Abstract
Background: Postoperative pain management after total knee arthroplasty (TKA) is crucial for promoting early recovery. Advances in pain management techniques have significantly improved outcomes after TKA. Recently, multimodal analgesia has emerged as a key concept in pain management following TKA, using regional anaesthesia to reduce narcotic use and minimise narcotic-related side effects. This Bayesian network meta-analysis compared different treatment options for the management of postoperative pain following primary TKA. Methods: This study was conducted following the 2020 PRISMA statement. In January 2025, all randomised controlled trials (RCTs) related to postoperative pain management following TKA were accessed. Pain reported on postoperative days (PODs) 1–3 was evaluated. Results: Data from 7199 patients were retrieved. Of these, 63.2% (4232 of 6691) were women, and the mean age was 66.7 ± 3.1 years. The mean length of follow-up was 10.2 ± 18.3 weeks. At baseline, comparability was confirmed for age (p = 0.1), BMI (p = 0.8), and visual analogue scale (VAS, p = 0.1). On POD 1, single-shot SNB/three-in-one block was associated with a lower VAS, followed by continuous intra-articular analgesia/local infiltration analgesia (LIA)/posterior capsule infiltration (PCI) and continuous femoral nerve block (FNB)/intermittent SNB. On POD 2, continuous intra-articular analgesia/LIA/PCI was associated with a lower VAS, followed by continuous FNB/PCI and single-shot femoral triangle block (FTB)/single-shot infiltration between the popliteal artery and capsule of the knee (IPACK). On POD 3, continuous ACB was associated with a lower VAS, followed by continuous intra-articular analgesia/LIA/PCI and continuous FNB/PCI. Conclusions: Continuous intra-articular analgesia/LIA/PCI was associated with the best pain control following primary TKA. Multimodal analgesia, which incorporates peripheral nerve blockade and periarticular injections, has become a key concept in contemporary pain management following TKA.
1. Introduction
Total knee arthroplasty (TKA) is a widely performed surgical procedure for patients with end-stage osteoarthritis (OA) or rheumatic arthritis to improve mobility and alleviate joint pain [1,2,3,4]. TKA is one of the most effective surgeries in musculoskeletal medicine [5,6,7,8,9]. However, despite its effectiveness, TKA often results in moderate to severe postoperative pain, which can be challenging to manage [10]. In some cases, patients experience extreme immediate postoperative pain [11], which can significantly hinder rehabilitation efforts, reduce patient satisfaction, and adversely impact the overall outcomes of the procedure. Severe postoperative pain can lead to prolonged hospital stays, increased readmissions, and higher opioid consumption, often accompanied by nausea and vomiting. These factors reduce patient satisfaction and increase healthcare costs [11,12]. Therefore, effective postoperative pain management is crucial for prompting early recovery and improving patient outcomes. In the past, perioperative pain management for TKA primarily relied on opioids [13]. However, the use of opioids is associated with adverse effects, such as risk of addiction and adverse side effects, limiting their routine application in clinical practice. Advances in pain management techniques, especially in the last two decades, have significantly improved the outcomes and practice of TKA. Recently, multimodal analgesia has emerged as a key concept in pain management following TKA. This approach incorporates techniques such as local infiltration analgesia (LIA), posterior capsule infiltration (PCI), peripheral nerve blocks such as adductor canal block (ACB), femoral nerve block (FNB) and sciatic nerve block (SNB), intravenous patient-controlled analgesia (PCA), epidural anaesthesia, and the use of various pain medications [10,11,14].
There is a growing trend toward multimodal approaches using regional anaesthesia to reduce narcotic use and minimise narcotic-related side effects [11]. However, these techniques also have limitations, such as suboptimal pain control and unwanted side effects, and no gold standard has been established yet. Therefore, this Bayesian network meta-analysis aimed to compare the different treatment options in managing postoperative pain following primary TKA.
2. Methods
2.1. Search Strategy
This Bayesian network meta-analysis was conducted according to the PRISMA extension statement for reporting systematic reviews incorporating network meta-analyses of healthcare interventions [15]. The following framework (PICOTD) was used for the search:
- P (Problem): TKA;
- I (Intervention): postoperative pain control;
- C (Comparison): different strategies to manage pain control;
- O (Outcomes): visual analogue scale;
- T (Timing): hospitalisation;
- D (Design): randomised controlled trials.
PubMed, Web of Science, and Embase were accessed in January 2025 without additional filters or temporal constraints. The Medical Subject Headings (MeSHs) used in the database are reported as Supplementary Materials.
2.2. Eligibility Criteria
All randomised controlled trials (RCTs) concerning postoperative pain management following TKA were considered. Eligible studies were required to be published in peer-reviewed journals. According to the authors’ capabilities, only articles in the following languages were considered: Italian, Spanish, German, English, or French. Only studies with levels I to II of evidence, according to the Oxford Centre of Evidence-Based Medicine (OCEBM) [16], were considered. Studies that evaluated other non-pharmacological analgesia modalities were not considered. Opinions, letters, editorials, and reviews were excluded. Additionally, studies involving animals, computational analyses, biomechanical assessments, in vitro experiments, or cadaveric research were disregarded. Only RCTs concerning pain management in TKA were included. Studies evaluating monocompartmental arthroplasty or those in revision settings were not eligible. Only studies reporting data on the visual analogue scale (VAS) [17] for each postoperative day (POD) were included.
2.3. Outcomes of Interest
Three authors (H.A.M.E, G.C., and T.B.) conducted data extraction. For each study, the following generalities were collected: author, year of publication, journal, study design, and length of follow-up. The following data at baseline were retrieved: number of patients, number of women, mean age, and mean BMI. Data concerning VAS were collected for PODs 0, 1, 2, and 3 and at discharge. Extraction was performed using Microsoft Office Excel version 16.0 (Microsoft Corporation, Redmond, WA, USA).
2.4. Methodology Quality Assessment
Three authors (H.A.M.E., G.C., and T.B.) performed the methodological quality assessment using the revised Risk of Bias assessment tool (RoB2) [18,19] of the Cochrane tool for assessing the Risk of Bias in randomised trials [20]. The following endpoints were considered: bias arising from the randomisation process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in measuring the outcome, and bias in selecting the reported result.
2.5. Statistical Analysis
The statistical analysis was performed by the main author (F.M.). STATA Software/MP (version 15, StataCorporation, College Station, TX, USA) was used for the statistical analyses. The baseline comparability was assessed using analysis of variance (ANOVA), with p-values > 0.1 considered satisfactory. The network meta-analyses were performed through the STATA routine using the inverse variance method for Bayesian hierarchical random-effects model analysis. The standardised mean difference (STD) was calculated for continuous data. The overall inconsistency was evaluated through the equation for global linearity via the Wald test. If the p-value > 0.1, the null hypothesis cannot be rejected, and the consistency assumption is accepted at the overall level of each treatment. Edge plots were performed to display direct and indirect comparisons and respective statistical weights. Interval plots were performed to rank each treatment according to their estimated effect. Both confidence (CI) and percentile (PrI) intervals were set at 95% in each interval plot. Funnel plots were performed to investigate the risk of bias related to each comparison. Greater plot asymmetry indicates greater data variability and is associated with a greater risk of bias.
3. Results
3.1. Search Result
The systematic literature search resulted in the identification of 3283 articles. After removing duplicates, the abstracts of 2196 articles were screened for eligibility. A total of 2004 articles were excluded for the following reasons: mismatch with the predefined study design criteria (n = 1123), full-text unavailability (n = 725), and language limitations (n = 156). Of the remaining 192 studies, another 115 were excluded after full-text evaluation. Consequently, a final selection of 77 studies [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96] was included in this systematic review. The results of the literature search are shown in Figure 1.
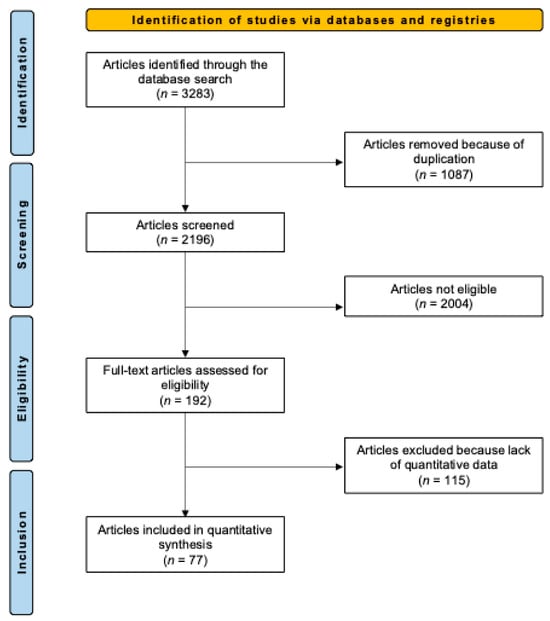
Figure 1.
Flowchart of the literature search.
3.2. Methodological Quality Assessment
The Cochrane Risk of Bias Assessment tool (ROB 2) was used to evaluate the 77 included RCTs. The analysis suggested a generally low to moderate risk of bias in the first three and the last domains: randomisation process, deviation from intended intervention, missing data, and selection of the reported result. Given the lack of blinded assessors, the outcome measurement, on the other hand, was at high risk in nearly half of the RCTs. The overall RoB was estimated to be low or moderate in more than half of the included RCTs, suggesting an acceptable methodological quality. However, caution must be paid to the potential bias in the outcome measurements. Figure 2 shows the bias risk distribution across the included RCTs.
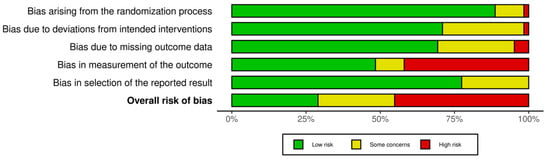
Figure 2.
Methodological quality assessment.
3.3. Patient Demographics
Data from 7199 patients were retrieved, 63.2% of whom (4232 of 6691) were women. The mean length of follow-up was 10.2 ± 18.3 weeks. The mean age was 66.7 ± 3.1 years, and the mean BMI was 28.6 ± 3.0 kg/m2. The ANOVA test found comparability in age (p = 0.1), BMI (p = 0.8), and VAS (p = 0.1) at baseline. Table 1 shows the generalities and demographics of the studies.

Table 1.
Characteristics and patient baseline of the included studies (LIA: local infiltration analgesia; PCI: posterior capsule infiltration; ACB: adductor canal block; FNB: femoral nerve block; SNB: sciatic nerve block; PCA: patient-controlled analgesia; FTB: femoral triangle block; IPACK: infiltration between the popliteal artery and capsule of the knee).
3.4. Outcomes of Interest
Sixty-two RCTs (5856 patients) reported data on POD 1 [21,22,23,25,26,27,28,29,30,31,34,35,36,38,39,42,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,66,67,68,69,70,71,72,73,74,75,76,77,78,80,83,84,86,87,88,89,90,91,92,93,94,95,96]. Single-shot SNB/three-in-one block was associated with a lower VAS (SMD −0.50; 95% CI −1.98 to 0.98), followed by continuous intra-articular analgesia/LIA/PCI (SMD −0.17; 95% CI 2.13 to 1.78) and continuous FNB/intermittent SNB (SMD −0.0; 95% CI −2.13 to 2.13).
Fifty-three RCTs (5695 patients) reported data on POD 2 [23,24,25,27,31,36,37,39,40,41,42,43,44,45,47,50,51,52,53,54,55,56,58,59,60,62,63,64,65,66,69,70,71,72,73,74,77,78,79,80,81,84,86,87,88,89,90,91,92,93,94,95,96]. Continuous intra-articular analgesia/LIA/PCI was associated with a lower VAS (SMD −0.76; 95% CI 2.40 to 0.87), followed by continuous FNB/PCI (SMD −0.50; 95% CI −1.73 to 0.74) and single-shot FTB/single-shot IPACK (SMD −0.35; 95% CI −2.08 to 1.38).
Twenty-nine RCTs (2101 patients) reported data on POD 3 [21,23,27,31,32,33,36,37,42,44,45,50,51,54,55,56,58,70,76,80,81,82,85,87,88,89,90,91,95]. Continuous ACB was associated with a lower VAS (SMD −1.00; 95% CI −3.62 to 1.62), followed by continuous intra-articular analgesia/LIA/PCI (SMD −0.70; 95% CI −2.51 to 1.11) and continuous FNB/PCI (SMD −0.52; 95% CI −2.33 to 1.29). Edge, funnel, and interval plots of each POD are reported as Supplementary Materials.
4. Discussion
Multimodal analgesia is a key concept in pain management following TKA. However, there is still debate regarding the most effective combination of techniques. According to the findings of the present Bayesian network meta-analysis, continuous intra-articular analgesia/LIA/PCI was associated with superior pain control after primary TKA. The lowest VAS scores were observed with single-shot SNB/three-in-one block on postoperative day 1, for continuous intra-articular analgesia/LIA/PCI on postoperative day 2, and continuous ACB on postoperative day 3.
Traditional general anaesthesia combined with postoperative epidural and patient-controlled opioid analgesia is associated with a high rate of undesirable adverse effects. In contrast, the emerging concept of multimodal anaesthesia for TKA offers superior pain control, minimises opioid-related side effects, enhances patient satisfaction, and reduces the risk of postoperative complications [97]. In addition to oral opioid and non-opioid medications during the perioperative and postoperative period, multimodal anaesthesia integrates elements of pre-emptive analgesia, neuraxial perioperative anaesthesia, peripheral nerve blockade (PNB), and periarticular injections (PAI) [97].
However, despite the availability of multimodal treatment options for pain management following TKA, there is still no consensus on the optimal method [13,14]. PNB, LIA, and PCA are the most commonly used techniques. PCA involves using a programmable device tailored to the analgesic, the patient’s physical characteristics, and baseline pain levels. A small amount of analgesic can be delivered when patients press a button to administer it as needed. While opioids, such as morphine, fentanyl, and hydromorphone, are commonly used in PCA, it is associated with some adverse effects [98]. However, these effects are generally less severe than those caused by conventional opioid treatment [99]. Additionally, PCA encourages early mobilisation and reduces the length of hospital stays after TKA [13]. Based on a systematic review and meta-analysis, the International Consensus on Anesthesia-Related Outcomes after Surgery (ICAROS) group recommends PNB use in THA/TKA for improved outcomes [100]. Among PNB, femoral nerve block (FNB) has been widely accepted as the gold standard for pain relief after TKA. It provides adequate postoperative analgesia and contributes to long-term functional recovery in patients undergoing TKA [101]. However, FNB also reduces quadriceps muscle strength, which limits knee extension [102,103] and is associated with potentially serious complications, such as blood vessel and nerve damage [104]. Because the knee is innervated by several nerves, including the femoral, sciatic, obturator, saphenous, and lateral femoral cutaneous nerve, pain in the posterior aspect of the knee is not adequately reduced by FNB alone. Combining FNB with a sciatic nerve block (SNB) could address this limitation effectively. However, a combination of SNB with FNB may cause postoperative muscle weakness and may delay rehabilitation in the early postoperative period. Besides FNB and SNB, ACB is becoming increasingly popular as it provides postoperative pain relief as effectively as FNB but without impairing quadriceps muscle strength [103,105]. With ACB, it may be possible to block the saphenous nerve while sparing the major motor branches of the femoral nerve [13]. Compared with FNB, patients receiving ACB experience better quadriceps muscle strength, improved early rehabilitation, longer ambulation distances, and shorter hospital stays [79,106]. However, ACB does not adequately relieve lateral knee pain in the early stages [79] and remains a relatively new regional anaesthesia technique for TKA, requiring further clinical and scientific evaluation. In a systematic review and meta-analysis by Sercia et al., continuous ACB significantly reduced 48 h pain scores but did not significantly decrease opioid consumption [107]. LIA has gained significant interest in recent years given its simplicity, low associated risk, and reduced likelihood of systemic toxicity from local anaesthetics [13]. Additionally, LIA can address posterior knee pain by enabling injections into the posterior joint capsule (PCI: posterior capsule infiltration).
Furthermore, ultrasound-guided infiltration of local anaesthetics in the interspace between the popliteal artery and the posterior capsule of the knee (IPACK) is a novel regional anaesthetic technique for posterior knee analgesia that has shown promising results [108]. LIA is regarded as a promising method for pain management due to its ability to facilitate early mobilisation without compromising quadriceps muscle strength [109]. For this reason, combining an LIA with FNB may be a more acceptable approach than combining SNB with FNB for pain management following TKA [14]. A meta-analysis by Zhang et al. demonstrated that LIA was as effective as FNB regarding VAS scores for pain control at 24, 48, and 72 h, total morphine consumption, range of motion, knee society scores, complications, and length of hospital stay [110]. Furthermore, LIA significantly improved postoperative pain relief and reduced opioid consumption compared with ACB [111]. In contrast to the present findings, a meta-analysis by Wang et al. showed that single-shot FNB might provide better pain relief in the early postoperative period compared with single-shot periarticular multimodal drug injection (PMDI)/LIA. At the same time, continuous PMDI/LIA offered postoperative analgesia comparable to continuous FNB [112]. When comparing the efficacy of LIA and SNB combined with single-shot and continuous FNB, Tanikawa et al. found that SNB was more effective than LIA in reducing pain immediately after surgery [81]. However, consistent with the present findings, SNB was less effective than LIA at 24 h post-surgery [81]. Interestingly, a recent network meta-analysis suggested that ACB combined with IPACK may be the optimal analgesic regimen for TKA patients [113]. The analysis included and compared FNB, ACB, IPACK, and genicular nerve block (GNB). ACB + IPACK was the most effective regimen for resting pain and movement pain relief (78% and 87%, respectively) and for reducing opioid consumption (90%) at 48 h. Meanwhile, FNB combined with IPACK was the most efficacious option for resting pain relief (42%) and reducing opioid consumption (68%) at 24 h. GNB was the most effective option for movement pain relief at 24 h (94%) [113]. These conflicting results may be attributed to the different analgesic methods included in the analysis.
This study has several limitations. The present Bayesian network meta-analysis encompasses a wide range of interventions. The strength of Bayesian network meta-analysis is its capability to integrate a variety of treatments while maintaining consistency and validity through the concept of transitivity. In the present investigation, the authors carefully examined the comparability of studies, ensuring that the included treatments were sufficiently similar regarding their overall treatment objectives, population characteristics, and methodological rigour. Using a random-effects model accounts for variability between studies, including differences in treatment regimens, dosages, and patient populations. This model explicitly acknowledges and incorporates between-study heterogeneity, thereby enabling the synthesis of diverse treatment arms without compromising the validity of the pooled estimates. While the authors recognise that combining diverse treatments requires careful consideration, the Bayesian framework offers a robust methodology for pooling results, provided that the studies meet established methodological criteria. This meta-analytic approach was intentionally designed to maximise the inclusivity of relevant studies, ensuring a comprehensive dataset for the network meta-analyses. A key principle of Bayesian network meta-analysis is that its reliability and robustness directly depend on the quantity and connectivity of the available data. By minimising restrictive eligibility criteria, the present study aimed to include the broadest possible range of high-quality RCTs, thereby enhancing the statistical power and transitivity of the network. This approach is particularly critical in Bayesian frameworks, where prior distributions and borrowing of strength across comparisons require a well-connected and sufficiently populated network to yield stable and generalisable results. Only short-term pain relief was evaluated in this analysis, making it unclear which modality provides the best long-term clinical outcome. Additionally, this meta-analysis included multiple studies that are heterogenous and differ in several aspects, such as participant characteristics (age, sex, activity, BMI, etc.), single- or multicentre study designs, cohort size (small-, medium- and large-sized studies), and the number and types of analyses conducted. Another limitation is that ACB can be categorised into proximal and distal blocks, which produce different effects [114]. However, these distinctions were not considered in the present analysis. Moreover, the concentration and volume of local anaesthetic varied between studies, potentially affecting the analgesic outcomes. This study compared different treatment options for managing postoperative pain following primary TKA. Other aspects, such as the use of various drugs with differing times of onset and duration of effect, mechanisms of action, and routes of administration, were not included in this study. Additionally, the type of implants (e.g., short- or standard-stem and dual- or single-mobility) and related variations (e.g., cementation and surgical access) were not considered. Furthermore, there were differences in postoperative rehabilitation protocols and concomitant procedures performed across studies. These differences were not accounted for in the final analysis, although they could influence the results.
The strength of the present study is the analysis of various analgesic methods. We included several local infiltration methods, peripheral nerve blocks, intravenous patient-controlled analgesia, epidural anaesthesia, and the use of pain medications.
5. Conclusions
Continuous intra-articular analgesia, LIA, or PCI was associated with the best pain control following primary TKA. However, there appears to be a shift toward multimodal approaches, using regional anaesthesia to minimise narcotic consumption. Multimodal analgesia, incorporating peripheral nerve blockade and periarticular injection elements, has become a cornerstone in pain management following TKA.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ph18040556/s1, File S1: Research Questions and Searching Strategy.
Author Contributions
Conceptualisation, F.M.; methodology, F.M., M.B., G.C., H.A.M.E., A.D. and T.B.; formal analysis, F.M.; writing—original draft preparation, H.A.M.E., F.M., M.B. and M.P.; writing—review and editing, F.M., F.H. and M.B.; project administration, F.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within this article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Migliorini, F.; Aretini, P.; Driessen, A.; El Mansy, Y.; Quack, V.; Tingart, M.; Eschweiler, J. Better outcomes after mini-subvastus approach for primary total knee arthroplasty: A Bayesian network meta-analysis. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 979–992. Correction in Eur. J. Orthop. Surg. Traumatol. 2021, 31, 1259. https://doi.org/10.1007/s00590-021-03026-9. [CrossRef] [PubMed]
- Migliorini, F.; Eschweiler, J.; Baroncini, A.; Tingart, M.; Maffulli, N. Better outcomes after minimally invasive surgeries compared to the standard invasive medial parapatellar approach for total knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2021, 29, 3608–3620. [Google Scholar] [CrossRef] [PubMed]
- Migliorini, F.; Eschweiler, J.; Mansy, Y.E.; Quack, V.; Schenker, H.; Tingart, M.; Driessen, A. Gap balancing versus measured resection for primary total knee arthroplasty: A meta-analysis study. Arch. Orthop. Trauma Surg. 2020, 140, 1245–1253. [Google Scholar] [CrossRef]
- Migliorini, F.; Eschweiler, J.; Niewiera, M.; El Mansy, Y.; Tingart, M.; Rath, B. Better outcomes with patellar resurfacing during primary total knee arthroplasty: A meta-analysis study. Arch. Orthop. Trauma Surg. 2019, 139, 1445–1454. [Google Scholar] [CrossRef]
- Migliorini, F.; Eschweiler, J.; Tingart, M.; Rath, B. Posterior-stabilized versus cruciate-retained implants for total knee arthroplasty: A meta-analysis of clinical trials. Eur. J. Orthop. Surg. Traumatol. 2019, 29, 937–946. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N. What are the best antithrombotic prophylaxes following total knee arthroplasty? Expert Opin. Drug Saf. 2024, 23, 1367–1369. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Cuozzo, F.; Pilone, M.; Elsner, K.; Eschweiler, J. No difference between mobile and fixed bearing in primary total knee arthroplasty: A meta-analysis. Knee Surg. Sports Traumatol. Arthrosc. 2022, 30, 3138–3154. [Google Scholar] [CrossRef]
- Migliorini, F.; Maffulli, N.; Schafer, L.; Simeone, F.; Bell, A.; Hofmann, U.K. Minimal clinically important difference (MCID), substantial clinical benefit (SCB), and patient-acceptable symptom state (PASS) in patients who have undergone total knee arthroplasty: A systematic review. Knee Surg. Relat. Res. 2024, 36, 3. [Google Scholar] [CrossRef]
- Migliorini, F.; Pilone, M.; Schafer, L.; Simeone, F.; Bell, A.; Maffulli, N. Functional alignment in robotic-assisted total knee arthroplasty: A systematic review. Arch. Orthop. Trauma Surg. 2024, 144, 1741–1749. [Google Scholar] [CrossRef] [PubMed]
- Ranawat, A.S.; Ranawat, C.S. Pain management and accelerated rehabilitation for total hip and total knee arthroplasty. J. Arthroplast. 2007, 22, 12–15. [Google Scholar] [CrossRef]
- Maheshwari, A.V.; Blum, Y.C.; Shekhar, L.; Ranawat, A.S.; Ranawat, C.S. Multimodal pain management after total hip and knee arthroplasty at the Ranawat Orthopaedic Center. Clin. Orthop. Relat. Res. 2009, 467, 1418–1423. [Google Scholar] [CrossRef] [PubMed]
- Indelli, P.F.; Grant, S.A.; Nielsen, K.; Vail, T.P. Regional anesthesia in hip surgery. Clin. Orthop. Relat. Res. 2005, 441, 250–255. [Google Scholar] [CrossRef]
- Li, J.W.; Ma, Y.S.; Xiao, L.K. Postoperative Pain Management in Total Knee Arthroplasty. Orthop. Surg. 2019, 11, 755–761. [Google Scholar] [CrossRef]
- Aso, K.; Izumi, M.; Sugimura, N.; Okanoue, Y.; Kamimoto, Y.; Yokoyama, M.; Ikeuchi, M. Additional benefit of local infiltration of analgesia to femoral nerve block in total knee arthroplasty: Double-blind randomized control study. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2368–2374. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef]
- Howick, J.C.I.; Glasziou, P.; Greenhalgh, T.; Carl Heneghan Liberati, A.; Moschetti, I.; Phillips, B.; Thornton, H.; Goddard, O.; Hodgkinson, M. The 2011 Oxford CEBM Levels of Evidence. Oxford Centre for Evidence-Based Medicine. 2011. Available online: https://www.cebm.net/index.aspx?o=5653 (accessed on 18 February 2025).
- Migliorini, F.; Maffulli, N.; Eschweiler, J.; Schenker, H.; Tingart, M.; Betsch, M. Arthroscopic versus mini-open rotator cuff repair: A meta-analysis. Surgeon 2023, 21, e1–e12. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savovic, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Chapter 8: Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.3; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., Eds.; Updated February 2022; Cochrane: Cochrane, AB, Canada, 2022; Available online: https://training.cochrane.org/handbook/current/chapter-08 (accessed on 18 February 2025).
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Adams, H.A.; Saatweber, P.; Schmitz, C.S.; Hecker, H. Postoperative pain management in orthopaedic patients: No differences in pain score, but improved stress control by epidural anaesthesia. Eur. J. Anaesthesiol. 2002, 19, 658–665. [Google Scholar] [CrossRef]
- Akesen, S.; Akesen, B.; Atıcı, T.; Gurbet, A.; Ermutlu, C.; Özyalçın, A. Comparison of efficacy between the genicular nerve block and the popliteal artery and the capsule of the posterior knee (IPACK) block for total knee replacement surgery: A prospective randomized controlled study. Acta Orthop. Traumatol. Turc. 2021, 55, 134–140. [Google Scholar] [CrossRef]
- Albrecht, E.; Morfey, D.; Chan, V.; Gandhi, R.; Koshkin, A.; Chin, K.J.; Robinson, S.; Frascarolo, P.; Brull, R. Single-injection or continuous femoral nerve block for total knee arthroplasty? Clin. Orthop. Relat. Res. 2014, 472, 1384–1393. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, T.; Doais, K.S.; Aljassir, F.; Alshaygy, I.; Albishi, W.; Terkawi, A.S. Randomized clinical trial of continuous femoral nerve block combined with sciatic nerve block versus epidural analgesia for unilateral total knee arthroplasty. J. Arthroplast. 2015, 30, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Ardon, A.E.; Clendenen, S.R.; Porter, S.B.; Robards, C.B.; Greengrass, R.A. Opioid consumption in total knee arthroplasty patients: A retrospective comparison of adductor canal and femoral nerve continuous infusions in the presence of a sciatic nerve catheter. J. Clin. Anesth. 2016, 31, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, A.; Raut, V.V.; Canty, S.J.; McLauchlan, G.J. Pain control after primary total knee replacement. A prospective randomised controlled trial of local infiltration versus single shot femoral nerve block. Knee 2013, 20, 324–327. [Google Scholar] [CrossRef]
- Bagry, H.; de la Cuadra Fontaine, J.C.; Asenjo, J.F.; Bracco, D.; Carli, F. Effect of a continuous peripheral nerve block on the inflammatory response in knee arthroplasty. Reg. Anesth. Pain. Med. 2008, 33, 17–23. [Google Scholar] [CrossRef]
- Bali, C.; Ozmete, O.; Eker, H.E.; Hersekli, M.A.; Aribogan, A. Postoperative analgesic efficacy of fascia iliaca block versus periarticular injection for total knee arthroplasty. J. Clin. Anesth. 2016, 35, 404–410. [Google Scholar] [CrossRef]
- Baranović, S.; Maldini, B.; Milosević, M.; Golubić, R.; Nikolić, T. Peripheral regional analgesia with femoral catheter versus intravenous patient controlled analgesia after total knee arthroplasty: A prospective randomized study. Coll. Antropol. 2011, 35, 1209–1214. [Google Scholar]
- Campbell, A.; McCormick, M.; McKinlay, K.; Scott, N.B. Epidural vs. lumbar plexus infusions following total knee arthroplasty: Randomized controlled trial. Eur. J. Anaesthesiol. 2008, 25, 502–507. [Google Scholar] [CrossRef]
- Canbek, U.; Akgun, U.; Aydogan, N.H.; Kilinc, C.Y.; Uysal, A.I. Continuous adductor canal block following total knee arthroplasty provides a better analgesia compared to single shot: A prospective randomized controlled trial. Acta Orthop. Traumatol. Turc. 2019, 53, 334–339. [Google Scholar] [CrossRef]
- Cappelleri, G.; Ghisi, D.; Fanelli, A.; Albertin, A.; Somalvico, F.; Aldegheri, G. Does continuous sciatic nerve block improve postoperative analgesia and early rehabilitation after total knee arthroplasty? A prospective, randomized, double-blinded study. Reg. Anesth. Pain. Med. 2011, 36, 489–492. [Google Scholar] [CrossRef]
- Carli, F.; Clemente, A.; Asenjo, J.F.; Kim, D.J.; Mistraletti, G.; Gomarasca, M.; Morabito, A.; Tanzer, M. Analgesia and functional outcome after total knee arthroplasty: Periarticular infiltration vs. continuous femoral nerve block. Br. J. Anaesth. 2010, 105, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, R.; Calixto, L.; Braganca, J.P. Effect of a single shot sciatic nerve block combined with a continuous femoral block on pain scores after knee arthroplasty. A randomized controlled trial. Open J. Anesthesiol. 2012, 2, 22253. [Google Scholar] [CrossRef][Green Version]
- Casati, A.; Vinciguerra, F.; Cappelleri, G.; Aldegheri, G.; Fanelli, G.; Putzu, M.; Chelly, J.E. Adding clonidine to the induction bolus and postoperative infusion during continuous femoral nerve block delays recovery of motor function after total knee arthroplasty. Anesth. Analg. 2005, 100, 866–872. [Google Scholar] [CrossRef]
- Chan, M.H.; Chen, W.H.; Tung, Y.W.; Liu, K.; Tan, P.H.; Chia, Y.Y. Single-injection femoral nerve block lacks preemptive effect on postoperative pain and morphine consumption in total knee arthroplasty. Acta Anaesthesiol. Taiwan. 2012, 50, 54–58. [Google Scholar] [CrossRef]
- Chan, E.Y.; Fransen, M.; Sathappan, S.; Chua, N.H.; Chan, Y.H.; Chua, N. Comparing the analgesia effects of single-injection and continuous femoral nerve blocks with patient controlled analgesia after total knee arthroplasty. J. Arthroplast. 2013, 28, 608–613. [Google Scholar] [CrossRef]
- Chaumeron, A.; Audy, D.; Drolet, P.; Lavigne, M.; Vendittoli, P.A. Periarticular injection in knee arthroplasty improves quadriceps function. Clin. Orthop. Relat. Res. 2013, 471, 2284–2295. [Google Scholar] [CrossRef]
- Cicekci, F.; Yildirim, A.; Önal, Ö.; Celik, J.B.; Kara, I. Ultrasound-guided adductor canal block using levobupivacaine versus periarticular levobupivacaine infiltration after totalknee arthroplasty: A randomized clinical trial. Sao Paulo Med. J. 2019, 137, 45–53. [Google Scholar] [CrossRef]
- Elkassabany, N.M.; Antosh, S.; Ahmed, M.; Nelson, C.; Israelite, C.; Badiola, I.; Cai, L.F.; Williams, R.; Hughes, C.; Mariano, E.R.; et al. The Risk of Falls After Total Knee Arthroplasty with the Use of a Femoral Nerve Block Versus an Adductor Canal Block: A Double-Blinded Randomized Controlled Study. Anesth. Analg. 2016, 122, 1696–1703. [Google Scholar] [CrossRef]
- Elkassabany, N.M.; Cai, L.F.; Badiola, I.; Kase, B.; Liu, J.; Hughes, C.; Israelite, C.L.; Nelson, C.L. A prospective randomized open-label study of single injection versus continuous adductor canal block for postoperative analgesia after total knee arthroplasty. Bone Jt. J. 2019, 101, 340–347. [Google Scholar] [CrossRef]
- Fritze, P.; Anderl, S.; Marouf, A.; Cumlivski, R.; Müller, C.; Pernicka, E.; Redl, G. Pain therapy using stimulating catheters after total knee arthroplasty. Schmerz 2009, 23, 292–298. [Google Scholar] [CrossRef]
- Gi, E.; Yamauchi, M.; Yamakage, M.; Kikuchi, C.; Shimizu, H.; Okada, Y.; Kawamura, S.; Suzuki, T. Effects of local infiltration analgesia for posterior knee pain after total knee arthroplasty: Comparison with sciatic nerve block. J. Anesth. 2014, 28, 696–701. [Google Scholar] [CrossRef] [PubMed]
- Good, R.P.; Snedden, M.H.; Schieber, F.C.; Polachek, A. Effects of a preoperative femoral nerve block on pain management and rehabilitation after total knee arthroplasty. Am. J. Orthop. 2007, 36, 554–557. [Google Scholar] [PubMed]
- Grosso, M.J.; Murtaugh, T.; Lakra, A.; Brown, A.R.; Maniker, R.B.; Cooper, H.J.; Macaulay, W.; Shah, R.P.; Geller, J.A. Adductor Canal Block Compared with Periarticular Bupivacaine Injection for Total Knee Arthroplasty: A Prospective Randomized Trial. J. Bone Jt. Surg. Am. 2018, 100, 1141–1146. [Google Scholar] [CrossRef] [PubMed]
- Kanadli, H.; Dogru, S.; Karaman, T.; Karaman, S.; Tapar, H.; Şahin, A.; Aşçi, M.; Kanadli, K.A.; Süren, M. Comparison of the efficacy of femoral nerve block and fascia iliaca compartment block in patients with total knee replacement. Minerva Anestesiol. 2018, 84, 1134–1141. [Google Scholar] [CrossRef]
- Kovalak, E.; Doğan, A.T.; Üzümcügil, O.; Obut, A.; Yıldız, A.S.; Kanay, E.; Tüzüner, T.; Özyuvacı, E. A comparison of continuous femoral nerve block and periarticular local infiltration analgesia in the management of early period pain developing after total knee arthroplasty. Acta Orthop. Traumatol. Turc. 2015, 49, 260–266. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; Dadheech, A.N.; Wakankar, H.M.; Ganjewar, N.V.; Hedgire, S.S.; Pandit, H.G. Randomized Prospective Comparative Study of Adductor Canal Block vs Periarticular Infiltration on Early Functional Outcome After Unilateral Total Knee Arthroplasty. J. Arthroplast. 2019, 34, 2360–2364. [Google Scholar] [CrossRef]
- Kurosaka, K.; Tsukada, S.; Seino, D.; Morooka, T.; Nakayama, H.; Yoshiya, S. Local Infiltration Analgesia Versus Continuous Femoral Nerve Block in Pain Relief After Total Knee Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2016, 31, 913–917. [Google Scholar] [CrossRef]
- Kutzner, K.P.; Paulini, C.; Hechtner, M.; Rehbein, P.; Pfeil, J. Postoperative analgesia after total knee arthroplasty: Continuous intra-articular catheter vs. continuous femoral nerve block. Orthopade 2015, 44, 566–573. [Google Scholar] [CrossRef]
- Li, C.; Xu, H.; Shen, B.; Yang, J.; Zhou, Z.; Kang, P.; Pei, F. Effect of continuous and single shot adductor canal blocks for postoperative analgesia and early rehabilitation after total knee arthroplasty. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2017, 31, 1049–1054. [Google Scholar] [CrossRef]
- Li, D.; Alqwbani, M.; Wang, Q.; Yang, Z.; Liao, R.; Kang, P. Ultrasound-guided adductor canal block combined with lateral femoral cutaneous nerve block for post-operative analgesia following total knee arthroplasty: A prospective, double-blind, randomized controlled study. Int. Orthop. 2021, 45, 1421–1429. [Google Scholar] [CrossRef]
- Li, D.; Alqwbani, M.; Wang, Q.; Liao, R.; Yang, J.; Kang, P. Efficacy of Adductor Canal Block Combined With Additional Analgesic Methods for Postoperative Analgesia in Total Knee Arthroplasty: A Prospective, Double-Blind, Randomized Controlled Study. J. Arthroplast. 2020, 35, 3554–3562. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Chen, Y.; Qian, J.; Li, S.; Chen, S.; Fu, P. Comparison of Femoral Triangle Block in Combination with IPACK to Local Periarticular Injection in Total Knee Arthroplasty. J. Knee Surg. 2023, 36, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Long, W.T.; Ward, S.R.; Dorr, L.D.; Raya, J.; Boutary, M.; Sirianni, L.E. Postoperative pain management following total knee arthroplasty: A randomized comparison of continuous epidural versus femoral nerve infusion. J. Knee Surg. 2006, 19, 137–143. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Yu, Q.P.; Zeng, W.N.; Xiao, Q.; Chen, X.; Wang, H.Y.; Zhou, Z. Adductor canal block combined with local infiltration analgesia with morphine and betamethasone show superior analgesic effect than local infiltration analgesia alone for total knee arthroplasty: A prospective randomized controlled trial. BMC Musculoskelet. Disord. 2022, 23, 468. [Google Scholar] [CrossRef]
- Macrinici, G.I.; Murphy, C.; Christman, L.; Drescher, M.; Hughes, B.; Macrinici, V.; Diab, G. Prospective, Double-Blind, Randomized Study to Evaluate Single-Injection Adductor Canal Nerve Block Versus Femoral Nerve Block: Postoperative Functional Outcomes After Total Knee Arthroplasty. Reg. Anesth. Pain. Med. 2017, 42, 10–16. [Google Scholar] [CrossRef]
- Marino, J.; Scuderi, G.; Dowling, O.; Farquhar, R.; Freycinet, B.; Overdyk, F. Periarticular Knee Injection With Liposomal Bupivacaine and Continuous Femoral Nerve Block for Postoperative Pain Management After Total Knee Arthroplasty: A Randomized Controlled Trial. J. Arthroplast. 2019, 34, 495–500. [Google Scholar] [CrossRef]
- Memtsoudis, S.G.; Yoo, D.; Stundner, O.; Danninger, T.; Ma, Y.; Poultsides, L.; Kim, D.; Chisholm, M.; Jules-Elysee, K.; Valle, A.G.; et al. Subsartorial adductor canal vs. femoral nerve block for analgesia after total knee replacement. Int. Orthop. 2015, 39, 673–680. [Google Scholar] [CrossRef]
- Mistraletti, G.; De La Cuadra-Fontaine, J.C.; Asenjo, F.J.; Donatelli, F.; Wykes, L.; Schricker, T.; Carli, F. Comparison of analgesic methods for total knee arthroplasty: Metabolic effect of exogenous glucose. Reg. Anesth. Pain Med. 2006, 31, 260–269. [Google Scholar] [CrossRef]
- Moreno, I.; Tsamassiottis, S.; Ettinger, M.; Fischer-Kumbruch, M.; Przemeck, M. Femoral nerve blockade versus local infiltration analgesia for primary knee arthroplasty. Randomised controlled trial. Anaesthesiol. Intensive Ther. 2022, 54, 387–392. [Google Scholar] [CrossRef]
- Mu, T.; Liu, D.; Gao, F. Butorphanol as an Adjuvant to Ropivacaine for Adductor Canal Blocks in Total Knee Arthroplasty Patients: A Randomized, Double, Blind Study. J. Healthc. Eng. 2022, 2022, 7718108. [Google Scholar] [CrossRef]
- Mudumbai, S.C.; Kim, T.E.; Howard, S.K.; Workman, J.J.; Giori, N.; Woolson, S.; Ganaway, T.; King, R.; Mariano, E.R. Continuous adductor canal blocks are superior to continuous femoral nerve blocks in promoting early ambulation after TKA. Clin. Orthop. Relat. Res. 2014, 472, 1377–1383. [Google Scholar] [CrossRef] [PubMed]
- Ng, F.Y.; Ng, J.K.; Chiu, K.Y.; Yan, C.H.; Chan, C.W. Multimodal periarticular injection vs. continuous femoral nerve block after total knee arthroplasty: A prospective, crossover, randomized clinical trial. J. Arthroplast. 2012, 27, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Nicolino, T.I.; Costantini, J.; Carbó, L. Complementary Saphenous Nerve Block to Intra-Articular Analgesia Reduces Pain After Total Knee Arthroplasty: A Prospective Randomized Controlled Trial. J. Arthroplast. 2020, 35, S168–S172. [Google Scholar] [CrossRef]
- Paauwe, J.J.; Thomassen, B.J.; Weterings, J.; van Rossum, E.; Ausems, M.E. Femoral nerve block using ropivacaine 0.025%, 0.05% and 0.1%: Effects on the rehabilitation programme following total knee arthroplasty: A pilot study. Anaesthesia 2008, 63, 948–953. [Google Scholar] [CrossRef]
- Parvataneni, H.K.; Shah, V.P.; Howard, H.; Cole, N.; Ranawat, A.S.; Ranawat, C.S. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: A prospective randomized study. J. Arthroplast. 2007, 22 (Suppl. S2), 33–38. [Google Scholar] [CrossRef]
- Patterson, M.E.; Vitter, J.; Bland, K.; Nossaman, B.D.; Thomas, L.C.; Chimento, G.F. The Effect of the IPACK Block on Pain After Primary TKA: A Double-Blinded, Prospective, Randomized Trial. J. Arthroplast. 2020, 35, S173–S177. [Google Scholar] [CrossRef]
- Rousseau-Saine, N.; Williams, S.R.; Girard, F.; Hébert, L.J.; Robin, F.; Duchesne, L.; Lavoie, F.; Ruel, M. The Effect of Adductor Canal Block on Knee Extensor Muscle Strength 6 Weeks After Total Knee Arthroplasty: A Randomized, Controlled Trial. Anesth. Analg. 2018, 126, 1019–1027. [Google Scholar] [CrossRef]
- Salinas, F.V.; Liu, S.S.; Mulroy, M.F. The effect of single-injection femoral nerve block versus continuous femoral nerve block after total knee arthroplasty on hospital length of stay and long-term functional recovery within an established clinical pathway. Anesth. Analg. 2006, 102, 1234–1239. [Google Scholar] [CrossRef]
- Sankineani, S.R.; Reddy, A.R.C.; Ajith Kumar, K.S.; Eachempati, K.K.; Reddy, A.V.G. Comparative analysis of influence of adductor canal block and multimodal periarticular infiltration versus adductor canal block alone on pain and knee range of movement after total knee arthroplasty: A prospective non-randomised study. Musculoskelet. Surg. 2018, 102, 173–177. [Google Scholar] [CrossRef]
- Sankineani, S.R.; Reddy, A.R.C.; Eachempati, K.K.; Jangale, A.; Gurava Reddy, A.V. Comparison of adductor canal block and IPACK block (interspace between the popliteal artery and the capsule of the posterior knee) with adductor canal block alone after total knee arthroplasty: A prospective control trial on pain and knee function in immediate postoperative period. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1391–1395. [Google Scholar] [CrossRef]
- Shah, N.A.; Jain, N.P. Is continuous adductor canal block better than continuous femoral nerve block after total knee arthroplasty? Effect on ambulation ability, early functional recovery and pain control: A randomized controlled trial. J. Arthroplast. 2014, 29, 2224–2229. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Jain, N.P.; Panchal, K.A. Adductor Canal Blockade Following Total Knee Arthroplasty-Continuous or Single Shot Technique? Role in Postoperative Analgesia, Ambulation Ability and Early Functional Recovery: A Randomized Controlled Trial. J. Arthroplast. 2015, 30, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Sites, B.D.; Beach, M.; Gallagher, J.D.; Jarrett, R.A.; Sparks, M.B.; Lundberg, C.J.F. A single injection ultrasound-assisted femoral nerve block provides side effect-sparing analgesia when compared with intrathecal morphine in patients undergoing total knee arthroplasty. Anesth. Analg. 2004, 99, 1539–1543. [Google Scholar] [CrossRef] [PubMed]
- Stathellis, A.; Fitz, W.; Schnurr, C.; Koeck, F.X.; Gebauer, M.; Huth, J.; Bauer, G.; Beckmann, J. Periarticular injections with continuous perfusion of local anaesthetics provide better pain relief and better function compared to femoral and sciatic blocks after TKA: A randomized clinical trial. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 2702–2707. [Google Scholar] [CrossRef]
- Tak, R.; Gurava Reddy, A.V.; Jhakotia, K.; Karumuri, K.; Sankineani, S.R. Continuous adductor canal block is superior to adductor canal block alone or adductor canal block combined with IPACK block (interspace between the popliteal artery and the posterior capsule of knee) in postoperative analgesia and ambulation following total knee arthroplasty: Randomized control trial. Musculoskelet. Surg. 2022, 106, 155–162. [Google Scholar] [CrossRef]
- Talmo, C.T.; Kent, S.E.; Fredette, A.N.; Anderson, M.C.; Hassan, M.K.; Mattingly, D.A. Prospective Randomized Trial Comparing Femoral Nerve Block With Intraoperative Local Anesthetic Injection of Liposomal Bupivacaine in Total Knee Arthroplasty. J. Arthroplast. 2018, 33, 3474–3478. [Google Scholar] [CrossRef]
- Tan, Z.; Kang, P.; Pei, F.; Shen, B.; Zhou, Z.; Yang, J. A comparison of adductor canal block and femoral nerve block after total-knee arthroplasty regarding analgesic effect, effectiveness of early rehabilitation, and lateral knee pain relief in the early stage. Medicine 2018, 97, e13391. [Google Scholar] [CrossRef]
- Tang, Q.; Li, X.; Yu, L.; Hao, Y.; Lu, G. Preoperative ropivacaine with or without tramadol for femoral nerve block in total knee arthroplasty. J. Orthop. Surg. 2016, 24, 183–187. [Google Scholar] [CrossRef]
- Tanikawa, H.; Sato, T.; Nagafuchi, M.; Takeda, K.; Oshida, J.; Okuma, K. Comparison of local infiltration of analgesia and sciatic nerve block in addition to femoral nerve block for total knee arthroplasty. J. Arthroplast. 2014, 29, 2462–2467. [Google Scholar] [CrossRef]
- Tanikawa, H.; Harato, K.; Ogawa, R.; Sato, T.; Kobayashi, S.; Nomoto, S.; Niki, Y.; Okuma, K. Local infiltration of analgesia and sciatic nerve block provide similar pain relief after total knee arthroplasty. J. Orthop. Surg. Res. 2017, 12, 109. [Google Scholar] [CrossRef][Green Version]
- Theodosiadis, P.; Sachinis, N.; Goroszeniuk, T.; Grosomanidis, V.; Chalidis, B. Ropivacaine versus bupivacaine for 3-in-1 block during total knee arthroplasty. J. Orthop. Surg. 2013, 21, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Thobhani, S.; Scalercio, L.; Elliott, C.E.; Nossaman, B.D.; Thomas, L.C.; Yuratich, D.; Bland, K.; Osteen, K.; Patterson, M.E. Novel Regional Techniques for Total Knee Arthroplasty Promote Reduced Hospital Length of Stay: An Analysis of 106 Patients. Ochsner J. 2017, 17, 233–238. [Google Scholar] [PubMed]
- Tsukada, S.; Wakui, M.; Hoshino, A. Postoperative epidural analgesia compared with intraoperative periarticular injection for pain control following total knee arthroplasty under spinal anesthesia: A randomized controlled trial. J. Bone Jt. Surg. Am. 2014, 96, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Wall, P.D.H.; Parsons, N.R.; Parsons, H.; Achten, J.; Balasubramanian, S.; Thompson, P.; Costa, M.L. A pragmatic randomised controlled trial comparing the efficacy of a femoral nerve block and periarticular infiltration for early pain relief following total knee arthroplasty. Bone Jt. J. 2017, 99, 904–911. [Google Scholar] [CrossRef]
- Wang, H.; Boctor, B.; Verner, J. The effect of single-injection femoral nerve block on rehabilitation and length of hospital stay after total knee replacement. Reg. Anesth. Pain. Med. 2002, 27, 139–144. [Google Scholar] [CrossRef]
- Wang, Q.; Yue, Y.; Li, D.; Yang, Z.; Yeersheng, R.; Kang, P. Efficacy of Single-Shot Adductor Canal Block Combined With Posterior Capsular Infiltration on Postoperative Pain and Functional Outcome After Total Knee Arthroplasty: A Prospective, Double-Blind, Randomized Controlled Study. J. Arthroplast. 2019, 34, 1650–1655. [Google Scholar] [CrossRef]
- Wang, C.G.; Ding, Y.L.; Wang, Y.Y.; Liu, J.Y.; Zhang, Q. Comparison of Adductor Canal Block and Femoral Triangle Block for Total Knee Arthroplasty. Clin. J. Pain 2020, 36, 558–561. [Google Scholar] [CrossRef]
- Wang, C.G.; Ma, W.H.; Liu, R.; Yang, M.Y.; Yang, Y.; Ding, Y.L. The effect of continuous adductor canal block combined with distal interspace between the popliteal artery and capsule of the posterior knee block for total knee arthroplasty: A randomized, double-blind, controlled trial. BMC Anesthesiol. 2022, 22, 175. [Google Scholar] [CrossRef]
- Wu, J.W.; Wong, Y.C. Elective unilateral total knee replacement using continuous femoral nerve blockade versus conventional patient-controlled analgesia: Perioperative patient management based on a multidisciplinary pathway. Hong Kong Med. J. 2014, 20, 45–51. [Google Scholar] [CrossRef]
- Yao, Y.Y.; Zhou, Q.H.; Yu, L.N.; Yan, M. Additional femoral nerve block analgesia does not reduce the chronic pain after total knee arthroplasty: A retrospective study in patients with knee osteoarthritis. Medicine 2019, 98, e14991. [Google Scholar] [CrossRef]
- Zaric, D.; Boysen, K.; Christiansen, C.; Christiansen, J.; Stephensen, S.; Christensen, B. A comparison of epidural analgesia with combined continuous femoral-sciatic nerve blocks after total knee replacement. Anesth. Analg. 2006, 102, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Y.; Zhang, E.F.; Bai, X.L.; Cheng, Z.J.; Jia, P.Y.; Li, Y.N.; Guo, Z.; Yang, J.X. Ultrasound-Guided Continuous Femoral Nerve Block with Dexmedetomidine Combined with Low Concentrations of Ropivacaine for Postoperative Analgesia in Elderly Knee Arthroplasty. Med. Princ. Pract. 2019, 28, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Zinkus, J.; Mockutė, L.; Gelmanas, A.; Tamošiūnas, R.; Vertelis, A.; Macas, A. Comparison of 2 Analgesia Modalities in Total Knee Replacement Surgery: Is There an Effect on Knee Function Rehabilitation? Med. Sci. Monit. 2017, 23, 3019–3025. [Google Scholar] [CrossRef]
- Zoratto, D.; Phelan, R.; Hopman, W.M.; Wood, G.C.A.; Shyam, V.; DuMerton, D.; Shelley, J.; McQuaide, S.; Kanee, L.; Ho, A.M.; et al. Adductor canal block with or without added magnesium sulfate following total knee arthroplasty: A multi-arm randomized controlled trial. Can. J. Anaesth. 2021, 68, 1028–1037. [Google Scholar] [CrossRef]
- Moucha, C.S.; Weiser, M.C.; Levin, E.J. Current Strategies in Anesthesia and Analgesia for Total Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2016, 24, 60–73. [Google Scholar] [CrossRef]
- Song, M.H.; Kim, B.H.; Ahn, S.J.; Yoo, S.H.; Kang, S.W.; Kim, Y.J.; Kim, D.H. Peri-articular injections of local anaesthesia can replace patient-controlled analgesia after total knee arthroplasty: A randomised controlled study. Int. Orthop. 2016, 40, 295–299. [Google Scholar] [CrossRef]
- Walder, B.; Schafer, M.; Henzi, I.; Tramèr, M.R. Efficacy and safety of patient-controlled opioid analgesia for acute postoperative pain. A quantitative systematic review. Acta Anaesthesiol. Scand. 2001, 45, 795–804. [Google Scholar] [CrossRef]
- Memtsoudis, S.G.; Cozowicz, C.; Bekeris, J.; Bekere, D.; Liu, J.; Soffin, E.M.; Mariano, E.R.; Johnson, R.L.; Go, G.; Hargett, M.J.; et al. Peripheral nerve block anesthesia/analgesia for patients undergoing primary hip and knee arthroplasty: Recommendations from the International Consensus on Anesthesia-Related Outcomes after Surgery (ICAROS) group based on a systematic review and meta-analysis of current literature. Reg. Anesth. Pain Med. 2021, 46, 971–985. [Google Scholar] [CrossRef]
- Dixit, V.; Fathima, S.; Walsh, S.M.; Seviciu, A.; Schwendt, I.; Spittler, K.H.; Briggs, D. Effectiveness of continuous versus single injection femoral nerve block for total knee arthroplasty: A double blinded, randomized trial. Knee 2018, 25, 623–630. [Google Scholar] [CrossRef]
- Charous, M.T.; Madison, S.J.; Suresh, P.J.; Sandhu, N.S.; Loland, V.J.; Mariano, E.R.; Donohue, M.C.; Dutton, P.H.; Ferguson, E.J.; Ilfeld, B.M. Continuous femoral nerve blocks: Varying local anesthetic delivery method (bolus versus basal) to minimize quadriceps motor block while maintaining sensory block. Anesthesiology 2011, 115, 774–781. [Google Scholar] [CrossRef]
- Li, D.; Ma, G.G. Analgesic efficacy and quadriceps strength of adductor canal block versus femoral nerve block following total knee arthroplasty. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.Y.; Fransen, M.; Parker, D.A.; Assam, P.N.; Chua, N. Femoral nerve blocks for acute postoperative pain after knee replacement surgery. Cochrane Database Syst. Rev. 2014, 2014, CD009941. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.S.; Kim, O.G.; Seo, J.H.; Kim, D.H.; Kim, Y.G.; Park, B.Y. Comparison of the Effect of Continuous Femoral Nerve Block and Adductor Canal Block after Primary Total Knee Arthroplasty. Clin. Orthop. Surg. 2017, 9, 303–309. [Google Scholar] [CrossRef]
- Karkhur, Y.; Mahajan, R.; Kakralia, A.; Pandey, A.P.; Kapoor, M.C. A comparative analysis of femoral nerve block with adductor canal block following total knee arthroplasty: A systematic literature review. J. Anaesthesiol. Clin. Pharmacol. 2018, 34, 433–438. [Google Scholar] [CrossRef]
- Sercia, Q.P.; Bergeron, J.J.; Pelet, S.; Belzile, É.L. Continuous vs. single-shot adductor canal block for pain management following primary total knee arthroplasty: A systematic review and meta-analysis of randomized controlled trials. Orthop. Traumatol. Surg. Res. 2022, 108, 103290. [Google Scholar] [CrossRef]
- D’Souza, R.S.; Langford, B.J.; Olsen, D.A.; Johnson, R.L. Ultrasound-Guided Local Anesthetic Infiltration Between the Popliteal Artery and the Capsule of the Posterior Knee (IPACK) Block for Primary Total Knee Arthroplasty: A Systematic Review of Randomized Controlled Trials. Local Reg. Anesth. 2021, 14, 85–98. [Google Scholar] [CrossRef]
- Thorsell, M.; Holst, P.; Hyldahl, H.C.; Weidenhielm, L. Pain control after total knee arthroplasty: A prospective study comparing local infiltration anesthesia and epidural anesthesia. Orthopedics 2010, 33, 75–80. [Google Scholar] [CrossRef]
- Zhang, L.K.; Ma, J.X.; Kuang, M.J.; Ma, X.L. Comparison of Periarticular Local Infiltration Analgesia With Femoral Nerve Block for Total Knee Arthroplasty: A Meta-Analysis of Randomized Controlled Trials. J. Arthroplast. 2018, 33, 1972–1978.e4. [Google Scholar] [CrossRef]
- Sardana, V.; Burzynski, J.M.; Scuderi, G.R. Adductor Canal Block or Local Infiltrate Analgesia for Pain Control After Total Knee Arthroplasty? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Arthroplast. 2019, 34, 183–189. [Google Scholar] [CrossRef]
- Wang, C.; Cai, X.Z.; Yan, S.G. Comparison of Periarticular Multimodal Drug Injection and Femoral Nerve Block for Postoperative Pain Management in Total Knee Arthroplasty: A Systematic Review and Meta-Analysis. J. Arthroplast. 2015, 30, 1281–1286. [Google Scholar] [CrossRef]
- Xue, X.; Lv, X.; Ma, X.; Zhou, Y.; Yu, N. Postoperative pain relief after total knee arthroplasty: A Bayesian network meta-analysis and systematic review of analgesic strategies based on nerve blocks. J. Clin. Anesth. 2024, 96, 111490. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Li, Y.; Xu, Z.; Geng, Z.-Y.; Wang, D.-X. Comparison of the ultrasound-guided single-injection femoral triangle block versus adductor canal block for analgesia following total knee arthroplasty: A randomized, double-blind trial. J. Anesth. 2020, 34, 702–711. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).