α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects
Abstract
1. Introduction
2. Results and Discussion
2.1. Inhibition Activity of Flavonoids on α-Glucosidase
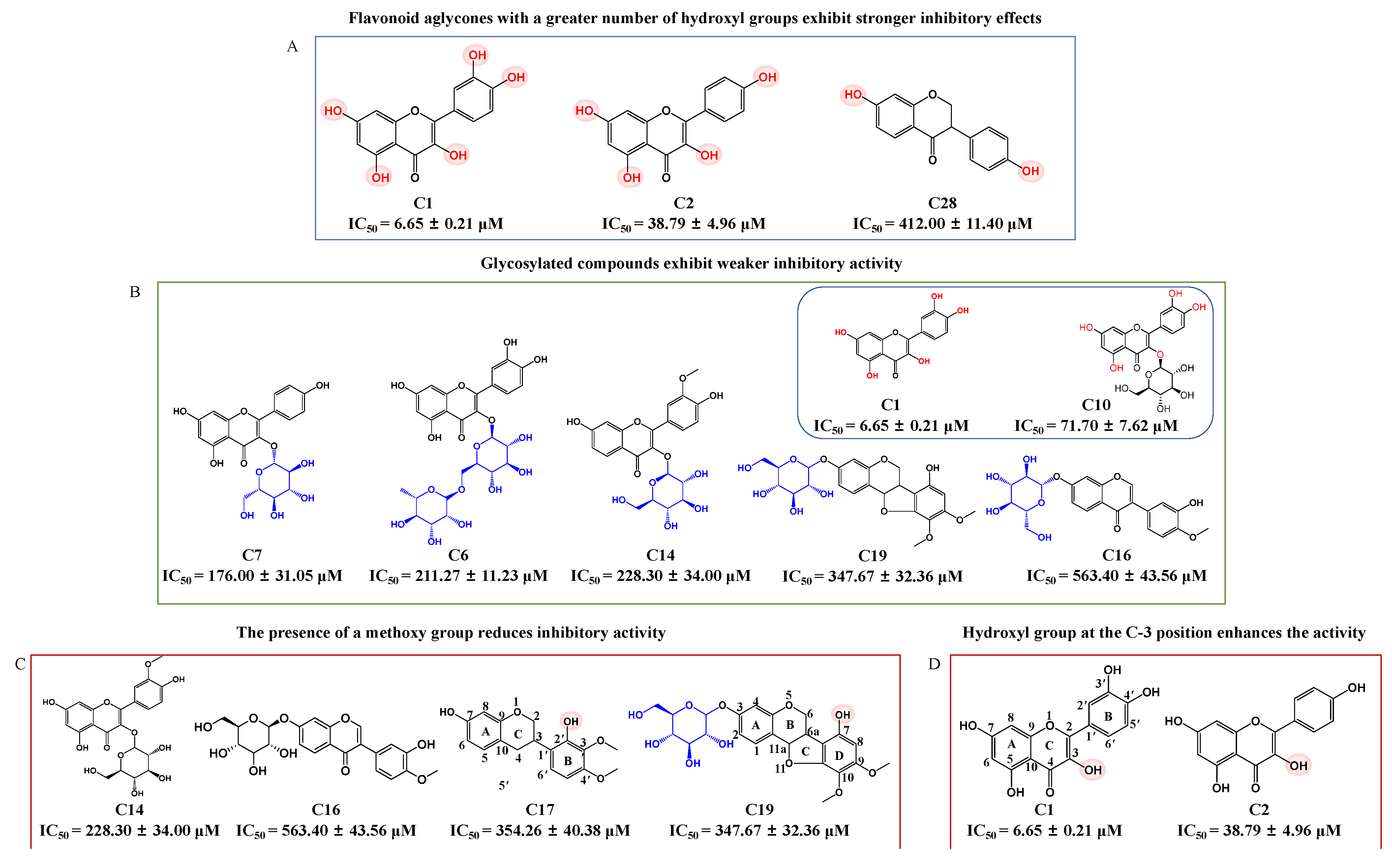
2.2. Structure–Activity Relationship (SAR) Analysis
2.3. Kinetic Type of Inhibition on α-Glucosidase
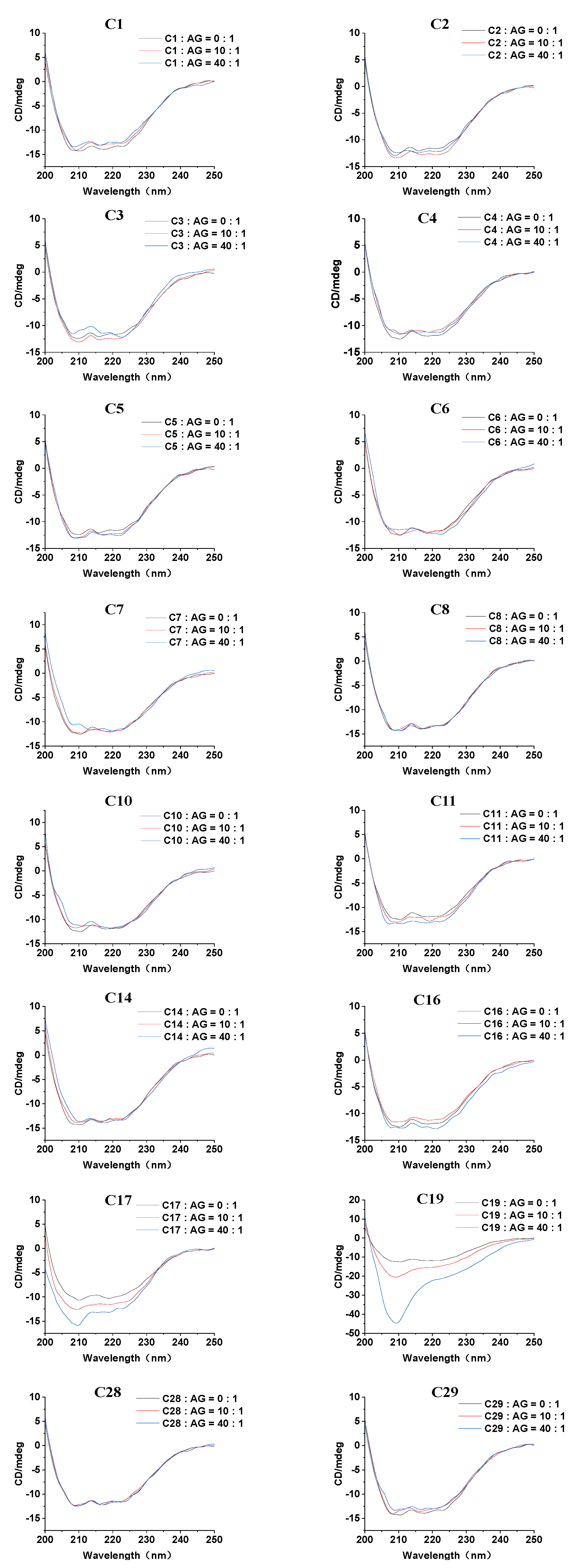
2.4. Circular Dichroism (CD) Spectra
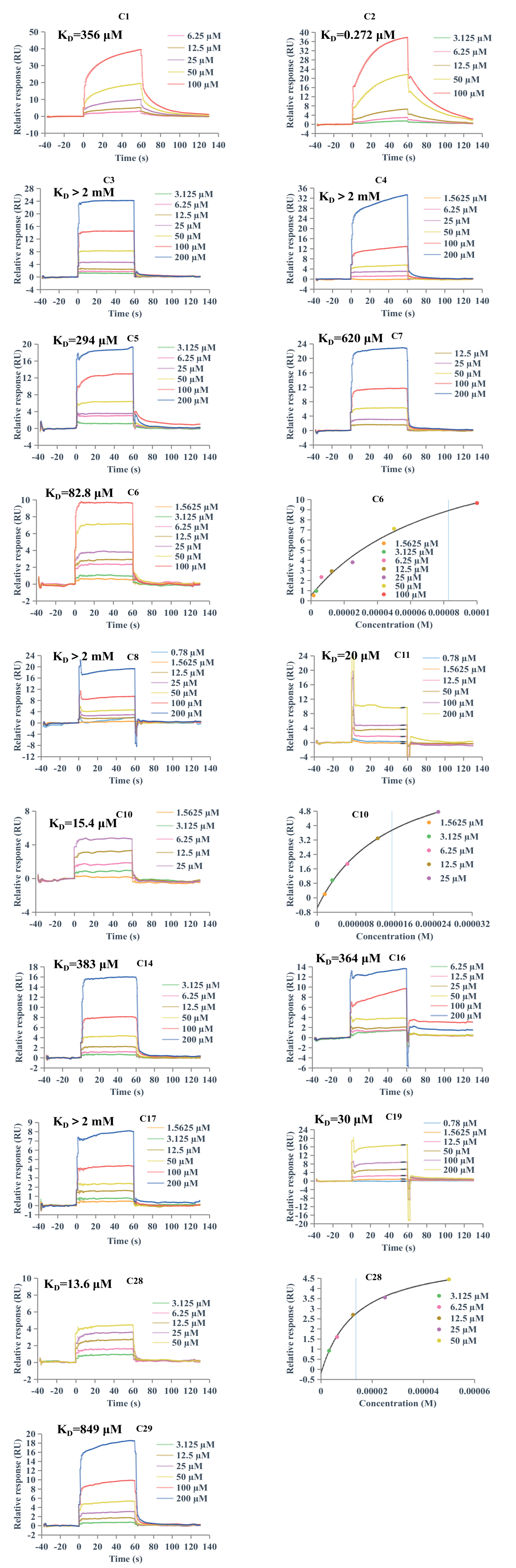
2.5. Surface Plasmon Resonance (SPR) Analysis
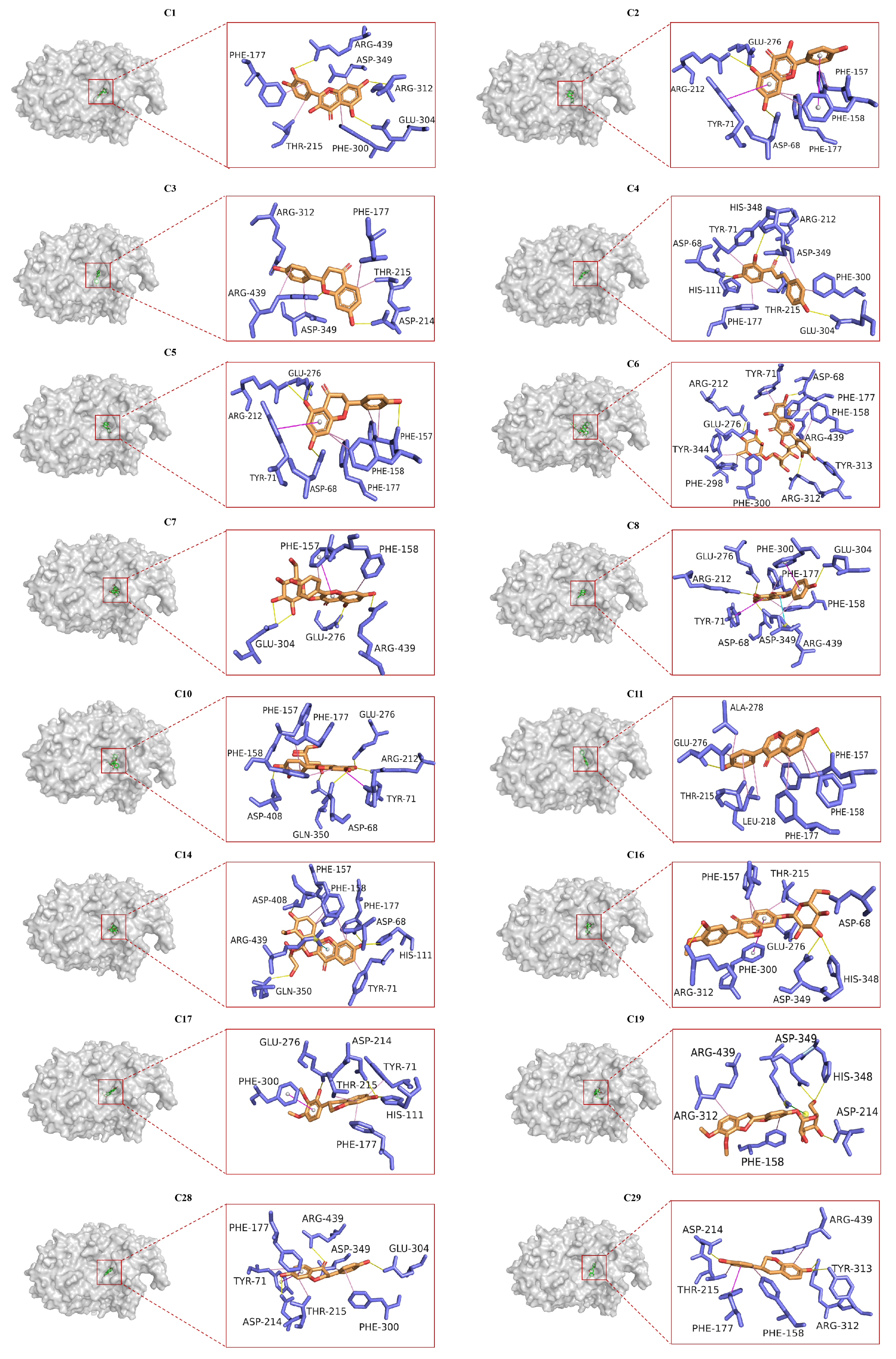
2.6. Molecular Docking Analysis
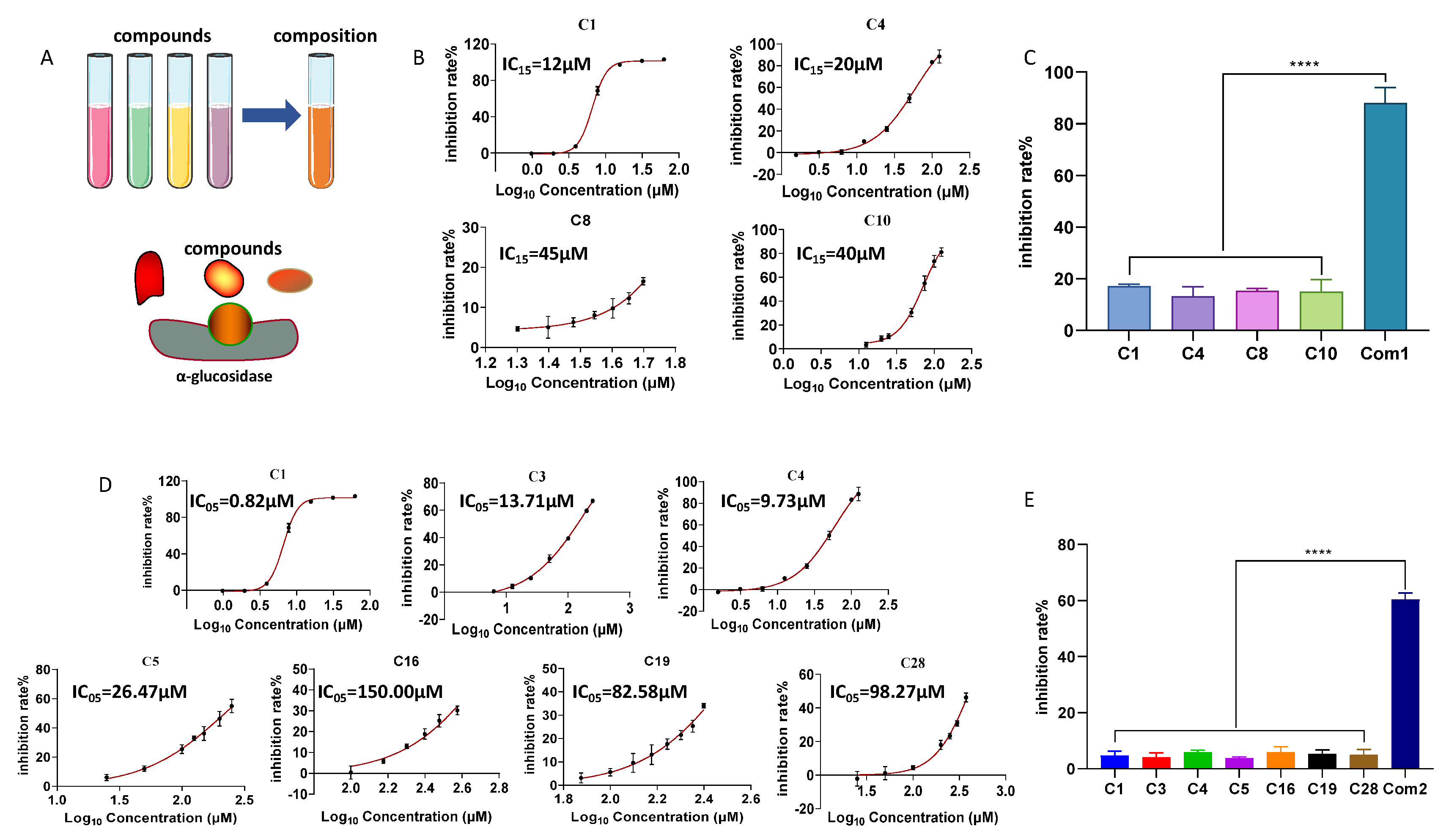
2.7. Inhibitory Effects of Combinations of Flavonoid Compounds on α-Glucosidase
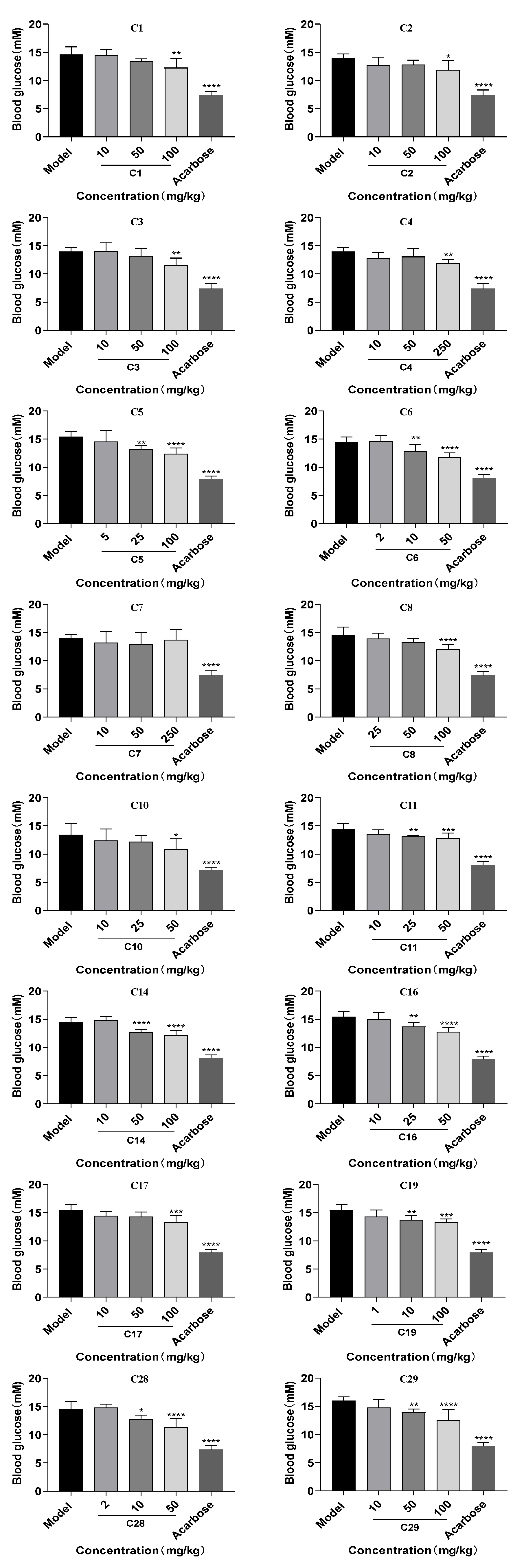
2.8. Effects of 16 Flavonoids on Postprandial Hyperglycemia in Normal Mice
3. Materials and Methods
3.1. Chemicals and Materials
3.2. α-Glucosidase Inhibition Assay
3.3. Inhibitory Kinetic Analysis
3.4. CD Spectroscopy
3.5. SPR Experiments
3.6. Molecular Docking
3.7. Mixture Effects
3.8. Experimental Animals
3.9. Blood Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AR | Astragali Radix |
| SAR | structure–activity relationship |
| CD | circular dichroism |
| SPR | surface plasmon resonance |
| DM | diabetes mellitus |
| IDF | International Diabetes Federation |
| pNPG | p-nitrophenyl-α-D-glucopyranoside |
| DMSO | dimethyl sulfoxide |
| PBS | phosphate-buffered saline |
| KD | dissociation constant |
| OSucTT | oral sucrose tolerance test |
| CMC-Na | sodium carboxymethyl cellulose |
| CI | combination index |
| PBS | phosphate buffered saline |
References
- Sanachai, K.; Chamni, S.; Nutho, B.; Khammuang, S.; Ratha, J.; Choowongkomon, K.; Puthongking, P. Mechanistic study of α-mangostin derivatives as potent α-glucosidase inhibitors. Mol. Divers. 2025; ahead of print. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Chen, C.; Wei, F.; Zhao, N.; Yang, W.Y.; Zhang, T.R.; Ren, G.Y.; Qiu, Z.J.; Zhang, B. Identification and Molecular Mechanism of Novel α-Glucosidase Inhibitory Peptides from the Hydrolysate of Hemp Seed Proteins: Peptidomic Analysis, Molecular Docking, and Dynamics Simulation. Int. J. Mol. Sci. 2025, 26, 2222. [Google Scholar] [CrossRef]
- Jiang, L.L.; Wang, Z.; Wang, X.Y.; Wang, S.J.; Cao, J.; Liu, Y. Exploring the inhibitory mechanism of piceatannol on α-glucosidase relevant to diabetes mellitus. RSC Adv. 2020, 10, 4529–4537. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.P.; Soegondo, S.; Schnell, O.; Sheu, W.H.H.; Grzeszczak, W.; Watada, H.; Yamamoto, N.; Kalra, S. Efficacy of acarbose in different geographical regions of the world: Analysis of a real-life database. Diabetes Metab. Res. Rev. 2015, 31, 155–1567. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, C.; Huang, Q.; Fu, X.; Liu, R.H. Investigation into the mechanisms of quercetin-3-O-glucuronide inhibiting α-glucosidase activity and non-enzymatic glycation by spectroscopy and molecular docking. Food. Funct. 2012, 12, 7825–7835. [Google Scholar] [CrossRef]
- Kim, G.J.; Jang, Y.; Kwon, K.T.; Kim, J.W.; Kang, S.; Ko, H.C.; Lee, J.Y.; Apostolidis, E.; Kwon, Y.I. Jeju Citrus (Citrus unshiu) leaf extract and hesperidin inhibit small intestinal α-glucosidase activities in vitro and postprandial hyperglycemia in animal model. Int. J. Mol. Sci. 2025, 25, 13721. [Google Scholar] [CrossRef]
- DeMarsilis, A.; Reddy, N.; Boutari, C.; Filippaios, A.; Sternthal, E.; Katsiki, N.; Mantzoros, C. Pharmacotherapy of type 2 diabetes: An update and future directions. Metabolism 2022, 137, 155332. [Google Scholar] [CrossRef]
- Shah, M.A.; Khalil, R.; Ul-Haq, Z.; Panichayupakaranant, P. α-Glucosidase inhibitory effect of rhinacanthins-rich extract from Rhinacanthus nasutus leaf and synergistic effect in combination with acarbose. J. Funct. Foods 2017, 36, 325–331. [Google Scholar] [CrossRef]
- Chinese Pharmacopoeia Commission. Pharmacopeia Commission of the People’s Republic of China, 2020 ed.; China Medical Science Press: Beijing, China, 2020; Volume 1, Available online: https://db.ouryao.com/yd2020/view.php?id=f02d674467 (accessed on 17 May 2025).
- Hou, J.L.; Li, A.P.; Wang, G.H.; Qin, X.M.; Liu, Y.T. Metabolomics analysis of Astragali Radix in Shanxi Province: Investigating the impact of various cultivation methods and growth years on metabolite profiles. Food Chem. 2025, 468, 142492. [Google Scholar] [CrossRef]
- Li, C.N.; Zhang, K.Y.; Liu, L.; Shen, J.M.; Wang, Y.L.; Tan, Y.Y.; Feng, X.Q.; Liu, W.J.; Zhang, H.; Sun, J.M. Study of the mechanism of Astragali Radix in treating type 2 diabetes mellitus and its renal protection based on enzyme activity, network pharmacology, and experimental verification. Molecules 2023, 28, 8030. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, X.C.; Liu, H.L.; Sun, B.G. Medicine and food homology substances: A review of bioactive ingredients, pharmacological effects and applications. Food Chem. 2025, 463, 141111. [Google Scholar] [CrossRef]
- Liu, Y.X.; Song, X.M.; Dan, L.W.; Tang, J.M.; Jiang, Y.; Deng, C.; Zhang, D.D.; Li, Y.Z.; Wang, W. Astragali Radix: Comprehensive review of its botany, phytochemistry, pharmacology and clinical application. Arch. Pharm. Res. 2024, 47, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, J.; Huang, J.; Wen, Q. Flavonoids intake and risk of type 2 diabetes mellitus: A meta-analysis of prospective cohort studies. Medicine 2018, 97, e0686. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.H.; Zhang, W.; Feng, F.J.; Zhang, Y.; Kang, W.Y. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci. Hum. Wellness 2014, 3, 136–174. [Google Scholar] [CrossRef]
- Pan, J.K.; Nawaz, M.; Liu, J.C.; Liu, H.; Lv, Z.Z.; Yang, W.B.; Jiao, Z.G.; Zhang, Q.G. Exploring synergistic inhibitory mechanisms of flavonoid mixtures on α-glucosidase by experimental analysis and molecular dynamics simulation. Food Chem. 2025, 464, 141560. [Google Scholar] [CrossRef]
- Han, L.; Wang, H.Q.; Cao, J.W.; Li, Y.L.; Jin, X.Y.; He, C.; Wang, M. Inhibition mechanism of α-glucosidase inhibitors screened from Tartary buckwheat and synergistic effect with acarbose. Food Chem. 2023, 420, 136102. [Google Scholar] [CrossRef]
- Geng, P.; Yang, Y.; Gao, Z.; Yu, Y.; Shi, Q.; Bai, G. Combined effect of total alkaloids from Feculae Bombycis and natural flavonoids on diabetes. J. Pharm. Pharmacol. 2007, 59, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Bronner, W.E.; Beecher, G.R. Extraction and measurement of prominent flavonoids in orange and grapefruit juice concentrates. J. Chromatogr. A 1995, 705, 247–256. [Google Scholar] [CrossRef]
- Careri, M.; Elviri, L.; Mangia, A.; Musci, M. Spectrophotometric and coulometric detection in the high-performance liquid chromatography of flavonoids and optimization of sample treatment for the determination of quercetin in orange juice. J. Chromatogr. A 2020, 881, 449–460. [Google Scholar] [CrossRef]
- Corradini, E.; Foglia, P.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Flavonoids: Chemical properties and analytical methodologies of identification and quantitation in foods and plants. Nat. Prod. Res. 2011, 25, 469–495. [Google Scholar] [CrossRef]
- Moradi-Afrapoli, F.; Asghari, B.; Saeidnia, S.; Ajani, Y.; Mirjani, M.; Malmir, M.; Dolatabadi Bazaz, R.; Hadjiakhoondi, A.; Salehi, P.; Hamburger, M.; et al. In Vitro α-glucosidase inhibitory activity of phenolic constituents from aerial parts of Polygonum hyrcanicum. DARU J. Pharm. Sci. 2012, 20, 37–42. [Google Scholar] [CrossRef]
- Li, Y.Q.; Zhou, F.C.; Gao, F.; Bian, J.S.; Shan, F. Comparative evaluation of quercetin, isoquercetin and rutin as inhibitors of alpha-glucosidase. J. Agric. Food Chem. 2009, 57, 11463–11468. [Google Scholar] [CrossRef] [PubMed]
- Quan, Y.S.; Zhang, X.Y.; Yin, X.M.; Wang, S.H.; Jin, L.L. Potential α-glucosidase inhibitor from Hylotelephium erythrostictum. Bioorg. Med. Chem. Lett. 2020, 30, 127665. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Z.; Zhang, B.; Tan, C.P.; Huang, Q. α-Glucosidase inhibitors: Consistency of in silico docking data with in vitro inhibitory data and inhibitory effect prediction of quercetin derivatives. Food. Funct. 2019, 10, 6312–6321. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.J.; Chen, X.A.; Xu, F.; Gu, C.H.; Zhu, K.X.; Zhang, Y.J.; Wu, G.; Wang, P.; Tan, L.H. Effects of hydroxylation at C3′ on the B ring and diglycosylation at C3 on the C ring on flavonols inhibition of α-glucosidase activity. Food Chem. 2023, 16, 135057. [Google Scholar] [CrossRef]
- Liu, X.L. Molecular Modification of Dihydroquercetin and Its Inhibitory Effect on α-Glucosidase. Master’s Thesis, Henan University of Science and Technology, Luoyang, China, 2021. [Google Scholar]
- Li, W.X.; Zhang, M.; Zhang, R.F.; Huang, F.; Dong, L.H.; Jia, X.C.; Zhang, M.W. Structural elucidation, binding sites exploration and biological activities of bound phenolics from Radix Puerariae Thomsonii. Food Chem. 2024, 450, 139323. [Google Scholar] [CrossRef]
- Fang, H.L.; Liu, M.L.; Li, S.Y.; Song, W.Q.; Ouyang, H.; Xiao, Z.P.; Zhu, H.L. Identification, potency evaluation, and mechanism clarification of α-glucosidase inhibitors from tender leaves of Lithocarpus polystachyus Rehd. Food Chem. 2022, 371, 131128. [Google Scholar] [CrossRef]
- Zhou, X.J.; Chen, Y.Q.; Yin, Z.P.; Liang, Q.; Zang, J.W.; Tang, D.B.; Chen, J.G. Inhibitory effect of naringenin on α-glucosidase and its mechanism. Food Ind. Technol. 2022, 43, 157–164. [Google Scholar] [CrossRef]
- Ghoran, S.H.; Abdjan, M.I.; Kristanti, A.N.; Aminah, N.S. Insights into in vitro and in silico studies of α-glucosidase inhibitors isolated from the leaves of Grewia optiva (Malvaceae). Int. J. Biol. Macromol. 2025, 287, 138590. [Google Scholar] [CrossRef]
- Huang, C.Y. Study on the Hypoglycemic Active Components and Fingerprint of Longan Leaves. Master’s Thesis, Guangxi University of Chinese Medicine, Nanning, China, 2021. [Google Scholar] [CrossRef]
- Li, D.S.; Li, S.H. Genistein, a soy isoflavone, is a potent α-glucosidase inhibitor. FEBS Lett. 2001, 501, 84–86. [Google Scholar] [CrossRef]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Yoo, D.S.; Kim, Y.S.; Yon, G.H.; Hong, K.S.; Kim, Y.H.; Ryu, S.Y. Yeast α-glucosidase inhibition by isoflavones from plants of leguminosae as an in vitro alternative to acarbose. J. Agric. Food Chem. 2010, 58, 9988–9993. [Google Scholar] [CrossRef]
- Chen, Y.G.; Li, P.; Li, P.; Yan, R.; Zhang, X.Q.; Wang, Y.; Zhang, X.T.; Ye, W.C.; Zhang, Q.W. α-Glucosidase inhibitory effect and simultaneous quantification of three major flavonoid glycosides in Microctis folium. Molecules 2013, 18, 4221–4232. [Google Scholar] [CrossRef] [PubMed]
- Han, L.L. Study on the Inhibitory Effect of Calycosin and Its Glycoside on α-Glucosidase and its Mechanism. Master’s Thesis, Shanxi Medical University, Taiyuan, China, 2022. [Google Scholar] [CrossRef]
- Ji, Y.H. Study on the Target Occupancy Model of Drugs Based on Intestinal Targets and Drug-Target Binding Kinetics. Master’s Thesis, Beijing University of Chinese Medicine, Beijing, China, 2020. [Google Scholar] [CrossRef]
- Hong, Y.; Liao, X.Y.; Chen, Z.L. Screening and characterization of potential α-glucosidase inhibitors from Cercis chinensis Bunge fruits using ultrafiltration coupled with HPLC-ESI-MS/MS. Food Chem. 2022, 372, 131316. [Google Scholar] [CrossRef]
- Ning, Z.W.; Zhai, L.X.; Huang, T.; Peng, J.; Hu, D.; Xiao, H.T.; Wen, B.; Lin, C.Y.; Zhao, L.; Bian, Z.X. Identification of α-glucosidase inhibitors from Cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking. Food Funct. 2019, 10, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.H.; Uddin, Z.; Jin, Y.M.; Li, Z.P.; Curtis-Long, M.J.; Kim, K.D.; Cho, J.K.; Park, K.H. Inhibition of protein tyrosine phosphatase (PTP1B) and α-glucosidase by geranylated flavonoids from Paulownia tomentosa. J. Enzym. Inhib. Med. Chem. 2017, 32, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Zhang, Y.R.; Karrar, E.; Jin, Q.Z.; Zhang, H.; Wu, G.C.; Wang, X.G. Mechanisms of sesamol and sesamin inhibiting α-glucosidase activity by spectroscopy and molecular docking. Food Biosci. 2023, 53, 102680. [Google Scholar] [CrossRef]
- Xu, Z.M.; Hileuskaya, K.; Kraskouski, A.; Yang, Y.J.; Huang, Z.; Zhao, Z.G. Inhibition of α-glucosidase activity and intestinal glucose transport to assess the in vivo anti-hyperglycemic potential of dodecyl-acylated phlorizin and polydatin derivatives. Food Funct. 2024, 15, 4785–4804. [Google Scholar] [CrossRef]
- He, Y.L. Inhibition of α-Glucosidase Activity and Advanced Glycation End Product Formation by Three Flavonoids. Master’s Thesis, Liaoning University, Shenyang, China, 2019. Available online: https://kns.cnki.net/kcms2/article/abstract?v=vG2M3utQQCegvpP6J-a0LbaXiVm8MuhFjA6576WLvhqtrxx5c-8g1gbpVHvwRS0HFLFLdJGOMC6YDyI9wahQzMFKQBjqLPh9PVJ9-sulZ00i-fwS2Dcg96w_vV4MQ9zPNrp2j17jZxzL-yq6qq9AhKUHhTlLmpTgCyDGlZz6Z5jBC_Awj2HdUJTldF331ULrh1Dc_wRr6rADhNL9bP7KMw==&uniplatform=NZKPT (accessed on 17 May 2025).
- Peng, Q. Study on the Inhibition of α-Glucosidase by Flavonoid Compounds and the Physicochemical Properties and Biological Activities of Their Nanoparticle Encapsulations. Master’s Thesis, Nanchang University, Nanchang, China, 2016. Available online: https://kns.cnki.net/kcms2/article/abstract?v=vG2M3utQQCcL5JUFh1giaeA1CgIzWFbVZ-Poot594_7Cjnu427nRLLHL9Eu9kGktFHjHo-E72a__K9Ctib8O9N8FdaBfMEeI1DfiZl5Z7yeBf4xXTgYo1byZdKOnLSiOZoFZFHJCPfZOEl810O9FLiLYstlZTEiWPESS2-AmWMQvlIhtf6JZf6wXvfLuz5RHTrYdXsW56BWd40h7jaGbRQ==&uniplatform=NZKPT (accessed on 17 May 2025).
- Yan, Z.X.; Lin, G.; Ye, Y.; Wang, Y.T.; Yan, R. A generic multiple reaction monitoring based approach for plant flavonoids profiling using a triple quadrupole linear ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2014, 25, 955–965. [Google Scholar] [CrossRef]
- Liu, Y.J.; Zhu, J.; Yu, J.M.; Chen, X.; Zhang, S.Y.; Cai, Y.X.; Li, L. A new functionality study of vanillin as the inhibitor for α-glucosidase and its inhibition kinetic mechanism. Food Chem. 2021, 353, 129448. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhao, D.G.; Zhang, R.Q.; He, X.; Li, B.Q.; Zhang, X.Z.; Wang, Z.J.; Zhang, K. Identification of bioactive compounds that contribute to the α-glucosidase inhibitory activity of rosemary. Food Funct. 2020, 11, 1692–1701. [Google Scholar] [CrossRef]
- Kiefer, F.; Arnold, K.; Künzli, M.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository and associated resources. Nucleic Acids Res. 2008, 37, D387–D392. [Google Scholar] [CrossRef]
- Jiang, W.Y.; Kan, H.; Li, P.D.; Liu, S.; Liu, Z.Y. Screening and structural characterization of potential α-glucosidase inhibitors from Radix Astragali flavonoids extract by ultrafiltration LCDAD-ESI-MSn. Anal. Methods 2015, 7, 123–128. [Google Scholar] [CrossRef]
- Xu, F.; Yang, D.H.; Shang, M.Y.; Wang, X.; Cai, S.Q. “Effective forms”, “additive effect”, and “toxicity scattering effect” of active ingredients in traditional Chinese medicine: Reflections triggered by in vivo metabolism studies. World Sci. Technol.-Mod. Tradit. Chin. Med. 2006, 16, 688–703. [Google Scholar]
- Tian, J.L.; Si, X.; Wang, Y.H.; Gong, E.S.; Xie, X.; Zhang, Y.; Li, B.; Shu, C. Bioactive flavonoids from Rubus corchorifolius inhibit α-glucosidase and α-amylase to improve postprandial hyperglycemia. Food Chem. 2021, 341, 128149. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xie, L.H.; Xie, J.H.; Liu, Y.; Chen, W. Pelargonidin-3-O-rutinoside as a novel α-glucosidase inhibitor for improving postprandial hyperglycemia. Chem. Commun. 2018, 55, 39–42. [Google Scholar] [CrossRef]
- Sun, H.; Scott, D.O. Impact of genetic polymorphisms of cytochrome P450 2 C (CYP2C) enzymes on the drug metabolism and design of antidiabetics. Chem. Biol. Interact. 2011, 194, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Santamarina, S.; Kuhn, M.; Devendran, S.; Maier, L.; Driessen, M.; Mateus, A.; Mastrorilli, E.; Brochado, A.R.; Savitski, M.M.; Patil, K.R.; et al. Emergence of community behaviors in the gut microbiota upon drug treatment. Cell 2024, 187, 6346–6357. [Google Scholar] [CrossRef]
- Zhu, C.S.; Niu, H.J.; Nie, A.Z.; Bian, M. Bioactivity-guided separation of potential α-glycosidase inhibitor from Clerodendranthus spicatus based on HSCCC coupled with molecular docking. Sci. Rep. 2021, 11, 6914. [Google Scholar] [CrossRef]
- Han, L.; Zhang, L.L.; Ma, W.F.; Li, D.; Shi, R.J.; Wang, M. Proanthocyanidin B2 attenuates postprandial blood glucose and its inhibitory effect on alpha-glucosidase: Analysis by kinetics, fluorescence spectra, atomic force microscope and molecular docking. Food Funct. 2018, 9, 4673–4682. [Google Scholar] [CrossRef]
- Xie, L.L.; Zhang, T.; Karrar, E.; Zheng, L.Y.; Xie, D.; Jin, J.; Chang, M.; Wang, X.G.; Jin, Q.Z. Insights into an α-glucosidase inhibitory profile of 4,4-dimethylsterols by multispectral techniques and molecular docking. J. Agric. Food Chem. 2021, 69, 15252–15260. [Google Scholar] [CrossRef]
- Ding, H.F.; Hu, X.; Xu, X.M.; Zhang, G.W.; Gong, D.M. Inhibitory mechanism of two allosteric inhibitors, oleanolic acid and ursolic acid on α-glucosidase. Int. J. Biol. Macromol. 2018, 107, 1844–1855. [Google Scholar] [CrossRef]
- Yamamoto, K.; Miyake, H.; Kusunoki, M.; Osaki, S. Crystal structures of isomaltase from Saccharomyces cerevisiae and in complex with its competitive inhibitor maltose. FEBS J. 2010, 277, 4205–4214. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef] [PubMed]
- GB/T 35892-2018; Laboratory Animal—Guideline for Ethical Review of Animal Welfare. Standardization Administration of the People’s Republic of China: Beijing, China, 2018. Available online: https://lac.cdmc.edu.cn/module/download/downfile.jsp?classid=0&filename=1803190833455154874.pdf (accessed on 17 May 2025).
- Rivera-Chávez, J.; González-Andrade, M.; del Carmen González, M.; Glenn, A.E.; Mata, R. Thielavins A, J and K: α-glucosidase inhibitors from MEXU27095, an endophytic fungus from Hintonia latiflora. Phytochemistry 2013, 94, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Xue, N.; He, B.N.; Jia, Y.T.; Yang, C.Q.; Wang, J.; Li, M. The mechanism of binding with the α-glucosidase in vitro and the evaluation on hypoglycemic effect in vivo: Cocrystals involving synergism of gallic acid and conformer. Eur. J. Pharm. Biopharm. 2020, 156, 64–74. [Google Scholar] [CrossRef]
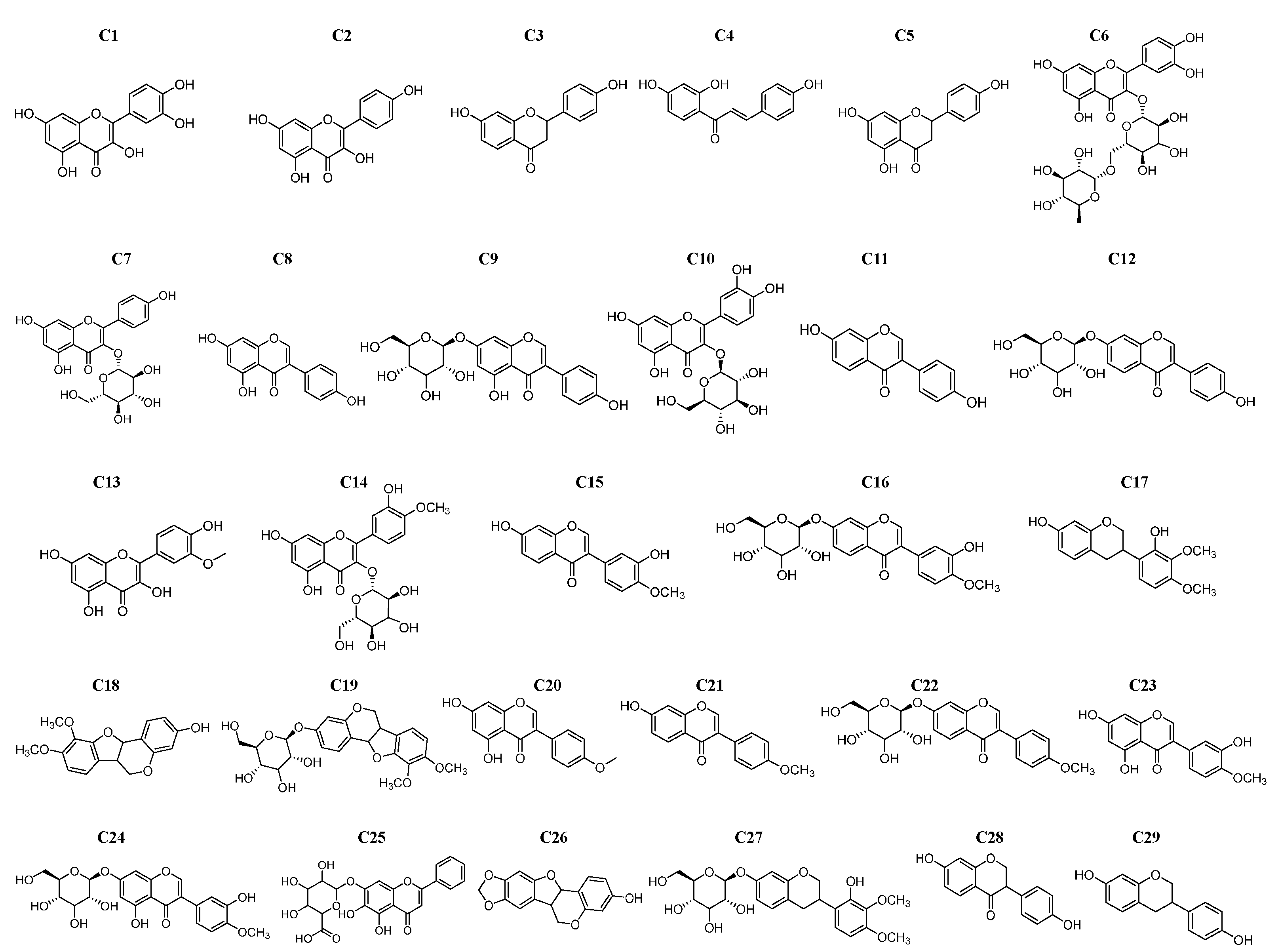
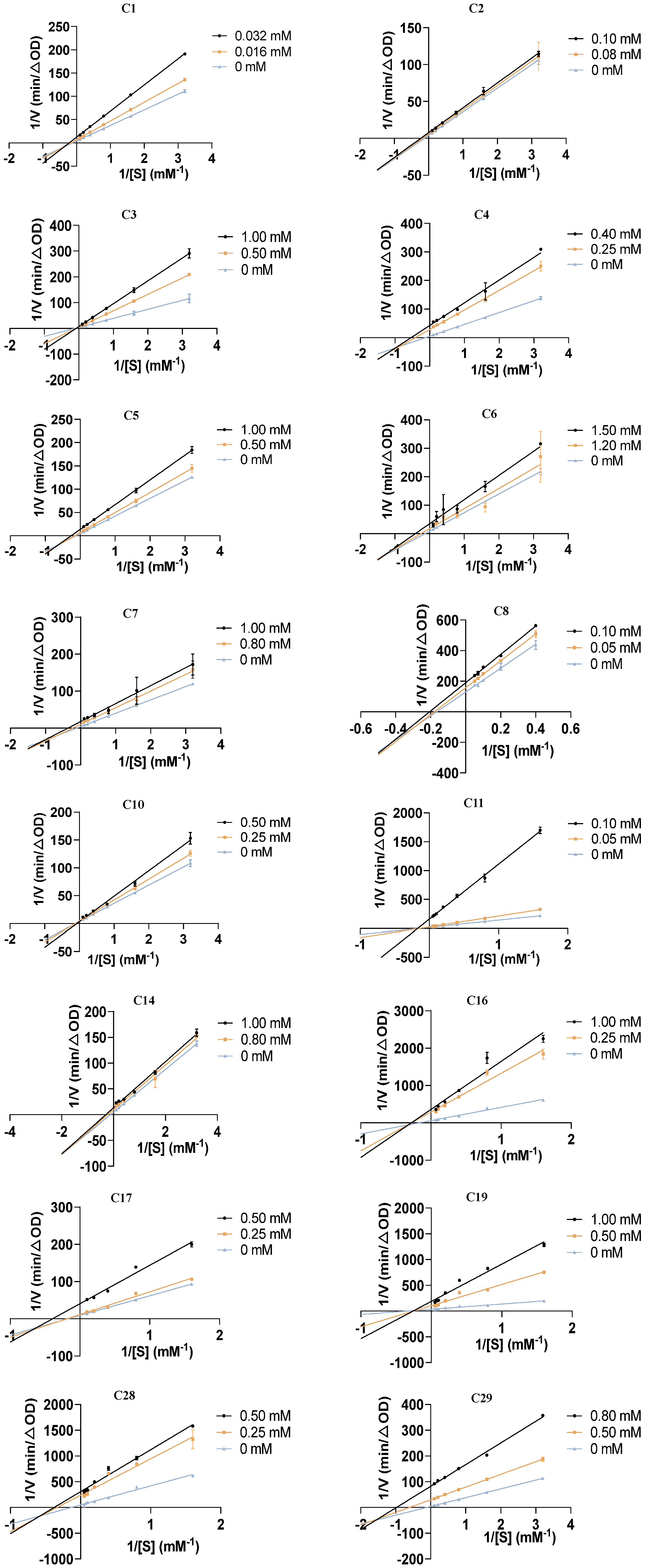
| No. | Name | IC50 (μM) | IC50 in References (μM) |
|---|---|---|---|
| C1 | quercetin | 6.65 ± 0.21 | 3.3 [22], 17 [23], 230.3 [24] |
| C2 | kaempferol | 38.79 ± 4.96 | 18.6 [25], 420.5 [24], 1.22 × 103 [26] |
| C3 | liquiritigenin | 160.77 ± 36.29 | - |
| C4 | isoliquiritigenin | 69.11 ± 10.66 | 36.29 [27], 1.81 × 103 [28] |
| C5 | naringenin | 225.83 ± 36.14 | 36.84 [29], 96.8 [25], 174 [30] |
| C6 | rutin | 211.27 ± 11.23 | 0.10 [25], 196 [25], 841.92 [31] |
| C7 | astragalin | 176.00 ± 31.05 | 114.6 [31], 519.21 [32] |
| C8 | genistein | 64.80 ± 24.24 | 50 [33], 70 [28], 150 [34] |
| C10 | isoquercitrin | 71.70 ± 7.62 | 47.40 [29], 185 [23] |
| C11 | daidzein | 83.66 ± 20.19 | 150 [28] |
| C14 | isorhamnetin-3-O-glucoside | 228.30 ± 34.00 | 275.4 [35] |
| C16 | calycosin-7-O-glucoside | 563.40 ± 43.56 | 174.04 [36] |
| C17 | isomucronulatol | 354.26 ± 40.38 | - |
| C19 | astrapterocarpan-3-O-glucoside | 347.67 ± 32.36 | - |
| C28 | dihydrodaidzein | 412.00 ± 11.40 | - |
| C29 | equol | 131.37 ± 7.65 | - |
| acarbose | 10.91 ± 0.36 nM | 8 × 10−3 [37], 15.49 × 10−3 [26], 68.20 [38], 1.1 × 103 [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, X.; Wang, P.; Zhang, J.; Lv, Y.; Zhao, Z.; Zhang, F.; Shang, M.; Liu, G.; Wang, X.; Cai, S.; et al. α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects. Pharmaceuticals 2025, 18, 744. https://doi.org/10.3390/ph18050744
Han X, Wang P, Zhang J, Lv Y, Zhao Z, Zhang F, Shang M, Liu G, Wang X, Cai S, et al. α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects. Pharmaceuticals. 2025; 18(5):744. https://doi.org/10.3390/ph18050744
Chicago/Turabian StyleHan, Xing, Pengpu Wang, Jing Zhang, Yang Lv, Zhigao Zhao, Fengxian Zhang, Mingying Shang, Guangxue Liu, Xuan Wang, Shaoqing Cai, and et al. 2025. "α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects" Pharmaceuticals 18, no. 5: 744. https://doi.org/10.3390/ph18050744
APA StyleHan, X., Wang, P., Zhang, J., Lv, Y., Zhao, Z., Zhang, F., Shang, M., Liu, G., Wang, X., Cai, S., & Xu, F. (2025). α-Glucosidase Inhibition Mechanism and Anti-Hyperglycemic Effects of Flavonoids from Astragali Radix and Their Mixture Effects. Pharmaceuticals, 18(5), 744. https://doi.org/10.3390/ph18050744






