The Adverse Effects and Use of Bevacizumab in Patients with Glioblastoma: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Results
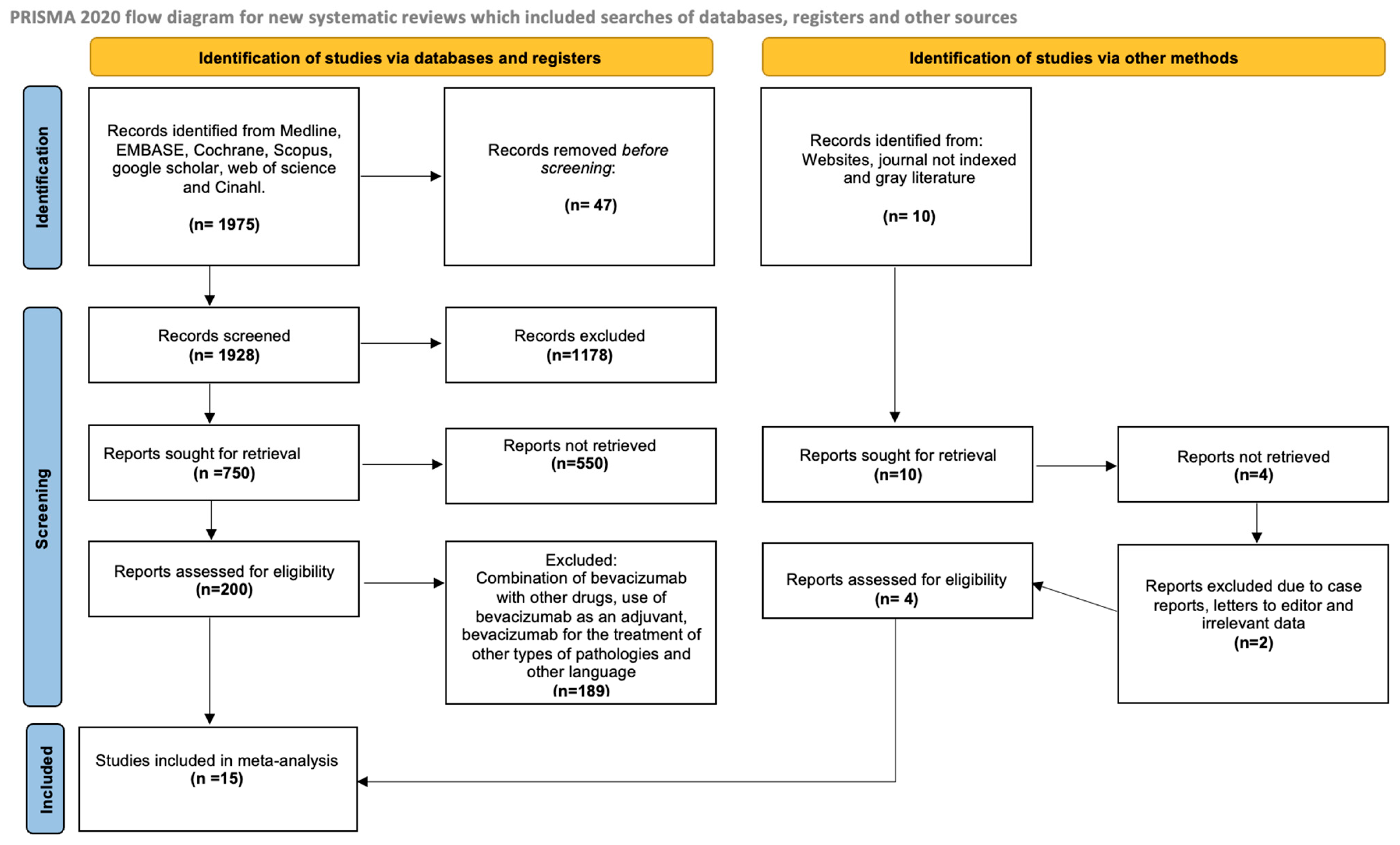
2.1. Study Selection
2.2. Study Characteristics
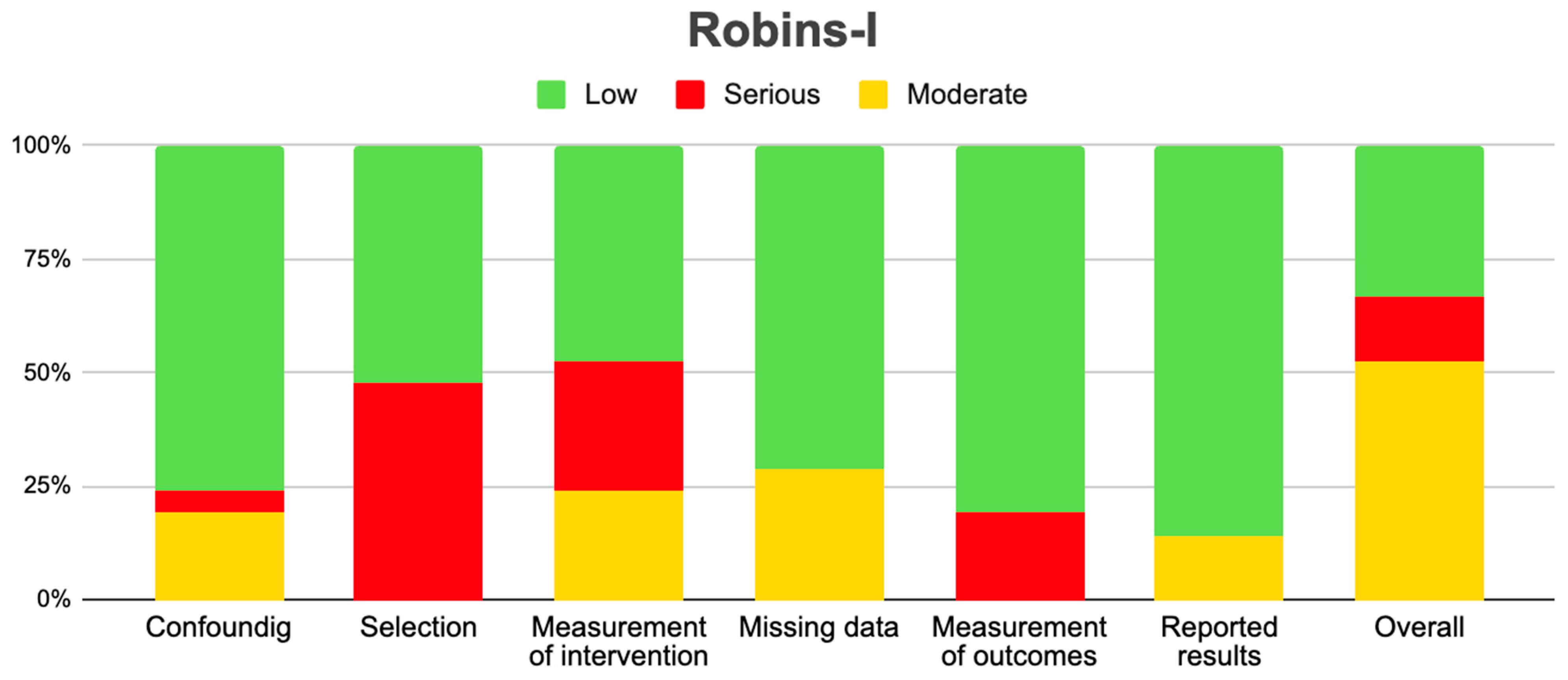
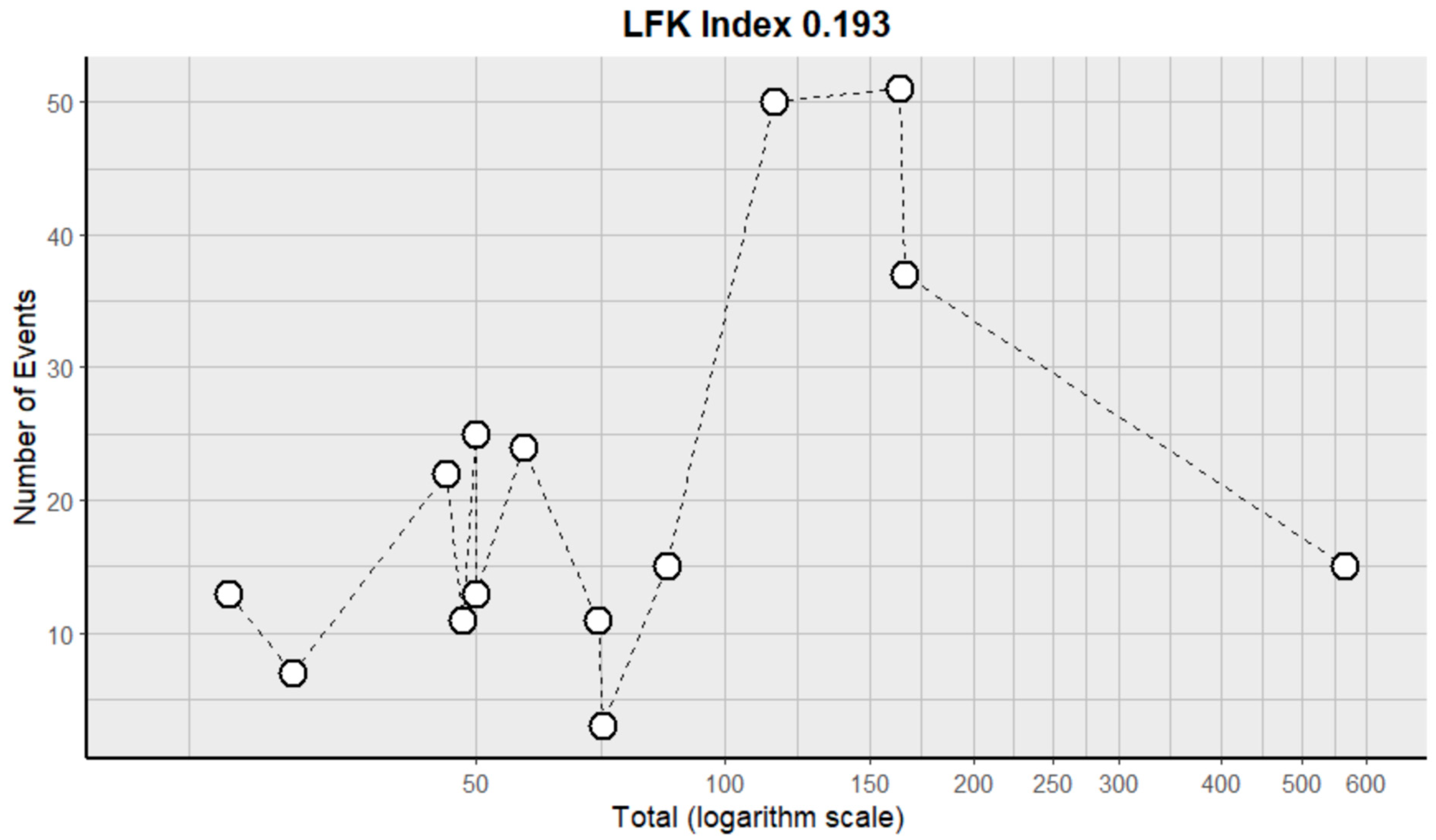
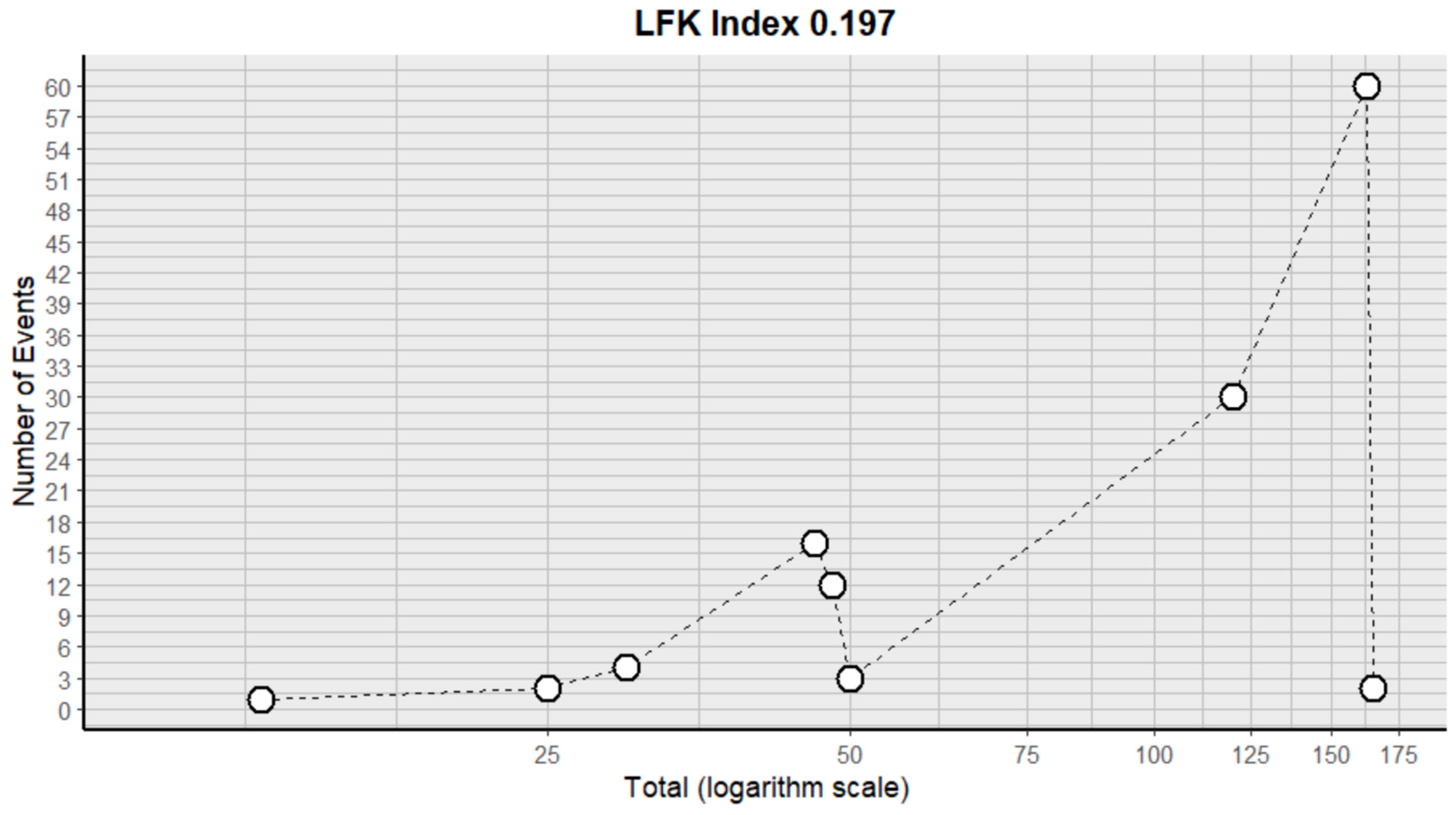
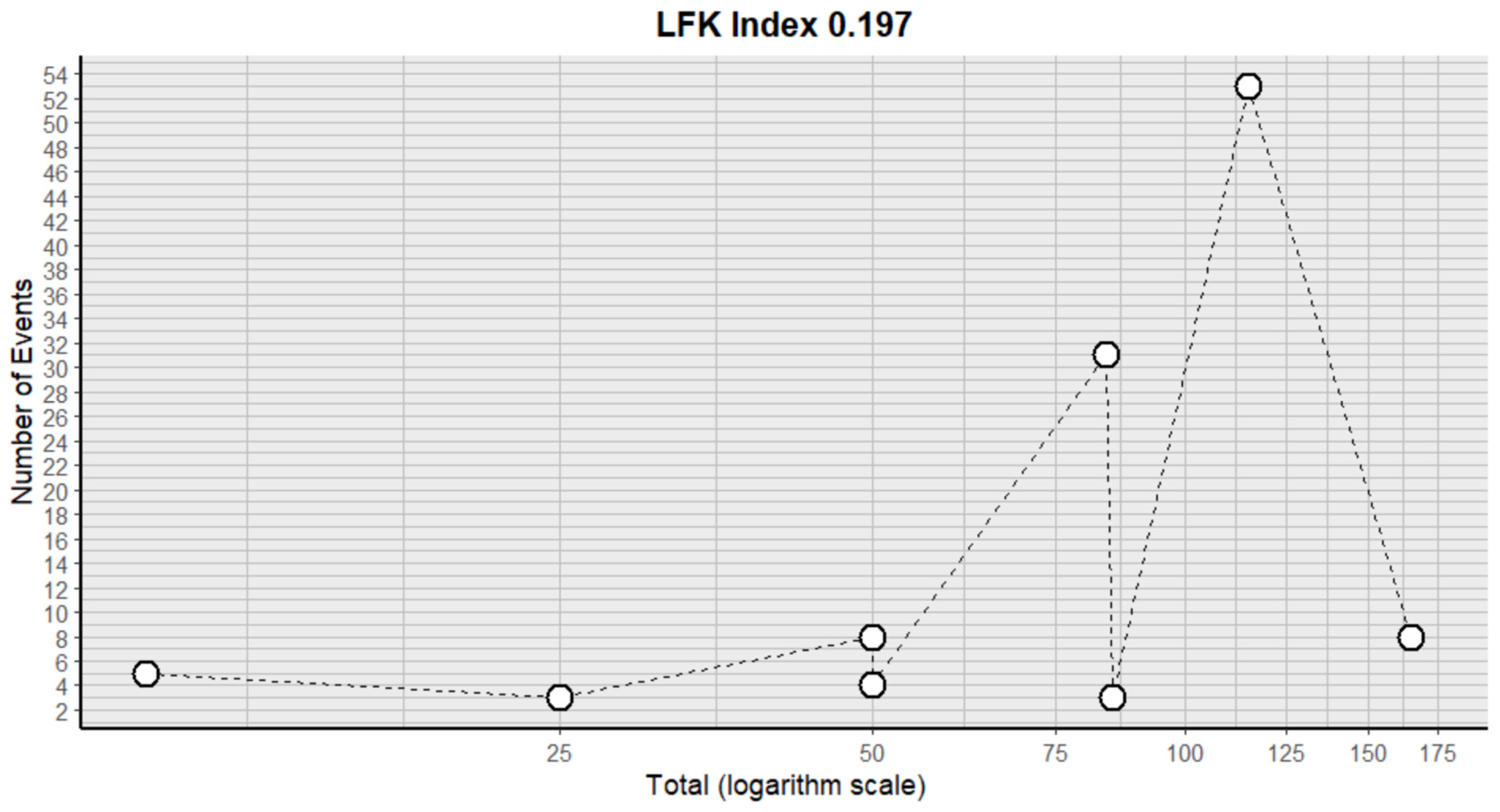
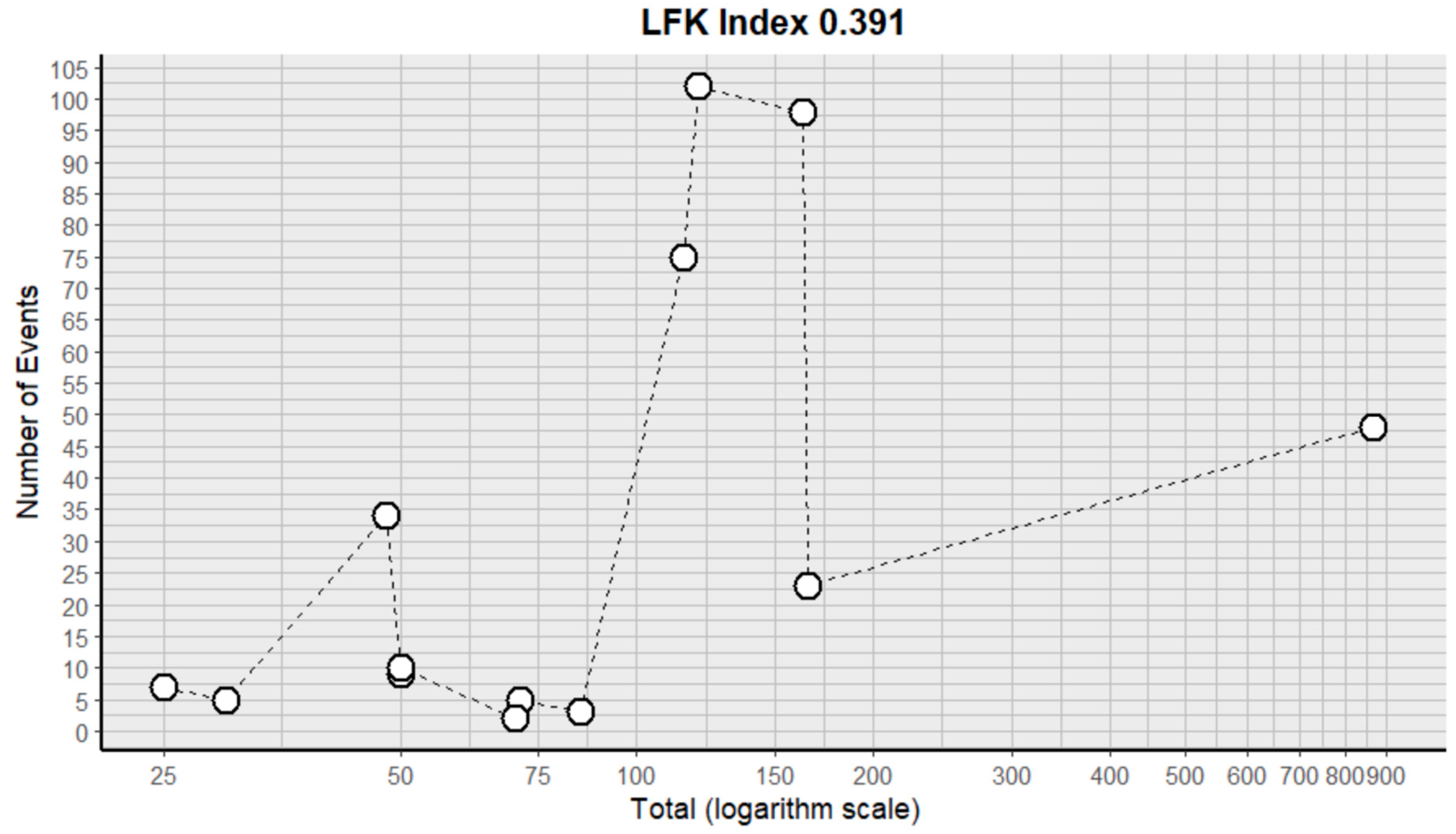
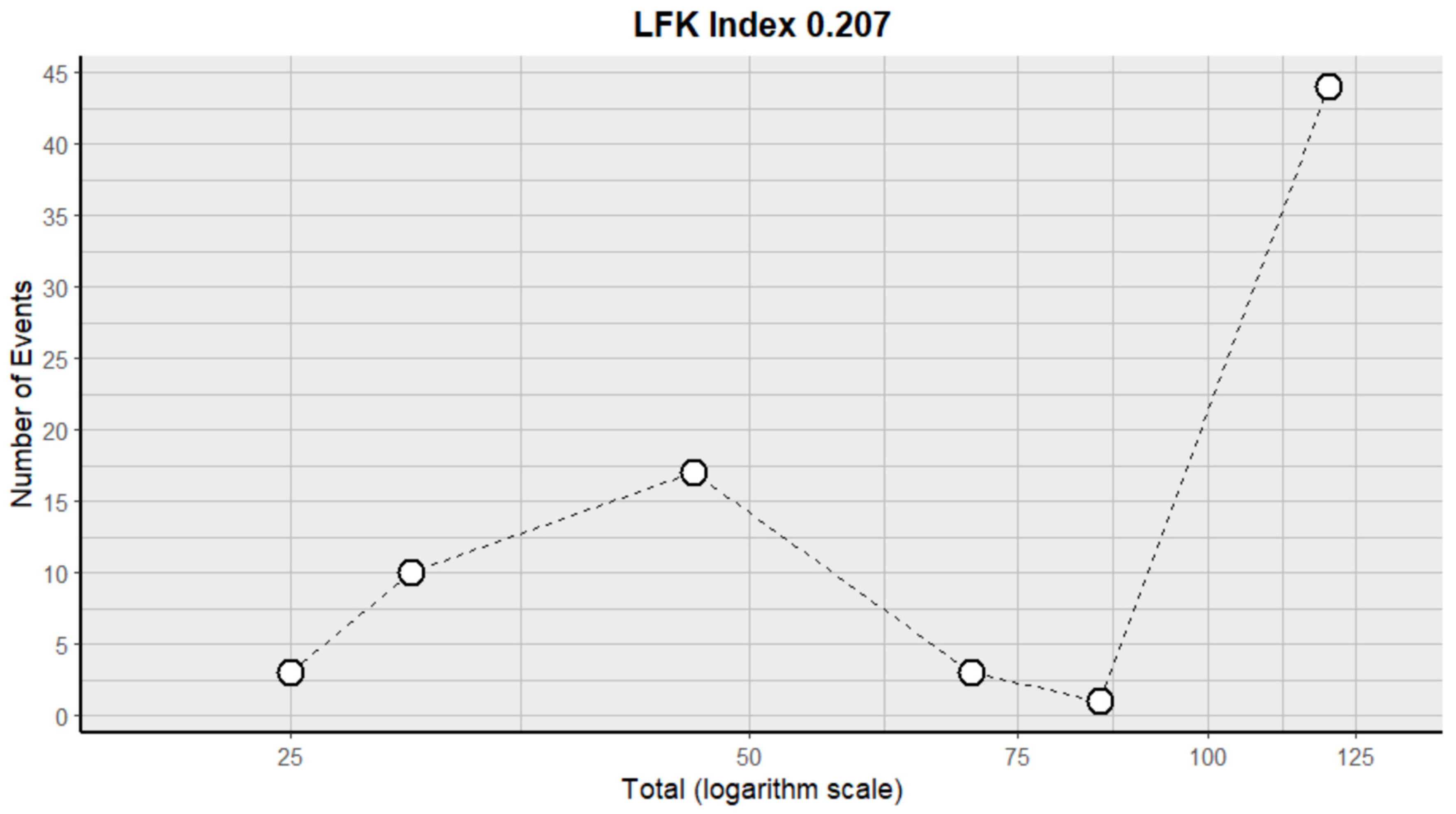
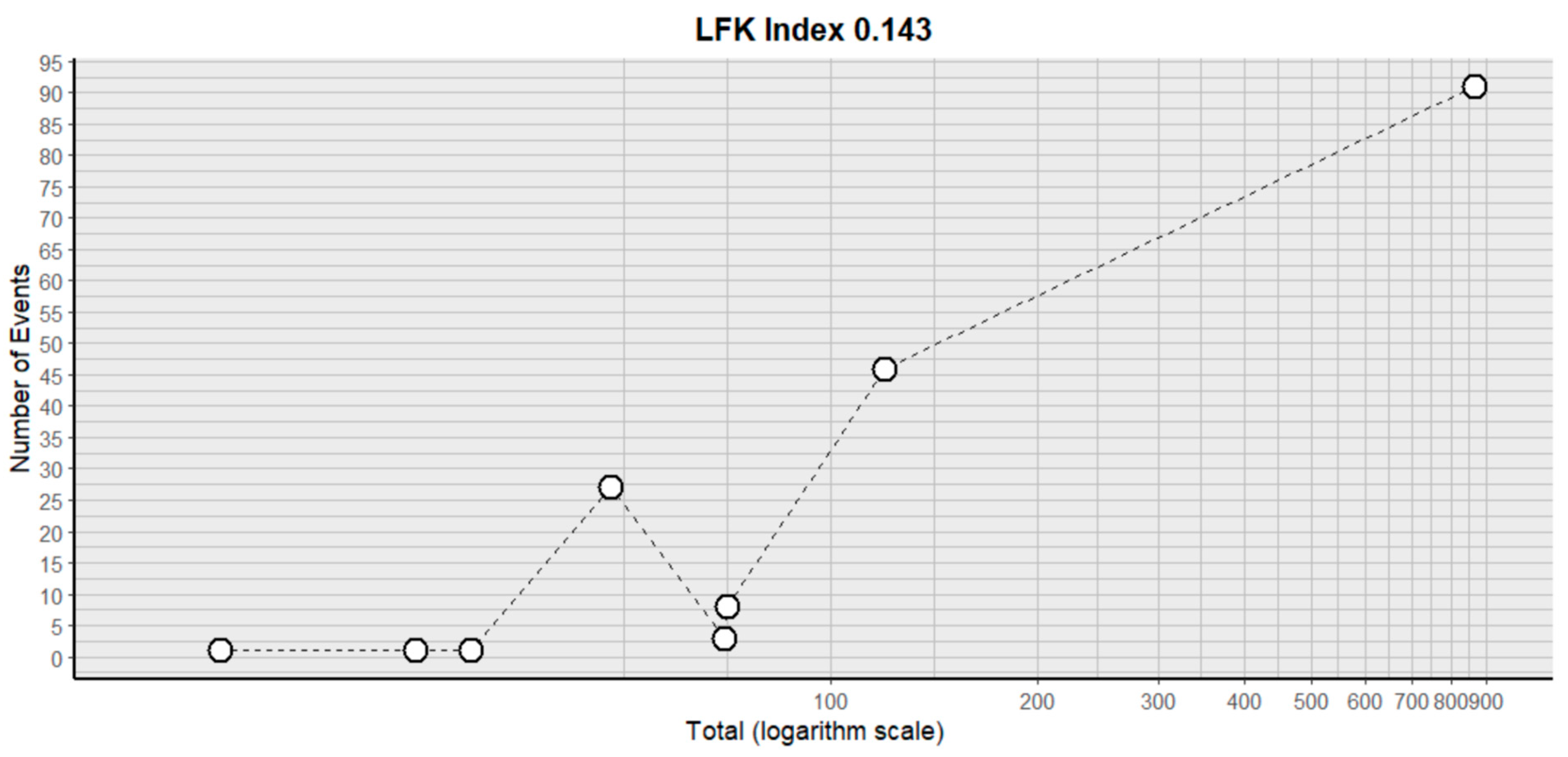
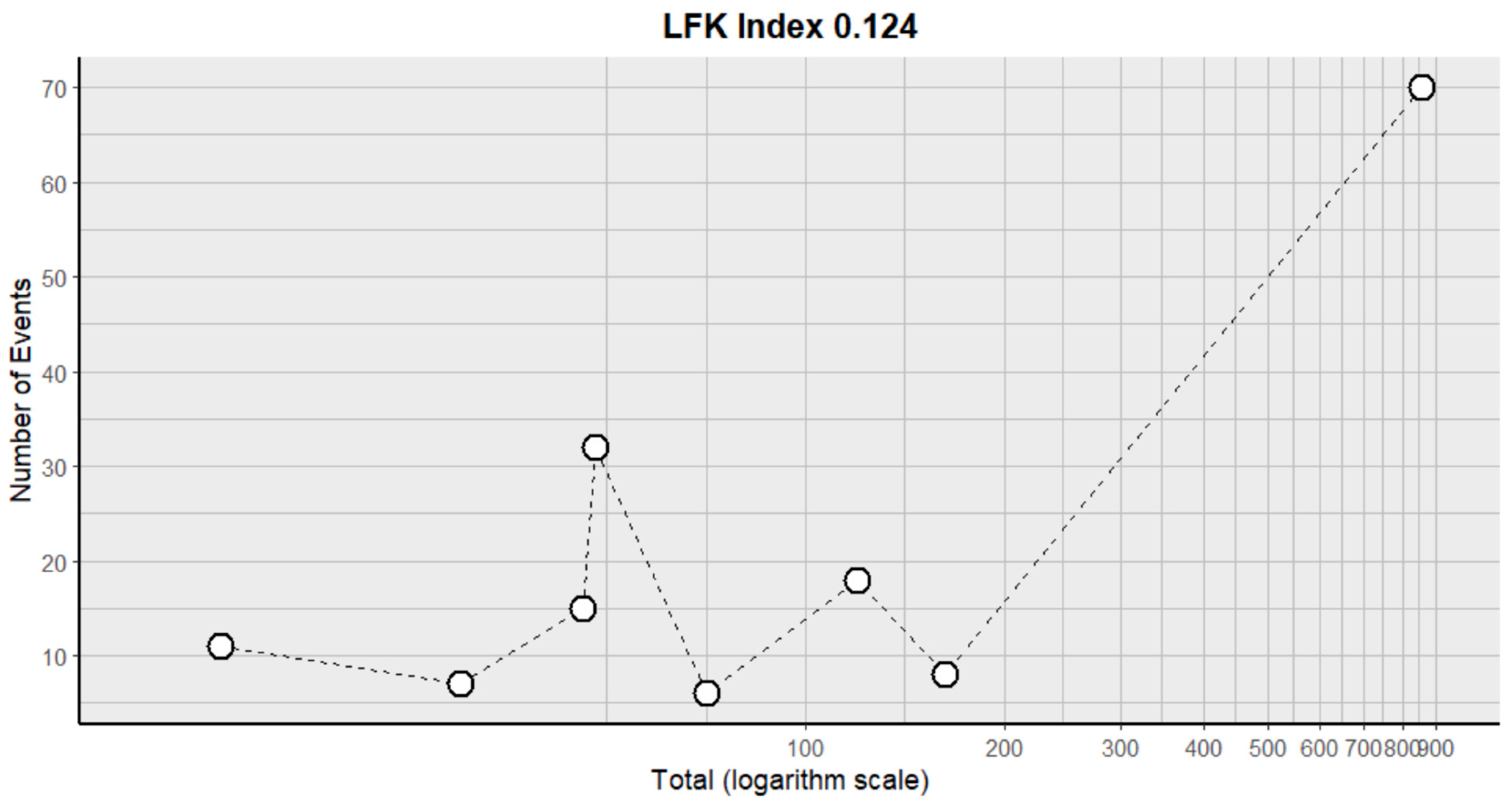
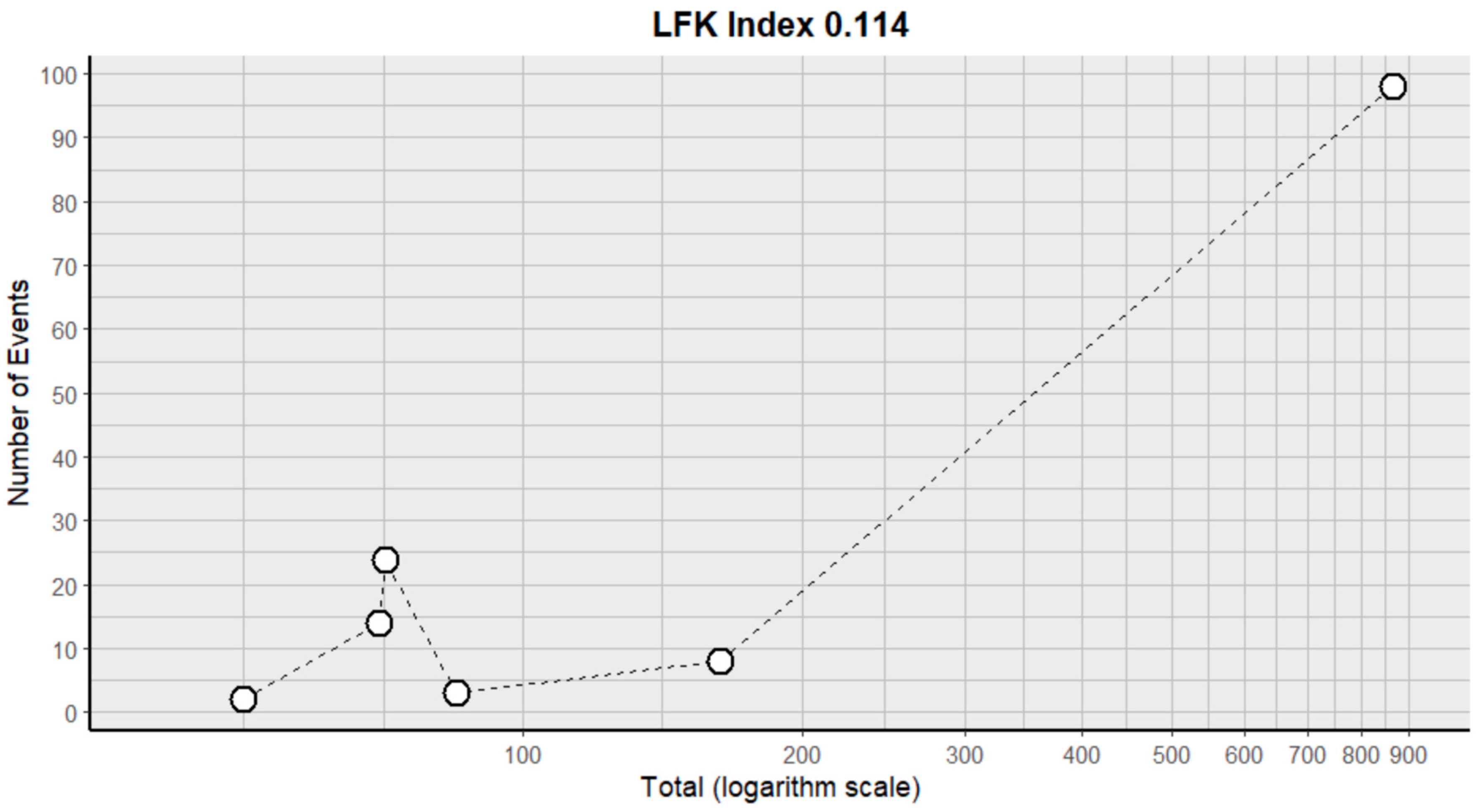
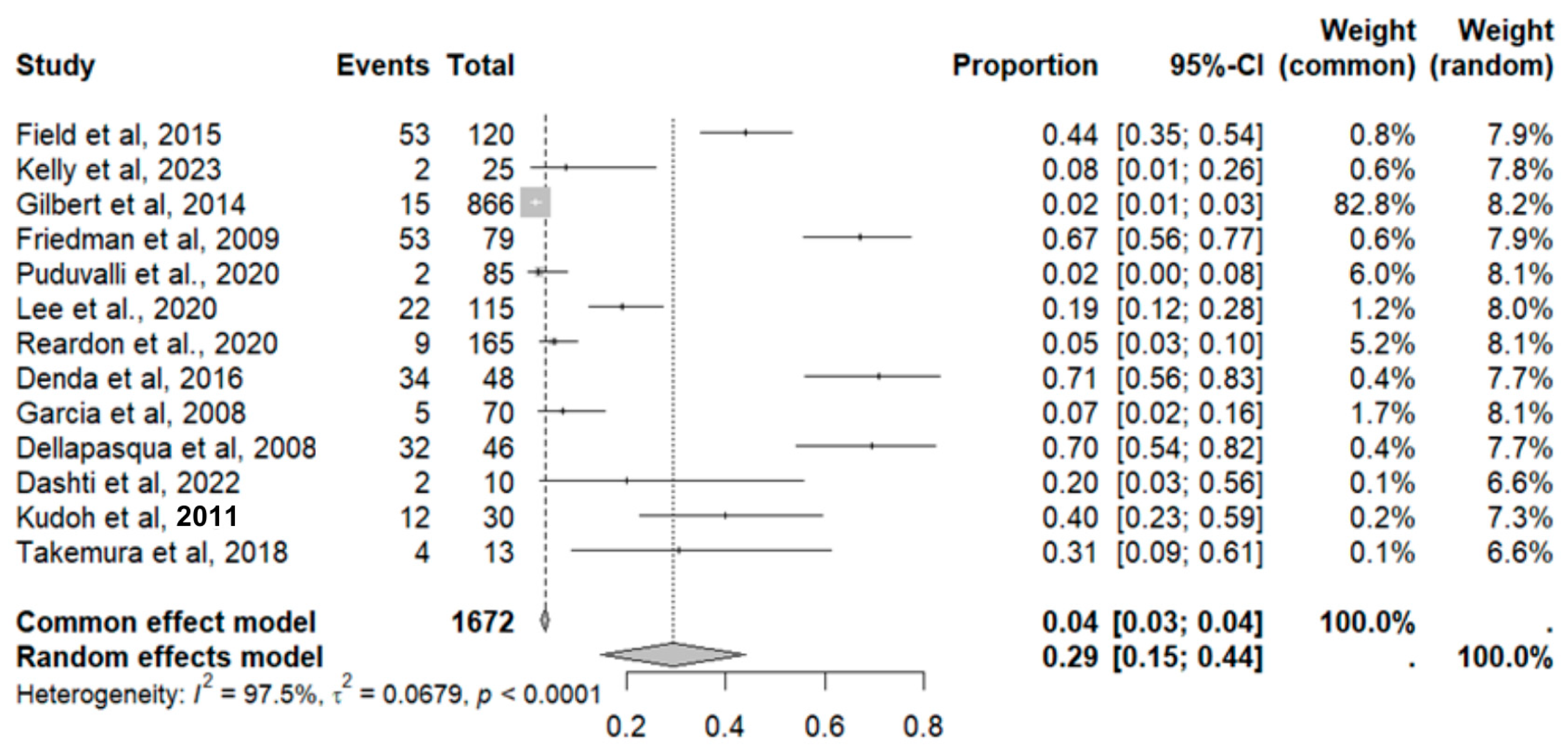
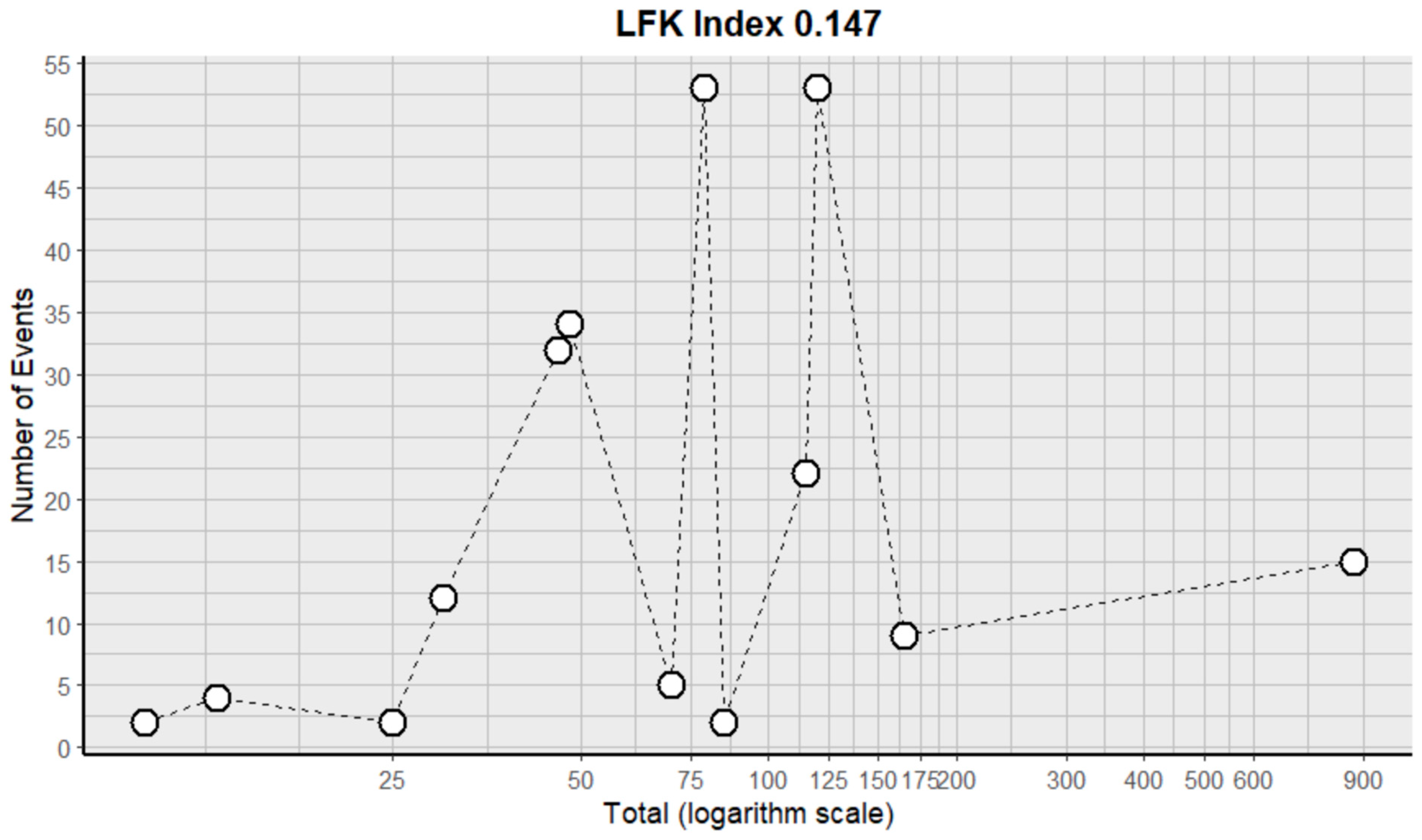
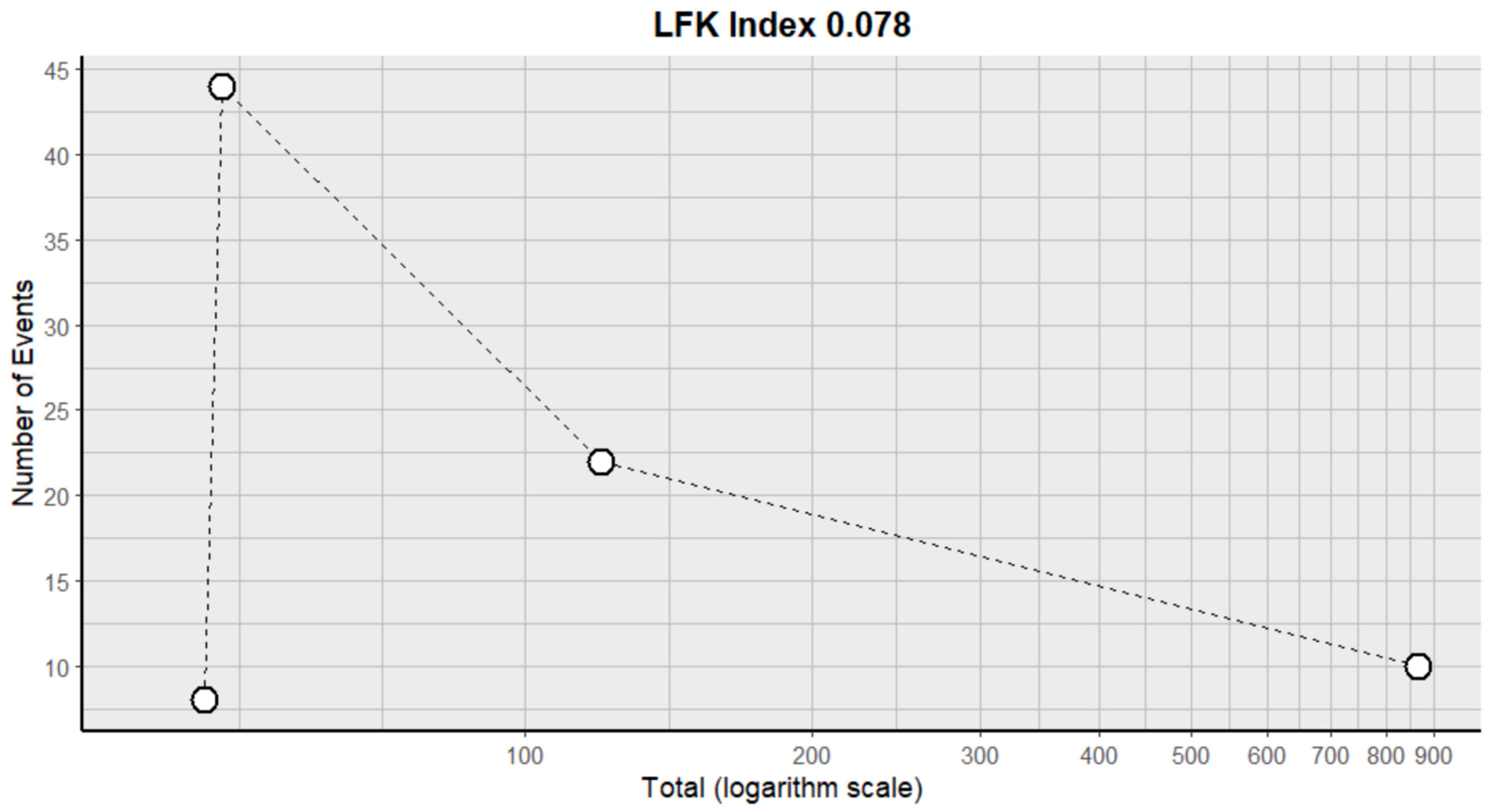
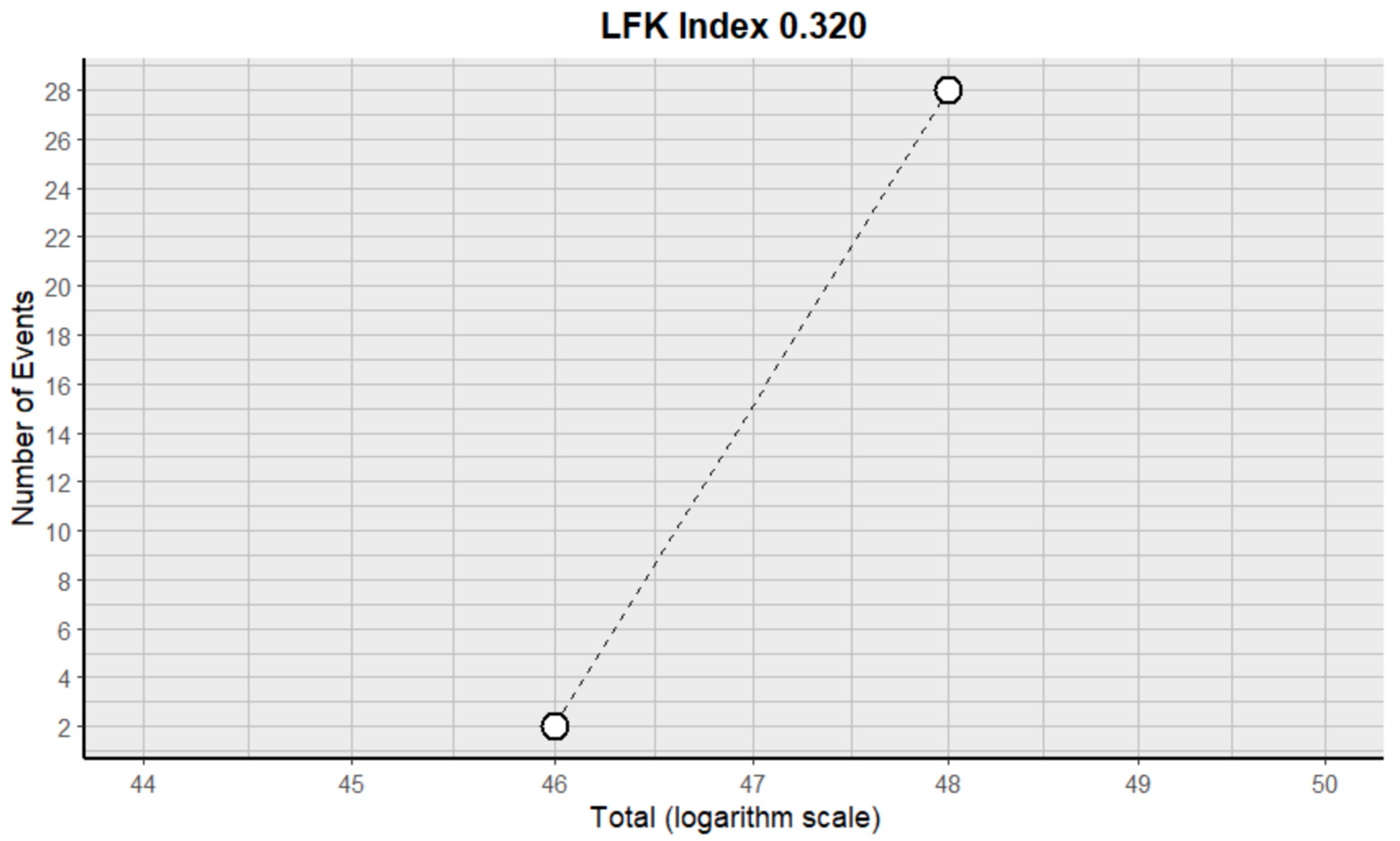
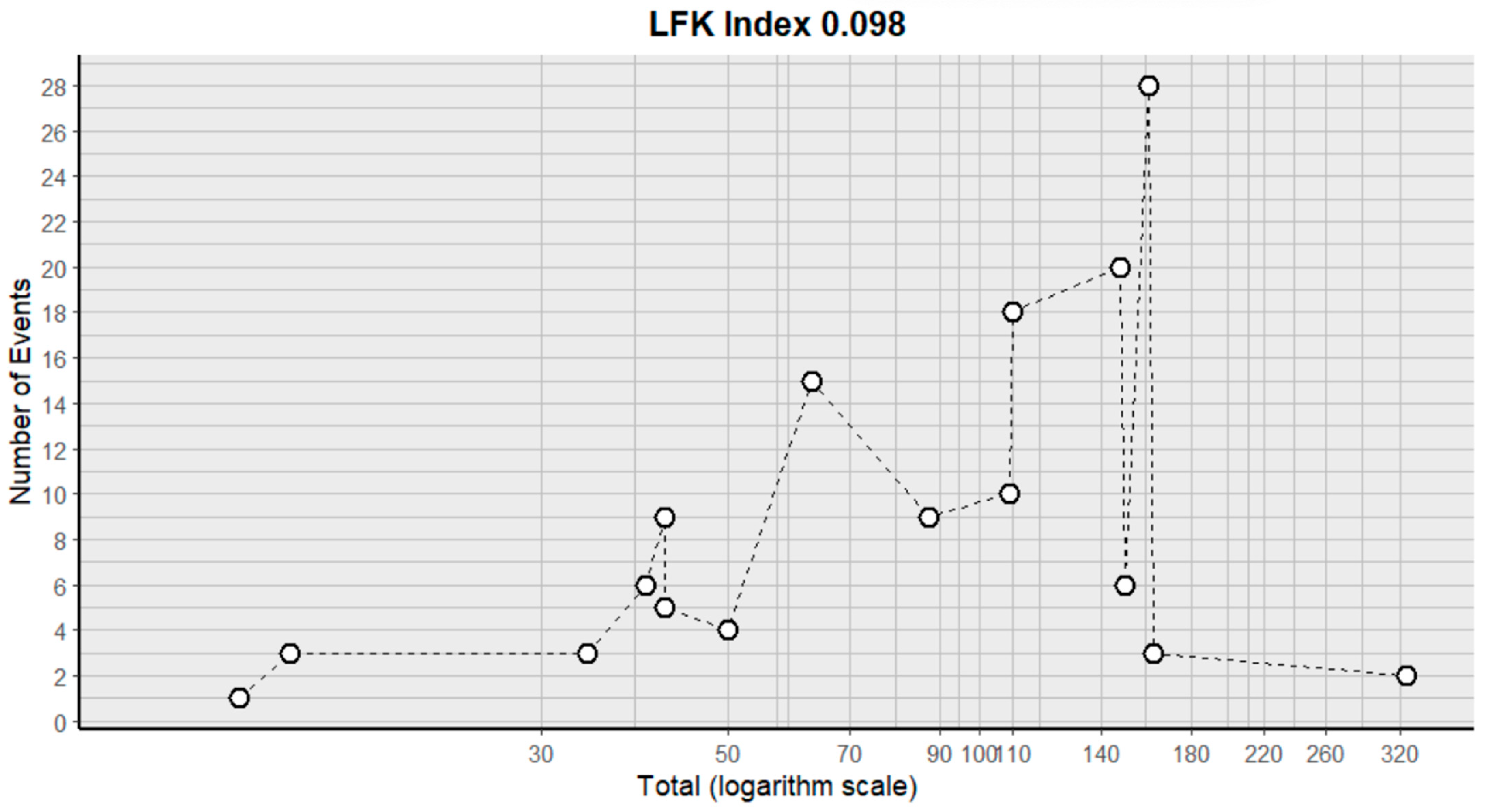
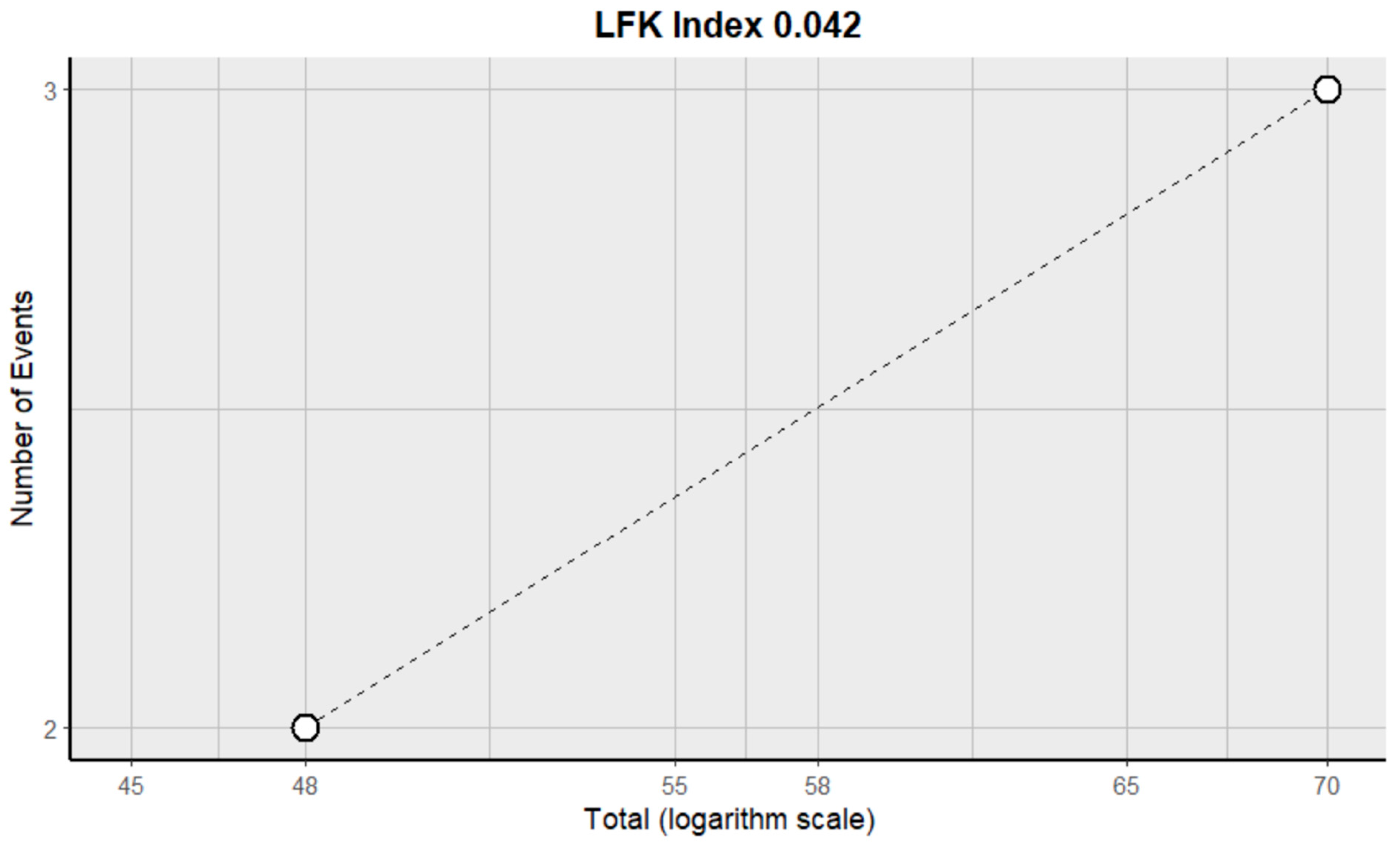
2.3. Risk of Bias Assessment in Individual Studies and Heterogeneity
2.4. BV with Another Drug vs. BV//BV with Another Drug vs. Another Drug
2.5. BV with Another Drug vs. Placebo
2.6. BV with Radiotherapy and Another Modality
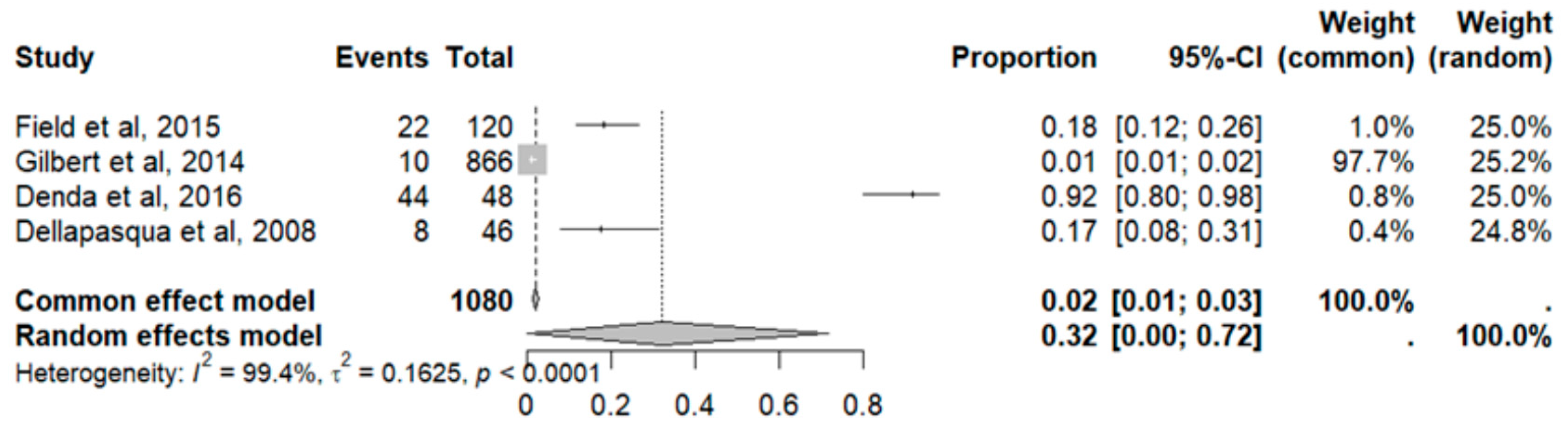
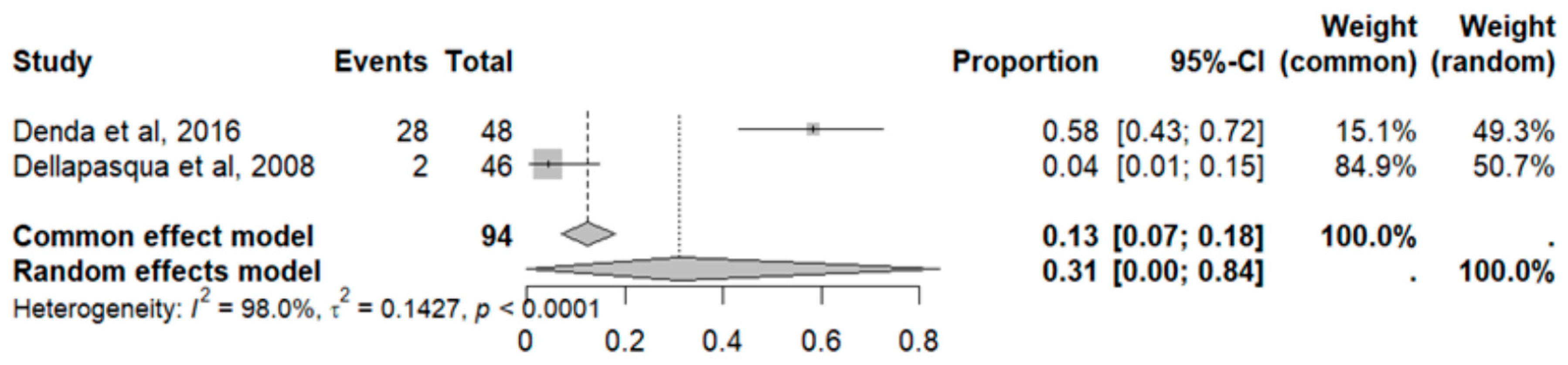
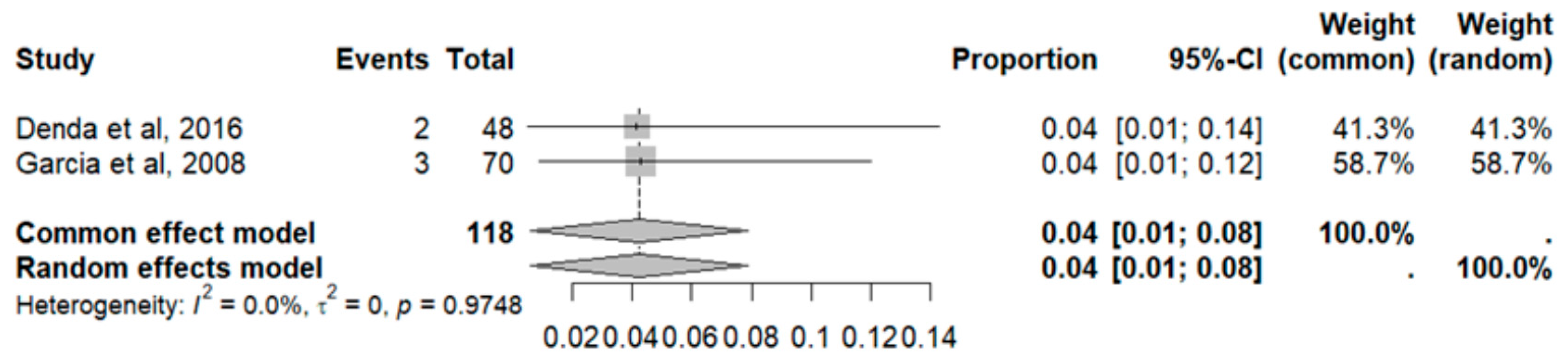
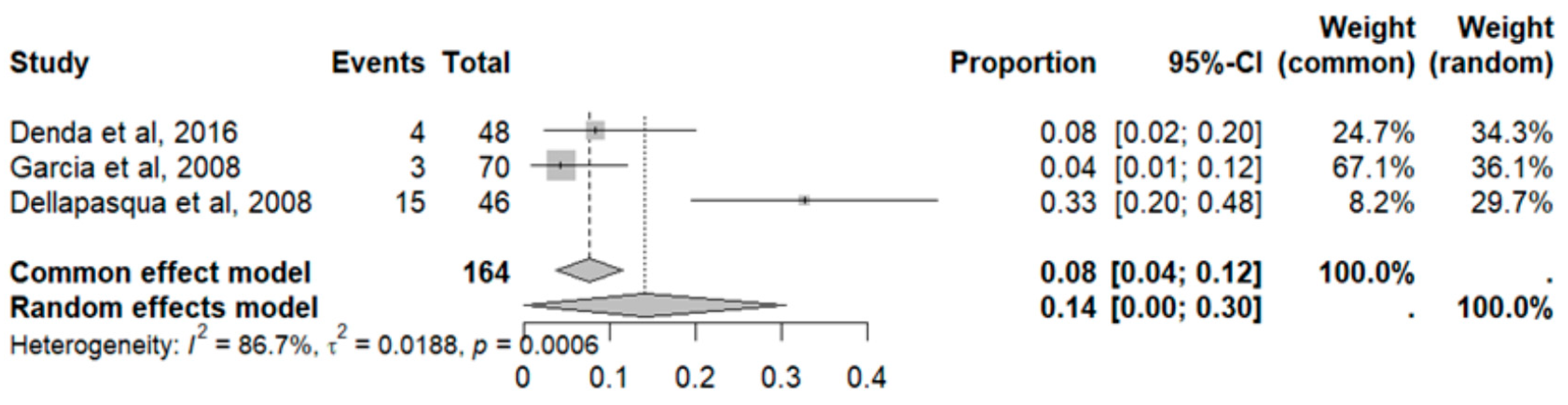
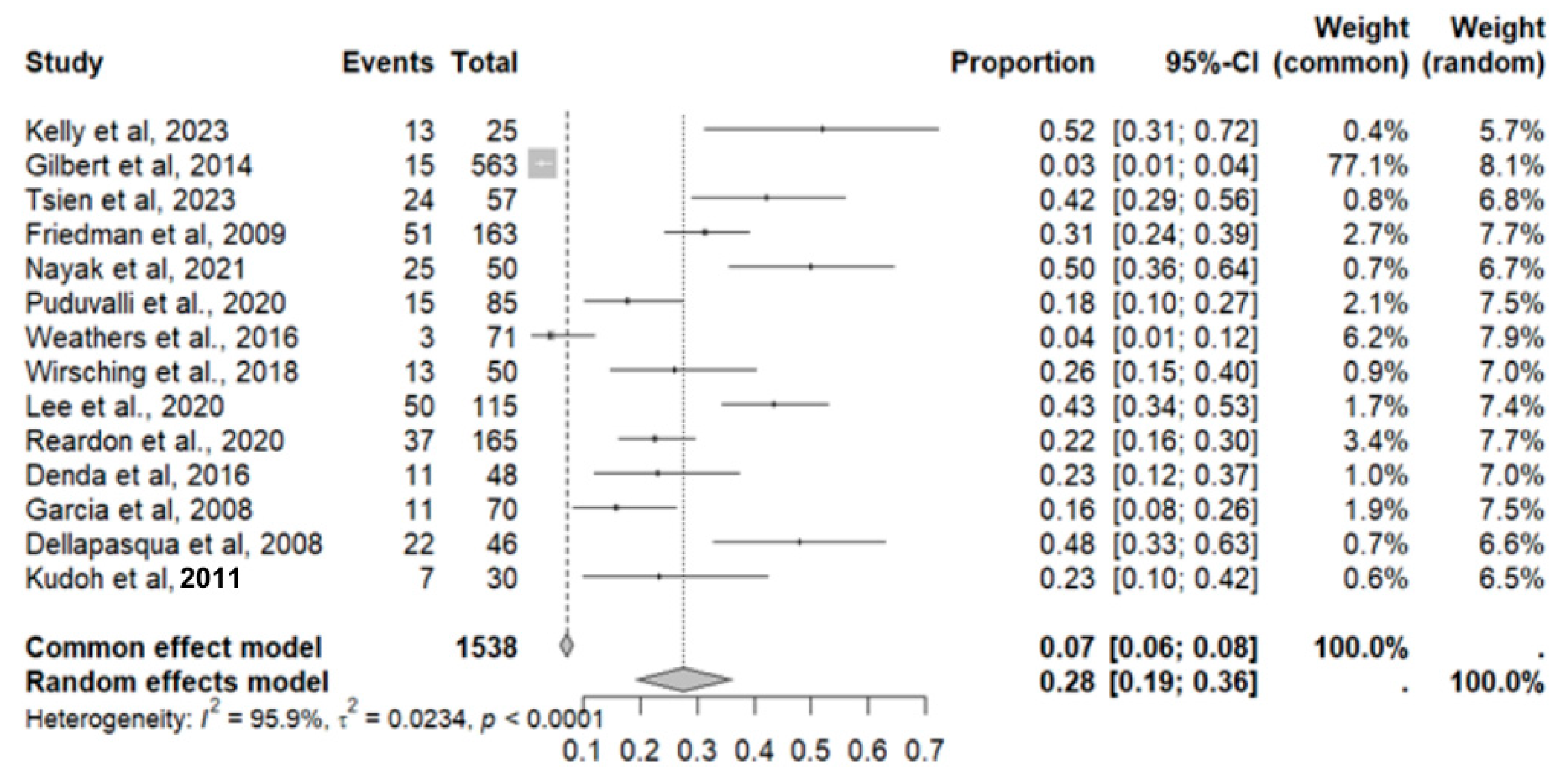
2.7. Adverse Effects in GBM
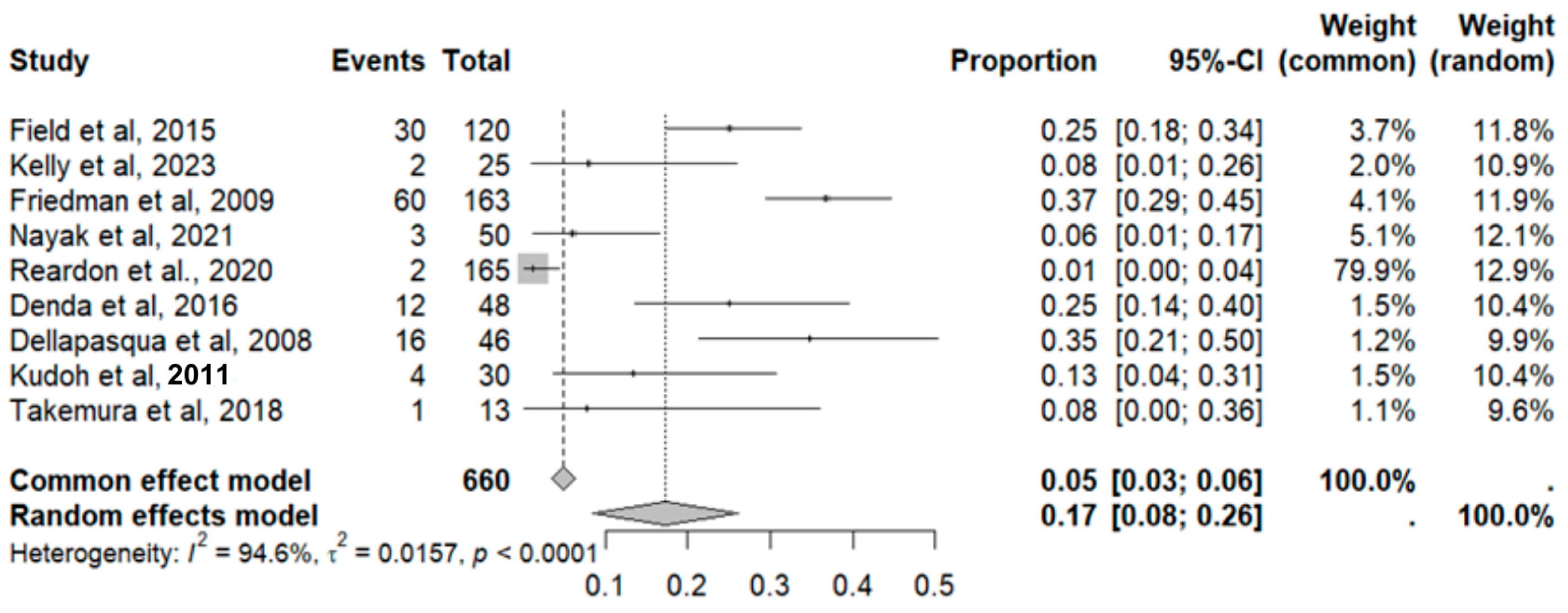
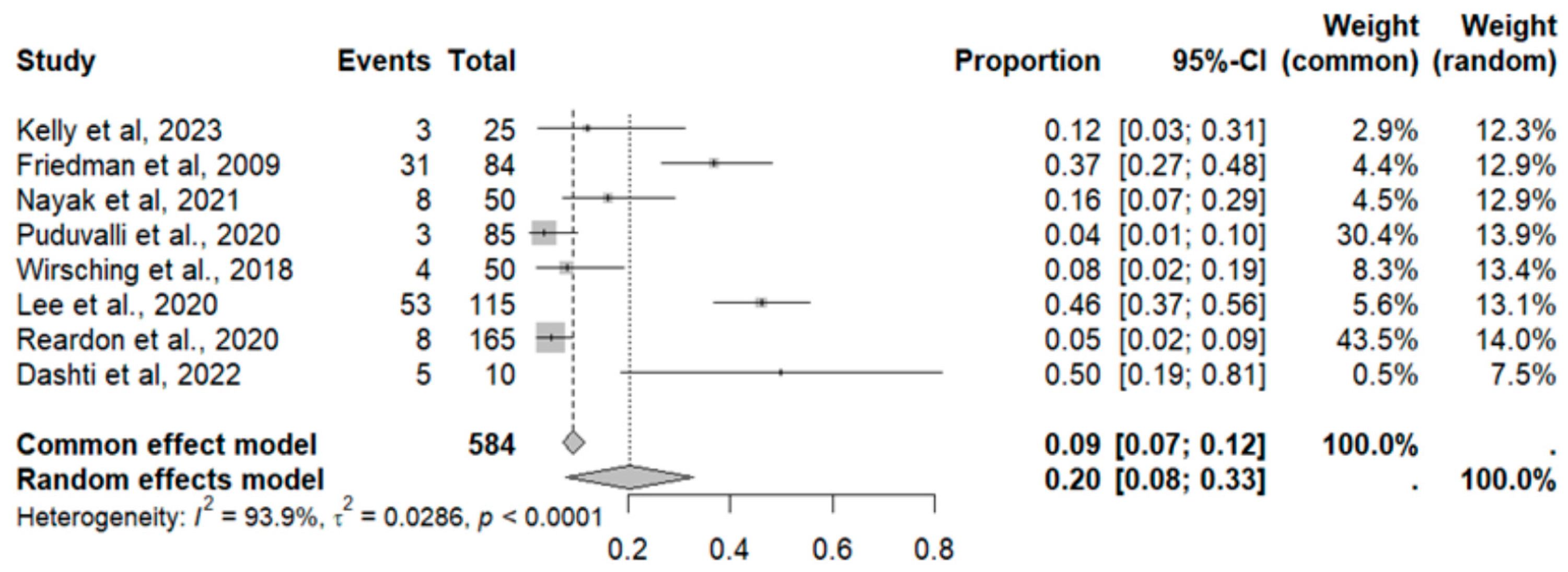
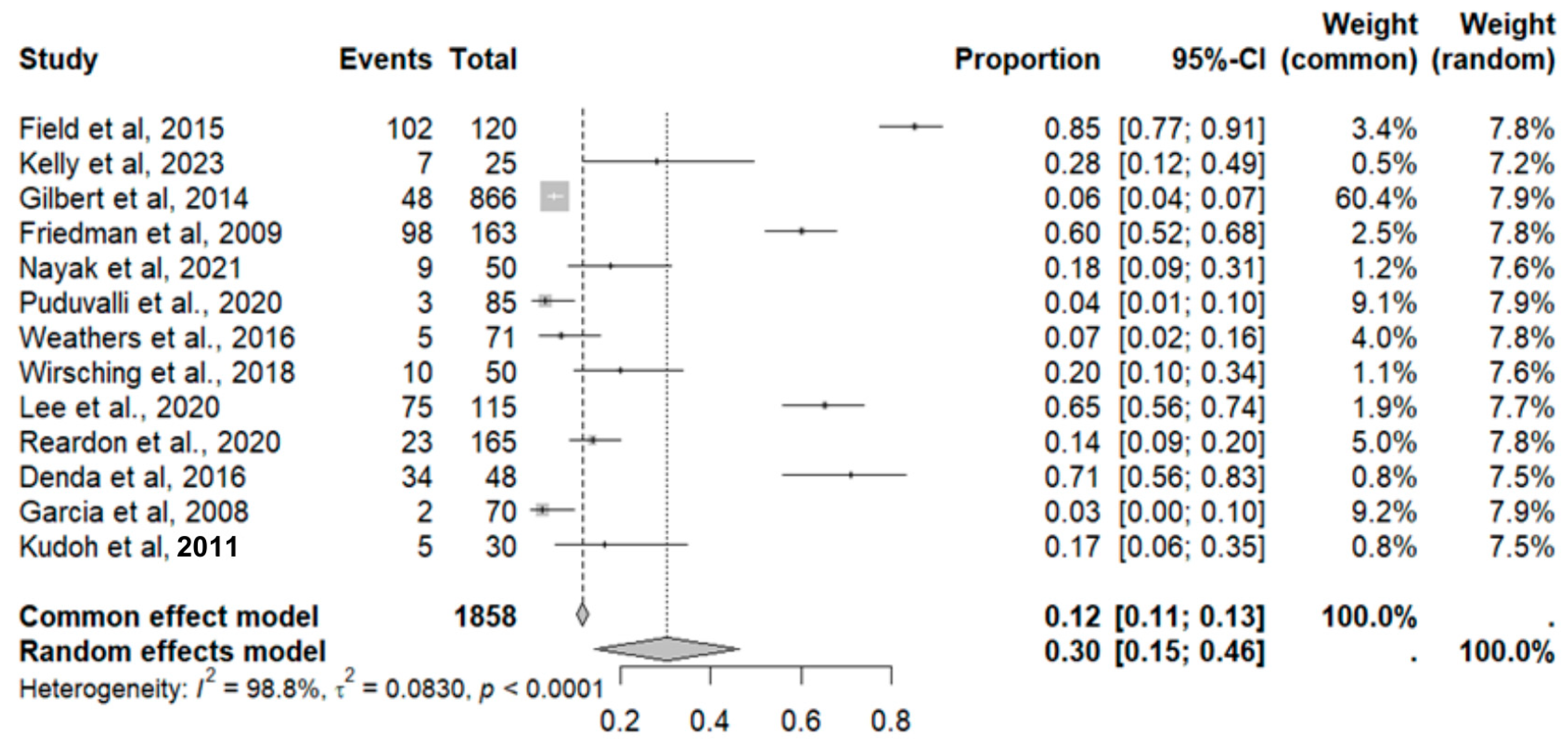
2.8. Adverse Event Statistics in GBM
2.9. Adverse Event Statistics Total
2.9.1. General Adverse Effects of Bevacizumab (In Other Types of Cancers)
2.9.2. Arterial Hypertension
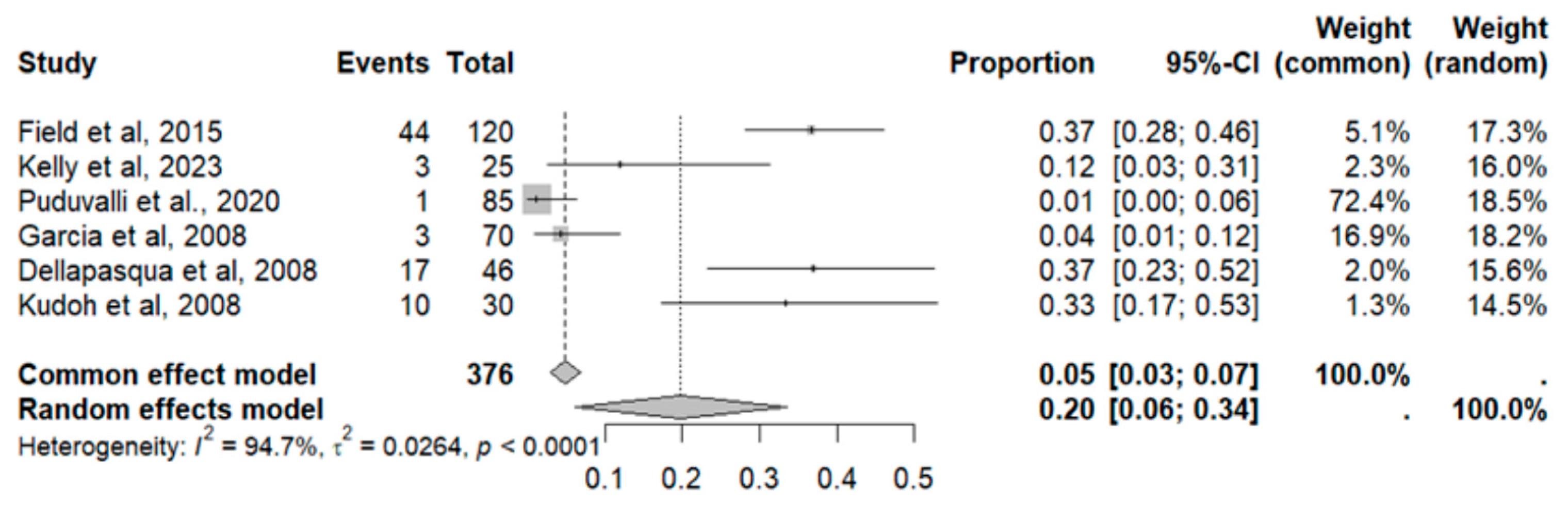
2.9.3. Diarrhea
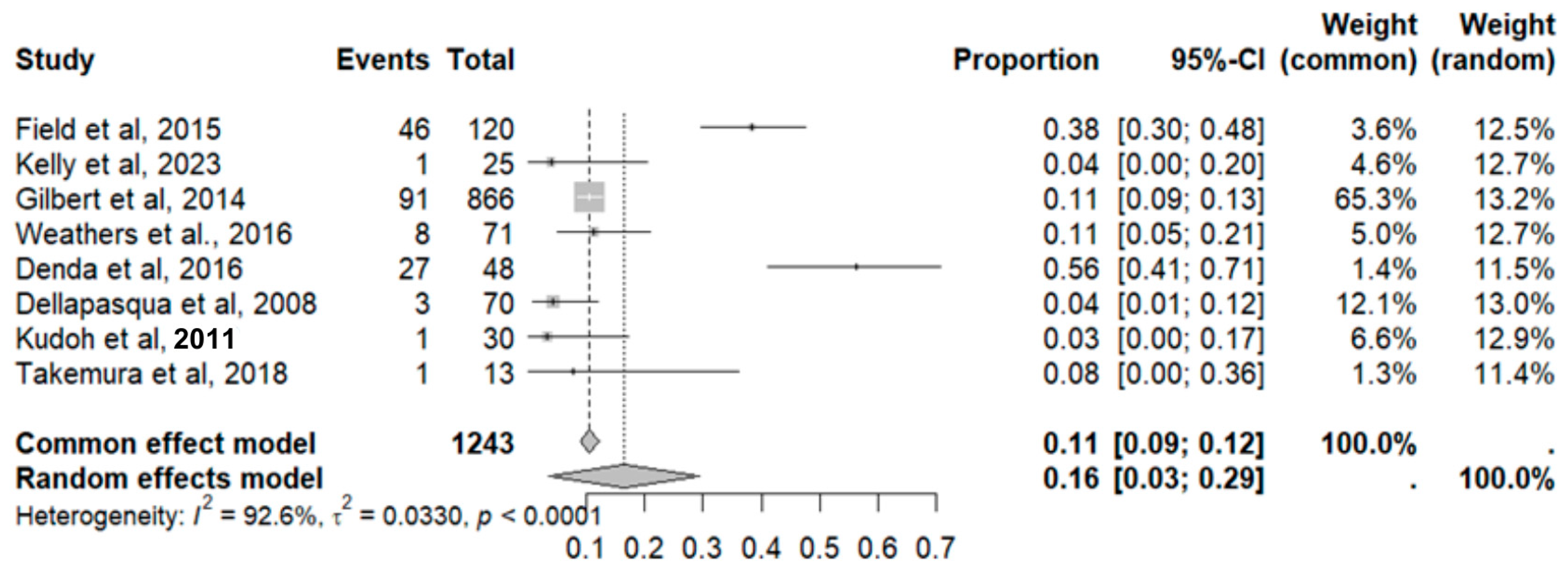
2.9.4. Headache
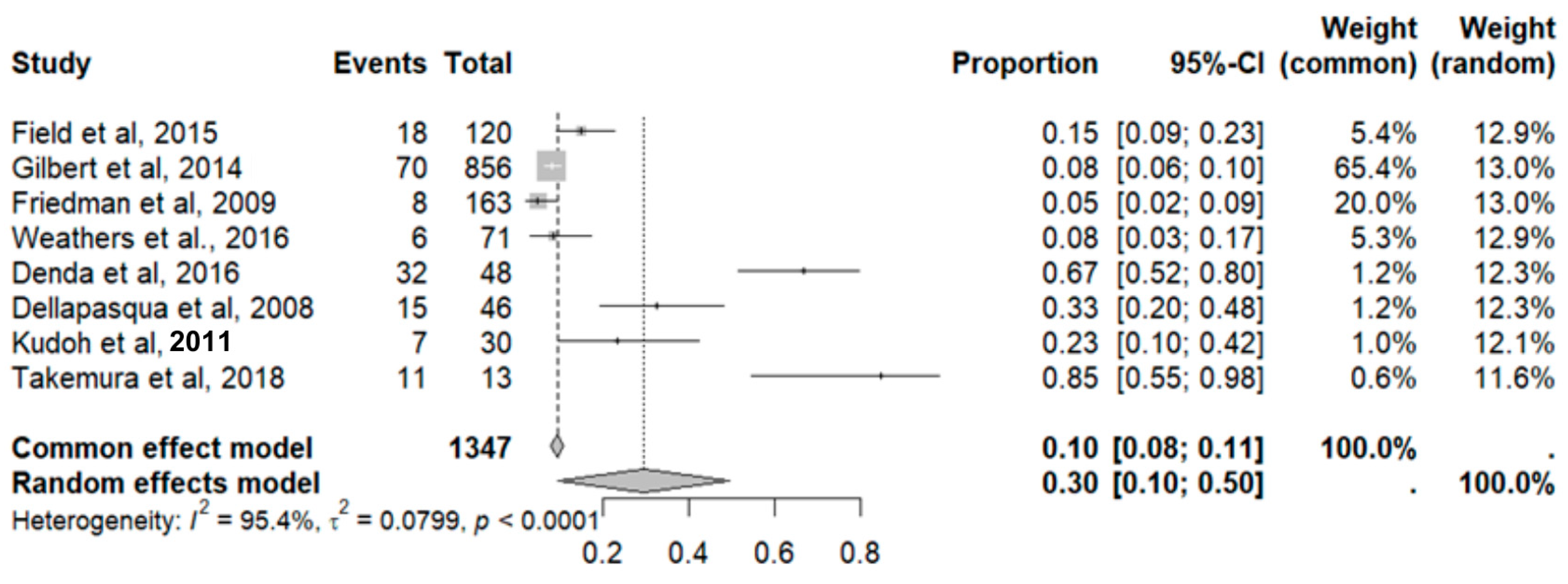
2.9.5. Fatigue
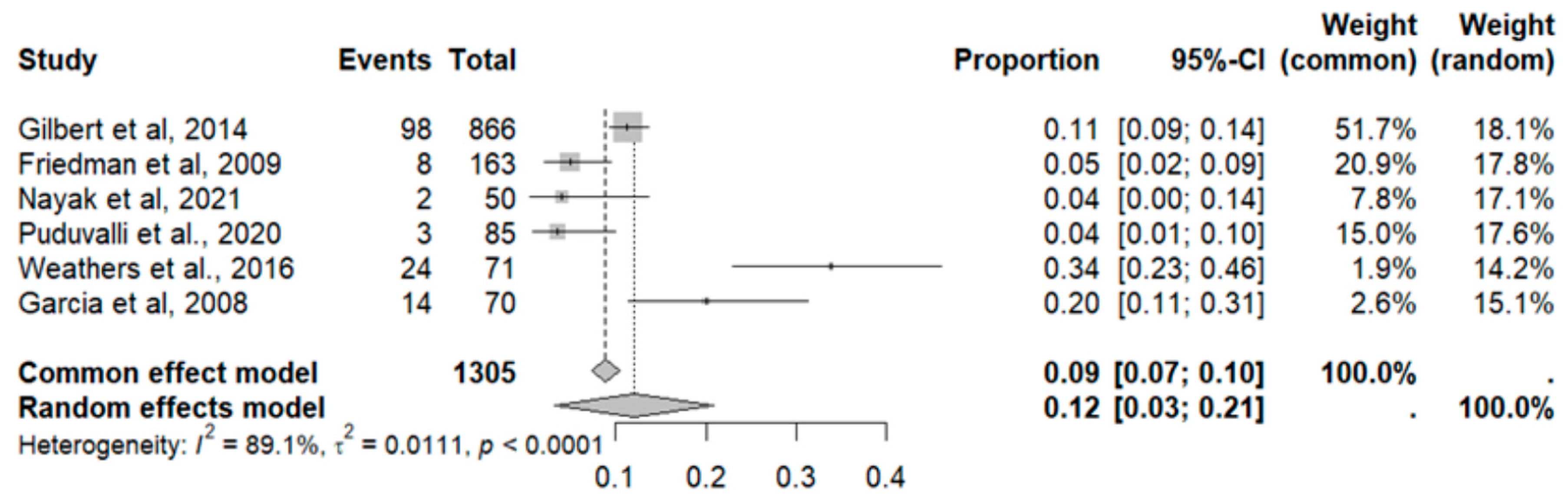
2.9.6. Constipation
2.9.7. Thrombocytopenia
2.9.8. Neutropenia
2.9.9. Lymphopenia
2.9.10. Nausea and Vomiting
2.9.11. Anemia
2.9.12. Anorexia
2.9.13. Thrombosis
2.9.14. Leukopenia
2.9.15. Proteinuria
3. Discussion
4. Materials and Methods
4.1. Protocol and Registration
4.2. Literature Search
4.3. Study Selection
4.4. Data Extraction and Quality Assessment
4.5. Statistical Methods
5. Conclusions
Limitations
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valenzuela-Fuenzalida, J.J.; Moyano-Valarezo, L.; Silva-Bravo, V.; Milos-Brandenberg, D.; Orellana-Donoso, M.; Nova-Baeza, P.; Suazo-Santibáñez, A.; Rodríguez-Luengo, M.; Oyanedel-Amaro, G.; Sanchis-Gimeno, J.; et al. Association between the anatomical location of Glioblastoma and its evaluation with clinical considerations: A systematic review and meta-analysis. J. Clin. Med. 2024, 13, 3460. [Google Scholar] [CrossRef]
- Urbańska, K.; Sokołowska, J.; Szmidt, M.; Sysa, P. Glioblastoma multiforme—An overview. Contemp. Oncol. 2014, 18, 307–312. [Google Scholar] [CrossRef]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef]
- Angel, R.-P.M. Glioblastoma Multiforme: Reporte de un caso. Lux Médica 2015, 10, 47–52. [Google Scholar]
- Sinning, M.; Fariña, A.; Valenzuela, R.; Bennett, C.; Riveros, R.; Torres, F.; Patricio Paredes, M.; Roger Gejman, E.; Ronny Muñoz, M.; Ríos, J.A. Consenso Chileno Para el Diagnóstico y Tratamiento De Gliomas del Adulto. Rev. Chil. Neuro-Psiquiatr. 2022, 60, 102–115. [Google Scholar] [CrossRef]
- Solís, S.T.; Ahicart, G.P.; Lozano, I.I.; Schmidt, C.d.Q.; Coello, A.F.; Panisello, C.H.; Urzaiz, L.L.; Romero, J.C.G.; Valle, R.D.; Sánchez, J.G.; et al. Consenso sobre guías de tratamiento de los glioblastomas elaborado por el Grupo de Trabajo de Neurooncología (GTNO) de la SENEC. Neurocirugia 2020, 31, 289–298. [Google Scholar] [CrossRef]
- Kazazi-Hyseni, F.; Beijnen, J.H.; Schellens, J.H.M. Bevacizumab. Oncologist 2010, 15, 819–825. [Google Scholar] [CrossRef]
- Wick, W.; Weller, M.; Bent, M.v.D.; Stupp, R. Bevacizumab and recurrent malignant gliomas: A European perspective. J. Clin. Oncol. 2010, 28, e188–e189. [Google Scholar] [CrossRef]
- Liao, Y.; Bai, X.; Cao, Y.; Zhang, M. Effect of low-dose Bevacizumab on health-related quality of life in patients with recurrent high-grade glioma: A retrospective clinical study. J. Clin. Neurosci. 2024, 120, 196–203. [Google Scholar] [CrossRef]
- Agencia Española de Medicamentos y Productos Sanitarios AE. Ficha Tecnica Avastin 25 Mg/Ml Concentrado Para Solucion Para Perfusion. Aemps.es. Available online: https://cima.aemps.es/cima/dochtml/ft/04300002/FT_04300002.html (accessed on 11 December 2024).
- Friedman, H.S.; Prados, M.D.; Wen, P.Y.; Mikkelsen, T.; Schiff, D.; Abrey, L.E.; Yung, W.A.; Paleologos, N.; Nicholas, M.K.; Jensen, R.; et al. Bevacizumab Alone and in Combination With Irinotecan in Recurrent Glioblastoma. J. Clin. Oncol. 2009, 27, 4733–4740. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Tsien, C.I.; Pugh, S.L.; Dicker, A.P.; Raizer, J.J.; Matuszak, M.M.; Lallana, E.C.; Huang, J.; Algan, O.; Deb, N.; Portelance, L.; et al. NRG Oncology/RTOG1205: A Randomized Phase II Trial of Concurrent Bevacizumab and Reirradiation Versus Bevacizumab Alone as Treatment for Recurrent Glioblastoma. J. Clin. Oncol. 2023, 41, 1285–1295. [Google Scholar] [CrossRef]
- Lee, E.Q.; Zhang, P.; Wen, P.Y.; Gerstner, E.R.; Reardon, D.A.; Aldape, K.D.; Degroot, J.F.; Pan, E.; Raizer, J.J.; Kim, L.J.; et al. NRG/RTOG 1122: A phase 2, double-blinded, placebo-controlled study of Bevacizumab with and without trebananib in patients with recurrent Glioblastoma or gliosarcoma. Cancer 2020, 126, 2821–2828. [Google Scholar] [CrossRef]
- Puduvalli, V.K.; Wu, J.; Yuan, Y.; Armstrong, T.S.; Vera, E.; Wu, J.; Xu, J.; Giglio, P.; Colman, H.; Walbert, T.; et al. A Bayesian adaptive randomized phase II multicenter trial of Bevacizumab with or without vorinostat in adults with recurrent Glioblastoma. Neuro-Oncology 2020, 22, 1505–1515. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients with Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. Available online: https://jamanetwork.com/journals/jamaoncology/fullarticle/2766213 (accessed on 15 May 2025). [CrossRef]
- Weathers, S.-P.; Han, X.; Liu, D.D.; Conrad, C.A.; Gilbert, M.R.; Loghin, M.E.; O’brien, B.J.; Penas-Prado, M.; Puduvalli, V.K.; Tremont-Lukats, I.; et al. A randomized phase II trial of standard dose Bevacizumab versus low dose BV plus lomustine (CCNU) in adults with recurrent Glioblastoma. J. Neurooncol. 2016, 129, 487–494. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Tabatabai, G.; Roelcke, U.; Hottinger, A.F.; Jörger, F.; Schmid, A.; Plasswilm, L.; Schrimpf, D.; Mancao, C.; Capper, D.; et al. Bevacizumab plus hypofractionated radiotherapy versus radiotherapy alone in elderly patients with Glioblastoma: The randomized, open-label, phase II ARTE trial. Ann. Oncol. 2018, 29, 1423–1430. Available online: https://www.annalsofoncology.org/article/S0923-7534(19)34900-2/fulltext (accessed on 15 May 2025). [CrossRef]
- Field, K.M.; Simes, J.; Nowak, A.K.; Cher, L.; Wheeler, H.; Hovey, E.J.; Brown, C.S.; Barnes, E.H.; Sawkins, K.; Livingstone, A.; et al. Randomized phase 2 study of carboplatin and Bevacizumab in recurrent Glioblastoma. Neuro-Oncology 2015, 17, 1504–1513. [Google Scholar] [CrossRef]
- Kelly, W.; Duque, A.E.D.; Michalek, J.; Konkel, B.; Caflisch, L.; Chen, Y.; Pathuri, S.C.; Madhusudanannair-Kunnuparampil, V.; Floyd, J.; Brenner, A. Phase II investigation of TVB-2640 (denifanstat) with BV in patients with first relapse high-grade astrocytoma. Clin. Cancer Res. 2023, 29, 2419–2425. [Google Scholar] [CrossRef]
- Ellingson, B.M.; E Abrey, L.; Garcia, J.; Chinot, O.; Wick, W.; Saran, F.; Nishikawa, R.; Henriksson, R.; Mason, W.P.; Harris, R.J.; et al. Post-chemoradiation volumetric response predicts survival in newly diagnosed GBM treated with radiation, temozolomide, and Bevacizumab or placebo. Neuro-Oncology 2018, 20, 1525–1535. [Google Scholar] [CrossRef]
- Wirsching, H.-G.; Roelcke, U.; Weller, J.; Hundsberger, T.; Hottinger, A.F.; von Moos, R.; Caparrotti, F.; Conen, K.; Remonda, L.; Roth, P.; et al. MRI and 18FET-PET Predict Survival Benefit from Bevacizumab Plus Radiotherapy in Patients with Isocitrate Dehydrogenase Wild-type GBM: Results from the Randomized ARTE Trial. Clin. Cancer Res. 2021, 27, 179–188. [Google Scholar] [CrossRef]
- Kickingereder, P.; Brugnara, G.; Hansen, M.B.; Nowosielski, M.; Pflüger, I.; Schell, M.; Isensee, F.; Foltyn, M.; Neuberger, U.; Kessler, T.; et al. Noninvasive Characterization of Tumor Angiogenesis and Oxygenation in Bevacizumab-treated Recurrent Glioblastoma by Using Dynamic Susceptibility MRI: Secondary Analysis of the European Organization for Research and Treatment of Cancer 26101 Trial. Radiology 2020, 297, 164–175. [Google Scholar] [CrossRef]
- Herrlinger, U.; Schäfer, N.; Steinbach, J.P.; Weyerbrock, A.; Hau, P.; Goldbrunner, R.; Friedrich, F.; Rohde, V.; Ringel, F.; Schlegel, U.; et al. Bevacizumab Plus Irinotecan Versus Temozolomide in Newly Diagnosed O6-Methylguanine–DNA Methyltransferase Nonmethylated Glioblastoma: The Randomized GLARIUS Trial. J. Clin. Oncol. 2016, 34, 1611–1619. [Google Scholar] [CrossRef]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of Bevacizumab for newly diagnosed Glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. Available online: https://www.ncbi.nlm.nih.gov/pubmed/24552317/ (accessed on 15 May 2025). [CrossRef]
- von Hippel, P.T. The heterogeneity statistic I(2) can be biased in small meta-analyses. BMC Med. Res. Methodol. 2015, 15, 35. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fu, R.; Gartlehner, G.; Grant, M.; Shamliyan, T.; Sedrakyan, A.; Wilt, T.J.; Griffith, L.; Oremus, M.; Raina, P.; Ismaila, A.; et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J. Clin. Epidemiol. 2011, 64, 1187–1197. [Google Scholar] [CrossRef]
- Furuya-Kanamori, L.; Barendregt, J.J.; Doi, S.A. A new improved graphical and quantitative method for detecting bias in meta-analysis. Int. J. Evid.-Based Healthc. 2018, 16, 195–203. [Google Scholar] [CrossRef]
- Denda, T.; Kanda, M.; Morita, Y.; Kim, H.M.; Kashiwada, T.; Matsuda, C.; Fujieda, S.; Nakata, K.; Murotani, K.; Oba, K.; et al. Pharmacokinetic dose adjustment of 5-FU in modified FOLFOX7 plus bevacizumab for metastatic colorectal cancer in Japanese patients: A-JUST phase II clinical trial. Cancer Chemother. Pharmacol. 2016, 78, 1253–1261. [Google Scholar] [CrossRef]
- Garcia, A.A.; Hirte, H.; Fleming, G.; Yang, D.; Tsao-Wei, D.D.; Roman, L.; Groshen, S.; Swenson, S.; Markland, F.; Gandara, D.; et al. Phase II clinical trial of bevacizumab and low-dose metronomic oral cyclophosphamide in recurrent ovarian cancer: A trial of the California, Chicago, and Princess Margaret Hospital phase II consortia. J. Clin. Oncol. 2008, 26, 76–82. [Google Scholar] [CrossRef]
- Dellapasqua, S.; Bertolini, F.; Bagnardi, V.; Campagnoli, E.; Scarano, E.; Torrisi, R.; Shaked, Y.; Mancuso, P.; Goldhirsch, A.; Rocca, A.; et al. Metronomic cyclophosphamide and capecitabine combined with bevacizumab in advanced breast cancer. J. Clin. Oncol. 2008, 26, 4899–4905. [Google Scholar] [CrossRef]
- Kim, S.W.; Ha, B.J.; Kim, E.K.; Tchah, H.; Kim, T.-I. The effect of topical bevacizumab on corneal neovascularization. Ophthalmology 2008, 115, e33–e38. [Google Scholar] [CrossRef]
- Dashti, S.R.; Kadner, R.J.; Folley, B.S.; Sheehan, J.P.; Han, D.Y.; Kryscio, R.J.; Carter, M.B.; Shields, L.B.E.; Plato, B.M.; La Rocca, R.V.; et al. Single low-dose targeted bevacizumab infusion in adult patients with steroid-refractory radiation necrosis of the brain: A phase II open-label prospective clinical trial. J. Neurosurg. 2022, 137, 1676–1686. [Google Scholar] [CrossRef]
- Spitzer, M.S.; Ziemssen, F.; Yoeruek, E.; Petermeier, K.; Aisenbrey, S.; Szurman, P. Efficacy of intravitreal bevacizumab in treating postoperative pseudophakic cystoid macular edema. J. Cataract. Refract. Surg. 2008, 34, 70–75. [Google Scholar] [CrossRef]
- Kudoh, K.; Takano, M.; Kouta, H.; Kikuchi, R.; Kita, T.; Miyamoto, M.; Watanabe, A.; Kato, M.; Goto, T.; Kikuchi, Y. Effects of bevacizumab and pegylated liposomal doxorubicin for the patients with recurrent or refractory ovarian cancers. Gynecol. Oncol. 2011, 122, 233–237. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Birrer, M.J.; Penson, R.T.; Huang, H.; Moore, D.H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; et al. Final survival analysis of topotecan and paclitaxel for first-line treatment of advanced cervical cancer: An NRG oncology randomized study. Gynecol. Oncol. 2023, 171, 141–150. [Google Scholar] [CrossRef]
- Dupuis-Girod, S.; Ambrun, A.; Decullier, E.; Samson, G.; Roux, A.; Fargeton, A.-E.; Rioufol, C.; Schwiertz, V.; Disant, F.; Chapuis, F.; et al. ELLIPSE Study: A Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia: A Phase 1 study evaluating the tolerance of bevacizumab nasal spray in the treatment of epistaxis in hereditary hemorrhagic telangiectasia. MAbs 2014, 6, 794–799. [Google Scholar] [CrossRef]
- Kennedy, K.A.; Mintz-Hittner, H.A. BEAT-ROP Cooperative Group. Medical and developmental outcomes of bevacizumab versus laser for retinopathy of prematurity. J. Am. Assoc. Pediatr. Ophthalmol. Strabismus 2018, 22, 61–65.e1. [Google Scholar] [CrossRef]
- Takemura, Y.; Chihara, Y.; Morimoto, Y.; Tanimura, K.; Imabayashi, T.; Seko, Y.; Kaneko, Y.; Date, K.; Ueda, M.; Arimoto, T.; et al. Feasibility study of sequentially alternating EGFR-TKIs and chemotherapy for patients with non-small cell lung cancer. Anticancer. Res. 2018, 38, 2385–2390. [Google Scholar] [CrossRef]
- Dongpo, S.; Zhengyao, Z.; Xiaozhuo, L.; Qing, W.; Mingming, F.; Fengqun, M.; Mei, L.; Qian, H.; Tong, C. Efficacy and safety of Bevacizumab combined with other therapeutic regimens for treatment of recurrent Glioblastoma: A network meta-analysis. World Neurosurg. 2022, 160, e61–e79. [Google Scholar] [CrossRef]
- Li, X.; Huang, R.; Xu, Z. Risk of adverse vascular events in newly diagnosed Glioblastoma multiforme patients treated with Bevacizumab: A systematic review and meta-analysis. Sci. Rep. 2015, 5, 14698. [Google Scholar] [CrossRef]
- Liu, D.; Li, Z. Survival prediction using apparent diffusion coefficient values in recurrent Glioblastoma under Bevacizumab treatment: An updated systematic review and meta-analysis. Diagn. Interv. Radiol. 2024, 30, 270–274. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, S.; Wang, Z. A meta-analysis of Bevacizumab alone and in combination with irinotecan in the treatment of patients with recurrent Glioblastoma multiforme. J. Clin. Neurosci. 2012, 19, 1636–1640. [Google Scholar] [CrossRef]
- Zhao, B.; Wu, J.; Xia, Y.; Li, H.; Wang, Y.; Qu, T.; Xing, H.; Wang, Y.; Ma, W. Comparative efficacy and safety of therapeutics for elderly glioblastoma patients: A Bayesian network analysis. Pharmacol. Res. 2022, 182, 106316. [Google Scholar] [CrossRef] [PubMed]
- Straube, C.; Kessel, K.A.; Zimmer, C.; Schmidt-Graf, F.; Schlegel, J.; Gempt, J.; Meyer, B.; Combs, S.E. A Second Course of Radiotherapy in Patients with Recurrent Malignant Gliomas: Clinical Data on Re-irradiation, Prognostic Factors, and Usefulness of Digital Biomarkers. Curr. Treat. Options Oncol. 2019, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Helmi, A.; Chan, A.; Towfighi, S.; Kapadia, A.; Perry, J.; Ironside, S.; Machnowska, M.; Symons, S.P.; Fox, A.J.; Sahgal, A.; et al. Incidence of Dural Venous Sinus Thrombosis in Patients with Glioblastoma and Its Implications. World Neurosurg. 2019, 125, e189–e197. [Google Scholar] [CrossRef] [PubMed]
- Lam, L.; Mumford, J.; Keber, B.; Flanagan, B. Hematologic Conditions: Leukopenia. FP Essent. 2019, 485, 11–16. [Google Scholar] [PubMed]
- Roa-Chamorro, R.; Torres-Quintero, L. Monitorización ambulatoria de la presión arterial en pacientes con enfermedades oncohematológicas [Ambulatory blood pressure monitoring in patients with onco-hematological diseases]. Hipertens. Riesgo Vasc. 2023, 40, 132–136. (In Spanish) [Google Scholar] [CrossRef] [PubMed]
- Valko, P.O.; Siddique, A.; Linsenmeier, C.; Zaugg, K.; Held, U.; Hofer, S. Prevalence and predictors of fatigue in glioblastoma: A prospective study. Neuro-Oncology 2015, 17, 274–281. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Scheer, K.G.; Ebert, L.M.; Samuel, M.S.; Bonder, C.S.; Gomez, G.A. Bevacizumab-Induced Hypertension in Glioblastoma Patients and Its Potential as a Modulator of Treatment Response. Hypertension 2023, 80, 1590–1597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brown, P.; Zhou, Y. Large expert-curated database for benchmarking document similarity detection in biomedical literature search. Database 2019, 2019, baz085. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 74, 790–799. [Google Scholar]
- Sterne, J.A.C.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Cheng, A.L.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Lim, H.Y.; Kudo, M.; Breder, V.; Merle, P.; et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J. Hepatol. 2022, 76, 862–873. [Google Scholar] [CrossRef]
- Qin, S.; Chen, M.; Cheng, A.L.; Kaseb, A.O.; Kudo, M.; Lee, H.C.; Yopp, A.C.; Zhou, J.; Wang, L.; Wen, X.; et al. Atezolizumab plus bevacizumab versus active surveillance in patients with resected or ablated high-risk hepatocellular carcinoma (IMbrave050): A randomised, open-label, multicentre, phase 3 trial. Lancet 2023, 402, 1835–1847. [Google Scholar] [CrossRef]
- Prager, G.W.; Taieb, J.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Pápai, Z.; et al. Trifluridine-Tipiracil and Bevacizumab in Refractory Metastatic Colorectal Cancer. N. Engl. J. Med. 2023, 388, 1657–1667. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef]
- Oaknin, A.; Gladieff, L.; Martínez-García, J.; Villacampa, G.; Takekuma, M.; De Giorgi, U.; Lindemann, K.; Woelber, L.; Colombo, N.; Duska, L.; et al. Atezolizumab plus bevacizumab and chemotherapy for metastatic, persistent, or recurrent cervical cancer (BEATcc): A randomised, open-label, phase 3 trial. Lancet 2024, 403, 31–43. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Rimini, M.; Tada, T.; Suda, G.; Shimose, S.; Kudo, M.; Cheon, J.; Finkelmeier, F.; Lim, H.Y.; Rimassa, L.; et al. Atezolizumab plus bevacizumab versus lenvatinib for unresectable hepatocellular carcinoma: A large real-life worldwide population. Eur. J. Cancer 2023, 180, 9–20. [Google Scholar] [CrossRef]
- Tewari, K.S.; Sill, M.W.; Penson, R.T.; Huang, H.; Ramondetta, L.M.; Landrum, L.M.; Oaknin, A.; Reid, T.J.; Leitao, M.M.; Michael, H.E.; et al. Bevacizumab for advanced cervical cancer: Final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240). Lancet 2017, 390, 1654–1663. [Google Scholar] [CrossRef]
- McDermott, D.F.; Huseni, M.A.; Atkins, M.B.; Motzer, R.J.; Rini, B.I.; Escudier, B.; Fong, L.; Joseph, R.W.; Pal, S.K.; Reeves, J.A.; et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat. Med. 2018, 24, 749–757. [Google Scholar] [CrossRef]
- Eroğlu, Y.; Baykara, M.; Perçinel Yazici, İ.; Utku Yazici, K.; Kürşad Poyraz, A. Evaluation of the corpus callosum using magnetic resonance imaging histogram analysis in autism spectrum disorder. Neuroradiol. J. 2022, 35, 751–757. [Google Scholar] [CrossRef]
- Vergote, I.; Van Nieuwenhuysen, E.; O’Cearbhaill, R.E.; Westermann, A.; Lorusso, D.; Ghamande, S.; Collins, D.C.; Banerjee, S.; Mathews, C.A.; Gennigens, C.; et al. Tisotumab Vedotin in Combination with Carboplatin, Pembrolizumab, or Bevacizumab in Recurrent or Metastatic Cervical Cancer: Results From the innovaTV 205/GOG-3024/ENGOT-cx8 Study. J. Clin. Oncol. 2023, 41, 5536–5549. [Google Scholar] [CrossRef]
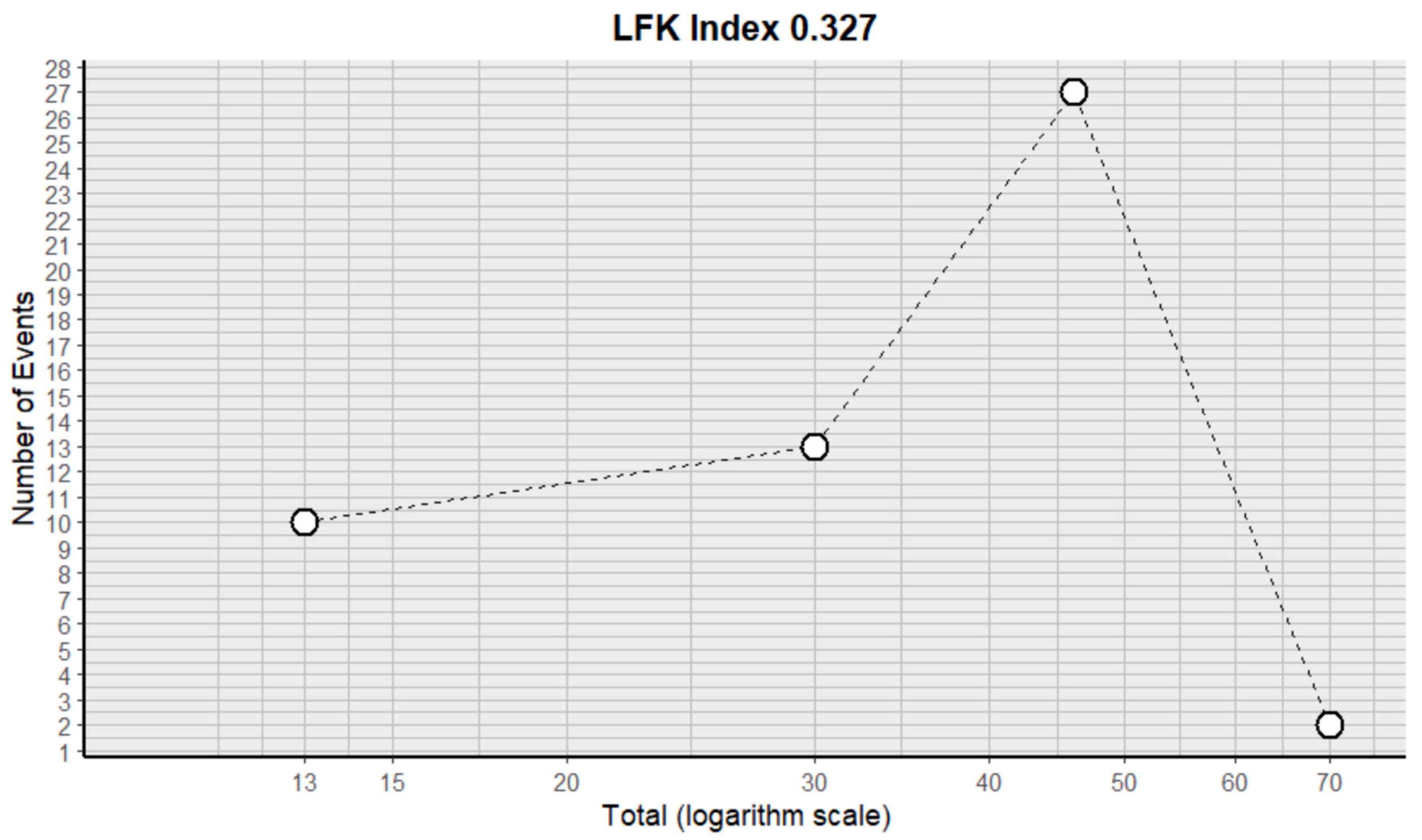
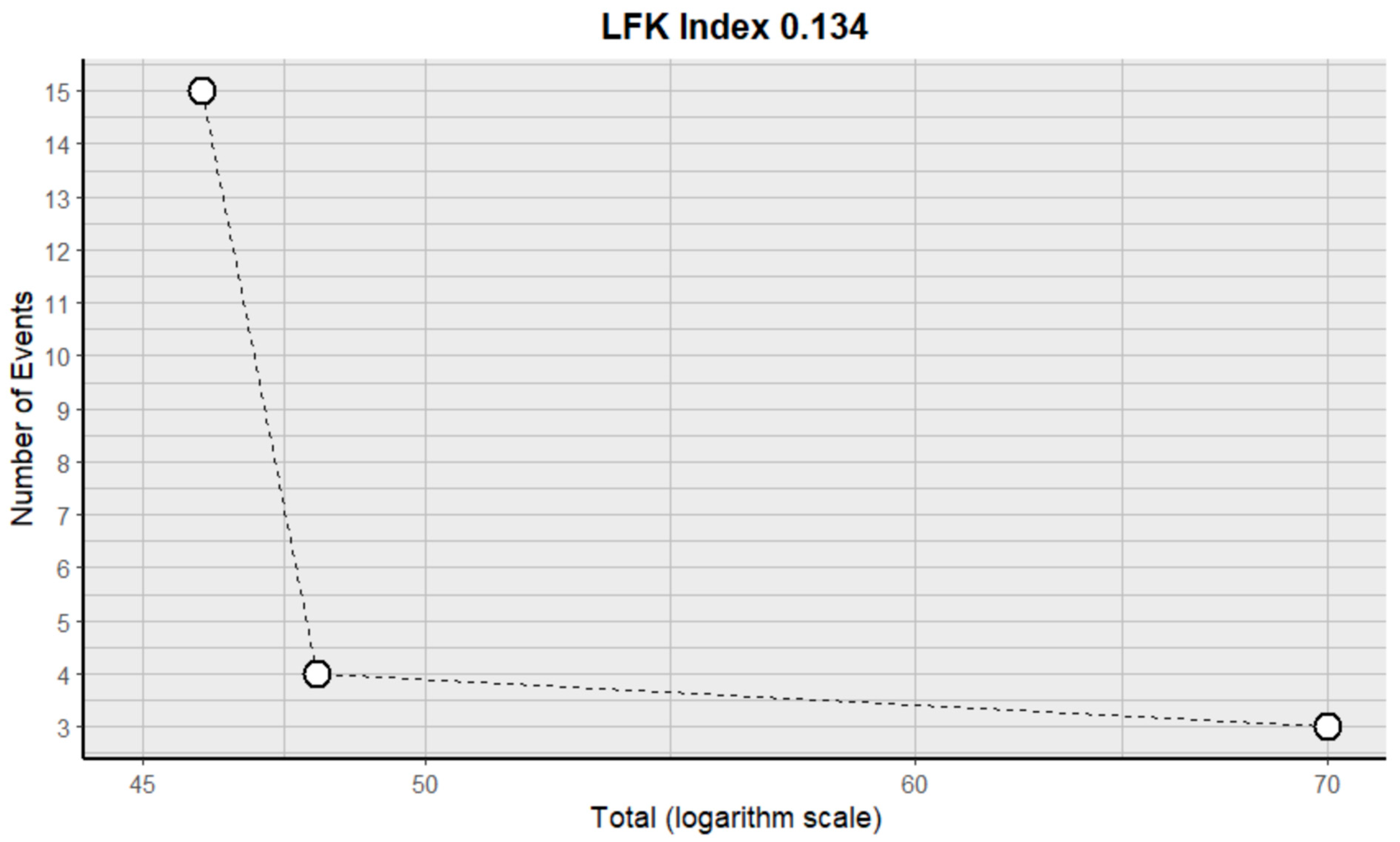
| Author | Country | Population | Intervention | Outcomes | Follow-Up | Results | ||
|---|---|---|---|---|---|---|---|---|
| Sample Size (n) | Patients’ Mean Age (SD) | Intervention | Characteristics/Dose | |||||
| Friedman et al., 2009 [11] | USA | CG: 82 EG: 85 | CG: 57 EG: 54 | CG: BV + CP-11 EG: BV |
|
| 2 months |
|
| Nayak et al., 2021 [12] | USA | CG: 30 EG: 50 | CG: Not mentioned EG: Not mentioned | CG: pembrolizumab EG: pembrolizumab + BV |
|
|
| |
| Tsien et al., 2023 [13] | USA | CG: 86 EG: 84 | CG: 60 EG: 57 | CG: BV + RT EG: BV |
| Not mentioned | Not mentioned | |
| Lee et al., 2020 [14] | USA | CG: 58 EG: 57 | CG: No average mentioned EG: No average mentioned | CG: BV + trebananib EG: BV + placebo |
| 6 months |
| |
| Puduvalli et al., 2020 [15] | USA | CG: 44 EG: 30 | CG: No average is mentioned EG: No average is mentioned | CG: BV + Vor EG: BV |
|
| 19.84 months average |
|
| Reardon et al., 2020 [16] | USA | CG: 182 EG: 165 | CG: 55.2 EG: 55 | CG: Nivolumab EG: BV |
| 9.5 months |
| |
| Weathers et al., 2016 [17] | USA | CG: 33 EG: 36 | CG: No average is mentioned EG: No average is mentioned | CG: BV + lomustine EG: BV |
| 6 months |
| |
| Wirsching et al., 2018 [18] | Suiza | CG: 25 EG: 50 | CG: 70 EG: 70 | CG: B (only radiotherapy) EG: A (radiotherapy + BV) See radiotherapy |
| Not mentioned |
| |
| Field et al., 2015 [19] | Australia | BV + ICE: 58. BV: 62. | BV + Carboplatin: 55. BV: 55. | BV + carboplatin BV |
| None | 32 months |
|
| Kelly et al., 2023 [20] | USA | EG: 60 | EG: 59 | A1: TVB-2640 + BV. A2: BV. |
| None | Not mentioned |
|
| Ellingson et al., 2018 [21] | USA | CG: 459 EG: 458 | CG: 56. EG: 55. | CG: Chemoradiation + placebo. EG: Chemoradiation + BV See radiotherapy |
|
| Not mentioned |
|
| Wirsching et al., 2021 [22] | Switzerland | BV + RTH: 44 RTH: 23 | BV + RTH: 70 RTH: 69 | BV + RTH RTH |
|
| 4 months |
|
| Kickingereder et al., 2020 [23] | Germany | CG: 93 EG: 161 | CG: 60 EG: 58 | BV groups: BV/BV + lomustina NO-BV group: Lomustina |
|
| Not mentioned |
|
| Herrlinger et al., 2016 [24] | Germany | CG: 54 EG: 116 | CG: 56 EG: 56 | CG: TMZ + RT EG: BV + RT + IRI |
|
| 12 weeks |
|
| Gilbert et al., 2014 [25] | USA | CG: 309. EG: 312. | None | CG: Placebo EG: BV |
|
| Patients evaluated weekly for adverse events had weekly complete blood counts and monthly blood chemical analyses during radiotherapy During maintenance phase of treatment, patients underwent hemograms and blood chemical analyses on days 21 and 28 of each cycle Imaging studies of tumors |
|
| Author | Confounding | Selection | Measurement of Intervention | Missing Data | Measurement of Outcomes | Reported Result | Overall |
|---|---|---|---|---|---|---|---|
| Friedman et al., 2009 [11] | Low | Serious | Serious | Moderate | Low | Low | Moderate |
| Gilbert et al., 2014 [25] | Low | Low | Low | Low | Low | Low | Low |
| Field et al., 2015 [19] | Low | Serious | Serious | Moderate | Low | Low | Moderate |
| Herrlinger et al., 2016 [24] | Low | Low | Low | Low | Low | Low | Low |
| Weathers et al., 2016 [17] | Moderate | Low | Low | Low | Low | Low | Low |
| Ellingson et al., 2018 [21] | Low | Serious | Serious | Moderate | Low | Low | Moderate |
| Wirsching et al., 2018 [18] | Low | Low | Low | Low | Low | Low | Moderate |
| Kickingereder et al., 2020 [23] | Moderate | Serious | Moderate | Low | Serious | Moderate | Serious |
| Lee et al., 2020 [14] | Moderate | Serious | Serious | Moderate | Low | Low | Moderate |
| Puduvalli et al., 2020 [15] | Serious | Serious | Serious | Moderate | Low | Low | Serious |
| Reardon et al., 2020 [16] | Low | Low | Low | Low | Low | Low | Low |
| Wirsching et al., 2021 [22] | Low | Serious | Moderate | Low | Serious | Moderate | Moderate |
| Nayak et al., 2021 [12] | Low | Serious | Serious | Moderate | Low | Low | Moderate |
| Kelly et al., 2023 [20] | Low | Low | Low | Low | Low | Low | Low |
| Tsien et al., 2023 [13] | Low | Serious | Moderate | Low | Serious | Moderate | Moderate |
| Author | Diarrhea | Hypophosphatemia | Nausea and Vomiting | Headache | Fatigue | Constipation | Thrombocytopenia | Neutropenia | Lymphopenia | Anemia | High Blood Pressure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Field et al., 2015 [19] | 24% (15/62) | Not reported | 39% (24/62) | Not reported | 84% (52/62) | 29% (18/62) | 23% (14/62) | 6% (4/62) | Not reported | 10% (6/62) | Not reported |
| Kelly et al., 2023 [20] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Ellingson et al., 2018 [21] | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used |
| Wirsching et al., 2021 [22] | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used |
| Kickingereder et al., 2020 [23] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Herrlinger et al., 2016 [24] | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used |
| Gilbert et al., 2014 [25] | Not reported | Not reported | 0.66% (2/303) | Not reported | 2.3% (7/303) | Not reported | 10.2% (31/303) | 7.3% (22/303) | 10.5% (32/303) | 0.66% (2/303) | Not reported |
| Tsien et al., 2023 [13] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | 42.2% (24/57) |
| Friedman et al., 2009 [11] | 1.2% (1/84) | Not reported | Not reported | 36.9% (31/84) | 45.2% (38/84) | Not reported | Not reported | 1.2% (1/84) | 2.4% (2/84) | Not reported | 29.8% (30/84) |
| Nayak et al., 2021 [12] | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used |
| Puduvalli et al., 2020 [15] | Not reported | Not reported | 5.2% (2/38) | 5.2% (2/38) | 2.6% (1/38) | 0% 0/38 | Not reported | Not reported | 2.6% (1/38) | Not reported | 23.7 (9/38) |
| Weathers et al., 2016 [17] | Not reported | Not reported | Not reported | Not reported | 11.1% (4/36) | Not reported | 0% (0/36) | 2.8% (1/36) | 22.2% (8/36) | Not reported | 5.6% (2/36) |
| Wirsching et al., 2018 [18] | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used | BV alone was not used |
| Lee et al., 2020 [14] | Not reported | 14% (8/57) | 24.6% (14/57) * | 57.9% (33/57) | 66.7% (38/57) | Not reported | Not reported | Not reported | Not reported | Not reported | 42.1% (24/57) |
| Reardon et al., 2020 [16] | 1.2% (2/165) | not reported | 5.5% (9/165) * | 4.8% (8/165) | 13.9% (23/165) | not reported | not reported | not reported | not reported | not reported | 22.4% (37/165) |
| Total percentage in the articles that reported the effects | 5.8% | 14% | 9.2% | 36.87% | 24.1% | 29% | 12.32% | 5.77% | 9.32% | 2.19% | 32.72% |
| Author | BV + X | Diarrhea | Hypophosphatemia | Nausea and Vomiting | Headache | Fatigue | Constipation | Thrombocytopenia | Neutropenia | Lymphopenia | Anemia | High Blood Pressure |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Field et al., 2015 [19] | BV + Carboplatin | 26% (15/58) | Not reported | 50% (29/58) | Not reported | 86% (50/58) | 45% (26/58) | 55% (32/58) | 24% (14/58) | Not reported | 28% (16/58) | Not reported |
| Kelly et al., 2023 [20] | BV + Denifanstat | 8% (2/25) | Not reported | 8% (2/25) (only vomiting) | 12% (3/25) | 0.28% (7/25) | 0.12% (3/25) | 4% (1/25) | Not reported | Not reported | Not reported | 52% (13/25) |
| Ellingson et al., 2018 [21] | BV + Radiotherapy + Temozolomide | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Wirsching et al., 2021 [22] | BV + Hypofractionated Radiotherapy | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Kickingereder et al., 2020 [23] | BV + Lomustina | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Herrlinger et al., 2016 [24] | BV + Radiotherapy + Irinotecan | Not reported | Not reported | 4.2% | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Gilbert et al., 2014 [25] | BV + Radiotherapy + Temozolomide | Not reported | Not reported | 0.66% (2/303 during chemotherapy) 4% (11/260 during adjuvant treatment) | Not reported | 2% (7/303 during chemotherapy) 13% (34/260 during adjuvant treatment) | Not reported | 10% (31/303 during chemotherapy) 11% (29/260 during adjuvant treatment) | 7% (22/303 during chemotherapy) 10% (26/250 during adjuvant treatment) | 11% (32/303 during chemotherapy) 13% (34/260 during adjuvant treatment) | 0.66% (2/303 during chemotherapy) 2% (6/260 during adjuvant treatment). | 1.32% (4/303 during chemotherapy) 4% (11/260 during adjuvant treatment) |
| Tsien et al., 2023 [13] | BV + Radiotherapy | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Friedman et al., 2009 [11] | BV + Irinotecan | 74.7% (59/79) | Not reported | 67.1% * (53/79) | Not reported | 75.9% (60/79) | Not reported | Not reported | 8.9% (7/79) | 7.6% (6/79) | Not reported | 26.6% (21/79) |
| Nayak et al., 2021 [12] | BV + Pembrolizumab | 6% (3/50) | Not reported | Not reported | 16% (8/50) | 18% (9/50) | Not reported | Not reported | Not reported | 4% (2/50) | Not reported | 50% (25/50) |
| Puduvalli et al., 2020 [15] | BV + Vorinostat (Suberoylanidized Acid) | Not reported | Not reported | 0.0% (0/47) nausea and 0.0% (0/47) vomiting | 2.1% (1/47) | 4% (2/47) | 2.1% (1/47) | Not reported | Not reported | 4% (2/47) | Not reported | 13% (6/47) |
| Weathers et al., 2016 [17] | BV + Lomustina | Not reported | Not reported | Not reported | Not reported | 3% (1/35) | Not reported | 23% (8/35) | 14% (5/35) | 46% (16/35) | Not reported | 0.03% (1/35) |
| Wirsching et al., 2018 [18] | BV + Radiotherapy | Not reported | Not reported | Not reported | 8% (4/50) | 20% (10/50) | Not reported | Not reported | Not reported | Not reported | Not reported | 26% (13/50) |
| Lee et al., 2020 [14] | BV + Trebananib | Not reported | 8.6% (5/58) | 13.8% (8/58) | 34.5% (20/58) | 63.8% (37/58) | Not reported | Not reported | Not reported | Not reported | Not reported | 44.8% (26/58) |
| Reardon et al., 2020 [16] | - | - | - | - | - | - | - | - | - | - | - | - |
| Total percentage in the articles that reported the effects | - | 15 | 8.6 | 13.37 | 15.65 | 22.48 | 23 | 14.83 | 10.2 | 11.88 | 3.86 | 13.2 |
| Author | Diarrhea | Anorexia | Nausea and Vomiting | Headache | Fatigue | Thrombosis | Thrombocytopenia | Neutropenia | Lymphopenia | Leukopenia | Anemia | High Blood Pressure | Proteinuria | Constipation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Denda et al., 2016 [29] | 12/48 25% | 28/48 58% | 24/48 50%, only nausea 10/48 21%, vomiting | Not reported | 34/48 71% | 2/48 4% | 27/48 56% | 32/48 67 | Not reported | Not reported | 44/48 92% | 11/48 23% | 4/48 8% | Not reported |
| Garcia et al., 2008 [30] | Not reported | Not reported | 2/70 2.9%, only nausea 3/70 4.3%, vomiting | Not reported | 6/70 8.6% | 3/70 4.3% | Not reported | Not reported | 14/70 20% | 2/70 2.9% | Not reported | 11/70 15.7% | 3/70 4.3% | 3/70 4.3% |
| Dellapasqua et al., 2008 [31] | 16/46 34.8% | 2/46 4.3% | 24/46 52.2%, only nausea 8/46 17.4%, vomiting | Not reported | Not reported | Not reported | 7/46 15.2% | 15/46 32.6% | Not reported | 27/46 58.7% | 8/46 17.4% | 22/46 47.8% | 15/46 32.6% | 17/46 37% |
| Kim et al., 2008 [32] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Dashti et al., 2022 [33] | Not reported | Not reported | 2/10 20% | 5/10 50% | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Spitzer et al., 2008 [34] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Kudoh et al., 2011 [35] | 4/30 13.3% | Not reported | 12/30 40%, only nausea 5/30 16.7%, vomiting | Not reported | 5/30 16.7% | Not reported | 1/30 3.3% | 7/30 23.3% | Not reported | 13/30 43.3% | Not reported | 7/30 23.3% | Not reported | 10/30 33.3% |
| Tewari et al., 2023 [36] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Dupuis-Girod et al., 2014 [37] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Kennedy et al., 2018 [38] | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported | Not reported |
| Takemura et al., 2018 [39] | 1/18 5.5% | Not reported | 4/18 22.2%, only nausea | Not reported | Not reported | Not reported | 1/18 5.5% | 11/18 61.1% | Not reported | 10/18 55.5% | Not reported | Not reported | Not reported | Not reported |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bruna-Mejías, A.; Silva-Bravo, V.; Moyano Valarezo, L.; Delgado-Retamal, M.F.; Nazar-Izquierdo, D.; Aguilar-Aguirre, I.; Nova-Baeza, P.; Orellana-Donoso, M.; Suazo-Santibáñez, A.; Gutiérrez-Espinoza, H.; et al. The Adverse Effects and Use of Bevacizumab in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 795. https://doi.org/10.3390/ph18060795
Bruna-Mejías A, Silva-Bravo V, Moyano Valarezo L, Delgado-Retamal MF, Nazar-Izquierdo D, Aguilar-Aguirre I, Nova-Baeza P, Orellana-Donoso M, Suazo-Santibáñez A, Gutiérrez-Espinoza H, et al. The Adverse Effects and Use of Bevacizumab in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(6):795. https://doi.org/10.3390/ph18060795
Chicago/Turabian StyleBruna-Mejías, Alejandro, Vicente Silva-Bravo, Laura Moyano Valarezo, María Fernanda Delgado-Retamal, Diego Nazar-Izquierdo, Isidora Aguilar-Aguirre, Pablo Nova-Baeza, Mathias Orellana-Donoso, Alejandra Suazo-Santibáñez, Héctor Gutiérrez-Espinoza, and et al. 2025. "The Adverse Effects and Use of Bevacizumab in Patients with Glioblastoma: A Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 6: 795. https://doi.org/10.3390/ph18060795
APA StyleBruna-Mejías, A., Silva-Bravo, V., Moyano Valarezo, L., Delgado-Retamal, M. F., Nazar-Izquierdo, D., Aguilar-Aguirre, I., Nova-Baeza, P., Orellana-Donoso, M., Suazo-Santibáñez, A., Gutiérrez-Espinoza, H., Sanchis Gimeno, J., Bastidas-Caldes, C., & Valenzuela Fuenzalida, J. J. (2025). The Adverse Effects and Use of Bevacizumab in Patients with Glioblastoma: A Systematic Review and Meta-Analysis. Pharmaceuticals, 18(6), 795. https://doi.org/10.3390/ph18060795