Skimmianine Modulates Tumor Proliferation and Immune Dynamics in Breast Cancer by Targeting PCNA and TNF-α
Abstract
1. Introduction
2. Results
2.1. Serum Tumor Marker Levels
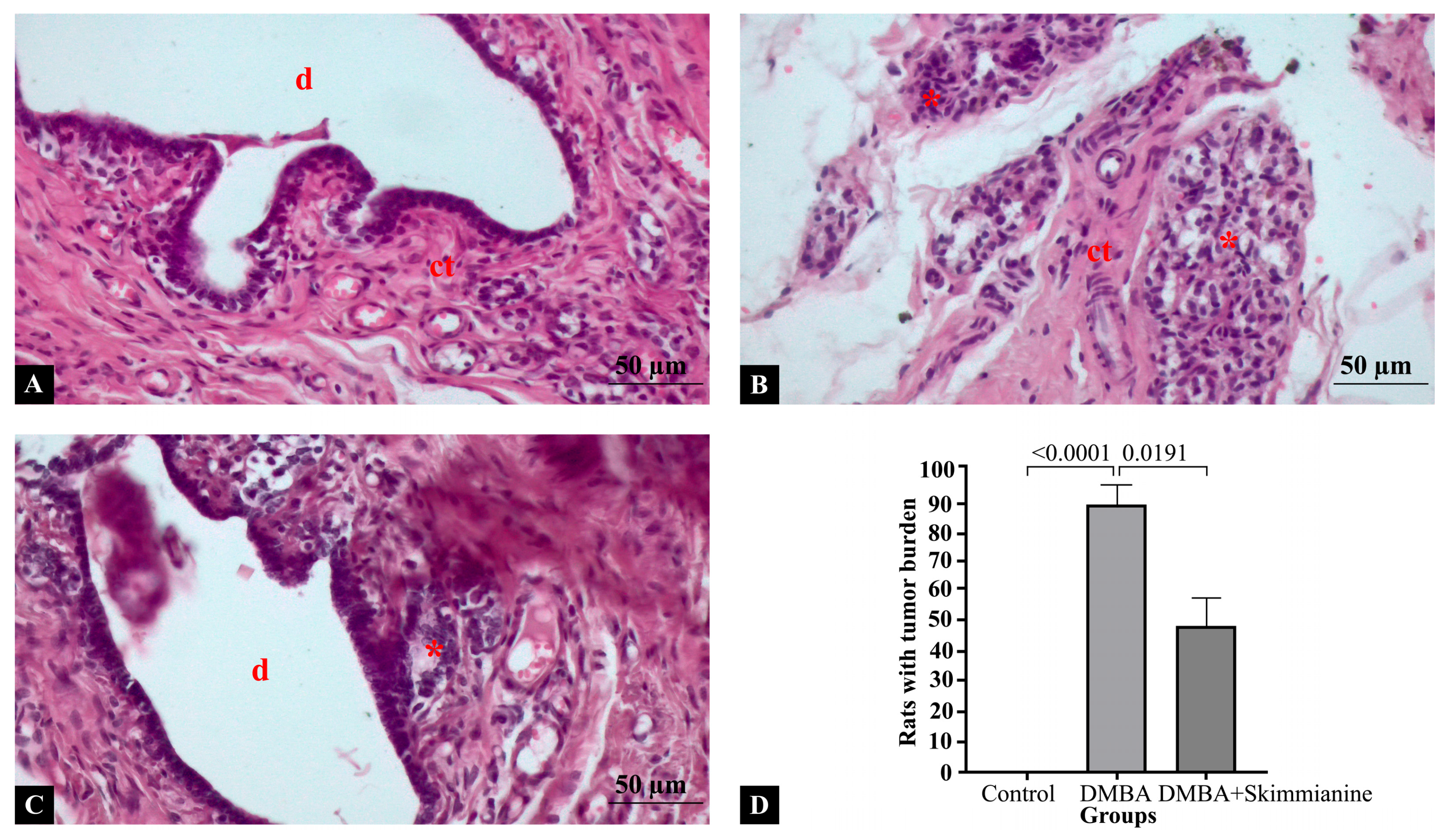
2.2. Histopathological Findings
2.3. PPI Network and Functional Annotation of Skimmianine-Associated PCNA and TNF-α Targets
2.4. Immunological Significance of Hub Proteins in the PPI Network of Skimmianine-Shared PCNA and TNF-α Targets
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Induction of Breast Cancer
- Control Group: Rats in this group were administered 1 mL of sesame oil orally for 16 weeks, followed by 1 mL of 5% carboxymethyl cellulose sodium aqueous solution intraperitoneally for 4 weeks;
- DMBA Group: Rats in this group were administered 50 mg/kg DMBA-containing sesame oil orally for 16 weeks and 1 mL of 5% carboxymethyl cellulose sodium aqueous solution intraperitoneally for the next 4 weeks;
- DMBA + Skimmianine Group: After DMBA administration, 40 mg/kg skimmianine dissolved in 1 mL of 5% carboxymethyl cellulose sodium aqueous solution was given intraperitoneally for 4 weeks.
4.3. Measurement of Serum CA15-3 Levels
4.4. Histological Staining
4.5. Semi-Quantitative Histological Scoring
4.6. Measurement of Tumor Burden
4.7. Integrative Network and Enrichment Analysis of PCNA, TNF-α, and Skimmianine
4.8. Immune Correlation and Infiltration Analysis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCNA | Proliferating Cell Nuclear Antigen |
| TNF-α | Tumor Necrosis Factor-alpha |
| DMBA | 7,12-Dimethylbenz[a]anthracene |
| CA15-3 | Cancer Antigen 15-3 |
| H&E | Hematoxylin and Eosin |
| PPI | Protein–Protein Interaction |
| TLR | Toll-like Receptor |
| NLR | Nucleotide-binding Domain Leucine-rich Repeat |
| TRIF | TIR-domain-containing Adapter-inducing Interferon-β |
| CDK | Cyclin-Dependent Kinase 2/4 |
| HDAC1 | Histone Deacetylase 1 |
| MDM2 | Mouse Double Minute 2 homolog |
| BCL2 | B-Cell Lymphoma 2 |
| IL2 | Interleukin-2 |
| CASP3 | Caspase-3 |
| MMP9 | Matrix Metallopeptidase 9 |
| TME | Tumor Microenvironment |
| NK cells | Natural Killer Cells |
| Nrf2 | Nuclear Factor Erythroid 2–Related Factor 2 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| HBXIP | Hepatitis B X-Interacting Protein |
| NF-κB | Nuclear Factor kappa Beta |
| MCC | Maximal Clique Centrality |
| TCGA | The Cancer Genome Atlas |
| GTEx | Genotype Tissue Expression |
| TISIDB | Tumor and Immune System Interaction Database |
| TIMER | Tumor Immune Estimation Resource |
References
- Korak, T.; Albayrak, M.G.B.; Kasap, M.; Akpinar, G. Thymoquinone and Metabolic Reprogramming in Breast Cancer: A New Dimension from Proteomic Analysis. J. Biochem. Mol. Toxicol. 2025, 39, e70124. [Google Scholar] [CrossRef] [PubMed]
- Yanar, S.; Özkan, A.D.; Albayrak, M.G.B.; Betts, Z. The Impact of Simultaneous Epigenetic and Epitranscriptomic Intervention in Breast Cancer Cells. Istanb. Gelisim Univ. J. Health Sci. 2024, 23, 505–521. [Google Scholar] [CrossRef]
- Li, M.-Y.; Gu, A.; Li, J.; Tang, N.; Matin, M.; Yang, Y.; Zengin, G.; Atanasov, A.G. Exploring Food and Medicine Homology: Potential Implications for Cancer Treatment Innovations. Acta Mater. Med. 2025, 4, 200–206. [Google Scholar] [CrossRef]
- Wang, J.; Liao, Z.-X. Research Progress of Microrobots in Tumor Drug Delivery. Food Med. Homol. 2024, 1, 9420025. [Google Scholar] [CrossRef]
- Yadav, D.; Malviya, R. Novel Nanomaterials as Photo-activated Cancer Diagnostics and Therapy. Med. Adv. 2023, 1, 190–209. [Google Scholar] [CrossRef]
- Szewczyk, A.; Pęczek, F. Furoquinoline Alkaloids: Insights into Chemistry, Occurrence, and Biological Properties. Int. J. Mol. Sci. 2023, 24, 12811. [Google Scholar] [CrossRef]
- Zuo, Y.; Pu, J.; Chen, G.; Shen, W.; Wang, B. Study on the Activity and Mechanism of Skimmianine against Human Non-Small Cell Lung Cancer. Nat. Prod. Res. 2019, 33, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Ilakiyalakshmi, M.; Napoleon, A.A. Review on Recent Development of Quinoline for Anticancer Activities. Arab. J. Chem. 2022, 15, 104168. [Google Scholar] [CrossRef]
- Molnar, J.; Ocsovszki, I.; Puskas, L.; Ghane, T.; Hohmann, J.; Zupko, I. Investigation of the Antiproliferative Action of the Quinoline Alkaloids Kokusaginine and Skimmianine on Human Cell Lines. Curr. Signal Transduct. Ther. 2013, 8, 148–155. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Sarker, S.D. Evaluation of the Chemopreventive Effect of Selected Medicinal Plants Extracts via Induction of the Nrf2 in a Modified Model of Breast Cancer Cells: Identification of Bioactive Lead Compounds. Eur. J. Cancer Prev. 2022, 31, 50–53. [Google Scholar] [CrossRef]
- Bishayee, A.; Mandal, A.; Bhattacharyya, P.; Bhatia, D. Pomegranate Exerts Chemoprevention of Experimentally Induced Mammary Tumorigenesis by Suppression of Cell Proliferation and Induction of Apoptosis. Nutr. Cancer 2016, 68, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Hamza, A.A.; Khasawneh, M.A.; Elwy, H.M.; Hassanin, S.O.; Elhabal, S.F.; Fawzi, N.M. Salvadora Persica Attenuates DMBA-Induced Mammary Cancer through Downregulation Oxidative Stress, Estrogen Receptor Expression and Proliferation and Augmenting Apoptosis. Biomed. Pharmacother. 2022, 147, 112666. [Google Scholar] [CrossRef] [PubMed]
- Malkas, L.H.; Herbert, B.S.; Abdel-Aziz, W.; Dobrolecki, L.E.; Liu, Y.; Agarwal, B.; Hoelz, D.; Badve, S.; Schnaper, L.; Arnold, R.J.; et al. A Cancer-Associated PCNA Expressed in Breast Cancer Has Implications as a Potential Biomarker. Proc. Natl. Acad. Sci. USA 2006, 103, 19472–19477. [Google Scholar] [CrossRef]
- Cai, X.; Cao, C.; Li, J.; Chen, F.; Zhang, S.; Liu, B.; Zhang, W.; Zhang, X.; Ye, L. Inflammatory Factor TNF-α Promotes the Growth of Breast Cancer via the Positive Feedback Loop of TNFR1/NF-ΚB (and/or P38)/p-STAT3/HBXIP/TNFR1. Oncotarget 2017, 8, 58338–58352. [Google Scholar] [CrossRef] [PubMed]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The Dual Role of Tumor Necrosis Factor-Alpha (TNF-α) in Breast Cancer: Molecular Insights and Therapeutic Approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Yang, T.-H.O.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- Isomiddinovich, S.Z.; Rahmanovich, M.Y.; Karimdjanovna, Y.L.; Furqatovna, R.N.; Bahodirovich, P.O.; Po’latovich, B.B. Toxicological Characteristics of Skimmianine Under Chronic Administration in White Rats. Eur. J. Mol. Clin. Med. 2021, 8, 302–307. [Google Scholar]
- Abba, M.C.; Zhong, Y.; Lee, J.; Kil, H.; Lu, Y.; Takata, Y.; Simper, M.S.; Gaddis, S.; Shen, J.; Aldaz, C.M. DMBA Induced Mouse Mammary Tumors Display High Incidence of Activating Pik3caH1047 and Loss of Function Pten Mutations. Oncotarget 2016, 7, 64289–64299. [Google Scholar] [CrossRef]
- Duffy, M.J.; Evoy, D.; McDermott, E.W. CA 15-3: Uses and Limitation as a Biomarker for Breast Cancer. Clin. Chim. Acta 2010, 411, 1869–1874. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, L.; Shi, S.-M.; Li, B.-J.; Zhang, Y.; Zhang, X.-Z.; Guo, X.-W.; Fu, G.; Zheng, G.-N.; Hao, H.; et al. Skimmianine as a Novel Therapeutic Agent Suppresses Proliferation and Migration of Human Esophageal Squamous Cell Carcinoma via Blocking the Activation of ERK1/2. Neoplasma 2022, 69, 571–582. [Google Scholar] [CrossRef]
- Gaobotse, G.; Venkataraman, S.; Brown, P.D.; Masisi, K.; Kwape, T.E.; Nkwe, D.O.; Rantong, G.; Makhzoum, A. The Use of African Medicinal Plants in Cancer Management. Front. Pharmacol. 2023, 14, 1122388. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Prasad, C.P.; Mathur, S.; Mathur, R. Derangement of Metabolic Homeostasis, Detoxifying Ability and CA 15-3 in Young Adult Female Rats by Fructose (15%) Drinking Is Akin to Known Carcinogens: A Missed Fiend? Indian J. Physiol. Pharmacol. 2023, 67, 163–171. [Google Scholar] [CrossRef]
- Maued, A.Z.F.; El-Tohamy, A.; Ashry, M. Anti-Tumor Effects of Dendronephthya Putteri Ethanolic Extract in DMBA- Induced Breast Cancer in Adult Female Rats. Egypt. J. Hosp. Med. 2023, 39, 7153–7158. [Google Scholar] [CrossRef]
- Wang, L.; Kong, W.; Liu, B.; Zhang, X. Proliferating Cell Nuclear Antigen Promotes Cell Proliferation and Tumorigenesis by Up-Regulating STAT3 in Non-Small Cell Lung Cancer. Biomed. Pharmacother. 2018, 104, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Karakurt, E.; Kuru, M.; Dağ, S.; Beytut, E.; Oral, H.; Nuhoğlu, H.; Yildiz, A. Immunohistochemically Evaluation of PCNA and MMP-9 Expressions in Different Types of Canine Mammary Carcinomas. Dicle Üniversitesi Vet. Fakültesi Derg. 2020, 13, 119–124. [Google Scholar] [CrossRef]
- Carvalho, M.I.; Pires, I.; Prada, J.; Lobo, L.; Queiroga, F.L. Ki-67 and PCNA Expression in Canine Mammary Tumors and Adjacent Nonneoplastic Mammary Glands. Vet. Pathol. 2016, 53, 1138–1146. [Google Scholar] [CrossRef]
- Ibrahim, M.Y.; Hashim, N.M.; Omer, F.A.A.; Abubakar, M.S.; Mohammed, H.A.; Salama, S.M.; Jayash, S.N. Potential Antitumor Effect of α-Mangostin against Rat Mammary Gland Tumors Induced by LA7 Cells. Int. J. Mol. Sci. 2023, 24, 10283. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z. Targeting the “Undruggable”: Small-Molecule Inhibitors of Proliferating Cell Nuclear Antigen (PCNA) in the Spotlight in Cancer Therapy. J. Med. Chem. 2025, 68, 2058–2088. [Google Scholar] [CrossRef]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.-P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a Cell-Penetrating Protein Targeting PCNA, Can Be Safely Administered as Intravenous Infusion in Patients and Shows Clinical Activity in a Phase 1 Study. Oncogene 2023, 42, 541–544. [Google Scholar] [CrossRef]
- Nkuimi, O.B.K.; Silihe, K.K.; Tabi, Y.O.; Pambe, J.C.N.; Njamen, D.; Zingue, S. Duguetia Confinis Engl. & Diels (Annonaceae) Inhibitory and Cytotoxic Effects on Breast Adenocarcinoma Growth Both in Vitro and in Vivo. Heliyon 2024, 10, e24410. [Google Scholar] [CrossRef]
- Vera, M.J.; Guajardo, F.; Urra, F.A.; Tobar, N.; Martínez, J. TNF-Alpha Promotes an Inflammatory Mammary Microenvironment That Favors Macrophage and Epithelial Migration in a CCL2-and Mitochondrial-ROS-Dependent Manner. Antioxidants 2023, 12, 813. [Google Scholar] [CrossRef] [PubMed]
- Katanov, C.; Lerrer, S.; Liubomirski, Y.; Leider-Trejo, L.; Meshel, T.; Bar, J.; Feniger-Barish, R.; Kamer, I.; Soria-Artzi, G.; Kahani, H.; et al. Regulation of the Inflammatory Profile of Stromal Cells in Human Breast Cancer: Prominent Roles for TNF-α and the NF-ΚB Pathway. Stem Cell Res. Ther. 2015, 6, 87. [Google Scholar] [CrossRef]
- Paik, P.K.; Luo, J.; Ai, N.; Kim, R.; Ahn, L.; Biswas, A.; Coker, C.; Ma, W.; Wong, P.; Buonocore, D.J.; et al. Phase I Trial of the TNF-α Inhibitor Certolizumab plus Chemotherapy in Stage IV Lung Adenocarcinomas. Nat. Commun. 2022, 13, 6095. [Google Scholar] [CrossRef]
- Thu, K.; Soria-Bretones, I.; Mak, T.; Cescon, D. Targeting the Cell Cycle in Breast Cancer: Towards the next Phase. Cell Cycle 2018, 17, 1871–1885. [Google Scholar] [CrossRef] [PubMed]
- Alexandrou, S.; George, S.M.; Ormandy, C.J.; Lim, E.; Oakes, S.R.; Caldon, C.E. The Proliferative and Apoptotic Landscape of Basal-like Breast Cancer. Int. J. Mol. Sci. 2019, 20, 667. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Yang, H.; Zhou, H.; Feng, P.; Zhou, X.; Wen, H.; Xie, X.; Shen, H.; Zhu, X. Reduced Expression of Toll-like Receptor 4 Inhibits Human Breast Cancer Cells Proliferation and Inflammatory Cytokines Secretion. J. Exp. Clin. Cancer Res. 2010, 29, 92. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Liu, S.; DeRubeis, D.; Zhang, D. Toll-like Receptors in Breast Cancer Immunity and Immunotherapy. Front. Immunol. 2024, 15, 1418025. [Google Scholar] [CrossRef]
- Fouad, T.M.; Kogawa, T.; Reuben, J.M.; Ueno, N.T. Inflammation and Cancer. Adv. Exp. Med. Biol. 2014, 816, 53–73. [Google Scholar] [CrossRef]
- Mercogliano, M.F.; Bruni, S.; Elizalde, P.V.; Schillaci, R. Tumor Necrosis Factor α Blockade: An Opportunity to Tackle Breast Cancer. Front. Oncol. 2020, 10, 584. [Google Scholar] [CrossRef]
- Wu, L.; Jin, Y.; Zhao, X.; Tang, K.; Zhao, Y.; Tong, L.; Yu, X.; Xiong, K.; Luo, C.; Zhu, J.; et al. Tumor Aerobic Glycolysis Confers Immune Evasion through Modulating Sensitivity to T Cell-Mediated Bystander Killing via TNF-α. Cell Metab. 2023, 35, 1580–1596.e9. [Google Scholar] [CrossRef] [PubMed]
- Kwan, A.; Mcdermott-Brown, I.; Muthana, M. Proliferating Cell Nuclear Antigen in the Era of Oncolytic Virotherapy. Viruses 2024, 16, 1264. [Google Scholar] [CrossRef] [PubMed]
- Brummer, T.; Zeiser, R. The Role of the MDM2/P53 Axis in Antitumor Immune Responses. Blood 2024, 143, 2701–2709. [Google Scholar] [CrossRef] [PubMed]
- Shanabag, A.; Armand, J.; Son, E.; Yang, H.W. Targeting CDK4/6 in Breast Cancer. Exp. Mol. Med. 2025, 57, 312–322. [Google Scholar] [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016, 6, a026831. [Google Scholar] [CrossRef]
- Li, C.; Wang, B.; Tu, J.; Liu, C.; Wang, Y.; Chen, J.; Huang, Y.; Liu, B.; Yuan, X. ATM Inhibition Enhance Immunotherapy by Activating STING Signaling and Augmenting MHC Class I. Cell Death Dis. 2024, 15, 519. [Google Scholar] [CrossRef]
- Gameiro, S.R.; Malamas, A.S.; Tsang, K.Y.; Ferrone, S.; Hodge, J.W. Inhibitors of Histone Deacetylase 1 Reverse the Immune Evasion Phenotype to Enhance T-Cell Mediated Lysis of Prostate and Breast Carcinoma Cells. Oncotarget 2016, 7, 7390–7402. [Google Scholar] [CrossRef]
- Zhang, W.; Ge, L.; Zhang, Y.; Zhang, Z.; Zhang, W.; Song, F.; Huang, P.; Xu, T. Targeted Intervention of Tumor Microenvironment with HDAC Inhibitors and Their Combination Therapy Strategies. Eur. J. Med. Res. 2025, 30, 69. [Google Scholar] [CrossRef]
- Zeng, Q.; Zeng, S.; Dai, X.; Ding, Y.; Huang, C.; Ruan, R.; Xiong, J.; Tang, X.; Deng, J. MDM2 Inhibitors in Cancer Immunotherapy: Current Status and Perspective. Genes Dis. 2024, 11, 101279. [Google Scholar] [CrossRef]
- Muhammad, S.; Fan, T.; Hai, Y.; Gao, Y.; He, J. Reigniting Hope in Cancer Treatment: The Promise and Pitfalls of IL-2 and IL-2R Targeting Strategies. Mol. Cancer 2023, 22, 121. [Google Scholar] [CrossRef]
- Gatti-Mays, M.E.; Balko, J.M.; Gameiro, S.R.; Bear, H.D.; Prabhakaran, S.; Fukui, J.; Disis, M.L.; Nanda, R.; Gulley, J.L.; Kalinsky, K.; et al. If We Build It They Will Come: Targeting the Immune Response to Breast Cancer. npj Breast Cancer 2019, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Yu, P.Y.; Liu, Y.Q.; Zhu, Y.; Li, F.C.; Wang, Y.Y.; Chen, X.Y.; Xiao, M. Recent Advances in Caspase-3, Breast Cancer, and Traditional Chinese Medicine: A Review. J. Chemother. 2024, 36, 370–388. [Google Scholar] [CrossRef]
- Varamini, P.; Javidnia, K.; Soltani, M.; Mehdipour, A.; Ghaderi, A. Cytotoxic Activity and Cell Cycle Analysis of Quinoline Alkaloids Isolated from Haplophyllum Canaliculatum Boiss. Planta Med. 2009, 75, 1509–1516. [Google Scholar] [CrossRef] [PubMed]
- Ninh, T.S. Skimmianine: Natural Occurrence, Biosynthesis, Synthesis, Pharmacology and Pharmacokinetics. Med. Chem. 2023, 19, 556–569. [Google Scholar] [CrossRef]
- Huang, A.; Xu, H.; Zhan, R.; Chen, W.; Liu, J.; Chi, Y.; Chen, D.; Ji, X.; Luo, C. Metabolic Profile of Skimmianine in Rats Determined by Ultra-Performance Liquid Chromatography Coupled with Quadrupole Time-of-Flight Tandem Mass Spectrometry. Molecules 2017, 22, 489. [Google Scholar] [CrossRef]
- Gu, L.; Li, M.; Li, C.M.; Haratipour, P.; Lingeman, R.; Jossart, J.; Gutova, M.; Flores, L.; Hyde, C.; Kenjić, N.; et al. Small Molecule Targeting of Transcription-Replication Conflict for Selective Chemotherapy. Cell Chem. Biol. 2023, 30, 1235–1247.e6. [Google Scholar] [CrossRef]
- Rosental, B.; Brusilovsky, M.; Hadad, U.; Oz, D.; Appel, M.Y.; Afergan, F.; Yossef, R.; Rosenberg, L.A.; Aharoni, A.; Cerwenka, A.; et al. Proliferating Cell Nuclear Antigen Is a Novel Inhibitory Ligand for the Natural Cytotoxicity Receptor NKp44. J. Immunol. 2011, 187, 5693–5702. [Google Scholar] [CrossRef] [PubMed]
- Montfort, A.; Dufau, C.; Colacios, C.; Andrieu-Abadie, N.; Levade, T.; Filleron, T.; Delord, J.-P.; Ayyoub, M.; Meyer, N.; Ségui, B. Anti-TNF, a Magic Bullet in Cancer Immunotherapy? J. Immunother. Cancer 2019, 7, 303. [Google Scholar] [CrossRef]
- Nassan, M.A.; Soliman, M.M.; Ismail, S.A.; El-Shazly, S. Effect of Taraxacum Officinale Extract on PI3K/Akt Pathway in DMBA-Induced Breast Cancer in Albino Rats. Biosci. Rep. 2018, 38, BSR20180334. [Google Scholar] [CrossRef]
- Karaaslanlı, A.; Tuncer, M.C.; Aşır, F.; Korak, T. Gallic Acid Showed Neuroprotection against Endoplasmic Reticulum Stress in Rats. Acta Cirúrgica Bras. 2025, 40, e400925. [Google Scholar] [CrossRef]
- Crowe, A.R.; Yue, W. Updated: Semi-Quantitative Determination of Protein Expression Using Immunohistochemistry Staining and Analysis. Bio-Protoc. 2019, 9, e3465. [Google Scholar] [CrossRef] [PubMed]
- Zdrazil, B.; Felix, E.; Hunter, F.; Manners, E.J.; Blackshaw, J.; Corbett, S.; de Veij, M.; Ioannidis, H.; Lopez, D.M.; Mosquera, J.F.; et al. The ChEMBL Database in 2023: A Drug Discovery Platform Spanning Multiple Bioactivity Data Types and Time Periods. Nucleic Acids Res. 2023, 52, D1180–D1192. [Google Scholar] [CrossRef]
- Xie, Z.; Bailey, A.; Kuleshov, M.V.; Clarke, D.J.B.; Evangelista, J.E.; Jenkins, S.L.; Lachmann, A.; Wojciechowicz, M.L.; Kropiwnicki, E.; Jagodnik, K.M.; et al. Gene Set Knowledge Discovery with Enrichr. Curr. Protoc. 2021, 1, e90. [Google Scholar] [CrossRef] [PubMed]
- Keşim, D.A.; Aşır, F.; Ayaz, H.; Korak, T. The Effects of Ellagic Acid on Experimental Corrosive Esophageal Burn Injury. Curr. Issues Mol. Biol. 2024, 46, 1579–1592. [Google Scholar] [CrossRef] [PubMed]
- Ru, B.; Wong, C.N.; Tong, Y.; Zhong, J.Y.; Zhong, S.S.W.; Wu, W.C.; Chu, K.C.; Wong, C.Y.; Lau, C.Y.; Chen, I.; et al. TISIDB: An Integrated Repository Portal for Tumor–Immune System Interactions. Bioinformatics 2019, 35, 4200–4202. [Google Scholar] [CrossRef]
- Korak, T.; Albayrak, M.G.B.; Kasap, M.; Akpinar, G.; Yanar, S. Unlocking the Potential of RARRES1: A Pan-Cancer Analysis for Prognosis, Diagnosis, Tumor Immunity and Drug Sensitivity. J. Biol. Res.-Thessalon. 2024, 31, 1–18. [Google Scholar] [CrossRef]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A Comprehensive, Interaction-friendly Clinical Bioinformatics Analysis Platform. Imeta 2022, 1, e36. [Google Scholar] [CrossRef]





| Control | DMBA | DMBA + Skimmianine | Multiple Comparisons | |
|---|---|---|---|---|
| CA15-3 (ng/mL) | 0.23 ± 0.06 | 8.57 ± 1.01 * | 3.72 ± 0.58 ** | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korak, T.; Ayaz, H.; Aşır, F. Skimmianine Modulates Tumor Proliferation and Immune Dynamics in Breast Cancer by Targeting PCNA and TNF-α. Pharmaceuticals 2025, 18, 756. https://doi.org/10.3390/ph18050756
Korak T, Ayaz H, Aşır F. Skimmianine Modulates Tumor Proliferation and Immune Dynamics in Breast Cancer by Targeting PCNA and TNF-α. Pharmaceuticals. 2025; 18(5):756. https://doi.org/10.3390/ph18050756
Chicago/Turabian StyleKorak, Tuğcan, Hayat Ayaz, and Fırat Aşır. 2025. "Skimmianine Modulates Tumor Proliferation and Immune Dynamics in Breast Cancer by Targeting PCNA and TNF-α" Pharmaceuticals 18, no. 5: 756. https://doi.org/10.3390/ph18050756
APA StyleKorak, T., Ayaz, H., & Aşır, F. (2025). Skimmianine Modulates Tumor Proliferation and Immune Dynamics in Breast Cancer by Targeting PCNA and TNF-α. Pharmaceuticals, 18(5), 756. https://doi.org/10.3390/ph18050756







