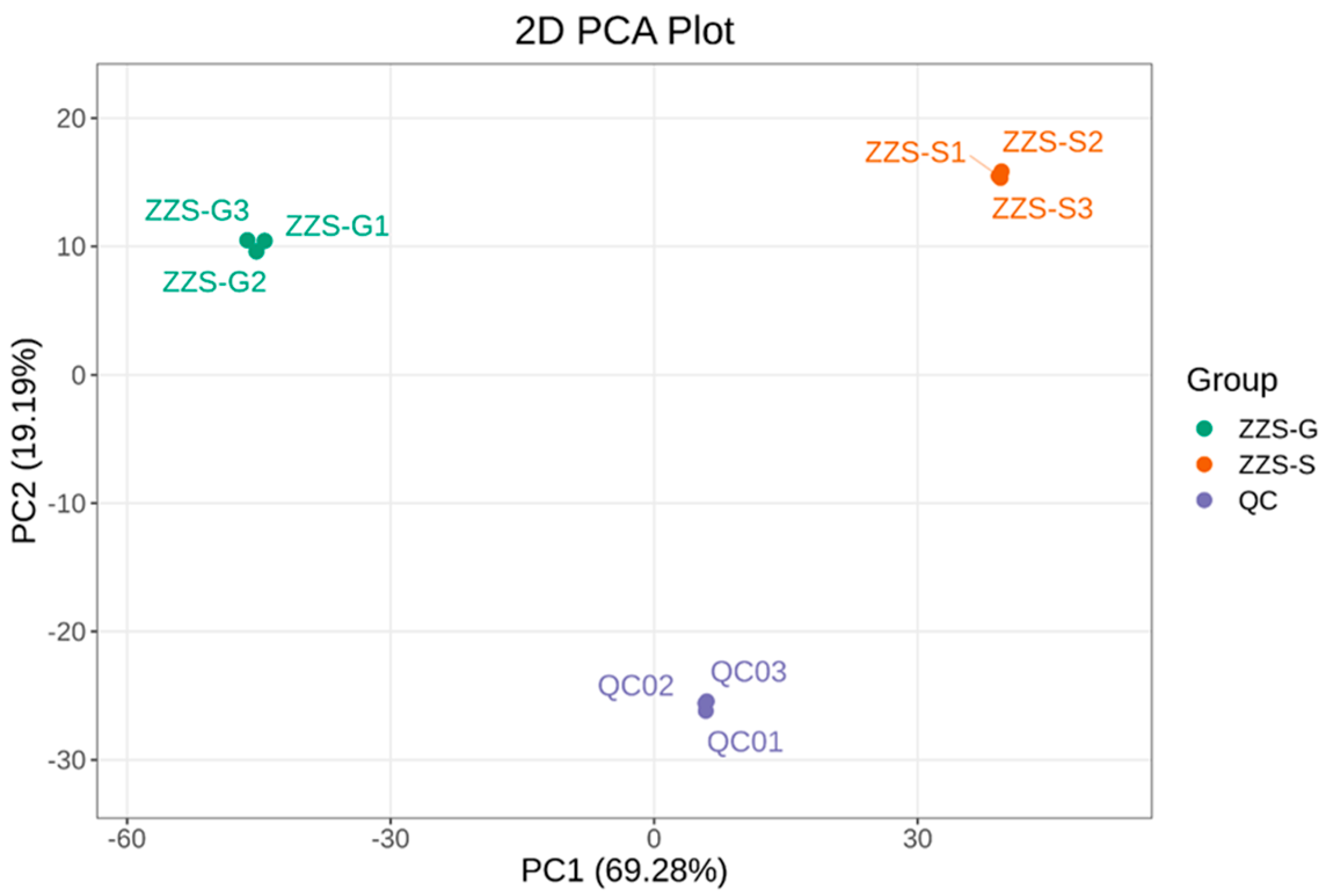
2.7. Analysis of Major Components and Differential Metabolites in Panax japonicus
2.8. Cell Viability Assays
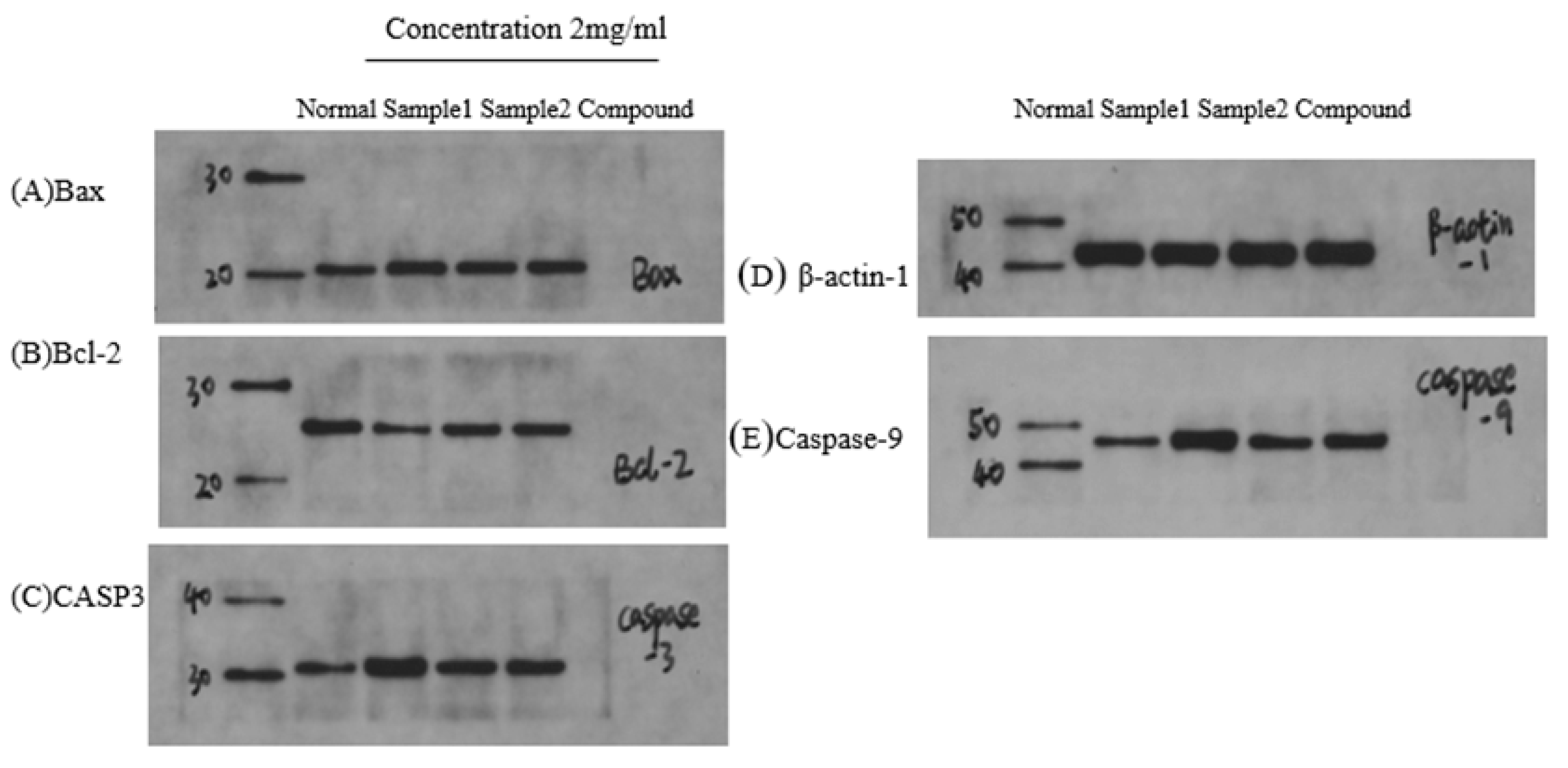
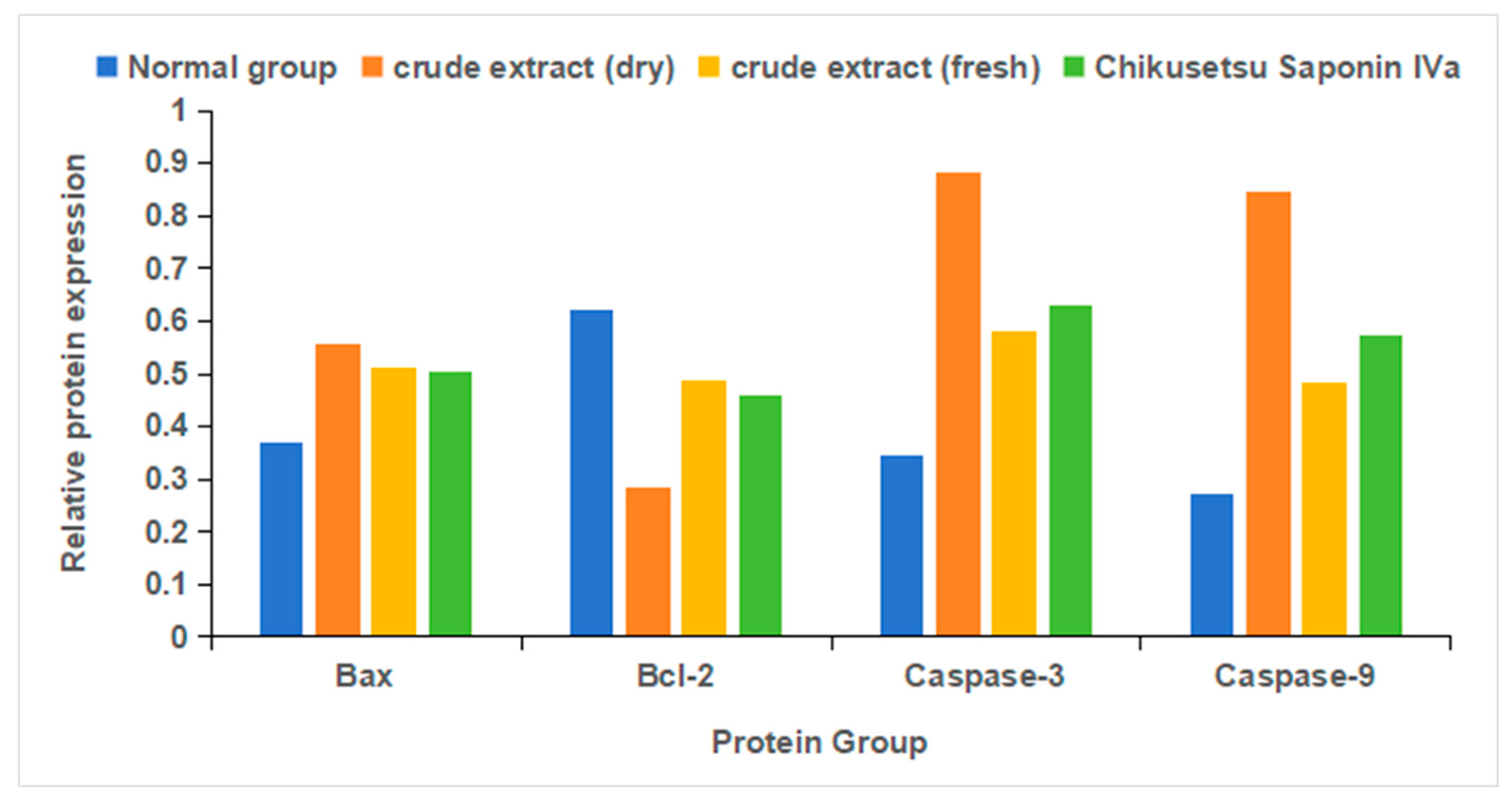
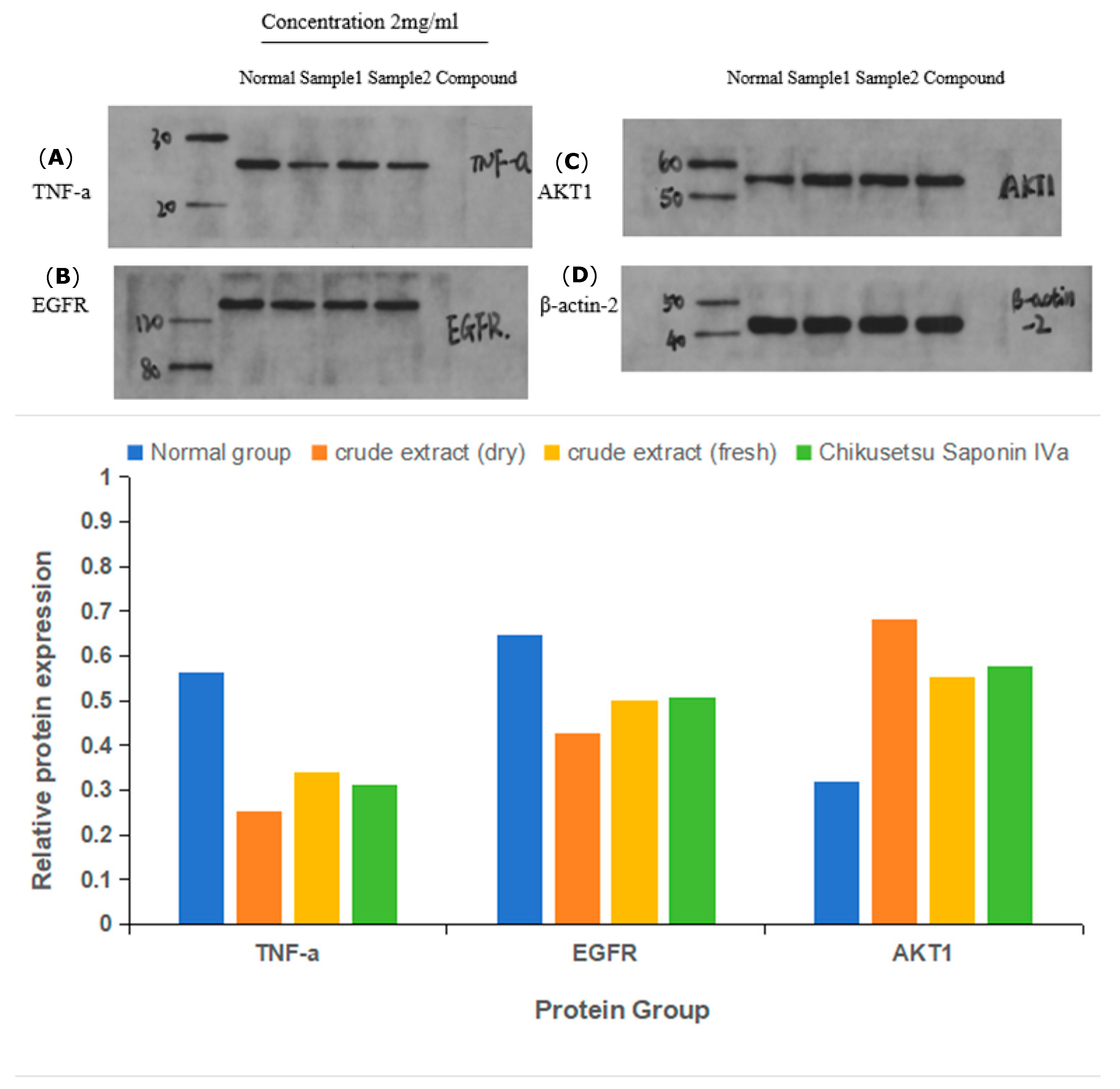
2.9. Immunoblot Assays
3. Discussion
4. Materials and Methods
4.1. Medicinal Materials and Chemicals
4.2. Collection of Main Active Compounds and Corresponding Targets
4.3. Collection of Disease-Related Targets
4.4. Protein–Protein Interaction (PPI) Network Analysis
4.5. Gene Ontology Term Enrichment and Kyoto Encyclopedia of Genes and Genomes Pathway Enrichment Analysis
4.6. Survival Analysis
4.7. Molecular Docking Analysis
4.8. Molecular Dynamics Simulations and Calculation of Binding Free Energies
4.9. Untargeted Metabolomics
4.10. MTS Cell Inhibition Rate
4.11. Immunoblotting
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| GC | Gastric cancer |
| ETCM | Encyclopedia of Traditional Chinese Medicine |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| MD | Molecular Dynamics |
| RMSD | Root-Mean-Square Deviation |
| RMSF | Root-Mean-Square Fluctuation |
| MMPBS | Molecular Mechanics Poisson-Boltzmann Surface Area |
| FBS | Fetal Bovine Serum |
| ECL | Enhanced Chemiluminescence |
| IPP | Image-Pro Plus |
| ZZS-G | Dried P. japonicus var. major Group |
| ZZS-S | Fresh P. japonicus var. major Group |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Ajani, J.A.; D’amico, T.A.; Bentrem, D.J.; Chao, J.; Cooke, D.; Corvera, C.; Das, P.; Enzinger, P.C.; Enzler, T.; Fanta, P.; et al. Pluchino, Gastric Cancer, Version 2.2022, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2022, 20, 167–192. [Google Scholar] [CrossRef] [PubMed]
- Vijayalakshmi, S.; Yoo, D.-S.; Kim, D.-G.; Chelliah, R.; Barathikannan, K.; Aloo, S.-O.; Tyagi, A.; Yan, P.; Shan, L.; Gebre, T.S.; et al. Fermented Perilla frutescens leaves and their untargeted metabolomics by UHPLC-QTOF-MS reveal anticancer and immunomodulatory effects. Food Biosci. 2023, 56, 103065. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Na, Z.; Liang, S.; Wu, F.; Xie, H.; Zhang, X.; Xu, W.; Wang, X. Network Pharmacology Analysis of Liquid-Cultured Armillaria ostoyae Mycelial Metabolites and Their Molecular Mechanism of Action against Gastric Cancer. Molecules 2024, 29, 1668. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Cao, W.; He, Z.; Liu, L.; Guo, J.; Dong, L.; Song, J.; Wu, Y.; Zhao, Y. Network medicine analysis for dissecting the therapeutic mechanism of consensus TCM formulae in treating hepatocellular carcinoma with different TCM syndromes. Front. Endocrinol. 2024, 15, 1373054. [Google Scholar] [CrossRef]
- Lu, D.; Yuan, L.; Ma, X.; Meng, F.; Xu, D.; Jia, S.; Wang, Z.; Li, Y.; Zhang, Z.; Nan, Y. The mechanism of polyphyllin in the treatment of gastric cancer was verified based on network pharmacology and experimental validation. Heliyon 2024, 10, e31452. [Google Scholar] [CrossRef]
- Ji, Y.; Liu, Y.; Hu, J.; Cheng, C.; Xing, J.; Zhu, L.; Shen, H. Exploring the Molecular Mechanism of Astragali Radix-Curcumae Rhizoma against Gastric Intraepithelial Neoplasia by Network Pharmacology and Molecular Docking. Evid.-Based Complement. Altern. Med. 2021, 2021, 8578615. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, X.; Tang, J.; Yin, L.; Wu, Z.; Zhang, J.; Gao, Y. Analysis of Network Pharmacology and Molecular Docking on Radix Pseudostellariae for Its Active Components on Gastric Cancer. Appl. Biochem. Biotechnol. 2022, 195, 1968–1982. [Google Scholar] [CrossRef]
- Zoi, V.; Galani, V.; Lianos, G.D.; Voulgaris, S.; Kyritsis, A.P.; Alexiou, G.A. The Role of Curcumin in Cancer Treatment. Biomedicines 2021, 9, 1086. [Google Scholar] [CrossRef]
- Duda-Madej, A.; Viscardi, S.; Szewczyk, W.; Topola, E. Natural Alkaloids in Cancer Therapy: Berberine, Sanguinarine and Chelerythrine against Colorectal and Gastric Cancer. Int. J. Mol. Sci. 2024, 25, 8375. [Google Scholar] [CrossRef]
- Sun, Y.-W.; Ju, Y.; Liu, C.-H.; Du, K.-C.; Meng, D.-L. Polyhydroxyl guaianolide terpenoids as potential NF-κB inhibitors induced cytotoxicity in human gastric adenocarcinoma cell line. Bioorganic Chem. 2020, 95, 103551. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Wilairatana, P.; Joshi, P.B.; Ahmad, Z.; Olatunde, A.; Hafeez, N.; Hemeg, H.A.; Mubarak, M.S. Revisiting luteolin: An updated review on its anticancer potential. Heliyon 2024, 10, e26701. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Sun, K.; Jin, X.; Wu, X.; Xia, M.; Sun, Y.; Feng, L.; Li, G.; Wan, X.; Chen, C. Review on active components and mechanism of natural product polysaccharides against gastric carcinoma. Heliyon 2024, 10, e27218. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Yin, J.; Bai, Z.; Zhang, Z. Effects of taxol resistance gene 1 on the cisplatin response in gastric cancer. Oncol. Lett. 2018, 15, 8287–8294. [Google Scholar] [CrossRef]
- Liu, J.; Liu, J.; Wang, D.; Luo, Q.; Liu, Z. Activity tracking isolation of Gelsemium elegans alkaloids and evaluation of their antihuman gastric cancer activity in vivo. Chin. J. Anal. Chem. 2021, 49, 91–102. [Google Scholar] [CrossRef]
- Ma, S.; Hu, Y.; Chen, J.; Wang, X.; Zhang, C.; Liu, Q.; Cai, G.; Wang, H.; Zheng, J.; Wang, Q.; et al. Marine fungus-derived alkaloid inhibits the growth and metastasis of gastric cancer via targeting mTORC1 signaling pathway. Chem. Interact. 2023, 382, 110618. [Google Scholar] [CrossRef]
- Li, H.Y.; Wang, H.Y.; Na, L.S.; Jin, C.H.; Wang, J.L. Ideal solution ranking based on principal component analysis, orthogonal partial least squares discriminant analysis and weighted approximation-gray Evaluating the quality of bead ginseng from different origins by correlation fusion modeling. Chin. Tradit. Herb. Drugs 2024, 55, 3116–3126. [Google Scholar]
- Zhang, D.; Dan, L.; Gao, T.; Zhang, Y.; Gong, C.; Liu, C.; Li, Y.; Song, X. Panacis majoris Rhizoma: A Comprehensive Review of Phytochemistry, Pharmacology and Clinical Applications. Rec. Nat. Prod. 2024, 18, 53–94. [Google Scholar] [CrossRef]
- Guo, X.; Ji, J.; Zhang, J.; Hou, X.; Fu, X.; Luo, Y.; Mei, Z.; Feng, Z. Anti-inflammatory and osteoprotective effects of Chikusetsusaponin IVa on rheumatoid arthritis via the JAK/STAT signaling pathway. Phytomedicine 2021, 93, 153801. [Google Scholar] [CrossRef]
- Li, M.; Li, X.; Zhou, L.; Jin, Y. Effects of total saponins from Panacis majoris Rhizoma and its degradation products on myocardial ischemia-reperfusion injury in rats. Biomed. Pharmacother. 2020, 130, 110538. [Google Scholar] [CrossRef]
- Zuo, T.; Zhang, Z.; Jiang, P.; Zhang, R.; Ni, D.; Yuan, Y.; Zhang, S. Characterization of chikusetsusaponin IV and V induced apoptosis in HepG2 cancer cells. Mol. Biol. Rep. 2022, 49, 4247–4255. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhou, R.; He, Y.; Meng, M.; Hu, J.; Liu, Y.; Pan, Y.; Tang, Z.; Yue, Z. Total saponins from Rhizoma Panacis Majoris inhibit proliferation, induce cell cycle arrest and apoptosis and influence MAPK signalling pathways on the colorectal cancer cell. Mol. Med. Rep. 2021, 24, 542. [Google Scholar] [CrossRef] [PubMed]
- Li, D.-J.; Chen, M.-H.; He, H.-B.; Li, X.-M.; Ding, Y.-H.; Zhang, Y.-F.; Xu, H.-Y.; Feng, M.-L.; Xiang, C.-Q.; Zhou, J.-G.; et al. Saponins from Rhizoma Panacis Majoris attenuate myocardial ischemia/reperfusion injury via the activation of the Sirt1/Foxo1/Pgc-1α and Nrf2/antioxidant defense pathways in rats. Pharmacogn. Mag. 2018, 14, 297–307. [Google Scholar] [CrossRef]
- Zhang, M.X.; Fang, F.; Yang, Y.; Fan, J.L.; Yuan, P. Protective effect of ginsenoside Rg1 on hypoxic-ischemic brain injury and neuronal apoptosis in young rats. J. Xi Jiaotong Univ. 2021, 5, 693–699. [Google Scholar] [CrossRef]
- Ai, Z.; Liu, S.; Zhang, J.; Hu, Y.; Tang, P.; Cui, L.; Wang, X.; Zou, H.; Li, X.; Liu, J.; et al. Ginseng Glucosyl Oleanolate from Ginsenoside Ro, Exhibited Anti-Liver Cancer Activities via MAPKs and Gut Microbiota In Vitro/Vivo. J. Agric. Food Chem. 2024, 72, 7845–7860. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zhu, M.; Zhu, Z.; Tang, W.; Zhang, H.; Chen, Y.; Liu, F.; Zhang, Y. Ginsenoside Re inhibits non-small cell lung cancer progression by suppressing macrophage M2 polarization induced by AMPKα1/STING positive feedback loop. Phytother Res. 2024, 38, 5088–5106. [Google Scholar] [CrossRef]
- Mansour, H.S.; El-Wahab, H.A.A.A.; Ali, A.M.; Aboul-Fadl, T. Inversion kinetics of some E/Z 3-(benzylidene)-2-oxo-indoline derivatives and their in silico CDK2 docking studies. RSC Adv. 2021, 11, 7839–7850. [Google Scholar] [CrossRef]
- Sargsyan, K.; Grauffel, C.; Lim, C. How Molecular Size Impacts RMSD Applications in Molecular Dynamics Simulations. J. Chem. Theory Comput. 2017, 13, 1518–1524. [Google Scholar] [CrossRef]
- Wang, E.; Sun, H.; Wang, J.; Wang, Z.; Liu, H.; Zhang, J.Z.H.; Hou, T. End-Point Binding Free Energy Calculation with MM/PBSA and MM/GBSA: Strategies and Applications in Drug Design. Chem. Rev. 2019, 119, 9478–9508. [Google Scholar] [CrossRef]
- Sharma, G.; Shukla, R.; Singh, T.R. Identification of small molecules against the NMDAR: An insight from virtual screening, density functional theory, free energy landscape and molecular dynamics simulation-based findings. Netw. Model. Anal. Health Inform. Bioinform. 2022, 11, 31. [Google Scholar] [CrossRef]
- Wiggins, T.; Kumar, S.; Markar, S.R.; Antonowicz, S.; Hanna, G.B. and Tryptophan in Gastroesophageal Malignancy: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2015, 24, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Li, Z.; Deng, X.-M.; Jiang, K.; Liu, D.; Zhang, H.-H.; Shi, T.; Liu, L.-Y.; Wen, H.-X.; Li, Q.-E.; et al. synthesis and bioactivity evaluation of isoquinoline alkaloids from Corydalis hendersonii Hemsl. against gastric cancer in vitro and in vivo. Bioorganic Med. Chem. 2022, 60, 116705. [Google Scholar] [CrossRef]
- Mochalski, P.; Leja, M.; Ślefarska-Wolak, D.; Mezmale, L.; Patsko, V.; Ager, C.; Królicka, A.; Mayhew, C.A.; Shani, G.; Haick, H. Identification of Key Volatile Organic Compounds Released by Gastric Tissues as Potential Non-Invasive Biomarkers for Gastric Cancer. Diagnostics 2023, 13, 335. [Google Scholar] [CrossRef] [PubMed]
- Bednarz-Misa, I.; Fleszar, M.G.; Fortuna, P.; Lewandowski, Ł.; Mierzchała-Pasierb, M.; Diakowska, D.; Krzystek-Korpacka, M. Altered L-Arginine Metabolic Pathways in Gastric Cancer: Potential Therapeutic Targets and Biomarkers. Biomolecules 2021, 11, 1086. [Google Scholar] [CrossRef]
- Wen, Y.; Liu, C.; Guo, J.; Yuan, Q.; Wang, R.; Li, X.Q.; Yuan, D.; Yuan, C.F.; Yumin, H.E. Research Progress on Non-Medicinal Parts of Zhujieshen (Panax japonicus) and Zhuzishen (Panax japonicus var. major). J. Tradit. Chin. Med. 2025, 43. [Google Scholar]
- Zhang, Z.Q.; Cao, L.; Song, L.; Zhao, R.; Wang, K.L. Historical evolution and application prospects of Panax japonicus var. major like medicinal herbs. Yunnan J. Tradit. Chin. Med. Mater. Medica 2012, 33, 68–71. [Google Scholar]
- Yan, X.; Inta, A.; Yang, X.; Pandith, H.; Disayathanoowat, T.; Yang, L. An Investigation of the Effect of the Traditional Naxi Herbal Formula Against Liver Cancer Through Network Pharmacology, Molecular Docking, and In Vitro Experiments. Pharmaceuticals 2024, 17, 1429. [Google Scholar] [CrossRef]
- Qi, S.; Liang, X.; Wang, Z.; Jin, H.; Zou, L.; Yang, J. Potential Mechanism of Tibetan Medicine Liuwei Muxiang Pills against Colorectal Cancer: Network Pharmacology and Bioinformatics Analyses. Pharmaceuticals 2024, 17, 429. [Google Scholar] [CrossRef]
- Men, D.; Dai, J.; Yuan, Y.; Jiang, H.; Wang, X.; Wang, Y.; Tao, L.; Sheng, J.; Tian, Y. Exploration of anti-osteoporotic peptides from Moringa oleifera leaf proteins by network pharmacology, molecular docking, molecular dynamics and cellular assay analyses. J. Funct. Foods 2024, 116, 106144. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Elkahoui, S.; Alshammari, A.M.; Patel, M.; Ghoniem, A.E.M.; Abdalla, R.A.H.; Dwivedi-Agnihotri, H.; Badraoui, R.; Adnan, M. Mechanistic Insights into the Anticancer Potential of Asparagus racemosus Willd. Against Triple-Negative Breast Cancer: A Network Pharmacology and Experimental Validation Study. Pharmaceuticals 2025, 18, 433. [Google Scholar] [CrossRef]
- Yu, F.; Zhao, L.X.; Chu, S. TCHH as a Novel Prognostic Biomarker for Patients with Gastric Cancer by Bioinformatics Analysis. Clin. Exp. Gastroenterol. 2024, 17, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Van Quickelberghe, E.; De Sutter, D.; van Loo, G.; Eyckerman, S.; Gevaert, K. A protein-protein interaction map of the TNF-induced NF-κB signal transduction pathway. Sci. Data 2018, 5, 180289. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, S.; Jiang, L.; Tan, Z.; Wang, J. A Systematic Pan-Cancer Analysis of CASP3 as a Potential Target for Immunotherapy. Front. Mol. Biosci. 2022, 9, 776808. [Google Scholar] [CrossRef]
- Shimoi, T.; Hashimoto, J.; Sudo, K.; Shimomura, A.; Noguchi, E.; Shimizu, C.; Yunokawa, M.; Yonemori, K.; Yoshida, H.; Yoshida, M.; et al. Hotspot mutation profiles of AKT1 in Asian women with breast and endometrial cancers. BMC Cancer 2021, 21, 1131. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Kikuchi, O.; Yamamoto, Y.; Sunami, T.; Wang, Y.; Fukuyama, K.; Saito, T.; Nakahara, H.; Minamiguchi, S.; Kanai, M.; et al. Colorectal cancer harboring EGFR kinase domain duplication response to EGFR tyrosine kinase inhibitors. Oncologist 2025, 30, oyae113. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, Z.; Yuan, L.; Chen, X.; Huang, H.; Cheng, F.; Shen, W. Hesperidin inhibits colon cancer progression by downregulating SLC5A1 to suppress EGFR phosphorylation. J. Cancer 2025, 16, 876–887. [Google Scholar] [CrossRef]
- Wang, J.; Chen, W.; Li, Q.; Yang, R.; Lin, X.; Han, P.; Huang, X.; Hu, H.; Luo, M. AKT1E17K-Interacting lncRNA SVIL-AS1 Promotes AKT1 Oncogenic Functions by Preferentially Blocking AKT1E17K Dephosphorylation. Adv. Sci. 2025, 12, 2500919. [Google Scholar] [CrossRef]
- National Pharmacopoeia Committee. Pharmacopoeia of the People’s Republic of China (I); China Pharmaceutical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Wang, S.; Chen, X.; Cheng, J.; Cai, T.; Wu, X.; Cheng, Z.; Qi, S.; Qi, Z. Calunduloside E inhibits HepG2 cell proliferation and migration via p38/JNK-HMGB1 signalling axis. J. Pharmacol. Sci. 2021, 147, 18–26. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Shi, Y.; Chen, T.; Xu, Z.; Wang, P.; Yu, M.; Chen, W.; Li, B.; Jing, Z.; et al. 0: An update with comprehensive resource and rich annotations for traditional Chinese medicine. Acta Pharm. Sin. B 2023, 13, 2559–2571. [Google Scholar] [CrossRef]
- Gfeller, D.; Grosdidier, A.; Wirth, M.; Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: A web server for target prediction of bioactive small molecules. Nucleic Acids Res. 2014, 42, W32–W38. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Stein, T.I.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2004, 33, D514–D517. [Google Scholar] [CrossRef] [PubMed]
- Otasek, D.; Morris, J.H.; Bouças, J.; Pico, A.R.; Demchak, B. Cytoscape Automation: Empowering workflow-based network analysis. Genome Biol. 2019, 20, 185. [Google Scholar] [CrossRef] [PubMed]
- Tambouratzis, T. An artificial neural network based approach for online string matching/filtering of large databases. Int. J. Intell. Syst. 2010, 25, 365–385. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Su, Q.; Lv, Q.; Wu, R. Mining expression and prognosis of FOLR1 in ovarian cancer by using Oncomine and Kaplan-Meier plotter. Pteridines 2019, 30, 158–164. [Google Scholar] [CrossRef]
- Wang, R.; Fang, X.; Lu, Y.; Wang, S. The PDB bind database: Collection of binding affinities for protein-ligand complexes with known three-dimensional structures. J. Med. Chem. 2004, 47, 2977–2980. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J.C. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1, 19–25. [Google Scholar] [CrossRef]








| Compound Name | Compound Structure | Degree | Betweenness | Closeness |
|---|---|---|---|---|
| Panaxjapyne C |  | 45.0 | 8980.48 | 0.23108666 |
| Panaxjapyne B | 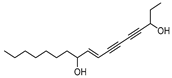 | 45.0 | 8888.03 | 0.22919509 |
| Cholesterol |  | 22.0 | 2596.21 | 0.20512821 |
| β-Sitosterol | 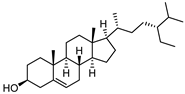 | 19.0 | 1565.01 | 0.20363636 |
| Stigmasterol | 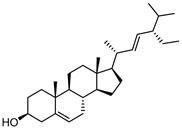 | 17.0 | 1001.37 | 0.20071685 |
| Notoginsenoside R2 | 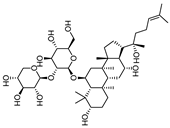 | 13.0 | 759.52 | 0.19421965 |
| 3,4,5-Trimethoxybenzoic Acid | 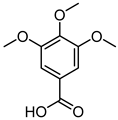 | 12.0 | 3169.00 | 0.19156215 |
| Chikusetsusaponin IVa |  | 12.0 | 1601.09 | 0.20664206 |
| Stipuleanoside R2 | 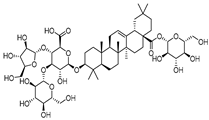 | 11.0 | 739.68 | 0.20023838 |
| Calenduloside E | 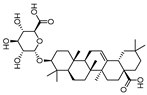 | 11.0 | 782.38 | 0.20715167 |
| Protein Name | Degree | Protein Name | Degree |
|---|---|---|---|
| AKT1 | 96 | MAPK14 | 61 |
| TNF | 93 | GSK3B | 59 |
| EGFR | 87 | MAPK1 | 59 |
| CASP3 | 84 | KDR | 58 |
| STAT3 | 84 | EP300 | 58 |
| JUN | 83 | HRAS | 58 |
| ESR1 | 78 | FGF2 | 56 |
| HSP90AA1 | 74 | PARP1 | 54 |
| ERBB2 | 73 | IL2 | 53 |
| MMP9 | 73 | ICAM1 | 48 |
| MAPK3 | 69 | CDK4 | 47 |
| MTOR | 69 | ABL1 | 46 |
| PTGS2 | 67 | AR | 44 |
| PPARG | 67 | ACE | 34 |
| PIK3CA | 62 |
| CASP3–Chikusetsu Saponin IVa | |
|---|---|
| Van der Waals force | −70.74 ± 1.28 |
| EEL | −54.74 ± 1.96 |
| EGB | 78.59 ± 4.05 |
| ESURF | −9.88 ± 0.09 |
| ΔGGAS | −125.48 ± 2.34 |
| ΔGSOLV | 68.70 ± 4.05 |
| ΔTotal | −56.77 ± 4.68 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Li, G.; Yan, X.; Disayathanoowat, T.; Inta, A.; Gao, L.; Yang, L. Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets. Pharmaceuticals 2025, 18, 823. https://doi.org/10.3390/ph18060823
Huang C, Li G, Yan X, Disayathanoowat T, Inta A, Gao L, Yang L. Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets. Pharmaceuticals. 2025; 18(6):823. https://doi.org/10.3390/ph18060823
Chicago/Turabian StyleHuang, Chao, Ge Li, Xiuxiang Yan, Terd Disayathanoowat, Angkhana Inta, Lu Gao, and Lixin Yang. 2025. "Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets" Pharmaceuticals 18, no. 6: 823. https://doi.org/10.3390/ph18060823
APA StyleHuang, C., Li, G., Yan, X., Disayathanoowat, T., Inta, A., Gao, L., & Yang, L. (2025). Molecular Mechanisms of Panax japonicus var. major Against Gastric Cancer: Metabolite Analysis, Signaling Pathways, and Protein Targets. Pharmaceuticals, 18(6), 823. https://doi.org/10.3390/ph18060823







