Efficacy and Safety of Novel Oral Anti-Cholestatic Agents for Primary Biliary Cholangitis: Meta-Analyses and Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Study Design
2.2. Eligibility Criteria
- Population: Adult patients diagnosed with PBC using established diagnostic criteria;
- Intervention: Novel oral anti-cholestatic agents (e.g., obeticholic acid, fibrates, nor-UDCA, etc.);
- Comparator: Placebo, UDCA monotherapy, or other oral anti-cholestatic agents;
- Outcomes: Efficacy (e.g., improvements in alkaline phosphatase [ALP], bilirubin levels, or pruritus) and safety (e.g., adverse events, tolerability);
- Study Design: Randomized controlled trials (RCTs), cohort studies, or case-control studies.
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Literature Search
3.2. Study Characteristics
3.3. Efficacy of Novel Cholestatic Drugs
3.4. Safety
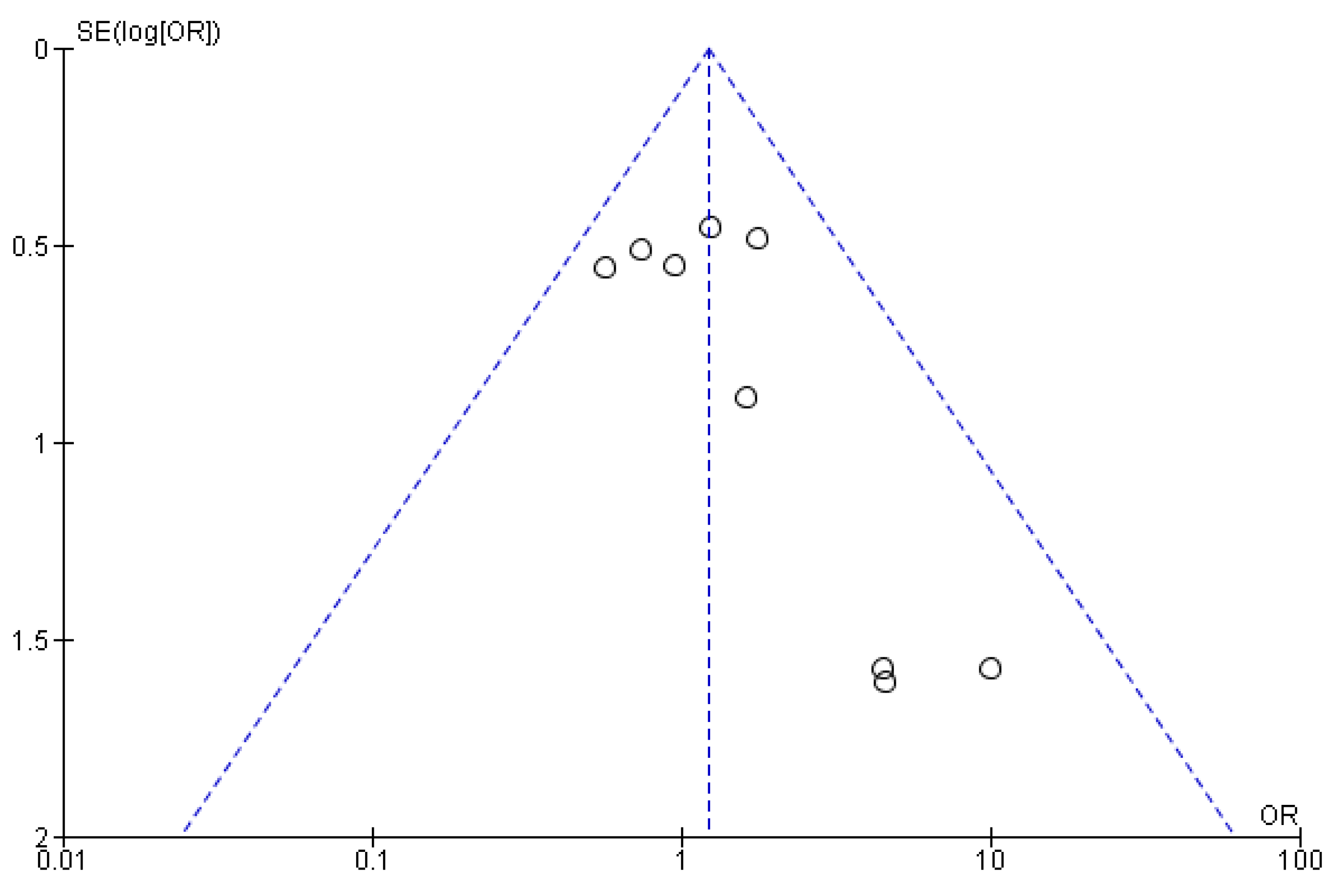
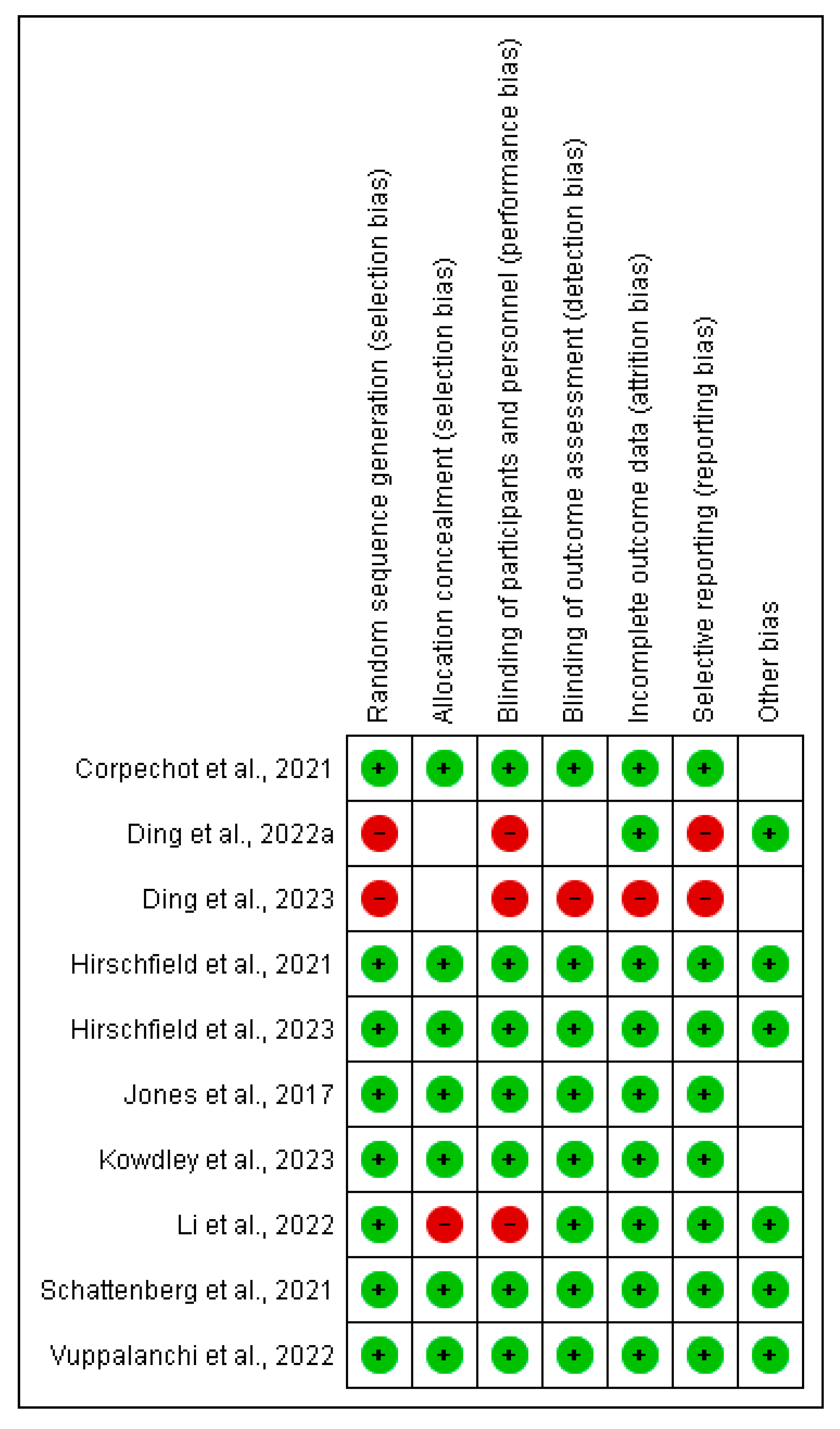
3.5. Risk of Bias Assessment
4. Discussion
5. Limitations
6. Future Directions
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Trivella, J.; John, B.V.; Levy, C. Primary biliary cholangitis: Epidemiology, prognosis, and treatment. Hepatol. Commun. 2023, 7, e0179. [Google Scholar] [CrossRef] [PubMed]
- Purohit, T.; Cappell, M.S. Primary biliary cirrhosis: Pathophysiology, clinical presentation and therapy. World J. Hepatol. 2015, 7, 926–941. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Takikawa, H.; Mochida, S.; Koike, K.; Miwa, H.; Shimosegawa, T. Changing nomenclature for PBC from ‘primary biliary cirrhosis’ to ‘primary biliary cholangitis’. J. Gastroenterol. 2016, 51, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Beuers, U.; Gershwin, M.E.; Gish, R.G.; Invernizzi, P.; Jones, D.E.J.; Lindor, K.; Ma, X.; Mackay, I.R.; Parés, A.; Tanaka, A.; et al. Changing nomenclature for PBC: From ‘cirrhosis’ to ‘cholangitis’. Gastroenterology 2015, 149, 1627–1629. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Ronca, V.; Bruno, S.; Invernizzi, P.; Mells, G.F. Toward precision medicine in primary biliary cholangitis. Dig. Liver Dis. 2016, 48, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Lv, T.; Chen, S.; Li, M.; Zhang, D.; Kong, Y.; Jia, J. Regional variation and temporal trend of primary biliary cholangitis epidemiology: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1423–1434. [Google Scholar] [CrossRef]
- Lu, M.; Zhou, Y.; Haller, I.V.; Romanelli, R.J.; VanWormer, J.J.; Rodriguez, C.V.; Anderson, H.; Boscarino, J.A.; Schmidt, M.A.; Daida, Y.G.; et al. Increasing prevalence of primary biliary cholangitis and reduced mortality with treatment. Clin. Gastroenterol. Hepatol. 2018, 16, 1342–1350.e1. [Google Scholar] [CrossRef] [PubMed]
- Lleo, A.; Jepsen, P.; Morenghi, E.; Carbone, M.; Moroni, L.; Battezzati, P.M.; Podda, M.; Mackay, I.R.; Gershwin, M.E.; Invernizzi, P. Evolving trends in female to male incidence and male mortality of primary biliary cholangitis. Sci. Rep. 2016, 6, 25906. [Google Scholar] [CrossRef] [PubMed]
- Selmi, C.; Mayo, M.J.; Bach, N.; Ishibashi, H.; Invernizzi, P.; Gish, R.G.; Gordon, S.C.; Wright, H.I.; Zweiban, B.; Podda, M.; et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: Genetics, epigenetics, and environment. Gastroenterology 2004, 127, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Gulamhusein, A.F.; Juran, B.D.; Lazaridis, K.N. GWAS in primary biliary cirrhosis. Semin. Liver Dis. 2015, 35, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Hirschfield, G.M. The immunogenetics of autoimmune cholestasis. Clin. Liver Dis. 2016, 20, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Juran, B.D.; Lazaridis, K.N. Environmental factors in primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Chrétien, Y.; Chazouillères, O.; Poupon, R. Demographic, lifestyle, medical and familial factors associated with primary biliary cirrhosis. J. Hepatol. 2010, 53, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Orphanet: Primary Biliary Cholangitis. Available online: https://www.orpha.net/en/disease/detail/186 (accessed on 28 November 2024).
- Khanna, A.; Leighton, J.; Lee Wong, L.; Jones, D.E. Symptoms of PBC—Pathophysiology and management. Best Pract. Res. Clin. Gastroenterol. 2018, 34–35, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Dyson, J.K.; Wilkinson, N.; Jopson, L.; Mells, G.; Bathgate, A.; Heneghan, M.A.; Neuberger, J.; Hirschfield, G.M.; Ducker, S.J.; Sandford, R.; et al. The inter-relationship of symptom severity and quality of life in 2055 patients with primary biliary cholangitis. Aliment. Pharmacol. Ther. 2016, 44, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Lammers, W.J.; van Buuren, H.R.; Hirschfield, G.M.; Janssen, H.L.A.; Invernizzi, P.; Mason, A.L.; Ponsioen, C.Y.; Floreani, A.; Corpechot, C.; Mayo, M.J.; et al. Levels of alkaline phosphatase and bilirubin are surrogate end points of outcomes of patients with primary biliary cirrhosis: An international follow-up study. Gastroenterology 2014, 147, 1338–1349.e5. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Lindor, K.D.; Bowlus, C.L.; Boyer, J.; Levy, C.; Mayo, M. Primary biliary cholangitis: 2021 practice guidance update from the American Association for the Study of Liver Diseases. Hepatology 2021, 75, 1012–1013. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Ma, X.; Yokosuka, O.; Weltman, M.; You, H.; Amarapurkar, D.N.; Kim, Y.J.; Abbas, Z.; Payawal, D.A.; Chang, M.-L.; et al. Autoimmune liver diseases in the Asia-Pacific region: Proceedings of APASL symposium on AIH and PBC 2016. Hepatol. Int. 2016, 10, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Ding, D.; Ren, P.; Guo, G.; Liu, Y.; Yang, C.; Zheng, L.; Jia, G.; Deng, J.; Sun, R.; Wang, X.; et al. Fenofibrate normalizes alkaline phosphatase and improves long-term outcomes in patients with advanced primary biliary cholangitis refractory to ursodeoxycholic acid. Gastroenterol. Hepatol. 2023, 46, 692–701. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.; Boudes, P.F.; Swain, M.G.; Bowlus, C.L.; Galambos, M.R.; Bacon, B.R.; Doerffel, Y.; Gitlin, N.; Gordon, S.C.; Odin, J.A.; et al. Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: A double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet Gastroenterol. Hepatol. 2017, 2, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Vuppalanchi, R.; Caldwell, S.H.; Pyrsopoulos, N.; deLemos, A.S.; Rossi, S.; Levy, C.; Goldberg, D.S.; Mena, E.A.; Sheikh, A.; Ravinuthala, R.; et al. Proof-of-concept study to evaluate the safety and efficacy of saroglitazar in patients with primary biliary cholangitis. J. Hepatol. 2022, 76, 75–85. [Google Scholar] [CrossRef]
- Li, C.; Zheng, K.; Chen, Y.; He, C.; Liu, S.; Yang, Y.; Li, M.; Zeng, X.; Wang, L.; Zhang, F. A randomized, controlled trial on fenofibrate in primary biliary cholangitis patients with incomplete response to ursodeoxycholic acid. Ther. Adv. Chronic Dis. 2022, 13, 20406223221114198. [Google Scholar] [CrossRef] [PubMed]
- Hirschfield, G.M.; Shiffman, M.L.; Gulamhusein, A.; Kowdley, K.V.; Vierling, J.M.; Levy, C.; Kremer, A.E.; Zigmond, E.; Andreone, P.; Gordon, S.C.; et al. Seladelpar efficacy and safety at 3 months in patients with primary biliary cholangitis: ENHANCE, a phase 3, randomized, placebo-controlled study. Hepatology 2023, 78, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Kowdley, K.V.; Bowlus, C.L.; Levy, C.; Akarca, U.S.; Alvares-da-Silva, M.R.; Andreone, P.; Arrese, M.; Corpechot, C.; Francque, S.M.; Heneghan, M.A.; et al. Efficacy and Safety of Elafibranor in Primary Biliary Cholangitis. N. Engl. J. Med. 2024, 390, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Schattenberg, J.M.; Pares, A.; Kowdley, K.V.; Heneghan, M.A.; Caldwell, S.; Pratt, D.; Bonder, A.; Hirschfield, G.M.; Levy, C.; Vierling, J.; et al. A randomized placebo-controlled trial of elafibranor in patients with primary biliary cholangitis and incomplete response to UDCA. J. Hepatol. 2021, 74, 1344–1354. [Google Scholar] [CrossRef]
- Ding, D.; Guo, G.; Liu, Y.; Zheng, L.; Jia, G.; Deng, J.; Sun, R.; Wang, X.; Guo, C.; Shang, Y.; et al. Efficacy and safety of fenofibrate addition therapy in patients with cirrhotic primary biliary cholangitis with incomplete response to ursodeoxycholic acid. Hepatol. Commun. 2022, 6, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Chazouillères, O.; Rousseau, A.; Le Gruyer, A.; Habersetzer, F.; Mathurin, P.; Goria, O.; Potier, P.; Minello, A.; Silvain, C.; et al. A Placebo-Controlled Trial of Bezafibrate in Primary Biliary Cholangitis. N. Engl. J. Med. 2018, 378, 2171–2181. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Beuers, U.; Kupcinskas, L.; Ott, P.; Bergquist, A.; Färkkilä, M.; Manns, M.P.; Parés, A.; Spengler, U.; Stiess, M.; et al. A placebo-controlled randomised trial of budesonide for PBC following an insufficient response to UDCA. J. Hepatol. 2021, 74, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Rupp, C.; Rössler, A.; Halibasic, E.; Sauer, P.; Weiss, K.-H.; Friedrich, K.; Wannhoff, A.; Stiehl, A.; Stremmel, W.; Trauner, M.; et al. Reduction in alkaline phosphatase is associated with longer survival in primary sclerosing cholangitis, independent of dominant stenosis. Aliment. Pharmacol. Ther. 2014, 40, 1292–1301. [Google Scholar] [CrossRef] [PubMed]
- Barba Bernal, R.; Ferrigno, B.; Medina Morales, E.; Castro, C.M.; Goyes, D.; Trivedi, H.; Patwardhan, V.R.; Bonder, A. Management of primary biliary cholangitis: Current treatment and future perspectives. Turk. J. Gastroenterol. 2023, 34, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Kremer, A.E.; Mayo, M.J.; Hirschfield, G.; Levy, C.; Bowlus, C.L.; Jones, D.E.; Steinberg, A.; McWherter, C.A.; Choi, Y.-J. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022, 42, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Angulo, P.; Jorgensen, R.A.; Keach, J.C.; Dickson, E.R.; Smith, C.; Lindor, K.D. Oral budesonide in the treatment of patients with primary biliary cirrhosis with a suboptimal response to ursodeoxycholic acid. Hepatology 2000, 31, 318–323. [Google Scholar] [CrossRef] [PubMed]
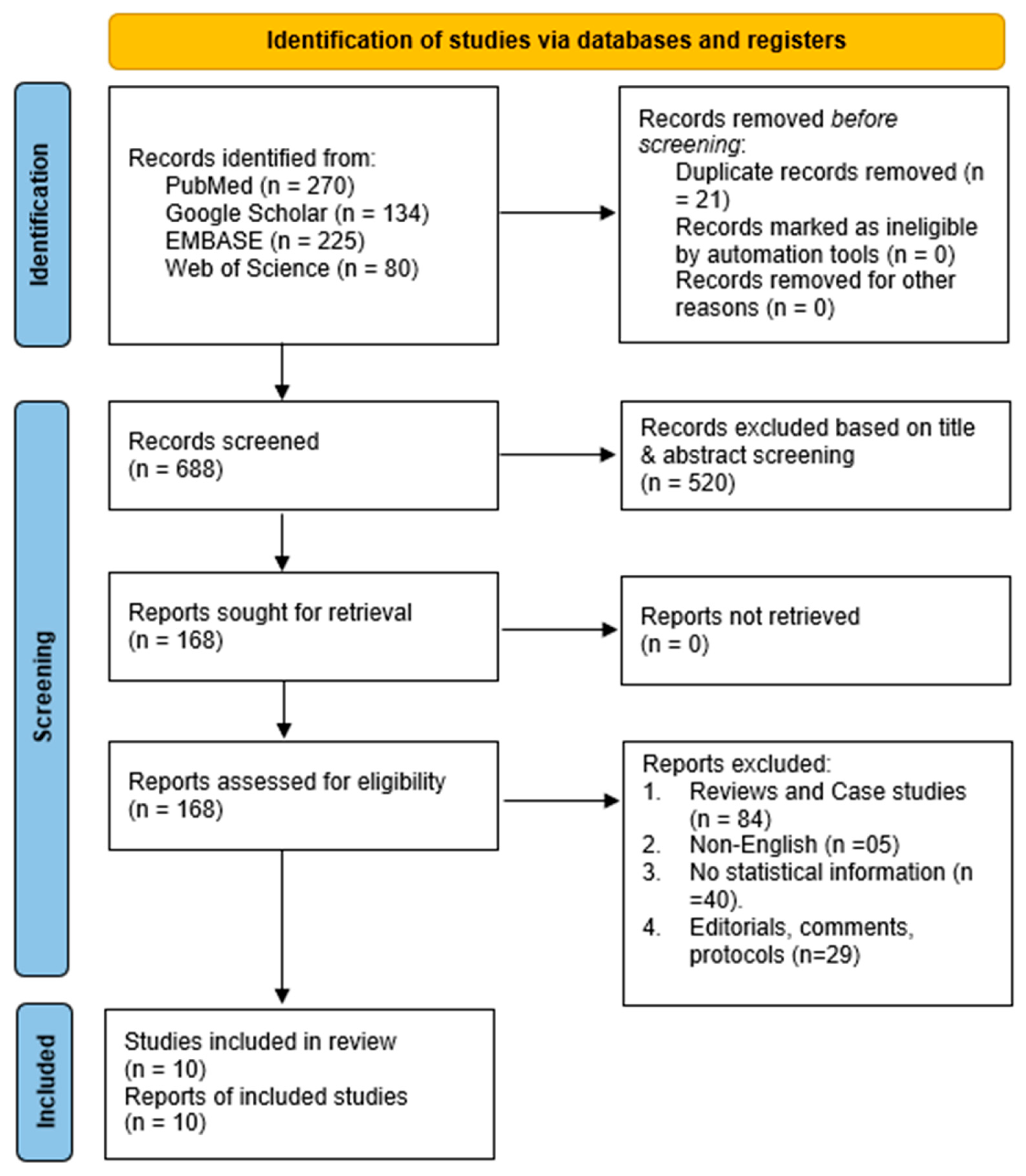
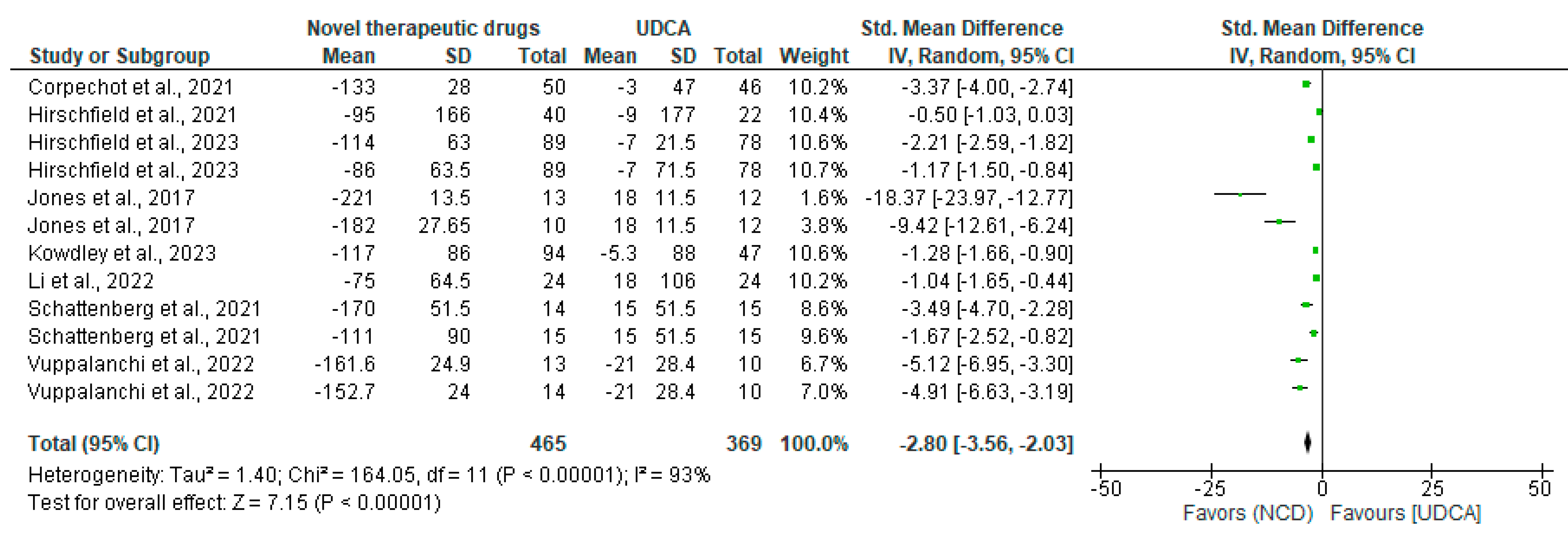
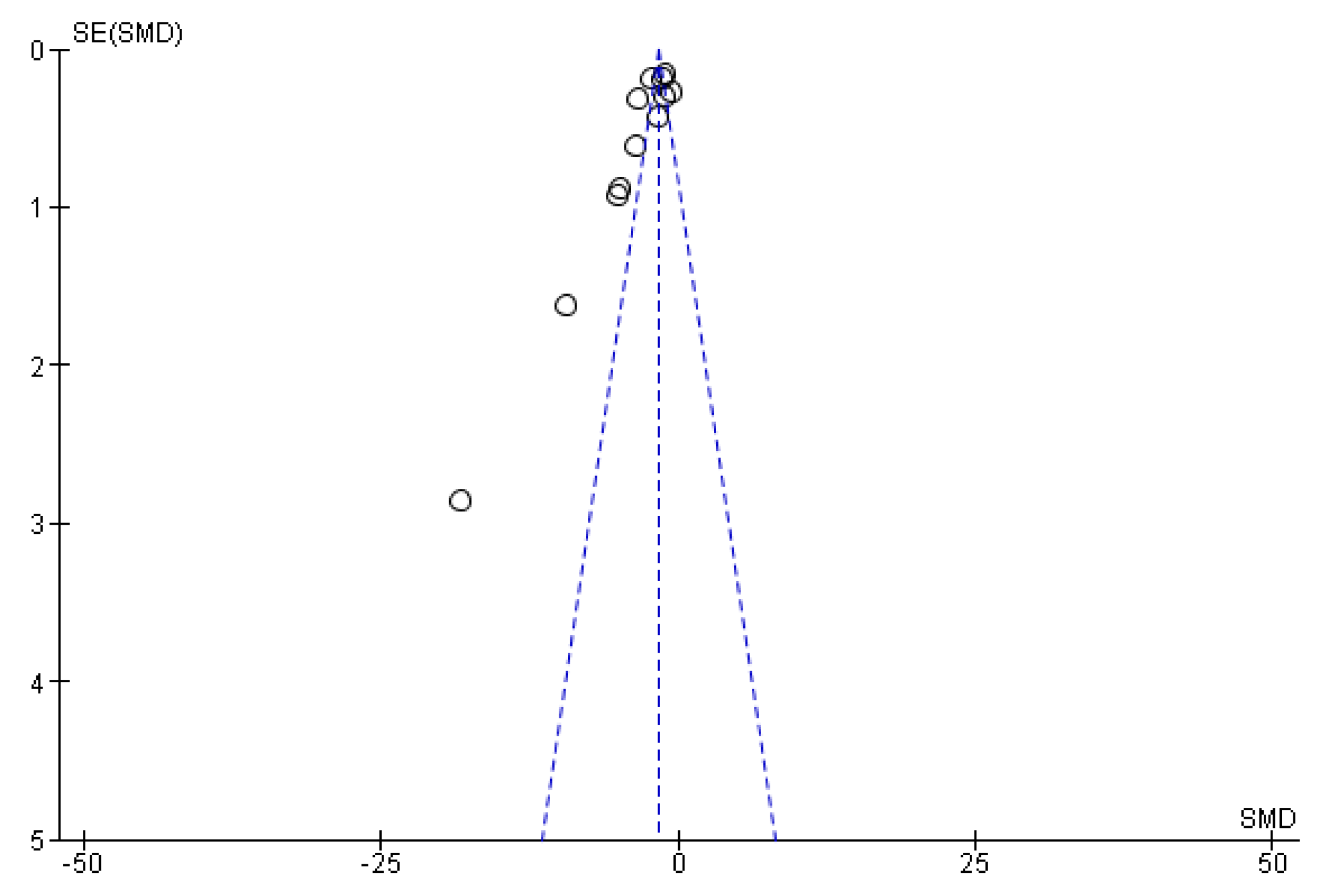
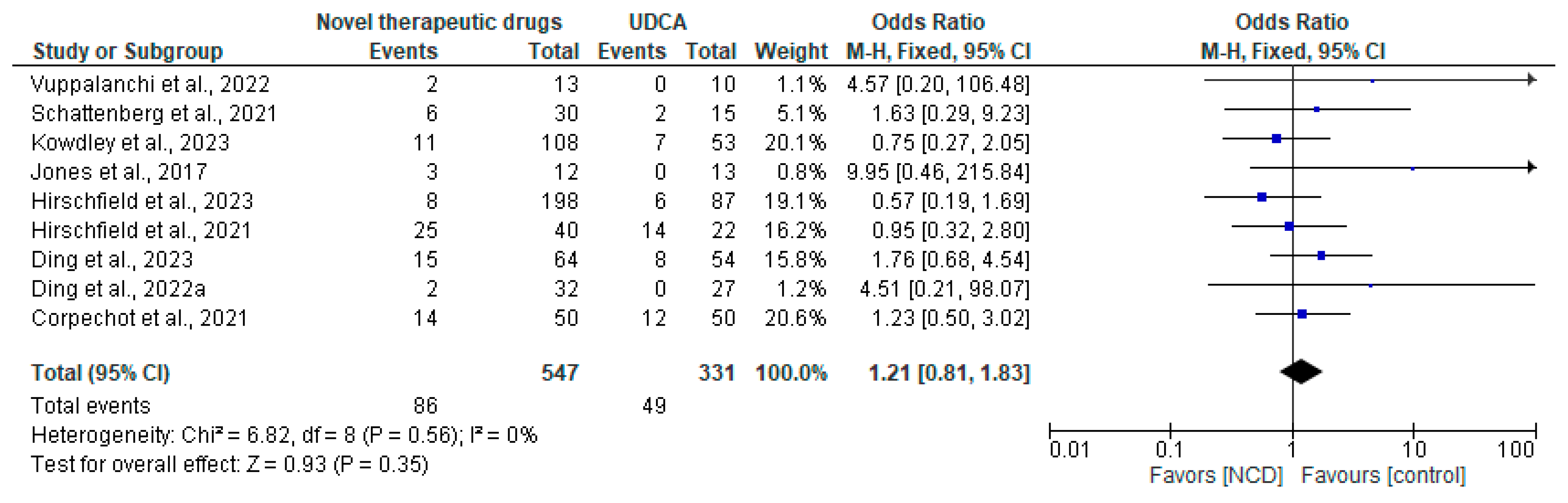
| Study | Population | Intervention | Comparator | Outcomes | Study Design |
|---|---|---|---|---|---|
| Ding et al., 2023 [21] | Adult patients with PBC | Fenofibrate | UDCA | ALP levels, long-term outcomes | Retrospective |
| Jones et al., 2017 [22] | Adult patients with PBC | Seladelpar (50 mg, 200 mg) | Placebo | ALP reduction, normalization rates | Phase 2 RCT |
| Vuppalanchi et al., 2022 [23] | Adult patients with PBC | Saroglitazar (2 mg, 4 mg) | Placebo | ALP levels, normalization rates | Phase 2 RCT |
| Li et al., 2022 [24] | Adult patients with PBC | Fenofibrate | Placebo | ALP levels | Open-label RCT |
| Hirschfield et al., 2023 [25] | Adult patients with PBC | Seladelpar (5 mg, 10 mg) | Placebo | ALP levels, safety outcomes | Phase 3 RCT |
| Kowdley et al., 2024 [26] | Adult patients with PBC | Elafibranor (80 mg) | Placebo | ALP levels, safety outcomes | Phase 3 RCT |
| Schattenberg et al., 2021 [27] | Adult patients with PBC | Elafibranor (80 mg, 120 mg) | Placebo | ALP levels, safety outcomes | Phase 3 RCT |
| Ding et al., 2022 [28] | Adult patients with advanced PBC | Fenofibrate | UDCA | ALP levels | Retrospective |
| Corpechot et al., 2018 [29] | Adult patients with PBC | Bezafibrate (400 mg) | Placebo | ALP levels, normalization rates | Phase 3 RCT |
| Hirschfield et al., 2021 [30] | Adult patients with PBC | Budesonide | Placebo | ALP levels, pruritus improvement | Phase 3 RCT |
| Author ID | Design | Country | NCD | N | Randomization | Study Duration | Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ALP Changes (U/l) | ALP Normalization | Pruritus | |||||||||||||||
| Ding et al., 2023 [21] | Retrospective | China | Fenofibrate | 118 | Fenofibrate | UDCA | 36 (24–48) weeks | Fenofibrate | UDCA | Fenofibrate | UDCA | Not reported | |||||
| 64 | 54 | 29 | 14 | 24 | 6 | ||||||||||||
| Jones et al., 2017 [22] | Phase 2 RCT | North America and Europe | Seladelpar | 41 | Seladelpar (50 g) | Seladelpar (200 mg) | Placebo | 12 weeks | Seladelpar (50 g) | Seladelpar (200 g) | Placebo | Seladelpar (50 g) | Seladelpar (200 g) | Placebo | Seladelpar (50 g) | Seladelpar (200 g) | Placebo |
| 13 | 13 | 12 | 53% (14) | 63% (8) | −2% (16) | 100% | 100% | 0 | 4 | 1 | 1 | ||||||
| Reduction in ALP levels by at least 15% | ALP normalization | ||||||||||||||||
| Vuppalanchi et al., 2022 [23] | Phase 2 RCT | USA | Saroglitazar | 37 | Saroglitazar (4 mg) | Saroglitazar (2 mg) | Placebo | 16 weeks | Saroglitazar (4 mg) | Saroglitazar (2 mg) | placebo | Saroglitazar (4 mg) | Saroglitazar (2 mg) | Placebo | Not reported | ||
| 13 | 14 | 10 | 84.60% | 92.90% | 20% | 38.50% | 50% | 0% | |||||||||
| Li et al., 2022 [24] | Open-label RCT | China | Fenofibrate | 48 | Fenofibrate | Placebo | 58 weeks | Not reported | Fenofibrate | Control | Not reported | ||||||
| 24 | 24 | 54.20% | 4.2% | ||||||||||||||
| Hirschfield et al., 2023 [25] | Phase 3 RCT | Multinational (21 countries) | Seladelpar | 265 | Seladelpar (5 mg) | Seladelpar (10 mg) | Placebo | 58 weeks | Not reported | Seladelpar (5 mg) | Seladelpar (10 mg) | placebo | Seladelpar (5 mg) | Seladelpar (10 mg) | Placebo | ||
| 89 | 89 | 87 | 5.40% | 27.30% | 0% | 3.40% | 11.20% | 12.60% | |||||||||
| Kowdley et al., 2024 [26] | Phase 3 RCT | Multinational | Elafibranor | 161 | Elafibranor (80 mg) | Placebo | 52 weeks | Elafibranor (80 mg) LS | Placebo | Elafibranor (80 mg) | Placebo | Elafibranor (80 mg) | Placebo | ||||
| 108 | 53 | −117.0 (−134.4–−99.6) | −5.3 (−30.4–19.7) | 15% | 0% | 20% | 26% | ||||||||||
| Shattenberg et al., 2021 [27] | Phase 3 RCT | USA and Europe | Elafibranor | 45 | Elafibranor (80 mg) | Elafibranor (120 mg) | Placebo | 12 weeks | Elafibranor (80 mg) | Elafibranor (120 mg) | Placebo | Elafibranor (80 mg) | Elafibranor (120 mg) | Placebo | Elafibranor (80 mg) | Elafibranor (120 mg) | Placebo |
| 15 | 15 | 15 | −48.3 ± 14.8% | −40.6 ± 17.4% | +3.2 ± 14.8% | 13.30% | 21.40% | 0% | 0 | 0 | 2 | ||||||
| Ding et al., 2022 [28] | Retrospective | China | Fenofibrate | 59 | Fenofibrate | N/A | UDCA monotherapy | 24 months | Fenofibrate | Placebo | Fenofibrate | UDCA | Not reported | ||||
| 32 | N/A | 27 | 25 | 23 | 10 | 0 | |||||||||||
| Median change from baseline | |||||||||||||||||
| Corpechot et al., 2018 [29] | Phase 3 RCT | France | Bezafibrate | 100 | Bezafibrate (400 mg) | Placebo | 24 months | Bezafibrate | Placebo | Bezafibrate | Placebo | Bezafibrate | Placebo | ||||
| 50 | 50 | −60 (−66–−46) | 0 (−14–20) | 67% | 2% | 8% | 14% | ||||||||||
| Mean change from baseline (SD) | Change from baseline | ||||||||||||||||
| Hirschfield et al., 2021 [30] | Phase 3 RCT | Multinational | Budesonide | 62 | Budesonide | Placebo | 32.9 months | Budesonide | Placebo | Budesonide | Placebo | Budesonide | Placebo | ||||
| 40 | 22 | ç95 (166) | −9 (177) | 35% | 9% | −0.3 (2.3) | 0.6 (3.1) | ||||||||||
| Study | Study Design | Quality Rating | Comments |
|---|---|---|---|
| Ding et al., 2023 [21] | Retrospective | Low | Limited sample size, potential bias in data collection. |
| Jones et al., 2017 [22] | Phase 2 RCT | Low | Early termination of the trial, safety concerns noted. |
| Vuppalanchi et al., 2022 [23] | Phase 2 RCT | Moderate | Small sample size, but clear outcome measures. |
| Li et al., 2022 [24] | Open-label RCT | Moderate | Short duration, but significant findings on ALP. |
| Hirschfield et al., 2023 [25] | Phase 3 RCT | Low | Potential bias due to early termination and safety concerns. |
| Kowdley et al., 2024 [26] | Phase 3 RCT | Moderate | Larger sample size, but variability in patient demographics. |
| Schattenberg et al., 2021 [27] | Phase 3 RCT | Moderate | Short duration, but clear outcome measures. |
| Ding et al., 2022 [28] | Retrospective | Low | Limited sample size, potential bias in data collection. |
| Corpechot et al., 2018 [29] | Phase 3 RCT | Moderate | Larger sample size, but variability in outcome measures. |
| Hirschfield et al., 2021 [30] | Phase 3 RCT | Moderate | Larger sample size, but potential issues with reporting. |
| NCD | Effect Size | Heterogeneity | |||
|---|---|---|---|---|---|
| SMD | 95% CI | p-Value | I2 | p-Value | |
| Elafibranor | −2.02 | (−3.11, −0.92) | 0.0003 | 83% | 0.003 |
| Seladelpar | −4.42 | (−6.24, −2.46) | <0.00001 | 96% | <0.00001 |
| Fenofibrate | −1.04 | (−1.65, −0.44) | 0.0007 | N/A | N/A |
| Bezafibrate | −3.37 | (−4.00, −2.74) | <0.00001 | N/A | N/A |
| Budesonide | −0.50 | (−1.03, 0.03) | 0.06 | N/A | N/A |
| Saroglitazar | −5.01 | (−6.26, 3.26) | <0.00001 | 0% | 0.87 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gadour, E.; Miutescu, B.; Bashir, H.; Ali, A.; Alanzi, S.; Al-Shahrani, A.A.; Almuhaidb, A.; Mohamed, S.; Abaalkhail, F.; Kuriry, H.; et al. Efficacy and Safety of Novel Oral Anti-Cholestatic Agents for Primary Biliary Cholangitis: Meta-Analyses and Systematic Review. Pharmaceuticals 2025, 18, 697. https://doi.org/10.3390/ph18050697
Gadour E, Miutescu B, Bashir H, Ali A, Alanzi S, Al-Shahrani AA, Almuhaidb A, Mohamed S, Abaalkhail F, Kuriry H, et al. Efficacy and Safety of Novel Oral Anti-Cholestatic Agents for Primary Biliary Cholangitis: Meta-Analyses and Systematic Review. Pharmaceuticals. 2025; 18(5):697. https://doi.org/10.3390/ph18050697
Chicago/Turabian StyleGadour, Eyad, Bogdan Miutescu, Hiba Bashir, Abubaker Ali, Salem Alanzi, Abdullah A. Al-Shahrani, Aymen Almuhaidb, Shahed Mohamed, Faisal Abaalkhail, Hadi Kuriry, and et al. 2025. "Efficacy and Safety of Novel Oral Anti-Cholestatic Agents for Primary Biliary Cholangitis: Meta-Analyses and Systematic Review" Pharmaceuticals 18, no. 5: 697. https://doi.org/10.3390/ph18050697
APA StyleGadour, E., Miutescu, B., Bashir, H., Ali, A., Alanzi, S., Al-Shahrani, A. A., Almuhaidb, A., Mohamed, S., Abaalkhail, F., Kuriry, H., & AlQahtani, M. S. (2025). Efficacy and Safety of Novel Oral Anti-Cholestatic Agents for Primary Biliary Cholangitis: Meta-Analyses and Systematic Review. Pharmaceuticals, 18(5), 697. https://doi.org/10.3390/ph18050697







