Benzyl Benzoate Isolation from Acridocarpus smeathmannii (DC.) Guill. & Perr Roots and Its Bioactivity on Human Prostate Smooth Muscle Contractions
Abstract
1. Introduction
2. Results and Discussion
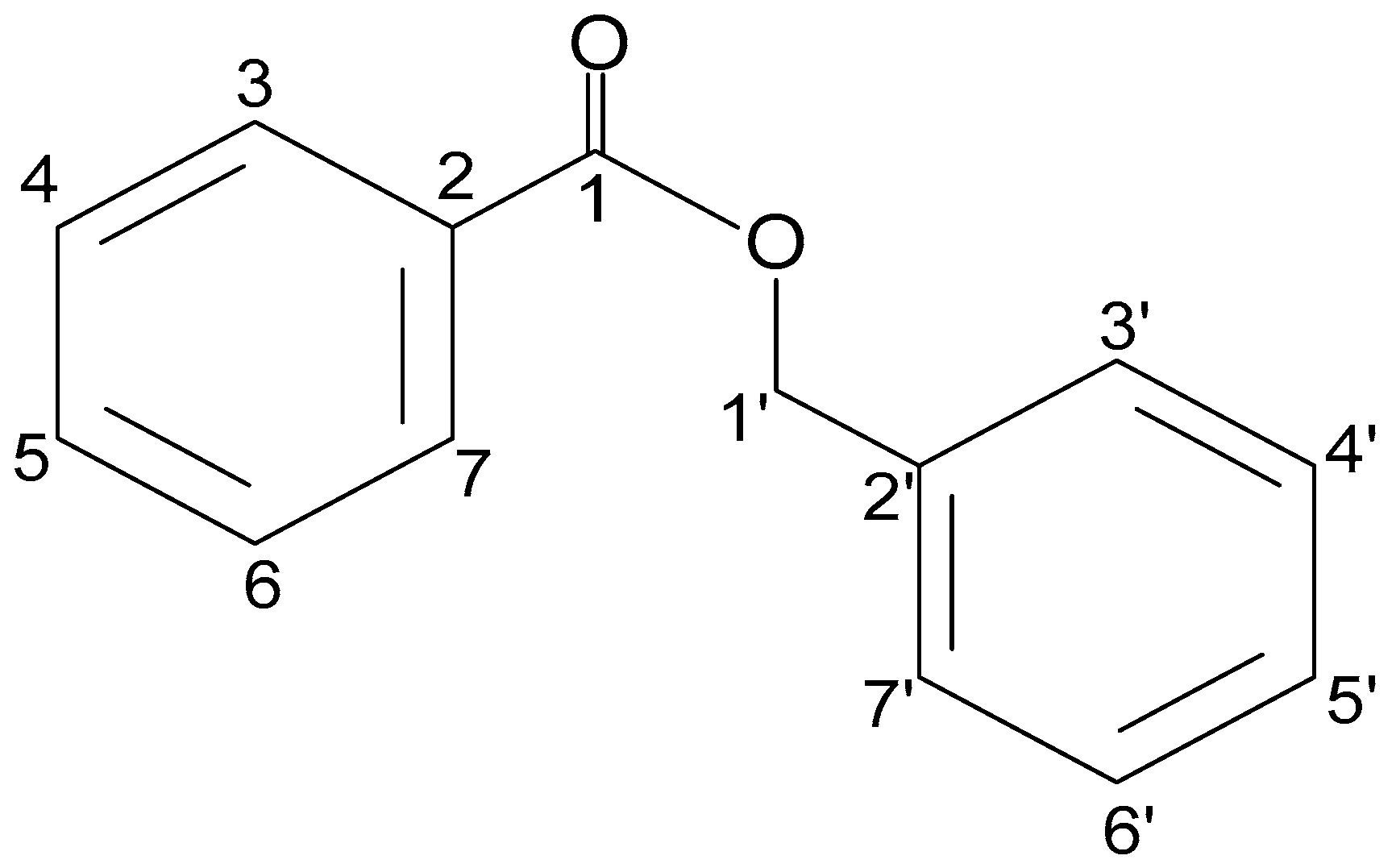
2.1. Structural Analysis of HLASFF12
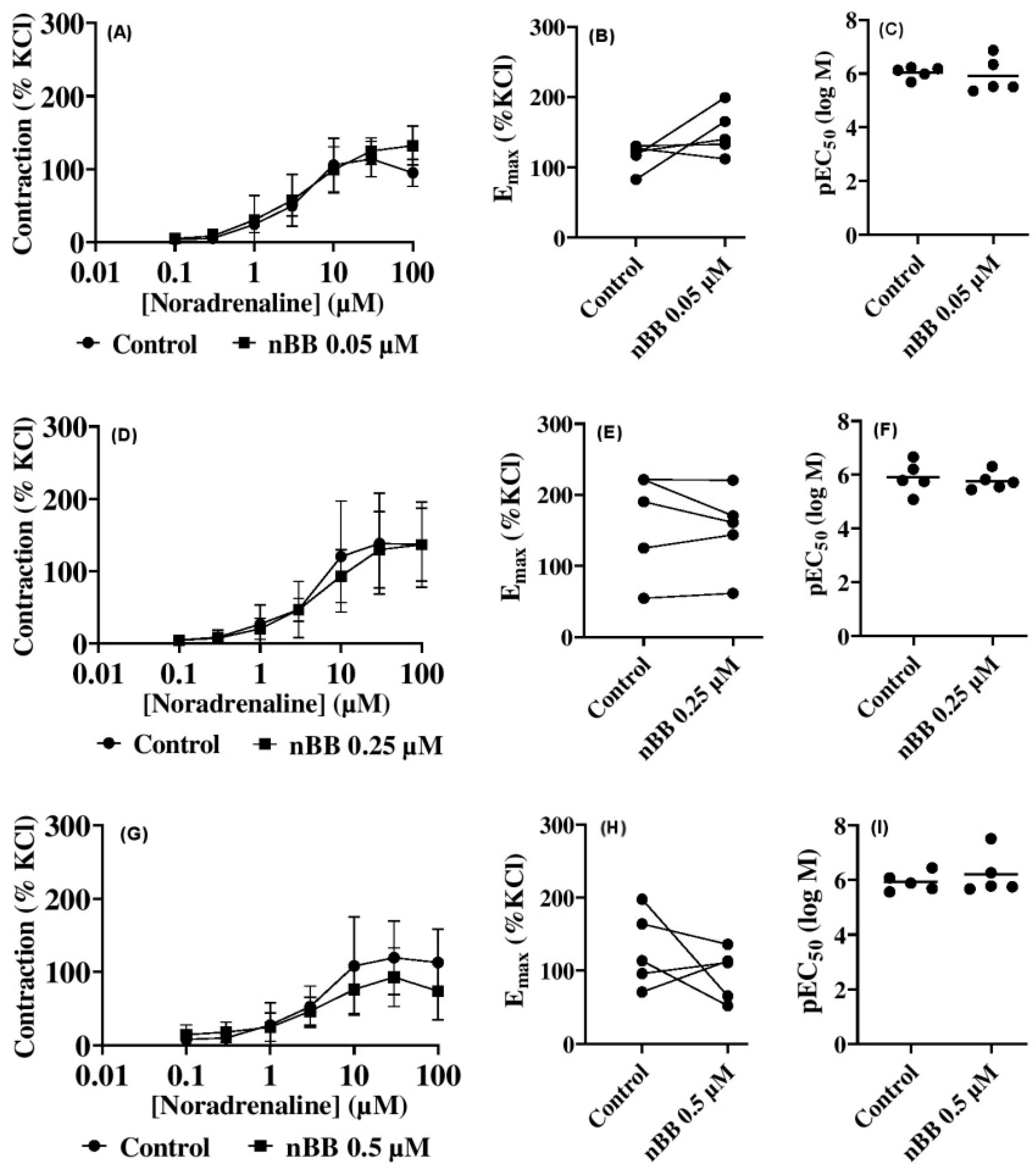
2.2. Effects of HLASFF12 on Noradrenaline-Induced Contractions of Human Prostate Tissues
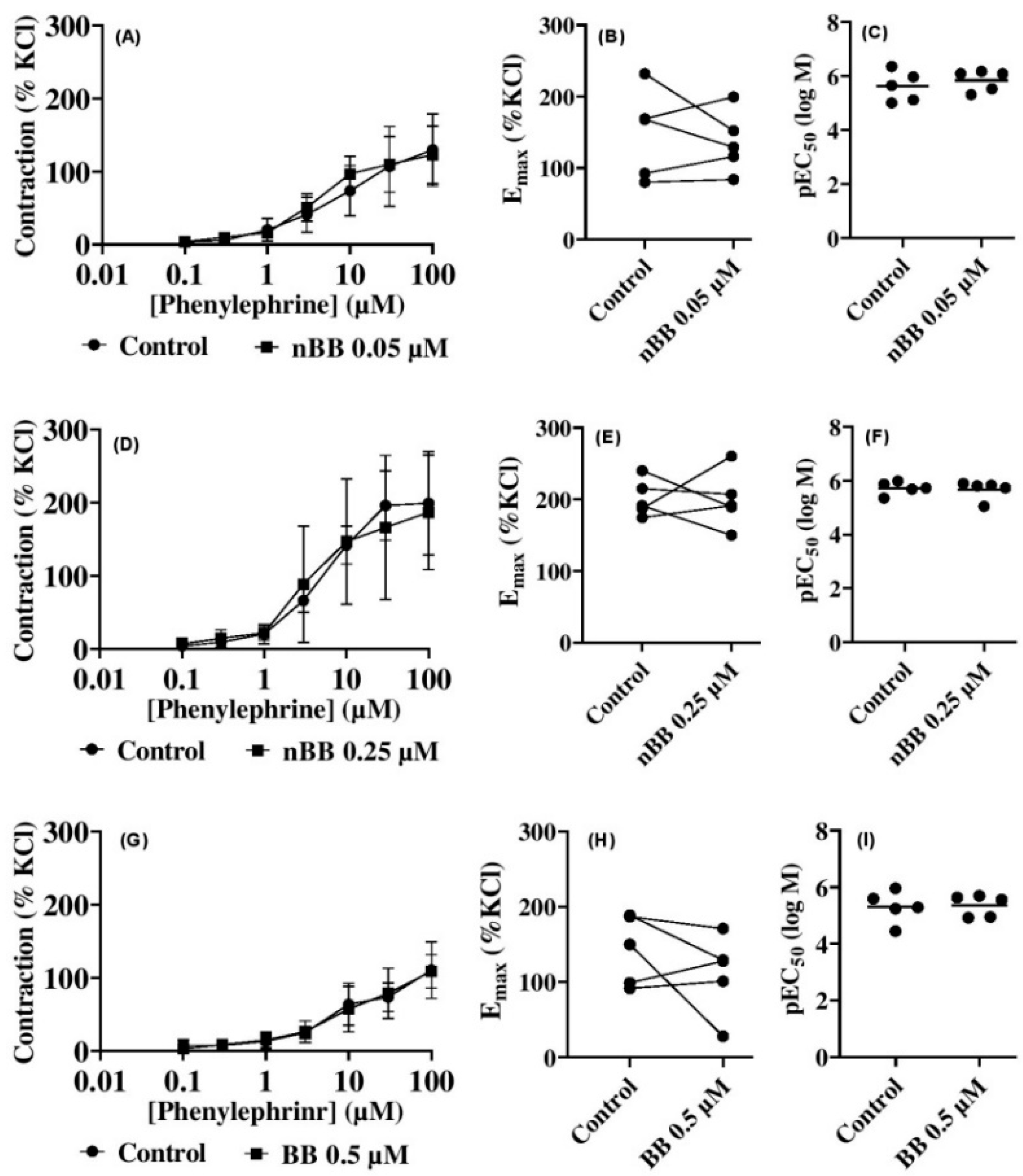
2.3. Effects of HLASFF12 on Phenylephrine-Induced Contractions of Human Prostate Tissues
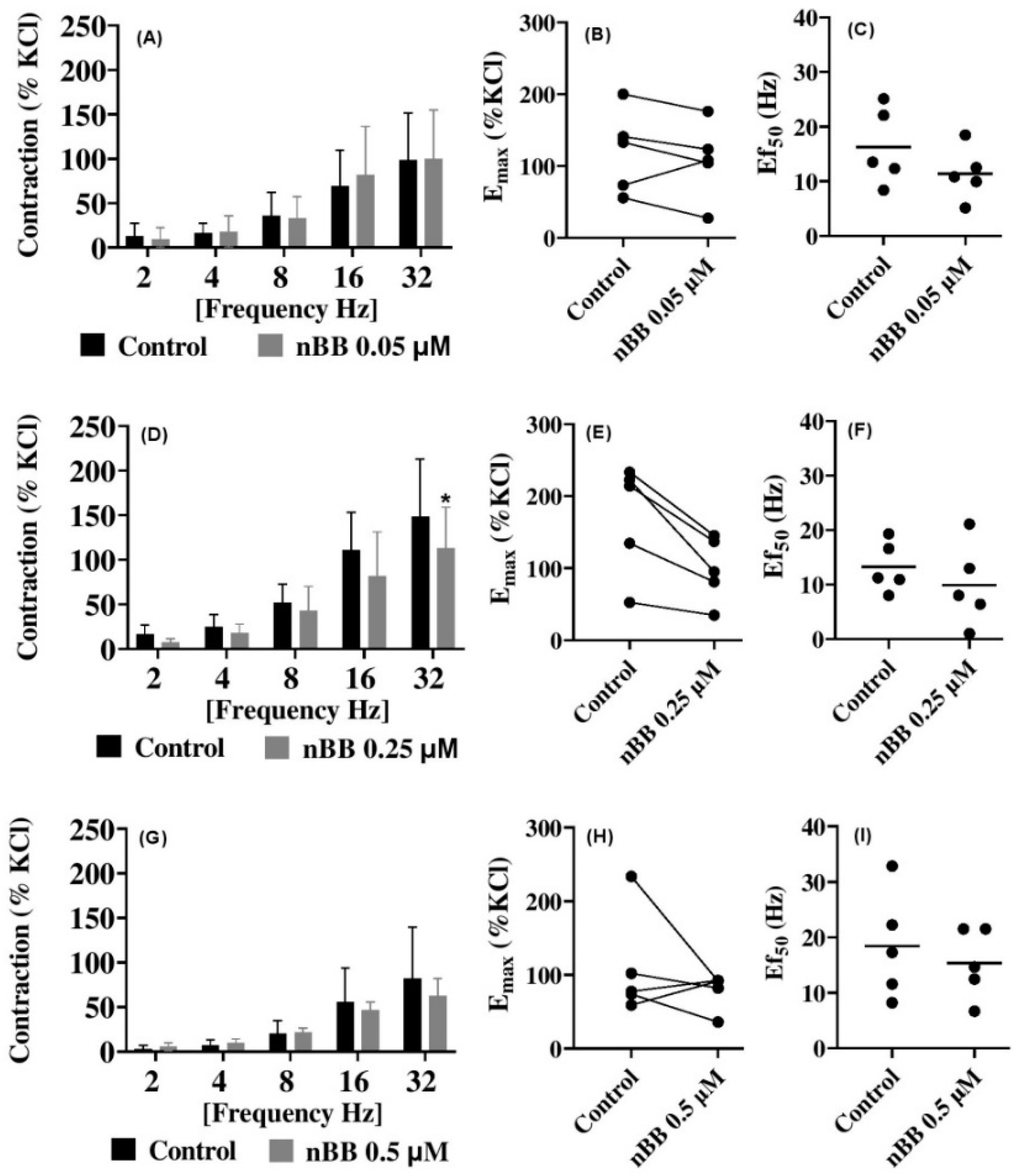
2.4. Effects of HLASFF12 on Electric Field Stimulation-Induced Contraction of Human Prostate Tissues
3. Materials and Methods
3.1. Drugs and Chemicals
3.2. Plant Collection, Authentication and Extraction
3.3. Chromatography Studies
3.4. Gas Chromatography–Mass Spectrometry Analysis
3.5. Nuclear Magnetic Resonance Analysis
3.6. High-Performance Column Chromatography (HPLC)
3.7. Biological Actions of HLASFF12 on Human Prostate Tissue Contraction
3.8. Effects of HLAFF12 on Prostate Smooth Muscle Contractile Activity
3.9. Electrical Field Stimulation
3.10. Data and Statistical Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A. smeathmannii (Acridocarpus smeathmannii) | (AS) |
| Benign prostatic hyperplasia | (BPH) |
| Benzyl benzoate | (BB) |
| Concentrations inducing 50% of maximum agonist-induced contraction | (EC50) |
| Electrical field stimulation | (EFS) |
| Frequencies (f) inducing 50% of maximum EFS-induced contraction | (Ef50) |
| Gas chromatography–mass spectrometry | (GC-MS) |
| High-performance liquid chromatography | (HPLC) |
| Lower layer of A. smeathmannii | (HLASF) |
| Lower layer of A. smeathmannii fraction | (HLASFF12) (nBB) |
| Lower urinary tract symptoms | (LUTSs) |
| Maximum possible contractions | (Emax) |
| Negative logarithms of the molar concentration for agonists | (pEC50) |
| Noradrenaline | (NA) |
| Nuclear magnetic resonance | (NMR) |
| Phenylephrine | (PHE) |
| Radical prostatectomy | (rPx) |
| Retention factor | (Rf) |
| Thin layer chromatography | (TLC) |
| Homonuclear correlation spectroscopy | (COSY) |
| Nuclear Overhauser Effect Spectroscopy | (NOESY) |
| Heteronuclear single quantum coherence | (HSQC) |
| Distortionless Enhancement by Polarization Transfer | (DEPT) |
| Heteronuclear Multiple Bond Correlation | (HMBC) |
References
- Souto, L.S.; Oliveira, D.M.T. Seed development in Malpighiaceae species with an emphasis on the relationships between nutritive tissues. Comptes Rendus. Biol. 2014, 337, 62–70. [Google Scholar] [CrossRef]
- Da Costa Santos, J.V.; Guesdon, I.R.; Amorim, A.M.A.; Meira, R.M.A.S. Does leaf morphoanatomy corroborate systematics and biogeographic events in the Paleotropical genus Acridocarpus (Malpighiaceae)? S. Afr. J. Bot. 2023, 163, 262–274. [Google Scholar] [CrossRef]
- De Almeida, R.F.; de Morais, I.L.; Alves-Silva, T.; Antonio-Domingues, H.; Pellegrini, M.O. A new classification system and taxonomic synopsis for Malpighiaceae (Malpighiales, Rosids) based on molecular phylogenetics, morphology, palynology, and chemistry. PhytoKeys 2024, 242, 69. [Google Scholar] [CrossRef]
- Catarino, L.; Havik, P.J.; Romeiras, M.M. Medicinal plants of Guinea-Bissau: Therapeutic applications, ethnic diversity and knowledge transfer. J. Ethnopharmacol. 2016, 183, 71–94. [Google Scholar] [CrossRef]
- Kale, O.E.; Awodele, O.; Akindele, A.J. Acridocarpus smeathmannii (DC.) Guill. & Perr. Root enhanced reproductive behavior and sexual function in male wistar rats: Biochemical and pharmacological mechanisms. J. Ethnopharmacol. 2019, 230, 95–108. [Google Scholar] [PubMed]
- Kale, O.E.; Awodele, O.; Akindele, A.J. Subacute and subchronic oral toxicity assessments of Acridocarpus smeathmannii (DC.) Guill. & Perr. root in Wistar rats. Toxicol. Rep. 2019, 6, 161–175. [Google Scholar]
- Chen, J.; Oi, D.H. Naturally occurring compounds/materials as alternatives to synthetic chemical insecticides for use in fire ant management. Insects 2020, 11, 758. [Google Scholar] [CrossRef]
- De Meneses, A.C.; Balen, M.; de Andrade Jasper, E.; Korte, I.; de Araújo, P.H.H.; Sayer, C.; de Oliveira, D. Enzymatic synthesis of benzyl benzoate using different acyl donors: Comparison of solvent-free reaction techniques. Process Biochem. 2020, 92, 261–268. [Google Scholar] [CrossRef]
- Soszka, N.; Hachuła, B.; Tarnacka, M.; Kamińska, E.; Grelska, J.; Jurkiewicz, K.; Geppert-Rybczyńska, M.; Wrzalik, R.; Grzybowska, K.; Pawlus, S.; et al. The impact of the length of alkyl chain on the behavior of benzyl alcohol homologues–the interplay between dispersive and hydrogen bond interactions. Phys. Chem. Chem. Phys. 2021, 23, 23796–23807. [Google Scholar] [CrossRef]
- Rivero-Cruz, B.; Rivero-Cruz, I.; Rodríguez-Sotres, R.; Mata, R. Effect of natural and synthetic benzyl benzoates on calmodulin. Phytochemistry 2007, 68, 1147–1155. [Google Scholar] [CrossRef]
- Orra, S.; Boyajian, M.K.; Bryant, J.R.; Talbet, J.H.; Mulliken, J.B.; Rogers, G.F.; Oh, A.K. Balsam of Peru: History and utility in plastic surgery. J. Cranio-fac. Surg. 2021, 32, 1209–1210. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Moreno-Toral, E. Pharmacy and fragrances: Traditional and current use of plants and their extracts. Cosmetics 2023, 10, 157. [Google Scholar] [CrossRef]
- Hayes, B.W.; Choi, H.W.; Rathore, A.P.; Bao, C.; Shi, J.; Huh, Y.; Kim, M.W.; Mencarelli, A.; Bist, P.; Ng, L.G.; et al. Recurrent infections drive persistent bladder dysfunction and pain via sensory nerve sprouting and mast cell activity. Sci. Immunol. 2024, 9, eadi5578. [Google Scholar] [CrossRef]
- Anuchatkidjaroen, S.; Phaechamud, T. Virgin coconut oil containing injectable vehicles for ibuprofen sustainable release. Key Eng. Mater. 2013, 545, 52–56. [Google Scholar] [CrossRef]
- Kazi, A.A.; Reddy, B.S.; Singh, L.R. Synthetic approaches to FDA approved drugs for asthma and COPD from 1969 to 2020. Bioorg. Med. Chem. 2021, 41, 116212. [Google Scholar] [CrossRef]
- Bezabh, S.A.; Tesfaye, W.; Christenson, J.K.; Carson, C.F.; Thomas, J. Antiparasitic activity of tea tree oil (TTO) and its components against medically important ectoparasites: A systematic review. Pharmaceutics 2022, 14, 1587. [Google Scholar] [CrossRef]
- Al-Akeel, R.A.; El-Kersh, T.A.; Al-Sheikh, Y.A.; Al-Ahmadey, Z.Z. Heparin-benzyl alcohol enhancement of biofilms formation and antifungal susceptibility of vaginal Candida species isolated from pregnant and nonpregnant Saudi women. Bioinformation 2013, 9, 357. [Google Scholar] [CrossRef]
- Vashishth, R.; Chuong, M.C.; Duarte, J.C.; Gharat, Y.; Kerr, S.G. Two Sustained Release Membranes Used in Formulating Low Strength Testosterone Reservoir Transdermal Patches. Curr. Drug Deliv. 2024, 21, 438–450. [Google Scholar] [CrossRef]
- Pang, D.S. Anesthetic and analgesic adjunctive drugs. In Veterinary Anesthesia and Analgesia: The Sixth Edition of Lumb and Jones; Lamont, L., Grimm, K., Robertson, S., Love, L., Schroeder, C., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2024; pp. 420–447. [Google Scholar]
- Kheir, G.B.; Verbakel, I.; Wyndaele, M.; Monaghan, T.F.; Sinha, S.; Larsen, T.H.; Van Laecke, E.; Birder, L.; Hervé, F.; Everaert, K. Lifelong LUTS: Understanding the bladder’s role and implications across transition phases, a comprehensive review. Neurourol. Urodyn. 2024, 43, 1066–1074. [Google Scholar] [CrossRef]
- Hennenberg, M.; Hu, S.; Tamalunas, A.; Stief, C.G. Genetic Predisposition to Benign Prostatic Hyperplasia: Where Do We Stand? Eur. Urol. Open Sci. 2024, 70, 154–157. [Google Scholar] [CrossRef]
- Zhang, W.; Ding, Z.; Peng, Y.; Wang, H.; Sun, Y.; Ke, H.; Su, D.; Wang, Q.; Xu, K. LUTS/BPH increases the risk of depressive symptoms among elderly adults: A 5-year longitudinal evidence from CHARLS. J. Affect. Disord. 2024, 367, 210–218. [Google Scholar] [CrossRef]
- Gravas, S.; Gacci, M.; Gratzke, C.; Herrmann, T.R.; Karavitakis, M.; Kyriazis, I.; Malde, S.; Mamoulakis, C.; Rieken, M.; Sakalis, V.I.; et al. Summary paper on the 2023 European Association of Urology guidelines on the management of non-neurogenic male lower urinary tract symptoms. Eur. Urol. 2023, 84, 207–222. [Google Scholar] [CrossRef]
- Katsimperis, S.; Kapriniotis, K.; Manolitsis, I.; Bellos, T.; Angelopoulos, P.; Juliebø-Jones, P.; Somani, B.; Skolarikos, A.; Tzelves, L. Early investigational agents for the treatment of benign prostatic hyperplasia. Expert Opin. Investig. Drugs 2024, 33, 359–370. [Google Scholar] [CrossRef]
- Arya, G.C.; Rathee, A.; Mehla, S.; Bisht, P.; Sharma, R. A Review of the Azasteroid-type 5-alpha Reductase Inhibitors for the Management of Benign Prostatic Hyperplasia. Lett. Drug Des. Discov. 2024, 21, 2271–2287. [Google Scholar] [CrossRef]
- Hennenberg, M.; Acevedo, A.; Wiemer, N.; Kan, A.; Tamalunas, A.; Wang, Y.; Yu, Q.; Rutz, B.; Ciotkowska, A.; Herlemann, A.; et al. Non-adrenergic, tamsulosin-insensitive smooth muscle contraction is sufficient to replace α1-adrenergic tension in the human prostate. Prostate 2017, 77, 697–707. [Google Scholar] [CrossRef]
- Michel, M.C.; Cardozo, L.; Chermansky, C.J.; Cruz, F.; Igawa, Y.; Lee, K.S.; Sahai, A.; Wein, A.J.; Andersson, K.E. Current and emerging pharmacological targets and treatments of urinary incontinence and related disorders. Pharmacol. Rev. 2023, 75, 554–674. [Google Scholar]
- Leisegang, K.; Jimenez, M.; Durairajanayagam, D.; Finelli, R.; Majzoub, A.; Henkel, R.; Agarwal, A. A systematic review of herbal medicine in the clinical treatment of benign prostatic hyperplasia. Phytomed. Plus 2022, 2, 100153. [Google Scholar] [CrossRef]
- Davis, C.C.; Choisy, P. Medicinal plants meet modern biodiversity science. Curr. Biol. 2024, 34, R158–R173. [Google Scholar] [CrossRef]
- Chihomvu, P.; Ganesan, A.; Gibbons, S.; Woollard, K.; Hayes, M.A. Phytochemicals in Drug Discovery—A Confluence of Tradition and Innovation. Int. J. Mol. Sci. 2024, 25, 8792. [Google Scholar] [CrossRef]
- Sharma, C.M.; Al Kaabi, J.M.; Nurulain, S.N.; Goyal, S.; Amjad Kamal, M.; Ojha, S. Polypharmacological properties and therapeutic potential of β-caryophyllene: A dietary phytocannabinoid of pharmaceutical promise. Curr. Pharm. Des. 2016, 22, 3237–3264. [Google Scholar] [CrossRef]
- Singh, M.K.; Savita, K.; Singh, S.; Mishra, D.; Rani, P.; Chanda, D.; Verma, R.S. Vasorelaxant property of 2-phenyl ethyl alcohol isolated from the spent floral distillate of damask rose (Rosa damascena Mill.) and its possible mechanism. J. Ethnopharmacol. 2023, 313, 116603. [Google Scholar] [CrossRef]
- Kodpinid, M.; Sadavongvivad, C.; Thebtaranonth, C.; Thebtaranonth, Y. Benzyl benzoates from the root of Uvaria purpurea. Phytochemistry 1984, 23, 199–200. [Google Scholar] [CrossRef]
- Ohno, O.; Ye, M.; Koyama, T.; Yazawa, K.; Mura, E.; Matsumoto, H.; Ichino, T.; Yamada, K.; Nakamura, K.; Ohno, T.; et al. Inhibitory effects of benzyl benzoate and its derivatives on angiotensin II-induced hypertension. Bioorg. Med. Chem. 2008, 16, 7843–7852. [Google Scholar] [CrossRef]
- Johnson, W.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.C.; Marks, J.G., Jr.; Shank, R.C.; Slaga, T.J.; Snyder, P.W.; et al. Safety assessment of benzyl alcohol, benzoic acid and its salts, and benzyl benzoate. Int. J. Toxicol. 2017, 36 (Suppl. S3), 5S–30S. [Google Scholar] [CrossRef]
- Diastuti, H.; Chasani, M.; Suwandri, S. Antibacterial activity of benzyl benzoate and crotepoxide from Kaempferia rotunda L. Rhizome. Indones. J. Chem. 2020, 20, 9–15. [Google Scholar] [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Emodin-A natural anthraquinone derivative with diverse pharmacological activities. Phytochemistry 2021, 190, 112854. [Google Scholar] [CrossRef]
- Nair, B. Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int. J. Toxicol. 2001, 20, 23–50. [Google Scholar]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef]
- Yin, W.; Kim, H.T.; Wang, S.; Gunawan, F.; Wang, L.; Kishimoto, K.; Zhong, H.; Roman, D.; Preussner, J.; Guenther, S.; et al. The potassium channel KCNJ13 is essential for smooth muscle cytoskeletal organization during mouse tracheal tubulogenesis. Nat. Commun. 2018, 9, 2815. [Google Scholar] [CrossRef]
- Yuan, J.H.; Goehl, T.J.; Abdo, K.; Clark, J.; Espinosa, O.; Bugge, C.; Garcia, D. Effects of gavage versus dosed feed administration on the toxicokinetics of benzyl acetate in rats and mice. Food Chem. Toxicol. 1995, 33, 151–158. [Google Scholar] [CrossRef]
- Zu, K.; Pizzurro, D.M.; Lewandowski, T.A.; Goodman, J.E. Pharmacokinetic data reduce uncertainty in the acceptable daily intake for benzoic acid and its salts. Regul. Toxicol. Pharmacol. 2017, 89, 83–94. [Google Scholar] [CrossRef]
- Amundsen, C.L.; Helmuth, M.E.; Smith, A.R.; DeLancey, J.O.; Bradley, C.S.; Flynn, K.E.; Kenton, K.S.; Lai, H.; Cella, D.; Griffith, J.W.; et al. Longitudinal changes in symptom-based female and male LUTS clusters. Neurourol. Urodyn. 2020, 39, 393–402. [Google Scholar] [CrossRef]
- Cavanaugh, D.; Urbanucci, A.; Mohamed, N.E.; Tewari, A.K.; Figueiro, M.; Kyprianou, N. Link between circadian rhythm and benign prostatic hyperplasia (BPH)/lower urinary tract symptoms (LUTS). Prostate 2024, 84, 417–425. [Google Scholar] [CrossRef]
- Adler, D.; Lindstrot, A.; Ellinger, J.; Rogenhofer, S.; Buettner, R.; Perner, S.; Wernert, N. The peripheral zone of the prostate is more prone to tumor development than the transitional zone: Is the ETS family the key? Mol. Med. Rep. 2012, 5, 313–316. [Google Scholar]
| Agonist Concentrations | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.1 μM | 0.3 μM | 1 μM | 3 μM | 10 μM | 30 μM | 100 μM | |||
| Prostate | nBB 0.05 | Noradrenaline | −1.3 [−39.3 to 37.6] | −3.3 [ −41.3 to 34.7] | −6.2 [ −44.2 to 31.8] | −7.8 [ −45.8 to 30.2] | 6.8 [ −31.2 to 44.8] | −11.1 [ −49.1 to 26.9] | −37.2 [ −75.2 to 0.77] |
| nBB 0.25 | 0.4 [ −35.8 to 36.4] | 1.1 [ −35.0 to 37.2] | 6.5 [ −29.6 to 42.6] | 0.5 [ −35.6 to 36.6] | 27.1 [ −9.0 to 63.2] | 8.5 [ −27.6 to 44.6] | 0.1 [ −36.0 to 36.2] | ||
| nBB 0.5 | −6.6 [ −75.1 to 61.8] | −8.1 [ −76.6 to 60.3] | 2.9 [ −65.6 to 71.3] | 6.2 [ −62.2 to 74.6] | 32.1 [ −36.3 to 100.5] | 26.4 [ −42.0 to 94.8] | 39.0 [ −29.4 to 107.5] | ||
| 0.1 Μm | 0.3 μM | 1 μM | 3 μM | 10 μM | 30 μM | 100 μM | |||
| Prostate | nBB 0.05 | Phenylephrine | −0.3 [ −50.6 to 50.1] | −4.2 [ −54.6 to 46.2] | 3.7 [ −46.7 to 54.1] | −9.2 [ −60.1 to 40.7] | −23.1 [ −73.5 to 27.3] | −2.7 [ −53.1 to 47.7] | 6.7 [ −43.7 to 57.1] |
| nBB 0.25 | −2.8 [ −86.3 to 80.8] | −6.4 [ −89.0 to 78.1] | −1.4 [ −85.0 to 82.1] | −22.6 [ −106.2 to 60.9] | −5.0 [ −88.5 to 78.6] | 30.0 [ −53.6 to 113.5] | 12.3 [ −71.2 to 95.9] | ||
| nBB 0.5 | 4.5 [ −24.0 to 33.0] | −0.9 [ −29.5 to 27.6] | −1.7 [ −30.3 to 26.8] | −1.6 [ −30.1 to 27.0] | −6.5 [ −22.0 to 35.0] | −5.2 [ −33.7 to 23.3] | 1.8 [ −26.8 to 30.3] | ||
| Neurogenic Stimulations | |||||||
|---|---|---|---|---|---|---|---|
| Prostate | Freq. | 2 Hz | 4 Hz | 8 Hz | 16 Hz | 32 Hz | |
| nBB 0.05 | EFS | 3.7 [ −17.8 to 25.1] | −1.4 [ −22.9 to 20.1] | 2.9 [ −18.6 to 24.4] | −12.2 [ −33.7 to 9.3] | −1.1 [ −22.6 to 20.4] | |
| nBB 0.25 | 10.5 [ −15.6 to 36.6] | 6.8 [ −19.3 to 32.9] | 12.7 [ −13.4 to 38.8] | 23.7 [ −2.35 to 49.8] | 41.3 [ 15.2 to 67.4] | ||
| nBB 0.5 | −2.6 [ −48.1 to 42.9] | −2.8 [ −48.3 to 42.7] | −1.5 [ −46.9 to 44.0] | 9.0 [ −36.5 to 54.5] | 19.5 [ −25.9 to 65.0] | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kale, O.E.; Rauanov, I.; Huber, C.; Tamalunas, A.; Stief, C.G.; Eisenreich, W.; Hennenberg, M. Benzyl Benzoate Isolation from Acridocarpus smeathmannii (DC.) Guill. & Perr Roots and Its Bioactivity on Human Prostate Smooth Muscle Contractions. Pharmaceuticals 2025, 18, 687. https://doi.org/10.3390/ph18050687
Kale OE, Rauanov I, Huber C, Tamalunas A, Stief CG, Eisenreich W, Hennenberg M. Benzyl Benzoate Isolation from Acridocarpus smeathmannii (DC.) Guill. & Perr Roots and Its Bioactivity on Human Prostate Smooth Muscle Contractions. Pharmaceuticals. 2025; 18(5):687. https://doi.org/10.3390/ph18050687
Chicago/Turabian StyleKale, Oluwafemi Ezekiel, Iskander Rauanov, Claudia Huber, Alexander Tamalunas, Christian G. Stief, Wolfgang Eisenreich, and Martin Hennenberg. 2025. "Benzyl Benzoate Isolation from Acridocarpus smeathmannii (DC.) Guill. & Perr Roots and Its Bioactivity on Human Prostate Smooth Muscle Contractions" Pharmaceuticals 18, no. 5: 687. https://doi.org/10.3390/ph18050687
APA StyleKale, O. E., Rauanov, I., Huber, C., Tamalunas, A., Stief, C. G., Eisenreich, W., & Hennenberg, M. (2025). Benzyl Benzoate Isolation from Acridocarpus smeathmannii (DC.) Guill. & Perr Roots and Its Bioactivity on Human Prostate Smooth Muscle Contractions. Pharmaceuticals, 18(5), 687. https://doi.org/10.3390/ph18050687









