Prebiotic-like Effects of Proanthocyanidin-Rich Aronia Extract Supplementation on Gut Microbiota Composition and Function in the Twin-M-SHIME® Model
Abstract
1. Introduction
2. Results
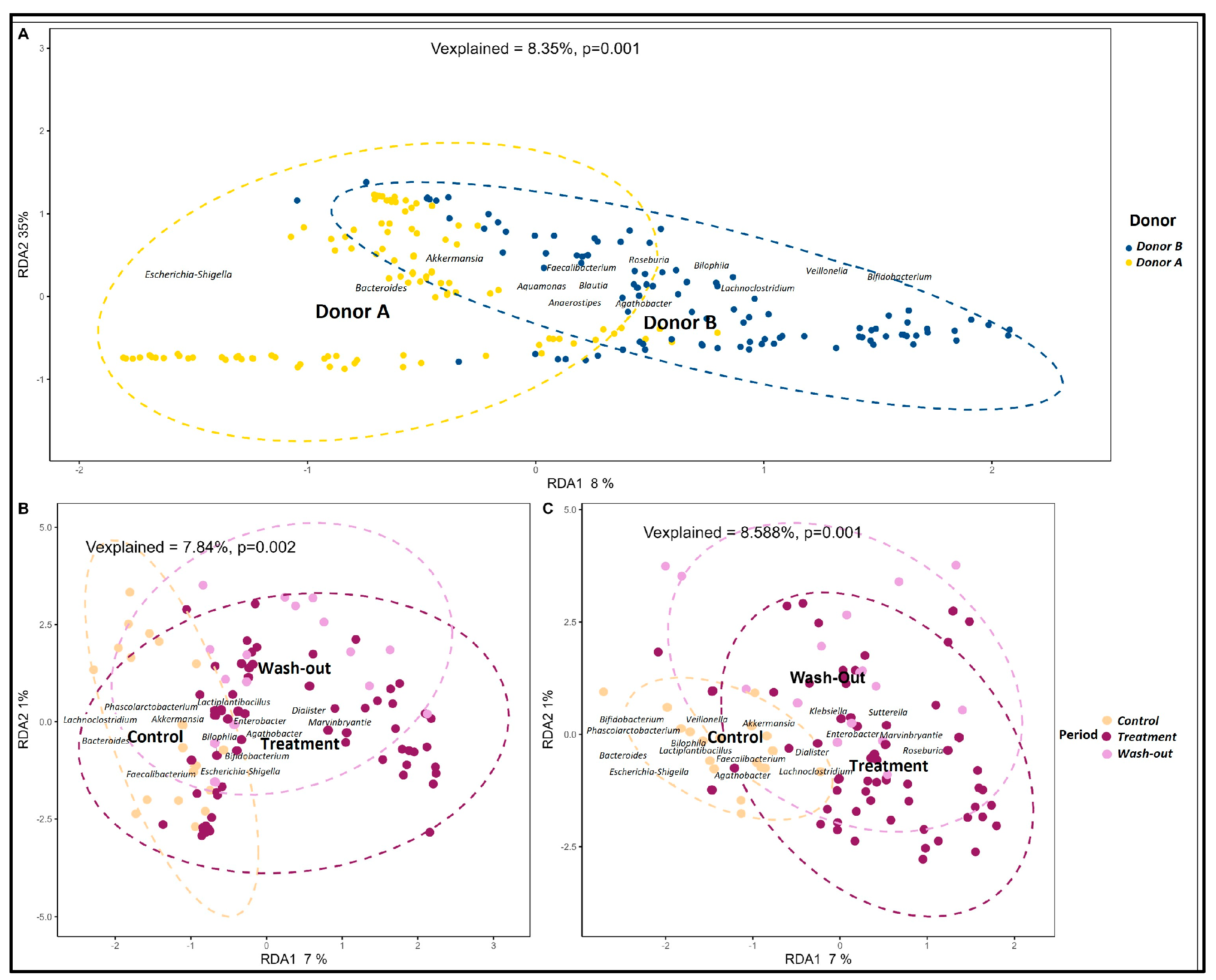
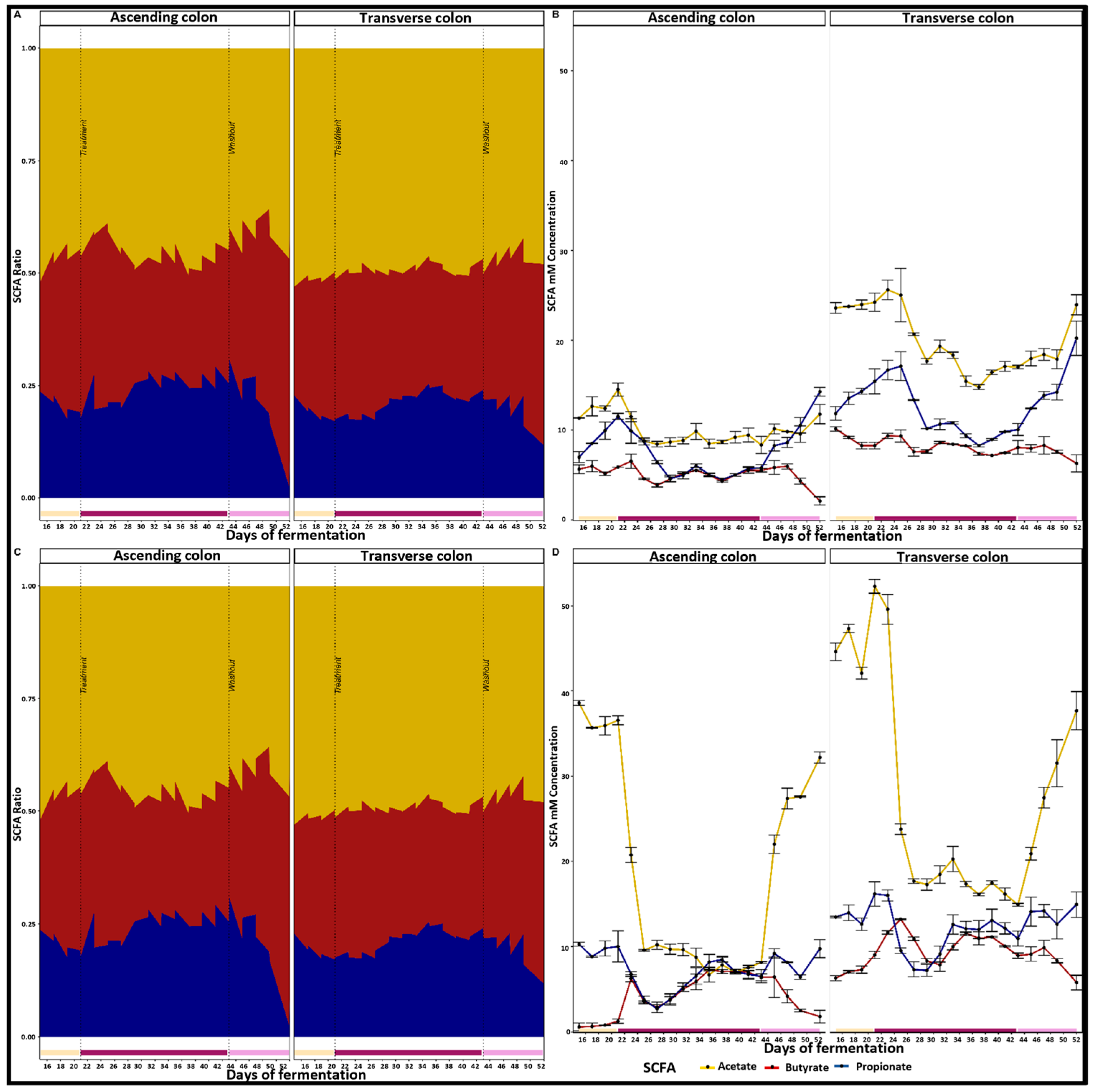
2.1. Inter-Individual Variability of Donors’ Microbiota and Their Production of SCFAs
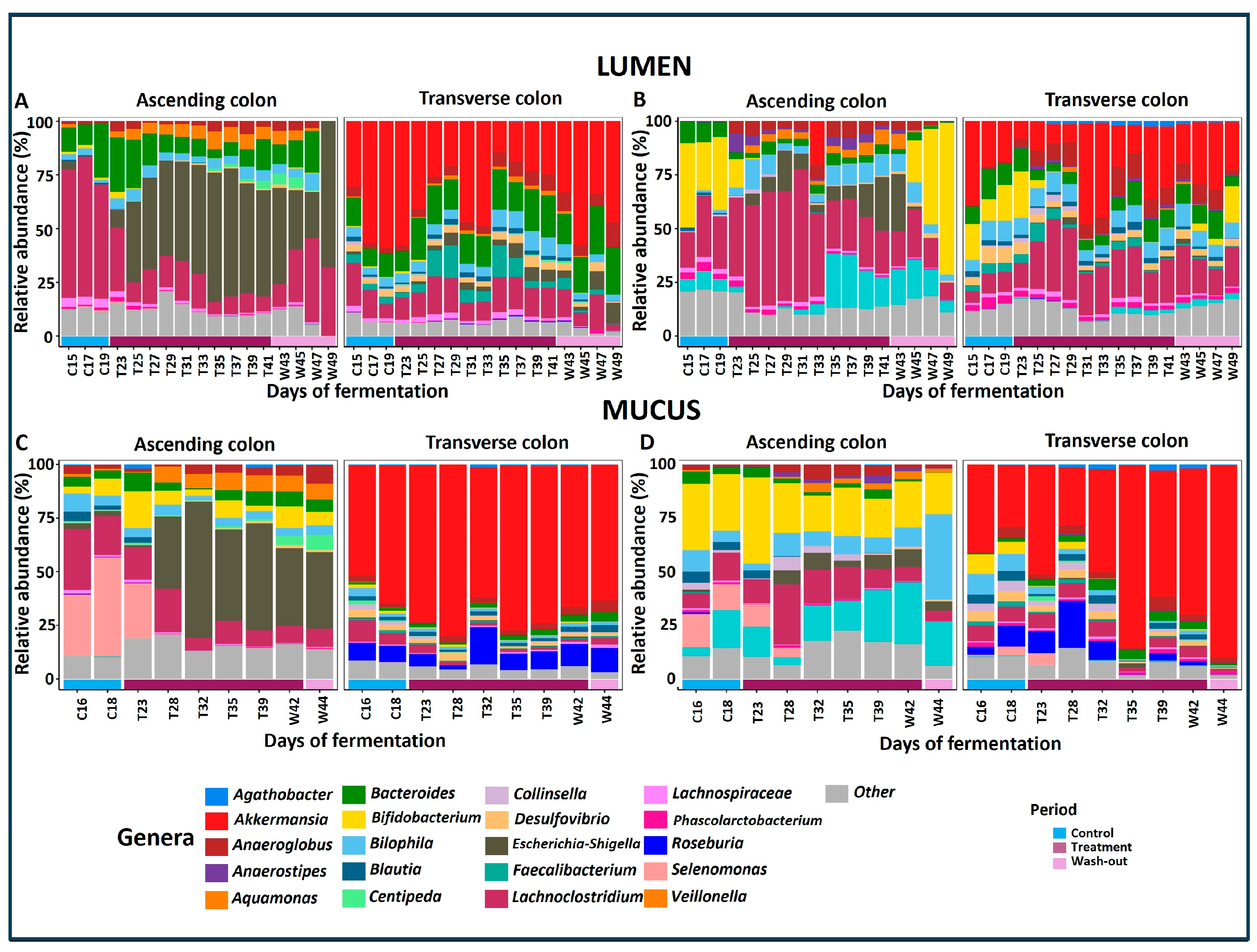
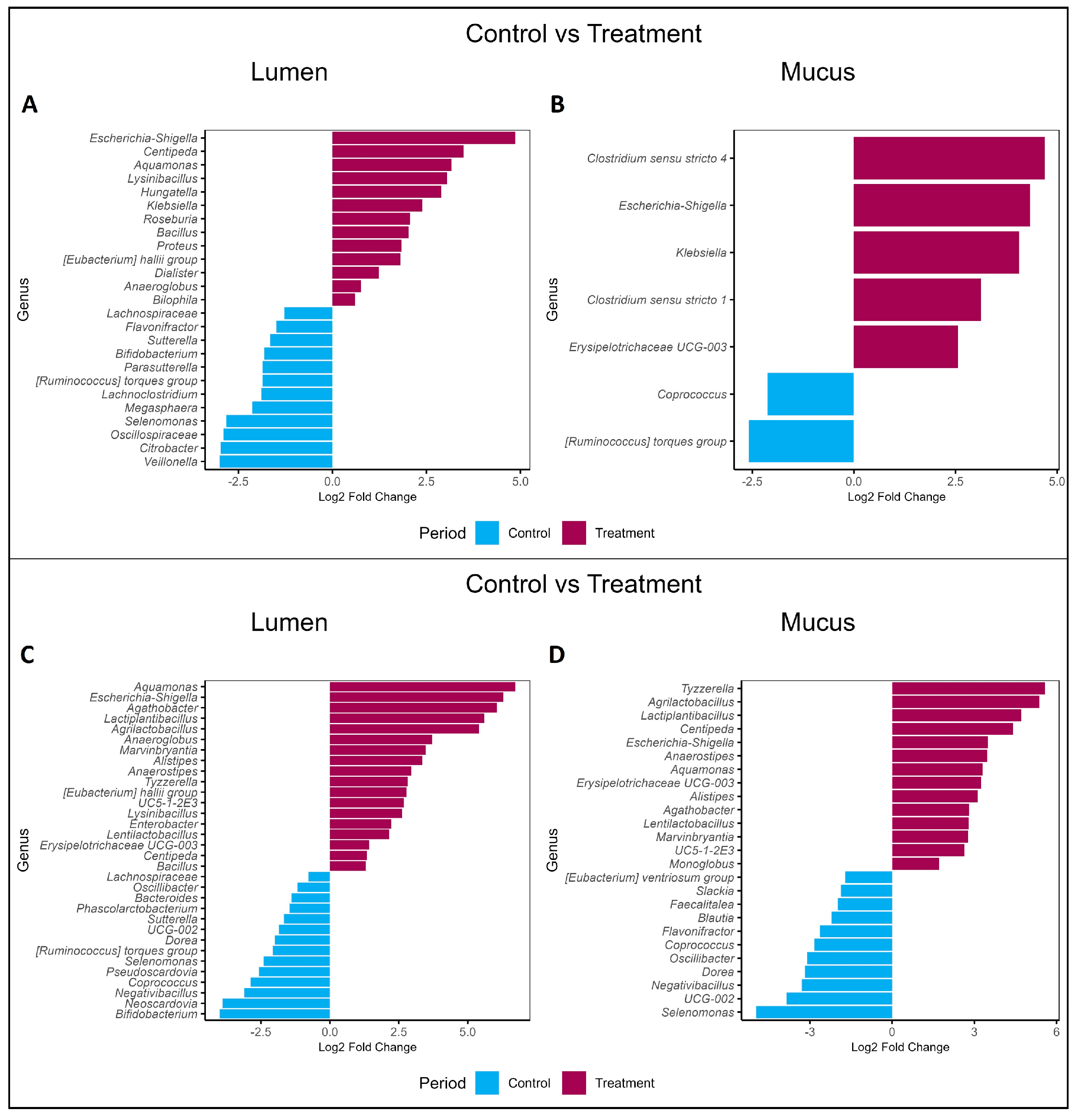
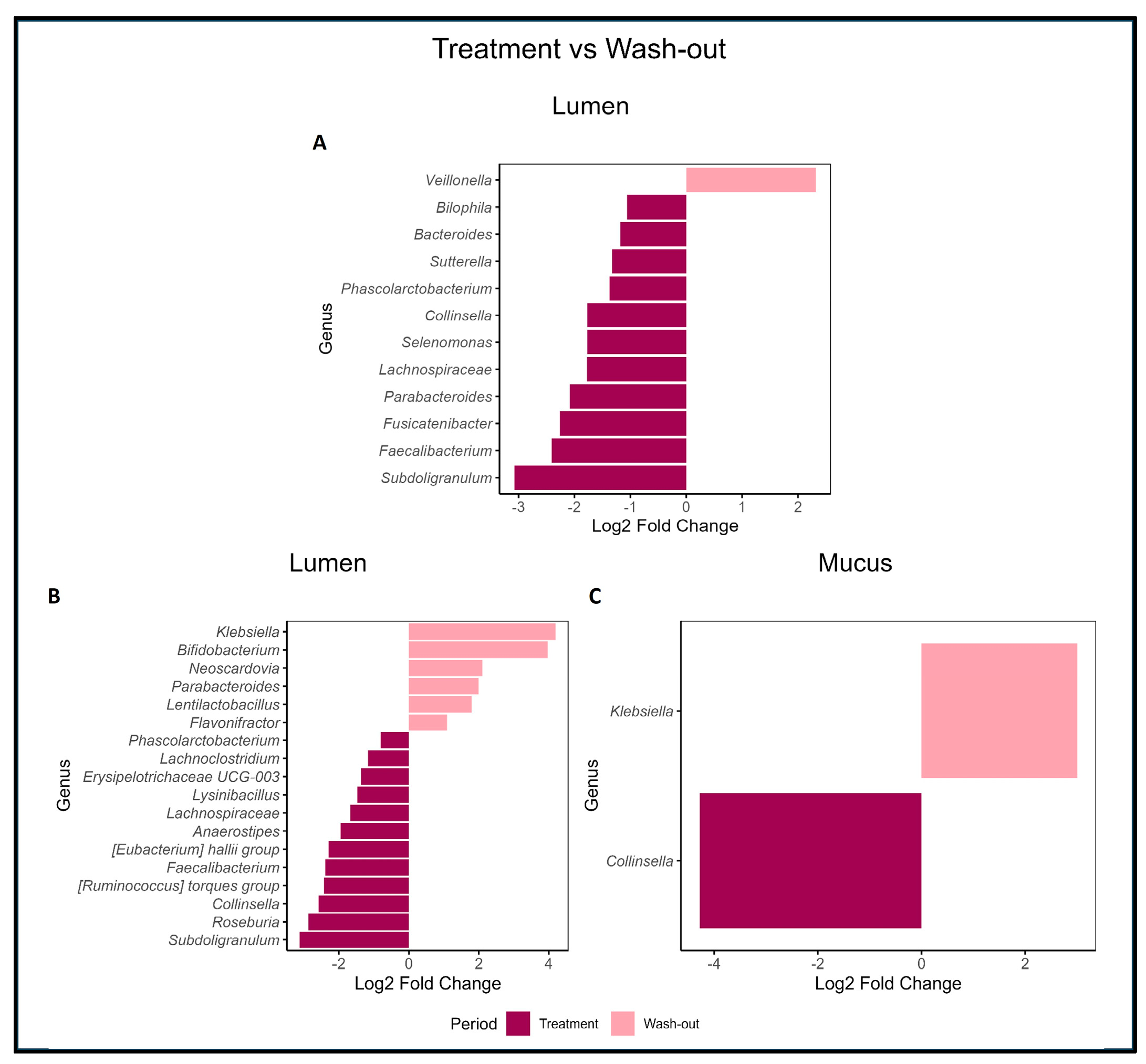
2.2. Effects of PAC-Rich Extract Supplementation on Microbiota Composition
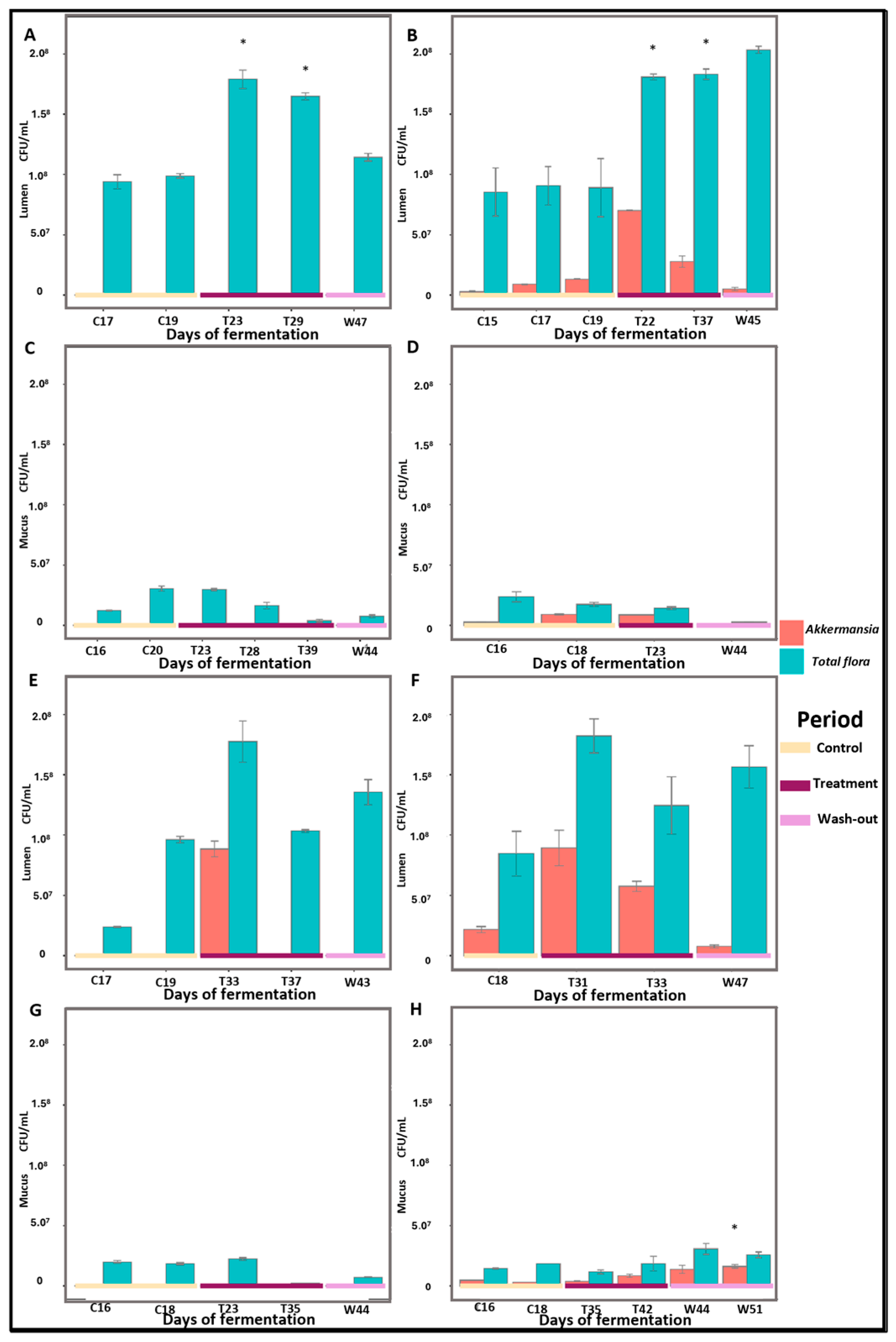
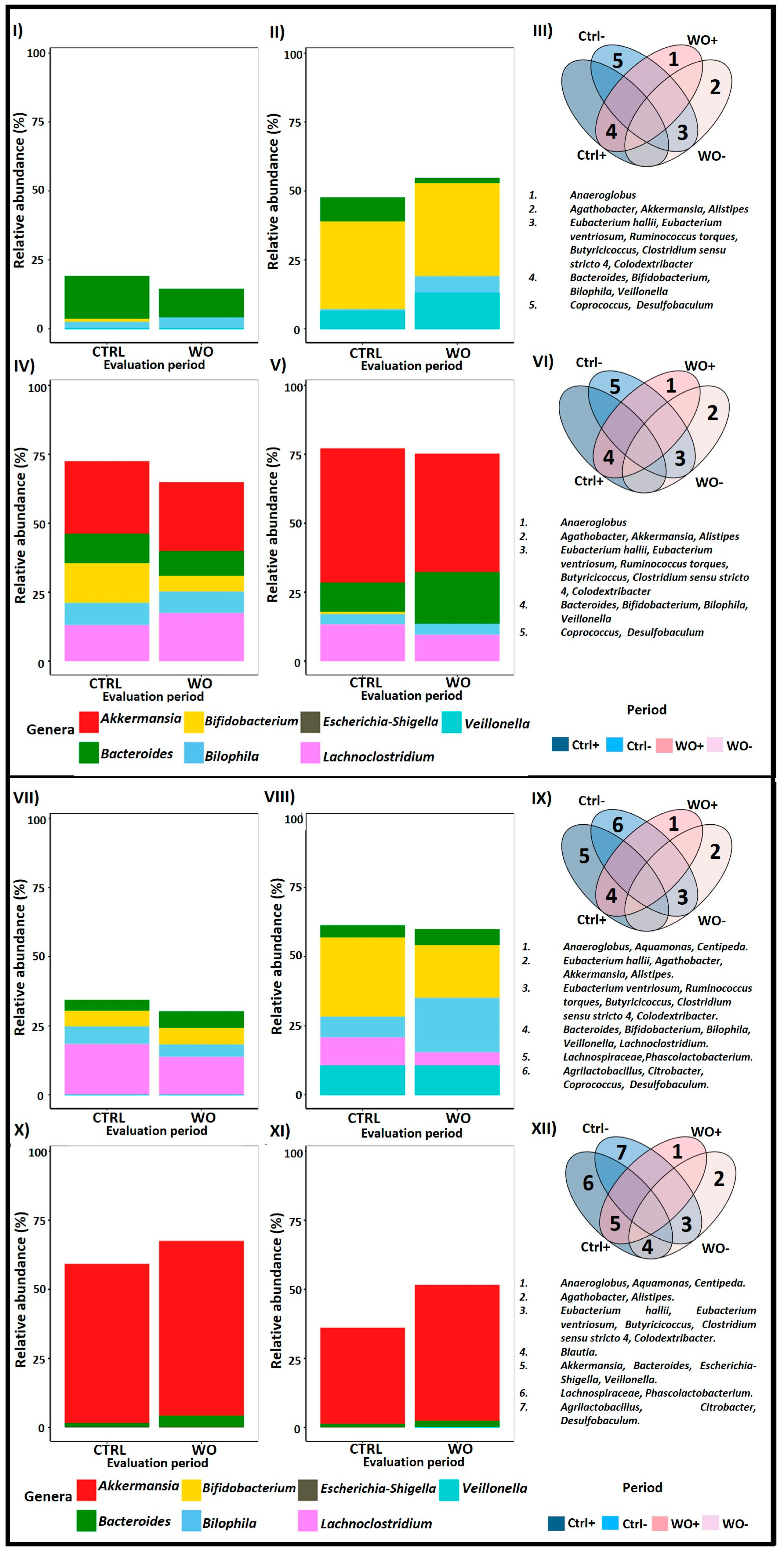
2.3. Supplementation with PAC-Rich Aronia Extract Maintains a High Relative Abundance of Akkermansia in Both Donors in the Transverse Colon Tested in Twin-M-SHIME®
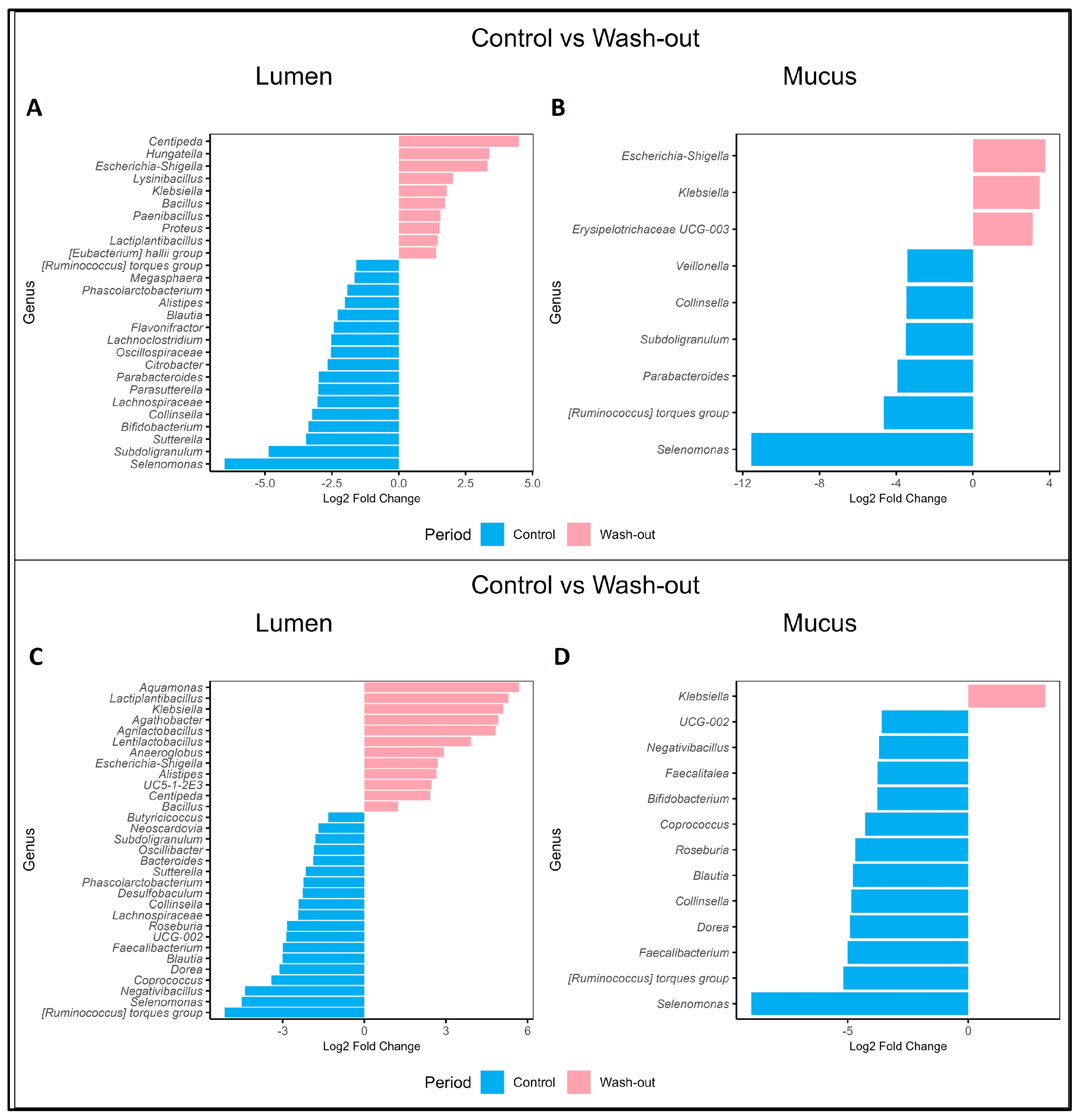
2.4. Contrasting Recovery: A Comparative Analysis of Control and Wash-Out Periods
3. Discussion
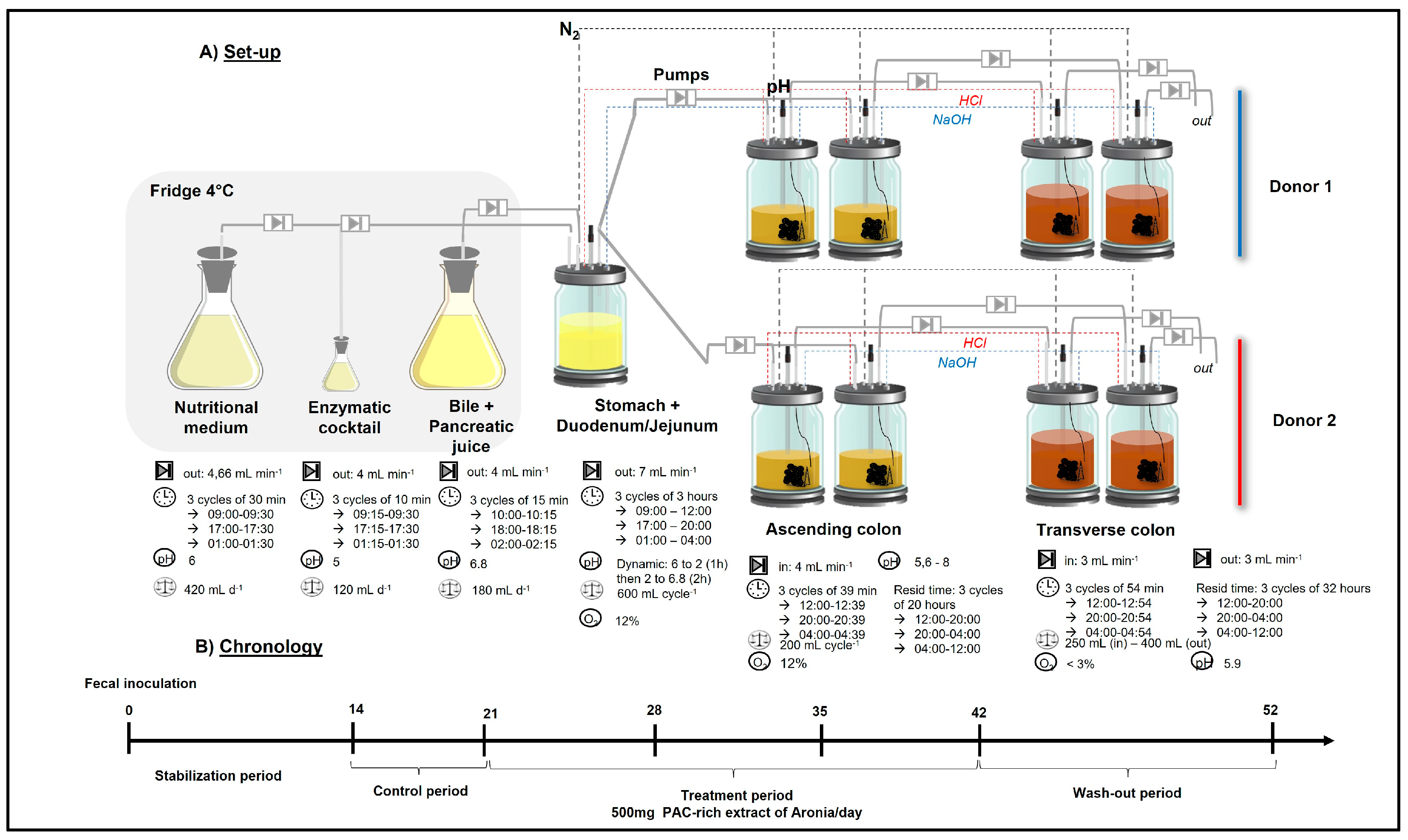
4. Materials and Methods
4.1. PAC-Rich Aronia Extract
4.2. Reagents
4.3. Twin-M-SHIME®
4.4. Short-Chain Fatty Acid Analysis
4.5. Metagenomics Analysis
4.6. Absolute Quantification of PCR Targets for Total Bacteria and Akkermansia muciniphila
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAC | Proanthocyanidins |
| GM | Gut microbiota |
| SCFA | Short-chain fatty acids |
| Twin-M-SHIME® | Twin Mucosal Simulator of the Human Intestinal Microbial Ecosystem |
| rRNA | Ribosomal ribonucleic acid |
| WO | Wash-out |
| DP | Degree of polymerization |
| Muc2 | Mucin 2 |
| PYY | Peptide tyrosine tyrosine |
| GLP-1 | Glucagon-like peptide-1 |
| TNF-α | Tumor necrosis factor alpha |
| IL | Interleukin |
| MCP-1 | SCFAs |
| PERMANOVA | Permutational multivariate analysis of variance |
| AC | Ascending colon |
| TC | Descending colon |
| db-RDA | Distance-based redundancy analysis |
| RDA | Redundancy analysis |
| mM | Millimolar |
| LEfSe | Linear discriminant analysis effect size |
| LDA | Linear discriminant analysis |
| DESeq | Differential gene expression analysis of RNA-seq data |
| ddPCR | Droplet digital polymerase chain reaction |
| CFU | Colony-forming unit |
| mL | Milliliter |
| ANOVA | Analysis of variance |
| ST | Stomach |
| SI | Small intestine |
| v/w | Volume/weight |
| DNA | Deoxynucleic acid |
| min | Minutes |
| °C | Degrees Celsius |
| µl | Microliter |
| ng | Nanogram |
| mg | Milligram |
| IBIS | Institut de Biologie Intégrative et des Systèmes |
| ASVs | Amplicon sequence variants |
| qPCR | Quantitative polymerase chain reaction |
References
- Wu, X.; Pittman, H.E.; Prior, R.L. Pelargonidin Is Absorbed and Metabolized Differently than Cyanidin after Marionberry Consumption in Pigs. J. Nutr. 2004, 134, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Grootaert, C.; Van den Abbeele, P.; Marzorati, M.; Broekaert, W.F.; Courtin, C.M.; Delcour, J.A.; Verstraete, W.; Van de Wiele, T. Comparison of Prebiotic Effects of Arabinoxylan Oligosaccharides and Inulin in a Simulator of the Human Intestinal Microbial Ecosystem: Arabinoxylan Oligosaccharides Compared with Inulin in Vitro. FEMS Microbiol. Ecol. 2009, 69, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, J.; Clark, C.; Kord Varkaneh, H.; Lakiang, T.; Vasanthan, L.T.; Onyeche, V.; Mousavi, S.M.; Zhang, Y. The Effect of Aronia Consumption on Lipid Profile, Blood Pressure, and Biomarkers of Inflammation: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 1981–1990. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Grootaert, C.; Pitart, J.; Vidovic, N.K.; Kamiloglu, S.; Possemiers, S.; Glibetic, M.; Smagghe, G.; Raes, K.; Van de Wiele, T.; et al. Aronia (Aronia Melanocarpa) Polyphenols Modulate the Microbial Community in a Simulator of the Human Intestinal Microbial Ecosystem (SHIME) and Decrease Secretion of Proinflammatory Markers in a Caco-2/Endothelial Cell Coculture Model. Mol. Nutr. Food Res. 2018, 62, 1800607. [Google Scholar] [CrossRef]
- Bijak, M.; Bobrowski, M.; Borowiecka, M.; Podsędek, A.; Golański, J.; Nowak, P. Anticoagulant Effect of Polyphenols-Rich Extracts from Black Chokeberry and Grape Seeds. Fitoterapia 2011, 82, 811–817. [Google Scholar] [CrossRef]
- Istas, G.; Wood, E.; Le Sayec, M.; Rawlings, C.; Yoon, J.; Dandavate, V.; Cera, D.; Rampelli, S.; Costabile, A.; Fromentin, E.; et al. Effects of Aronia Berry (Poly)Phenols on Vascular Function and Gut Microbiota: A Double-Blind Randomized Controlled Trial in Adult Men. Am. J. Clin. Nutr. 2019, 110, 316–329. [Google Scholar] [CrossRef]
- Malinowska, J.; Oleszek, W.; Stochmal, A.; Olas, B. The Polyphenol-Rich Extracts from Black Chokeberry and Grape Seeds Impair Changes in the Platelet Adhesion and Aggregation Induced by a Model of Hyperhomocysteinemia. Eur. J. Nutr. 2013, 52, 1049–1057. [Google Scholar] [CrossRef]
- Parzonko, A.; Oświt, A.; Bazylko, A.; Naruszewicz, M. Anthocyans-Rich Aronia Melanocarpa Extract Possesses Ability to Protect Endothelial Progenitor Cells against Angiotensin II Induced Dysfunction. Phytomedicine 2015, 22, 1238–1246. [Google Scholar] [CrossRef]
- Sikora, J.; Broncel, M.; Mikiciuk-Olasik, E. Aronia melanocarpa Elliot Reduces the Activity of Angiotensin I-Converting Enzyme— In Vitro and Ex Vivo Studies. Oxidative Med. Cell. Longev. 2014, 2014, Article. [Google Scholar] [CrossRef]
- Kędzierska, M.; Głowacki, R.; Czernek, U.; Szydłowska-Pazera, K.; Potemski, P.; Piekarski, J.; Jeziorski, A.; Olas, B. Changes in Plasma Thiol Levels Induced by Different Phases of Treatment in Breast Cancer; the Role of Commercial Extract from Black Chokeberry. Mol. Cell. Biochem. 2013, 372, 47–55. [Google Scholar] [CrossRef]
- Choi, H.S.; Kim, J.; Kim, S.; Deng, H.; Lee, D.; Kim, C.S.; Yun, B.; Lee, D. Catechol Derived from Aronia Juice through Lactic Acid Bacteria Fermentation Inhibits Breast Cancer Stem Cell Formation via Modulation Stat3/IL-6 Signaling Pathway. Mol. Carcinog. 2018, 57, 1467–1479. [Google Scholar] [CrossRef] [PubMed]
- Lala, G.; Malik, M.; Zhao, C.; He, J.; Kwon, Y.; Giusti, M.M.; Magnuson, B.A. Anthocyanin-Rich Extracts Inhibit Multiple Biomarkers of Colon Cancer in Rats. Nutr. Cancer 2006, 54, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Weever, F.; Hübner, F.; Humpf, H.-U.; Esselen, M. Characterization of Oligomeric Proanthocyanidin-Enriched Fractions from Aronia Melanocarpa (M ichx.) E lliott via High-Resolution Mass Spectrometry and Investigations on Their Inhibitory Potential on Human Topoisomerases. J. Agric. Food Chem. 2021, 69, 11053–11064. [Google Scholar] [CrossRef] [PubMed]
- Loo, B.-M.; Erlund, I.; Koli, R.; Puukka, P.; Hellström, J.; Wähälä, K.; Mattila, P.; Jula, A. Consumption of Chokeberry (Aronia Mitschurinii) Products Modestly Lowered Blood Pressure and Reduced Low-Grade Inflammation in Patients with Mildly Elevated Blood Pressure. Nutr. Res. 2016, 36, 1222–1230. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Łaniewska, I.; Millo, B.; Dłużniewski, M. Combination Therapy of Statin with Flavonoids Rich Extract from Chokeberry Fruits Enhanced Reduction in Cardiovascular Risk Markers in Patients after Myocardial Infraction (MI). Atherosclerosis 2007, 194, e179–e184. [Google Scholar] [CrossRef]
- Xie, L.; Vance, T.; Kim, B.; Lee, S.G.; Caceres, C.; Wang, Y.; Hubert, P.A.; Lee, J.-Y.; Chun, O.K.; Bolling, B.W. Aronia Berry Polyphenol Consumption Reduces Plasma Total and Low-Density Lipoprotein Cholesterol in Former Smokers without Lowering Biomarkers of Inflammation and Oxidative Stress: A Randomized Controlled Trial. Nutr. Res. 2017, 37, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Goh, A.R.; Youn, G.S.; Yoo, K.-Y.; Won, M.H.; Han, S.-Z.; Lim, S.S.; Lee, K.W.; Choi, S.Y.; Park, J. Aronia melanocarpa Concentrate Ameliorates Pro-Inflammatory Responses in HaCaT Keratinocytes and 12- O -Tetradecanoylphorbol-13-Acetate-Induced Ear Edema in Mice. J. Med. Food 2016, 19, 654–662. [Google Scholar] [CrossRef]
- Denev, P.; Číž, M.; Kratchanova, M.; Blazheva, D. Black Chokeberry (Aronia Melanocarpa) Polyphenols Reveal Different Antioxidant, Antimicrobial and Neutrophil-Modulating Activities. Food Chem. 2019, 284, 108–117. [Google Scholar] [CrossRef]
- Lackner, S.; Sconocchia, T.; Ziegler, T.; Passegger, C.; Meier-Allard, N.; Schwarzenberger, E.; Wonisch, W.; Lahousen, T.; Kohlhammer-Dohr, A.; Mörkl, S.; et al. Immunomodulatory Effects of Aronia Juice Polyphenols—Results of a Randomized Placebo-Controlled Human Intervention Study and Cell Culture Experiments. Antioxidants 2022, 11, 1283. [Google Scholar] [CrossRef]
- Lackner, S.; Mahnert, A.; Moissl-Eichinger, C.; Madl, T.; Habisch, H.; Meier-Allard, N.; Kumpitsch, C.; Lahousen, T.; Kohlhammer-Dohr, A.; Mörkl, S.; et al. Interindividual Differences in Aronia Juice Tolerability Linked to Gut Microbiome and Metabolome Changes—Secondary Analysis of a Randomized Placebo-Controlled Parallel Intervention Trial. Microbiome 2024, 12, 49. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Cao, G.; Muccitelli, H.U.; Sánchez-Moreno, C.; Prior, R.L. Anthocyanins Are Absorbed in Glycated Forms in Elderly Women: A Pharmacokinetic Study. Am. J. Clin. Nutr. 2001, 73, 920–926. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Chen, C.-Y.O.; Zampariello, C.A.; Blumberg, J.B. Flavonoids and Phenolic Acids from Cranberry Juice Are Bioavailable and Bioactive in Healthy Older Adults. Food Chem. 2015, 168, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, H.; Zhang, W.; Ding, Y.; Zhao, T.; Zhang, M.; Mao, G.; Feng, W.; Wu, X.; Yang, L. Bioaccessibility and Biotransformation of Anthocyanin Monomers Following in Vitro Simulated Gastric-Intestinal Digestion and in Vivo Metabolism in Rats. Food Funct. 2019, 10, 6052–6061. [Google Scholar] [CrossRef] [PubMed]
- Keppler, K.; Humpf, H.-U. Metabolism of Anthocyanins and Their Phenolic Degradation Products by the Intestinal Microflora. Bioorganic Med. Chem. 2005, 13, 5195–5205. [Google Scholar] [CrossRef]
- Taheri, R.; Connolly, B.A.; Brand, M.H.; Bolling, B.W. Underutilized Chokeberry (Aronia Melanocarpa, Aronia Arbutifolia, Aronia Prunifolia) Accessions Are Rich Sources of Anthocyanins, Flavonoids, Hydroxycinnamic Acids, and Proanthocyanidins. J. Agric. Food Chem. 2013, 61, 8581–8588. [Google Scholar] [CrossRef]
- Selma, M.V.; Espín, J.C.; Tomás-Barberán, F.A. Interaction between Phenolics and Gut Microbiota: Role in Human Health. J. Agric. Food Chem. 2009, 57, 6485–6501. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A Comprehensive Review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Redondo-Castillejo, R.; Garcimartín, A.; Hernández-Martín, M.; López-Oliva, M.E.; Bocanegra, A.; Macho-González, A.; Bastida, S.; Benedí, J.; Sánchez-Muniz, F.J. Proanthocyanidins: Impact on Gut Microbiota and Intestinal Action Mechanisms in the Prevention and Treatment of Metabolic Syndrome. IJMS 2023, 24, 5369. [Google Scholar] [CrossRef]
- Zhang, X.; Song, X.; Hu, X.; Chen, F.; Ma, C. Health Benefits of Proanthocyanidins Linking with Gastrointestinal Modulation: An Updated Review. Food Chem. 2023, 404, 134596. [Google Scholar] [CrossRef]
- Cattero, V.; Roussel, C.; Lessard-Lord, J.; Roy, D.; Desjardins, Y. Supplementation with a Cranberry Extract Favors the Establishment of Butyrogenic Guilds in the Human Fermentation SHIME System. Microbiome Res. Rep. 2024, 3, 34. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of Tea Phenolics and Their Aromatic Fecal Bacterial Metabolites on Intestinal Microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Tripathi, K.; Harun, M.; Krishnan, V.; Kaushik, R.; Chawla, G.; Shakil, N.A.; Verma, M.K.; Dahuja, A.; Sachdev, A.; et al. Unravelling the Effect of Extraction on Anthocyanin Functionality and Prebiotic Potential. Heliyon 2024, 10, e31780. [Google Scholar] [CrossRef]
- Verediano, T.A.; Stampini Duarte Martino, H.; Dias Paes, M.C.; Tako, E. Effects of Anthocyanin on Intestinal Health: A Systematic Review. Nutrients 2021, 13, 1331. [Google Scholar] [CrossRef]
- Casanova-Martí, À.; González-Abuín, N.; Serrano, J.; Blay, M.T.; Terra, X.; Frost, G.; Pinent, M.; Ardévol, A. Long Term Exposure to a Grape Seed Proanthocyanidin Extract Enhances L-Cell Differentiation in Intestinal Organoids. Mol. Nutr. Food Res. 2020, 64, 2000303. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhao, S.; Wang, J.; Shi, J.; Sun, Y.; Wang, W.; Ning, G.; Hong, J.; Liu, R. Grape Seed Proanthocyanidin Extract Ameliorates Inflammation and Adiposity by Modulating Gut Microbiota in High-Fat Diet Mice. Mol. Nutr. Food Res. 2017, 61, 1601082. [Google Scholar] [CrossRef]
- Karioti, A.; Sokovic, M.; Ciric, A.; Koukoulitsa, C.; Bilia, A.R.; Skaltsa, H. Antimicrobial Properties of Quercus Ilex L. Proanthocyanidin Dimers and Simple Phenolics: Evaluation of Their Synergistic Activity with Conventional Antimicrobials and Prediction of Their Pharmacokinetic Profile. J. Agric. Food Chem. 2011, 59, 6412–6422. [Google Scholar] [CrossRef]
- Roussel, C.; Chabaud, S.; Lessard-Lord, J.; Cattero, V.; Pellerin, F.-A.; Feutry, P.; Bochard, V.; Bolduc, S.; Desjardins, Y. UPEC Colonic-Virulence and Urovirulence Are Blunted by Proanthocyanidins-Rich Cranberry Extract Microbial Metabolites in a Gut Model and a 3D Tissue-Engineered Urothelium. Microbiol. Spectr. 2022, 10, e02432-21. [Google Scholar] [CrossRef]
- Zang, X.; Shang, M.; Xu, F.; Liang, J.; Wang, X.; Mikage, M.; Cai, S. A-Type Proanthocyanidins from the Stems of Ephedra Sinica (Ephedraceae) and Their Antimicrobial Activities. Molecules 2013, 18, 5172–5189. [Google Scholar] [CrossRef]
- Rodríguez-Daza, M.C.; Pulido-Mateos, E.C.; Lupien-Meilleur, J.; Guyonnet, D.; Desjardins, Y.; Roy, D. Polyphenol-Mediated Gut Microbiota Modulation: Toward Prebiotics and Further. Front. Nutr. 2021, 8, 689456. [Google Scholar] [CrossRef]
- Stephens, M. False Discovery Rates: A New Deal. Biostat 2016, kxw041. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What Is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Van Hul, M.; Cani, P.D.; Petitfils, C.; De Vos, W.M.; Tilg, H.; El-Omar, E.M. What Defines a Healthy Gut Microbiome? Gut 2024, 73, 1893–1908. [Google Scholar] [CrossRef]
- Déprez, S. Biomarquage de Tanins Condenses et Etude de leur Biodisponibilite dans L’organisme Humain. Doctoral Dissertation, Institut National Agronomique Paris-Grignon, Paris, France, 1999. Available online: https://www.sudoc.abes.fr/cbs//DB=2.1/SET=2/TTL=1/SHW?FRST=2 (accessed on 29 April 2025).
- Déprez, S.; Mila, I.; Lapierre, C.; Brezillon, C.; Rabot, S.; Philippe, C.; Scalbert, A. Polymeric Proanthocyanidins Are Catabolized by Human Colonic Microflora into Low-Molecular-Weight Phenolic Acids. J. Nutr. 2000, 130, 2733–2738. [Google Scholar] [CrossRef]
- Kahle, K.; Kraus, M.; Scheppach, W.; Richling, E. Colonic Availability of Apple Polyphenols—A Study in Ileostomy Subjects. Mol. Nutr. Food Res. 2005, 49, 1143–1150. [Google Scholar] [CrossRef]
- Kahle, K.; Huemmer, W.; Kempf, M.; Scheppach, W.; Erk, T.; Richling, E. Polyphenols Are Intensively Metabolized in the Human Gastrointestinal Tract after Apple Juice Consumption. J. Agric. Food Chem. 2007, 55, 10605–10614. [Google Scholar] [CrossRef]
- Manach, C.; Williamson, G.; Morand, C.; Scalbert, A.; Rémésy, C. Bioavailability and Bioefficacy of Polyphenols in Humans. I. Review of 97 Bioavailability Studies. Am. J. Clin. Nutr. 2005, 81, 230S–242S. [Google Scholar] [CrossRef]
- Ou, K.; Gu, L. Absorption and Metabolism of Proanthocyanidins. J. Funct. Foods 2014, 7, 43–53. [Google Scholar] [CrossRef]
- Lessard-Lord, J.; Roussel, C.; Guay, V.; Desjardins, Y. Characterization of the Interindividual Variability Associated with the Microbial Metabolism of (−)-Epicatechin. J. Agric. Food Chem. 2023, 71, 13814–13827. [Google Scholar] [CrossRef]
- Lessard-Lord, J.; Roussel, C.; Guay, V.; Desjardins, Y. Assessing the Gut Microbiota’s Ability to Metabolize Oligomeric and Polymeric Flavan-3-ols from Aronia and Cranberry. Mol. Nutr. Food Res. 2024, 68, 2300641. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Patán, F.; Tabasco, R.; Monagas, M.; Requena, T.; Peláez, C.; Moreno-Arribas, M.V.; Bartolomé, B. Capability of Lactobacillus Plantarum IFPL935 To Catabolize Flavan-3-Ol Compounds and Complex Phenolic Extracts. J. Agric. Food Chem. 2012, 60, 7142–7151. [Google Scholar] [CrossRef]
- Kohri, T.; Matsumoto, N.; Yamakawa, M.; Suzuki, M.; Nanjo, F.; Hara, Y.; Oku, N. Metabolic Fate of (-)-[4-(3)H]Epigallocatechin Gallate in Rats after Oral Administration. J. Agric. Food Chem. 2001, 49, 4102–4112. [Google Scholar] [CrossRef] [PubMed]
- Casanova-Martí, À.; Serrano, J.; Portune, K.J.; Sanz, Y.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. Grape Seed Proanthocyanidins Influence Gut Microbiota and Enteroendocrine Secretions in Female Rats. Food Funct. 2018, 9, 1672–1682. [Google Scholar] [CrossRef]
- Taguri, T.; Tanaka, T.; Kouno, I. Antimicrobial Activity of 10 Different Plant Polyphenols against Bacteria Causing Food-Borne Disease. Biol. Pharm. Bull. 2004, 27, 1965–1969. [Google Scholar] [CrossRef]
- Ivanov, M.; Novović, K.; Malešević, M.; Dinić, M.; Stojković, D.; Jovčić, B.; Soković, M. Polyphenols as Inhibitors of Antibiotic Resistant Bacteria—Mechanisms Underlying Rutin Interference with Bacterial Virulence. Pharmaceuticals 2022, 15, 385. [Google Scholar] [CrossRef]
- Cladis, D.P.; Simpson, A.M.R.; Cooper, K.J.; Nakatsu, C.H.; Ferruzzi, M.G.; Weaver, C.M. Blueberry Polyphenols Alter Gut Microbiota & Phenolic Metabolism in Rats. Food Funct. 2021, 12, 2442–2456. [Google Scholar] [CrossRef]
- Mayta-Apaza, A.C.; Pottgen, E.; De Bodt, J.; Papp, N.; Marasini, D.; Howard, L.; Abranko, L.; Van De Wiele, T.; Lee, S.-O.; Carbonero, F. Impact of Tart Cherries Polyphenols on the Human Gut Microbiota and Phenolic Metabolites in Vitro and in Vivo. J. Nutr. Biochem. 2018, 59, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Kemperman, R.A.; Gross, G.; Mondot, S.; Possemiers, S.; Marzorati, M.; Van De Wiele, T.; Doré, J.; Vaughan, E.E. Impact of Polyphenols from Black Tea and Red Wine/Grape Juice on a Gut Model Microbiome. Food Res. Int. 2013, 53, 659–669. [Google Scholar] [CrossRef]
- Bialonska, D.; Ramnani, P.; Kasimsetty, S.G.; Muntha, K.R.; Gibson, G.R.; Ferreira, D. The Influence of Pomegranate By-Product and Punicalagins on Selected Groups of Human Intestinal Microbiota. Int. J. Food Microbiol. 2010, 140, 175–182. [Google Scholar] [CrossRef]
- Whitman, J.A.; Doherty, L.A.; Pantoja-Feliciano De Goodfellow, I.G.; Racicot, K.; Anderson, D.J.; Kensil, K.; Karl, J.P.; Gibson, G.R.; Soares, J.W. In Vitro Fermentation Shows Polyphenol and Fiber Blends Have an Additive Beneficial Effect on Gut Microbiota States. Nutrients 2024, 16, 1159. [Google Scholar] [CrossRef] [PubMed]
- Van Den Abbeele, P.; Duysburgh, C.; Ghyselinck, J.; Goltz, S.; Berezhnaya, Y.; Boileau, T.; De Blaiser, A.; Marzorati, M. Fructans with Varying Degree of Polymerization Enhance the Selective Growth of Bifidobacterium Animalis Subsp. Lactis BB-12 in the Human Gut Microbiome In Vitro. Appl. Sci. 2021, 11, 598. [Google Scholar] [CrossRef]
- Dong, Y.; Han, M.; Fei, T.; Liu, H.; Gai, Z. Utilization of Diverse Oligosaccharides for Growth by Bifidobacterium and Lactobacillus Species and Their in Vitro Co-Cultivation Characteristics. Int. Microbiol. 2023, 27, 941–952. [Google Scholar] [CrossRef]
- Wang, H.; Huang, X.; Tan, H.; Chen, X.; Chen, C.; Nie, S. Interaction between Dietary Fiber and Bifidobacteria in Promoting Intestinal Health. Food Chem. 2022, 393, 133407. [Google Scholar] [CrossRef]
- Hiippala, K.; Kainulainen, V.; Kalliomäki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef]
- Li, F.; Han, Y.; Cai, X.; Gu, M.; Sun, J.; Qi, C.; Goulette, T.; Song, M.; Li, Z.; Xiao, H. Dietary Resveratrol Attenuated Colitis and Modulated Gut Microbiota in Dextran Sulfate Sodium-Treated Mice. Food Funct. 2020, 11, 1063–1073. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in Gut Microbiota Control Inflammation in Obese Mice through a Mechanism Involving GLP-2-Driven Improvement of Gut Permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [PubMed]
- Schwiertz, A.; Hold, G.L.; Duncan, S.H.; Gruhl, B.; Collins, M.D.; Lawson, P.A.; Flint, H.J.; Blaut, M. Anaerostipes Caccae Gen. Nov., Sp. Nov., a New Saccharolytic, Acetate-Utilising, Butyrate-Producing Bacterium from Human Faeces. Syst. Appl. Microbiol. 2002, 25, 46–51. [Google Scholar] [CrossRef]
- Yu, W.; Gao, J.; Hao, R.; Yang, J.; Wei, J. Effects of Simulated Digestion on Black Chokeberry (Aronia Melanocarpa (Michx.) Elliot) Anthocyanins and Intestinal Flora. J. Food Sci. Technol. 2021, 58, 1511–1523. [Google Scholar] [CrossRef]
- Holmes, Z.C.; Villa, M.M.; Durand, H.K.; Jiang, S.; Dallow, E.P.; Petrone, B.L.; Silverman, J.D.; Lin, P.-H.; David, L.A. Microbiota Responses to Different Prebiotics Are Conserved within Individuals and Associated with Habitual Fiber Intake. Microbiome 2022, 10, 114. [Google Scholar] [CrossRef]
- Schupack, D.A.; Mars, R.A.T.; Voelker, D.H.; Abeykoon, J.P.; Kashyap, P.C. The Promise of the Gut Microbiome as Part of Individualized Treatment Strategies. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Bresciani, L.; Brindani, N.; Ludwig, I.A.; Pereira-Caro, G.; Angelino, D.; Llorach, R.; Calani, L.; Brighenti, F.; Clifford, M.N.; et al. Phenyl-γ-Valerolactones and Phenylvaleric Acids, the Main Colonic Metabolites of Flavan-3-Ols: Synthesis, Analysis, Bioavailability, and Bioactivity. Nat. Prod. Rep. 2019, 36, 714–752. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Sugamoto, K.; Matsuura, Y.; Kunitake, H.; Shimoda, K.; Morishita, K. Inhibition of Adult T-cell Leukemia Cell Proliferation by Polymerized Proanthocyanidin from Blueberry Leaves through JAK Proteolysis. Cancer Sci. 2022, 113, 1406–1416. [Google Scholar] [CrossRef]
- Andersen-Civil, A.I.S.; Leppä, M.M.; Thamsborg, S.M.; Salminen, J.-P.; Williams, A.R. Structure-Function Analysis of Purified Proanthocyanidins Reveals a Role for Polymer Size in Suppressing Inflammatory Responses. Commun. Biol. 2021, 4, 896. [Google Scholar] [CrossRef]
- Goya-Jorge, E.; Gonza, I.; Bondue, P.; Druart, G.; Al-Chihab, M.; Boutaleb, S.; Douny, C.; Taminiau, B.; Daube, G.; Scippo, M.-L.; et al. Unveiling the Influence of a Probiotic Combination of Heyndrickxia Coagulans and Lacticaseibacillus Casei on Healthy Human Gut Microbiota Using the TripleSHIME® System. Microbiol. Res. 2024, 285, 127778. [Google Scholar] [CrossRef]
- Koper, J.E.; Troise, A.D.; Loonen, L.M.; Vitaglione, P.; Capuano, E.; Fogliano, V.; Wells, J.M. Tryptophan Supplementation Increases the Production of Microbial-Derived AhR Agonists in an In Vitro Simulator of Intestinal Microbial Ecosystem. J. Agric. Food Chem. 2022, 70, 3958–3968. [Google Scholar] [CrossRef]
- Van den Abbeele, P.; Venema, K.; Van De Wiele, T.; Verstraete, W.; Possemiers, S. Different Human Gut Models Reveal the Distinct Fermentation Patterns of Arabinoxylan versus Inulin. J. Agric. Food Chem. 2013, 61, 9819–9827. [Google Scholar] [CrossRef]
- Fragiadakis, G.K.; Wastyk, H.C.; Robinson, J.L.; Sonnenburg, E.D.; Sonnenburg, J.L.; Gardner, C.D. Long-Term Dietary Intervention Reveals Resilience of the Gut Microbiota despite Changes in Diet and Weight. Am. J. Clin. Nutr. 2020, 111, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Hutkins, R.; Walter, J.; Gibson, G.R.; Bedu-Ferrari, C.; Scott, K.; Tancredi, D.J.; Wijeyesekera, A.; Sanders, M.E. Classifying Compounds as Prebiotics—Scientific Perspectives and Recommendations. Nat. Rev. Gastroenterol. Hepatol. 2025, 22, 54–70. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Campaniello, D.; Speranza, B.; Racioppo, A.; Sinigaglia, M.; Corbo, M.R. An Update on Prebiotics and on Their Health Effects. Foods 2024, 13, 446. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Daza, M.-C.; Daoust, L.; Boutkrabt, L.; Pilon, G.; Varin, T.; Dudonné, S.; Levy, É.; Marette, A.; Roy, D.; Desjardins, Y. Wild Blueberry Proanthocyanidins Shape Distinct Gut Microbiota Profile and Influence Glucose Homeostasis and Intestinal Phenotypes in High-Fat High-Sucrose Fed Mice. Sci. Rep. 2020, 10, 2217. [Google Scholar] [CrossRef]
- Hartman, A.L.; Lough, D.M.; Barupal, D.K.; Fiehn, O.; Fishbein, T.; Zasloff, M.; Eisen, J.A. Human Gut Microbiome Adopts an Alternative State Following Small Bowel Transplantation. Proc. Natl. Acad. Sci. USA 2009, 106, 17187–17192. [Google Scholar] [CrossRef]
- Cao, Y.; Dong, Q.; Wang, D.; Zhang, P.; Liu, Y.; Niu, C. microbiomeMarker: An R/Bioconductor Package for Microbiome Marker Identification and Visualization. Bioinformatics 2022, 38, 4027–4029. [Google Scholar] [CrossRef]

| GM | Donor | Colon | Period | Chao Index (104) | Shannon Index | Simpson Index |
|---|---|---|---|---|---|---|
| Lumen | A | AC | Control | 18.22 ± 7.28 | 3.97 ± 0.33 | 0.85 ± 0.03 |
| Treatment | 18.33 ± 9.20 | 4.12 ± 0.21 | 0.88 ± 0.03 | |||
| Wash-out | 50.06 ± 8.10 | 5.07 ± 1.26 *** | 0.93 ± 0.03 | |||
| TC | Control | 9.86 ± 3.89 | 4.74 ± 0.44 | 0.90 ± 0.04 | ||
| Treatment | 9.25 ± 3.39 | 5.26 ± 0.38 * | 0.95 ± 0.03 | |||
| Wash-out | 78.31 ± 10.50 ** | 5.70 ± 1.52 | 0.93 ± 0.07 | |||
| B | AC | Control | 18.11 ± 4.71 | 4.64 ± 0.27 | 0.93 ± 0.02 | |
| Treatment | 13.40 ± 6.31 * | 4.79 ± 0.40 | 0.94 ± 0.03 | |||
| Wash-out | 20.21 ± 13.46 | 4.66 ± 0.37 | 0.93 ± 0.03 | |||
| TC | Control | 16.84 ± 12.01 | 5.15 ± 0.24 | 0.96 ± 0.01 | ||
| Treatment | 9.13 ± 2.88 | 5.47 ± 0.36* | 0.96 ± 0.03 | |||
| Wash-out | 25.77 ± 3.89 | 5.42 ± 1.05 | 0.94 ± 0.06 | |||
| Mucus | A | AC | Control | 17.61 ± 5.12 | 4.48 ± 0.56 | 0.92 ± 0.03 |
| Treatment | 35.57 ± 57.08 | 4.40 ± 1.11 | 0.89 ± 0.06 | |||
| Wash-out | 23.97 ± 21.04 | 4.60 ± 0.28 | 0.93 ± 0.02 | |||
| TC | Control | 39.03 ± 12.48 | 4.26 ± 0.32 | 0.86 ± 0.04 | ||
| Treatment | 36.61 ± 42.12 | 3.41 ± 0.57 ** | 0.73 ± 0.10 | |||
| Wash-out | 119.03 ± 13.33 | 5.30 ± 2.89 | 0.85 ± 0.20 | |||
| B | AC | Control | 15.92 ± 10.83 | 4.44 ± 0.77 | 0.92 ± 0.06 | |
| Treatment | 38.15 ± 6.08 | 4.95 ± 0.89 | 0.95 ± 0.02 | |||
| Wash-out | 115.83 ± 14.07 | 6.29 ± 1.90 | 0.98 ± 0.01 | |||
| TC | Control | 22.72 ± 0.56 | 4.92 ± 0.16 | 0.95 ± 0.01 | ||
| Treatment | 49.93 ± 7.75 | 4.73 ± 1.63 | 0.85 ± 0.15 | |||
| Wash-out | 119.99 ± 13.51 | 5.24 ± 3.36 | 0.81 ± 0.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Álvarez, B.E.; Cattero, V.; Desjardins, Y. Prebiotic-like Effects of Proanthocyanidin-Rich Aronia Extract Supplementation on Gut Microbiota Composition and Function in the Twin-M-SHIME® Model. Pharmaceuticals 2025, 18, 793. https://doi.org/10.3390/ph18060793
Ruiz-Álvarez BE, Cattero V, Desjardins Y. Prebiotic-like Effects of Proanthocyanidin-Rich Aronia Extract Supplementation on Gut Microbiota Composition and Function in the Twin-M-SHIME® Model. Pharmaceuticals. 2025; 18(6):793. https://doi.org/10.3390/ph18060793
Chicago/Turabian StyleRuiz-Álvarez, Blanca Elizabeth, Valentina Cattero, and Yves Desjardins. 2025. "Prebiotic-like Effects of Proanthocyanidin-Rich Aronia Extract Supplementation on Gut Microbiota Composition and Function in the Twin-M-SHIME® Model" Pharmaceuticals 18, no. 6: 793. https://doi.org/10.3390/ph18060793
APA StyleRuiz-Álvarez, B. E., Cattero, V., & Desjardins, Y. (2025). Prebiotic-like Effects of Proanthocyanidin-Rich Aronia Extract Supplementation on Gut Microbiota Composition and Function in the Twin-M-SHIME® Model. Pharmaceuticals, 18(6), 793. https://doi.org/10.3390/ph18060793






