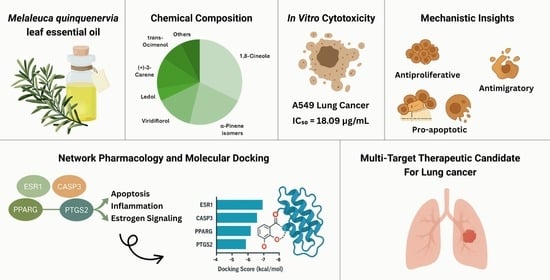
Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking
Abstract
1. Introduction
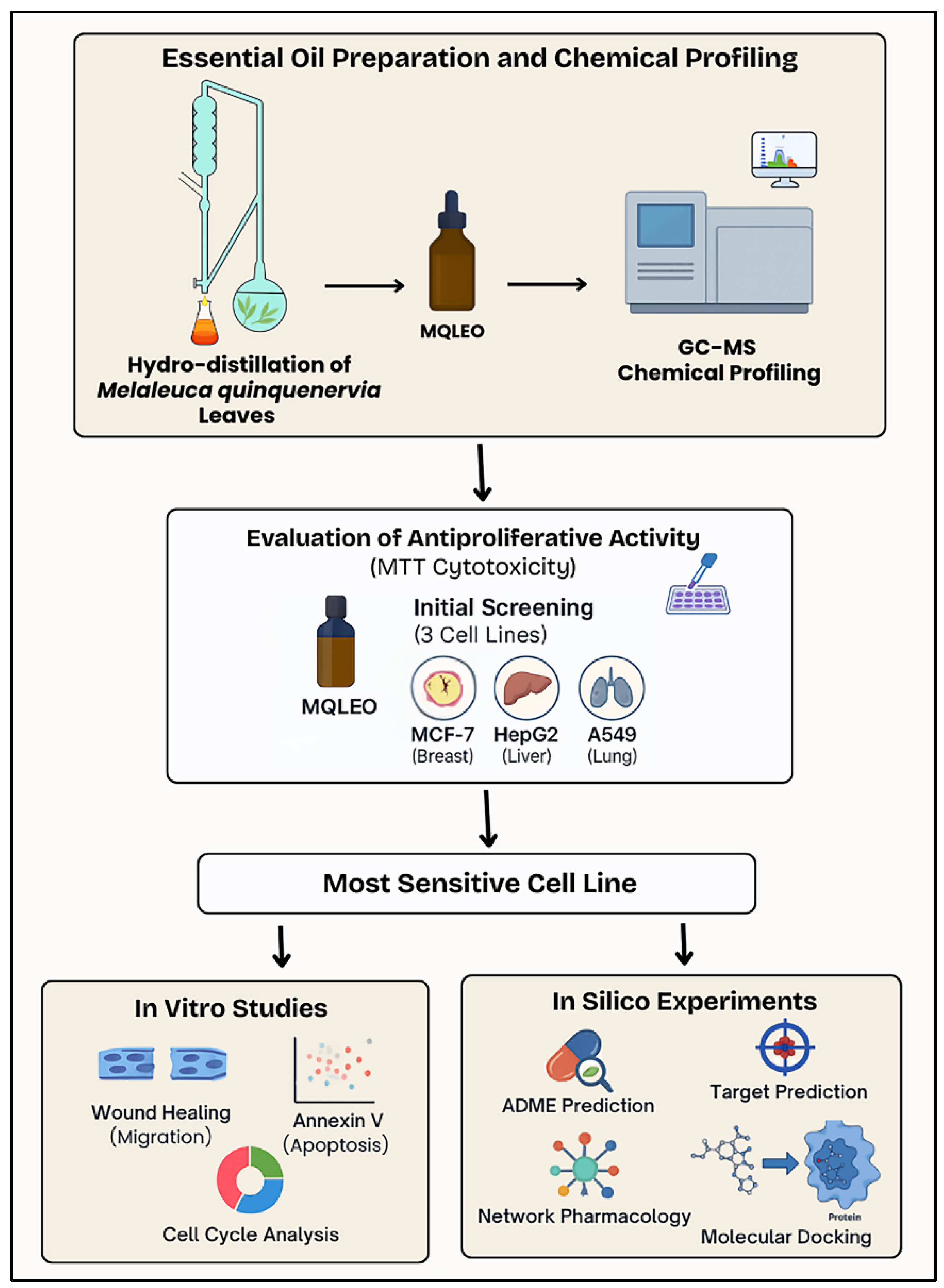
2. Results
2.1. Chemical Composition of MQLEO
2.2. In Vitro Assessments
2.2.1. Cytotoxic Potential and Selectivity of MQLEO
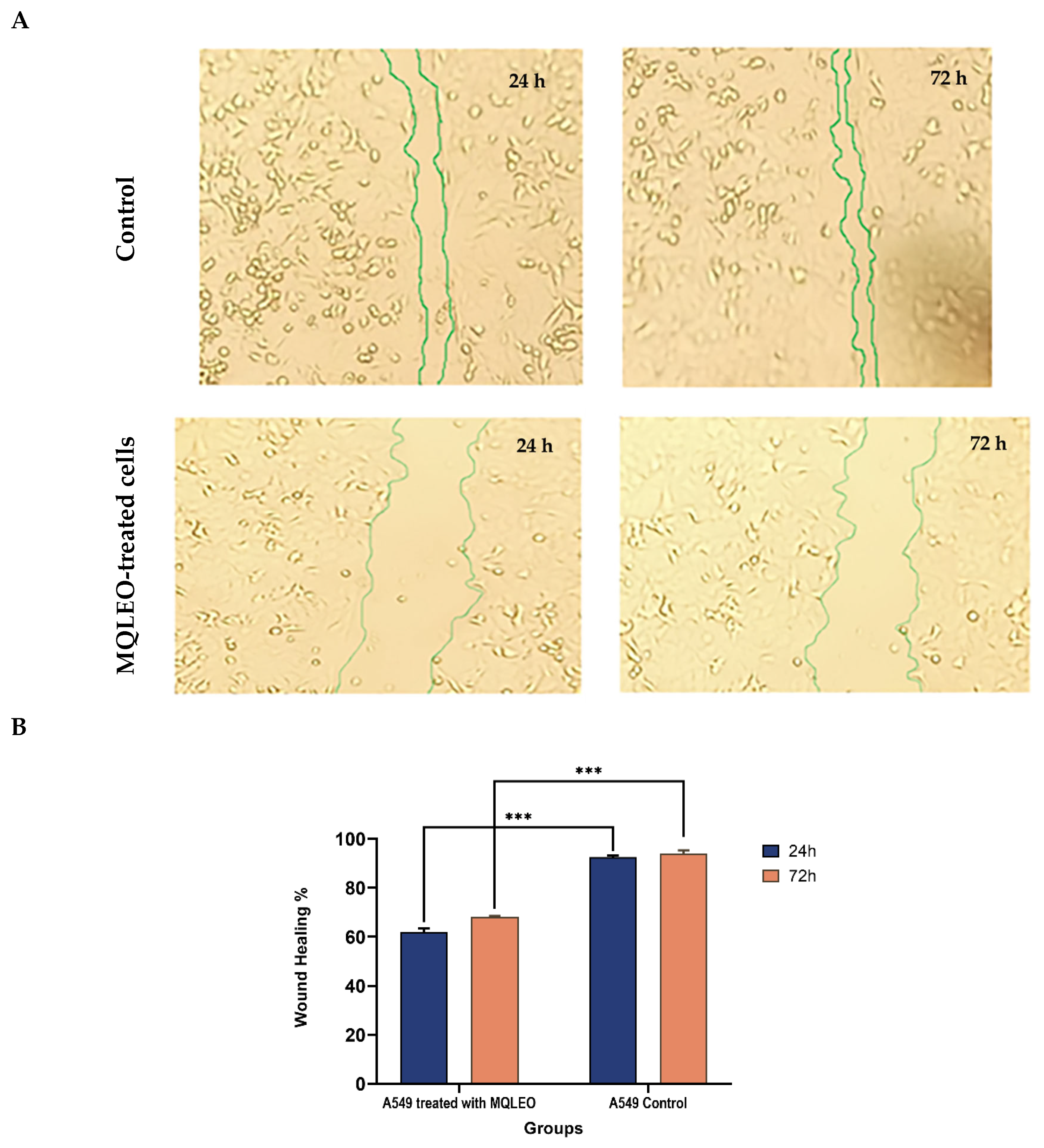
2.2.2. MQLEO-Mediated Suppression of Wound Healing and Metastatic Migration in A549 Lung Cancer Cells
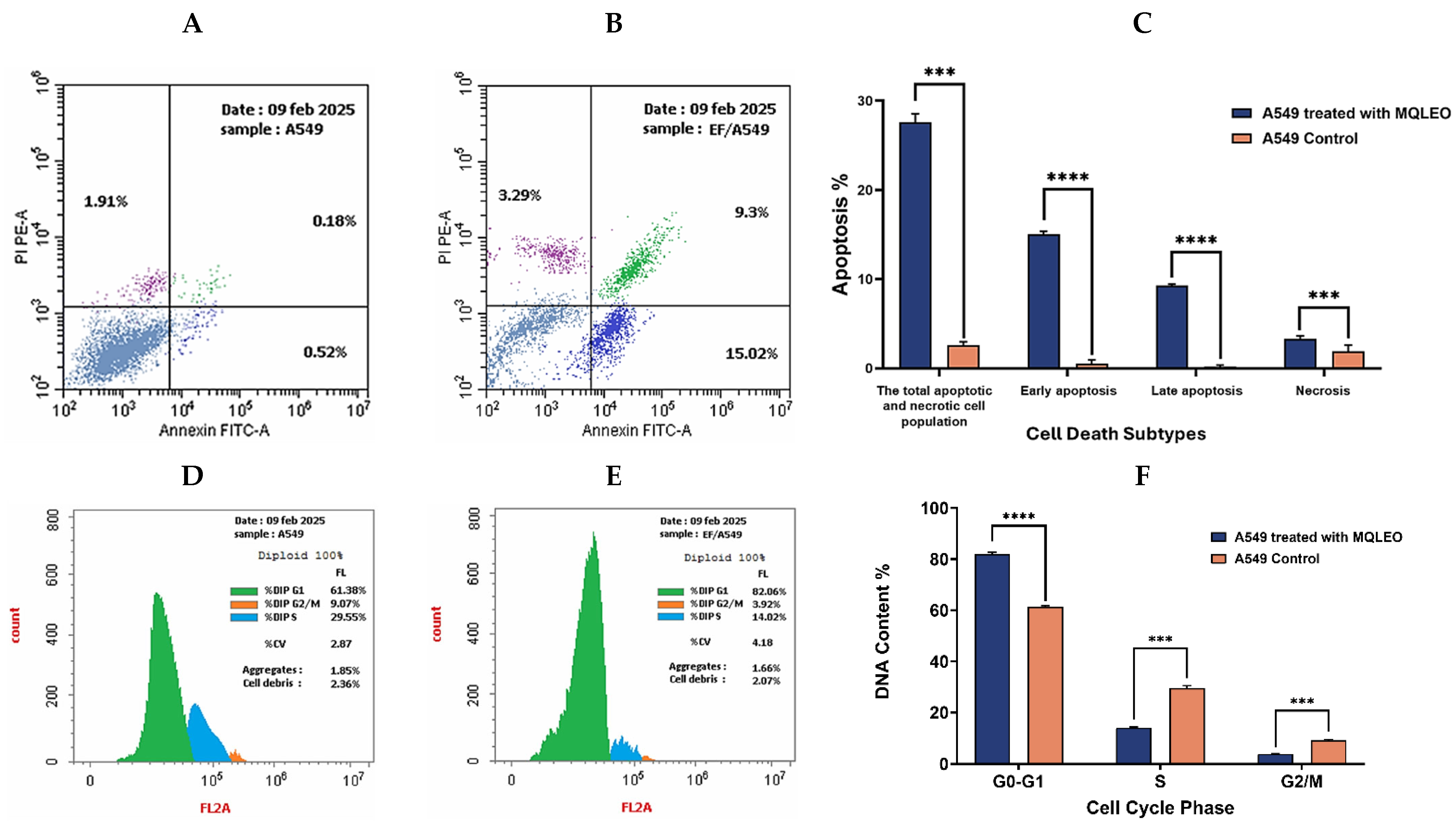
2.2.3. Apoptosis and Cell Cycle Analysis of A549 Cells in Response to MQLEO
Apoptosis Induction by MQLEO
MQLEO-Induced Cell Cycle Arrest
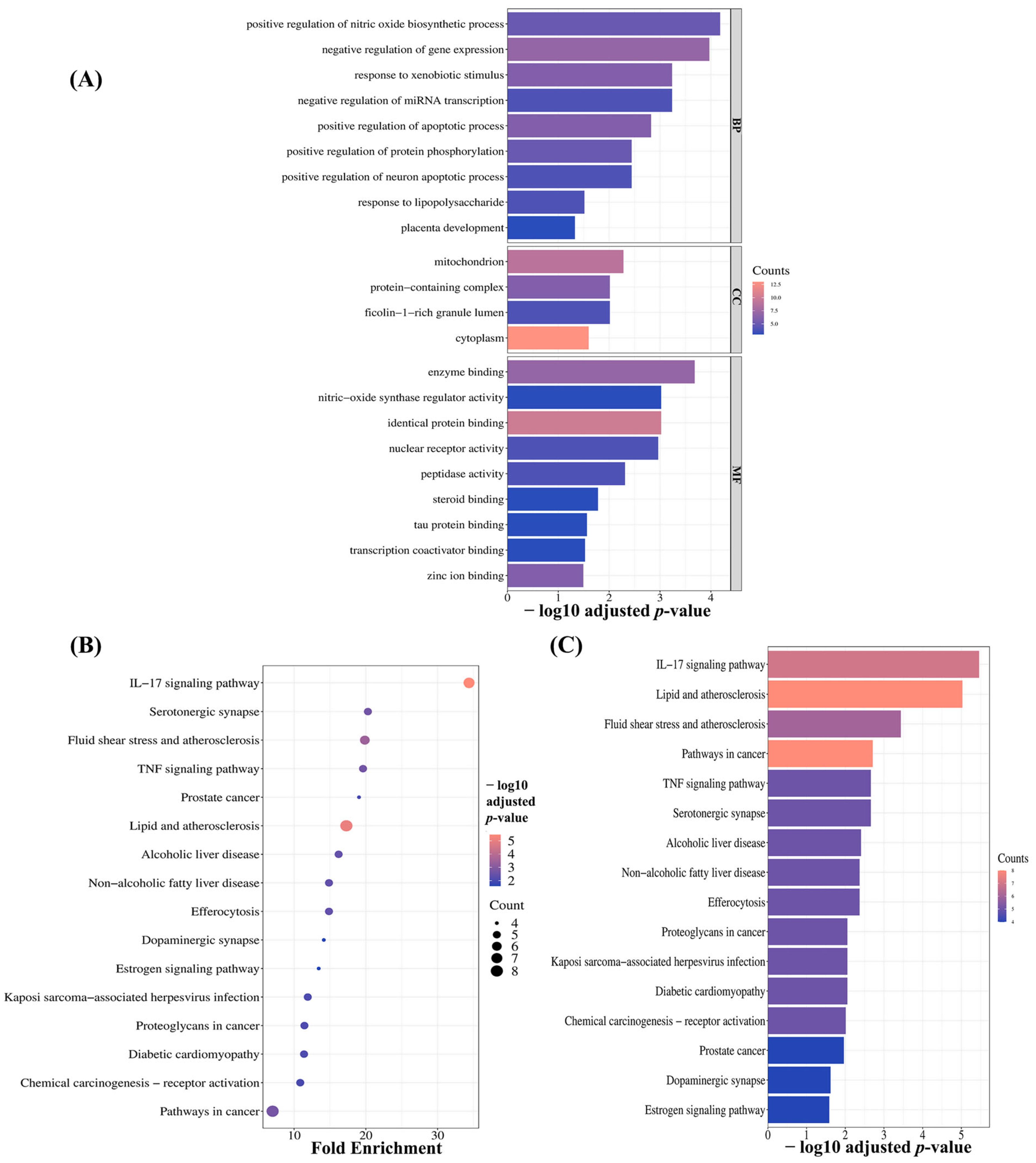
2.3. Network Pharmacology-Guided Mechanistic Analysis
2.3.1. Drug-likeness Profiling of Bioactive Constituents and Target Exploration
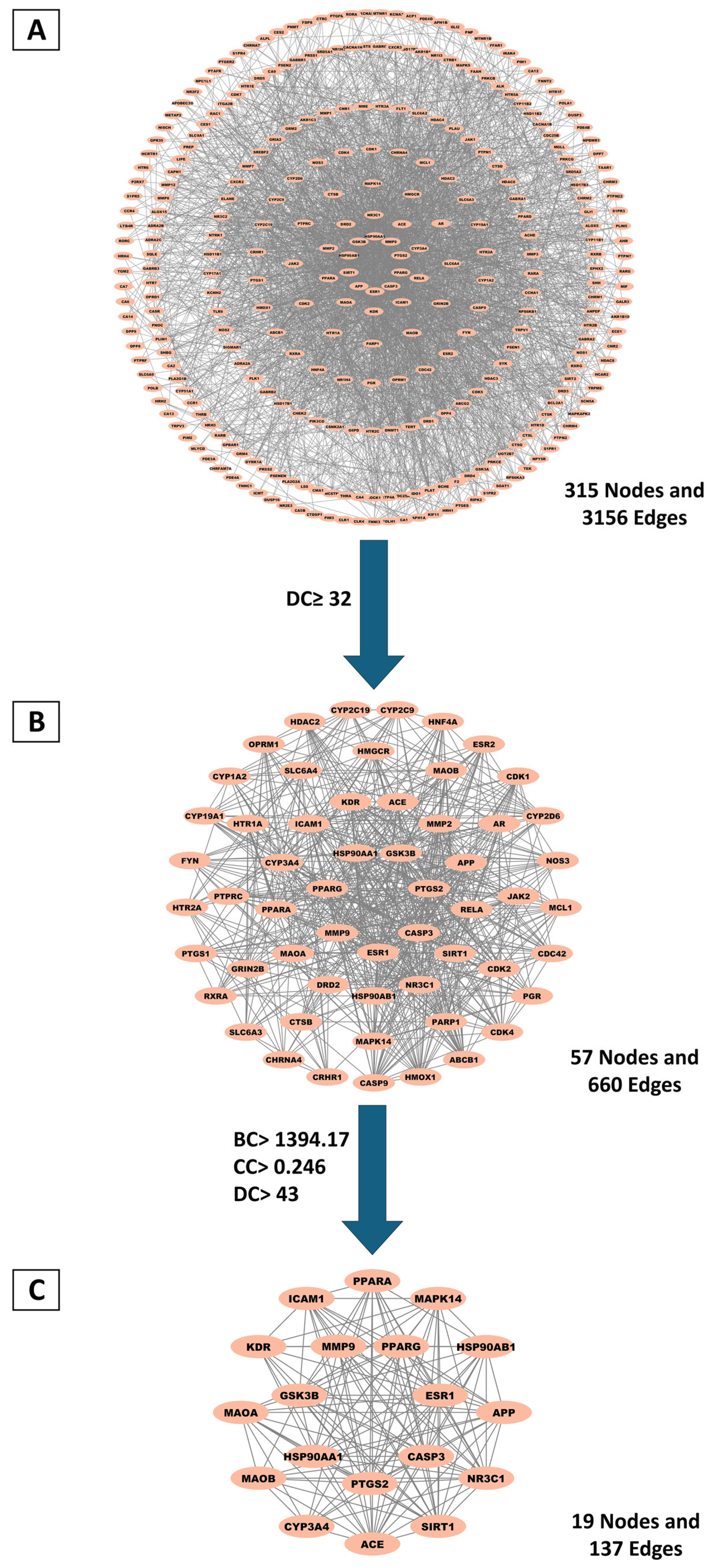
2.3.2. Identification and Topological Characterization of Hub Genes via Protein–Protein Interaction (PPI) Network Analysis
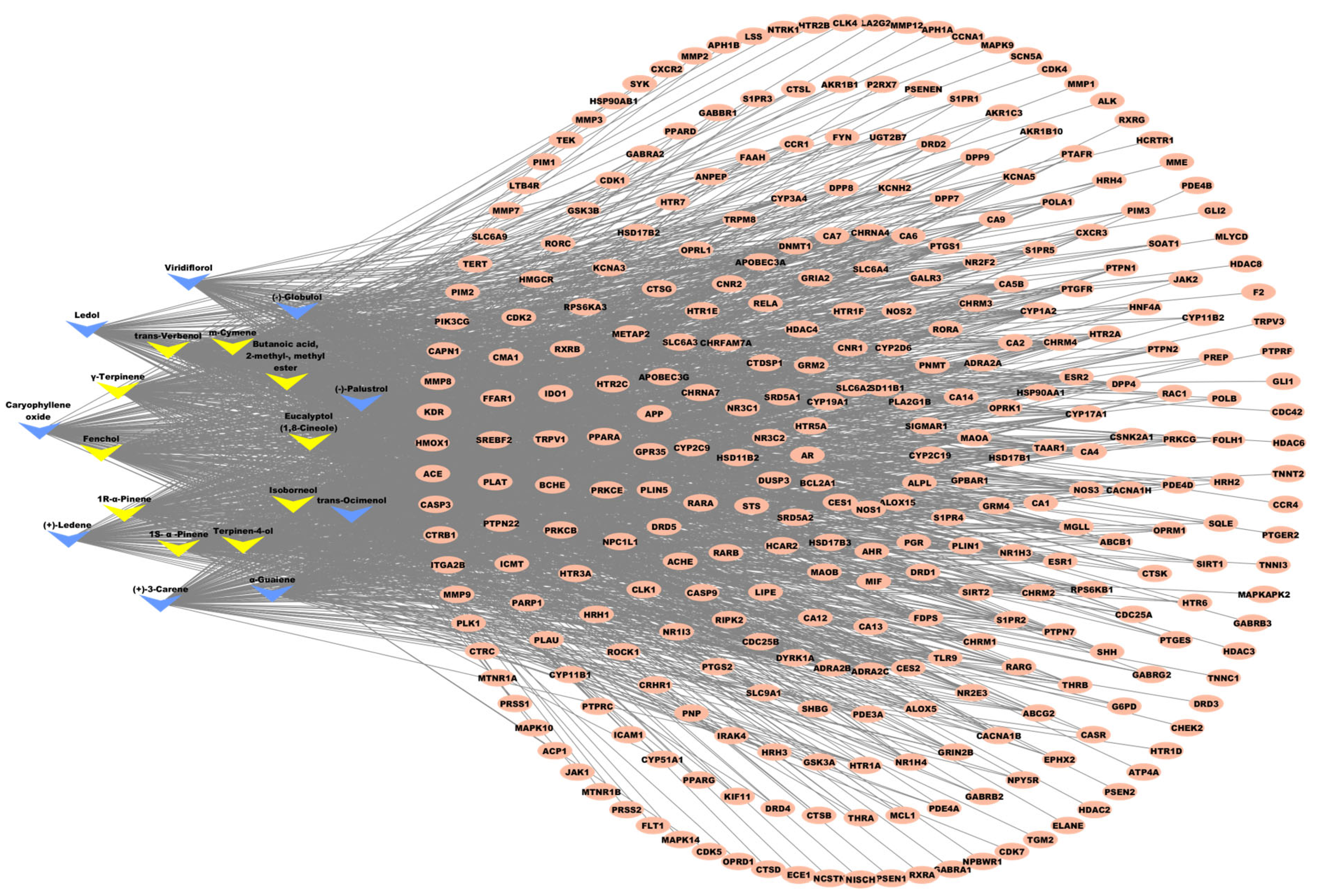
2.3.3. Key MQLEO Bioactive Constituents Linked to Lung Cancer Targets
2.3.4. Pathway Enrichment Analysis of MQLEO’s Core Targets in Lung Cancer
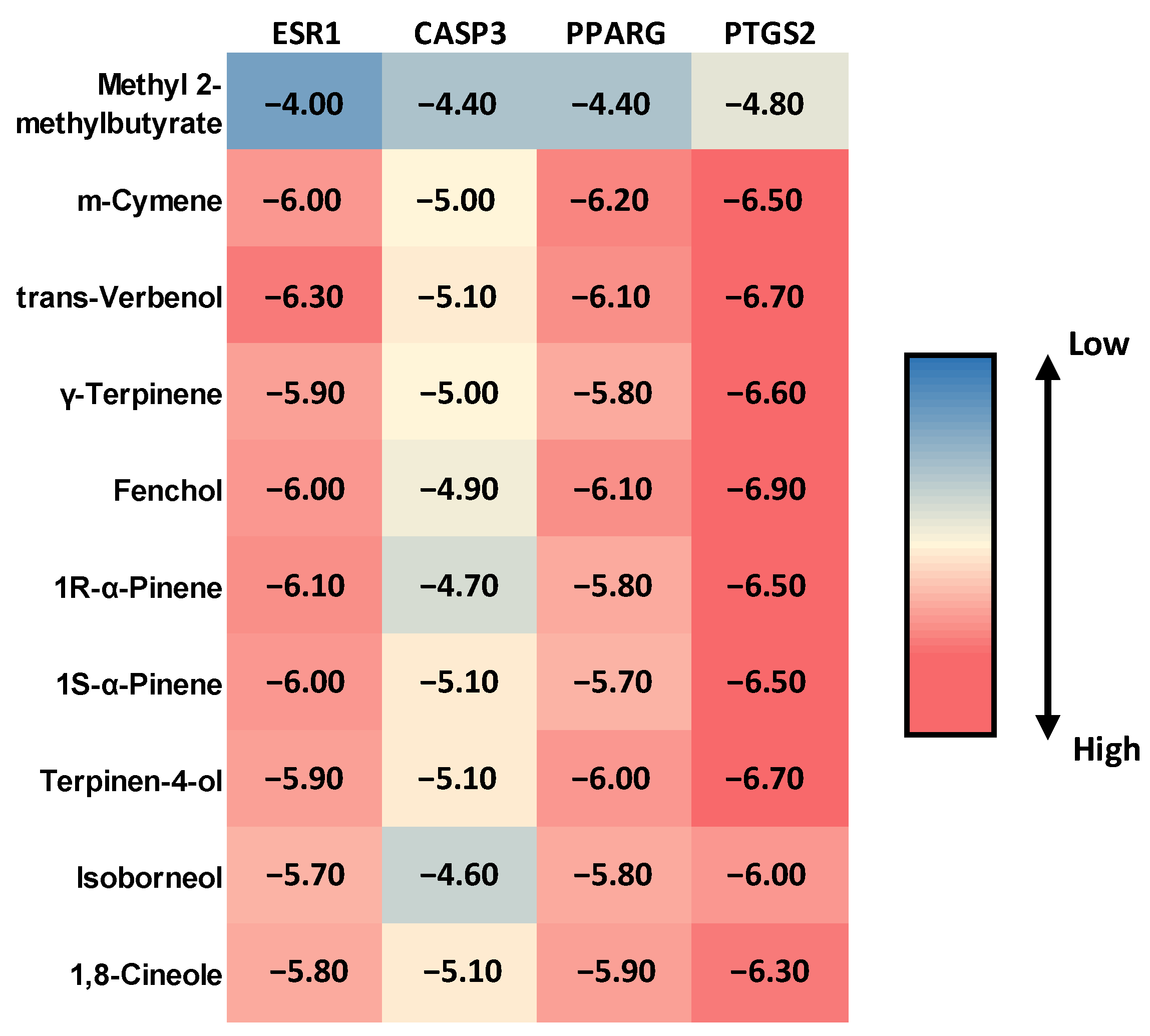
2.4. Molecular Docking Evaluation of MQLEO Compounds Against Lung Cancer Targets
3. Discussion
4. Materials and Methods
4.1. Botanical Specimen Collection and Processing Methodology
4.2. GC–MS Characterization of MQLEO
4.3. In Vitro Validations
4.3.1. Cytotoxicity Screening
Cell Culture and Treatment
MTT Assay Protocol
Data Analysis and Statistical Evaluation
Selectivity Index (SI)
4.3.2. Evaluation of Anti-Migratory Effects of MQLEO on A549 Tumor Cells
4.3.3. Apoptosis and Cell Cycle Analysis of A549 Cells Treated with MQLEO
Statistical Analysis
4.4. Network Pharmacology
4.4.1. Pharmacokinetic Evaluation of MQLEO Phytoconstituents
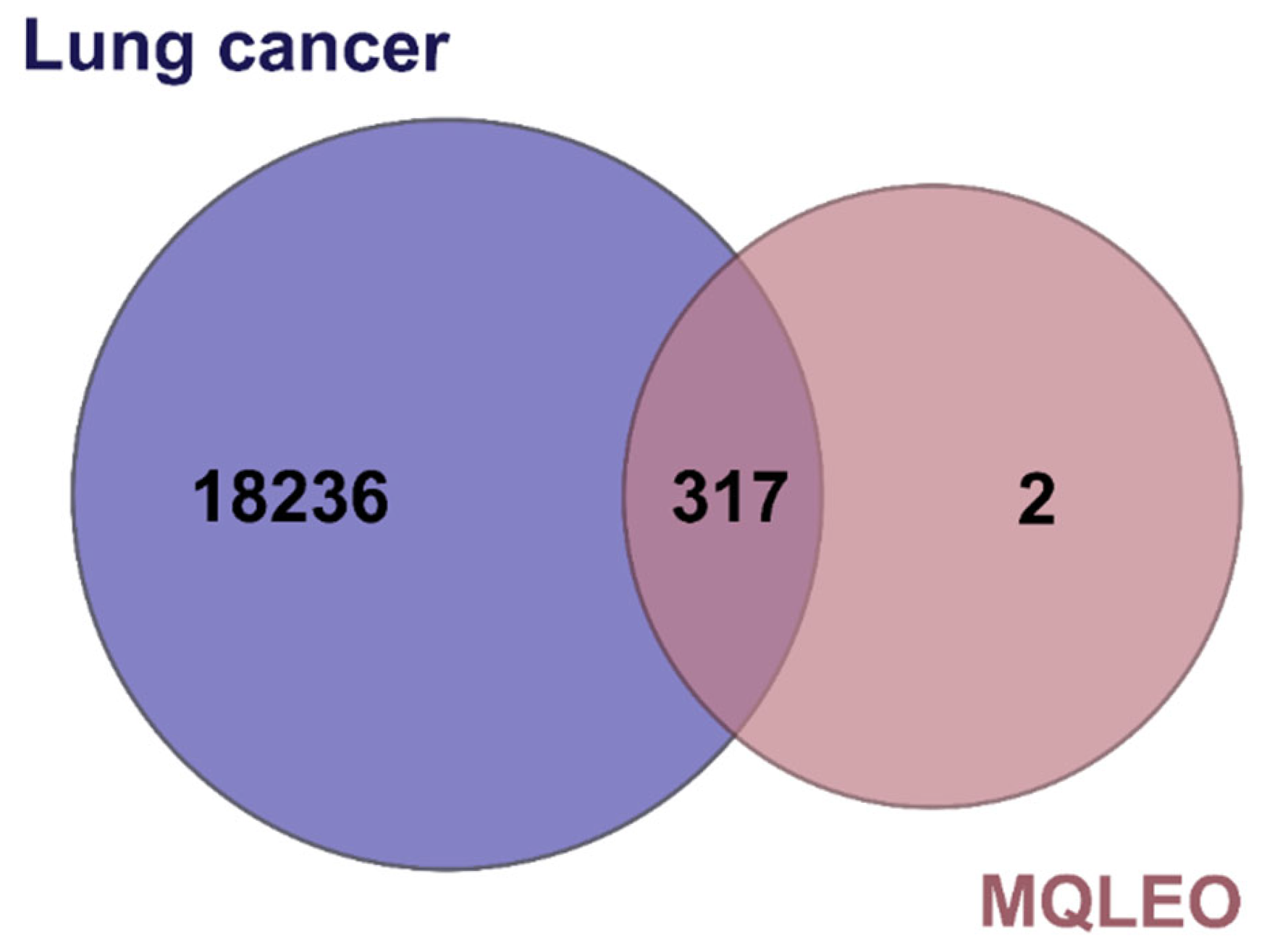
4.4.2. Identification of Intersection Genes Between Lung Cancer and MQLEO Bioactive Compounds
4.4.3. PPI Network Construction
4.4.4. Compound–Target Interaction Network Construction
4.4.5. Functional Annotation and Pathway Enrichment
4.5. Molecular Docking Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-549 | Human Lung Cancer Cell Line |
| ATCC | American Type Culture Collection |
| BC | Betweenness Centrality |
| BP | Biological Process |
| CASP3 | Caspase-3 |
| CASTp | Computed Atlas of Surface Topography of Proteins |
| CC | Closeness Centrality/Cellular Component |
| CC50 | 50% Cytotoxic Concentration |
| CDK | Cyclin-Dependent Kinase |
| CO2 | Carbon Dioxide |
| DAVID | Database for Annotation, Visualization, and Integrated Discovery |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | Dimethyl Sulfoxide |
| eV | Electron Volt |
| EO | Essential Oil |
| ESR1 | Estrogen Receptor 1 (a nuclear receptor involved in hormone signaling) |
| FBS | Fetal Bovine Serum |
| FITC | Fluorescein Isothiocyanate |
| G0-G1 Phase | Gap 0/Growth 1 Phase of the Cell Cycle |
| GC-MS | Gas Chromatography–Mass Spectrometry |
| GO | Gene Ontology |
| HepG-2 | Human Liver Cancer Cell Line |
| IC25 | 25% Inhibitory Concentration |
| IC50 | Half-maximal Inhibitory Concentration |
| IL-17 | Interleukin 17 |
| kcal/mol | Kilocalories per Mole |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK/ERK | Mitogen-Activated Protein Kinase/Extracellular Signal-Regulated Kinase |
| MCF-7 | Human Breast Cancer Cell Line |
| MF | Molecular Function |
| MMP | Matrix Metalloproteinase |
| MQLEO | Melaleuca quinquenervia Leaf Essential Oil |
| MS | Mass Spectrometry |
| mA | Milliampere |
| m/z | Mass-to-Charge Ratio |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide |
| NCI | National Cancer Institute |
| NO | Nitric Oxide |
| NSCLC | Non-Small Cell Lung Cancer |
| OD | Optical Density |
| OMIM | Online Mendelian Inheritance in Man |
| PDB | Protein Data Bank |
| PBS | Phosphate-Buffered Saline |
| PI | Propidium Iodide |
| PPARG | Peroxisome Proliferator-Activated Receptor Gamma |
| PPI | Protein–Protein Interaction |
| PS | Phosphatidylserine |
| PTGS2 | Prostaglandin-Endoperoxide Synthase 2 (COX-2) |
| RI | Retention Index |
| RIExp. | Experimental Retention Index |
| RILit. | Literature Retention Index |
| RMSD | Root Mean Square Deviation |
| RNase A | Ribonuclease A |
| RPM | Revolutions Per Minute |
| Rt | Retention Time In Minutes |
| S Phase | DNA Synthesis Phase of the Cell Cycle |
| SD | Standard Deviation |
| SI | Selectivity Index |
| STP | Swiss Target Prediction |
| TNF | Tumor Necrosis Factor |
| VERO | Normal African Green Monkey Kidney Cells |
References
- Pina-Sanchez, P.; Chavez-Gonzalez, A.; Ruiz-Tachiquin, M.; Vadillo, E.; Monroy-Garcia, A.; Montesinos, J.J.; Grajales, R.; Gutierrez de la Barrera, M.; Mayani, H. Cancer biology, epidemiology, and treatment in the 21st century: Current status and future challenges from a biomedical perspective. Cancer Control 2021, 28, 10732748211038735. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Das, S.K.; Sinha, K.; Ghosh, B.; Sen, K.; Ghosh, N.; Sil, P.C. The Emerging Role of Natural Products in Cancer Treatment. Arch. Toxicol. 2024, 98, 2353–2391. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Decker, C.C.; Zechner, L.; Krstin, S.; Wink, M. In vitro wound healing of tumor cells: Inhibition of cell migration by selected cytotoxic alkaloids. BMC Pharmacol. Toxicol. 2019, 20, 4. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Georgescu, M.; Marinas, I.C.; Ustundag, C.B.; Bertesteanu, G.; Pinteală, M.; Maier, S.S.; Al-Matarneh, C.M.; Angheloiu, M.; Chifiriuc, M.C. Therapeutic management of malignant wounds: An update. Curr. Treat. Options Oncol. 2024, 25, 97–126. [Google Scholar] [CrossRef]
- Tian, X.; Srinivasan, P.R.; Tajiknia, V.; Uruchurtu, A.F.S.S.; Seyhan, A.A.; Carneiro, B.A.; De La Cruz, A.; Pinho-Schwermann, M.; George, A.; Zhao, S.; et al. Targeting apoptotic pathways for cancer therapy. J. Clin. Investig. 2024, 134, e179570. [Google Scholar] [CrossRef]
- Wong, R.S. Apoptosis in cancer: From pathogenesis to treatment. J. Exp. Clin. Cancer Res. 2011, 30, 87. [Google Scholar] [CrossRef]
- Bai, J.; Li, Y.; Zhang, G. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar]
- Li, C.-X.; Wang, J.-S.; Wang, W.-N.; Xu, D.-K.; Zhou, Y.-T.; Sun, F.-Z.; Li, Y.-Q.; Guo, F.-Z.; Ma, J.-L.; Zhang, X.-Y.; et al. Expression dynamics of periodic transcripts during cancer cell cycle progression and their correlation with anticancer drug sensitivity. Mil. Med. Res. 2022, 9, 71. [Google Scholar] [CrossRef]
- Sharma, M.; Grewal, K.; Jandrotia, R.; Batish, D.R.; Singh, H.P.; Kohli, R.K. Essential oils as anticancer agents: Potential role in malignancies, drug delivery mechanisms, and immune system enhancement. Biomed. Pharmacother. 2022, 146, 112514. [Google Scholar] [CrossRef]
- Wilson, P.G. Myrtaceae. In Flowering Plants. Eudicots: Sapindales, Cucurbitales, Myrtaceae; Springer: Berlin/Heidelberg, Germany, 2010; pp. 212–271. [Google Scholar]
- Barbosa, L.C.A.; Silva, C.J.; Teixeira, R.R.; Meira, R.M.S.A.; Pinheiro, A.L. Chemistry and biological activities of essential oils from Melaleuca L. species. Agric. Conspec. Sci. 2013, 78, 11–23. [Google Scholar]
- Tran, P.H.; Vu, T.T.T.; Phan, T.D.T.; Nguyen, V.M.; Ngo, T.N.M.; Le, C.V.C.; Ton, T.H.D. Chemical compositions and biological properties of the leaf essential oil of three Melaleuca species. World Acad. Sci. J. 2024, 6, 67. [Google Scholar] [CrossRef]
- Liu, X.; Zu, Y.; Fu, Y.; Yao, L.; Gu, C.; Wang, W.; Efferth, T. Antimicrobial activity and cytotoxicity towards cancer cells of Melaleuca alternifolia (tea tree) oil. Eur. Food Res. Technol. 2009, 229, 247–253. [Google Scholar] [CrossRef]
- Acha, E.; Ahounou Aikpe, J.; Adovelande, J.; Assogba, M.F.; Agossou, G.; Sezan, A.; Dansou, H.P.; Gbenou, J. Anti-inflammatory properties of Melaleuca quinquenervia (Cav.) ST Blake Myrtaceae (Niaouli) leaves’ essential oil. Int. J. Curr. Res. Chem. Pharm. Sci. 2019, 6, 30–40. [Google Scholar]
- Joshi, A.; Prakash, O.; Pant, A.K.; Kumar, R.; Szczepaniak, L.; Kucharska-Ambrożej, K. Methyl eugenol, 1, 8-cineole and nerolidol rich essential oils with their biological activities from three Melaleuca species growing in Tarai region of North India. Braz. Arch. Biol. Technol. 2022, 64, e21210186. [Google Scholar] [CrossRef]
- Monzote, L.; Scherbakov, A.M.; Scull, R.; Satyal, P.; Cos, P.; Shchekotikhin, A.E.; Gille, L.; Setzer, W.N. Essential oil from Melaleuca leucadendra: Antimicrobial, antikinetoplastid, antiproliferative and cytotoxic assessment. Molecules 2020, 25, 5514. [Google Scholar] [CrossRef]
- Bhagat, M.; Sangral, M.; Pandita, S.; Gupta, S.; Bindu, K. Pleiotropic chemodiversity in extracts and Essential oil of Melaleuca viminalis and Melaleuca armillaris of Myrtaceae Family. J. Explor. Res. Pharmacol. 2017, 2, 113–120. [Google Scholar] [CrossRef]
- Albayrak, İ.G.; Gültekin, S.K.; Konuk, M. Effect of Melaleuca alternifolia oil on cytotoxicity and neuropeptide y gene expression. İstanbul J. Pharm. 2023, 53, 58–62. [Google Scholar] [CrossRef]
- Byahatti, S.; Bogar, C.; Bhat, K.; Dandagi, G. Evaluation of anticancer activity of Melaleuka Alternifolia.(ie tea tree oil) on Breast cancer cell line (MDA MB)-An invitro study. Int. J. Med. Microbiol. Trop. Dis. 2018, 4, 176–180. [Google Scholar]
- Chabir, N.; Romdhane, M.; Valentin, A.; Moukarzel, B.; Marzoug, H.N.B.; Brahim, N.B.; Mars, M.; Bouajila, J. Chemical study and antimalarial, antioxidant, and anticancer activities of Melaleuca armillaris (Sol Ex Gateau) Sm essential oil. J. Med. Food 2011, 14, 1383–1388. [Google Scholar] [CrossRef]
- Govaerts, R.; Sobral, M.; Ashton, P.; Barrie, F.; Holst, B.K.; Landrum, L.L.; Matsumoto, K.; Mazine, F.F.; Lughadha, E.N.; Proneça, C. World Checklist of Myrtaceae; Royal Botanic Gardens, Kew: Richmond, UK, 2008. [Google Scholar]
- Ireland, B.; Hibbert, D.; Goldsack, R.; Doran, J.; Brophy, J. Chemical variation in the leaf essential oil of Melaleuca quinquenervia (Cav.) ST Blake. Biochem. Syst. Ecol. 2002, 30, 457–470. [Google Scholar] [CrossRef]
- Chaverri, C.; F Cicció, J. Chemical composition of essential oils of the tree Melaleuca quinquenervia (Myrtaceae) cultivated in Costa Rica. Cuad. Investig. UNED 2021, 13, e3327. [Google Scholar] [CrossRef]
- Aboutabl, E.; Tohamy, S.E.; De Footer, H.; De Buyck, L. A comparative study of the essential oils from three Melaleuca species growing in Egypt. Flavour Fragr. J. 1991, 6, 139–141. [Google Scholar] [CrossRef]
- Luro, F.; Garcia Neves, C.; Costantino, G.; da Silva Gesteira, A.; Paoli, M.; Ollitrault, P.; Tomi, F.; Micheli, F.; Gibernau, M. Effect of Environmental Conditions on the Yield of Peel and Composition of Essential Oils from Citrus Cultivated in Bahia (Brazil) and Corsica (France). Agronomy 2020, 10, 1256. [Google Scholar] [CrossRef]
- Dakhlaoui, S.; Bourgou, S.; Zar Kalai, F.; Bachkouel, S.; Ippolito, N.M.; Msaada, K. Eucalyptus essential oils: Chemical profiling and pharmacological potential for sustainable forest cultivation. Euro-Mediterr. J. Environ. Integr. 2024. [Google Scholar] [CrossRef]
- Waseem, R.; Low, K.H. Advanced analytical techniques for the extraction and characterization of plant-derived essential oils by gas chromatography with mass spectrometry. J. Sep. Sci. 2015, 38, 483–501. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, S.; Mishra, S.; Shankar, U.; Maurya, A.; Verma, R.S. Recent Advances in Extraction, Analysis, Value Addition, and Applications of Essential Oils. In Medicinal and Aromatic Plants: Current Research Status, Value-Addition to Their Waste, and Agro-Industrial Potential (Volome I); Kumar, L., Bharadvaja, N., Singh, R., Anand, R., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 75–96. [Google Scholar]
- Chao, W.-W.; Su, C.-C.; Peng, H.-Y.; Chou, S.-T. Melaleuca quinquenervia essential oil inhibits α-melanocyte-stimulating hormone-induced melanin production and oxidative stress in B16 melanoma cells. Phytomedicine 2017, 34, 191–201. [Google Scholar] [CrossRef]
- Nishida, A.; Andoh, A. The Role of Inflammation in Cancer: Mechanisms of Tumor Initiation, Progression, and Metastasis. Cells 2025, 14, 488. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Buttery, R.G.; Flath, R.A. Volatile constituents of Asian pear (Pyrus serotina). J. Agric. Food Chem. 1992, 40, 1925–1929. [Google Scholar] [CrossRef]
- Carasek, E.; Pawliszyn, J. Screening of tropical fruit volatile compounds using solid-phase microextraction (SPME) fibers and internally cooled SPME fiber. J. Agric. Food Chem. 2006, 54, 8688–8696. [Google Scholar] [CrossRef] [PubMed]
- Hoskovec, M.; Grygarová, D.; Cvačka, J.; Streinz, L.; Zima, J.; Verevkin, S.P.; Koutek, B. Determining the vapour pressures of plant volatiles from gas chromatographic retention data. J. Chromatogr. A 2005, 1083, 161–172. [Google Scholar] [CrossRef]
- Fikry, E.; Orfali, R.; Tawfeek, N.; Perveen, S.; Ghafar, S.; El-Domiaty, M.M.; El-Shafae, A.M. Unveiling the Bioactive Efficacy of Cupressus sempervirens ‘Stricta’ Essential Oil: Composition, In Vitro Activities, and In Silico Analyses. Pharmaceuticals 2024, 17, 1019. [Google Scholar] [CrossRef]
- Allegrone, G.; Belliardo, F.; Cabella, P. Comparison of Volatile Concentrations in Hand-Squeezed Juices of Four Different Lemon Varieties. J. Agric. Food Chem. 2006, 54, 1844–1848. [Google Scholar] [CrossRef]
- Medina, A.L.; Lucero, M.E.; Holguin, F.O.; Estell, R.E.; Posakony, J.J.; Simon, J.; O’Connell, M.A. Composition and antimicrobial activity of Anemopsis californica leaf oil. J. Agric. Food Chem. 2005, 53, 8694–8698. [Google Scholar] [CrossRef]
- Siani, A.C.; Garrido, I.S.; Monteiro, S.S.; Carvalho, E.S.; Ramos, M.F.S. Protium icicariba as a source of volatile essences. Biochem. Syst. Ecol. 2004, 32, 477–489. [Google Scholar] [CrossRef]
- Paolini, J.; Muselli, A.; Bernardini, A.-F.; Bighelli, A.; Casanova, J.; Costa, J. Thymol derivatives from essential oil of Doronicum corsicum L. Flavour Fragr. J. 2007, 22, 479–487. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the Essential Oils of Thymus and Origanum Species from Algeria and Their Antioxidant and Antimicrobial Activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Tayoub, G.; Schwob, I.; Bessière, J.-M.; Masotti, V.; Rabier, J.; Ruzzier, M.; Viano, J. Composition of volatile oils of Styrax (Styrax officinalis L.) leaves at different phenological stages. Biochem. Syst. Ecol. 2006, 34, 705–709. [Google Scholar] [CrossRef]
- Fikry, E.; Orfali, R.; Elbaramawi, S.S.; Perveen, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Chamaecyparis lawsoniana leaf essential oil as a potential anticancer agent: Experimental and computational studies. Plants 2023, 12, 2475. [Google Scholar] [CrossRef]
- Mohanty, D.; Padhee, S.; Priyadarshini, A.; Champati, B.B.; Das, P.K.; Jena, S.; Sahoo, A.; Panda, P.C.; Nayak, S.; Ray, A. Elucidating the anti-cancer potential of Cinnamomum tamala essential oil against non-small cell lung cancer: A multifaceted approach involving GC-MS profiling, network pharmacology, and molecular dynamics simulations. Heliyon 2024, 10, e28026. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, J.; Liu, Q.; Ge, J.; Ma, Z.; Mou, J.; Wang, L. Biological, functional and network pharmacological exploration of essential oils in treatment and healthcare of human diseases. Future Integr. Med. 2023, 2, 23–31. [Google Scholar] [CrossRef]
- Olmedo, R.H.; Asensio, C.M.; Grosso, N.R. Thermal stability and antioxidant activity of essential oils from aromatic plants farmed in Argentina. Ind. Crops Prod. 2015, 69, 21–28. [Google Scholar] [CrossRef]
- Abdelmohsen, U.R.; Elmaidomy, A.H. Exploring the therapeutic potential of essential oils: A review of composition and influencing factors. Front. Nat. Prod. 2025, 4, 1490511. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Mahanta, B.P.; Bora, P.K.; Kemprai, P.; Borah, G.; Lal, M.; Haldar, S. Thermolabile essential oils, aromas and flavours: Degradation pathways, effect of thermal processing and alteration of sensory quality. Food Res. Int. 2021, 145, 110404. [Google Scholar] [CrossRef]
- Barra, A. Factors affecting chemical variability of essential oils: A review of recent developments. Nat. Prod. Commun. 2009, 4, 1934578X0900400827. [Google Scholar] [CrossRef]
- Kocabaş Oğuz, I.; Kaplan, M. The effect of altitude and soil properties on the essential oil components of Turkish sage (Salvia fruticosa Mill.). Bol. Latinoam. Caribe Plantas Med. Aromát. 2023, 22, 204–213. [Google Scholar] [CrossRef]
- Malsy, M.; Bitzinger, D.; Graf, B.; Bundscherer, A. Staurosporine induces apoptosis in pancreatic carcinoma cells PaTu 8988t and Panc-1 via the intrinsic signaling pathway. Eur. J. Med. Res. 2019, 24, 5. [Google Scholar] [CrossRef]
- Montalvão, M.M.; Felix, F.B.; Propheta Dos Santos, E.W.; Santos, J.F.; de Lucca Júnior, W.; Farias, A.S.; de Souza Ribeiro, A.; Cavaleiro, C.; Machado, S.M.F.; Scher, R.; et al. Cytotoxic activity of essential oil from Leaves of Myrcia splendens against A549 Lung Cancer cells. BMC Complement. Med. Ther. 2023, 23, 139. [Google Scholar] [CrossRef]
- Alqudah, M.A.; Yaseen, M.M.; Alzoubi, K.H.; Al-Husein, B.A.; Bardaweel, S.K.; Abuhelwa, A.Y.; Semreen, A.M.; Zenati, R.A.; El-Awady, R.; Shara, M.; et al. Metabolomic Analysis, Antiproliferative, Anti-Migratory, and Anti-Invasive Potential of Amlodipine in Lung Cancer Cells. Drug Des. Dev. Ther. 2025, 19, 1215–1229. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.K.; Azmi, M.N.; In, L.L.; Awang, K.; Nagoor, N.H. Anti-proliferative, apoptotic induction, and anti-migration effects of hemi-synthetic 1′ S-1′-acetoxychavicol acetate analogs on MDA-MB-231 breast cancer cells. Drug Des. Dev. Ther. 2017, 11, 2763–2776. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, M.; Bu, Q.; Zhao, C. Signaling Pathways Driving MSC Osteogenesis: Mechanisms, Regulation, and Translational Applications. Int. J. Mol. Sci. 2025, 26, 1311. [Google Scholar] [CrossRef]
- Selvi, H.; Brüning-Richardson, A.; Danovi, D. Systematic Review of Pre-Clinical Systems Using Artificial Microenvironments and Anti-Migratory Drugs to Control Migration of Glioblastoma Cells. Expert Rev. Mol. Med. 2025, 27, e6. [Google Scholar] [CrossRef]
- Maltese, W.A.; Overmeyer, J.H. Non-apoptotic cell death associated with perturbations of macropinocytosis. Front. Physiol. 2015, 6, 38. [Google Scholar] [CrossRef]
- Lataster, L.; Huber, H.M.; Böttcher, C.; Föller, S.; Takors, R.; Radziwill, G. Cell Cycle Control by Optogenetically Regulated Cell Cycle Inhibitor Protein p21. Biology 2023, 12, 1194. [Google Scholar] [CrossRef]
- Lossaint, G.; Horvat, A.; Gire, V.; Bačević, K.; Mrouj, K.; Charrier-Savournin, F.; Georget, V.; Fisher, D.; Dulić, V. Reciprocal regulation of p21 and Chk1 controls the cyclin D1-RB pathway to mediate senescence onset after G2 arrest. J. Cell Sci. 2022, 135, jcs259114. [Google Scholar] [CrossRef]
- Gandhi, D.S.; Gupta, I.; Menia, R.; Kumar, R. Perspective Chapter: Molecular Pathology of Lung Cancer. In Molecular Histopathology and Cytopathology; Kara, A., Gelen, V., Kara, H., Eds.; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Hsu, R.; Leyba, A.; Chen, D.; Krause, H.B.; Elliott, A.; Cozen, W.; Roussos Torres, E.T.; Nagasaka, M.; Mamdani, H.; Lopes, G.; et al. Survival and mutational differences based on ESR1 and ESR2 expression in non-small cell lung cancer (NSCLC). J. Clin. Oncol. 2024, 42, 16. [Google Scholar] [CrossRef]
- Hamilton, D.H.; Griner, L.M.; Keller, J.M.; Hu, X.; Southall, N.; Marugan, J.; David, J.M.; Ferrer, M.; Palena, C. Targeting estrogen receptor signaling with fulvestrant enhances immune and chemotherapy-mediated cytotoxicity of human lung cancer. Clin. Cancer Res. 2016, 22, 6204–6216. [Google Scholar] [CrossRef]
- Li, Q.; Li, Z.; Luo, T.; Shi, H. Targeting the PI3K/AKT/mTOR and RAF/MEK/ERK pathways for cancer therapy. Mol. Biomed. 2022, 3, 47. [Google Scholar] [CrossRef]
- Eskandari, E.; Negri, G.L.; Tan, S.; MacAldaz, M.E.; Ding, S.; Long, J.; Nielsen, K.; Spencer, S.E.; Morin, G.B.; Eaves, C.J. Dependence of human cell survival and proliferation on the CASP3 prodomain. Cell Death Discov. 2024, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, E.; Eaves, C.J. Paradoxical roles of caspase-3 in regulating cell survival, proliferation, and tumorigenesis. J. Cell Biol. 2022, 221, e202201159. [Google Scholar] [CrossRef]
- Gökhan, A. Evaluation of cytotoxic, membrane damaging and apoptotic effects of Origanum majorana essential oil on lung cancer and epidermoid carcinoma cells. Evaluation 2022, 7, 201–206. [Google Scholar] [CrossRef]
- Saini, D.; Chaudhary, P.K.; Chaudhary, J.K.; Kaur, H.; Verma, G.K.; Pramanik, S.D.; Roy, P.; Mirza-Shariff, A.A.; Prasad, R. Molecular mechanisms of antiproliferative and pro-apoptotic effects of essential oil active constituents in MCF7 and T24 cancer cell lines: In vitro insights and in silico modelling of proapoptotic gene product-compound interactions. Apoptosis 2024, 30, 805–825. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, M.; Shang, J. PPARγ Modulators in Lung Cancer: Molecular Mechanisms, Clinical Prospects, and Challenges. Biomolecules 2024, 14, 190. [Google Scholar] [CrossRef]
- Xu, R.; Luo, X.; Ye, X.; Li, H.; Liu, H.; Du, Q.; Zhai, Q. SIRT1/PGC-1α/PPAR-γ correlate with hypoxia-induced chemoresistance in non-small cell lung cancer. Front. Oncol. 2021, 11, 682762. [Google Scholar] [CrossRef]
- Lin, X.-M.; Luo, W.; Wang, H.; Li, R.-Z.; Huang, Y.-S.; Chen, L.-K.; Wu, X.-P. The Role of Prostaglandin-Endoperoxide Synthase-2 in Chemoresistance of Non-Small Cell Lung Cancer. Front. Pharmacol. 2019, 10, 836. [Google Scholar] [CrossRef]
- Lai, H.; Liu, Y.; Wu, J.; Cai, J.; Jie, H.; Xu, Y.; Deng, S. Targeting cancer-related inflammation with non-steroidal anti-inflammatory drugs: Perspectives in pharmacogenomics. Front. Pharmacol. 2022, 13, 1078766. [Google Scholar] [CrossRef]
- Wang, D.; Xia, D.; DuBois, R.N. The crosstalk of PTGS2 and EGF signaling pathways in colorectal cancer. Cancers 2011, 3, 3894–3908. [Google Scholar] [CrossRef]
- Seol, G.H.; Kim, K.Y. Eucalyptol and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 929, 389–398. [Google Scholar]
- Singh, P.P.; Jaiswal, A.K.; Kumar, A.; Gupta, V.; Prakash, B. Untangling the multi-regime molecular mechanism of verbenol-chemotype Zingiber officinale essential oil against Aspergillus flavus and aflatoxin B1. Sci. Rep. 2021, 11, 6832. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Trytek, M.; Król, S.K.; Kud, J.; Frant, M.; Kandefer-Szerszeń, M.; Fiedurek, J. Biological activity of terpene compounds produced by biotechnological methods. Pharm. Biol. 2016, 54, 1096–1107. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.A.; Cieśla, Ł.M.; Waksmundzka-Hajnos, M. Approach to determination a structure–antioxidant activity relationship of selected common terpenoids evaluated by ABTS•+ radical cation assay. Nat. Prod. Commun. 2018, 13, 1934578X1801300308. [Google Scholar] [CrossRef]
- Salehi, B.; Upadhyay, S.; Erdogan Orhan, I.; Kumar Jugran, A.; L.D. Jayaweera, S.; A. Dias, D.; Sharopov, F.; Taheri, Y.; Martins, N.; Baghalpour, N.; et al. Therapeutic Potential of α- and β-Pinene: A Miracle Gift of Nature. Biomolecules 2019, 9, 738. [Google Scholar] [CrossRef]
- Deen, J.I.; Zawad, A.S.; Uddin, M.; Chowdhury, M.A.H.; Al Araby, S.Q.; Rahman, M.A. Terpinen-4-ol, A volatile terpene molecule, extensively electrifies the biological systems against the oxidative stress-linked pathogenesis. Adv. Redox Res. 2023, 9, 100082. [Google Scholar] [CrossRef]
- Yadav, N.; Chandra, H. Suppression of inflammatory and infection responses in lung macrophages by eucalyptus oil and its constituent 1, 8-cineole: Role of pattern recognition receptors TREM-1 and NLRP3, the MAP kinase regulator MKP-1, and NFκB. PLoS ONE 2017, 12, e0188232. [Google Scholar] [CrossRef]
- Gao, K.; Wu, C.; Li, Y.; Lu, J.; Jiang, Y. Transcriptome Analysis Reveals the Anti-Tumor Mechanism of Eucalyptol Treatment on Neuroblastoma Cell Line SH-SY5Y. Neurochem. Res. 2022, 47, 3854–3862. [Google Scholar] [CrossRef]
- Hu, T.; Shi, R.; Xu, Y.; Xu, T.; Fang, Y.; Gu, Y.; Zhou, Z.; Shu, Y. Multi-omics and single-cell analysis reveals machine learning-based pyrimidine metabolism-related signature in the prognosis of patients with lung adenocarcinoma. Int. J. Med. Sci. 2025, 22, 1375–1392. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, T.; Liu, J.; Sun, R.; Yu, Z.; Shen, G. Exploring the Mechanism of Comfrey in Lung Cancer Based on Network Pharmacology and Molecular Docking. SAS J. Med. 2024, 9, 973–982. [Google Scholar] [CrossRef]
- Pervin, S.; Singh, R.; Sen, S.; Chaudhuri, G. Dual role of nitric oxide in cancer biology. In Nitric Oxide (NO) and Cancer: Prognosis, Prevention, and Therapy; Springer: New York, NY, USA, 2010; pp. 39–57. [Google Scholar]
- Lee, C.B.; Choi, H.G.; Gurmessa, S.K.; Jang, I.-T.; Kumar, N.; Jiang, Z.; Kaushik, N.K.; Kim, H.-J. Enhancing antitumor immunity in Lewis lung cancer through plasma-treated medium-induced activation of dendritic cells. Cancer Cell Int. 2024, 24, 389. [Google Scholar] [CrossRef]
- Singh, P.; Lim, B. Targeting apoptosis in cancer. Curr. Oncol. Rep. 2022, 24, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.E.; Zhang, G.; Akcora, C.; Lin, Y.; Fang, B.; Koomen, J.; Haura, E.B.; Grimes, M. Network models of protein phosphorylation, acetylation, and ubiquitination connect metabolic and cell signaling pathways in lung cancer. PLoS Comput. Biol. 2023, 19, e1010690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Leung, P.-C.; Cho, W.C.-S.; Wong, C.-K.; Wang, D. Targeting PI3K signaling in Lung Cancer: Advances, challenges and therapeutic opportunities. J. Transl. Med. 2025, 23, 184. [Google Scholar] [CrossRef]
- Xia, M.; Zhang, Y.; Jin, K.; Lu, Z.; Zeng, Z.; Xiong, W. Communication between mitochondria and other organelles: A brand-new perspective on mitochondria in cancer. Cell Biosci. 2019, 9, 27. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Ponneri Babuharisankar, A.; Lin, Y.-C.; Lien, H.-W.; Lo, Y.K.; Chou, H.-Y.; Tangeda, V.; Cheng, L.-C.; Cheng, A.N.; Lee, A.Y.-L. Mitochondrial oxidative stress in the tumor microenvironment and cancer immunoescape: Foe or friend? J. Biomed. Sci. 2022, 29, 74. [Google Scholar] [CrossRef]
- Zhou, Q.; Cao, T.; Li, F.; Zhang, M.; Li, X.; Zhao, H.; Zhou, Y. Mitochondria: A new intervention target for tumor invasion and metastasis. Mol. Med. 2024, 30, 129. [Google Scholar] [CrossRef]
- Wang, S.-F.; Tseng, L.-M.; Lee, H.-C. Role of mitochondrial alterations in human cancer progression and cancer immunity. J. Biomed. Sci. 2023, 30, 61. [Google Scholar] [CrossRef]
- Harris, J.R.; Marles-Wright, J. Macromolecular Protein Complexes IV: Structure and Function, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 118. [Google Scholar] [CrossRef]
- Keshet, R.; Erez, A. Arginine and the metabolic regulation of nitric oxide synthesis in cancer. Dis. Models Mech. 2018, 11, dmm033332. [Google Scholar] [CrossRef]
- Martins, F.; Rosspopoff, O.; Carlevaro-Fita, J.; Forey, R.; Offner, S.; Planet, E.; Pulver, C.; Pak, H.; Huber, F.; Michaux, J.; et al. A Cluster of Evolutionarily Recent KRAB Zinc Finger Proteins Protects Cancer Cells from Replicative Stress–Induced Inflammation. Cancer Res. 2024, 84, 808–826. [Google Scholar] [CrossRef]
- Guo, H.; Wang, S.; Zhang, H.; Li, J.; Wang, C.; Liu, Z.; Chen, J.; Wang, K.; Wei, X.; Wei, Q.; et al. Research progress on the molecular structure, function, and application in tumor therapy of zinc transporter ZIP4. Int. J. Biol. Sci. 2024, 20, 5910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Gui, Y.; Cai, J.; Deng, X. Biometallic ions and derivatives: A new direction for cancer immunotherapy. Mol. Cancer 2025, 24, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Lan, T.; Chiari, C.; Ye, X.; Wang, K.; Chen, J. The role of interleukin-17 in inflammation-related cancers. Front. Immunol. 2025, 15, 1479505. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The role of tumor necrosis factor in manipulating the immunological response of tumor microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef]
- Webster, J.D.; Vucic, D. The balance of TNF mediated pathways regulates inflammatory cell death signaling in healthy and diseased tissues. Front. Cell Dev. Biol. 2020, 8, 365. [Google Scholar] [CrossRef]
- Ahrens, T.D.; Bang-Christensen, S.R.; Jørgensen, A.M.; Løppke, C.; Spliid, C.B.; Sand, N.T.; Clausen, T.M.; Salanti, A.; Agerbæk, M.Ø. The role of proteoglycans in cancer metastasis and circulating tumor cell analysis. Front. Cell Dev. Biol. 2020, 8, 749. [Google Scholar] [CrossRef]
- Glaviano, A.; Lau, H.S.-H.; Carter, L.M.; Lee, E.H.C.; Lam, H.Y.; Okina, E.; Tan, D.J.J.; Tan, W.; Ang, H.L.; Carbone, D.; et al. Harnessing the tumor microenvironment: Targeted cancer therapies through modulation of epithelial-mesenchymal transition. J. Hematol. Oncol. 2025, 18, 6. [Google Scholar] [CrossRef]
- Amos, S.E.; Choi, Y.S. The cancer microenvironment: Mechanical challenges of the metastatic cascade. Front. Bioeng. Biotechnol. 2021, 9, 625859. [Google Scholar] [CrossRef]
- Yuan, Z.; Li, Y.; Zhang, S.; Wang, X.; Dou, H.; Yu, X.; Zhang, Z.; Yang, S.; Xiao, M. Extracellular matrix remodeling in tumor progression and immune escape: From mechanisms to treatments. Mol. Cancer 2023, 22, 48. [Google Scholar] [CrossRef]
- Ravegnini, G.; Sammarini, G.; Hrelia, P.; Angelini, S. Key genetic and epigenetic mechanisms in chemical carcinogenesis. Toxicol. Sci. 2015, 148, 2–13. [Google Scholar] [CrossRef]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 2017, 198, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Caner, A. Immune Escape Mechanism of Cancer. Curr. Mol. Biol. Rep. 2024, 10, 9–19. [Google Scholar] [CrossRef]
- Chatatikun, M.; Pattaranggoon, N.C.; Sama-ae, I.; Ranteh, O.; Poolpirom, M.; Pantanakong, O.; Chumworadet, P.; Kawakami, F.; Imai, M.; Tedasen, A. Mechanistic exploration of bioactive constituents in Gnetum gnemon for GPCR-related cancer treatment through network pharmacology and molecular docking. Sci. Rep. 2024, 14, 25738. [Google Scholar] [CrossRef]
- Cory, A.H.; Owen, T.C.; Barltrop, J.A.; Cory, J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991, 3, 207–212. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M.; Mahmmoud, E.A.; Elaasser, M.M. Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd. 2015, 51, 1030–1038. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, W357–W364. [Google Scholar] [CrossRef]
- Yao, Z.J.; Dong, J.; Che, Y.J.; Zhu, M.F.; Wen, M.; Wang, N.N.; Wang, S.; Lu, A.P.; Cao, D.S. TargetNet: A web service for predicting potential drug-target interaction profiling via multi-target SAR models. J. Comput. Aided Mol. Des. 2016, 30, 413–424. [Google Scholar] [CrossRef]
- Hamosh, A.; Scott, A.F.; Amberger, J.S.; Bocchini, C.A.; McKusick, V.A. Online Mendelian Inheritance in Man (OMIM), a knowledgebase of human genes and genetic disorders. Nucleic Acids Res. 2005, 33, D514–D517. [Google Scholar] [CrossRef]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. TIG 1997, 13, 163. [Google Scholar] [CrossRef]
- Safran, M.; Dalah, I.; Alexander, J.; Rosen, N.; Iny Stein, T.; Shmoish, M.; Nativ, N.; Bahir, I.; Doniger, T.; Krug, H.; et al. GeneCards Version 3: The human gene integrator. Database 2010, 2010, baq020. [Google Scholar] [CrossRef] [PubMed]
- Zaru, R.; Orchard, S.; Consortium, U. Uniprot tools: BLAST, align, peptide search, and ID mapping. Curr. Protoc. 2023, 3, e697. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein–protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Tang, Y.; Li, M.; Wang, J.; Pan, Y.; Wu, F.X. CytoNCA: A cytoscape plugin for centrality analysis and evaluation of protein interaction networks. Biosystems 2015, 127, 67–72. [Google Scholar] [CrossRef]
- Chin, C.-H.; Chen, S.-H.; Wu, H.-H.; Ho, C.-W.; Ko, M.-T.; Lin, C.-Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for annotation, visualization, and integrated discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Vajdos, F.F.; Hoth, L.R.; Geoghegan, K.F.; Simons, S.P.; LeMotte, P.K.; Danley, D.E.; Ammirati, M.J.; Pandit, J. The 2.0 Å crystal structure of the ERα ligand-binding domain complexed with lasofoxifene. Protein Sci. 2007, 16, 897–905. [Google Scholar] [CrossRef]
- Maciag, J.J.; Mackenzie, S.H.; Tucker, M.B.; Schipper, J.L.; Swartz, P.; Clark, A.C. Tunable allosteric library of caspase-3 identifies coupling between conserved water molecules and conformational selection. Proc. Natl. Acad. Sci. USA 2016, 113, E6080–E6088. [Google Scholar] [CrossRef]
- Arifi, S.; Marschner, J.A.; Pollinger, J.; Isigkeit, L.; Heitel, P.; Kaiser, A.; Obeser, L.; Höfner, G.; Proschak, E.; Knapp, S.; et al. Targeting the alternative vitamin E metabolite binding site enables noncanonical PPARγ modulation. J. Am. Chem. Soc. 2023, 145, 14802–14810. [Google Scholar] [CrossRef]
- Lucido, M.J.; Orlando, B.J.; Vecchio, A.J.; Malkowski, M.G. Crystal structure of aspirin-acetylated human cyclooxygenase-2: Insight into the formation of products with reversed stereochemistry. Biochemistry 2016, 55, 1226–1238. [Google Scholar] [CrossRef] [PubMed]
- Burley, S.K.; Berman, H.M.; Kleywegt, G.J.; Markley, J.L.; Nakamura, H.; Velankar, S. Protein Data Bank (PDB): The Single Global Macromolecular Structure Archive. In Protein Crystallography: Methods and Protocols; Wlodawer, A., Dauter, Z., Jaskolski, M., Eds.; Springer: New York, NY, USA, 2017; pp. 627–641. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Fikry, E.; Orfali, R.; El-Sayed, S.S.; Perveen, S.; Ghafar, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Potential Hepatoprotective Effects of Chamaecyparis lawsoniana against Methotrexate-Induced Liver Injury: Integrated Phytochemical Profiling, Target Network Analysis, and Experimental Validation. Antioxidants 2023, 12, 2118. [Google Scholar] [CrossRef]
- Tian, W.; Chen, C.; Lei, X.; Zhao, J.; Liang, J. CASTp 3.0: Computed atlas of surface topography of proteins. Nucleic Acids Res. 2018, 46, W363–W367. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef]
- Biovia, D.S. Discovery Studio Visualizer, v21. 1.0. 20298; Dassault Systèmes: San Diego, CA, USA, 2021.
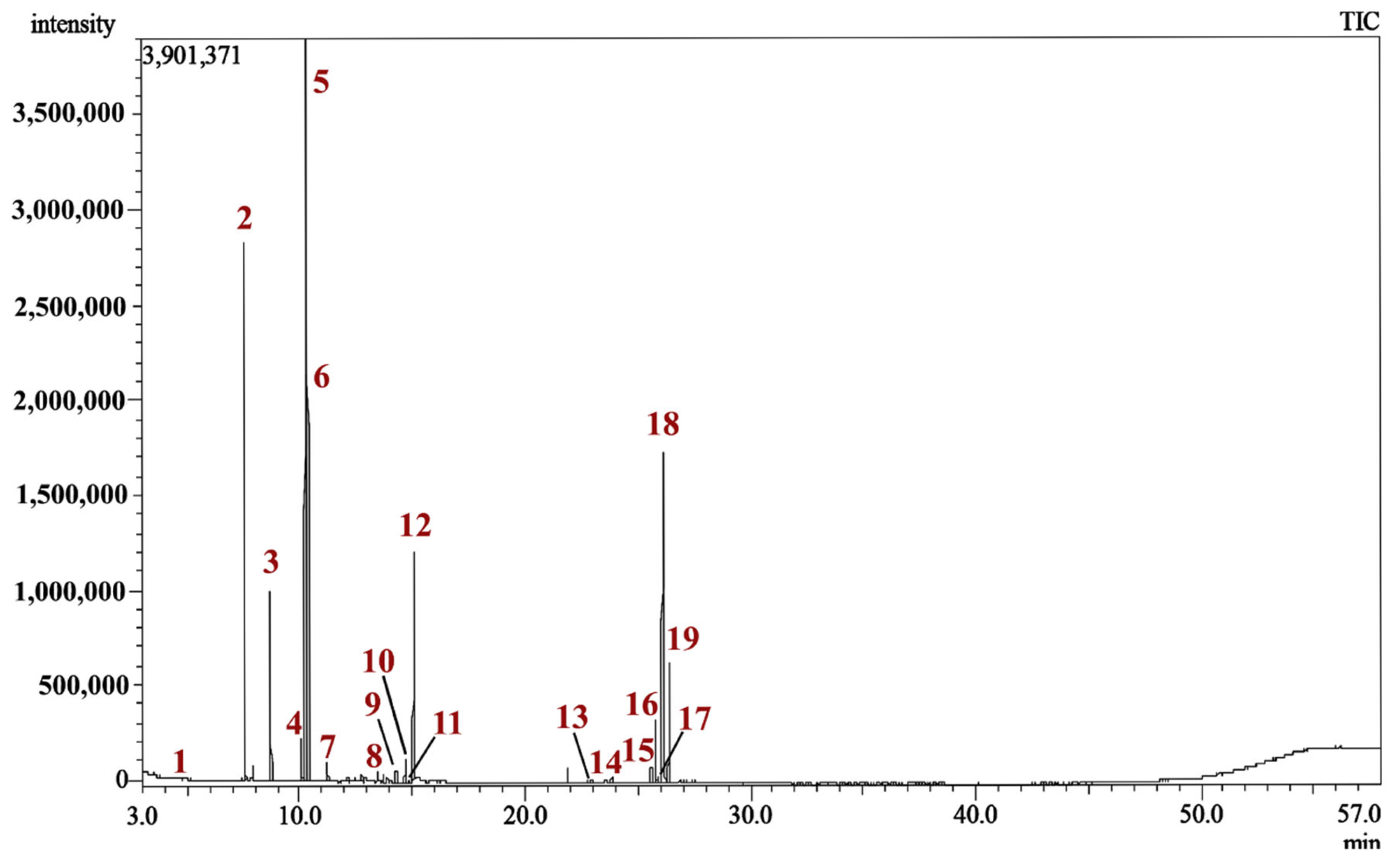

| Peak | Rt | Compound Name | Chemical Class | RIExp. a | RILit. b | Area % | Identification c |
|---|---|---|---|---|---|---|---|
| 1. | 3.540 | Methyl 2-methylbutyrate (Butanoic acid, 2-methyl-, methyl ester) | Fatty acid ester | 769 | 767 | 0.04 | MS, RI |
| 2. | 7.565 | 1R-α-Pinene (D-α-Pinene) | Bicyclic monoterpene hydrocarbon | 923 | 922 | 15.97 | MS, RI |
| 3. | 8.725 | 1S-α-Pinene (L-α-Pinene) | Bicyclic monoterpene hydrocarbon | 962 | 963 | 5.29 | MS, RI |
| 4. | 10.075 | m-Cymene | Aromatic monoterpene hydrocarbon | 1006 | 1005 | 1.54 | MS, RI |
| 5. | 10.305 | 1,8-Cineole (Eucalyptol) | Monocyclic monoterpene oxide | 1013 | 1013 | 31.57 | MS, RI |
| 6. | 10.370 | (+)-3-Carene | Aromatic monoterpene hydrocarbon | 1015 | 1015 | 11.57 | MS, RI |
| 7. | 11.230 | γ-Terpinene | Monocyclic monoterpene hydrocarbon | 1043 | 1042 | 0.58 | MS, RI |
| 8. | 12.750 | Fenchol | Monocyclic monoterpene alcohol | 1091 | 1097 | 0.24 | MS, RI |
| 9. | 14.275 | Isoborneol | Bicyclic monoterpene alcohol | 1141 | 1147 | 0.48 | MS, RI |
| 10. | 14.680 | Terpinen-4-ol | Monocyclic monoterpene alcohol | 1154 | 1152 | 0.80 | MS, RI |
| 11. | 14.950 | trans-Verbenol | Bicyclic monoterpene alcohol | 1163 | 1155 | 0.11 | MS, RI |
| 12. | 15.065 | trans-Ocimenol | Acyclic monoterpene alcohol | 1167 | 1169 | 8.26 | MS, RI |
| 13. | 22.955 | α-Guaiene | Bicyclic sesquiterpene hydrocarbon | 1445 | 1440 | 0.16 | MS, RI |
| 14. | 23.860 | (+)-Ledene | Bicyclic sesquiterpene hydrocarbon | 1480 | 1482 | 0.24 | MS, RI |
| 15. | 25.590 | (−)-Palustrol | Bicyclic sesquiterpene alcohol | 1561 | 1562 | 0.56 | MS, RI |
| 16. | 25.795 | Caryophyllene oxide | Tricyclic Sesquiterpene oxide | 1572 | 1578 | 2.23 | MS, RI |
| 17. | 25.930 | (−)-Globulol | Tricyclic sesquiterpene alcohol | 1578 | 1580 | 0.25 | MS, RI |
| 18. | 26.135 | Viridiflorol | Tricyclic sesquiterpene alcohol | 1589 | 1587 | 13.65 | MS, RI |
| 19. | 26.390 | Ledol | Tricyclic sesquiterpene alcohol | 1601 | 1599 | 4.55 | MS, RI |
| Total identified | 98.05 | ||||||
| Monoterpenes hydrocarbons | 34.95 | ||||||
| Oxygenated monoterpenes | 41.46 | ||||||
| Sesquiterpene hydrocarbons | 0.40 | ||||||
| Oxygenated sesquiterpenes | 21.24 | ||||||
| Vero | MCF-7 | HepG-2 | A-549 | ||||
|---|---|---|---|---|---|---|---|
| CC50 | IC50 | SI | IC50 | SI | IC50 | SI | |
| MQLEO | 77.76 ± 3.96 | 27.74 ± 1.41 | 2.80 | 66.04 ± 3.36 | 1.18 | 18.09 ± 0.92 | 4.30 |
| Staurosporine | 24.20 ± 1.23 | 4.62 ± 0.24 | 5.24 | 9.51 ± 0.48 | 2.54 | 3.92 ± 0.2 | 6.17 |
| Target Name | BC | CC | DC |
|---|---|---|---|
| ESR1 | 8287.888526 | 0.26860565 | 99 |
| CASP3 | 4448.232593 | 0.2670068 | 93 |
| PPARG | 5617.635482 | 0.2652027 | 88 |
| PTGS2 | 5493.159146 | 0.26430976 | 85 |
| HSP90AA1 | 3015.111312 | 0.26079734 | 82 |
| MMP9 | 3298.324704 | 0.25950413 | 82 |
| GSK3B | 3013.523609 | 0.26036484 | 71 |
| HSP90AB1 | 1607.859991 | 0.25570033 | 69 |
| SIRT1 | 2215.048411 | 0.25737705 | 68 |
| APP | 3729.932474 | 0.25632653 | 63 |
| KDR | 2429.196004 | 0.25220884 | 57 |
| ICAM1 | 1813.331077 | 0.25445705 | 56 |
| CYP3A4 | 2092.784051 | 0.25160256 | 55 |
| ACE | 2263.673069 | 0.25322581 | 54 |
| NR3C1 | 2162.884329 | 0.25570033 | 54 |
| PPARA | 2315.896888 | 0.25160256 | 52 |
| MAOA | 2097.54489 | 0.25039872 | 51 |
| MAOB | 1518.447362 | 0.24666143 | 48 |
| MAPK14 | 1912.303096 | 0.24782952 | 45 |
| Rank | Name | Score |
|---|---|---|
| 1 | Methyl 2-methylbutyrate | 169 |
| 2 | m-Cymene | 169 |
| 3 | trans-Verbenol | 154 |
| 4 | γ-Terpinene | 146 |
| 5 | Fenchol | 143 |
| 6 | 1R-α-Pinene | 141 |
| 7 | 1S-α-Pinene | 141 |
| 8 | Terpinen-4-ol | 139 |
| 9 | Isoborneol | 134 |
| 10 | 1,8-Cineole (Eucalyptol) | 133 |
| 11 | (+)-3-Carene | 129 |
| 12 | α-Guaiene | 127 |
| 13 | trans-Ocimenol | 120 |
| 14 | (−)-Palustrol | 101 |
| 15 | (−)-Globulol | 98 |
| 16 | Viridiflorol | 98 |
| 17 | Ledol | 98 |
| 18 | Caryophyllene oxide | 97 |
| 19 | (+)-Ledene | 96 |
| Ligand/Protein | Interacted Amino Acids at the Active Site | |||
|---|---|---|---|---|
| ESR1 | CASP3 | PPARG | PTGS2 | |
| Methyl 2-methylbutyrate | - | - | - | - |
| m-Cymene | - pi-pi t-shaped with PHE99. - Alkyl with LEU41, LEU44, LEU45, MET83, LEU86, LEU123. - pi-Alkyl with LEU82, LEU86, PHE99. | - | - pi-Anion with GLU59. - pi-pi t-shaped with PHE64. - Alkyl with ILE62. - pi-Alkyl with ARG80. | - Alkyl with VAL318, LEU353, MET491, VAL492. - pi-Alkyl with PHE48, LEU321, TRP356. |
| trans-Verbenol | - Alkyl with ALA45 (two), LEU82 (two), LEU86, LEU79 | - Conventional hydrogen bond with TYR236, TYR238. - Alkyl with LEU240 (two). | - Alkyl with ALA92, ILE96, MET129 (two). - pi-Alkyl with PHE26. | - Carbon hydrogen bond with VAL492, GLY495. - Alkyl with VAL318 (two), LEU321 (two), VAL492, ALA496. - pi-Alkyl with TYR317. |
| γ-Terpinene | - Alkyl with LEU86. - pi-Alkyl with LEU41, ALA45, LEU82, PHE99. | - | - Amide-pi stacked with ARG80. - Alkyl with ILE141, MET148. - pi-Alkyl withILE62, ILE81. | - Alkyl with VAL318, LEU353, VAL492. - pi-Alkyl with LEU321, TYR354, TRP356, VAL492. |
| Fenchol | - Alkyl with ALA45, LEU82, LEU86. | - | - Conventional hydrogen bond with ARG95. - Alkyl with LEU28, MET129, LEU133. | - Alkyl with LEU321, VAL492, ALA496. |
| 1R-α-Pinene | - Alkyl with LEU41, ALA45 (three), LEU79, LEU82, LEU220. | - | - Alkyl with ALA92 (two bond), ILE96 (one bond), MET129 (two), LEU133 (one). - pi-Alkyl with PHE26 (one). | - Alkyl with VAL318 (two), LEU321, VAL492, ALA496 (three). |
| 1S-α-Pinene | - Alkyl with ALA45 (three), LEU79 (two), LEU82 (three). | - pi-Sigma with TYR236. - Alkyl with MET11 and LEU240 (two). - pi-Alkyl with TYR236, TYR238. | - Alkyl with ALA92 (two bond), ILE96 (one bond), MET129 (three), LEU133 (one). - pi-Alkyl with PHE26 (one). | - Alkyl with VAL318 (two), LEU321 (two), ALA496 (two). |
| Terpinen-4-ol | - Alkyl with LEU41, MET116, ILE119. - pi-Alkyl with PHE99. | - Conventional hydrogen bond with SER8. - Carbon hydrogen bond with SER8. - pi-Sigma with TYR236. - Alkyl with LEU240. - pi-Alkyl with TYR236, TYR238. | - Carbon hydrogen bond with GLY84, ILE81. - Unfavorable donor-donor with CYS85. - Alkyl with ILE62, ARG88, ILE141(two). | - Carbon hydrogen bond with VAL492. - Alkyl with LEU321, LEU353, VAL492. - pi-Alkyl with TRP354, TRP356. |
| Isoborneol | - Alkyl with LEU41, ALA45. - pi-Alkyl with PHE99. | - | - Alkyl with ALA92, MET129, LEU133. | - Alkyl with LEU321, VAL492, ALA496. - pi-Alkyl with PHE487. |
| 1,8-Cineole | - Alkyl with ALA45. | - Conventional hydrogen bond with SER8. - Alkyl with LEU240. | - Alkyl with ALA92, MET129, LEU133. | - Carbon hydrogen bond with ALA496. - Alkyl with VAL318, LEU321, ALA496. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fikry, E.; Orfali, R.; Perveen, S.; Ghaffar, S.; El-Shafae, A.M.; El-Domiaty, M.M.; Tawfeek, N. Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking. Pharmaceuticals 2025, 18, 771. https://doi.org/10.3390/ph18060771
Fikry E, Orfali R, Perveen S, Ghaffar S, El-Shafae AM, El-Domiaty MM, Tawfeek N. Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking. Pharmaceuticals. 2025; 18(6):771. https://doi.org/10.3390/ph18060771
Chicago/Turabian StyleFikry, Eman, Raha Orfali, Shagufta Perveen, Safina Ghaffar, Azza M. El-Shafae, Maher M. El-Domiaty, and Nora Tawfeek. 2025. "Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking" Pharmaceuticals 18, no. 6: 771. https://doi.org/10.3390/ph18060771
APA StyleFikry, E., Orfali, R., Perveen, S., Ghaffar, S., El-Shafae, A. M., El-Domiaty, M. M., & Tawfeek, N. (2025). Chemical Composition and Anti-Lung Cancer Activities of Melaleuca quinquenervia Leaf Essential Oil: Integrating Gas Chromatography–Mass Spectrometry (GC/MS) Profiling, Network Pharmacology, and Molecular Docking. Pharmaceuticals, 18(6), 771. https://doi.org/10.3390/ph18060771