Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care
Abstract
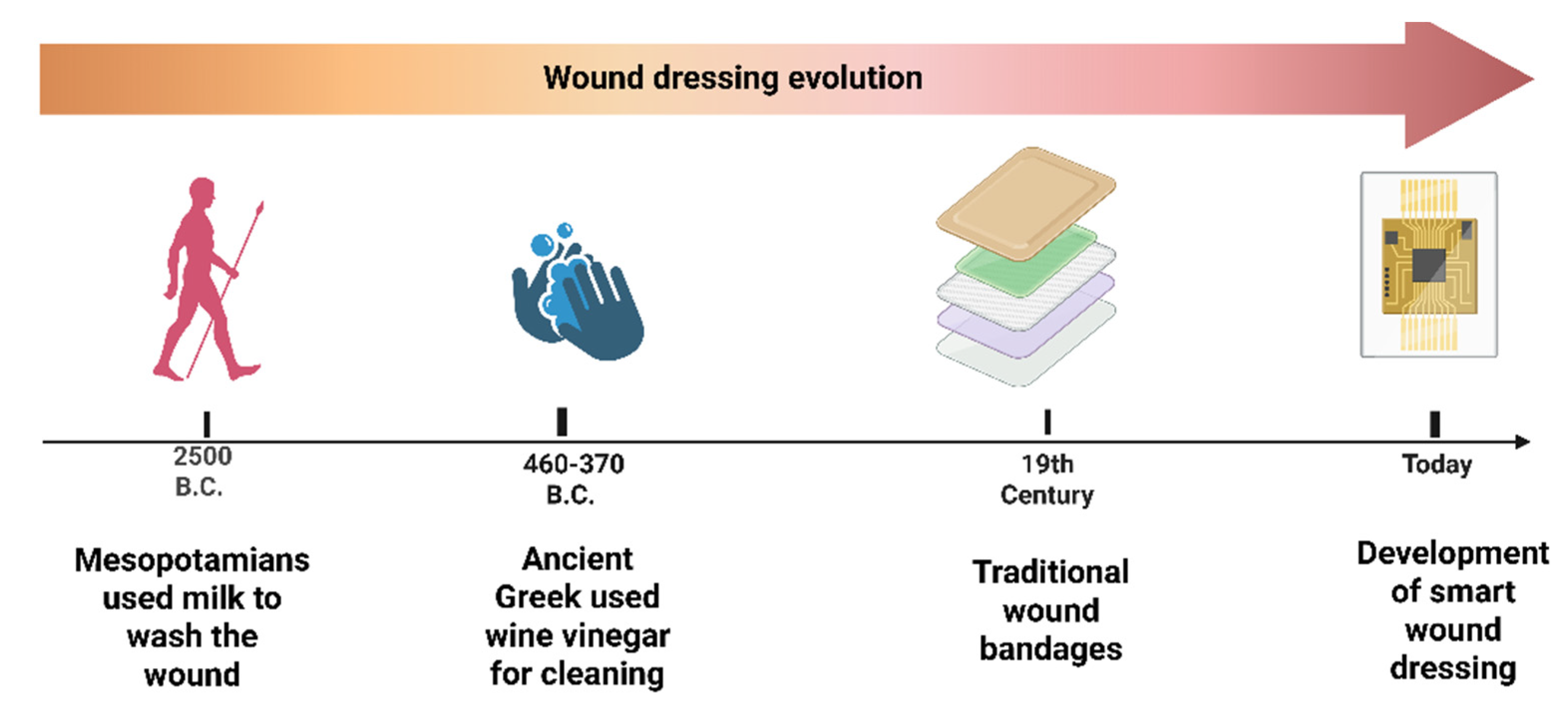
1. Introduction
2. Methodology
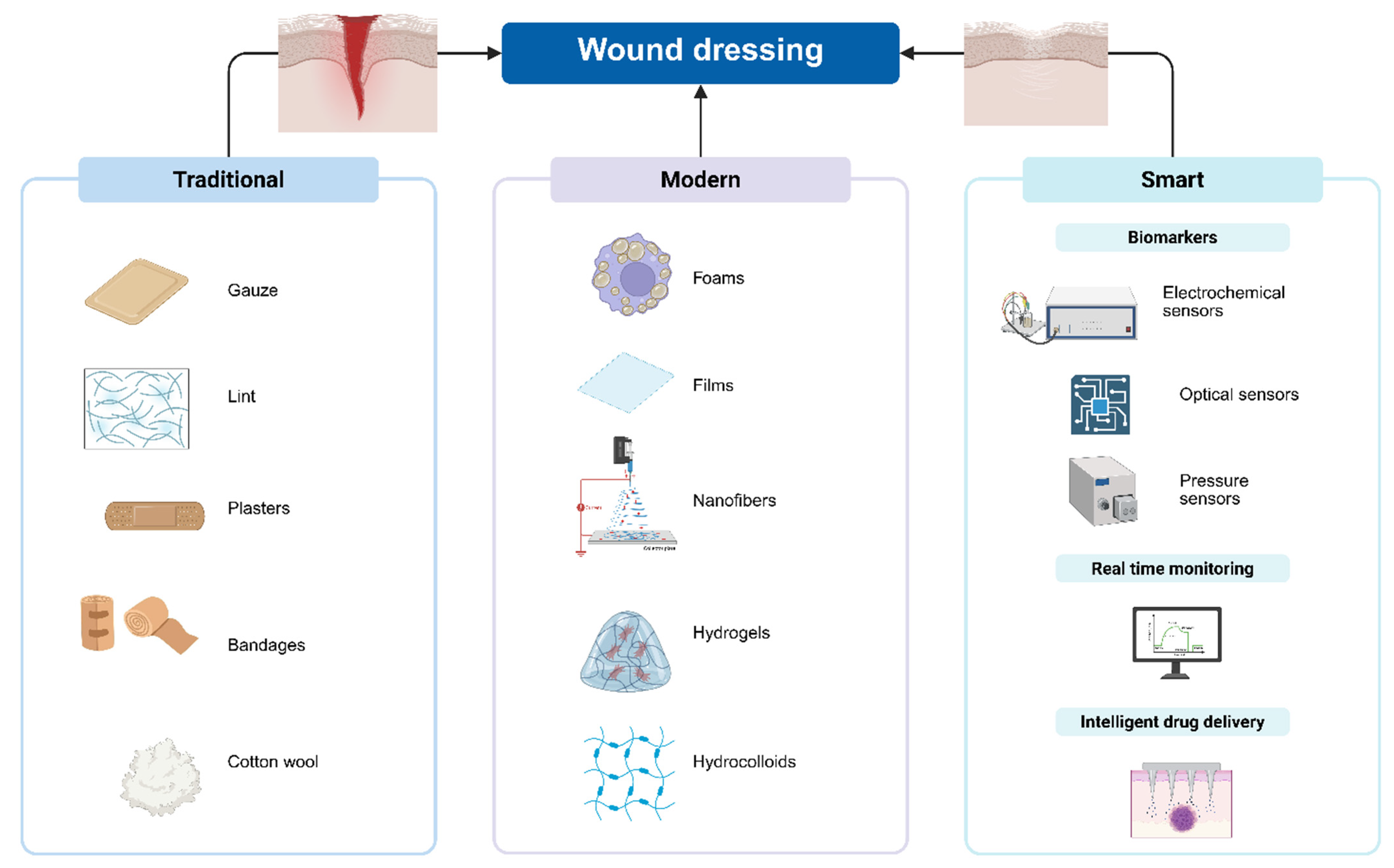
3. Traditional Wound Dressings and Their Limitations in Combating Antibiotic-Resistant Bacteria
4. The Concepts of Modern Dressings
4.1. Films
4.2. Hydrocolloids
4.3. Foams
4.4. Hydrogels
4.5. Nanofibres Dressings
5. Transition from Modern Dressings to Smart Dressings
5.1. Wound-Related Biomarkers
5.1.1. Electrochemical Sensors
5.1.2. Optical Sensors
5.1.3. Pressure Sensors
5.2. Wound Dressing with Intelligent Drug Delivery
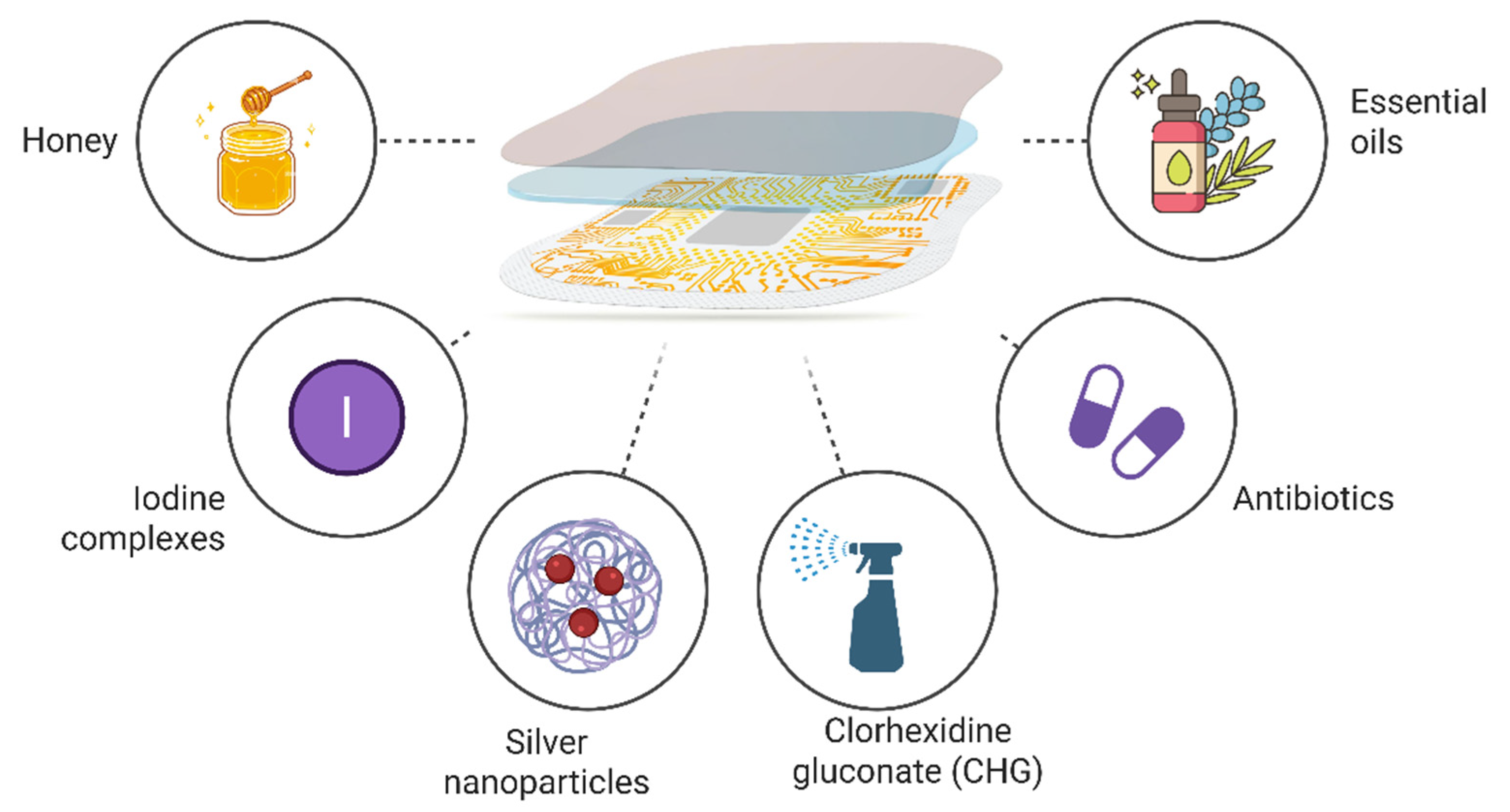
6. Types of Antimicrobial Agents Used in Smart Dressings
6.1. Antibiotics
6.2. Honey
6.3. Essential Oils
6.4. Iodine and Iodine Complexes
6.5. Chlorhexidine Gluconate (CHG)
6.6. Silver
7. Challenges and Prospects in the Development of Smart Dressings
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Before Christ |
| BCE | Before Christian Era |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MDR | Multidrug-resistant |
| PHMB | Polyhexamethylene biguanide |
| AMPs | Antimicrobial peptides |
| PTT | Photothermal therapy |
| PDT | Photodynamic therapy |
| ECM | Extracellular matrix |
| PLA | Polylactic acid |
| PLGA | Poly(lactic-co-glycolic acid) |
| PCL | Polycaprolactone |
| PVA | Polyvinyl alcohol |
| MPO | Myeloperoxidase |
| DNA | Deoxyribonucleic acid |
| E-DNA | Ultrasensitive electrochemical DNA |
| ISDPR | Isothermal strand-displacement polymerization reaction |
| CaP NPs | Calcium phosphate nanoparticles |
| TOB | Tobramycin |
| OD | Oxidized dextran |
| QCS | Quaternized chitosan |
| GelMA | Methacrylated gelatin |
| MUG | 4-Methylumphulone beta-D-glucoside |
| CFU | Colony-forming unit |
| CuS NPs | Copper sulfide nanoparticles |
| UCNPs@TiO2 | Up-converted nanoparticles coated with titanium dioxide |
| CIP | Ciprofloxacin |
| GS | Gentamicin sulfate |
| CMC | Carboxymethyl chitosan |
| COL | Collagen |
| EOs | Essential oils |
| PVP-I | Povidone-iodine |
| VEGF | Vascular endothelial growth factor |
| CHG | Chlorhexidine gluconate |
| SEM | Scanning electron microscopy |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| AgNPs | Silver nanoparticles |
References
- Robson, M.C.; Steed, D.L.; Franz, M.G. Wound Healing: Biologic Features and Approaches to Maximize Healing Trajectories. Curr. Probl. Surg. 2001, 38, A1–A140. [Google Scholar] [CrossRef] [PubMed]
- Las Heras, K.; Igartua, M.; Santos-Vizcaino, E.; Hernandez, R.M. Chronic Wounds: Current Status, Available Strategies and Emerging Therapeutic Solutions. J. Control. Release 2020, 328, 532–550. [Google Scholar] [CrossRef] [PubMed]
- Eming, S.A.; Hammerschmidt, M.; Krieg, T.; Roers, A. Interrelation of Immunity and Tissue Repair or Regeneration. Semin. Cell Dev. Biol. 2009, 20, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Fonder, M.A.; Lazarus, G.S.; Cowan, D.A.; Aronson-Cook, B.; Kohli, A.R.; Mamelak, A.J. Treating the Chronic Wound: A Practical Approach to the Care of Nonhealing Wounds and Wound Care Dressings. J. Am. Acad. Dermatol. 2008, 58, 185–206. [Google Scholar] [CrossRef]
- Han, G.; Ceilley, R. Chronic Wound Healing: A Review of Current Management and Treatments. Adv. Ther. 2017, 34, 599–610. [Google Scholar] [CrossRef]
- Thomas, S.; Uzun, M. Testing Dressings and Wound Management Materials. In Advanced Textiles for Wound Care; Elsevier: Amsterdam, The Netherlands, 2019; pp. 23–54. ISBN 978-0-08-102192-7. [Google Scholar]
- Brumberg, V.; Astrelina, T.; Malivanova, T.; Samoilov, A. Modern Wound Dressings: Hydrogel Dressings. Biomedicines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Pranantyo, D.; Yeo, C.K.; Wu, Y.; Fan, C.; Xu, X.; Yip, Y.S.; Vos, M.I.G.; Mahadevegowda, S.H.; Lim, P.L.K.; Yang, L.; et al. Hydrogel Dressings with Intrinsic Antibiofilm and Antioxidative Dual Functionalities Accelerate Infected Diabetic Wound Healing. Nat. Commun. 2024, 15, 954. [Google Scholar] [CrossRef]
- Popovich, K.; Tohm, P.; Hurd, T. Skin and Wound Care Excellence: Integrating Best-Practice Evidence. Healthc. Q 2010, 13, 42–46. [Google Scholar] [CrossRef][Green Version]
- Collignon, P. Clinical Impact of Antimicrobial Resistance in Humans. Rev. Sci. Tech. OIE 2012, 31, 211–220. [Google Scholar] [CrossRef]
- Yang, C.; Yang, C.; Chen, Y.; Liu, J.; Liu, Z.; Chen, H.-J. The Trends in Wound Management: Sensing, Therapeutic Treatment, and “Theranostics”. J. Sci. Adv. Mater. Devices 2023, 8, 100619. [Google Scholar] [CrossRef]
- Bowler, P.G. Antibiotic Resistance and Biofilm Tolerance: A Combined Threat in the Treatment of Chronic Infections. J. Wound Care 2018, 27, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C.; Teot, L.; Vanscheidt, W. Wound Bed Preparation: A Systematic Approach to Wound Management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Kirketerp-Møller, K.; Jensen, P.Ø.; Fazli, M.; Madsen, K.G.; Pedersen, J.; Moser, C.; Tolker-Nielsen, T.; Høiby, N.; Givskov, M.; Bjarnsholt, T. Distribution, Organization, and Ecology of Bacteria in Chronic Wounds. J. Clin. Microbiol. 2008, 46, 2717–2722. [Google Scholar] [CrossRef] [PubMed]
- Awuor, S.O.; Omwenga, E.O.; Mariita, R.M.; Musila, J.M.; Musyoki, S. Monitoring the Battleground: Exploring Antimicrobial Resistance and Virulence Factors in Wound Bacterial Isolates. Access Microbiol. 2023, 5, 000613-v6. [Google Scholar] [CrossRef]
- Howell-Jones, R.S.; Wilson, M.J.; Hill, K.E.; Howard, A.J.; Price, P.E.; Thomas, D.W. A Review of the Microbiology, Antibiotic Usage and Resistance in Chronic Skin Wounds. J. Antimicrob. Chemother. 2005, 55, 143–149. [Google Scholar] [CrossRef]
- Wolcott, R.D.; Hanson, J.D.; Rees, E.J.; Koenig, L.D.; Phillips, C.D.; Wolcott, R.A.; Cox, S.B.; White, J.S. Analysis of the Chronic Wound Microbiota of 2,963 Patients by 16S rDNA Pyrosequencing. Wound Repair Regen. 2016, 24, 163–174. [Google Scholar] [CrossRef]
- Smith, D.M.; Snow, D.E.; Rees, E.; Zischkau, A.M.; Hanson, J.D.; Wolcott, R.D.; Sun, Y.; White, J.; Kumar, S.; Dowd, S.E. Evaluation of the Bacterial Diversity of Pressure Ulcers Using bTEFAP Pyrosequencing. BMC Med. Genom. 2010, 3, 41. [Google Scholar] [CrossRef]
- Kolar, M.; Cermak, P.; Hobzova, L.; Bogdanova, K.; Neradova, K.; Mlynarcik, P.; Bostik, P. Antibiotic Resistance in Nosocomial Bacteria Isolated from Infected Wounds of Hospitalized Patients in Czech Republic. Antibiotics 2020, 9, 342. [Google Scholar] [CrossRef]
- Filius, P.M.; Gyssens, I.C. Impact of Increasing Antimicrobial Resistance on Wound Management. Am. J. Clin. Dermatol. 2002, 3, 1–7. [Google Scholar] [CrossRef]
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable Deaths and Disability-Adjusted Life-Years Caused by Infections with Antibiotic-Resistant Bacteria in the EU and the European Economic Area in 2015: A Population-Level Modelling Analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef]
- Edwards-Jones, V. Antimicrobial Stewardship in Wound Care. Br. J. Nurs. 2020, 29, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Guest, J.F.; Ayoub, N.; McIlwraith, T.; Uchegbu, I.; Gerrish, A.; Weidlich, D.; Vowden, K.; Vowden, P. Health Economic Burden That Different Wound Types Impose on the UK ’s National Health Service. Int. Wound J. 2017, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Woodmansey, E.J.; Roberts, C.D. Appropriate Use of Dressings Containing Nanocrystalline Silver to Support Antimicrobial Stewardship in Wounds. Int. Wound J. 2018, 15, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Chin, J.D.; Zhao, L.; Mayberry, T.G.; Cowan, B.C.; Wakefield, M.R.; Fang, Y. Photodynamic Therapy, Probiotics, Acetic Acid, and Essential Oil in the Treatment of Chronic Wounds Infected with Pseudomonas aeruginosa. Pharmaceutics 2023, 15, 1721. [Google Scholar] [CrossRef]
- Pang, Q.; Yang, F.; Jiang, Z.; Wu, K.; Hou, R.; Zhu, Y. Smart Wound Dressing for Advanced Wound Management: Real-Time Monitoring and on-Demand Treatment. Mater. Des. 2023, 229, 111917. [Google Scholar] [CrossRef]
- Pirouzzadeh, M.; Moraffah, F.; Samadi, N.; Sharifzadeh, M.; Motasadizadeh, H.; Vatanara, A. Enhancement of Burn Wound Healing Using Optimized Bioactive Probiotic-Loaded Alginate Films. Int. J. Biol. Macromol. 2025, 301, 140454. [Google Scholar] [CrossRef]
- Wolcott, R. Are Chronic Wounds, Chronic Infections? J. Wound Care 2016, 25, S3. [Google Scholar] [CrossRef]
- Hurlow, J.; Bowler, P.G. Acute and Chronic Wound Infections: Microbiological, Immunological, Clinical and Therapeutic Distinctions. J. Wound Care 2022, 31, 436–445. [Google Scholar] [CrossRef]
- Percival, S.L.; McCarty, S.M.; Lipsky, B. Biofilms and Wounds: An Overview of the Evidence. Adv. Wound Care 2015, 4, 373–381. [Google Scholar] [CrossRef]
- Sen, C.K.; Roy, S.; Mathew-Steiner, S.S.; Gordillo, G.M. Biofilm Management in Wound Care. Plast. Reconstr. Surg. 2021, 148, 275e–288e. [Google Scholar] [CrossRef]
- Zhao, G.; Usui, M.L.; Lippman, S.I.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Biofilms and Inflammation in Chronic Wounds. Adv. Wound Care 2013, 2, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Watters, C.; Fleming, D.; Bishop, D.; Rumbaugh, K.P. Host Responses to Biofilm. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2016; Volume 142, pp. 193–239. ISBN 978-0-12-809385-6. [Google Scholar]
- Verderosa, A.D.; Totsika, M.; Fairfull-Smith, K.E. Bacterial Biofilm Eradication Agents: A Current Review. Front. Chem. 2019, 7, 824. [Google Scholar] [CrossRef] [PubMed]
- Huemer, M.; Mairpady Shambat, S.; Brugger, S.D.; Zinkernagel, A.S. Antibiotic Resistance and Persistence—Implications for Human Health and Treatment Perspectives. EMBO Rep. 2020, 21, e51034. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, R.; Shukla, A. Highly Sensitive Label-Free Dual Sensor Array for Rapid Detection of Wound Bacteria. Biosens. Bioelectron. 2017, 92, 425–433. [Google Scholar] [CrossRef]
- Magee, E.; Yusufu, D.; Rice, C.J.; Skvortsov, T.; Mills, A.; Gilmore, B.F. A Smart Sensor for Monitoring Antimicrobial Interventions in Wound Infections. Sens. Actuators B Chem. 2024, 405, 135179. [Google Scholar] [CrossRef]
- Fang, H.; Wang, J.; Li, L.; Xu, L.; Wu, Y.; Wang, Y.; Fei, X.; Tian, J.; Li, Y. A Novel High-Strength Poly(ionic liquid)/PVA Hydrogel Dressing for Antibacterial Applications. Chem. Eng. J. 2019, 365, 153–164. [Google Scholar] [CrossRef]
- Zhou, J.; Yao, D.; Qian, Z.; Hou, S.; Li, L.; Jenkins, A.T.A.; Fan, Y. Bacteria-Responsive Intelligent Wound Dressing: Simultaneous In Situ Detection and Inhibition of Bacterial Infection for Accelerated Wound Healing. Biomaterials 2018, 161, 11–23. [Google Scholar] [CrossRef]
- Huang, T.; Sun, Z.; Heath, D.E.; O’Brien-Simpson, N.; O’Connor, A.J. 3D Printed and Smart Alginate Wound Dressings with pH-Responsive Drug and Nanoparticle Release. Chem. Eng. J. 2024, 492, 152117. [Google Scholar] [CrossRef]
- Huang, Y.; Mu, L.; Zhao, X.; Han, Y.; Guo, B. Bacterial Growth-Induced Tobramycin Smart Release Self-Healing Hydrogel for Pseudomonas aeruginosa-Infected Burn Wound Healing. ACS Nano 2022, 16, 13022–13036. [Google Scholar] [CrossRef]
- Yang, J.; He, Y.; Li, Z.; Yang, X.; Gao, Y.; Chen, M.; Zheng, Y.; Mao, S.; Shi, X. Intelligent Wound Dressing for Simultaneous in Situ Detection and Elimination of Pathogenic Bacteria. Acta Biomater. 2024, 174, 177–190. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, M.; Zhao, S.; Li, H.; Wang, W.; Cheng, J.; Jin, L.; Wang, Y. Janus Amphiphilic Nanofiber Membranes Synergistically Drive Antibacterial and Anti-Inflammatory Strategies for Skin Wound Healing. Mater. Des. 2023, 227, 111778. [Google Scholar] [CrossRef]
- Hu, S.; Cai, X.; Qu, X.; Yu, B.; Yan, C.; Yang, J.; Li, F.; Zheng, Y.; Shi, X. Preparation of Biocompatible Wound Dressings with Long-Term Antimicrobial Activity through Covalent Bonding of Antibiotic Agents to Natural Polymers. Int. J. Biol. Macromol. 2019, 123, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Zenati, F.; Benbelaid, F.; Khadir, A.; Bellahsene, C.; Bendahou, M. Antimicrobial Effects of Three Essential Oils on Multidrug Resistant Bacteria Responsible for Urinary Infections. J. App. Pharm. Sci. 2014, 4, 15–18. [Google Scholar] [CrossRef]
- Cheung, H.-Y.; Wong, M.M.-K.; Cheung, S.-H.; Liang, L.Y.; Lam, Y.-W.; Chiu, S.-K. Differential Actions of Chlorhexidine on the Cell Wall of Bacillus subtilis and Escherichia coli. PLoS ONE 2012, 7, e36659. [Google Scholar] [CrossRef] [PubMed]
- Kapanya, A.; Somsunan, R.; Molloy, R.; Jiranusornkul, S.; Leewattanapasuk, W.; Jongpaiboonkit, L.; Kong, Y. Synthesis of Polymeric Hydrogels Incorporating Chlorhexidine Gluconate as Antibacterial Wound Dressings. J. Biomater. Sci. Polym. Ed. 2020, 31, 895–909. [Google Scholar] [CrossRef]
- Shi, S.-S.; Lei, S.-J.; Fu, C. Studies of the Properties of CHG-Loaded Alginate Fibers for Medical Application. Polym. Test. 2020, 83, 106141. [Google Scholar] [CrossRef]
- Składanowski, M.; Golinska, P.; Rudnicka, K.; Dahm, H.; Rai, M. Evaluation of Cytotoxicity, Immune Compatibility and Antibacterial Activity of Biogenic Silver Nanoparticles. Med. Microbiol. Immunol. 2016, 205, 603–613. [Google Scholar] [CrossRef]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented Silver Resistance in Clinically Isolated Enterobacteriaceae: Major Implications for Burn and Wound Management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef]
- Krishnan, N.; Velramar, B.; Ramatchandirin, B.; Abraham, G.C.; Duraisamy, N.; Pandiyan, R.; Velu, R.K. Effect of Biogenic Silver Nanocubes on Matrix Metalloproteinases 2 and 9 Expressions in Hyperglycemic Skin Injury and Its Impact in Early Wound Healing in Streptozotocin-Induced Diabetic Mice. Mater. Sci. Eng. C 2018, 91, 146–152. [Google Scholar] [CrossRef]
- Prader, J.; Rumpler, M.; Kamolz, L.P.; Hajnsek, M. Myeloperoxidase-Based in-Vitro Test Strip Sensor for Early Detection of Wound Infections at the Patient’s Bedside. Sens. Actuators B Chem. 2022, 372, 132628. [Google Scholar] [CrossRef]
- Thirabowonkitphithan, P.; Phuengmaung, P.; Leelahavanichkul, A.; Laiwattanapaisal, W. MWCNTs/PVA Hydrogel-Modified Electrochemical Sensors for Ex Vivo and In Vivo Detection of Pyocyanin Biomarker for Pseudomonas aeruginosa Wound Infection. ACS Appl. Electron. Mater. 2023, 5, 821–831. [Google Scholar] [CrossRef]
- Sismaet, H.J.; Banerjee, A.; McNish, S.; Choi, Y.; Torralba, M.; Lucas, S.; Chan, A.; Shanmugam, V.K.; Goluch, E.D. Electrochemical Detection of Pseudomonas in Wound Exudate Samples from Patients with Chronic Wounds. Wound Repair Regen. 2016, 24, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhang, Z.; Li, Y.; Xie, G. Amplified Electrochemical Detection of mecA Gene in Methicillin-Resistant Staphylococcus aureus Based on Target Recycling Amplification and Isothermal Strand-Displacement Polymerization Reaction. Sens. Actuators B Chem. 2015, 221, 148–154. [Google Scholar] [CrossRef]
- Mirani, B.; Pagan, E.; Currie, B.; Siddiqui, M.A.; Hosseinzadeh, R.; Mostafalu, P.; Zhang, Y.S.; Ghahary, A.; Akbari, M. An Advanced Multifunctional Hydrogel-Based Dressing for Wound Monitoring and Drug Delivery. Adv. Healthc. Mater. 2017, 6, 1700718. [Google Scholar] [CrossRef]
- Sta. Agueda, J.R.; Lim, J.; Mondragon, J.M.; Madrid, J.; Belen, M.G.; Eustaquio, G.M.Y.; Monjardin, J.G.; Salud, N. Rapid Prototyping of a Temperature, Humidity, and Pressure Monitor Electronic Layer for Pressure Ulcer Wound Patch. J. Phys. Conf. Ser. 2021, 2071, 012024. [Google Scholar] [CrossRef]
- Hickle, K.; Slamin, R.; Baez, A.; Sen, D.; Evan-Browning, E.; Tessier, H.; Mendelson, Y.; McNeill, J.; Dunn, R. Wireless Pressure Ulcer Sensor: Validation in an Animal Model. Ann. Plast. Surg. 2019, 82, S215–S221. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound Healing Dressings and Drug Delivery Systems: A Review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Farahani, M.; Shafiee, A. Wound Healing: From Passive to Smart Dressings. Adv. Healthc. Mater. 2021, 10, 2100477. [Google Scholar] [CrossRef]
- Borda, L.J.; Macquhae, F.E.; Kirsner, R.S. Wound Dressings: A Comprehensive Review. Curr. Derm. Rep. 2016, 5, 287–297. [Google Scholar] [CrossRef]
- Ghanim, Z.; Alkotaji, M.; Qazzaz, M. Insight into Topical Preparations for Wound Healing: Traditional and Modern Dressings. Al-Anbar Med. J. AMJ 2023, 19, 89–97. [Google Scholar] [CrossRef]
- Gao, Y.; Elhadad, A.; Choi, S. Janus Paper-Based Wound Dressings for Effective Exudate Absorption and Antibiotic Delivery. Adv. Eng. Mater. 2024, 26, 2301422. [Google Scholar] [CrossRef]
- Slater, M. Does Moist Wound Healing Influence the Rate of Infection? Br. J. Nurs. 2008, 17, S4–S15. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, C.; Hu, J.; Liu, C.; Ning, Y.; Lu, F. Current Strategies for Monitoring and Controlling Bacterial Biofilm Formation on Medical Surfaces. Ecotoxicol. Environ. Saf. 2024, 282, 116709. [Google Scholar] [CrossRef]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound Microbiology and Associated Approaches to Wound Management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef]
- Lin, P.; He, K.; Luo, J.; Wang, J.; Liu, Y.; Zhang, J.; Fan, H.; Huang, S.; Lan, W.; Wang, W.; et al. Multifunctional Nanofiber System with Photothermal-Controlled Drug Delivery and Motion Monitoring Capabilities as Intelligent Wound Dressing. Chem. Eng. J. 2025, 503, 158544. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef]
- Ventola, C.L. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Jones, V.; Grey, J.E.; Harding, K.G. Wound Dressings. BMJ 2006, 332, 777–780. [Google Scholar] [CrossRef]
- Li, F.; Qin, Y.; Lee, J.; Liao, H.; Wang, N.; Davis, T.P.; Qiao, R.; Ling, D. Stimuli-Responsive Nano-Assemblies for Remotely Controlled Drug Delivery. J. Control. Release 2020, 322, 566–592. [Google Scholar] [CrossRef]
- Jia, X.; Dou, Z.; Zhang, Y.; Li, F.; Xing, B.; Hu, Z.; Li, X.; Liu, Z.; Yang, W.; Liu, Z. Smart Responsive and Controlled-Release Hydrogels for Chronic Wound Treatment. Pharmaceutics 2023, 15, 2735. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver Nanoparticles: The Powerful Nanoweapon against Multidrug-Resistant Bacteria: Activity of Silver Nanoparticles against MDR Bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano Based Drug Delivery Systems: Recent Developments and Future Prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef]
- Cedillo-Cortezano, M.; Martinez-Cuevas, L.R.; López, J.A.M.; Barrera López, I.L.; Escutia-Perez, S.; Petricevich, V.L. Use of Medicinal Plants in the Process of Wound Healing: A Literature Review. Pharmaceuticals 2024, 17, 303. [Google Scholar] [CrossRef] [PubMed]
- Majtan, J. Honey: An Immunomodulator in Wound Healing. Wound Repair Regen. 2014, 22, 187–192. [Google Scholar] [CrossRef]
- Meuleneire, F. A Vapour-Permeable Film Dressing Used on Superficial Wounds. Br. J. Nurs. 2014, 23, S36–S43. [Google Scholar] [CrossRef] [PubMed]
- Ottaviano, L.; Buoso, S.; Zamboni, R.; Sotgiu, G.; Posati, T. Natural Protein Films from Textile Waste for Wound Healing and Wound Dressing Applications. J. Funct. Biomater. 2025, 16, 20. [Google Scholar] [CrossRef]
- Li, X.; Yang, X.; Wang, Z.; Liu, Y.; Guo, J.; Zhu, Y.; Shao, J.; Li, J.; Wang, L.; Wang, K. Antibacterial, Antioxidant and Biocompatible Nanosized Quercetin-PVA Xerogel Films for Wound Dressing. Colloids Surf. B Biointerfaces 2022, 209, 112175. [Google Scholar] [CrossRef]
- Kim, W.I.; Ko, Y.-G.; Park, M.R.; Jung, K.H.; Kwon, O.H. Preparation and Characterization of Polyurethane Foam Dressings Containing Natural Antimicrobial Agents for Wound Healing. Polymer 2018, 42, 806–812. [Google Scholar] [CrossRef]
- Li, J.; Xu, X.; Ma, X.; Cui, M.; Wang, X.; Chen, J.; Zhu, J.; Chen, J. Antimicrobial Nonisocyanate Polyurethane Foam Derived from Lignin for Wound Healing. ACS Appl. Bio Mater. 2024, 7, 1301–1310. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, P.; Gordon, I.; Chao, J.H.; Zehtabchi, S. A Systematic Review of Foam Dressings for Partial Thickness Burns. Am. J. Emerg. Med. 2019, 37, 1184–1190. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Ma, X.; Qiu, S.; Chen, J.; Lu, G.; Jia, Z.; Zhu, J.; Yang, Q.; Chen, J.; et al. Antimicrobial Lignin-Based Polyurethane/Ag Composite Foams for Improving Wound Healing. Biomacromolecules 2022, 23, 1622–1632. [Google Scholar] [CrossRef] [PubMed]
- Boyar, V. Successful Management of Complex Pediatric and Neonatal Wounds With Methylene Blue and Gentian Violet Foam Dressings. Wounds 2021, 33, 253–259. [Google Scholar] [CrossRef]
- Kamińska, M.S.; Cybulska, A.M.; Skonieczna-Żydecka, K.; Augustyniuk, K.; Grochans, E.; Karakiewicz, B. Effectiveness of Hydrocolloid Dressings for Treating Pressure Ulcers in Adult Patients: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 7881. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.J.; Kim, J.-H.; Moon, B.M.; Chao, J.R.; Yoon, J.; Ju, H.W.; Lee, J.M.; Park, H.J.; Kim, D.W.; Kim, S.J.; et al. Fabrication and Characterization of Hydrocolloid Dressing with Silk Fibroin Nanoparticles for Wound Healing. Tissue Eng. Regen. Med. 2016, 13, 218–226. [Google Scholar] [CrossRef]
- Jafari, D.; Moosazadeh Moghaddam, M.; Fallah Tafti, M.; Mirnejad, R. Fabrication of an Antibacterial Hydrocolloid Dressing for the Management of Wound Infection Caused by Antibiotic-Resistant Bacteria: In Vitro Study. Mater. Today Commun. 2025, 42, 111165. [Google Scholar] [CrossRef]
- Yanagibayashi, S.; Kishimoto, S.; Ishihara, M.; Murakami, K.; Aoki, H.; Takikawa, M.; Fujita, M.; Sekido, M.; Kiyosawa, T. Novel Hydrocolloid-Sheet as Wound Dressing to Stimulate Healing-Impaired Wound Healing in Diabetic Db/Db Mice. Bio-Med. Mater. Eng. 2012, 22, 301–310. [Google Scholar] [CrossRef]
- Abraham, S.; Harsha, G.G.S.; Desai, K.; Furtado, S.; Srinivasan, B. Nano Calcium Oxide Incorporated Hydrocolloid Dressings for Wound Care. J. Pharm. Innov. 2022, 17, 215–226. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef]
- Bashir, S.; Hina, M.; Iqbal, J.; Rajpar, A.H.; Mujtaba, M.A.; Alghamdi, N.A.; Wageh, S.; Ramesh, K.; Ramesh, S. Fundamental Concepts of Hydrogels: Synthesis, Properties, and Their Applications. Polymers 2020, 12, 2702. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Feng, X.; Pei, Y.; Wang, J.; Ding, J.; Chen, L. α-Cyclodextrin Concentration-Controlled Thermo-Sensitive Supramolecular Hydrogels. Mater. Sci. Eng. C 2018, 82, 25–28. [Google Scholar] [CrossRef]
- Li, H.; Yang, J.; Hu, X.; Liang, J.; Fan, Y.; Zhang, X. Superabsorbent Polysaccharide Hydrogels Based on Pullulan Derivate as Antibacterial Release Wound Dressing. J. Biomed. Mater. Res. 2011, 98A, 31–39. [Google Scholar] [CrossRef]
- Keshvardoostchokami, M.; Majidi, S.S.; Huo, P.; Ramachandran, R.; Chen, M.; Liu, B. Electrospun Nanofibers of Natural and Synthetic Polymers as Artificial Extracellular Matrix for Tissue Engineering. Nanomaterials 2020, 11, 21. [Google Scholar] [CrossRef]
- AL-Jbour, N.D.; Beg, M.D.; Gimbun, J.; Alam, A.K.M.M. An Overview of Chitosan Nanofibers and Their Applications in the Drug Delivery Process. Curr. Drug Deliv. 2019, 16, 272–294. [Google Scholar] [CrossRef]
- Jayakumar, A.; Radoor, S.; Kim, J.T.; Rhim, J.W.; Parameswaranpillai, J.; Siengchin, S. Functionalized Nanofiber for Wound Healing and Wound Dressing Applications. In Functionalized Nanofibers; Elsevier: Amsterdam, The Netherlands, 2023; pp. 253–276. ISBN 978-0-323-99461-3. [Google Scholar]
- Zhou, Z.; Li, C.; Zeng, Y.; Huang, T.; Jiang, X.; Yu, D.-G.; Wang, K. Natural Polymer Nanofiber Dressings for Effective Management of Chronic Diabetic Wounds: A Comprehensive Review. Int. J. Biol. Macromol. 2024, 282, 136688. [Google Scholar] [CrossRef]
- Kannon, G.A.; Garrett, A.B. Moist Wound Healing with Occlusive Dressings: A Clinical Review. Dermatol. Surg. 1995, 21, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Firlar, I.; Altunbek, M.; McCarthy, C.; Ramalingam, M.; Camci-Unal, G. Functional Hydrogels for Treatment of Chronic Wounds. Gels 2022, 8, 127. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; San Miguel, L. Are Modern Wound Dressings a Clinical and Cost-Effective Alternative to the Use of Gauze? J. Wound Care 2006, 15, 65–69. [Google Scholar] [CrossRef]
- Thomas, S. Hydrocolloid Dressings in the Management of Acute Wounds: A Review of the Literature. Int. Wound J. 2008, 5, 602–613. [Google Scholar] [CrossRef]
- Nguyen, N.; Dulai, A.S.; Adnan, S.; Khan, Z.; Sivamani, R.K. Narrative Review of the Use of Hydrocolloids in Dermatology: Applications and Benefits. J. Clin. Med. 2025, 14, 1345. [Google Scholar] [CrossRef]
- Sibbald, R.G.; Coutts, P.; Woo, K.Y. Reduction of Bacterial Burden and Pain in Chronic Wounds Using a New Polyhexamethylene Biguanide Antimicrobial Foam Dressing-Clinical Trial Results. Adv. Ski. Wound Care 2011, 24, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Chaiarwut, S.; Choipang, C.; Sangsanoh, P.; Niyompanich, J.; Supaphol, P. Using Natural Extracts to Promote the Antibacterial and Anti-Inflammatory Performance of Polyurethane Foams. J. Polym. Environ. 2023, 31, 1668–1678. [Google Scholar] [CrossRef]
- Hunter, A.M.; Grigson, C.; Wade, A. Influence of Topically Applied Menthol Cooling Gel on Soft Tissue Thermodynamics and Arterial and Cutaneous Blood Flow at Rest. Int. J. Sports Phys. Ther. 2018, 13, 483–492. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Chan, J.M.W.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial Hydrogels: A New Weapon in the Arsenal against Multidrug-Resistant Infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, W.; Xu, Q.; Zheng, Y. Progress in Antibacterial Hydrogel Dressing. Gels 2022, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, D.; Li, Y.; Zhou, X.; Hui, Z.; Lei, X.; Qiu, L.; Bai, Y.; Wang, C.; Xia, J.; et al. Collagen Hydrogel with Multiple Antimicrobial Mechanisms as Anti-Bacterial Wound Dressing. Int. J. Biol. Macromol. 2023, 232, 123413. [Google Scholar] [CrossRef]
- Jiang, S.; Deng, J.; Jin, Y.; Qian, B.; Lv, W.; Zhou, Q.; Mei, E.; Neisiany, R.E.; Liu, Y.; You, Z.; et al. Breathable, Antifreezing, Mechanically Skin-like Hydrogel Textile Wound Dressings with Dual Antibacterial Mechanisms. Bioact. Mater. 2023, 21, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xu, H.; Zhang, M.; Yu, D.-G. Electrospun Medicated Nanofibers for Wound Healing: Review. Membranes 2021, 11, 770. [Google Scholar] [CrossRef]
- Clark, M. Alginates in Dressings and Wound Management. In Alginates and Their Biomedical Applications; Rehm, B.H.A., Moradali, M.F., Eds.; Springer Series in Biomaterials Science and Engineering; Springer: Singapore, 2018; Volume 11, pp. 213–222. ISBN 978-981-10-6909-3. [Google Scholar]
- Berechet, M.D.; Gaidau, C.; Miletic, A.; Pilic, B.; Râpă, M.; Stanca, M.; Ditu, L.-M.; Constantinescu, R.; Lazea-Stoyanova, A. Bioactive Properties of Nanofibres Based on Concentrated Collagen Hydrolysate Loaded with Thyme and Oregano Essential Oils. Materials 2020, 13, 1618. [Google Scholar] [CrossRef]
- Chen, H.; Shen, Y.; Zhang, H.; Long, X.; Deng, K.; Xu, T.; Li, Y. Clinical Application of Polylactic Acid/Gelatin Nanofibre Membrane in Hard-to-Heal Lower Extremity Venous Ulcers. J. Wound Care 2022, 31, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Yukseloglu, S.M.; Sokmen, N.; Canoglu, S. Biomaterial Applications of Silk Fibroin Electrospun Nanofibres. Microelectron. Eng. 2015, 146, 43–47. [Google Scholar] [CrossRef]
- Sahoo, R.; Dash, B.P.; Panda, P.K. Polyacrylonitrile and Polylactic Acid Blend Nanofibre Spinning Using Needleless Electrospinning Technique. Indian J. Fibre Text. Res. (IJFTR) 2023, 48, 117–123. [Google Scholar] [CrossRef]
- Ma, W.; Zhou, M.; Dong, W.; Zhao, S.; Wang, Y.; Yao, J.; Liu, Z.; Han, H.; Sun, D.; Zhang, M. A Bi-Layered Scaffold of a Poly(lactic-co-glycolic acid) Nanofiber Mat and an Alginate–Gelatin Hydrogel for Wound Healing. J. Mater. Chem. B 2021, 9, 7492–7505. [Google Scholar] [CrossRef]
- Jirkovec, R.; Erben, J.; Samkova, A.; Chaloupek, J.; Chvojka, J. The Effect of the Electrospinning Setup on the Surface Energy of Polycaprolactone Nanofibre Layers. J. Ind. Text. 2022, 51, 8517S–8527S. [Google Scholar] [CrossRef]
- Fertah, M.; Belfkira, A.; Dahmane, E.M.; Taourirte, M.; Brouillette, F. Extraction and Characterization of Sodium Alginate from Moroccan Laminaria digitata Brown Seaweed. Arab. J. Chem. 2017, 10, S3707–S3714. [Google Scholar] [CrossRef]
- Yang, C.H.; Wang, M.X.; Haider, H.; Yang, J.H.; Sun, J.-Y.; Chen, Y.M.; Zhou, J.; Suo, Z. Strengthening Alginate/Polyacrylamide Hydrogels Using Various Multivalent Cations. ACS Appl. Mater. Interfaces 2013, 5, 10418–10422. [Google Scholar] [CrossRef]
- Topuz, F.; Henke, A.; Richtering, W.; Groll, J. Magnesium Ions and Alginate Do Form Hydrogels: A Rheological Study. Soft Matter 2012, 8, 4877. [Google Scholar] [CrossRef]
- Aderibigbe, B.; Buyana, B. Alginate in Wound Dressings. Pharmaceutics 2018, 10, 42. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial Biohybrid Nanofibers for Wound Dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Lu, X.; Zhou, L.; Song, W. Recent Progress of Electrospun Nanofiber Dressing in the Promotion of Wound Healing. Polymers 2024, 16, 2596. [Google Scholar] [CrossRef] [PubMed]
- Hodaei, H.; Esmaeili, Z.; Erfani, Y.; Esnaashari, S.S.; Geravand, M.; Adabi, M. Preparation of Biocompatible Zein/Gelatin/Chitosan/PVA Based Nanofibers Loaded with Vitamin E-TPGS via Dual-Opposite Electrospinning Method. Sci. Rep. 2024, 14, 23796. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Durán, N.; Durán, M.; De Jesus, M.B.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver Nanoparticles: A New View on Mechanistic Aspects on Antimicrobial Activity. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 789–799. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer—Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef]
- Sill, T.J.; Von Recum, H.A. Electrospinning: Applications in Drug Delivery and Tissue Engineering. Biomaterials 2008, 29, 1989–2006. [Google Scholar] [CrossRef]
- Ousey, K.; Rippon, M.G.; Rogers, A.A.; Totty, J.P. Considerations for an Ideal Post-Surgical Wound Dressing Aligned with Antimicrobial Stewardship Objectives: A Scoping Review. J. Wound Care 2023, 32, 334–347. [Google Scholar] [CrossRef]
- Bagherabadi, M.; Feuilloley, C.; Cameron, P.J.; Andrieu-Brunsen, A. Simultaneous Bacteria Sensing and On-Demand Antimicrobial Peptide Release. ACS Appl. Bio Mater. 2025, 8, 2365–2376. [Google Scholar] [CrossRef] [PubMed]
- Derakhshandeh, H.; Aghabaglou, F.; McCarthy, A.; Mostafavi, A.; Wiseman, C.; Bonick, Z.; Ghanavati, I.; Harris, S.; Kreikemeier-Bower, C.; Moosavi Basri, S.M.; et al. A Wirelessly Controlled Smart Bandage with 3D-Printed Miniaturized Needle Arrays. Adv. Funct. Mater. 2020, 30, 1905544. [Google Scholar] [CrossRef]
- Tang, N.; Zheng, Y.; Cui, D.; Haick, H. Multifunctional Dressing for Wound Diagnosis and Rehabilitation. Adv. Healthc. Mater. 2021, 10, 2101292. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and Future Directions in the Antibacterial Wound Dressings—A Review. J. Biomed. Mater. Res. 2021, 109, 703–716. [Google Scholar] [CrossRef]
- Pusta, A.; Tertiș, M.; Cristea, C.; Mirel, S. Wearable Sensors for the Detection of Biomarkers for Wound Infection. Biosensors 2021, 12, 1. [Google Scholar] [CrossRef]
- Mota, F.A.R.; Pereira, S.A.P.; Araújo, A.R.T.S.; Passos, M.L.C.; Saraiva, M.L.M.F.S. Biomarkers in the Diagnosis of Wounds Infection: An Analytical Perspective. TrAC Trends Anal. Chem. 2021, 143, 116405. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Xiao, J.; Nie, C. Conformable Electrochemical Devices for Closed-Loop Wound Management. Front. Bioeng. Biotechnol. 2023, 11, 1331567. [Google Scholar] [CrossRef]
- Chen, Z.; Dong, Y.; Fu, J.; Bai, Y.; Gao, Q.; Qin, Z.; Wang, J.; Li, S. Collaborative Biofluid Analysis Based Multi-Channel Integrated Wearable Detection System for the Monitoring of Wound Infection. Biosens. Bioelectron. X 2024, 17, 100443. [Google Scholar] [CrossRef]
- Permpoka, K.; Purinai, P.; Cheerasiri, C.; Rojpalakorn, W.; Nilaratanakul, V.; Laiwattanapaisal, W. Smartphone-Enabled 3D Origami-Fluidic Paper-Based Electrochemical Detection of Myeloperoxidase Activity for Assessing Wound Infection. Sens. Actuators B Chem. 2024, 398, 134712. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, Y.; Ma, C.; Yuan, Q.; Wang, X.; Wan, H.; Wang, P. A Review of Recent Advances in Flexible Wearable Sensors for Wound Detection Based on Optical and Electrical Sensing. Biosensors 2021, 12, 10. [Google Scholar] [CrossRef]
- Landsman, A.S.; Barnhart, D.; Sowa, M. Near-Infrared Spectroscopy Imaging for Assessing Skin and Wound Oxygen Perfusion. Clin. Podiatr. Med. Surg. 2018, 35, 343–355. [Google Scholar] [CrossRef]
- Li, Q.; Qi, P.; Wang, Y.; Fu, S.; Zhang, H.; Li, S.; Wang, L.; He, C.; Chen, S.; Hou, P. Rapid-Response near-Infrared Fluorescence Probe for Colorimetric Detection of HClO and Its Applications in Environmental Monitoring and Biological Imaging. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2024, 320, 124613. [Google Scholar] [CrossRef]
- Rasheed, S.; Kanwal, T.; Ahmad, N.; Fatima, B.; Najam-ul-Haq, M.; Hussain, D. Advances and Challenges in Portable Optical Biosensors for Onsite Detection and Point-of-Care Diagnostics. TrAC Trends Anal. Chem. 2024, 173, 117640. [Google Scholar] [CrossRef]
- Casey, V.; McAree, B.; Moloney, M.C.; Grace, P. Wearable Sub-Bandage Pressure Measurement System. In Proceedings of the 2010 IEEE Sensors Applications Symposium (SAS), Limerick, Ireland, 23–25 February 2010; IEEE: Limerick, Ireland, 2010; pp. 41–45. [Google Scholar]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and Imaging for Wound Healing: A Review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef]
- Xiao, Y.; Lai, R.Y.; Plaxco, K.W. Preparation of Electrode-Immobilized, Redox-Modified Oligonucleotides for Electrochemical DNA and Aptamer-Based Sensing. Nat. Protoc. 2007, 2, 2875–2880. [Google Scholar] [CrossRef]
- Saghazadeh, S.; Rinoldi, C.; Schot, M.; Kashaf, S.S.; Sharifi, F.; Jalilian, E.; Nuutila, K.; Giatsidis, G.; Mostafalu, P.; Derakhshandeh, H.; et al. Drug Delivery Systems and Materials for Wound Healing Applications. Adv. Drug Deliv. Rev. 2018, 127, 138–166. [Google Scholar] [CrossRef]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Butcher, M. Introducing a New Paradigm for Bioburden Management. J. Wound Care 2011, 20, 4–19. [Google Scholar] [CrossRef]
- Rippon, M.G.; Rogers, A.A.; Ousey, K. Estrategias de Protección Antimicrobiana En El Cuidado de Heridas: Evidencia Para El Uso de Apósitos Recubiertos Con DACC. J. Wound Care 2021, 30, 21–35. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Jiang, F.; Li, Y.; Chen, W.; Zhou, T. Preparation of Bacterial Cellulose/Acrylic Acid-Based pH-Responsive Smart Dressings by Graft Copolymerization Method. J. Biomater. Sci. Polym. Ed. 2024, 35, 2767–2789. [Google Scholar] [CrossRef]
- Deng, P.; Shi, Z.; Fang, F.; Xu, Y.; Zhou, L.; Liu, Y.; Jin, M.; Chen, T.; Wang, Y.; Cao, Y.; et al. Wireless Matrix Metalloproteinase-9 Sensing by Smart Wound Dressing with Controlled Antibacterial Nanoparticles Release toward Chronic Wound Management. Biosens. Bioelectron. 2025, 268, 116860. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, B.; Lou, Z.; Han, W.; Wang, L. The Advancement of Intelligent Dressings for Monitoring Chronic Wound Infections. Chem. Eng. J. 2024, 484, 149643. [Google Scholar] [CrossRef]
- Echague, C.G.; Hair, P.S.; Cunnion, K.M. A Comparison of Antibacterial Activity against Methicillin-Resistant Staphylococcus aureus and Gram-Negative Organisms for Antimicrobial Compounds in a Unique Composite Wound Dressing. Adv. Ski. Wound Care 2010, 23, 406–413. [Google Scholar] [CrossRef]
- Ilyas, F.; James, A.; Khan, S.; Haider, S.; Ullah, S.; Darwish, G.; Taqvi, S.A.H.R.; Ali, R.; Younas, Q.; Rehman, A. Multidrug-Resistant Pathogens in Wound Infections: A Systematic Review. Cureus 2024, 16, e58760. [Google Scholar] [CrossRef]
- Falcone, M.; De Angelis, B.; Pea, F.; Scalise, A.; Stefani, S.; Tasinato, R.; Zanetti, O.; Dalla Paola, L. Challenges in the Management of Chronic Wound Infections. J. Glob. Antimicrob. Resist. 2021, 26, 140–147. [Google Scholar] [CrossRef]
- Pogue, J.M.; Kaye, K.S.; Cohen, D.A.; Marchaim, D. Appropriate Antimicrobial Therapy in the Era of Multidrug-Resistant Human Pathogens. Clin. Microbiol. Infect. 2015, 21, 302–312. [Google Scholar] [CrossRef]
- Hemmati, J.; Azizi, M.; Asghari, B.; Arabestani, M.R. Multidrug-Resistant Pathogens in Burn Wound, Prevention, Diagnosis, and Therapeutic Approaches (Conventional Antimicrobials and Nanoparticles). Can. J. Infect. Dis. Med. Microbiol. 2023, 2023, 8854311. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M.M. Antimicrobial Resistance and Recent Alternatives to Antibiotics for the Control of Bacterial Pathogens with an Emphasis on Foodborne Pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- Shrestha, G.; Raphael, J.; Leavitt, S.D.; St. Clair, L.L. In Vitro Evaluation of the Antibacterial Activity of Extracts from 34 Species of North American Lichens. Pharm. Biol. 2014, 52, 1262–1266. [Google Scholar] [CrossRef]
- Molan, P.C. The Evidence Supporting the Use of Honey as a Wound Dressing. Int. J. Low Extrem. Wounds 2006, 5, 40–54. [Google Scholar] [CrossRef]
- Jull, A.B.; Rodgers, A.; Walker, N. Honey as a Topical Treatment for Wounds. Cochrane Database Syst. Rev. 2008, 2008, CD005083. [Google Scholar] [CrossRef]
- Cooper, R. Using Honey to Inhibit Wound Pathogens. Nurs. Times 2008, 104, 46–49. [Google Scholar]
- McLoone, P.; Oluwadun, A.; Warnock, M.; Fyfe, L. Honey: A Therapeutic Agent for Disorders of the Skin. Cent. Asian J. Glob. Health 2016, 5, 241. [Google Scholar] [CrossRef]
- Cooper, R. Honey in Wound Care: Antibacterial Properties. GMS Krankenhaushygiene Interdiszip. 2007, 2, Doc51. [Google Scholar]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.-G. Plant Essential Oils as Active Antimicrobial Agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Chemical Composition, Antimicrobial and Antibiotic Potentiating Activity of Essential Oils from 10 Tropical Medicinal Plants from Mauritius. J. Herb. Med. 2016, 6, 88–95. [Google Scholar] [CrossRef]
- Kavoosi, G.; Tafsiry, A.; Ebdam, A.A.; Rowshan, V. Evaluation of Antioxidant and Antimicrobial Activities of Essential Oils from Carum copticum Seed and Ferula assafoetida Latex. J. Food Sci. 2013, 78, T356–T361. [Google Scholar] [CrossRef]
- Rai, M.; Paralikar, P.; Jogee, P.; Agarkar, G.; Ingle, A.P.; Derita, M.; Zacchino, S. Synergistic Antimicrobial Potential of Essential Oils in Combination with Nanoparticles: Emerging Trends and Future Perspectives. Int. J. Pharm. 2017, 519, 67–78. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Development of Bacterial Resistance to Several Biocides and Effects on Antibiotic Susceptibility. J. Hosp. Infect. 2003, 55, 98–107. [Google Scholar] [CrossRef]
- Ferguson, A.W. Comparison of 5% Povidone-Iodine Solution against 1% Povidone-Iodine Solution in Preoperative Cataract Surgery Antisepsis: A Prospective Randomised Double Blind Study. Br. J. Ophthalmol. 2003, 87, 163–167. [Google Scholar] [CrossRef]
- Leaper, D. Topical Antiseptics in Wound Care: Time for Reflection. Int. Wound J. 2011, 8, 547–549. [Google Scholar] [CrossRef]
- Gupta, S.; Shinde, R.K.; Shinde, S. Comparison of the Outcomes of Cadexomer Iodine and Povidone-Iodine Ointments in Wound Management. Cureus 2022, 14, e24667. [Google Scholar] [CrossRef] [PubMed]
- Kekul, Ö.; Üstün, B.; Kömürcü, Ö.; Bi̇Lgi̇N, S.; Karakaya, D. Skin Reaction Related to Povidone Iodine Use. JECM 2021, 38, 389–392. [Google Scholar] [CrossRef]
- Eming, S.A.; Smola-Hess, S.; Kurschat, P.; Hirche, D.; Krieg, T.; Smola, H. A Novel Property of Povidon-Iodine: Inhibition of Excessive Protease Levels in Chronic Non-Healing Wounds. J. Investig. Dermatol. 2006, 126, 2731–2733. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Shinde, S.; Shinde, R.K. Topical Management of Wound: A Narrative Review of Cadexomer Iodine Ointment Versus Povidone Iodine Ointment. Cureus 2022, 14, e24598. [Google Scholar] [CrossRef]
- Aramwit, P.; Muangman, P.; Namviriyachote, N.; Srichana, T. In Vitro Evaluation of the Antimicrobial Effectiveness and Moisture Binding Properties of Wound Dressings. Int. J. Mol. Sci. 2010, 11, 2864–2874. [Google Scholar] [CrossRef]
- Van Den Poel, B.; Saegeman, V.; Schuermans, A. Increasing Usage of Chlorhexidine in Health Care Settings: Blessing or Curse? A Narrative Review of the Risk of Chlorhexidine Resistance and the Implications for Infection Prevention and Control. Eur. J. Clin. Microbiol. Infect. Dis. 2022, 41, 349–362. [Google Scholar] [CrossRef]
- Konop, M.; Damps, T.; Misicka, A.; Rudnicka, L. Certain Aspects of Silver and Silver Nanoparticles in Wound Care: A Minireview. J. Nanomater. 2016, 2016, 7614753. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a Therapeutic Tool to Combat Microbial Resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Hermans, M.H. Silver-Containing Dressings and the Need for Evidence. AJN Am. J. Nurs. 2006, 106, 60–68. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Hartemann, P. Silver as an Antimicrobial: Facts and Gaps in Knowledge. Crit. Rev. Microbiol. 2013, 39, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Dong, D.; Feng, S.; Guo, Y.; Yu, J.; Gan, C.; Hu, X.; Qin, Z.; Liu, Y.; Gao, Y. Metal-Based Antimicrobial Agents in Wound Dressings: Infection Management and the Challenge of Antibiotic Resistance. Chem. Eng. J. 2025, 507, 160726. [Google Scholar] [CrossRef]
- Dror, Y.; Ophir, C.; Freeman, A. Silver–Enzyme Hybrids as Wide-Spectrum Antimicrobial Agents. In Innovations and Emerging Technologies in Wound Care; Elsevier: Amsterdam, The Netherlands, 2020; pp. 293–307. ISBN 978-0-12-815028-3. [Google Scholar]
- Zhang, W.; Hu, J.; Wu, H.; Lin, X.; Cai, L. Stimuli-Responsive Hydrogel Dressing for Wound Healing. APL Mater. 2025, 13, 010601. [Google Scholar] [CrossRef]
- Ehtesabi, H.; Kalji, S.-O.; Movsesian, L. Smartphone-Based Wound Dressings: A Mini-Review. Heliyon 2022, 8, e09876. [Google Scholar] [CrossRef] [PubMed]
- Shu, W.; Wang, Y.; Zhang, X.; Li, C.; Le, H.; Chang, F. Functional Hydrogel Dressings for Treatment of Burn Wounds. Front. Bioeng. Biotechnol. 2021, 9, 788461. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Robles, J.; Cuartas-Gómez, E.; Dynes, S.; Utomo, E.; Anjani, Q.K.; Detamornrat, U.; Donnelly, R.F.; Moreno-Castellanos, N.; Larrañeta, E. Poly(caprolactone)/Lignin-Based 3D-Printed Dressings Loaded with a Novel Combination of Bioactive Agents for Wound-Healing Applications. Sustain. Mater. Technol. 2023, 35, e00581. [Google Scholar] [CrossRef]
- Unalan, I.; Schruefer, S.; Schubert, D.W.; Boccaccini, A.R. 3D-Printed Multifunctional Hydrogels with Phytotherapeutic Properties: Development of Essential Oil-Incorporated ALG-XAN Hydrogels for Wound Healing Applications. ACS Biomater. Sci. Eng. 2023, 9, 4149–4167. [Google Scholar] [CrossRef]
| Method | Test | References |
|---|---|---|
| In vitro | Antimicrobial tests (on Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli, and Streptococcus pyogenes) | [36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51] |
| Sensor array operation in vitro in simulated infected wound models | [36] | |
| Test strip sensor for early and accurate detection of wound infection at the bedside | [52] | |
| Electrochemical behavior sensors | [53,54,55] | |
| Mechanical Test | [38,44,56] | |
| Dehydration and Hydration Tests | [38,44,48,56,57] | |
| Viability/Cytotoxicity Assay | [38,39,40,41,43,44,47,49,56] | |
| Biocompatibility test | [44,49,56] | |
| Drug release kinetics | [40,41,43,44,47] | |
| Antioxidant activity | [41] | |
| Detection of bacterial fluorescence | [42] | |
| Ex-vivo | Biofilm formation on ex vivo porcine skin | [37] |
| Ex Vivo Bacterial Detection Tests | [56] | |
| In vivo | Animal model (bacterial inhibition, reduction angiogenesis, anti-inflammatory activity) | [38,39,42,43,44,51,53,58] |
| Type of Dressing | Actions | Application/Indications | Advantages | Desavantages | References |
|---|---|---|---|---|---|
| Films | Autolytic debridement | Superficial wounds with minimal exudate: minor burns, stage I and stage II pressure ulcers | Transparent-easy to visualize the wound Highly flexible Impermeable to liquid or bacteria | May adhere to wounds Not suitable for heavily draining wounds May promote macerations due to its occlusive nature No absorptive capacity | [27,79,80,81] |
| Foams | Absorb fluids Moisture control Flexible to the wound bed | Exudative wounds, deep wounds, burns, chronic wounds, lower leg ulcers | Existing low-adherent versions available for patients with fragile skin Great absorption | Not for use in dry or necrotic wounds | [82,83,84,85,86] |
| Hydrocolloids | Absorb fluids Promote autolytic debridement | Clean, low to moderate exuding wounds: Average thickness wounds, burns, chronic ulcers | Long wear-time Absorbent Occlusive Protects wound from contamination | May cause maceration | [87,88,89,90,91] |
| Hydrogels | Promote autolytic debridement Rehydrated wound bed Moisture control | Low to moderate exuding wound: Partial-thickness burns, dry chronic wounds, necrotic wounds, minor ulcerations, exposed bone | Provides moisture Easy removal Cooling effect | Expensive Biocompatibility issues May cause maceration | [7,92,93,94,95] |
| Nanofibers | Absorb exudate Promote autolytic debridement Moisture control | Surgical wounds, bleeding wounds, high-exudate chronic ulcers, sinus tracts | Excellent exudate absorbtion | Not for use in dry or necrotic wounds (can cause bleeding) | [67,96,97,98,99] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cristea, A.-G.; Lisă, E.-L.; Iacob, S.; Dragostin, I.; Ștefan, C.S.; Fulga, I.; Anghel, A.M.; Dragan, M.; Morariu, I.D.; Dragostin, O.-M. Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals 2025, 18, 825. https://doi.org/10.3390/ph18060825
Cristea A-G, Lisă E-L, Iacob S, Dragostin I, Ștefan CS, Fulga I, Anghel AM, Dragan M, Morariu ID, Dragostin O-M. Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals. 2025; 18(6):825. https://doi.org/10.3390/ph18060825
Chicago/Turabian StyleCristea (Hohotă), Alina-Georgiana, Elena-Lăcrămioara Lisă, Simona Iacob (Ciobotaru), Ionut Dragostin, Claudia Simona Ștefan, Iuliu Fulga, Andra Monica Anghel (Ștefan), Maria Dragan, Ionela Daniela Morariu, and Oana-Maria Dragostin. 2025. "Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care" Pharmaceuticals 18, no. 6: 825. https://doi.org/10.3390/ph18060825
APA StyleCristea, A.-G., Lisă, E.-L., Iacob, S., Dragostin, I., Ștefan, C. S., Fulga, I., Anghel, A. M., Dragan, M., Morariu, I. D., & Dragostin, O.-M. (2025). Antimicrobial Smart Dressings for Combating Antibiotic Resistance in Wound Care. Pharmaceuticals, 18(6), 825. https://doi.org/10.3390/ph18060825







