Abstract
Poly(lactide-co-glycolide) (PLGA) implants have become a cornerstone in drug delivery and regenerative medicine due to their biocompatibility, tunable degradation, and capacity for sustained, localized therapeutic release. Recent innovations in polymer design, fabrication methods, and functional modifications have expanded their utility across diverse clinical domains, including oncology, neurology, orthopedics, and ophthalmology. This review provides a comprehensive analysis of PLGA implant properties, fabrication strategies, and biomedical applications, while addressing key challenges such as burst release, incomplete drug release, manufacturing complexity, and inflammatory responses. Emerging solutions—such as 3D printing, in situ forming systems, predictive modeling, and patient-specific customization—are improving implant performance and clinical translation. Emphasis is placed on scalable production, long-term biocompatibility, and personalized design to support the next generation of precision therapeutics.
1. Introduction
PLGA is a pivotal material in biomedical engineering, extensively utilized for its biocompatibility, tunable degradation, and adaptability in drug delivery and tissue engineering. As an FDA-approved polymer, PLGA facilitates sustained and controlled drug release through implants that degrade into lactic acid and glycolic acid—non-toxic byproducts naturally metabolized by the body [1,2,3]. Its versatility, governed by variations in the lactic-to-glycolic acid ratio, molecular weight, and structural modifications, enables the design of implants tailored for diverse therapeutic domains including oncology, endocrinology, orthopedics, and neurology [4,5,6,7].
Critical implant characteristics such as degradation kinetics, drug release profiles, and mechanical performance are modulated through the polymer composition and incorporation of functional additives. Modifications with poly(ethylene glycol) (PEG), poloxamer, and surfactants improve biocompatibility, reduce burst release, and fine-tune release behavior [2,8,9,10,11,12]. These advancements have enabled prolonged drug delivery over periods ranging from days to several months, supporting applications in chronic disease management, localized chemotherapy, and regenerative therapies [6,13,14].
Despite these benefits, PLGA-based implants encounter key limitations. The initial burst release can compromise therapeutic efficacy, especially in sensitive systems such as ocular and neural delivery routes [15,16]. The acidic microenvironment produced during degradation can destabilize labile biomolecules, while high manufacturing costs and technical complexities hinder large-scale production [17,18,19]. Additionally, variability in polymer composition and environmental conditions may lead to inconsistent release profiles, highlighting the need for stringent quality control and predictive modeling to ensure clinical reliability [12,20,21].
This review presents a comprehensive evaluation of PLGA-based implants, emphasizing material characteristics, fabrication strategies, and application-specific progress. It further addresses prevailing challenges and discusses emerging directions aimed at optimizing PLGA systems for improved therapeutic performance. By integrating interdisciplinary insights, this work seeks to foster continued innovation and collaboration across biomedical fields.
2. Essential Materials for PLGA Implant Design
The versatility and efficacy of PLGA-based implants derive from the precise engineering of their constituent materials, including the polymer matrix, therapeutic agents, functional additives, and structural components. Strategic selection and integration of these materials enable the optimization of drug release kinetics, biocompatibility, and mechanical performance across a broad range of biomedical applications.
2.1. PLGA as a Base Polymer
PLGA is a leading biodegradable polymer for implantable systems due to its tunable degradation rate and drug release profile. These properties can be tailored by adjusting the lactic-to-glycolic acid ratio (e.g., 50:50 or 75:25) and molecular weight (15–53 kDa) [1,5,22,23,24,25,26,27,28,29]. A 50:50 ratio typically results in faster degradation and earlier drug release due to higher hydrophilicity and rapid water uptake, making it suitable for applications requiring quick therapeutic onset, such as oncology or anti-infective therapies [22,26,27]. In contrast, a 75:25 ratio slows water ingress and polymer erosion, enabling more prolonged and sustained release, which is beneficial in chronic indications like hepatitis B or ocular delivery [1,5,30].
Further control over degradation kinetics and drug release behavior is achieved through end-group modifications, such as acid and ester end-capping, which influence surface roughness, mechanical strength, and early release rates [21,30]. The LA:GA ratio also affects implant morphology; faster-degrading 50:50 PLGA generates porosity more quickly and lowers local pH through acidic byproducts, which may be suboptimal for sensitive tissues like the inner ear [21]. In such cases, formulations using higher lactic content or PEG-PLGA blends help delay degradation and stabilize the microenvironment [21,24]. Blending PLGA with other biodegradable polymers like polylactic acid (PLA) or polycaprolactone (PCL) enhances mechanical strength and flexibility, broadening its applicability in drug delivery and regenerative medicine [31,32].
2.2. Therapeutic Agents Delivered via PLGA
PLGA-based implants support controlled delivery of a wide spectrum of therapeutic agents. For inflammation-associated disorders, dexamethasone offers prolonged release in ocular and cochlear applications [16,23,33]. Anticancer agents such as doxorubicin, paclitaxel, and cisplatin are incorporated into PLGA systems for localized delivery in cancers, including gliomas and breast cancer [6,13,26,27]. In regenerative medicine, growth factors like basic fibroblast growth factor (bFGF), recombinant human bone morphogenetic protein-2 (rhBMP-2), and vascular endothelial growth factor (VEGF) facilitate angiogenesis, neurogenesis, and osteogenesis when delivered through PLGA-based scaffolds and microspheres [34,35,36]. Additionally, PLGA serves as a delivery matrix for antibiotics, such as amoxicillin and vancomycin, in dental and ocular infections [29,37] and antiangiogenic agents like lupeol and corosolic acid in treating diabetic retinopathy and macular degeneration [38,39].
2.3. Additives, Nanocarriers, and Structural Enhancements
To optimize drug encapsulation, stability, and release profiles, various additives and structural elements are employed. PEG and acetyltributyl citrate (ATBC) are used to regulate flexibility, swelling, and diffusion, thereby improving the overall implant performance [33,40,41]. Stabilizers such as trehalose and beta-cyclodextrin (β-CD) enhance protein stability and hydrophilicity, supporting the delivery of sensitive biomolecules, including monoclonal antibodies [12,42]. In infection-prone environments, antibacterial agents like nanosilver and copper–selenium nanoparticles are incorporated to bolster antimicrobial activity and prolong implant function [43,44,45].
PLGA-based delivery platforms also utilize nanoparticles, microspheres, and scaffolds to achieve localized delivery and regenerative outcomes. Gold nanoparticles conjugated with antagomiR204, for example, enhance osteogenesis in diabetic patients [46]. Similarly, chitosan-based nanoparticles and PLGA microspheres loaded with doxorubicin or exendin-4 improve encapsulation efficiency and therapeutic outcomes in breast cancer and type 2 diabetes mellitus (T2DM)-associated dental applications [47,48].
Scaffolds integrating β-tricalcium phosphate (β-TCP) or hydroxyapatite (HA) with PLGA improve bone regeneration and osseointegration, key for orthopedic applications [32,49]. For cartilage and soft tissue repair, PLGA scaffolds combined with fibrin gels or human embryonic stem cells demonstrate enhanced mechanical stability and regenerative potential [50,51,52].
2.4. Solvents and Processing Aids
The successful fabrication of PLGA implants depends on the use of suitable solvents and processing aids. Hydrophilic solvents such as N-methyl-pyrrolidone (NMP) and glycofurol are essential in in situ forming systems for creating controlled-release matrices [23,53,54]. Dimethyl sulfoxide (DMSO) facilitates rapid solidification in injectable formulations, while agents like triacetin and ethyl heptanoate adjust viscosity and mitigate burst release, contributing to a more predictable and sustained drug release profile [55,56].
Table 1 examines the detailed composition of PLGA-based implants, including additives like PEG, PVA, and excipients. It links composition choices to their effects on implant properties, degradation, and release kinetics. Strategic integration of additives within PLGA systems allows for precise control over key characteristics, such as mechanical stability, biocompatibility, and drug release profiles. Excipients like PEG improve hydrophilicity, promoting uniform drug release, while surfactants reduce burst effects, ensuring steady delivery. Antimicrobial agents enhance biocompatibility and implant sterilization potential. The choice of lactide-to-glycolide ratios influences degradation times, allowing formulations to be customized for short-term or extended therapies. This table highlights how material design in PLGA implants enables the creation of safe, stable, and effective systems, supporting a range of therapeutic applications.

Table 1.
Impact of composition and additives on PLGA implant properties.
3. Key Properties of PLGA Implants for Medical Applications
PLGA-based implants are characterized by their tunable material and structural properties, enabling precise control over drug release, mechanical performance, and biocompatibility. These implants are engineered by integrating therapeutic agents, functional additives, and design strategies to meet specific clinical needs. Core attributes such as release kinetics, porosity, degradation behavior, and functional integration are tailored to optimize therapeutic outcomes across diverse medical applications.
3.1. Controlled Release Kinetics
Sustained drug release over extended durations is a hallmark of PLGA implants, reducing administration frequency while enhancing treatment efficacy. Rilpivirine-loaded systems sustain HIV therapy for 42 days [2], and ciprofloxacin hydrochloride implants maintain therapeutic levels for up to 65 days [82]. Drug release profiles range from biphasic to triphasic, accommodating both small molecules like ibuprofen and proteins such as cytochrome C [25,83]. Additives, including albumin–oleic acid conjugates (AOC) [62] and PEG [3], minimize burst release and promote near-zero-order kinetics, as observed in paclitaxel-loaded microspheres. Additionally, shape-controlled implants enhance release uniformity by maintaining consistent surface-to-volume ratios [84].
3.2. Porosity and Morphology
Implant porosity and surface morphology critically influence drug diffusion and tissue compatibility. Porous structures facilitate water uptake and degradation, accelerating drug release, as demonstrated in hydrogel–PLGA systems [85]. Stabilizing agents like MPEG and shellac protect labile proteins and peptides while maintaining controlled release [11,86]. Shellac functions as a pH-responsive barrier, shielding acid-labile biomolecules from the acidic microenvironment created by PLGA degradation and enabling delayed, more complete release once the matrix pH rises [11]. PEG, particularly in MPEG-PLGA diblock copolymers, enhances water absorption and matrix swelling, improving diffusion and uniform degradation, which reduces protein aggregation and supports sustained release [86]. In contrast, denser morphologies prolong degradation, supporting long-term drug delivery for conditions such as infections, prostate enlargement, and substance use disorders [57,61,87]. In situ forming implants (ISFIs) employ phase inversion to dynamically regulate porosity and control release kinetics [9].
3.3. Swelling and Degradation Behavior
Swelling and biodegradation directly impact drug release rates and mechanical integrity. PLGA systems can swell by up to 1700%, facilitating controlled diffusion of encapsulated agents [22]. The customization of lactic/glycolic content, molecular weight, and environmental pH allows precise modulation of degradation. For example, leuprolide acetate implants utilize closed-pore architectures to regulate hormonal therapy [88]. As shown in Figure 1, methotrexate (MTX) release from PLGA/PLA-coated chitosan implants correlates with structural swelling, underlining the importance of morphology in drug delivery performance.
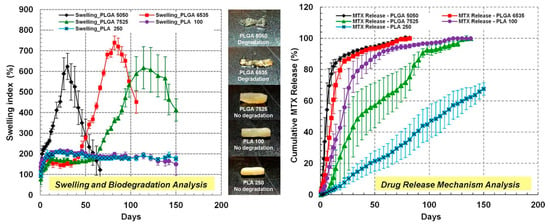
Figure 1.
The swelling index and cumulative MTX drug release from PLGA/PLA-coated chitosan-based micro-implants. The figure shows pictures of different combinations of PLGA/PLA-coated chitosan–MTX micro-implants: PLGA 5050, PLGA 6535, PLGA 7525, PLA 100, and PLA 250. These implants provided MTX release for up to 5 months and a delayed swelling and biodegradation of the micro-implants. Adopted with permission [76].
3.4. Thermal and Mechanical Stability
Thermal and mechanical resilience is essential for the functionality and durability of PLGA implants during processing and implantation. The incorporation of hydroxyapatite (HA) or β-tricalcium phosphate (β-TCP) significantly improves mechanical strength, elasticity, and environmental durability—key requirements for orthopedic applications [82]. For instance, HA-grafted PLGA formulations demonstrate more than double the tensile strength of unmodified PLGA, and β-TCP/PLGA scaffolds maintain structural integrity in wet environments, supporting guided bone repair [32,49].
3.5. Biocompatibility and Reduced Inflammation
Biocompatibility is a critical determinant of implant success. PLGA systems are engineered to reduce immune responses and promote tissue integration. Scaffolds composed of small intestinal submucosa (SIS) exhibit reduced inflammation compared to conventional PLGA materials [50]. In neural applications, PLGA coatings mitigate glial scar formation, enhancing implant–tissue compatibility [89]. Furthermore, PLGA-coated titanium and ZrO2 implants improve osteoblast proliferation and antibacterial performance, validating their utility in dental and orthopedic settings [90,91].
3.6. Multifunctionality and Enhanced Therapeutic Outcomes
PLGA-based implants increasingly serve multifunctional roles, delivering multiple therapeutic benefits beyond controlled release. Dual drug–particulate systems enable concurrent delivery of vaccines and therapeutics, improving efficacy in immunological and infectious disease contexts [92]. MRI-visible implants support real-time tracking of degradation and drug release, advancing precision diagnostics [93]. Antiangiogenic systems delivering lupeol or corosolic acid show comparable efficacy to Bevacizumab in retinal disease models [38,39]. Hybrid PLGA–gelatin systems target lymphatic pathways, offering improved delivery for metastatic conditions [73].
The therapeutic utility of PLGA systems extends across numerous clinical applications. Curcumin-loaded formulations demonstrate anti-inflammatory and survival benefits in cancer models [31], while titanium implants coated with testosterone–alendronate enhance bone integration [94]. In diabetic bone regeneration, bFGF-loaded microspheres improve osteogenesis and implant contact [36]. Optimal pore sizes (300–500 µm) in PLGA scaffolds facilitate lamellar bone formation and collagen alignment, reinforcing their value in regenerative medicine [95].
Table 2 highlights the critical physicochemical properties of PLGA-based implants, such as porosity, molecular weight, degradation profiles, and release kinetics, which are essential for tailored drug delivery and therapeutic efficacy. These properties are meticulously engineered, linking structural and functional components to achieve optimized therapeutic outcomes. For example, controlled porosity and molecular weight influence degradation rates and drug release patterns, while hydrophilicity and polymer ratios affect swelling and drug mobility. Stability under physiological conditions ensures long-term effectiveness, while structural integrity supports applications in bone repair and tissue scaffolding.

Table 2.
Critical physicochemical traits influencing PLGA implant performance.
4. Fabrication Techniques for Tailored PLGA Implants
The versatility of PLGA-based implants stems from a broad array of fabrication techniques that allow precise control over drug release, mechanical strength, and biological interactions. These methods integrate structural, therapeutic, and functional elements, enabling custom-tailored systems for specific clinical applications.
4.1. Solvent-Based Fabrication Techniques
Solvent-based methods are extensively employed in PLGA implant design. Phase separation using solvents such as dimethyl sulfoxide (DMSO) enables precise drug incorporation and controlled solidification [61,82]. Coacervation with dichloromethane improves drug encapsulation efficiency, while solvent exchange systems with N-methyl-2-pyrrolidone (NMP) and glycofurol facilitate sustained in situ formation [23,53,82]. A novel reversed-phase separation/coacervation approach produced flexible ciprofloxacin-loaded PLGA implants capable of 65-day antibiotic release [82]. Microfabrication technologies like UV-LIGA have been utilized to engineer cisplatin implants with micro-chamber structures for precise release modulation [113].
4.2. Hot Melt Extrusion (HME) for Controlled Release
Hot melt extrusion (HME) offers a solvent-free fabrication route, promoting uniform drug distribution and mechanical stability. Plasticizers improve porosity and drug release rates, as demonstrated in implants loaded with ibuprofen [4], dexamethasone [22,68], and BSA [85]. Mini-scale HME also supports bioactive protein encapsulation [104]. Figure 2 shows the morphological evolution of a dexamethasone-loaded PLGA implant over 21 days in PBS [33]. HME has also proven effective for creating long-acting drug delivery systems, including ovalbumin-based proteins, monoclonal antibodies [42], and gentamicin sulfate implants for osteomyelitis, with encapsulation efficiencies of 85–115% and sustained profiles comparable to PMMA-based Septopal® [70].
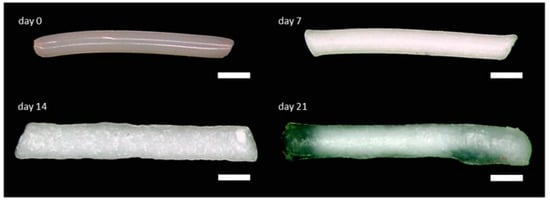
Figure 2.
The morphology of a biodegradable dexamethasone-loaded PLGA implant prepared by hot melt extrusion, shown at day 0 and after incubation in PBS at day 7, day 14, and day 21, that provided controlled 80% of intracochlear drug release for up to 3 weeks. Scale bar indicates 500 µm. Adopted with permission [33].
4.3. In Situ Forming and Injectable Implants
In situ forming implants (ISFIs) use solvent exchange or phase inversion to solidify PLGA in vivo, enabling minimally invasive and sustained drug delivery. Leuprolide acetate ISFIs reduce burst release while maintaining prolonged release [64,88,101]. Solvents like NMP and DMSO control solidification and long-term kinetics, particularly in oncology and psychiatric treatments [15,55,114]. Injectable formulations form gel matrices upon contact with body fluids, offering tunable release profiles [115]. For instance, ISFIs incorporating rosuvastatin and copper–selenium nanoparticles achieved combined antimicrobial and anticancer activity for breast cancer treatment [43].
4.4. Advanced 3D Printing Techniques
Three-dimensional printing enables precise control over implant geometry, porosity, and drug loading. Technologies such as fused deposition modeling (FDM) and Arburg Plastic Freeforming (APF) are used to create ibuprofen-loaded PLGA meshes with controlled release characteristics [17,59]. Figure 3 shows optical macroscopy images of these printed meshes [59]. Comparative studies show that FDM yields more porous structures with faster release, whereas APF enables extended profiles. Infill density is a critical parameter affecting release kinetics, particularly in bone regeneration scaffolds [106].
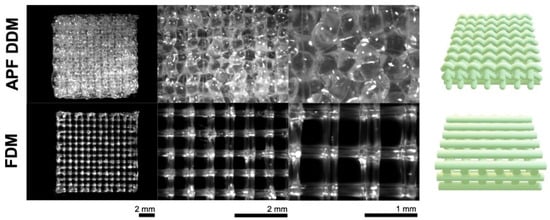
Figure 3.
Optical macroscopy pictures of ibuprofen-loaded PLGA implants (meshes) prepared with the use of Droplet Deposition Modeling (APF DDM) or Fused Deposition Modeling (FDM), before being exposed to the release medium. On the right-hand side are illustrations of the real structures of the mesh-shaped implants. Adopted with permission [59].
4.5. Microspheres, Nanoparticles, and Scaffold Fabrication
Microspheres and nanoparticles allow precise drug encapsulation and controlled release. Oil/water emulsion techniques support the loading of proteins and growth factors such as cytochrome C and bFGF [36,83,116]. Microfluidic emulsification improves monodispersity and release control [107], while spray drying and freeze milling ensure stability and mechanical integrity in wafers and particles [10,86]. Hybrid PLGA particles have been engineered for enhanced therapeutic effects, including pH-responsive curcumin nanoparticles via nanoprecipitation and lipid–PLGA systems for antipsychotic delivery [117]. Melt-grafted PLGA with hydroxyapatite (HA) or β-tricalcium phosphate (β-TCP) improves mechanical properties and regenerative potential [49,99]. Scaffolds and plugs are fabricated through direct printing or extrusion methods for applications in bone regeneration and annular defect repair [95,118]. PLGA/fibrin scaffolds support nerve ingrowth, particularly in disk degeneration therapies [52].
4.6. Surface Engineering and Hybrid Systems
Surface coating and layering techniques optimize implant performance through improved biocompatibility and drug delivery. Electrospraying and dip-coating methods are used to apply uniform PLGA layers on titanium and chitosan substrates for targeted antibiotic or growth factor release [35,90,102]. Ultrasonic spray coating allows testosterone–alendronate integration for orthopedic use [94]. Microsphere attachment at room temperature enhances antibacterial efficacy on titanium surfaces without compromising osteoblast function [119]. Microneedle-based implants fabricated via casting-mold techniques enable self-administered levonorgestrel (LNG) release with strong mechanical properties [75,109].
4.7. Real-Time Monitoring and Structural Optimization
Innovative designs and imaging tools enable real-time feedback and structural optimization of PLGA implants. Honeycomb-like microstructures fabricated using microlithography support linear drug release patterns [120]. MRI and EPR spectroscopy allow non-invasive tracking of degradation and drug distribution, facilitating the development of more predictable delivery systems [93].
Table 3 details the processing methods and manufacturing techniques used for PLGA-based implants, focusing on techniques like solvent evaporation, microfluidics, and hot melt extrusion, and their impacts on drug encapsulation and implant properties. Processing methods play a pivotal role in defining the efficacy of PLGA-based implants. Solvent evaporation ensures uniform particle size, while microfluidics enhances drug loading efficiency. Techniques like hot melt extrusion allow for high-temperature processing, supporting controlled release. Innovations like 3D printing enable implants tailored to individual anatomical needs. These methods also optimize implant integrity, shape, and biocompatibility, demonstrating the industry’s emphasis on precision engineering.

Table 3.
Manufacturing techniques and their role in PLGA implant design.
5. Testing and Validation of PLGA Implants
Comprehensive testing and validation are critical for ensuring the safety, efficacy, and clinical applicability of PLGA-based implants. Evaluations encompass physicochemical characterization, drug release behavior, biocompatibility, degradation kinetics, and therapeutic performance across diverse biomedical contexts.
5.1. Drug Release and Kinetics
Drug release assessments are foundational in validating implant performance. In vitro and in vivo studies characterize initial burst, sustained release, and long-term stability [1,40,76,96]. Pharmacokinetic evaluations, including plasma concentration analysis and in vitro-in vivo correlation (IVIVC), confirm the reliability of drug delivery [20,134,137]. Additionally, swelling and degradation studies inform drug stability and therapeutic window optimization [22,54,128].
5.2. Material Properties and Fabrication
Material characterization techniques are essential for assessing implant structure–function relationships. Thermal and mechanical properties are analyzed using differential scanning calorimetry (DSC), X-ray diffraction (XRD), and tensile testing [16,30,82]. Surface morphology and porosity—key to coating stability and release kinetics—are examined via scanning electron microscopy (SEM) and gravimetric analysis [21,29,57]. For precision manufacturing, 3D printing and microfabrication outputs are validated for geometric accuracy and release control [42,106,120]. Nanoparticles and microspheres are evaluated for particle size, zeta potential, and encapsulation efficiency to ensure consistency and bioavailability [3,108,138].
5.3. Degradation and Stability Studies
PLGA degradation studies evaluate implant longevity and functional stability. Polymer composition, additives, and environmental factors influence degradation rates, assessed through mass loss, pH shifts, and erosion analysis [95,139,140]. Both real-time and accelerated degradation protocols provide insights into behavior under physiological and stress conditions [111,141]. In vivo subcutaneous and intrathecal models further confirm structural integrity and degradation in biological systems [142,143].
5.4. Biocompatibility and Toxicity
Biocompatibility is validated via histological analysis and cytotoxicity assays to ensure minimal inflammatory response and optimal tissue integration [28,144,145]. Functional enhancements, including anti-inflammatory coatings and bioactive agents, contribute to reduced tissue reactivity. Long-term toxicity studies assess systemic and localized safety in ocular and systemic therapies [112,123,137]. In regenerative contexts, histomorphometric analyses evaluate tissue integration, fibrosis suppression, and adipose tissue formation [141,146].
5.5. Application-Specific Evaluations and Therapeutic Performance
PLGA implants are rigorously tested for efficacy in their target medical applications. In bone regeneration, micro-computed tomography (micro-CT) and histological methods assess osteogenic differentiation, bone–implant contact, and peri-implant growth [36,48,147]. Cartilage repair studies focus on glycosaminoglycan (GAG) content, collagen fiber alignment, and osteochondral integration [148,149]. Figure 4 illustrates the impact of continuous passive motion combined with acellular PLGA implants on osteochondral healing in rabbits [148].
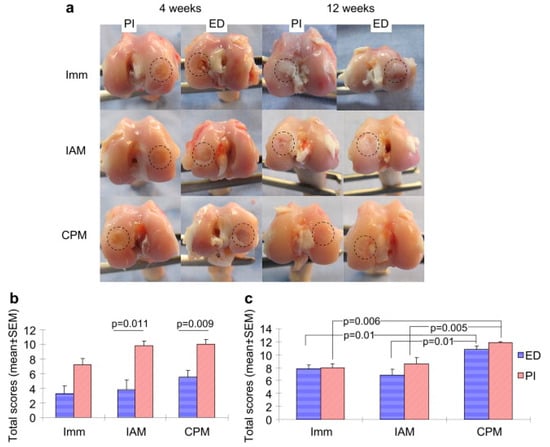
Figure 4.
The gross appearances of osteochondral regeneration in rabbit in three treatments: continuous passive motion (CPM), immobilization (Imm) and intermittent active motion (IAM) with PLGA implant (PI) and without PLGA implant as empty defect (ED) at weeks 4 and 12 postoperatively. The circle on the diagram shows the area of focus for osteochondral regeneration for each treatment. (a) Shows the quantitative scores of gross appearances at 4 weeks. (b) Shows the quantitative scores of gross appearances at 12 weeks. (c) Shows the quantitative scores of gross appearances after surgery. Adopted with permission [148].
In neural applications, disk height index, vascular proliferation, and functional recovery are evaluated through spinal repair models [52,118,150]. Antimicrobial efficacy is tested using infection models involving nanosilver or vancomycin-loaded systems [29,37,45]. Functional assessments such as tail-flick, paw-withdrawal, and locomotor recovery quantify therapeutic impact in spinal and intrathecal delivery [143,150,151]. Antiangiogenic activity is analyzed through CAM assays and HUVEC migration, particularly in ophthalmology and oncology [38,39]. Clinical outcomes, complication rates, and overall effectiveness are evaluated in patient trials [67,152,153].
5.6. Imaging and Monitoring Techniques
Advanced imaging supports real-time analysis of implant behavior. MRI, micro-CT, and X-ray imaging provide structural and bioactivity data critical for tissue integration studies [33,148,152]. Molecular characterization tools such as FTIR and HPLC-UV elucidate drug–polymer interactions and degradation profiles. Immunofluorescence imaging captures cellular responses, aiding the validation of therapeutic efficacy and biocompatibility [39,103,125].
5.7. Statistical and Computational Approaches
Computational modeling and statistical analysis facilitate implant design and performance validation. Finite element modeling simulates drug diffusion, polymer degradation, and mechanical behavior. Statistical methods, including Box–Behnken design and ANOVA, identify optimal formulation parameters and ensure reproducibility [55,84,136]. These approaches support precision engineering and predictive evaluation of PLGA systems.
Table 4 lists the diverse testing and evaluation methodologies employed for PLGA-based implants, including in vitro, in vivo, and imaging-based tests to validate their release profiles, biocompatibility, and therapeutic efficacy. These methods evaluate how polymer matrices, additives, and therapeutic agents work together to optimize implant performance. In vitro studies assess encapsulation efficiency, release kinetics, and degradation rates, while in vivo analyses confirm biocompatibility and therapeutic outcomes. Advanced imaging techniques, such as MRI and fluorescence microscopy, provide real-time insights into implant behavior. Biomechanical and histological tests ensure implant integration and tissue compatibility. This comprehensive validation guarantees that PLGA implants meet clinical and regulatory standards, ensuring their safety and efficacy for patient use.

Table 4.
Validation methods for PLGA implant safety and efficacy.
6. Tailored Therapeutic Effects of PLGA Implants
PLGA implants can be precisely engineered to respond to a range of physical and biological stimuli, enabling controlled drug release, site-specific action, and improved therapeutic outcomes. This section presents studies that highlight how PLGA implants are tuned to exogenous and endogenous triggers to optimize clinical performance.
6.1. Exogenous Stimuli
External stimuli such as ultrasound, mechanical forces, temperature, magnetic fields, and light have been used to enhance drug release dynamics, guide tissue regeneration, and modulate implant degradation. These approaches offer non-invasive and controllable strategies for improving treatment efficacy. However, their clinical feasibility depends on factors such as safety, regulatory clearance, and device compatibility.
Ultrasound: Ultrasound enhances PLGA degradation and swelling, enabling externally modulated drug release. Ultrasound-assisted imaging has been used to monitor phase inversion, correlating it with drug release kinetics [5]. In fluorescein-loaded PLGA systems, ultrasound improved drug penetration by 1.7- to 5.6-fold in soft tissue-mimicking environments, but not in PBS, indicating its effectiveness in biological matrices [158]. When combined with high-intensity focused ultrasound (HIFU), PLGA implants releasing doxorubicin improved tumor penetration, apoptosis, and shrinkage over HIFU treatment alone [159]. Notably, these applications utilize clinically established modalities, and ultrasound’s non-invasive nature and real-time imaging compatibility make it one of the most translatable external triggers.
Magnetic Fields: Magnetic field-responsive PLGA systems enable localized therapy with high precision. A PLGA(Ag-Fe3O4)-coated dental implant promoted osteogenesis and antibacterial activity under magnetic stimulation [44]. Injectable PLGA/Fe3O4 implants carrying cisplatin generated localized hyperthermia under alternating magnetic fields (AMF), leading to tumor necrosis and immune activation [72]. Similarly, phase-transitional PLGA-Fe implants responded to AMF by triggering doxorubicin release and tumor cell death, with no leakage and visibility under CT and ultrasound, meeting essential safety and monitoring requirements [74]. These systems demonstrate clinical potential through their integration with imaging tools and effective retention at target sites [159].
Light-Responsiveness: Light-activated PLGA systems allow spatiotemporally controlled drug release. Methacrylated alginate (MA)-PLGA ISFIs were photocrosslinked to achieve sustained intravitreal peptide delivery, with excellent biocompatibility and suitability for ophthalmic applications [108]. Such technologies are promising for minimally invasive and localized drug delivery, particularly where external light application is already routine, such as in ophthalmology and dermatology.
Mechanical Forces: Mechanical properties affect drug diffusion and implant behavior. Larger implant diameters slow drug diffusion by enhancing mechanical stability [8]. Continuous passive motion (CPM) combined with acellular PLGA scaffolds enhanced osteochondral regeneration in rabbits, with improved collagen expression and bone formation [148]. A PLGA–graphene scaffold increased bone volume by 50% in rats [129], while a bilayered PVA/PLGA shuttle supported deeper neural probe insertion for chronic electrophysiology [160]. Shape design, such as the “basket-in-tube” structure, stabilized release kinetics by minimizing implant movement [84]. Electrospun PLGA nanofibers co-loaded with fusidic acid and rifampicin prevented MRSA and S. epidermidis colonization on titanium implants [128].
Temperature Modulation: Temperature affects polymer porosity and drug diffusion. While heat exposure altered crystalline content in ibuprofen-loaded PLGA, it had a minimal impact on overall release [4]. Higher temperatures increased porosity and reduced burst release, while lower temperatures caused denser matrices and faster initial release [9]. Temperature also influenced microclimate pH, impacting hydrophilic drug stability during degradation [18].
In summary, while all stimuli-responsive systems offer promising control over drug release, ultrasound and magnetic field-based systems currently show the highest clinical feasibility. This is due to their non-invasive nature, established use in clinical imaging and therapy, and compatibility with real-time monitoring tools. Importantly, these modalities often leverage FDA-cleared devices, potentially reducing regulatory barriers. Their integration with implantable drug delivery systems has demonstrated both preclinical efficacy and controlled, localized drug activation. Continued progress in device integration, standardized protocols, and regulatory alignment will be essential to fully realize their clinical potential.
6.2. Endogenous Stimuli
PLGA implants are also responsive to biological stimuli such as enzymes, pH, inflammatory signals, and disease-specific environments. These factors influence degradation, drug release behavior, and host interaction, allowing for context-specific therapeutic adaptation.
Enzyme Sensitivity: Endogenous enzymes modulate PLGA degradation and drug release kinetics. Rilpivirine- and paclitaxel-loaded implants showed enzyme-mediated alterations in release profiles [2,6,13]. Lysozyme accelerated degradation in PLGA-PEG block copolymers, though co-encapsulated MgCO3 buffered acidic byproducts and preserved protein integrity [24]. BMP-2 release from layer-by-layer coated PLGA implants was enzyme-regulated, enhancing osteoblast activity [97].
pH Sensitivity: PLGA degradation produces acidic byproducts that affect stability. Dexamethasone-loaded PLGA systems exhibited pH-dependent release governed by glycolic acid content and end-group chemistry [68]. Acidic environments caused protein aggregation in PLGA matrices, countered by shellac incorporation for stabilization [11]. Swelling-induced osmotic pressure led to up to 1700% volume expansion, delaying release due to hydrophobic resistance to water infiltration [22]. PLGA/PLA nanoparticles modified with pH-sensitive surfactants improved antibacterial activity and corrosion resistance [71].
Disease Microenvironment Sensitivity: Implant behavior varies with disease context. In spinal cord injury models, scaffold stiffness influenced inflammation and neuroprotection, with softer scaffolds promoting regeneration [157]. In stroke models, VEGF-loaded PLGA microparticles with hNSCs enhanced vascularization and neural growth, though some hypervascularization was noted [34]. In Alzheimer’s disease, VEGF-loaded nanospheres increased hippocampal neurogenesis [79]. In cancer, doxorubicin-loaded implants improved local tumor suppression in osteosarcoma [161], and 5-FU-loaded PLGA systems sustained peritoneal concentrations in colon cancer while minimizing systemic toxicity [162].
Inflammatory Response and Immune Modulation: PLGA interactions with immune cells influence biocompatibility. SIS scaffolds elicited less inflammation than PLGA, while SIS/PLGA hybrids showed intermediate responses [50]. PLGA/PVA hydrogels with dexamethasone extended anti-inflammatory release for up to 95 days [146]. Predegraded dexamethasone microspheres reduced foreign body reactions when integrated into sutures [69].
Bone Regeneration and Osseointegration in Disease Contexts: PLGA implants support bone healing in compromised environments. bFGF-loaded microspheres improved osseointegration in diabetic rats [36], and exendin-4-loaded chitosan–PLGA microspheres promoted osteogenesis in diabetic models [48]. Zoledronate-loaded PLGA microcapsules reduced bone loss and inflammation in periodontitis by modulating cytokine expression [163]. A PLGA-coated implant releasing testosterone and alendronate enhanced mineralization and bone–implant contact [94].
Ocular and Neurological Applications: PLGA systems provide sustained release for chronic ocular diseases. Tacrolimus-loaded PLGA implants delivered the drug over six weeks with no ocular toxicity [137]. Lupeol-loaded PLGA systems suppressed endothelial proliferation and neovascularization in macular degeneration [38]. For glioblastoma, paclitaxel-loaded implants released the drug over 42 days, reducing tumor volume by 30-fold with 5 mm diffusion from the implant site [131].
7. Applications and Benefits of PLGA-Based Implants
PLGA-based implants are pivotal in modern biomedicine, offering precision-controlled drug release, support for tissue regeneration, and effective infection management. Their tunable design—enabled by structural modifications, functional additives, and drug–polymer integration—has expanded their clinical utility across diverse therapeutic domains.
7.1. Sustained and Controlled Drug Release
PLGA implants enable prolonged, programmable drug release, reducing dosing frequency and improving patient adherence. Customizable release profiles incorporating burst, diffusion, and erosion phases support precise therapeutic control [1,2,15,82,115]. For instance, rilpivirine implants extend HIV-1 therapy to 42 days [2], while entecavir implants maintain therapeutic levels for hepatitis B with 77.84% drug entrapment efficiency [1]. Tailoring molecular weight and additives like PEG and poloxamer allows optimization of drug kinetics across applications [3,10,12,164].
7.2. Localized and Minimally Invasive Drug Delivery
PLGA implants enhance site-specific delivery while minimizing systemic side effects. Ibuprofen-loaded implants offer localized analgesia [4], and tamsulosin implants release the drug over 10 days with reduced systemic exposure [61]. In oncology, PLGA-based systems deliver paclitaxel, carboplatin, and doxorubicin directly to tumor sites, improving efficacy and reducing toxicity [6,47,165]. VEGF-loaded PLGA implants support neurogenesis in stroke and Alzheimer’s models [34,79].
Innovative platforms like in situ forming implants (ISFIs) bypass gastric degradation and improve insulin bioavailability in diabetes therapy [166]. Microneedle-based PLGA systems enable long-term contraceptive delivery through minimally invasive methods [109]. These delivery routes have also been applied in glioblastoma and colon cancer treatments [6,122,165].
7.3. Mitigation of Burst Release and Drug Stability
PLGA formulations are engineered to minimize initial burst release and enhance the stability of labile drugs. Excipients like albumin–oleic acid conjugates and beta-cyclodextrin improve release profiles and reduce toxicity [12,62,167]. PLGA also stabilizes sensitive biologics, including human growth hormone (hGH) and insulin, preserving therapeutic efficacy [166,168]. Among various techniques, end-group modification—such as using ester-terminated PLGA—has shown significant promise by reducing hydrophilicity and early water uptake, thereby lowering burst release in clinically relevant formulations [2]. Phase inversion control, through manipulation of temperature and solvent systems, also minimizes burst by forming denser matrix structures during implant formation [9,56,88]. Additionally, PEG-based copolymer blends like PLGA-PEG-PLGA reduce burst release and align well with pharmacokinetic profiles of marketed products, supporting their clinical translatability [127]. Figure 5 shows a schematic of a PLGA-based implant incorporating shellac as a protective excipient for encapsulating the model protein ovalbumin (OVA), alongside a graph illustrating the cumulative release profile over 98 days. The triphasic release behavior, enabled by the integration of shellac as a material component, demonstrates how such modifications optimize drug delivery. Implants without shellac, in contrast, show significantly reduced protein release over the same period [11].
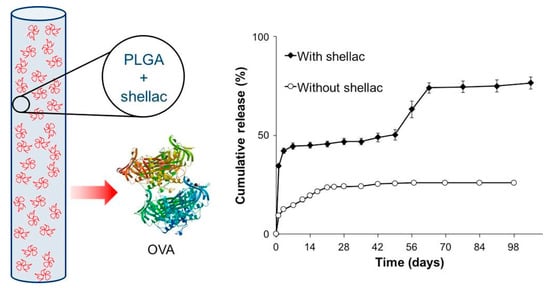
Figure 5.
PLGA-based implants for the acid-labile model protein ovalbumin with the use of shellac polymer as a protective excipient. This implant demonstrated a triphasic release profile providing a slow diffusion phase over 7 weeks and an erosion-controlled dissolution phase for 3 weeks. Adopted with permission [11].
7.4. Advances in Regenerative Medicine
PLGA scaffolds are widely applied in bone and dental tissue engineering. The integration of β-TCP and hydroxyapatite enhances bone regeneration and osseointegration, supporting femoral defect and dental repair [65,99]. Antibacterial agents such as nanosilver and Ag-Fe3O4 nanoparticles improve implant safety and promote osteoblastic activity [44,45]. In neural applications, flexible PLGA-coated devices reduce glial scarring and inflammation, promoting neural interface integration [89]. Growth factor-loaded scaffolds containing BDNF or VEGF accelerate regeneration in spinal cord injury models, demonstrating promise in neurorehabilitation [151,157].
7.5. Specialized Applications
PLGA systems address a range of specialized medical needs. PEG-modified PLGA implants enhance radiosensitization in hypoxic tumors, optimizing therapeutic outcomes in cancer [3]. Antibiotic-loaded PLGA implants, including Eudragit-modified systems, extend treatment efficacy against Pseudomonas aeruginosa for up to 12 days [87]. The precise delivery of chemotherapeutics and growth factors positions PLGA as a leading material for treating gliomas, breast cancer, and neurovascular disorders [6,47,165].
7.6. Enhancing Biocompatibility and Safety
Biocompatibility is enhanced through surface engineering and additive incorporation. PEG-modified PLGA and SIS scaffolds mitigate the acidic byproducts of degradation, reducing inflammatory responses [24,50,68]. These strategies are especially relevant in neural and ocular applications, where inflammation is a major concern. PLGA-PEG copolymers buffer local acidity and support sustained protein release with reduced immune activation [24], while SIS blending significantly attenuates host tissue responses compared to PLGA alone [50]. Surface coatings, such as PLGA-Parylene C, and shape-optimized designs improve mechanical integrity while refining drug release characteristics [84,103,119]. Additionally, attaching PLGA microspheres to implant surfaces enables localized drug delivery with antibacterial effects and promotes cell adhesion, further supporting long-term biocompatibility [119].
Table 5 emphasizes the proven benefits of PLGA-based implants, from controlled drug release and biocompatibility to enhanced patient compliance, highlighting their contributions to diverse therapeutic and diagnostic advancements. Their versatility arises from the ability to tailor structural and functional components to meet specific clinical needs. PLGA-based implants offer unparalleled advantages, including extended drug release, reduced dosing frequency, and localized treatment with minimal systemic effects. Their biocompatibility ensures safe degradation into metabolizable components. Through customized designs, such as modifying shape, surface characteristics, and release profiles, these implants are optimized for precision medicine approaches, addressing chronic and complex diseases across oncology, neurology, and orthopedics. Customizable properties, like shape and release profiles, enable precision medicine approaches. Moreover, their integration of diagnostic and therapeutic capabilities (theranostics) demonstrates the cutting-edge potential of PLGA implants. By combining therapeutic effects with diagnostic functionality, PLGA implants represent a cutting-edge solution for modern healthcare challenges, significantly improving patient outcomes and quality of life.

Table 5.
Advances in therapeutic and diagnostic benefits of PLGA implants.
Table 6 explores the diverse application areas of PLGA-based implants across medical fields. It categorizes implants based on therapeutic domains like oncology, endocrinology, neurology, and orthopedics, providing insights into how these implants support targeted treatments and long-term drug delivery. PLGA-based implants are versatile, addressing chronic conditions requiring sustained delivery systems. Oncology benefits from localized therapies that minimize systemic side effects, while endocrinology employs implants for hormonal regulation. Neurology applications focus on neural repair and inflammation control, and orthopedics utilizes implants for tissue regeneration and infection prevention. The ability to customize PLGA systems through the incorporation of functional materials ensures their adaptability to unique medical challenges, while their biodegradability ensures minimal residual toxicity, reinforcing their role as trusted systems for systemic and local treatments.

Table 6.
Therapeutic applications of PLGA implants across medical fields.
8. Customization of Implants for Individual Patients
Customized PLGA implants play a critical role in advancing personalized medicine by addressing patient-specific variables such as age, gender, disease condition, tissue type, and defect size. Recent advances in fabrication methods, computational modeling, imaging techniques, and smart materials have enabled the development of biomimetic implants tailored for individualized treatment.
8.1. Personalized Medicine Factors: Age, Gender, Health, and Defect Characteristics
Bone Regeneration and Healing Response: Bone healing capacity varies based on factors such as age, metabolic condition, and tissue density. Insulin-loaded PLGA microspheres enhanced peri-implant bone regeneration in diabetic conditions, demonstrating the value of customized drug release in compromised healing environments [7]. β-TCP/PLGA root analogs showed successful bone-like tissue formation after three months, supporting the application of personalized dental implants [65]. In diabetic models, PLGA-gold nanoparticle (AuNP) systems delivering antagomiR204 improved osseointegration, addressing disease-specific healing deficiencies through gene-modulated therapy [46]. Exendin-4-loaded chitosan–PLGA microspheres also enhanced osteogenic differentiation, further confirming the utility of targeted drug release for metabolic bone disorders [48].
Optimized Drug Delivery for Individual Needs: Customized drug release kinetics can significantly impact therapeutic efficacy. A Box–Behnken design optimized an injectable risperidone PLGA implant, minimizing burst release and achieving 51.08% drug release over 40 days—an alternative to conventional microsphere-based formulations for schizophrenia [55]. Similarly, PLGA-loaded mitomycin C (MMC) implants supported glaucoma surgery by sustaining intraocular pressure reduction [175]. Structural variations in PLGA-based dexamethasone intravitreal implants influenced release behavior, underlining the need for physiologically relevant test models [140].
8.2. Biomimetic Design: Scaffold Rigidity, Pore Size, and Implant Shape
Tissue-Specific Rigidity Optimization: PLGA scaffold stiffness plays a pivotal role in tissue-specific regeneration. In spinal cord injury (SCI) repair, soft scaffolds promoted neuroprotection, while stiffer versions induced mesodermal differentiation, highlighting the need for application-specific rigidity [157]. In cartilage engineering, MSC-seeded PLGA scaffolds exhibited time-dependent regeneration, reinforcing the importance of patient- and defect-specific implant design [173]. Flexible PLGA scaffolds seeded with chondrocytes supported meniscal healing under both static and dynamic conditions in a mouse model [176].
Optimizing Pore Size and Surface Characteristics: Pore size and surface architecture are pivotal in guiding tissue integration and regeneration. PLGA scaffolds with pores in the range of 300–500 µm have been shown to optimally support lamellar bone formation and promote collagen and hydroxyapatite deposition, outperforming both smaller and larger pore sizes [95]. Complementarily, surface-engineered implants—such as PLGA films deposited on TiO2 nanotube arrays—enable sustained release of osteogenic factors like rhBMP-2. A 250 nm thick PLGA layer demonstrated controlled release over four weeks while significantly enhancing osteoblast adhesion, proliferation, and differentiation, underscoring the potential of nanoscale modifications for targeted bone healing [35].
8.3. Biomechanical and Environmental Influences on Custom Regeneration
Exercise-Based Enhancement of Tissue Healing: Mechanical stimulation can significantly enhance implant performance. Acellular PLGA scaffolds combined with treadmill activity improved cartilage regeneration in minipigs [149]. Similarly, early treadmill exercise in rabbits accelerated cartilage repair, reduced inflammation, and enhanced trabecular integration when used with PLGA scaffolds [78].
Customized Pain Management: For postoperative applications, PLGA implants delivering racemic bupivacaine or Novabupi® provided 30-day pain relief. Variations in polymer composition modulated release kinetics, validating the importance of personalized implant formulations for analgesia [139].
8.4. Advanced Technologies for Personalized PLGA Implants
Computational Modeling for Implant Design: Finite element modeling has been used to simulate drug transport in PLGA nanofiber systems, allowing for precise prediction of release profiles [136]. A systematic review introduced a “12 Factor System” to optimize in situ forming PLGA implants, offering a standardized framework for personalized drug delivery customization [167].
Imaging Technologies for Implant Optimization: Imaging advances facilitate non-invasive, real-time monitoring of implant behavior. MRI and EPR spectroscopy enabled the tracking of degradation and drug release in vivo [93]. Ultrasound imaging further aided in monitoring swelling and degradation profiles in injectable systems [5]. Fluorescence imaging helped assess microclimate pH inside PLGA implants, improving understanding of in vivo drug stability [18].
8.5. Integration of Functionalized Nanoparticles for Patient-Specific Needs
Nanoparticles for Disease-Specific Applications: Functionalized nanoparticles enhance biocompatibility and targeted release for individual patients. Light-responsive PLGA hybrid nanoparticles enabled controlled peptide release in intravitreal therapy, improving retention and compliance in ocular disease management [108]. For cancer therapy, DOX-loaded PLGA implants were adapted for intrahepatic administration, achieving high local drug levels while minimizing systemic exposure [26]. Cannabidiol (CBD)-loaded PLGA implants provided sustained release for one month and exhibited antiangiogenic effects in tumor models, supporting patient-specific cannabinoid-based therapies [178].
9. Challenges in the Development of PLGA Implants
Despite significant advancements, PLGA-based implants still face critical challenges that limit their clinical adoption and long-term effectiveness. These include inconsistencies in drug release, inflammatory responses, structural and mechanical limitations, and difficulties in achieving long-term therapeutic efficacy. Understanding these barriers—along with insights from unsuccessful or limited studies—can inform future innovation in PLGA implant technology.
9.1. Challenges in Drug Release Consistency and Predictability
Burst Release and Protein Aggregation: One of the most prominent issues in PLGA systems is burst release, particularly problematic in sensitive applications like ocular or cancer therapy [2,15,16,54,55,57,62,101,164]. Incomplete protein release is also common due to aggregation in acidic microenvironments. Ovalbumin (OVA)-loaded implants demonstrated limited diffusion caused by polymer interactions, while the incorporation of shellac improved release efficiency by neutralizing acidic degradation products [11]. Interactions between proteins and the PLGA matrix further contributed to incomplete release, emphasizing the need for formulation strategies to mitigate these effects and promote bioavailability [85].
Unexpected Burst and Lag Times in ISFIs: Controlling release kinetics remains challenging in in situ forming implants (ISFIs). Dexamethasone-loaded PLGA microspheres initially exhibited rapid release due to predegradation, followed by reduced delivery after two weeks, complicating sustained release strategies [40]. Similarly, PLGA-based ISFIs with medium molecular weight exhibited higher burst release due to increased porosity [101]. Solvent interactions and polymer composition significantly affect release patterns, limiting reproducibility [53].
IVIVC Challenges: Establishing predictive in vitro–in vivo models remain difficult. In risperidone ISFIs, higher molecular weight PLGA and lactide content extended drug release, but IVIVC failed due to unpredictable phase separation [20]. In tamsulosin-loaded PLGA implants, the inclusion of Tween and Span affected kinetics, but inconsistent release profiles hindered predictability, underlining the need for improved modeling tools [61].
9.2. Manufacturing Complexity and Cost Barriers
Advanced techniques like hot melt extrusion, electrospraying, and 3D printing improve precision but require specialized equipment and expertise, raising production costs and limiting scalability [4,11,17,59,72,102,108,111,128]. Efforts to replace solvents like NMP in ISFIs with PEG 400 or triethyl citrate improved syringeability, but drugs like ibuprofen still exhibited burst release, while chlorhexidine showed lag phases, revealing ongoing formulation challenges [58].
9.3. Limitations in Bone Regeneration and Tissue Engineering
Suboptimal Bone Integration Outcomes: While widely applied in orthopedic contexts, PLGA sometimes fails to deliver expected outcomes. Lithium-incorporated PLGA implants aimed at Wnt signaling modulation did not enhance early bone growth [14]. Comparisons of nano- and microsized β-TCP in PLGA revealed limited osteointegration and lower mechanical strength versus titanium alloy implants [99]. Additionally, β-TCP/PLGA spacers used in knee osteoarthritis demonstrated some clinical benefits but were limited by degradation inconsistencies and mechanical instability under load [67].
Short-Term Regenerative Effects of Cell-Seeded Scaffolds: Mesenchymal stromal cell (MSC)-seeded PLGA scaffolds showed declining effectiveness in cartilage repair over 12 months [173]. In chondrocyte-seeded PLGA scaffolds, fibrocartilaginous repair occurred under static and dynamic conditions, but the results highlighted the critical influence of biomechanical stimulation [176]. Similarly, preadipocyte-seeded PLGA scaffolds initially supported adipose tissue formation, but by 5–12 months, both implant and tissue had degraded, indicating poor long-term viability [141].
9.4. Inflammatory Reactions and Biocompatibility Issues
Persistent Inflammatory Responses: PLGA scaffolds can trigger notable inflammatory reactions. Studies comparing PLGA to porcine small intestinal submucosa (SIS) showed milder inflammation in SIS scaffolds, suggesting a need for surface modifications or material hybrids to improve host response [50]. In intracerebral applications, carboplatin-loaded PLGA microspheres induced localized inflammation [165], while larger PLGA-based neural microelectrodes caused tissue adhesion, reinforcing the importance of structural refinements [89].
Excessive Vascularization and Unintended Outcomes: In some cases, PLGA-based implants cause unintended biological effects. VEGF-releasing PLGA microparticles used in stroke models increased vascularization but also led to hypervascularization in certain areas, requiring careful dose regulation [34]. In another study, PLGA/fibrin gel scaffolds implanted in intervertebral disks resulted in increased nerve fiber ingrowth, potentially contributing to pain rather than healing [52].
9.5. Structural and Mechanical Limitations of PLGA Implants
Mechanical Weakness in Load-Bearing Applications: Mechanical insufficiency remains a limitation in orthopedic use. While PLGA microparticles in calcium phosphate (CaP) cement improved bone ingrowth, mechanical strength was inadequate for load-bearing roles [147]. Attempts to enhance PLGA surface properties via CO2 laser modification improved degradation behavior, but high exposure levels caused excessive degradation and weakened tensile strength, restricting use in high-stress environments [111].
10. Opportunities and Future Directions in PLGA Implant Research
Despite current challenges, ongoing advancements in polymer science, fabrication techniques, and functional design are rapidly expanding the future clinical potential of PLGA-based implants. Innovations such as polymer blending, multilayered architectures, and functional coatings are enabling better control over drug release, reducing burst effects, and improving therapeutic stability and long-term efficacy [19,57,88,121,164,167].
Looking ahead, a number of strategic opportunities are emerging. There is growing interest in engineering implants that respond to local microenvironments—such as pH shifts or enzymatic activity—or to exogenous stimuli like ultrasound and magnetic fields, enabling on-demand therapeutic modulation. Functional integration with biosensors or imaging agents could lead to theranostic platforms, paving the way for intelligent and responsive implants. In parallel, materials science is converging with biomedical engineering to design next-generation PLGA composites tailored for specialized applications in the brain, inner ear, and ocular compartments.
Scalability is being addressed through cost-effective manufacturing techniques, including advanced 3D printing and microfluidic platforms, which are making production more accessible and customizable [4,13,17,59,72]. However, scaling these technologies for clinical use presents challenges. In 3D printing, differences in deposition methods such as Droplet Deposition Modeling (DDM) and Fused Deposition Modeling (FDM) significantly affect implant porosity and drug release, even with identical designs [17]. High filling densities limit drug diffusion, while low-density meshes support faster release [59]. Thermal processes like hot melt extrusion can also alter polymer and drug stability, requiring tight process control [4]. Microfluidics offers precise particle fabrication but faces limitations in throughput and scalability [13]. Despite these barriers, such methods remain promising for personalized PLGA-based therapies with continued optimization.
Enhancing biocompatibility remains a core objective. Future efforts will increasingly rely on integrating biocompatible additives such as chitosan, hydroxyapatite, PEG, and anti-inflammatory agents to mitigate immune responses and promote tissue integration [43,50,102,127]. Hybrid designs and surface-engineered coatings, including electrosprayed PLGA films, hold promise for improving implant–host interactions and reducing inflammation or thrombosis [102].
Multifunctional systems are also gaining momentum, combining therapeutic and diagnostic capabilities. For instance, in situ PLGA-PDMS-forming implants co-loaded with rosuvastatin and copper–selenium nanoparticles have shown dual antimicrobial and cytotoxic effects in breast cancer models, highlighting the potential of integrated therapy platforms [43]. PLGA-PEG triblock copolymers offer enhanced release control for sensitive molecules like naltrexone [127], while PLGA-embedded gelatin sponges show promise for targeted lymphatic drug delivery and metastatic disease control [73].
Predictive modeling and advanced in vitro platforms are becoming essential tools for implant development. Finite element modeling (FEM) is increasingly used to simulate drug diffusion and degradation in nanofiber or hydrogel systems, enabling rational design and formulation optimization [136]. Gel-based platforms now better replicate subcutaneous environments and allow for real-time pH monitoring and accelerated release modeling, supporting in vitro–in vivo correlation (IVIVC) and regulatory translation [93,114]. Non-invasive modalities such as benchtop MRI and EPR spectroscopy offer continuous in vivo tracking of implant behavior [93], while release profiling techniques like the “tubule” sample-separate method show strong IVIVC (R2 > 0.99), facilitating robust quality control [155].
Clinically, PLGA-based systems are expected to play an increasingly central role in treating chronic conditions, rare diseases, and hormone-related disorders. Applications span long-term contraception, immunosuppression, antiangiogenic therapies, and site-specific oncology treatments [36,38,39,96,156]. The integration of bioactive components—such as dexamethasone-loaded hydrogels and PLGA/PHB nanocomposites—further supports their utility in regenerative medicine and orthopedics [137,144,150]. To fully realize this potential, future efforts must focus on large animal and clinical studies to evaluate long-term safety, biodegradation kinetics, and therapeutic efficacy [144,150,173]. Additionally, the convergence of PLGA with gene and cell therapies, as well as stimuli-responsive systems, is likely to define the next generation of implantable medical technologies.
11. Conclusions
PLGA-based implants represent a transformative convergence of materials engineering and biomedical science, delivering advanced solutions for controlled drug release, tissue repair, and disease-specific therapy. Their success lies in the tunability of polymer characteristics, the integration of bioactive agents, and innovative structural designs that collectively enable personalized and localized treatment strategies.
Although challenges persist—including burst release, degradation variability, and manufacturing complexity—recent advances in surface modification, predictive modeling, and multifunctional systems offer promising solutions. The development of hybrid materials, multilayered configurations, and computationally optimized implants marks a significant step forward in enhancing PLGA performance and expanding their clinical reach.
As testing methodologies and design technologies evolve, PLGA implants are positioned to set new standards in precision medicine, regenerative therapies, and chronic disease management. Future progress will depend on sustained interdisciplinary collaboration, rigorous translational research, and continued investment in scalable, patient-specific solutions. Ultimately, PLGA technology is poised to play a central role in the future of personalized healthcare, advancing long-term, targeted, and effective therapeutic platforms.
Author Contributions
The authors confirm contribution to the paper as follows: conceptualization, writing, review and editing, H.O.; investigation, review and editing, R.L.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors partly used OpenAI Large-Scale Language Model to maximize accuracy, clarity, and organization.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ayoub, M.M.; Jasti, B.; Elantouny, N.G.; Elnahas, H.; Ghazy, F.E. Comparative Study of PLGA in-situ Implant and Nanoparticle Formulations of Entecavir; in-vitro and in-vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 56, 101585. [Google Scholar] [CrossRef]
- Ulianova, Y.; Ermolenko, Y.; Tkachenko, S.; Trukhan, V.; Morozov, A.; Gelperina, S. Tuning the release rate of rilpivirine from PLGA-based in situ forming implants. Polym. Bull. 2022, 80, 11401–11420. [Google Scholar] [CrossRef]
- Wang, F.; Lee, T.; Wang, C.H. PEG modulated release of etanidazole from implantable PLGA/PDLA discs. Biomaterials 2002, 23, 3555–3566. [Google Scholar] [CrossRef] [PubMed]
- Bassand, C.; Benabed, L.; Verin, J.; Danede, F.; Lefol, L.A.; Willart, J.F.; Siepmann, F.; Siepmann, J. Hot melt extruded PLGA implants loaded with ibuprofen: How heat exposure alters the physical drug state. J. Drug. Deliv. Sci. Technol. 2022, 73, 103432. [Google Scholar] [CrossRef]
- Solorio, L.; Olear, A.M.; Hamilton, J.I.; Patel, R.B.; Beiswenger, A.C.; Wallace, J.E.; Zhou, H.; Exner, A.A. Noninvasive characterization of the effect of varying PLGA molecular weight blends on in situ forming implant behavior using ultrasound imaging. Theranostics 2012, 2, 1064–1077. [Google Scholar] [CrossRef]
- Amini-Fazl, M.S. Biodegradation study of PLGA as an injectable in situ depot-forming implant for controlled release of paclitaxel. Polym. Bull. 2021, 79, 2763–2776. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Qi, F.; Zhao, J. The effect of a single injection of uniform-sized insulin-loaded PLGA microspheres on peri-implant bone formation. RSC Adv. 2018, 8, 40417–40425. [Google Scholar] [CrossRef]
- Bassand, C.; Freitag, J.; Benabed, L.; Verin, J.; Siepmann, F.; Siepmann, J. PLGA implants for controlled drug release: Impact of the diameter. Eur. J. Pharm. Biopharm. 2022, 177, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, K.; Bazraei, S.; Mashak, A.; Mobedi, H. The Impact of Temperature on the Formation, Release Mechanism, and Degradation of PLGA-based In-Situ Forming Implants. J. Polym. Environ. 2024, 32, 3591–3608. [Google Scholar] [CrossRef]
- Hamoudi-Ben Yelles, M.C.; Tran Tan, V.; Danede, F.; Willart, J.F.; Siepmann, J. PLGA implants: How Poloxamer/PEO addition slows down or accelerates polymer degradation and drug release. J. Control. Release 2017, 253, 19–29. [Google Scholar] [CrossRef]
- Duque, L.; Korber, M.; Bodmeier, R. Improving release completeness from PLGA-based implants for the acid-labile model protein ovalbumin. Int. J. Pharm. 2018, 538, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Mobedi, H.; Behnamghader, A. In situ-forming PLGA implants loaded with leuprolide acetate/β-cyclodextrin complexes: Mathematical modelling and degradation. J. Microencapsul. 2016, 33, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ranganath, S.H.; Kee, I.; Krantz, W.B.; Chow, P.K.; Wang, C.H. Hydrogel matrix entrapping PLGA-paclitaxel microspheres: Drug delivery with near zero-order release and implantability advantages for malignant brain tumour chemotherapy. Pharm. Res. 2009, 26, 2101–2114. [Google Scholar] [CrossRef] [PubMed]
- Thorfve, A.; Bergstrand, A.; Ekstrom, K.; Lindahl, A.; Thomsen, P.; Larsson, A.; Tengvall, P. Gene expression profiling of peri-implant healing of PLGA-Li+ implants suggests an activated Wnt signaling pathway in vivo. PLoS ONE 2014, 9, e102597. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; El-Megrab, N.A.; El-Nahas, H.M. An overview of PLGA in-situ forming implants based on solvent exchange technique: Effect of formulation components and characterization. Pharm. Dev. Technol. 2021, 26, 709–728. [Google Scholar] [CrossRef]
- Saraf, I.; Kushwah, V.; Alva, C.; Koutsamanis, I.; Rattenberger, J.; Schroettner, H.; Mayrhofer, C.; Modhave, D.; Braun, M.; Werner, B.; et al. Influence of PLGA End Groups on the Release Profile of Dexamethasone from Ocular Implants. Mol. Pharm. 2023, 20, 1307–1322. [Google Scholar] [CrossRef]
- Bassand, C.; Benabed, L.; Charlon, S.; Verin, J.; Freitag, J.; Siepmann, F.; Soulestin, J.; Siepmann, J. 3D printed PLGA implants: APF DDM vs. FDM. J. Control. Release 2023, 353, 864–874. [Google Scholar] [CrossRef]
- Schadlich, A.; Kempe, S.; Mader, K. Non-invasive in vivo characterization of microclimate pH inside in situ forming PLGA implants using multispectral fluorescence imaging. J. Control. Release 2014, 179, 52–62. [Google Scholar] [CrossRef]
- Yang, F.; Stahnke, R.; Lawal, K.; Mahnen, C.; Duffy, P.; Xu, S.; Durig, T. Development of poly (lactic-co-glycolic acid) (PLGA) based implants using hot melt extrusion (HME) for sustained release of drugs: The impacts of PLGA’s material characteristics. Int. J. Pharm. 2024, 663, 124556. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Q.; Wang, R.; Kwok, O.; Maurus, K.; Wang, Y.; Qin, B.; Burgess, D.J. In situ forming risperidone implants: Effect of PLGA attributes on product performance. J. Control. Release 2023, 361, 777–791. [Google Scholar] [CrossRef]
- Lehner, E.; Liebau, A.; Menzel, M.; Schmelzer, C.E.H.; Knolle, W.; Scheffler, J.; Binder, W.H.; Plontke, S.K.; Mäder, K. Characterization of PLGA versus PEG-PLGA intracochlear drug delivery implants: Degradation kinetics, morphological changes, and pH alterations. J. Drug Deliv. Sci. Technol. 2024, 99, 105972. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Fivez, A.; Siepmann, F.; Siepmann, J. Often neglected: PLGA/PLA swelling orchestrates drug release: HME implants. J. Control. Release 2019, 306, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Bode, C.; Kranz, H.; Kruszka, A.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants: How additives affect swelling and drug release. J. Drug Deliv. Sci. Technol. 2019, 53, 10. [Google Scholar] [CrossRef]
- Vesna Milacic, V.M.; Schwendeman, S.P. Lysozyme release and polymer erosion behavior of injectable implants prepared from PLGA-PEG block copolymers and PLGA/PLGA-PEG blends. Pharm. Res. 2014, 31, 436–448. [Google Scholar] [CrossRef]
- Bassand, C.; Verin, J.; Lamatsch, M.; Siepmann, F.; Siepmann, J. How agarose gels surrounding PLGA implants limit swelling and slow down drug release. J. Control. Release 2022, 343, 255–266. [Google Scholar] [CrossRef]
- Gao, L.; Li, Q.; Zhang, J.; Huang, Y.; Deng, L.; Li, C.; Tai, G.; Ruan, B. Local penetration of doxorubicin via intrahepatic implantation of PLGA based doxorubicin-loaded implants. Drug Deliv. 2019, 26, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- KadioĞLu, Y.; AtİLa, A.; Vural, İ.; ÇEtİN, M. Preparation and characterization of anticancer drug-loaded implantable PLGA microparticles. Turk. J. Chem. 2010, 34, 509–516. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, L.; Qin, J.; Sun, X.L.; Yang, T.T.; Ni, Y.X.; Zhou, Y.M. New Biodegradable Implant Material Containing Hydrogel with Growth Factors of Lyophilized PRF in Combination with an nHA/PLGA Scaffold. J. Hard Tissue Biol. 2015, 24, 54–60. [Google Scholar] [CrossRef]
- Kazek-Kesik, A.; Nosol, A.; Plonka, J.; Smiga-Matuszowicz, M.; Golda-Cepa, M.; Krok-Borkowicz, M.; Brzychczy-Wloch, M.; Pamula, E.; Simka, W. PLGA-amoxicillin-loaded layer formed on anodized Ti alloy as a hybrid material for dental implant applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 998–1008. [Google Scholar] [CrossRef]
- Ghaffari, A.A.; Matter, B.A.; Hartman, R.R.; Bourne, D.W.A.; Wang, Y.; Choi, S.; Kompella, U.B. Hot-Melt Extrusion-Based Dexamethasone-PLGA Implants: Physicochemical, Physicomechanical, and Surface Morphological Properties and In Vitro Release Corrected for Drug Degradation. Pharmaceutics 2024, 16, 895. [Google Scholar] [CrossRef]
- Kasinathan, N.; Amirthalingam, M.; Reddy, N.D.; Vanthi, M.B.; Volety, S.M.; Rao, J.V. Polycaprolactone-based in situ implant containing curcumin-PLGA nanoparticles prepared using the multivariate technique. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Won, J.Y.; Park, C.Y.; Bae, J.H.; Ahn, G.; Kim, C.; Lim, D.H.; Cho, D.W.; Yun, W.S.; Shim, J.H.; Huh, J.B. Evaluation of 3D printed PCL/PLGA/β-TCP versus collagen membranes for guided bone regeneration in a beagle implant model. Biomed. Mater. 2016, 11, 055013. [Google Scholar] [CrossRef] [PubMed]
- Lehner, E.; Gundel, D.; Liebau, A.; Plontke, S.; Mader, K. Intracochlear PLGA based implants for dexamethasone release: Challenges and solutions. Int. J. Pharm. X 2019, 1, 100015. [Google Scholar] [CrossRef] [PubMed]
- Bible, E.; Qutachi, O.; Chau, D.Y.; Alexander, M.R.; Shakesheff, K.M.; Modo, M. Neo-vascularization of the stroke cavity by implantation of human neural stem cells on VEGF-releasing PLGA microparticles. Biomaterials 2012, 33, 7435–7446. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Zeng, D.; Zhang, S.; Zhang, F.; Yu, W. PLGA film/Titanium nanotubues as a sustained growth factor releasing system for dental implants. J. Mater. Sci. Mater. Med. 2018, 29, 141. [Google Scholar] [CrossRef]
- Zou, G.K.; Song, Y.L.; Zhou, W.; Yu, M.; Liang, L.H.; Sun, D.C.; Li, D.H.; Deng, Z.X.; Zhu, W.Z. Effects of local delivery of bFGF from PLGA microspheres on osseointegration around implants in diabetic rats. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 284–289. [Google Scholar] [CrossRef]
- Resende, A.F.C.; Pereira, A.F.; Moreira, T.P.; Patricio, P.S.O.; Fialho, S.L.; Cunha, G.M.F.; Silva-Cunha, A.; Magalhaes, J.T.; Silva, G.R. PLGA Implants containing vancomycin and dexamethasone: Development, characterization and bactericidal effects. Pharmazie 2016, 71, 439–446. [Google Scholar] [CrossRef]
- Soares, D.C.F.; de Paula Oliveira, D.C.; Barcelos, L.S.; Barbosa, A.S.; Vieira, L.C.; Townsend, D.M.; Rubello, D.; de Barros, A.L.B.; Duarte, L.P.; Silva-Cunha, A. Antiangiogenic activity of PLGA-Lupeol implants for potential intravitreal applications. Biomed. Pharmacother. 2017, 92, 394–402. [Google Scholar] [CrossRef]
- Toledo, C.R.; Pereira, V.V.; Andrade, G.F.; Silva-Cunha, A. PLGA-corosolic acid implants for potential application in ocular neovascularization diseases. Braz. J. Pharm. Sci. 2020, 56, e18484. [Google Scholar] [CrossRef]
- Hickey, T.; Kreutzer, D.; Burgess, D.J.; Moussy, F. Dexamethasone/PLGA microspheres for continuous delivery of an anti-inflammatory drug for implantable medical devices. Biomaterials 2002, 23, 1649–1656. [Google Scholar] [CrossRef]
- Do, M.P.; Neut, C.; Metz, H.; Delcourt, E.; Siepmann, J.; Mader, K.; Siepmann, F. Mechanistic analysis of PLGA/HPMC-based in-situ forming implants for periodontitis treatment. Eur. J. Pharm. Biopharm. 2015, 94, 273–283. [Google Scholar] [CrossRef]
- Carlier, E.; Marquette, S.; Peerboom, C.; Amighi, K.; Goole, J. Development of mAb-loaded 3D-printed (FDM) implantable devices based on PLGA. Int. J. Pharm. 2021, 597, 120337. [Google Scholar] [CrossRef] [PubMed]
- Maged, A.; Mabrouk, M.; Nour El-Din, H.T.; Osama, L.; Badr-Eldin, S.M.; Mahmoud, A.A. PLGA and PDMS-based in situ forming implants loaded with rosuvastatin and copper-selenium nanoparticles: A promising dual-effect formulation with augmented antimicrobial and cytotoxic activity in breast cancer cells. Front. Pharmacol. 2024, 15, 1397639. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ren, S.; Zhang, X.; Yu, Y.; Liu, C.; Yang, J.; Miao, L. Safety and efficacy of PLGA(Ag-Fe3O4)-coated dental implants in inhibiting bacteria adherence and osteogenic inducement under a magnetic field. Int. J. Nanomed. 2018, 13, 3751–3762. [Google Scholar] [CrossRef]
- Geng, Z.; Dong, R.; Li, X.; Xu, X.; Chen, L.; Han, X.; Liu, D.; Liu, Y. Study on the Antibacterial Activity and Bone Inductivity of Nanosilver/PLGA-Coated TI-CU Implants. Int. J. Nanomed. 2024, 19, 6427–6447. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, N.; Zhou, Y.; Wei, H.; Ren, S.; Yu, F.; Chen, H.; Jia, C.; Yang, G.; Song, Y. Delivery of antagomiR204-conjugated gold nanoparticles from PLGA sheets and its implication in promoting osseointegration of titanium implant in type 2 diabetes mellitus. Int. J. Nanomed. 2017, 12, 7089–7101. [Google Scholar] [CrossRef]
- Kefayat, A.; Vaezifar, S. Biodegradable PLGA implants containing doxorubicin-loaded chitosan nanoparticles for treatment of breast tumor-bearing mice. Int. J. Biol. Macromol. 2019, 136, 48–56. [Google Scholar] [CrossRef]
- Shi, S.; Song, S.; Liu, X.; Zhao, G.; Ding, F.; Zhao, W.; Zhang, S.; Song, Y.; Ma, W. Construction and performance of exendin-4-loaded chitosan-PLGA microspheres for enhancing implant osseointegration in type 2 diabetic rats. Drug Deliv. 2022, 29, 548–560. [Google Scholar] [CrossRef]
- Park, J.W.; Hwang, J.U.; Back, J.H.; Jang, S.W.; Kim, H.J.; Kim, P.S.; Shin, S.; Kim, T. High strength PLGA/Hydroxyapatite composites with tunable surface structure using PLGA direct grafting method for orthopedic implants. Compos. Part B Eng. 2019, 178, 107449. [Google Scholar] [CrossRef]
- Kim, M.S.; Ahn, H.H.; Shin, Y.N.; Cho, M.H.; Khang, G.; Lee, H.B. An in vivo study of the host tissue response to subcutaneous implantation of PLGA- and/or porcine small intestinal submucosa-based scaffolds. Biomaterials 2007, 28, 5137–5143. [Google Scholar] [CrossRef]
- Bai, H.Y.; Chen, G.A.; Mao, G.H.; Song, T.R.; Wang, Y.X. Three step derivation of cartilage like tissue from human embryonic stem cells by 2D-3D sequential culture in vitro and further implantation in vivo on alginate/PLGA scaffolds. J. Biomed. Mater. Res. A 2010, 94, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Xu, W.; Yu, L.; Fan, S.; Wang, W.; Yu, F.; Wang, Z. Effects of annulus defects and implantation of poly(lactic-co-glycolic acid) (PLGA)/fibrin gel scaffolds on nerves ingrowth in a rabbit model of annular injury disc degeneration. J. Orthop. Surg. Res. 2017, 12, 73. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, R.E.; Kost, J. Characterization of a polymeric PLGA-injectable implant delivery system for the controlled release of proteins. J. Biomed. Mater. Res. 2000, 50, 388–396. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. In-situ forming PLGA implants for intraocular dexamethasone delivery. Int. J. Pharm. 2018, 548, 337–348. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; El-Megrab, N.A.; El-Nahas, H.M. Optimization of injectable PLGA in-situ forming implants of anti-psychotic risperidone via Box-Behnken Design. J. Drug Deliv. Sci. Technol. 2020, 58, 101803. [Google Scholar] [CrossRef]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Yazdian-Robati, R.; Haghbin, A.; Zohuri, G. An in-situ forming implant formulation of naltrexone with minimum initial burst release using mixture of PLGA copolymers and ethyl heptanoate as an additive: In-vitro, ex-vivo, and in-vivo release evaluation. J. Drug Deliv. Sci. Technol. 2018, 47, 95–105. [Google Scholar] [CrossRef]
- Bakhshi, R.; Vasheghani-Farahani, E.; Mobedi, H.; Jamshidi, A.; Khakpour, M. The effect of additives on naltrexone hydrochloride release and solvent removal rate from an injectable in situ forming PLGA implant. Polym. Adv. Technol. 2006, 17, 354–359. [Google Scholar] [CrossRef]
- Ramos, F.; Willart, J.F.; Neut, C.; Agossa, K.; Siepmann, J.; Siepmann, F. In-situ forming PLGA implants: Towards less toxic solvents. Int. J. Pharm. 2024, 657, 124121. [Google Scholar] [CrossRef]
- Bassand, C.; Siepmann, F.; Benabed, L.; Verin, J.; Freitag, J.; Charlon, S.; Soulestin, J.; Siepmann, J. 3D printed PLGA implants: How the filling density affects drug release. J. Control. Release 2023, 363, 1–11. [Google Scholar] [CrossRef]
- Riggin, C.N.; Qu, F.; Kim, D.H.; Huegel, J.; Steinberg, D.R.; Kuntz, A.F.; Soslowsky, L.J.; Mauck, R.L.; Bernstein, J. Electrospun PLGA Nanofiber Scaffolds Release Ibuprofen Faster and Degrade Slower After In Vivo Implantation. Ann. Biomed. Eng. 2017, 45, 2348–2359. [Google Scholar] [CrossRef]
- Elias-Al-Mamun, M.; Khan, H.A.; Dewan, I.; Jalil, R.U. In vitro study on tamsulosin release kinetics from biodegradable PLGA in situ implants. Pak. J. Pharm. Sci. 2009, 22, 360–367. [Google Scholar] [PubMed]
- Yu, Y.; Ngo, H.V.; Jin, G.; Tran, P.H.L.; Tran, T.T.D.; Nguyen, V.H.; Park, C.; Lee, B.J. Double-Controlled Release of Poorly Water-Soluble Paliperidone Palmitate from Self-Assembled Albumin-Oleic Acid Nanoparticles in PLGA in situ Forming Implant. Int. J. Nanomed. 2021, 16, 2819–2831. [Google Scholar] [CrossRef]
- Desai, K.G.; Olsen, K.F.; Mallery, S.R.; Stoner, G.D.; Schwendeman, S.P. Formulation and in vitro-in vivo evaluation of black raspberry extract-loaded PLGA/PLA injectable millicylindrical implants for sustained delivery of chemopreventive anthocyanins. Pharm. Res. 2010, 27, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Enayati, M.; Mobedi, H.; Hojjati-Emami, S.; Mirzadeh, H.; Jafari-Nodoushan, M. In situ forming PLGA implant for 90 days controlled release of leuprolide acetate for treatment of prostate cancer. Polym. Adv. Technol. 2017, 28, 867–875. [Google Scholar] [CrossRef]
- Kokovic, V.; Todorovic, L. Preimplantation filling of tooth socket with β-tricalcium phosphate/polylactic-polyglycolic acid (β-TCP/PLGA) root analogue: Clinical and histological analysis in a patient. Vojnosanit. Pregl. 2011, 68, 366–371. [Google Scholar] [CrossRef]
- Prosolov, K.A.; Komarova, E.G.; Kazantseva, E.A.; Luginin, N.A.; Kashin, A.D.; Uvarkin, P.V.; Sharkeev, Y.P. Enhanced Corrosion Resistance and Mechanical Durability of the Composite PLGA/CaP/Ti Scaffolds for Orthopedic Implants. Polymers 2024, 16, 826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, S.; Yin, Y.; Guo, J.; Chen, W.; Hou, Z.; Zhang, Y. Open-Wedge HTO with Absorbable β-TCP/PLGA Spacer Implantation and Proximal Fibular Osteotomy for Medial Compartmental Knee Osteoarthritis: New Technique Presentation. J. Investig. Surg. 2021, 34, 653–661. [Google Scholar] [CrossRef]
- Wachowiak, S.; Danede, F.; Willart, J.F.; Siepmann, F.; Siepmann, J.; Hamoudi, M. PLGA implants for controlled dexamethasone delivery: Impact of the polymer chemistry. J. Drug Deliv. Sci. Technol. 2023, 86, 104648. [Google Scholar] [CrossRef]
- Hickey, T.; Kreutzer, D.; Burgess, D.J.; Moussy, F. In vivo evaluation of a dexamethasone/PLGA microsphere system designed to suppress the inflammatory tissue response to implantable medical devices. J. Biomed. Mater. Res. 2002, 61, 180–187. [Google Scholar] [CrossRef]
- Gosau, M.; Muller, B.W. Release of gentamicin sulphate from biodegradable PLGA-implants produced by hot melt extrusion. Pharmazie 2010, 65, 487–492. [Google Scholar] [CrossRef]
- Ahmadi, H.; Haddadi-Asl, V.; Mohammadloo, H.E. Advancing anticorrosion and antibacterial performance of mg AZ31 implants using novel pH-responsive polymeric surfactant for preparing PLGA nanoparticles. Surf. Coat. Technol. 2024, 482, 130738. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Zheng, K.; Deng, L.; Yang, L.; Zhang, N.; Xu, C.; Ran, H.; Wang, Z.; Wang, Z.; et al. Injectable PLGA/Fe3O4 implants carrying cisplatin for synergistic magnetic hyperthermal ablation of rabbit VX2 tumor. PLoS ONE 2017, 12, e0177049. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Meisner, D.; Kwong, E.; Wu, X.Y.; Johnston, M.R. A novel trans-lymphatic drug delivery system: Implantable gelatin sponge impregnated with PLGA-paclitaxel microspheres. Biomaterials 2007, 28, 3236–3244. [Google Scholar] [CrossRef]
- Gao, W.; Zheng, Y.; Wang, R.; Chen, H.; Cai, X.; Lu, G.; Chu, L.; Xu, C.; Zhang, N.; Wang, Z.; et al. A smart, phase transitional and injectable DOX/PLGA-Fe implant for magnetic-hyperthermia-induced synergistic tumor eradication. Acta Biomater. 2016, 29, 298–306. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, S.; Yang, G.; Zhou, Z.; Gao, Y. Exploring Trehalose on the Release of Levonorgestrel from Implantable PLGA Microneedles. Polymers 2020, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Donnell, A.M.; Kaval, N.; Al-Rjoub, M.F.; Augsburger, J.J.; Banerjee, R.K. Improved design and characterization of PLGA/PLA-coated Chitosan based micro-implants for controlled release of hydrophilic drugs. Int. J. Pharm. 2018, 547, 122–132. [Google Scholar] [CrossRef]
- Holmkvist, A.D.; Agorelius, J.; Forni, M.; Nilsson, U.J.; Linsmeier, C.E.; Schouenborg, J. Local delivery of minocycline-loaded PLGA nanoparticles from gelatin-coated neural implants attenuates acute brain tissue responses in mice. J. Nanobiotechnol. 2020, 18, 27. [Google Scholar] [CrossRef]
- Chang, N.J.; Lin, C.C.; Shie, M.Y.; Yeh, M.L.; Li, C.F.; Liang, P.I.; Lee, K.W.; Shen, P.H.; Chu, C.J. Positive effects of cell-free porous PLGA implants and early loading exercise on hyaline cartilage regeneration in rabbits. Acta Biomater. 2015, 28, 128–137. [Google Scholar] [CrossRef]
- Herran, E.; Perez-Gonzalez, R.; Igartua, M.; Pedraz, J.L.; Carro, E.; Hernandez, R.M. Enhanced Hippocampal Neurogenesis in APP/Ps1 Mouse Model of Alzheimer’s Disease After Implantation of VEGF-loaded PLGA Nanospheres. Curr. Alzheimer Res. 2015, 12, 932–940. [Google Scholar] [CrossRef]
- Bode, C.; Kranz, H.; Siepmann, F.; Siepmann, J. Coloring of PLGA implants to better understand the underlying drug release mechanisms. Int. J. Pharm. 2019, 569, 118563. [Google Scholar] [CrossRef]
- Agossa, K.; Lizambard, M.; Rongthong, T.; Delcourt-Debruyne, E.; Siepmann, J.; Siepmann, F. Physical key properties of antibiotic-free, PLGA/HPMC-based in-situ forming implants for local periodontitis treatment. Int. J. Pharm. 2017, 521, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Park, P.I.; Makoid, M.; Jonnalagadda, S. The design of flexible ciprofloxacin-loaded PLGA implants using a reversed phase separation/coacervation method. Eur. J. Pharm. Biopharm. 2011, 77, 233–239. [Google Scholar] [CrossRef]
- Jain, R.A.; Rhodes, C.T.; Railkar, A.M.; Malick, A.W.; Shah, N.H. Comparison of various injectable protein-loaded biodegradable poly(lactide-co-glycolide) (PLGA) devices: In-situ-formed implant versus in-situ-formed microspheres versus isolated microspheres. Pharm. Dev. Technol. 2000, 5, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Fassihi, R. Release rate determination from in situ gel forming PLGA implant: A novel ‘shape-controlled basket in tube’ method. J. Pharm. Pharmacol. 2020, 72, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Ghalanbor, Z.; Korber, M.; Bodmeier, R. Interdependency of protein-release completeness and polymer degradation in PLGA-based implants. Eur. J. Pharm. Biopharm. 2013, 85, 624–630. [Google Scholar] [CrossRef]
- Kim, M.S.; Seo, K.S.; Hyun, H.; Kim, S.K.; Khang, G.; Lee, H.B. Sustained release of bovine serum albumin using implantable wafers prepared by MPEG-PLGA diblock copolymers. Int. J. Pharm. 2005, 304, 165–177. [Google Scholar] [CrossRef]
- Karp, F.; Turino, L.N.; Helbling, I.M.; Islan, G.A.; Luna, J.A.; Estenoz, D.A. In situ Formed Implants, Based on PLGA and Eudragit Blends, for Novel Florfenicol Controlled Release Formulations. J. Pharm. Sci. 2021, 110, 1270–1278. [Google Scholar] [CrossRef]
- Astaneh, R.; Erfan, M.; Barzin, J.; Mobedi, H.; Moghimi, H. Effects of Ethyl Benzoate on Performance, Morphology, and Erosion of PLGA Implants Formed In Situ. Adv. Polym. Technol. 2008, 27, 17–26. [Google Scholar] [CrossRef]
- Darlot, F.; Villard, P.; Salam, L.A.; Rousseau, L.; Piret, G. Glial scarring around intra-cortical MEA implants with flexible and free microwires inserted using biodegradable PLGA needles. Front. Bioeng. Biotechnol. 2024, 12, 1408088. [Google Scholar] [CrossRef]
- Lee, J.H.; Moon, S.K.; Kim, K.M.; Kim, K.N. Modification of TiO2 nanotube surfaces by electro-spray deposition of amoxicillin combined with PLGA for bactericidal effects at surgical implantation sites. Acta Odontol. Scand. 2013, 71, 168–174. [Google Scholar] [CrossRef]
- Noothongkaew, S.; Ariyachaokun, K.; Pansri, S. Enhanced bioactivity and antibacterial properties of anodized ZrO2 implant coatings via optimized nanoscale morphology and timed antibiotic release through PLGA overcoat. Ceram. Int. 2021, 47, 33775–33787. [Google Scholar] [CrossRef]
- Thalhauser, S.; Peterhoff, D.; Wagner, R.; Breunig, M. Silica particles incorporated into PLGA-based in situ-forming implants exploit the dual advantage of sustained release and particulate delivery. Eur. J. Pharm. Biopharm. 2020, 156, 1–10. [Google Scholar] [CrossRef]
- Kempe, S.; Metz, H.; Pereira, P.G.; Mader, K. Non-invasive in vivo evaluation of in situ forming PLGA implants by benchtop magnetic resonance imaging (BT-MRI) and EPR spectroscopy. Eur. J. Pharm. Biopharm. 2010, 74, 102–108. [Google Scholar] [CrossRef] [PubMed]
- van de Ven, C.; Bakker, N.E.C.; Link, D.P.; Geven, E.J.W.; Gossen, J.A. Sustained release of ancillary amounts of testosterone and alendronate from PLGA coated pericard membranes and implants to improve bone healing. PLoS ONE 2021, 16, e0251864. [Google Scholar] [CrossRef]
- Penk, A.; Forster, Y.; Scheidt, H.A.; Nimptsch, A.; Hacker, M.C.; Schulz-Siegmund, M.; Ahnert, P.; Schiller, J.; Rammelt, S.; Huster, D. The pore size of PLGA bone implants determines the de novo formation of bone tissue in tibial head defects in rats. Magn. Reson. Med. 2013, 70, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.L.; Chen, Y.F.; Lu, W.J.; Zhang, Q.; Gao, S.; Sun, L.F.; Chen, S.Q.; Hu, R.F. A biodegradable long-term contraceptive implant with steady levonorgestrel release based on PLGA microspheres embedded in PCL-coated implant. J. Drug Deliv. Sci. Technol. 2022, 67, 102955. [Google Scholar] [CrossRef]
- Eawsakul, K.; Tancharoen, S.; Nasongkla, N. Combination of dip coating of BMP-2 and spray coating of PLGA on dental implants for osseointegration. J. Drug Deliv. Sci. Technol. 2021, 61, 102296. [Google Scholar] [CrossRef]
- Boimvaser, S.; Mariano, R.N.; Turino, L.N.; Vega, J.R. In vitro bulk/surface erosion pattern of PLGA implant in physiological conditions: A study based on auxiliary microsphere systems. Polym. Bull. 2016, 73, 209–227. [Google Scholar] [CrossRef]
- Kulkova, J.; Moritz, N.; Suokas, E.O.; Strandberg, N.; Leino, K.A.; Laitio, T.T.; Aro, H.T. Osteointegration of PLGA implants with nanostructured or microsized β-TCP particles in a minipig model. J. Mech. Behav. Biomed. Mater. 2014, 40, 190–200. [Google Scholar] [CrossRef]
- Bassand, C.; Benabed, L.; Freitag, J.; Verin, J.; Siepmann, F.; Siepmann, J. How bulk fluid renewal can affect in vitro drug release from PLGA implants: Importance of the experimental set-up. Int. J. Pharm. X 2022, 4, 100131. [Google Scholar] [CrossRef]
- Astaneh, R.; Erfan, M.; Moghimi, H.; Mobedi, H. Changes in morphology of in situ forming PLGA implant prepared by different polymer molecular weight and its effect on release behavior. J. Pharm. Sci. 2009, 98, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.B.; Park, C.H.; Park, K.; Chung, D.J.; Han, D.K. Biodegradable PLGA Polymer Coating on Biomedical Metal Implants Using Electrospraying. Polymer-Korea 2009, 33, 620–624. [Google Scholar]
- Golda-Cepa, M.; Chorylek, A.; Chytrosz, P.; Brzychczy-Wloch, M.; Jaworska, J.; Kasperczyk, J.; Hakkarainen, M.; Engvall, K.; Kotarba, A. Multifunctional PLGA/Parylene C Coating for Implant Materials: An Integral Approach for Biointerface Optimization. ACS Appl. Mater. Interfaces 2016, 8, 22093–22105. [Google Scholar] [CrossRef] [PubMed]
- Cosse, A.; Konig, C.; Lamprecht, A.; Wagner, K.G. Hot Melt Extrusion for Sustained Protein Release: Matrix Erosion and In Vitro Release of PLGA-Based Implants. AAPS PharmSciTech 2017, 18, 15–26. [Google Scholar] [CrossRef]
- Youssef, S.H.; Kim, S.; Khetan, R.; Afinjuomo, F.; Song, Y.M.; Garg, S. The development of 5-fluorouracil biodegradable implants: A comparative study of PCL/PLGA blends. J. Drug Deliv. Sci. Technol. 2023, 81, 104300. [Google Scholar] [CrossRef]
- Annaji, M.; Mita, N.; Poudel, I.; Boddu, S.H.S.; Fasina, O.; Babu, R.J. Three-Dimensional Printing of Drug-Eluting Implantable PLGA Scaffolds for Bone Regeneration. Bioengineering 2024, 11, 259. [Google Scholar] [CrossRef]
- Zhang, Z.; Ekanem, E.E.; Nakajima, M.; Bolognesi, G.; Vladisavljevic, G.T. Monodispersed Sirolimus-Loaded PLGA Microspheres with a Controlled Degree of Drug-Polymer Phase Separation for Drug-Coated Implantable Medical Devices and Subcutaneous Injection. ACS Appl. Bio Mater. 2022, 5, 3766–3777. [Google Scholar] [CrossRef]
- Bisht, R.; Jaiswal, J.K.; Oliver, V.F.; Eurtivong, C.; Reynisson, J.; Rupenthal, I.D. Preparation and evaluation of PLGA nanoparticle-loaded biodegradable light-responsive injectable implants as a promising platform for intravitreal drug delivery. J. Drug Deliv. Sci. Technol. 2017, 40, 142–156. [Google Scholar] [CrossRef]
- He, M.; Yang, G.; Zhao, X.; Zhang, S.; Gao, Y. Intradermal Implantable PLGA Microneedles for Etonogestrel Sustained Release. J. Pharm. Sci. 2020, 109, 1958–1966. [Google Scholar] [CrossRef]
- Santovena, A.; Alvarez-Lorenzo, C.; Concheiro, A.; Llabres, M.; Farina, J.B. Rheological properties of PLGA film-based implants: Correlation with polymer degradation and SPf66 antimalaric synthetic peptide release. Biomaterials 2004, 25, 925–931. [Google Scholar] [CrossRef]
- Kobielarz, M.; Tomanik, M.; Mroczkowska, K.; Szustakiewicz, K.; Oryszczak, M.; Mazur, A.; Antończak, A.; Filipiak, J. Laser-modified PLGA for implants: In vitro degradation and mechanical properties. Acta Bioeng. Biomech. 2020, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Fernandes-Cunha, G.M.; Fialho, S.L.; da Silva, G.R.; Silva-Cunha, A.; Zhao, M.; Behar-Cohen, F. Ocular safety of Intravitreal Clindamycin Hydrochloride Released by PLGA Implants. Pharm. Res. 2017, 34, 1083–1092. [Google Scholar] [CrossRef]
- Li, W.F.; Wang, X.P.; Wang, K.; Chen, T.N.; Chen, X.; Niu, X.F. A Novel of Biodegradable Implants Based on PLGA for Control Delivery of Cisplatin. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 368–373. [Google Scholar] [CrossRef]
- Li, Z.; Mu, H.; Weng Larsen, S.; Jensen, H.; Ostergaard, J. An in vitro gel-based system for characterizing and predicting the long-term performance of PLGA in situ forming implants. Int. J. Pharm. 2021, 609, 121183. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.H.; Railkar, A.S.; Chen, F.C.; Tarantino, R.; Kumar, S.; Murjani, M.; Palmer, D.; Infeld, M.H.; Malick, A.W. A Biodegradable Injectable Implant for Delivering Micromolecules and Macromolecules Using Poly(lactic-co-glycolic) Acid (PLGA) Copolymers. J. Control. Release 1993, 27, 139–147. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, B.; Burgess, D.J. Microspheres prepared with PLGA blends for delivery of dexamethasone for implantable medical devices. Pharm. Res. 2014, 31, 373–381. [Google Scholar] [CrossRef]
- Dong, S.; Wang, S.; Zheng, C.; Liang, W.; Huang, Y. An in situ-forming, solid lipid/PLGA hybrid implant for long-acting antipsychotics. Soft Matter 2011, 7, 5873–5878. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, C.; Zhong, F.; Fan, S.; Wang, W.; Wang, Z. Minimal invasive annulotomy for induction of disc degeneration and implantation of poly (lactic-co-glycolic acid) (PLGA) plugs for annular repair in a rabbit model. Eur. J. Med. Res. 2016, 21, 7. [Google Scholar] [CrossRef] [PubMed]
- Xiao, D.Q.; Liu, Q.; Wang, D.W.; Xie, T.; Guo, T.L.; Duan, K.; Weng, J. Room-temperature attachment of PLGA microspheres to titanium surfaces for implant-based drug release. Appl. Surf. Sci. 2014, 309, 112–118. [Google Scholar] [CrossRef]
- Yang, R.; Chen, T.N.; Chen, H.L.; Wang, W.J. Microfabrication of biodegradable (PLGA) honeycomb-structures and potential applications in implantable drug delivery. Sens. Actuator B Chem. 2005, 106, 506–511. [Google Scholar] [CrossRef]
- Dorta, M.J.; Santovena, A.; Llabres, M.; Farina, J.B. Potential applications of PLGA film-implants in modulating in vitro drugs release. Int. J. Pharm. 2002, 248, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Dorta, M.J.; Oliva, A.; Munguia, O.; Llabres, M.; Farina, J.B. In-vitro release of fluoropyrimidines from PLGA film implants. J. Pharm. Pharmacol. 2002, 54, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Donnell, A.M.; Faraj, R.Q.C.; Riemann, B.I.; Riemann, C.D.; Augsburger, J.J.; Correa, Z.M.; Banerjee, R.K. Pharmacokinetics and Toxicity Evaluation of a PLGA and Chitosan-Based Micro-Implant for Sustained Release of Methotrexate in Rabbit Vitreous. Pharmaceutics 2021, 13, 1227. [Google Scholar] [CrossRef]
- Ramchandani, M.; Robinson, D. In vitro and in vivo release of ciprofloxacin from PLGA 50:50 implants. J. Control. Release 1998, 54, 167–175. [Google Scholar] [CrossRef]
- Fernandes-Cunha, G.M.; Rezende, C.M.; Mussel, W.N.; da Silva, G.R.; Gomes, E.C.d.L.; Yoshida, M.I.; Fialho, S.L.; Goes, A.M.; Gomes, D.A.; de Almeida Vitor, R.W.; et al. Anti-Toxoplasma activity and impact evaluation of lyophilization, hot molding process, and gamma-irradiation techniques on CLH-PLGA intravitreal implants. J. Mater. Sci. Mater. Med. 2016, 27, 10. [Google Scholar] [CrossRef] [PubMed]
- Tavares, H.S.; Cardoso, J.F.; Almeida, T.C.; Marques, M.B.F.; Mussel, W.N.; Lopes, M.C.P.; Orefice, R.L.; Andrade, S.N.; Varotti, F.P.; Silva, G.N.; et al. Spiramyin-loaded PLGA implants for the treatment of ocular toxoplasmosis: Development, characterization, biocompatibility, and anti-toxoplasma activity. Pharmazie 2021, 76, 68–76. [Google Scholar] [CrossRef]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Mohajeri, S.A.; Nazari, A.; Jafarian, A.H. Comparison of in-situ forming composite using PLGA-PEG-PLGA with in-situ forming implant using PLGA: In-vitro, ex-vivo, and in-vivo evaluation of naltrexone release. J. Drug Deliv. Sci. Technol. 2019, 50, 188–200. [Google Scholar] [CrossRef]
- Gilchrist, S.E.; Lange, D.; Letchford, K.; Bach, H.; Fazli, L.; Burt, H.M. Fusidic acid and rifampicin co-loaded PLGA nanofibers for the prevention of orthopedic implant associated infections. J. Control. Release 2013, 170, 64–73. [Google Scholar] [CrossRef]
- Newby, S.D.; Forsynth, C.; Bow, A.J.; Bourdo, S.E.; Hung, M.; Cheever, J.; Moffat, R.; Gross, A.J.; Licari, F.W.; Dhar, M.S. Xenogenic Implantation of Human Mesenchymal Stromal Cells Using a Novel 3D-Printed Scaffold of PLGA and Graphene Leads to a Significant Increase in Bone Mineralization in a Rat Segmental Femoral Bone Defect. Nanomaterials 2023, 13, 1149. [Google Scholar] [CrossRef]
- Ju, Y.M.; Yu, B.; West, L.; Moussy, Y.; Moussy, F. A dexamethasone-loaded PLGA microspheres/collagen scaffold composite for implantable glucose sensors. J. Biomed. Mater. Res. A 2010, 93, 200–210. [Google Scholar] [CrossRef]
- Ranganath, S.H.; Fu, Y.; Arifin, D.Y.; Kee, I.; Zheng, L.; Lee, H.S.; Chow, P.K.; Wang, C.H. The use of submicron/nanoscale PLGA implants to deliver paclitaxel with enhanced pharmacokinetics and therapeutic efficacy in intracranial glioblastoma in mice. Biomaterials 2010, 31, 5199–5207. [Google Scholar] [CrossRef]
- Razzaghi, M.; Kasiri-Asgarani, M.; Bakhsheshi-Rad, H.R.; Ghayour, H. In vitro bioactivity and corrosion of PLGA/hardystonite composite-coated magnesium-based nanocomposite for implant applications. Int. J. Miner. Metall. Mater. 2020, 28, 168–178. [Google Scholar] [CrossRef]
- Komarova, E.G.; Senkina, E.I.; Lozhkomoev, A.S.; Kazantseva, E.A.; Prosolov, K.A.; Kazantsev, S.O.; Akimova, E.B.; Tolkacheva, T.; Khimich, M.A.; Sharkeev, Y.P. Controlled anticancer 5-Fluorouracil release from functionalized 5-FU/PLGA/CaP coating on titanium implants: Characterization, in vitro drug delivery and cytotoxicity. Mater. Today Commun. 2024, 39, 109332. [Google Scholar] [CrossRef]
- Kamali, H.; Khodaverdi, E.; Hadizadeh, F.; Mohajeri, S.A.; Kamali, Y.; Jafarian, A.H. In-vitro, ex-vivo, and in-vivo release evaluation of in situ forming buprenorphine implants using mixture of PLGA copolymers and additives. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 965–977. [Google Scholar] [CrossRef]
- Shen, J.; Burgess, D.J. Accelerated in vitro release testing of implantable PLGA microsphere/PVA hydrogel composite coatings. Int. J. Pharm. 2012, 422, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Milosevic, M.; Stojanovic, D.; Simic, V.; Milicevic, B.; Radisavljevic, A.; Uskokovic, P.; Kojic, M. A Computational Model for Drug Release from PLGA Implant. Materials 2018, 11, 2416. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.C.; Fialho, S.L.; Souza, P.A.; Fulgencio, G.O.; Da Silva, G.R.; Silva-Cunha, A. Tacrolimus-loaded PLGA implants: In vivo release and ocular toxicity. Curr. Eye Res. 2014, 39, 99–102. [Google Scholar] [CrossRef]
- Ahmed, O.A.; Zidan, A.S.; Khayat, M. Mechanistic analysis of Zein nanoparticles/PLGA triblock in situ forming implants for glimepiride. Int. J. Nanomed. 2016, 11, 543–555. [Google Scholar] [CrossRef]
- Castro, M.A.d.; Cunha, G.M.F.; Andrade, G.F.; Yoshida, M.I.; Faria, A.L.d.; Silva-Cunha, A. Development and characterization of PLGA-Bupivacaine and PLGA-S75:R25 Bupivacaine (Novabupi®) biodegradable implants for postoperative pain. Braz. J. Pharm. Sci. 2022, 58, 10. [Google Scholar] [CrossRef]
- Costello, M.A.; Liu, J.; Kuehster, L.; Wang, Y.; Qin, B.; Xu, X.; Li, Q.; Smith, W.C.; Lynd, N.A.; Zhang, F. Role of PLGA Variability in Controlled Drug Release from Dexamethasone Intravitreal Implants. Mol. Pharm. 2023, 20, 6330–6344. [Google Scholar] [CrossRef]
- Patrick, C.W., Jr.; Zheng, B.; Johnston, C.; Reece, G.P. Long-term implantation of preadipocyte-seeded PLGA scaffolds. Tissue Eng. 2002, 8, 283–293. [Google Scholar] [CrossRef]
- Wang, X.; Qi, F.; Xing, H.; Zhang, X.; Lu, C.; Zheng, J.; Ren, X. Uniform-sized insulin-loaded PLGA microspheres for improved early-stage peri-implant bone regeneration. Drug Deliv. 2019, 26, 1178–1190. [Google Scholar] [CrossRef]
- Sendil, D.; Bonney, I.M.; Carr, D.B.; Lipkowski, A.W.; Wise, D.L.; Hasirci, V. Antinociceptive effects of hydromorphone, bupivacaine and biphalin released from PLGA polymer after intrathecal implantation in rats. Biomaterials 2003, 24, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Krucinska, I.; Zywicka, B.; Komisarczyk, A.; Szymonowicz, M.; Kowalska, S.; Zaczynska, E.; Struszczyk, M.; Czarny, A.; Jadczyk, P.; Uminska-Wasiluk, B.; et al. Biological Properties of Low-Toxicity PLGA and PLGA/PHB Fibrous Nanocomposite Implants for Osseous Tissue Regeneration. Part I: Evaluation of Potential Biotoxicity. Molecules 2017, 22, 2092. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yuan, Z.; Kao, W.; Miller, D.; Li, S.K.; Park, Y.C. Size-Exclusive Nanoporous Biodegradable PLGA Capsules for Drug Delivery Implants and In Vivo Stability in the Posterior Segment. ACS Appl. Bio Mater. 2020, 3, 1722–1729. [Google Scholar] [CrossRef]
- Bhardwaj, U.; Sura, R.; Papadimitrakopoulos, F.; Burgess, D.J. PLGA/PVA hydrogel composites for long-term inflammation control following s.c. implantation. Int. J. Pharm. 2010, 384, 78–86. [Google Scholar] [CrossRef]
- Link, D.P.; van den Dolder, J.; Jurgens, W.J.; Wolke, J.G.; Jansen, J.A. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials 2006, 27, 4941–4947. [Google Scholar] [CrossRef]
- Chang, N.J.; Lin, C.C.; Li, C.F.; Wang, D.A.; Issariyaku, N.; Yeh, M.L. The combined effects of continuous passive motion treatment and acellular PLGA implants on osteochondral regeneration in the rabbit. Biomaterials 2012, 33, 3153–3163. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Chu, C.J.; Chou, P.H.; Liang, C.H.; Liang, P.I.; Chang, N.J. Beneficial Therapeutic Approach of Acellular PLGA Implants Coupled With Rehabilitation Exercise for Osteochondral Repair: A Proof of Concept Study in a Minipig Model. Am. J. Sports Med. 2020, 48, 2796–2807. [Google Scholar] [CrossRef]
- Stefani, R.M.; Lee, A.J.; Tan, A.R.; Halder, S.S.; Hu, Y.; Guo, X.E.; Stoker, A.M.; Ateshian, G.A.; Marra, K.G.; Cook, J.L.; et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020, 102, 326–340. [Google Scholar] [CrossRef]
- Wen, Y.; Yu, S.; Wu, Y.; Ju, R.; Wang, H.; Liu, Y.; Wang, Y.; Xu, Q. Spinal cord injury repair by implantation of structured hyaluronic acid scaffold with PLGA microspheres in the rat. Cell Tissue Res. 2016, 364, 17–28. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Schug, J. Observations on healing of human tooth extraction sockets implanted with bioabsorbable polylactic-polyglycolic acids (PLGA) copolymer root replicas: A clinical, radiographic, and histologic follow-up report of 8 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 97, 559–569. [Google Scholar] [CrossRef]
- Landes, C.A.; Ballon, A.; Roth, C. Maxillary and mandibular osteosyntheses with PLGA and P(L/DL)LA implants: A 5-year inpatient biocompatibility and degradation experience. Plast. Reconstr. Surg. 2006, 117, 2347–2360. [Google Scholar] [CrossRef] [PubMed]
- Hild, N.; Tawakoli, P.N.; Halter, J.G.; Sauer, B.; Buchalla, W.; Stark, W.J.; Mohn, D. pH-dependent antibacterial effects on oral microorganisms through pure PLGA implants and composites with nanosized bioactive glass. Acta Biomater. 2013, 9, 9118–9125. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Hu, M.; Liu, W.; Hou, N.; Yin, K.; Shen, C.; Shang, Q. Fabrication of PLGA in situ forming implants and study on their correlation of in vitro release profiles with in vivo performances. J. Biomater. Sci. Polym. Ed. 2021, 32, 994–1008. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, E.; Eissa, N.G.; Ibrahim, T.M.; El-Bassossy, H.M.; El-Nahas, H.M.; Ayoub, M.M. Development of depot PLGA-based in-situ implant of Linagliptin: Sustained release and glycemic control. Saudi. Pharm. J 2023, 31, 499–509. [Google Scholar] [CrossRef]
- Han, I.B.; Thakor, D.K.; Ropper, A.E.; Yu, D.; Wang, L.; Kabatas, S.; Zeng, X.; Kim, S.W.; Zafonte, R.D.; Teng, Y.D. Physical impacts of PLGA scaffolding on hMSCs: Recovery neurobiology insight for implant design to treat spinal cord injury. Exp. Neurol. 2019, 320, 112980. [Google Scholar] [CrossRef]
- Manaspon, C.; Hernandez, C.; Nittayacharn, P.; Jeganathan, S.; Nasongkla, N.; Exner, A.A. Increasing Distribution of Drugs Released from In Situ Forming PLGA Implants Using Therapeutic Ultrasound. Ann. Biomed. Eng. 2017, 45, 2879–2887. [Google Scholar] [CrossRef]
- Li, J.; Krupka, T.; Yao, J.; Wang, R.; Jiang, L.; Zhou, Y.; Zuo, G.; Wang, Z.; Dai, L.; Ren, J.; et al. Liquid-solid phase-inversion PLGA implant for the treatment of residual tumor tissue after HIFU ablation. PLoS ONE 2015, 10, e0117358. [Google Scholar] [CrossRef]
- Pas, J.; Rutz, A.L.; Quilichini, P.P.; Slezia, A.; Ghestem, A.; Kaszas, A.; Donahue, M.J.; Curto, V.F.; O’Connor, R.P.; Bernard, C.; et al. A bilayered PVA/PLGA-bioresorbable shuttle to improve the implantation of flexible neural probes. J. Neural Eng. 2018, 15, 065001. [Google Scholar] [CrossRef]
- He, P.; Xu, S.; Guo, Z.; Yuan, P.; Liu, Y.; Chen, Y.; Zhang, T.; Que, Y.; Hu, Y. Pharmacodynamics and pharmacokinetics of PLGA-based doxorubicin-loaded implants for tumor therapy. Drug Deliv. 2022, 29, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, C.; Zhou, J. Effective sustained release of 5-FU-loaded PLGA implant for improving therapeutic index of 5-FU in colon tumor. Int. J. Pharm. 2018, 550, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Zhang, Q.; Li, J.; She, P.; Kong, F.; Du, Y.; Zhang, F. Implantable zoledronate-PLGA microcapsules ameliorate alveolar bone loss, gingival inflammation and oxidative stress in an experimental periodontitis rat model. J. Biomater. Appl. 2021, 35, 569–578. [Google Scholar] [CrossRef]
- Parent, M.; Nouvel, C.; Koerber, M.; Sapin, A.; Maincent, P.; Boudier, A. PLGA in situ implants formed by phase inversion: Critical physicochemical parameters to modulate drug release. J. Control. Release 2013, 172, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; He, J.; Olson, J.J.; Lu, D.R. Carboplatin-Loaded PLGA Microspheres for Intracerebral Implantation: In Vivo Characterization. Drug Deliv. 1997, 4, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Dsa, J.; Bekal, A.; Goswami, M. Microfabrication and Characterization of Chemically Actuated Implantable PLGA Reservoir-based Device for Controlled Drug Delivery. IETE J. Res. 2023, 70, 2941–2948. [Google Scholar] [CrossRef]
- Bakhrushina, E.O.; Sakharova, P.S.; Konogorova, P.D.; Pyzhov, V.S.; Kosenkova, S.I.; Bardakov, A.I.; Zubareva, I.M.; Krasnyuk, I.I.; Krasnyuk, I.I., Jr. Burst Release from In Situ Forming PLGA-Based Implants: 12 Effectors and Ways of Correction. Pharmaceutics 2024, 16, 115. [Google Scholar] [CrossRef]
- Santoveña, A.; Alvarez-Lorenzo, C.; Llabrés, M.; Concheiro, A.; Fariña, J.B. hGH release from directly compressed hGH-PLGA biodegradable implantable tablets: Influence of physicomechanical factors. Eur. Polym. J. 2009, 45, 2830–2838. [Google Scholar] [CrossRef]
- Amann, L.C.; Gandal, M.J.; Lin, R.; Liang, Y.; Siegel, S.J. In vitro-in vivo correlations of scalable PLGA-risperidone implants for the treatment of schizophrenia. Pharm. Res. 2010, 27, 1730–1737. [Google Scholar] [CrossRef]
- He, P.; Que, Y.K.; Li, S.; Chen, Y.; Xiao, L.Z.; Wang, H.M.; Guo, Z.H.; Wang, S.L.; Hu, Y. Enhanced anti-rheumatic efficacy of PLGA-based methotrexate-loaded implants in adjuvant-induced arthritis rat model. J. Drug Deliv. Sci. Technol. 2023, 88, 104939. [Google Scholar] [CrossRef]
- Zlomke, C.; Barth, M.; Mader, K. Polymer degradation induced drug precipitation in PLGA implants–Why less is sometimes more. Eur. J. Pharm. Biopharm. 2019, 139, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, L.; Pan, S.; Zhang, L.; Zhang, W.; Yi, H.; Niu, Y. Three-dimensional simulated microgravity culture improves the proliferation and odontogenic differentiation of dental pulp stem cell in PLGA scaffolds implanted in mice. Mol. Med. Rep. 2017, 15, 873–878. [Google Scholar] [CrossRef]
- Caminal, M.; Moll, X.; Codina, D.; Rabanal, R.M.; Morist, A.; Barrachina, J.; Garcia, F.; Pla, A.; Vives, J. Transitory improvement of articular cartilage characteristics after implantation of polylactide:polyglycolic acid (PLGA) scaffolds seeded with autologous mesenchymal stromal cells in a sheep model of critical-sized chondral defect. Biotechnol. Lett. 2014, 36, 2143–2153. [Google Scholar] [CrossRef] [PubMed]
- Onishi, H.; Takahashi, M.; Machida, Y. PLGA implant tablet of ketoprofen: Comparison of in vitro and in vivo releases. Biol. Pharm. Bull. 2005, 28, 2011–2015. [Google Scholar] [CrossRef]
- Rodriguez-Agirretxe, I.; Vega, S.C.; Rezola, R.; Vecino, E.; Mendicute, J.; Suarez-Cortes, T.; Acera, A. The PLGA implant as an antimitotic delivery system after experimental trabeculectomy. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5227–5235. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.J.; Bichara, D.A.; Zhao, X.; Randolph, M.A.; Gill, T.J. Implant-assisted meniscal repair in vivo using a chondrocyte-seeded flexible PLGA scaffold. J. Biomed. Mater. Res. A 2011, 99, 102–108. [Google Scholar] [CrossRef]
- Gad, H.A.; El-Nabarawi, M.A.; Abd El-Hady, S.S. Formulation and evaluation of PLA and PLGA in situ implants containing secnidazole and/or doxycycline for treatment of periodontitis. AAPS PharmSciTech 2008, 9, 878–884. [Google Scholar] [CrossRef]
- Lozza, I.; Martin-Sabroso, C.; Torres-Suarez, A.I.; Fraguas-Sanchez, A.I. In situ forming PLA and PLGA implants for the parenteral administration of Cannabidiol. Int. J. Pharm. 2024, 661, 124468. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).