Pharmacological and Non-Pharmacological Interventions for Polycystic Ovary Syndrome (PCOS) in Indian Women: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
1.1. Polycystic Ovarian Syndrome (PCOS)
1.2. Current Treatment Options
1.3. Rationale for Systematic Review
2. Objectives of Review
- To determine different pharmacological interventions available and tested for the management of different manifestations of PCOS among Indian women;
- To determine different non-pharmacological intervention approaches tested for the management of different manifestations of PCOS among Indian women;
- To identify knowledge gaps in PCOS management in India.
3. Methods
3.1. Study Design and Registration
3.2. Data Sources and Search Strategy
3.3. Inclusion/Exclusion Criteria for Studies
3.4. Study Selection and Screening
3.5. Data Extraction
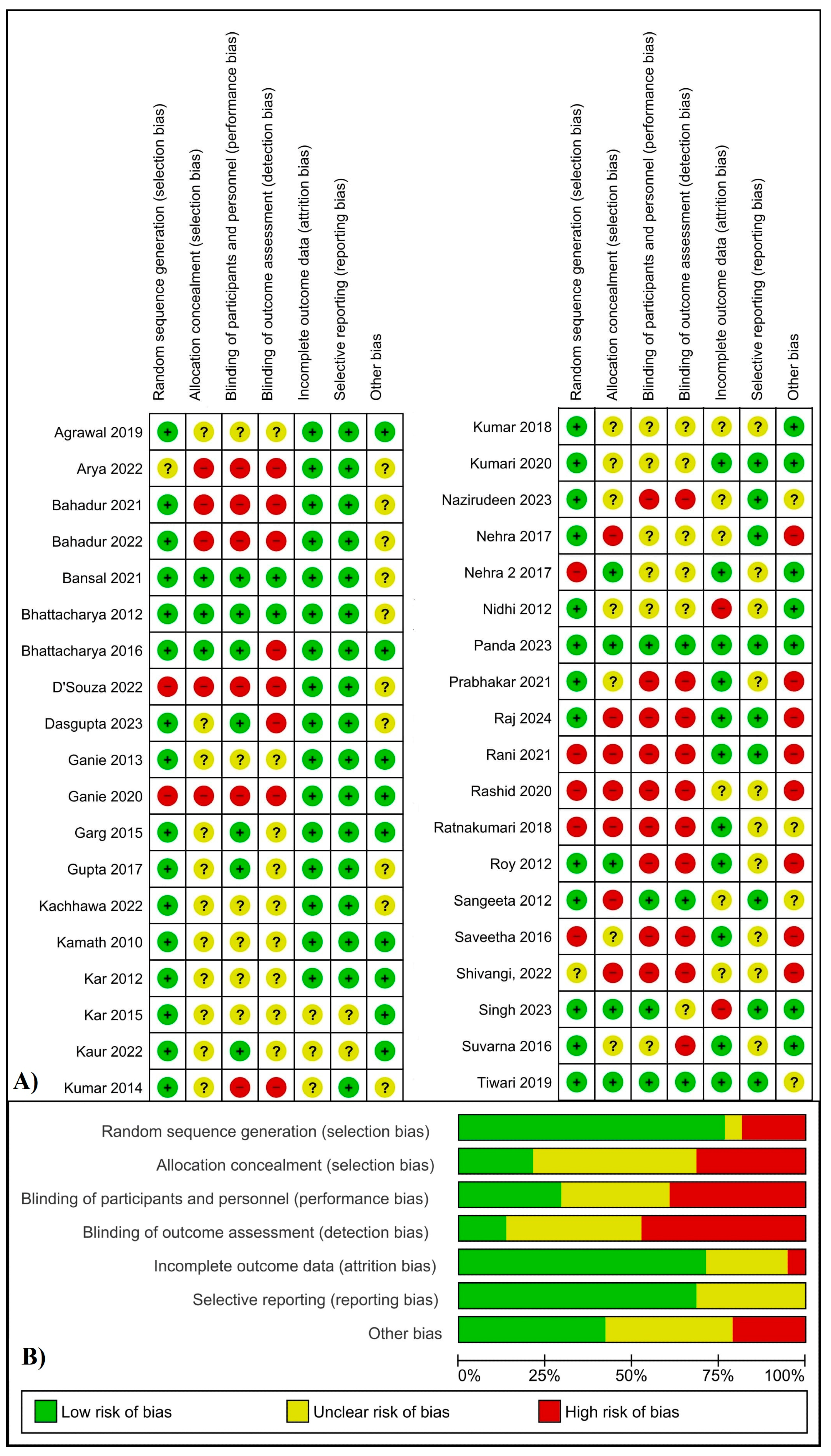
3.6. Assessment of Risk of Bias
3.7. Data Synthesis and Meta-Analysis
4. Results
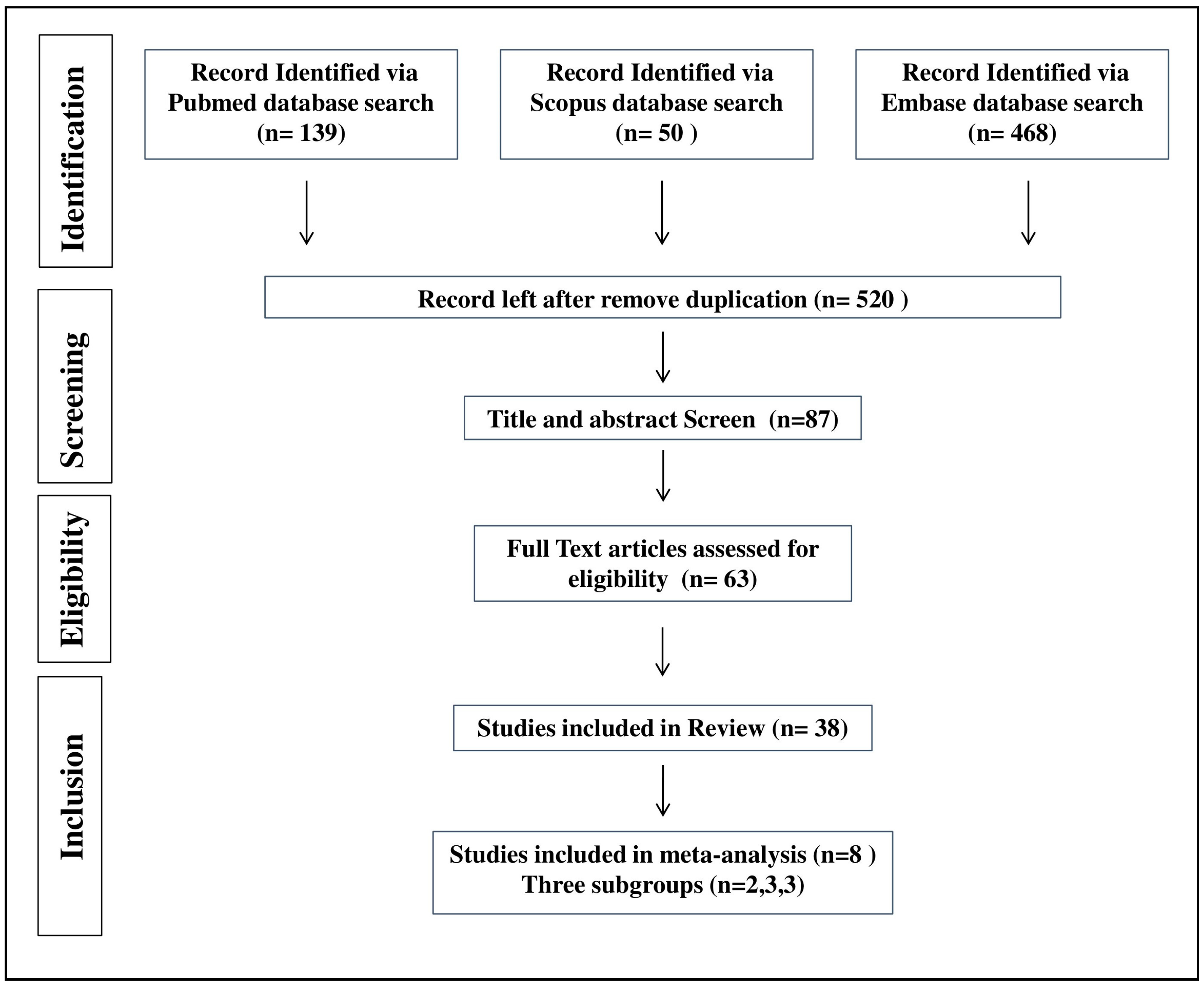
4.1. Study Selection
4.2. Description of Included Studies
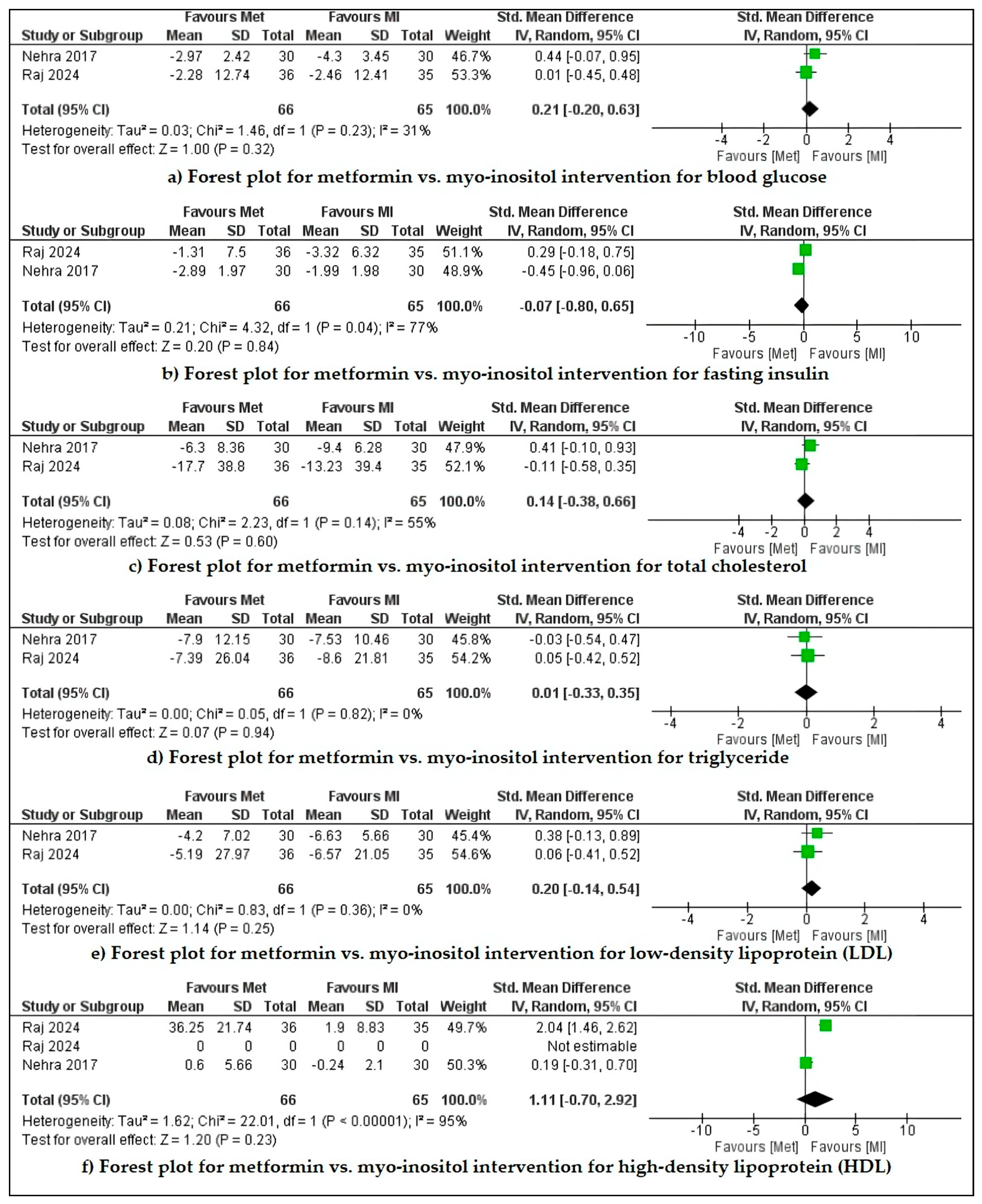
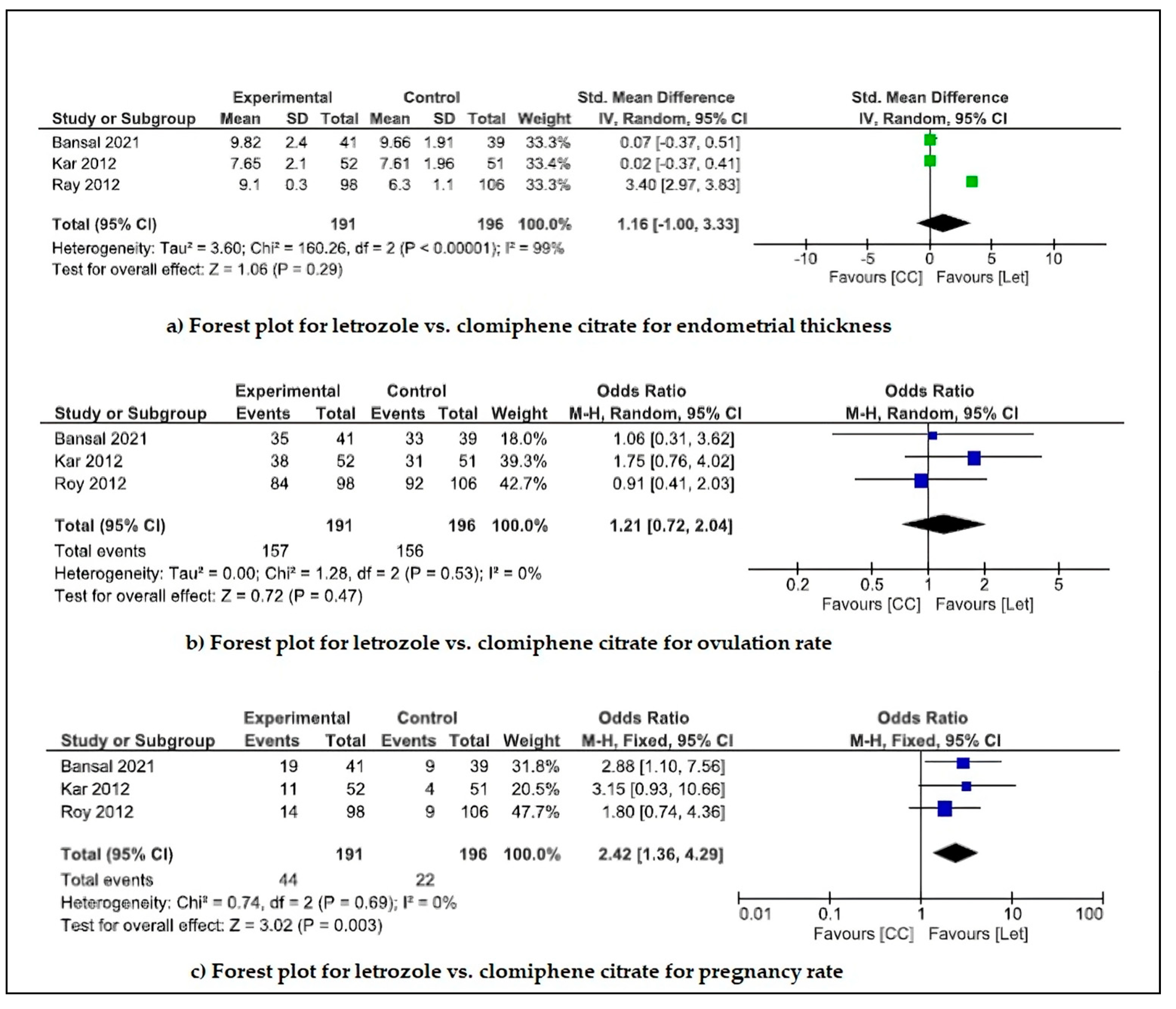
4.3. Outcomes
- (a)
- Metformin vs. inositol
- (b)
- Metformin vs. combination of metformin and inositol
- (c)
- Ovulation induction drugs
4.4. Risk of Bias
5. Discussion
5.1. Pharmacological Treatment
- (a)
- Insulin sensitizers and anti-androgen drugs
- (b)
- Anti-obesity drugs
- (c)
- Oral contraceptive pills (OCPs)
- (d)
- Ovulation induction drugs
5.2. Non-Pharmacological Treatment
- (a)
- Lifestyle modification (physical exercise and yoga)
- (b)
- Supplements such as vitamin D (Vit D)
- (c)
- Probiotics and herbal treatment
6. Limitations in the Current Landscape of Research for PCOS Management in India
- ➢
- Heterogeneity in study designs and interventions: The included studies employed a wide range of interventions targeting different outcomes, with significant variability in participant characteristics, drug dosages, and treatment durations. This high heterogeneity complicates result interpretation and limits direct comparisons;
- ➢
- Small sample sizes: Most of the studies had small sample sizes, with only two studies enrolling 100 participants per group. This restricts the statistical power and robustness of the meta-analysis findings;
- ➢
- Lack of multi-center trials: Most studies are single-center trials, which reduce the generalizability of the results to the broader population;
- ➢
- High risk of bias: Maximum studies exhibited a high risk of bias, affecting the external validity of their results;
- ➢
- Short-term follow-up: All the included studies assessed outcomes over only 3–12 months. Given that PCOS is a chronic condition, long-term studies are crucial to evaluate the sustainability of treatments and their impact on the overall health of women.
7. Future Perspective
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Li, R.; Zhang, Q.; Yang, D.; Li, S.; Lu, S.; Wu, X.; Wei, Z.; Song, X.; Wang, X.; Fu, S.; et al. Prevalence of polycystic ovary syndrome in women in China: A large community-based study. Hum. Reprod. 2013, 28, 2562–2569. [Google Scholar] [CrossRef] [PubMed]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Bharali, M.D.; Rajendran, R.; Goswami, J.; Singal, K.; Rajendran, V. Prevalence of Polycystic Ovarian Syndrome in India: A Systematic Review and Meta-Analysis. Cureus 2022, 14, e32351. [Google Scholar] [CrossRef] [PubMed]
- Dumesic, D.A.; Oberfield, S.E.; Stener-Victorin, E.; Marshall, J.C.; Laven, J.S.; Legro, R.S. Scientific Statement on the Diagnostic Criteria, Epidemiology, Pathophysiology, and Molecular Genetics of Polycystic Ovary Syndrome. Endocr. Rev. 2015, 36, 487–525. [Google Scholar] [CrossRef]
- Rempert, A.N.; Sarria, I.; Standeven, L.R.; Nylander, E.; Segars, J.; Singh, B. A Systematic Review of the Psychosocial Impact of Polycystic Ovarian Syndrome Before and After Treatment. Reprod. Sci. 2023, 30, 3153–3178. [Google Scholar] [CrossRef] [PubMed]
- Simon, V.; Peigné, M.; Dewailly, D. The Psychosocial Impact of Polycystic Ovary Syndrome. Reprod. Med. 2023, 4, 57–64. [Google Scholar] [CrossRef]
- Gautam, R.; Prambil, A.M.; Patel, A.K.; Arora, T. Emerging pollutants in etiology and pathophysiology of polycystic ovary syndrome. Reprod. Toxicol. 2024, 123, 108515. [Google Scholar] [CrossRef]
- Christ, J.P.; Cedars, M.I. Current Guidelines for Diagnosing PCOS. Diagnostics 2023, 13, 1113. [Google Scholar] [CrossRef]
- Fauser, B.C.J.M. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil. Steril. 2004, 81, 19–25. [Google Scholar] [CrossRef]
- Kiran, Z. Diagnostic criteria for polycystic ovary syndrome. In Polycystic Ovary Syndrome: Basic Science to Clinical Advances Across the Lifespan; Blackwell Scientific: Boston, MA, USA, 2023; pp. 61–74. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Dewailly, D.; Diamanti-Kandarakis, E.; Escobar-Morreale, H.F.; Futterweit, W.; Janssen, O.E.; Legro, R.S.; Norman, R.J.; Taylor, A.E.; et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil. Steril. 2009, 91, 456–488. [Google Scholar] [CrossRef]
- Teede, H.J.; Misso, M.L.; Costello, M.F.; Dokras, A.; Laven, J.; Moran, L.; Piltonen, T.; Norman, R.J.; Andersen, M.; Azziz, R.; et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertil. Steril. 2018, 110, 364–379. [Google Scholar] [CrossRef]
- Rocha, A.L.; Oliveira, F.R.; Azevedo, R.C.; Silva, V.A.; Peres, T.M.; Candido, A.L.; Gomes, K.B.; Reis, F.M. Recent advances in the understanding and management of polycystic ovary syndrome. F1000Research 2019, 8, 565. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. ACOG practice bulletin No. 108: Polycystic ovary syndrome. Obstet. Gynecol. 2009, 114, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Teede, H.J.; Misso, M.L.; Deeks, A.A.; Moran, L.J.; Stuckey, B.G.A.; Wong, J.L.A.; Norman, R.J.; Costello, M.F. Assessment and management of polycystic ovary syndrome: Summary of an evidence-based guideline. Med. J. Aust. 2011, 195, S65–S112. [Google Scholar] [CrossRef] [PubMed]
- Goodman, N.F.; Cobin, R.H.; Futterweit, W.; Glueck, J.S.; Legro, R.S.; Carmina, E. American association of clinical endocrinologists, American college of endocrinology, and androgen excess and pcos society disease state clinical review: Guide to the best practices in the evaluation and treatment of polycystic ovary syndrome—Part 1. Endocr. Pract. 2015, 21, 1291–1300. [Google Scholar] [CrossRef]
- Legro, R.S.; Arslanian, S.A.; Ehrmann, D.A.; Hoeger, K.M.; Murad, M.H.; Pasquali, R.; Welt, C.K. Diagnosis and treatment of polycystic ovary syndrome: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2013, 98, 4565–4592. [Google Scholar] [CrossRef]
- Cunha, A.; Póvoa, A.M. Infertility management in women with polycystic ovary syndrome: A review. Porto Biomed. J. 2021, 6, e116. [Google Scholar] [CrossRef]
- Al Wattar, B.H.; Fisher, M.; Bevington, L.; Talaulikar, V.; Davies, M.; Conway, G.; Yasmin, E. Clinical Practice Guidelines on the Diagnosis and Management of Polycystic Ovary Syndrome: A Systematic Review and Quality Assessment Study. J. Clin. Endocrinol. Metab. 2021, 106, 2436–2446. [Google Scholar] [CrossRef]
- Cooper, D.B.; Patel, P.; Mahdy, H. Oral Contraceptive Pills. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022; pp. 224–231. Available online: https://www.ncbi.nlm.nih.gov/books/NBK430882/ (accessed on 22 February 2024).
- Glendining, K.A.; Campbell, R.E. Recent advances in emerging PCOS therapies. Curr. Opin. Pharmacol. 2023, 68, 102345. [Google Scholar] [CrossRef]
- Diaz Ayllon, H.; Hernandez, O.L.; Nagi, T.; Cespedes, C.M. Severe Ovarian Hyperstimulation Syndrome in the Setting of In Vitro Fertilization Treatment. Cureus 2023, 15, e39939. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. Pravila PRISMA 2020.: Ažurirane smjernice za izvještavanje u sustavnim pregledima. Med. Flum. 2021, 57, 444–465. [Google Scholar] [CrossRef]
- Schiavo, J.H. PROSPERO: An International Register of Systematic Review Protocols. Med. Ref. Serv. Q. 2019, 38, 171–180. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; Handbook; Cochrane Training: London, UK, 2011; Available online: https://training.cochrane.org/handbook/current (accessed on 24 January 2024).
- Raj, P.; Samal, S.; Chandrasekaran, S.; Prabhu, K.; Sen, M. Comparison of Metformin and Myoinositol on Clinical, Hormonal and Metabolic Profile of Patients with Polycystic Ovarian Syndrome: An Open-label Randomised Clinical Trial. J. Clin. Diagn. Res. 2024, 18, QC18–QC22. [Google Scholar] [CrossRef]
- Nazirudeen, R.; Sridhar, S.; Priyanka, R.; Sumathi, B.; Natarajan, V.; Subbiah, E.; Raghavan, K.S.; Sangumani, J. A randomized controlled trial comparing myoinositol with metformin versus metformin monotherapy in polycystic ovary syndrome. Clin. Endocrinol. 2023, 99, 198–205. [Google Scholar] [CrossRef]
- Arya, T.S. A Study of Potential Comparison of N-Acetyl Cysteine with Metformin on Clinical Profile in an Ovulatory Infertile Woman with PCOS. Int. J. Pharm. Clin. Res. 2022, 14, 707–712. [Google Scholar]
- Bahadur, A.; Arora, H.; Ravi, A.K.; Naithani, M.; Bahurupi, Y.; Chaturvedi, J.; Ajmani, M.; Mundhra, R. Comparison of Clinical, Metabolic and Hormonal Effects of Metformin Versus Combined Therapy of Metformin With Myoinositol Plus D-Chiro-Inositol in Women With Polycystic Ovary Syndrome (PCOS): A Randomized Controlled Trial. Cureus 2021, 13, e15510. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.; Mahey, R.; Gupta, M.; Khadgawat, R.; Kachhawa, G.; Sharma, J.B.; Vanamail, P.; Kumari, R.; Bhatla, N. Impact of myoinositol with metformin and myoinositol alone in infertile PCOS women undergoing ovulation induction cycles—Randomized controlled trial. Gynecol. Endocrinol. 2021, 37, 332–336. [Google Scholar] [CrossRef]
- Kumari, A.; Sinha, J.; Sinha, S. A prospective, comparative study of N-acetyl cysteine with metformin on clinical profile in an ovulatory infertile woman with PCOS. Eur. J. Mol. Clin. Med. 2020, 7, 4003–4008. [Google Scholar]
- Rashid, A.; Ganie, M.A.; Wani, I.A.; Bhat, G.A.; Shaheen, F.; Wani, I.A.; Shrivastava, M.; Shah, Z.A. Differential Impact of Insulin Sensitizers vsAnti-Androgen on Serum Leptin Levels in Vitamin D Replete PCOS Women: A Six Month Open Labeled Randomized Study. Horm. Metab. Res. 2020, 52, 89–94. [Google Scholar] [CrossRef]
- Ganie, M.A.; Rashid, A.; Sood, M.; Sofi, N.Y.; Wani, I.A.; Nisar, S.; Butt, T.P.; Gupta, N.; Bhat, D.; Choh, N.; et al. Coadministration of metformin or spironolactone enhances efficacy of rosiglitazone in management of PCOS. Gynecol. Endocrinol. 2020, 36, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Mahey, R.; Kachhawa, G.; Khadgawat, R.; Vanamail, P.; Kriplani, A. Comparison of metformin plus myoinositol vs metformin alone in PCOS women undergoing ovulation induction cycles: Randomized controlled trial. Gynecol. Endocrinol. 2019, 35, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Pasrija, S.; Jain, S. Randomised controlled trial to study the efficacy of exercise with and without metformin on women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.A.; George, P.S.G.; Dasari, A.; Shanmugasundaram, P. A single-blinded randomized trial to evaluate the efficacy of N-acetyl cysteine over metformin in patients with polycystic ovarian syndrome. Drug Invent. Today 2018, 10, 241–243. [Google Scholar]
- Nehra, J.; Kaushal, J.; Singhal, S.R.; Ghalaut, V.S. Comparision of Myo-Inositol Versus Metformin on biochemical Profile in Polycystic Ovarian Syndrome in Women. Int. J. Pharm. Sci. Res. 2017, 8, 1664–1670. [Google Scholar]
- Nehra, J.; Kaushal, J.; Singhal, S.R.; Ghalaut, V.S. Comparision of Myo-Inositol Versus Metformin on Anthropometric Parameters in Polycystic Ovarian Syndrome in Women. Int. J. Pharm. Pharm. Sci. 2017, 9, 144–148. [Google Scholar] [CrossRef]
- Ganie, M.A.; Khurana, M.L.; Nisar, S.; Shah, P.A.; Shah, Z.A.; Kulshrestha, B.; Gupta, N.; Zargar, M.A.; Wani, T.A.; Mudasir, S.; et al. Improved efficacy of low-dose spironolactone and metformin combination than either drug alone in the management of women with polycystic ovary syndrome (PCOS): A six-month, open-label randomized study. J. Clin. Endocrinol. Metab. 2013, 98, 3599–3607. [Google Scholar] [CrossRef]
- Sangeeta, S. Metformin and pioglitazone in polycystic ovarian syndrome: A comparative study. J. Obstet. Gynecol. India 2012, 62, 551–556. [Google Scholar] [CrossRef]
- Rani, N.; Kumar, P.; Mishra, A.; Sankuratri, B.; Sethi, S.; Gelada, K.; Tiwari, H. Efficacy of Spironolactone in Adult Acne in Polycystic Ovary Syndrome Patients an Original Research. J. Pharm. Bioallied Sci. 2021, 13, S1659–S1663. [Google Scholar] [CrossRef]
- Saveetha, V.; Soma Sundaram, I.; Shanmugasundaram, P. A prospective study on the efficacy of metformin in obese and non-obese patients with polycystic ovary syndrome. Asian J. Pharm. Clin. Res. 2016, 9, 145–147. [Google Scholar]
- Kumar, P.; Arora, S. Orlistat in polycystic ovarian syndrome reduces weight with improvement in lipid profile and pregnancy rates. J. Hum. Reprod. Sci. 2014, 7, 255–261. [Google Scholar] [CrossRef]
- Dasgupta, S.; Mondal, J.; Goswami, B.; Dasgupta, J. Randomized Control Trial to Compare Effects of Ultra-Low Dose (Ethinylestradiol 20 μg or 15 μg) With Low Dose (Ethinylestradiol 30 μg) Hormonal Pills on Lipid Discordance in Non-Obese PCOS Women. Obstet. Gynecol. Surv. 2024, 79, 208–210. [Google Scholar] [CrossRef]
- Kachhawa, G.; Senthil Kumar, K.V.; Kulshrestha, V.; Khadgawat, R.; Mahey, R.; Bhatla, N. Efficacy of myo-inositol and d-chiro-inositol combination on menstrual cycle regulation and improving insulin resistance in young women with polycystic ovary syndrome: A randomized open-label study. Int. J. Gynecol. Obstet. 2022, 158, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Shivangi; Savita Rani Singhal, L. Effect of combined oral contraceptives and cyperoterone acetate-ethinyl estradiol combination on metabolic syndrome in polycystic ovarian syndrome (PCOS). Eur. J. Mol. Clin. Med. 2022, 9, 1571–1578. [Google Scholar]
- Suvarna, Y.; Maity, N.; Kalra, P.; Shivamurthy, M.C. Comparison of efficacy of metformin and oral contraceptive combination of ethinyl estradiol and drospirenone in polycystic ovary syndrome. J. Turk. Ger. Gynecol. Assoc. 2016, 17, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.M.; Jha, A.; Dasmukhopadhyay, L. Comparison of two contraceptive pills containing drospirenone and 20 μg or 30 μg ethinyl estradiol for polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2016, 132, 210–213. [Google Scholar] [CrossRef]
- Bhattacharya, S.M.; Ghosh, M.; Basu, R. Effects of ethinyl estradiol and desogestrel on clinical and metabolic parameters in Indian patients with polycystic ovary syndrome. J. Obstet. Gynaecol. Res. 2012, 38, 285–290. [Google Scholar] [CrossRef]
- Panda, S.R.; Sharmila, V.; Kalidoss, V.K.; Hota, S. A triple-blind, randomized controlled trial, comparing combined letrozole and clomiphene versus only letrozole for ovulation induction in women with polycystic ovarian syndrome. Int. J. Gynecol. Obstet. 2023, 161, 63–70. [Google Scholar] [CrossRef]
- Bansal, S.; Goyal, M.; Sharma, C.; Shekhar, S. Letrozole versus clomiphene citrate for ovulation induction in anovulatory women with polycystic ovarian syndrome: A randomized controlled trial. Int. J. Gynecol. Obstet. 2021, 152, 345–350. [Google Scholar] [CrossRef]
- Kar, S.; Sanchita, S. Clomiphene citrate, metformin or a combination of both as the first line ovulation induction drug for Asian Indian women with polycystic ovarian syndrome: A randomized controlled trial. J. Hum. Reprod. Sci. 2015, 8, 197–201. [Google Scholar] [CrossRef]
- Roy, K.; Baruah, J.; Singla, S.; Sharma, J.; Singh, N.; Jain, S.; Goyal, M. A prospective randomized trial comparing the efficacy of Letrozole and Clomiphene citrate in induction of ovulation in polycystic ovarian syndrome. J. Hum. Reprod. Sci. 2012, 5, 20–25. [Google Scholar] [CrossRef]
- Kar, S. Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J. Hum. Reprod. Sci. 2012, 5, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.S.; Aleyamma, T.K.; Chandy, A.; George, K. Aromatase inhibitors in women with clomiphene citrate resistance: A randomized, double-blind, placebo-controlled trial. Fertil. Steril. 2010, 94, 2857–2859. [Google Scholar] [CrossRef]
- D’Souza, P.D.; Rodrigues, D.E.; Kaipangala, R.G.; Leena, K.C. Effectiveness of Multimodular Interventions of Lifestyle Modification on Symptoms of Polycystic Ovarian Syndrome and Quality of Life among Women—A Quasi-experimental Study. J. Clin. Diagn. Res. 2022, 16, LC27–LC31. [Google Scholar] [CrossRef]
- Ratnakumari, M.; Manavalan, N.; Sathyanath, D.; Ayda, Y.; Reka, K. Study to evaluate the changes in polycystic ovarian morphology after naturopathic and yogic interventions. Int. J. Yoga 2018, 11, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Nidhi, R.; Padmalatha, V.; Nagarathna, R.; Ram, A. Effect of a yoga program on glucose metabolism and blood lipid levels in adolescent girls with polycystic ovary syndrome. Int. J. Gynecol. Obstet. 2012, 118, 37–41. [Google Scholar] [CrossRef]
- Bahadur, A.; Yadav, A.; Mundhra, R.; Chawla, L.; Naithani, M.; Chaturvedi, J. Effect of Two Different Doses of Vitamin D Supplementation on Clinical, Metabolic, and Hormonal Profiles of Patients with Polycystic Ovary Syndrome (PCOS) with Insulin Resistance: A Randomized Controlled Trial. J. South Asian Fed. Obstet. Gynaecol. 2022, 14, 307–312. [Google Scholar] [CrossRef]
- Gupta, T.; Rawat, M.; Gupta, N.; Arora, S. Study of Effect of Vitamin D Supplementation on the Clinical, Hormonal and Metabolic Profile of the PCOS Women. J. Obstet. Gynecol. India 2017, 67, 349–355. [Google Scholar] [CrossRef]
- Garg, G.; Kachhawa, G.; Ramot, R.; Khadgawat, R.; Tandon, N.; Sreenivas, V.; Kriplani, A.; Gupta, N. Effect of vitamin D supplementation on insulin kinetics and cardiovascular risk factors in polycystic ovarian syndrome: A pilot study. Endocr. Connect. 2015, 4, 108–116. [Google Scholar] [CrossRef]
- Kaur, I.; Suri, V.; Sachdeva, N.; Rana, S.V.; Medhi, B.; Sahni, N.; Ahire, J.; Singh, A. Efficacy of multi-strain probiotic along with dietary and lifestyle modifications on polycystic ovary syndrome: A randomised, double-blind placebo-controlled study. Eur. J. Nutr. 2022, 61, 4145–4154. [Google Scholar] [CrossRef]
- Singh, A.; Gainder, S.; Banerjee, P.; Goel, A.; Kumar, P.; Mondal, B.; Banik, S.P.; Bagchi, D. Efficacy of a Proprietary Fenugreek Seed Extract (Trigonella foenum-graecum, Furocyst®) in Women with Polycystic Ovary Syndrome (PCOS): A Randomized, Double-Blind, Placebo-Controlled Study. J. Am. Nutr. Assoc. 2023, 42, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Verma, P.; Maan, P.; Gautam, R.; Arora, T. Unveiling the Role of Artificial Intelligence (AI) in Polycystic Ovary Syndrome (PCOS) Diagnosis: A Comprehensive Review. Reprod. Sci. 2024, 31, 2901–2915. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Papavassiliou, A.G. Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 2006, 12, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Guigas, B.; Sanz Garcia, N.; Leclerc, J.; Foretz, M.; Andreelli, F. Cellular and molecular mechanisms of metformin: An overview. Clin. Sci. 2012, 122, 253–270. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Economou, F.; Palimeri, S.; Christakou, C. Metformin in polycystic ovary syndrome. Ann. N. Y. Acad. Sci. 2010, 1205, 192–198. [Google Scholar] [CrossRef]
- Teede, H.J.; Tay, C.T.; Laven, J.J.E.; Dokras, A.; Moran, L.J.; Piltonen, T.T.; Costello, M.F.; Boivin, J.; Redman, L.M.; Boyle, J.A.; et al. Recommendations From the 2023 International Evidence-based Guideline for the Assessment and Management of Polycystic Ovary Syndrome* on behalf of the International PCOS Network. J. Clin. Endocrinol. Metab. 2023, 108, 2447–2469. [Google Scholar] [CrossRef]
- Shurrab, N.T.; Arafa, E.S.A. Metformin: A review of its therapeutic efficacy and adverse effects. Obes. Med. 2020, 17, 100186. [Google Scholar] [CrossRef]
- Randeva, H.S.; Tan, B.K.; Weickert, M.O.; Lois, K.; Nestler, J.E.; Sattar, N.; Lehnert, H. Cardiometabolic aspects of the polycystic ovary syndrome. Endocr. Rev. 2012, 33, 812–841. [Google Scholar] [CrossRef]
- Larner, J.; Brautigan, D.L.; Thorner, M.O. D-chiro-inositol glycans in insulin signaling and insulin resistance. Mol. Med. 2010, 16, 543–551. [Google Scholar] [CrossRef]
- Hauner, H. The mode of action of thiazolidinediones. Diabetes. Metab. Res. Rev. 2002, 18, S10–S15. [Google Scholar] [CrossRef]
- Day, C. Thiazolidinediones: A new class of antidiabetic drugs. Diabet. Med. 1999, 16, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Melin, J.M.; Forslund, M.; Alesi, S.J.; Piltonen, T.; Romualdi, D.; Spritzer, P.M.; Tay, C.T.; Pena, A.S.; Witchel, S.F.; Mousa, A.; et al. Effects of different insulin sensitisers in the management of polycystic ovary syndrome: A systematic review and meta-analysis. Clin. Endocrinol. 2024, 100, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, M.A.; Shah, N.; Deshmukh, H.; Sahebkar, A.; Östlundh, L.; Al-Rifai, R.H.; Atkin, S.L.; Sathyapalan, T. The Effect of Thiazolidinediones in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Adv. Ther. 2024, 41, 2168–2195. [Google Scholar] [CrossRef] [PubMed]
- Armanini, D.; Andrisani, A.; Bordin, L.; Sabbadin, C. Spironolactone in the treatment of polycystic ovary syndrome. Expert Opin. Pharmacother. 2016, 17, 1713–1715. [Google Scholar] [CrossRef]
- Sabbadin, C.; Andrisani, A.; Zermiani, M.; Donà, G.; Bordin, L.; Ragazzi, E.; Boscaro, M.; Ambrosini, G.; Armanini, D. Spironolactone and intermenstrual bleeding in polycystic ovary syndrome with normal BMI. J. Endocrinol. Investig. 2016, 39, 1015–1021. [Google Scholar] [CrossRef]
- Bashir, R.; Asrar, M.M.; Shah, I.A.; Wani, I.A.; Ganie, M.A. Do Pleiotropic Effects of Spironolactone in Women with PCOS Make it More thanan Anti-androgen? Evidence from a Systematic Review and Meta-analysis. Curr. Pharm. Des. 2023, 29, 1486–1496. [Google Scholar] [CrossRef]
- Zeng, H.; Zhang, Y.; Huang, S.; Wu, J.; Ren, W.; Zhou, L.; Huang, L.; Ye, Y. Metformin combined with spironolactone vs. metformin alone in polycystic ovary syndrome: A meta-analysis. Front. Endocrinol. 2023, 14, 1223768. [Google Scholar] [CrossRef]
- Alesi, S.; Forslund, M.; Melin, J.; Romualdi, D.; Peña, A.; Tay, C.T.; Witchel, S.F.; Teede, H.; Mousa, A. Efficacy and safety of anti-androgens in the management of polycystic ovary syndrome: A systematic review and meta-analysis of randomised controlled trials. eClinicalMedicine 2023, 63, 102162. [Google Scholar] [CrossRef]
- Liu, J.; Su, H.; Jin, X.; Wang, L.; Huang, J. The effects of N-acetylcysteine supplement on metabolic parameters in women with polycystic ovary syndrome: A systematic review and meta-analysis. Front. Nutr. 2023, 10, 1209614. [Google Scholar] [CrossRef]
- Devi, N.; Boya, C.; Chhabra, M.; Bansal, D. N-acetyl-cysteine as adjuvant therapy in female infertility: A systematic review and meta-analysis. J. Basic Clin. Physiol. Pharmacol. 2020, 32, 899–910. [Google Scholar] [CrossRef]
- Shahveghar Asl, Z.; Parastouei, K.; Eskandari, E. The effects of N-acetylcysteine on ovulation and sex hormones profile in women with polycystic ovary syndrome: A systematic review and meta-analysis. Br. J. Nutr. 2023, 130, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; Yanovski, J.A.; Calis, K.A. Orlistat, a new lipase inhibitor for the management of obesity. Pharmacotherapy 2000, 20, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Graff, S.K.; Mario, F.M.; Ziegelmann, P.; Spritzer, P.M. Effects of orlistat vs. metformin on weight loss-related clinical variables in women with PCOS: Systematic review and meta-analysis. Int. J. Clin. Pract. 2016, 70, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.F.; Wu, Y.; Zhu, Y.H.; Ding, T.; Batterham, R.L.; Qu, F.; Hardiman, P.J. Pharmacologic therapy to induce weight loss in women who have obesity/overweight with polycystic ovary syndrome: A systematic review and network meta-analysis. Obes. Rev. 2018, 19, 1424–1445. [Google Scholar] [CrossRef]
- Goldberg, A.; Graca, S.; Liu, J.; Rao, V.; Witchel, S.F.; Pena, A.; Li, R.; Mousa, A.; Tay, C.T.; Pattuwage, L.; et al. Anti-obesity pharmacological agents for polycystic ovary syndrome: A systematic review and meta-analysis to inform the 2023 international evidence-based guideline. Obes. Rev. 2024, 25, e13704. [Google Scholar] [CrossRef]
- Shah, D.; Patil, M.; National PCOS Working Group. Consensus Statement on the Use of Oral Contraceptive Pills in Polycystic Ovarian Syndrome Women in India. J. Hum. Reprod. Sci. 2018, 11, 96–118. [Google Scholar] [CrossRef]
- Schindler, A.E. Non-Contraceptive Benefits of Oral Hormonal Contraceptives. Int. J. Endocrinol. Metab. 2013, 11, 41–47. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, Z. Effects of oral contraceptives plus orlistat in patients with polycystic ovary syndrome and overweight/obesity: A meta-analysis. J. Obstet. Gynaecol. Res. 2022, 48, 1399–1408. [Google Scholar] [CrossRef]
- Forslund, M.; Melin, J.; Alesi, S.; Piltonen, T.; Romualdi, D.; Tay, C.T.; Witchel, S.; Pena, A.; Mousa, A.; Teede, H. Different kinds of oral contraceptive pills in polycystic ovary syndrome: A systematic review and meta-analysis. Eur. J. Endocrinol. 2023, 189, S1–S16. [Google Scholar] [CrossRef]
- Rashid, R.; Mir, S.A.; Kareem, O.; Ali, T.; Ara, R.; Malik, A.; Amin, F.; Bader, G.N. Polycystic ovarian syndrome-current pharmacotherapy and clinical implications. Taiwan. J. Obstet. Gynecol. 2022, 61, 40–50. [Google Scholar] [CrossRef]
- Yang, A.M.; Cui, N.; Sun, Y.F.; Hao, G.M. Letrozole for Female Infertility. Front. Endocrinol. 2021, 12, 676133. [Google Scholar] [CrossRef] [PubMed]
- Cole, P.A.; Robinson, C.H. Mechanism and Inhibition of Cytochrome P-450 Aromatase. J. Med. Chem. 1990, 33, 2933–2942. [Google Scholar] [CrossRef]
- Kerin, J.F.; Liu, J.H.; Phillipou, G.; Yen, S.S.C. Evidence for a Hypothalamic Site of Action of Clomiphene Citrate in Women. J. Clin. Endocrinol. Metab. 1985, 61, 265–268. [Google Scholar] [CrossRef]
- Liu, Z.; Geng, Y.; Huang, Y.; Hu, R.; Li, F.; Song, Y.; Zhang, M. Letrozole Compared With Clomiphene Citrate for Polycystic Ovarian Syndrome: A Systematic Review and Meta-analysis. Obstet. Gynecol. 2023, 141, 523–534. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Gari, A.; Sabban, H.; Alshahrani, M.S.; Khadawardi, K.; Badghish, E.; AlSghan, R.; Bukhari, I.A.; Alyousef, A.; Abuzaid, M.; et al. Comparison of Letrozole and Clomiphene Citrate in Pregnancy Outcomes in Patients with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis. Reprod. Sci. 2024, 31, 883–905. [Google Scholar] [CrossRef]
- Abdolahian, S.; Tehrani, F.R.; Amiri, M.; Ghodsi, D.; Yarandi, R.B.; Jafari, M.; Majd, H.A.; Nahidi, F. Effect of lifestyle modifications on anthropometric, clinical, and biochemical parameters in adolescent girls with polycystic ovary syndrome: A systematic review and meta-analysis. BMC Endocr. Disord. 2020, 20, 71. [Google Scholar] [CrossRef] [PubMed]
- Gautam, R.; Maan, P.; Jyoti, A.; Kumar, A.; Malhotra, N.; Arora, T. The Role of Lifestyle Interventions in PCOS Management: A Systematic Review. Nutrients 2025, 17, 310. [Google Scholar] [CrossRef]
- Boden, G.; Sargrad, K.; Homko, C.; Mozzoli, M.; Stein, T.P. Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes. Ann. Intern. Med. 2005, 142, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, G.; Giacco, R.; Rivellese, A.A. Dietary fat, insulin sensitivity and the metabolic syndrome. Clin. Nutr. 2004, 23, 447–456. [Google Scholar] [CrossRef]
- Innes, K.E.; Bourguignon, C.; Gill Taylor, A. Risk Indices Associated with the Insulin Resistance Syndrome, Cardiovascular Disease, and Possible Protection with Yoga: A Systematic Review. J. Am. Board Fam. Med. 2005, 18, 491–519. [Google Scholar] [CrossRef]
- Chu, P.; Gotink, R.A.; Yeh, G.Y.; Goldie, S.J.; Hunink, M.G.M. The effectiveness of yoga in modifying risk factors for cardiovascular disease and metabolic syndrome: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Prev. Cardiol. 2016, 23, 291–307. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Lee, S.H. Effectiveness of Lifestyle Modification in Polycystic Ovary Syndrome Patients with Obesity: A Systematic Review and Meta-Analysis. Life 2022, 12, 308. [Google Scholar] [CrossRef] [PubMed]
- Al Wattar, B.H.; Hussain, N.M.; Khan, K.S. Lifestyle interventions in women with polycystic ovary syndrome: A scoping systematic review of randomised evidence. Med. Fam. Semer. 2022, 48, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Alesi, S.; Ee, C.; Moran, L.J.; Rao, V.; Mousa, A. Nutritional Supplements and Complementary Therapies in Polycystic Ovary Syndrome. Adv. Nutr. 2022, 13, 1243–1266. [Google Scholar] [CrossRef]
- Han, Y.; Cao, Q.; Qiao, X.; Huang, W. Effect of vitamin D supplementation on hormones and menstrual cycle regularization in polycystic ovary syndrome women: A systemic review and meta-analysis. J. Obstet. Gynaecol. Res. 2023, 49, 2232–2244. [Google Scholar] [CrossRef]
- Yang, M.; Shen, X.; Lu, D.; Peng, J.; Zhou, S.; Xu, L.; Zhang, J. Effects of vitamin D supplementation on ovulation and pregnancy in women with polycystic ovary syndrome: A systematic review and meta-analysis. Front. Endocrinol. 2023, 14, 1148556. [Google Scholar] [CrossRef]
- Tabrizi, R.; Ostadmohammadi, V.; Akbari, M.; Lankarani, K.B.; Vakili, S.; Peymani, P.; Karamali, M.; Kolahdooz, F.; Asemi, Z. The Effects of Probiotic Supplementation on Clinical Symptom, Weight Loss, Glycemic Control, Lipid and Hormonal Profiles, Biomarkers of Inflammation, and Oxidative Stress in Women with Polycystic Ovary Syndrome: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Probiotics Antimicrob. Proteins 2022, 14, 1–14. [Google Scholar] [CrossRef]
- Lakshmi, J.N.; Babu, A.N.; Kiran, S.S.M.; Nori, L.P.; Hassan, N.; Ashames, A.; Bhandare, R.R.; Shaik, A.B. Herbs as a Source for the Treatment of Polycystic Ovarian Syndrome: A Systematic Review. BioTech 2023, 12, 4. [Google Scholar] [CrossRef]
- Manouchehri, A.; Abbaszadeh, S.; Ahmadi, M.; Nejad, F.K.; Bahmani, M.; Dastyar, N. Polycystic ovaries and herbal remedies: A systematic review. J. Bras. Reprod. Assist. 2023, 27, 85–91. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
| Study and Citation | Study Design | Diagnostic Criteria for PCOS | PCOS Women Characteristics | Location | Interventions | N (Finally Analyzed Patient) | Duration of Intervention (Months) | Outcomes Measured | Outcome in Which Significant Difference Observed (Better-Performing Group) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pharmacological Treatment | |||||||||||
| Insulin Sensitizers | |||||||||||
| 1 | Raj et al., 2024 | Open-label RCT | Rotterdam 2003 | Age: 18–40 years | SRM Medical College Hospital and Research Centre, Kattankulathur, Tamil Nadu, Chengalpattu, India | 1 = metformin (500 mg), TDS; 2 = myo-inositol (1 g/day) | 1 = 36, 2 = 35 | 3 months | MC, P, FBS, FI, L, HDL, LDL, C, TAGs, FSH, LH, LH/FSH | P (50% vs. 26.6%), HDL (1), side effects (1 > 2) | [27] |
| 2 | Nazirudeen et al., 2023 | RCT | Rotterdam 2003 | Age: 18–35 years, BMI > 23 kg/m2 | Government Rajaji Hospital, Madurai, Tamil Nadu, India | 1 = metformin (500 mg), BD; 2 = metformin (500 mg) + myo-inositol (1.1 g) + D-chiro-inositol (27.6 mg), BD | 1 = 27, 2 = 26 | 6 months | MC, APs, FBS, FI, IR, L, T, SHBG, LH, FSH, AMH, USG, P, QoL | MC (2) | [28] |
| 3 | Arya et al., 2022 | RCT | Rotterdam 2003 | Age: 18 to 37 years | M.G.M. Medical College, Kishanganj, Bihar, India | 1 = metformin (500 mg), TDS; 2 = N acetyl cysteine (600 mg), TDS | 1 = 50, 2 =50 | 6 months | APs, MC, FG, T, TSH, Pr, LH, FSH, FBS, FI, USG | BMI, WHR, T (2) | [29] |
| 4 | Bahadur et al., 2021 | RCT | Rotterdam 2003 | Age: 18 to 45 years | AIIMS Rishikesh, Uttrakhand, India | 1 = metformin (500 mg), BD; 2 = metformin (500 mg) + myo-inositol (550 mg) + D-chiro-inositol (150 mg) | 1 = 36, 2 = 36 | 6 months | MC, FG, APs, LH/FSH, LH, FSH, T, DHEAS, HDL, LDL, TAGs, C, PI, FI, IR | A, MC, LH/FSH, HDL, LDL, C, PI (2) | [30] |
| 5 | Prabhakar et al., 2021 | RCT | Rotterdam 2003 | Age: 20–38 years, infertile, BMI < 30 kg/m2 | AIIMS, New Delhi, India | 1 = metformin (500 mg), TDS + myo-inositol (2 g), BD; 2 = myo-inositol (2 g), BD; both groups: after 3 months, three cycles of ovulation induction (letrozole, 2.5 mg, day 2–6 of menstrual cycle) + intrauterine insemination | 1 = 57, 2 = 59 | 6 months | BMI, L, FBS, FI, IR, LH, FSH, TSH, Pr, T, SHBG | P (45.5% vs. 42%, p > 0.05) (2 > 1), side effects (1 > 2) | [31] |
| 6 | Kumari et al., 2020 | Prospective study, randomly assigned | Rotterdam 2003 | Age: 18 to 37 years | NSMCH, Bihta, Patna, Bihar, India | 1 = metformin (500 mg), TDS; 2 = N acetyl cysteine (600 mg), TDS | 1 = 50, 2 = 50 | 6 months | APs, MC, FG, T, TSH, Pr, LH, FSH, FBS, FI, USG | FG, FI (1 = 2), BMI, WHR (0.08), T (0.23 nmol/L) (2 > 1) | [32] |
| 7 | Rashid et al., 2020 | Open-label RCT | Rotterdam 2003 | Age: 18–40 years | Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, J&K, India | 1 = spironolactone (50 mg) + Vit D 4000 IU; 2 = metformin (1000 mg) + Vit D 4000 IU; 3 = pioglitazone (30 mg) + Vit D 4000 IU OD | 1 = 30, 2 = 30, 3 = 30 | 6 months | MC, FG, FBS, IR, FI, T, C, TAGs, LDL, HDL | MC, LDL, Vit D, Ca (1 = 2 = 3), W (1.66 kg), FG (2.5), FBS (7.04 mg/dL), FI (4.99 mg/dL), IR (1.13), HDL, LH, leptin (2.97 ng/mL) (3 > 2 and 1: p < 0.05) | [33] |
| 8 | Ganie et al., 2020 | Prospective non-randomized study | Rotterdam 2003 | NM * | Sher-i-Kashmir Institute of Medical Sciences, Srinagar, J&K, India | 1 = rosiglitazone (4 mg), OD; 2 = rosiglitazone (4 mg), OD + spironolactone (50 mg), OD; 3 = rosiglitazone (4 mg), OD + metformin (500 mg), BD | 1 = 30, 2 = 40, 3 = 34 | 6 months | APs, FG, LH, FSH, T, C, TAGs, C, IR, FBS, PI, MC | MC, FG, IR, PI (1 = 2 = 3), T (30.21 ng/dL) (2 > 3 and 1: p < 0.05), W (2.26 kg), BMI (1.01 kg/m2), WHR (0.03), FBS (9.97 mg/dL), FI (10.1 mg/dL) (3 > 2 and 1: p < 0.05) | [34] |
| 9 | Agrawal et al., 2019 | RCT | Rotterdam 2003 | Age: 20–38 years, infertile, BMI < 30 kg/m2 | AIIMS, New Delhi, India | 1 = metformin (500 mg) + myo-inositol (600 mg), TDS; 2 = metformin (500 mg), TDS; both groups: after 3 months, three cycles of ovulation induction (letrozole, 2.5 mg, day 2–6 of menstrual cycle) + intrauterine insemination | 1 = 60, 2 = 60 | 6 months | LBR, MC, P, OI, OHS, BMI, FG, A, LH, FSH, T, SHBG, AMH, FBS, FI, IR, C, LDL, HDL | MC, P, IR, LBR (1) | [35] |
| 10 | Tiwari et al., 2018 | RCT | Rotterdam 2003 | NM * | Dr. Baba Saheb Ambedkar Medical College, New Delhi, India | 1 = fixed exercise + placebo; 2 = fixed exercise + metformin (850 mg), BD | 1 = 33, 2 = 33 | 6 months | MC, W, BMI, W:H, WC, FG, T, TAGs, C, GTT | MC (55.17% vs. 83.33%), W (1.08 kg vs. 2.5 kg), WC (2.56 cm vs. 4.75 cm), W:H (0.02 vs. 0.04) (2 > 1: p < 0.05) | [36] |
| 11 | Kumar et al., 2018 | RCT | NM * | Age: 18 to 37 years, Married | SRM Medical College Hospital and Research Centre, Tamil Nadu, India | 1 = metformin (500 mg), TDS; 2 = N acetyl cysteine (600 mg), TDS | 1 = 50, 2 = 50 | 3 months | FBS, FI, G:I, LH, FSH | FBS, FI, G:I, LH, FSH (1 = 2), side effect (1 > 2) | [37] |
| 12 | Nehra at al., 2017 | Open-label RCT | Androgen Excess Society 2006 | Age: 15 to 45 years, Married | PGIMS, Rohtak, Haryana, India | 1 = myo-inositol (1 g), BD 2 = metformin (500 mg), TDS | 1 = 30, 2 = 30 | 6 months | FG, FI, G:I, IR, L, LH, FSH, T | G:I, IR, L, LH, FSH, T (1 = 2) | [38] |
| 13 | Nehra at al., 2017 | Open-label RCT | Androgen Excess Society 2006 | Age: 15 to 45 years, Married | PGIMS, Rohtak, Haryana, India | 1 = myo-inositol (1 g), BD; 2 = metformin (500 mg), TDS | 1 = 30, 2 = 30 | 6 months | W, BMI, WHR | W, BMI, WHR (1 = 2) | [39] |
| 14 | Ganie et al., 2013 | Open-label RCT | Androgen Excess Society 2006 | NM * | Sher-i-Kashmir Institute of Medical Sciences (SKIMS), Srinagar, J&K, India | 1 = metformin (1000 mg/d); 2 = spironolactone (50 mg/d); 3 = metformin (1000 mg/d) + spironolactone (50 mg/d) | 1 = 56, 2 = 51, 3 = 62 | 6 months | MC, FG, BMI, APs, BP, LH, FSH, T, FBS, FI, IR | MC (6.13 ± 2.54 to 11.86 ± 3.20 cycles/y), FG (4.02), T (1.52 nmol/L), FBS (6.16 mg/dL), FI (7.6 µIU/mL) (3 > 2, 3 > 1: p = 0.01) | [40] |
| 15 | Sangeeta et al., 2012 | Double-blind RCT | Rotterdam 2003 | Age:18–30 years | Gandhi Hospital, Hyderabad, Andhra Pradesh, India | 1 = metformin (500 mg), BD; 2 = pioglitazone (15 mg) OD | 1 = 43, 2 = 42 | 6 months | MC, FG, HDL, VLDL, C, PI, IR, SHBG, FAI, LH, FSH | MC, FG, OR (1 = 2), C (6.4% vs. 20.67%), HDl (17% vs. 64%), VLDL (15% vs. 32%), IR (15% vs. 55%), SHBG (21% vs. 80%), FAI (22.6% vs. 47%), L/H ratio (0.045 vs. 0.736), OI (2 > 1 (p < 0.05) | [41] |
| Anti-obesity drugs | |||||||||||
| 16 | Rani et al., 2021 | Prospective clinical trial | Presence of chronic anovulation, one or more signs of clinical hyperandrogenism, and/or endocrinological abnormalities | Age: 18–32 years, treatment-naïve | Medinirai Medical College and Hospital, Palamu, Jharkhand, India | Spironolactone (100 mg/day) + food restriction (1400 kCal/day); 1 = lean (BMI < 24.5 kg/m2);2 = overweight (BMI > 25 kg/m2) | 1 = 13, 2 = 12 | 12 months | FG, BP, BMI, TAGs, C, HDL, FBS, GTT, I, IR | TA (−22%, p < 0.05), G, IR (2 > 1), HDL (24%, p < 0.05) (1 > 2), FG (1 = 2) | [42] |
| 17 | Saveetha et al., 2016 | Prospective study | PCOS diagnosed by USG and irregular cycles and anovulation | Age: 18 to 45 years | ESI Hospital, Ayanavaram, Chennai, Tamil Nadu, India | Metformin (500 mg), BD; 1 = obese (BMI > 29.9); 2 = non-obese (BMI = 18–24.5) | 1 = 37, 2 = 53 | 12 months | BP, BMI, FBS, PBS, C | BMI (1.41 kg/m2, p = 0.005), W (3.37 kg, p = 0.04), BP (6.6 mmHg, p 0.032), FBS (5.4 mg/dL, p = 0.037), PBS (5.44 mg/dl, p = 0.042), C (6.38 mm/dL, p = 0.047) (1 > 2), not significant effect for 2 | [43] |
| 18 | Kumar et al., 2014 | RCT | Rotterdam 2003 | Age: <40 years, BMI > 23 kg/m2, infertile | Manipal Assisted Reproduction Center, Kasturba Medical College, Manipal University, Manipal Karnataka, India | 1 = metformin (stepwise, max to 500 mg TDS) + fertility fitness program (diet, exercise, and lifestyle intervention); 2 = orlistat (120 mg BD) + fertility fitness program (diet, exercise, and lifestyle intervention); 3 = fertility fitness program (diet, exercise, and lifestyle intervention) | 1 = 30, 2 = 30, 3 = 30 | 3 months | MC, FG, A, APs, LH, FSH, DHEAS, SHBG, FAI, FI, FBS, HOMA-IR, HDL, LDL, C, TAGs, UAG | APs, W (1 = 2), side effects (1 > 2) | [44] |
| OCPs | |||||||||||
| 19 | Dasgupta et al., 2024 | RCT | Rotterdam 2003 | Non-obese, reproductive age | Rampurhat Government Medical College and Hospital, Birbhum, West Bengal, India | 1 = ethinylestradiol 20 µg + drosperinone 3 mg; 2 = ethinylestradiol 15 µg + gestodene 60 µg; 3 = ethinylestradiol 30 µg + desogestrel 150 µg | 1 = 51, 2 = 51, 3 = 51 | 1 year | BMI, FG, TSH, C, TC, HDL, TC/HDL, LDL, IR, T | TAGs (1.60 mg/dL), C (10.14 mg/dL), LDL (8.76 mg/dL) (2 > 1 and 3) (p = 0.01), TSH (1.6 mg/dL; p = 0.01) (1 > 2 and 3), T (1.38 ng/mL, p = 0.00), IR (0.28, p = 0.003) (3 > 1 and 2) | [45] |
| 20 | Kachhawa et al., 2022 | Open-label RCT | Rotterdam 2003 | Age: 15–24 years | AIIMS, New Delhi, India | 1 = myo-inositol (500 mg) and D-chiro-inositol (150 mg), BD; 2 = ethinyl estradiol (20 µg) + drospirenone (3 mg), OD | 1 = 33, 2 = 34 | 6 months | BMI, WHR, AMH, LH, FSH, T, IR, MC, FI | FI (3.82 µU/m, p = 0.05), IR (0.82, p < 0.05) (1 > 2), LH (1.17, mIU/mL), T (0.04, ng/mL), AMH (2.21 ng/mL), MC (27.27% vs. 100%) (2 > 1) | [46] |
| 21 | Shivangi et al., 2022 | RCT | Rotterdam 2003 | PCOS | PGIMS, Rohtak, Haryana, India | 1 = combined oral contraceptives (COCPs); 2 = cyperoterone acetate + ethinyl estradiol | 1 = 50, 2 = 50 | 6 months | BP, HDL, TAGs, FBS | Glucose level deranged in both groups (1 > 2), HDL, TAGs deranged in both groups (1 < 2) | [47] |
| 22 | Suvarna et al., 2016 | Prospective study, physician’s discretion | Rotterdam 2003 | Age: 18 to 45 years | M.S. Ramaiah Medical College and Hospitals, Karnataka, India | 1 = metformin (1 g) BD 2 = OCPs (drospirenone 3 mg + ethinyl estradiol 30 μg), OD, on days 1–21 of the menstrual cycle | 1 = 11, 2 = 11 | 6 months | MC, BMI, T, DHEA | MC (100% and 72%) (2 > 1), BMI (2.2 kg/m2 vs. 2.11 kg/m2), T (9.66 vs. 6.85 ng/dL), DHEA (31.71 vs. 30.8 mcg/dL) (1 = 2) | [48] |
| 23 | Bhattacharya et al., 2016 | RCT | Rotterdam 2003 | PCOS | S.C. Das Memorial Medical and Research Centre, Kolkata, India | 1 = drospirenone (3 mg) + ethinyl estradiol (30 μg), on days 1–21 of the menstrual cycle with no treatment on days 22–28 (21 + 7 regim); 2 = drospirenone (3 mg) + ethinyl estradiol (20 μg) (24 + 4 regimen) | 1 = 46, 2 = 48 | 6 and 12 months | SHBG, A, BMI, APs, FG, FAI, T, PBS, PI, PBS:PI | SHBG(2) at 6 and 12 months, PBS:PI (2) at 6 months | [49] |
| 24 | Bhattacharya et al., 2012 | RCT | Androgen Excess Society 2006 | Age: 18–35 years | S.C. Das Memorial Medical and Research Centre, Kolkata, India, | 1 = desogestrel (30/150 mg); 2 = cyproterone acetate (35/2000 mg); 3 = drospirenone (30/3000 mg)21 days followed by a 7-day gap, cyclically | 1 = 49, 2 = 51, 3 = 53 | 12 months | FG, SHBG, FAI, | FG (−5.29 vs.−1.69 vs. −2.12), SHBG (142.91 vs. 99.53 vs. 131.52 nmol/L), FAI (10.57 vs. 5.58 vs. 7.89) (2 > 3 > 1) | [50] |
| Ovulation Induction Drugs | |||||||||||
| 25 | Panda et al., 2023 | Triple-blind RCT | Rotterdam 2003 | Infertile, age: 20–35 years | AIIMS, Mangalagiri, Andhra Pradesh, India | 1 = letrozole (2.5 mg) + placebo; 2 = letrozole (2.5 mg) + clomiphene citrate (50 mg), OD, 3–7 days of menstrual cycle | 1 = 40, 2 = 40 | 1 treatment cycle | OI, P | OI (73% vs. 38%; p = 0.003) (2 > 1) | [51] |
| 26 | Bansal et al., 2021 | Assessor-masked RCT | Rotterdam 2003 | Age: 18–35 years, anovulatory infertility | AIIMS, Jodhpur, Rajasthan, India | 1 = letrozole (2.5 mg/day), OD, 2–6 days of menstrual cycle, stepwise increase to 7.5 mg/day in subsequent menstrual cycles; 2 = clomiphene citrate (50 mg/day) 2–6 days of menstrual cycle, stepwise increase to 150 mg/day in subsequent menstrual cycles | 1 = 41, 2 =39 | 3 cycles or until conception | ET, OI, MF, P, DT | P, DT, MF, OI (1) | [52] |
| 27 | Kar et al., 2015 | RCT | Rotterdam 2003 | Infertility, treatment-naïve | Kar Clinic and Hospital Pvt. Ltd., Bhubaneswar, Odisha, India | 1 = clomiphene citrate (50 mg/day) 2–6 days of menstrual cycle, stepwise increase to 150 mg/day in subsequent menstrual cycles; 2= metformin (850 mg/day increase to 1700 mg/day after 2 weeks); 3 = CC (50–150 mg/day) + metformin (850–1700 mg/day) | 1 = 32, 2 = 24, 3 = 24 | 6 months or until CC-resistant | P, LBR, OI, M, MF | LBR (1 < 2 < 3), MF (2), OI (27.1%: p = 0.03) (3 > 2 and 1) | [53] |
| 28 | Roy KK et al., 2012 | Prospective RCT | Rotterdam 2003 | Age: 20–35 years | AIIMS, New Delhi, India | 1= letrozole (2.5–5 mg); 2 = clomiphene citrate (50–100 mg);orally from days 3–7 of menstrual cycle | 1 = 98, 2 = 106 | 3 treatment cycles | OR, ET, P, LBR | ET, P, LBR (2) | [54] |
| 29 | Kar et al., 2012 | RCT | Rotterdam 2003 | Infertility, treatment-naïve | Kar Clinic and Hospital Pvt. Ltd., Bhubaneswar, Odisha, India | 1 = clomiphene citrate (100 mg/day) 2–6 days of menstrual cycle, stepwise increase to 150 mg/day in subsequent menstrual cycles; 2 = letrozole (2.5 mg/day) | 1 = 51, 2 = 52 | 1 treatment cycle | OR, ET, MF, OI, P, M | MF, P (2) | [55] |
| 30 | Kamath et al., 2010 | RCT | Rotterdam 2003 | CC resistance (200 mg) | Reproductive Medicine Unit, Christian Medical College, Vellore, Tamilnadu, India | 1 = letrozole, 2.5 mg, OD, 2–6 days of the menstrual cycle; 2 = placebo | 1 = 17, 2 = 17 | 1 treatment cycle | FDR, Pro, ET, BMI, FSH, BR, P, OR, MF | FDR (27% vs. 0%; p = 0.015), Pro (24.42 vs. 1.66 nmol/L; p = 0.014), OR (33.3% vs. 0%; p = 0.006) (1 > 2) | [56] |
| Lifestyle Modification | |||||||||||
| 31 | D’Souza et al., 2022 | Purposive sampling, prospective intervention study | NM * | Age: 18–30 years | Father Muller College of Nursing, Mangaluru, Karnataka, India | 1 = regular medical treatment + diet + walk + exercise; (a) insulin resistance diet (healthy fat and protein, few carbohydrates), prescribed by dietician; (b) brisk walk (30 min) in first month, jogging from next month (30 min); (c) core muscle exercise (half push-ups and burpees), 20 min/day, 5 days/week; 2 = regular medical treatment | 1 = 15, 2 = 15 | 6 months | APs, BMI, WHR, FG, A, FBS, MC, QoL | WHR (z-value = 3.328, p < 0.001), FG (z-value 2.296, p < 0.022), A, QoL (1) | [57] |
| Yoga | |||||||||||
| 32 | Ratnakumari et al., 2018 | Single-blinded prospective study, pre–post clinical trial | Rotterdam 2003 | Age: 18–35 years | Government Yoga and Naturopathy Medical College, Arumbakkam, Chennai, Tamil Nadu, India | 1 = yoga (asanas, pranayama, relaxation technique, kriyas, etc.) 20 min + naturopathy (hydrotherapy, mud therapy, massage therapy, fasting, and natural diet), 6 days/week, excluding menstrual days; 2 = control | 1 = 22, 2 = 22 | 3 months | USG, APs, MC | PCOM, APs (1) | [58] |
| 33 | Nidhi et al., 2012 | RCT | Rotterdam 2003 | Age: 15–18 years | Residential school in Anantpur, Andhra Pradesh, India | 1 = group lecture, Surya Namaskara, prone asanas, standing asanas, supine asanas, sitting asanas, guided relaxation, breathing techniques, OM meditation; 2 = group lecture, brisk walk, standing exercises, supine exercises, sitting exercises, supine rest, normal breathing | 1 = 35, 2 = 36 | 12 weeks | FI, FBS, IR, HDL, C, BMI, WC, HC | FI (−1.30 ± 4.65 vs. 1.60 ± 8.19 pmol/L; p < 0.05), FBS (−4.26 ± 6.97 vs. 0.64 ± 7.94 mmlo/L; p < 0.01), IR (0.38 ± 0.92 vs. 0.29 ± 1.56; p < 0.05), TAGs (12.94 ± 10.72 vs. 6.44 ± 10.80 mmol/L; p < 0.05), HDL, LDL (8.20 ± 9.83 vs. 2.85 ± 15.14 mmol/L; p < 0.05), VLDL (2.40 ± 1.97 vs. 1.34 ± 2.23 mmol/L; p < 0.05), C (9.37 ± 11.30 vs. 2.86 ± 17.75 mmol/L; p < 0.05) (2 > 1) | [59] |
| Vit D Supplement | |||||||||||
| 34 | Bahadur et al., 2022 | RCT | NM | Age 20–35 years, insulin resistance (HOMA-IR > 2.5), Vit D < 20 ng/mL, BMI < 30 kg/m2 | AIIMS, Rishikesh, Uttarakhand, India | 1 = metformin (500 mg), BD + Vit D3 (1000 IU/day); 2 = metformin (500 mg), BD + Vit D3 (4000 IU/day) | 1 = 36, 2 = 36 | 3 months | APs, FG, A, FBS, FI, PI, L, LH, FSH, T, DHEAS, IR, MC | FG (1.19 vs. 2.81; p = 0.027), PBS (32.89. 49.03; p = 0.005), FI (6.87 vs. 12.44, p = 0.040), MC, IR (1.79 vs. 4.38; p = 0.002) (2 > 1), MC (25% and 27.81%), A (2.42 and 2.81) (1 = 2) | [60] |
| 35 | Gupta et al., 2017 | Double-blind RCT | Rotterdam 2003 | Age: 18–45 years | ESI PGIMSR Basaidarapur, New Delhi, India. | 1 = Vit D (60,000 IU/week); 2 = placebo | 1 = 25, 2 = 25 | 3 months | LH, FSH, Pr, Cor, Est, T, TSH, FI, Vit D, Ca, FBS, PBS, C, TAGs, HDL, C, IR | FBS (5.88 mg/dL; p = 0.041), IR (1.38; p = 0.003), FI (5.34 mg/dL; p = 0.021), HDL (26.34 mg/dL; p = 0.035), QUICKI (0.024; p = 0.001), MC (20% to 48%) (1 > 2) | [61] |
| 36 | Garg et al., 2015 | Double-blind RCT | Rotterdam 2003 | Age: 18–35 years | AIIMS, New Delhi, India | 1 = metformin (1500 mg/day) + Vit D (4000 IU/day) (given once a month 120,000 IU oral dose); 2 = metformin (1500 mg/day) + placebo | 1 = 15, 2 = 17 | 6 months | IR, G, DI, I, BP, L, | No significant change | [62] |
| Probiotics | |||||||||||
| 37 | Kaur et al., 2022 | Double-blind RCT | Rotterdam 2003 | Age: 18–40 years | PGIMER, Chandigarh, India | 1 = Multi-strain probiotic oral capsule (10 billion CFU) (2 months OD then 4 months BD) (Lactobacillus acidophilus UBLA-34, L. rhamnosus UBLR-58, L. reuteri UBLRu-87 (each of 2 billion CFU); L. plantarum UBLP-40, L. casei UBLC-42, L. fermentum UBLF-31, Bifidobacterium bifidum UBBB-55 (each of 1 billion CFU); and fructo-oligosaccharides (100 mg)); 2 = placebo; both groups: dietary (carbohydrate (55–60%), fat (20%), and proteins (20–25%)) + exercise (two sets of exercise plans (phase I and II, 3 months each) | 1 = 48, 2 = 49 | 6 months | USG, T, DHEAS, I, FBS, LH, FSH, HOMA-IR, W, WHR, BMI, QoL | MC (18.8% vs. 6.1%; p = 0.23), T (0.3 vs. 0.29 nmol/L; p = 0.043), WHR (0.02 vs. 0.01; p = 0.027), WC (3.9 vs. 2.3 cm; p = 0.030), QoL (p = 0.034) (1) | [63] |
| Herbal Treatment | |||||||||||
| 38 | Singh et al., 2023 | Double-blind RCT | Rotterdam 2003 | Age: 18–45 years, BMI < 42 kg/m2 | PGIMER, Chandigarh, India | 1 = placebo; 2 = furocyst (500 mg capsule), BD (fenugreek; Trigonella foenum graecum extract); both groups: convential health diet with carbohydrate (40–50%), fat (30%), and protein (20–25%) | 1 = 95, 2 = 113 | 3 months | FBS, FI, SGOT, U, Cr, HDL, LDL, TAGs, LH, FSH, TSH, Pr, USG, SHBG, T, MC, IR, GTT, | FBS (16.63 mmol/L, p = 0.001), LH (26.4%, p < 0.01), FSH (21.1%, p < 0.01), TSH (0.47 mU/L, p = 0.01), Pr (2.51 ng/mL, p = 0.017), MC (41.15%), FG (27.1%), Cy [37.3% (right), 38.2% (left)], G, IR, C (35.76 mg/dL, p = 0.001), LDL (18.05 mg/dL, p = 0.001), TAGs (20.6 mg/dL, p = 0.015), T (0.38 ng/dL, p = 0.001) (2 > 1) | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maan, P.; Gautam, R.; Vasudevan, S.; Menon, G.R.; Arora, A.; Nair, A.; Jabbar, P.K.; Arora, T. Pharmacological and Non-Pharmacological Interventions for Polycystic Ovary Syndrome (PCOS) in Indian Women: A Systematic Review and Meta-Analysis. Pharmaceuticals 2025, 18, 680. https://doi.org/10.3390/ph18050680
Maan P, Gautam R, Vasudevan S, Menon GR, Arora A, Nair A, Jabbar PK, Arora T. Pharmacological and Non-Pharmacological Interventions for Polycystic Ovary Syndrome (PCOS) in Indian Women: A Systematic Review and Meta-Analysis. Pharmaceuticals. 2025; 18(5):680. https://doi.org/10.3390/ph18050680
Chicago/Turabian StyleMaan, Pratibha, Rohit Gautam, Sudharsan Vasudevan, Geetha R. Menon, Amit Arora, Abilash Nair, Puthiyaveettil Khadar Jabbar, and Taruna Arora. 2025. "Pharmacological and Non-Pharmacological Interventions for Polycystic Ovary Syndrome (PCOS) in Indian Women: A Systematic Review and Meta-Analysis" Pharmaceuticals 18, no. 5: 680. https://doi.org/10.3390/ph18050680
APA StyleMaan, P., Gautam, R., Vasudevan, S., Menon, G. R., Arora, A., Nair, A., Jabbar, P. K., & Arora, T. (2025). Pharmacological and Non-Pharmacological Interventions for Polycystic Ovary Syndrome (PCOS) in Indian Women: A Systematic Review and Meta-Analysis. Pharmaceuticals, 18(5), 680. https://doi.org/10.3390/ph18050680










