Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods
Abstract
1. Introduction
2. Results and Discussions
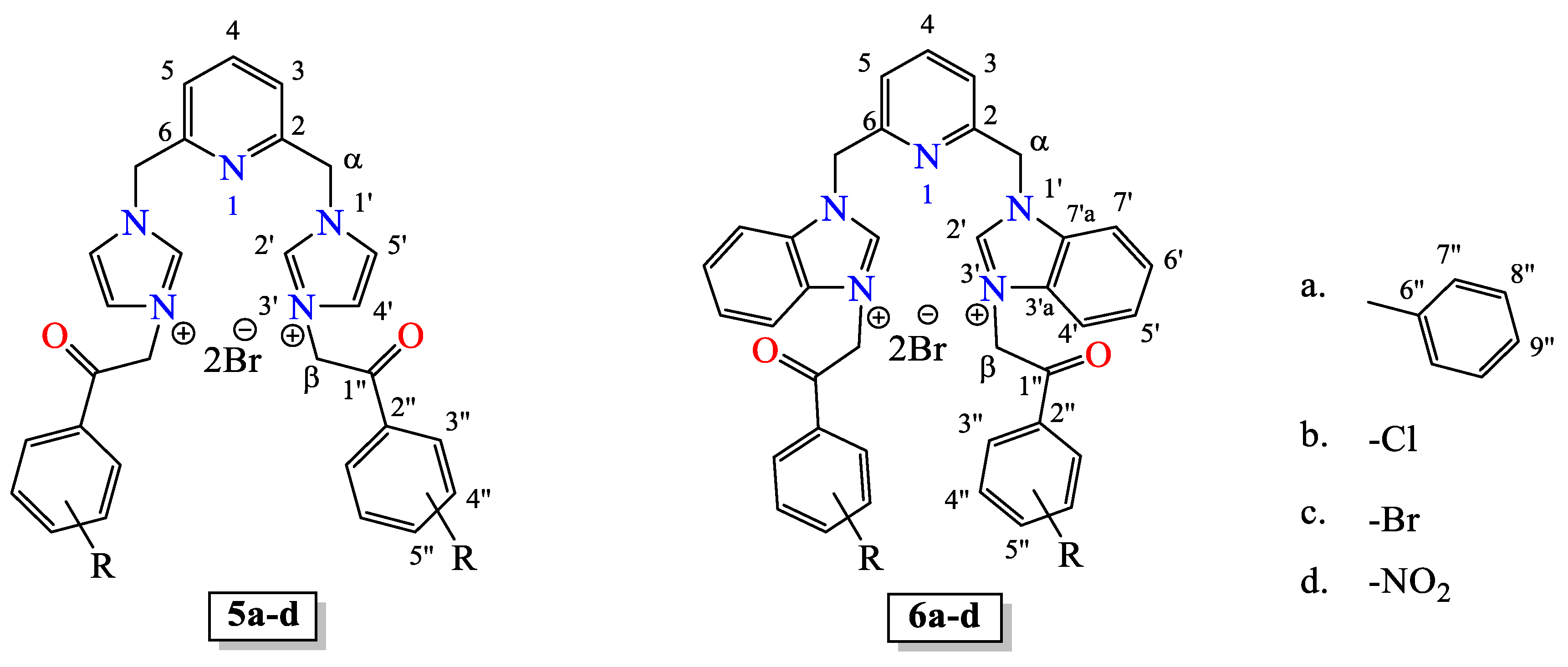
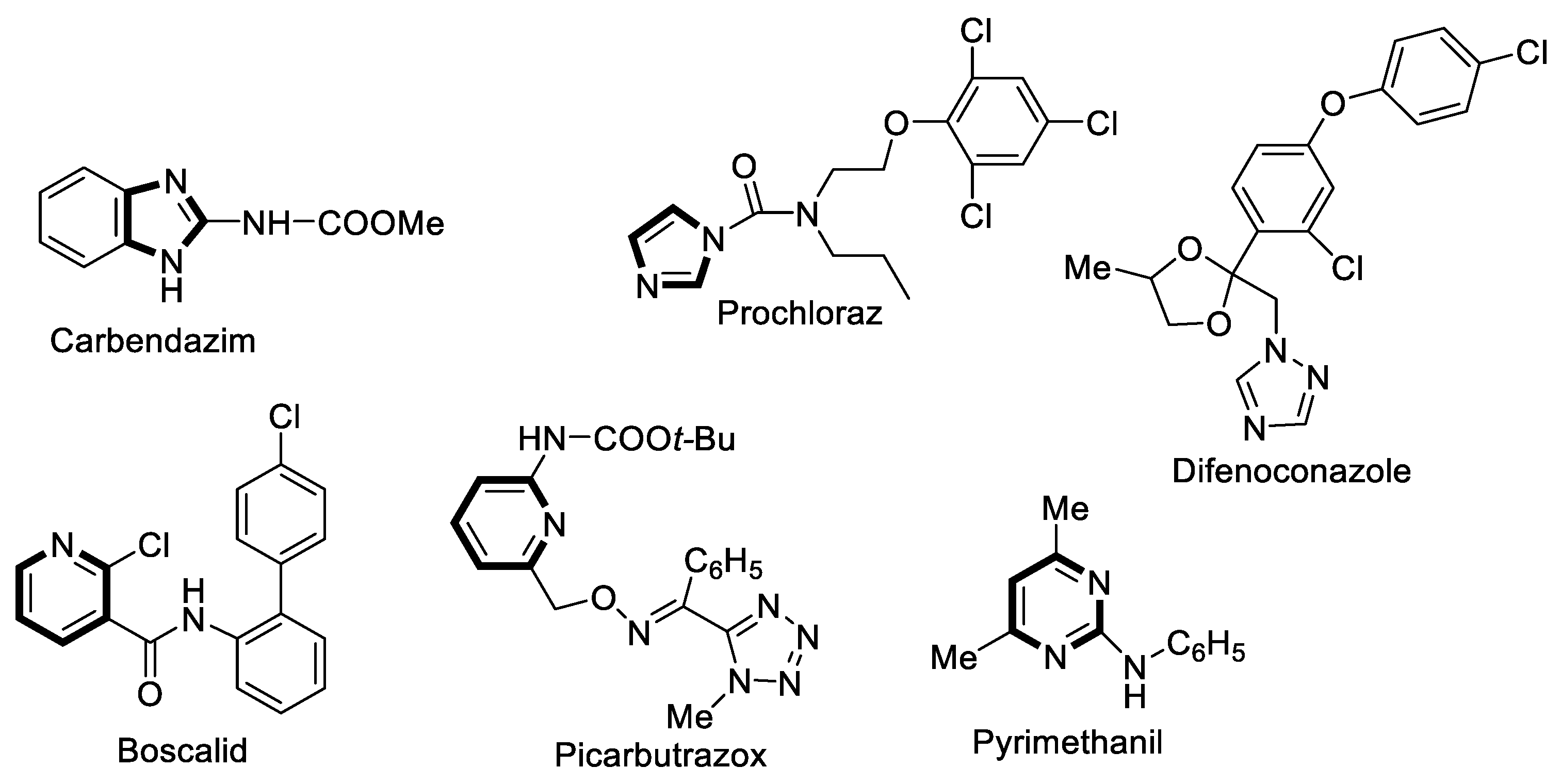
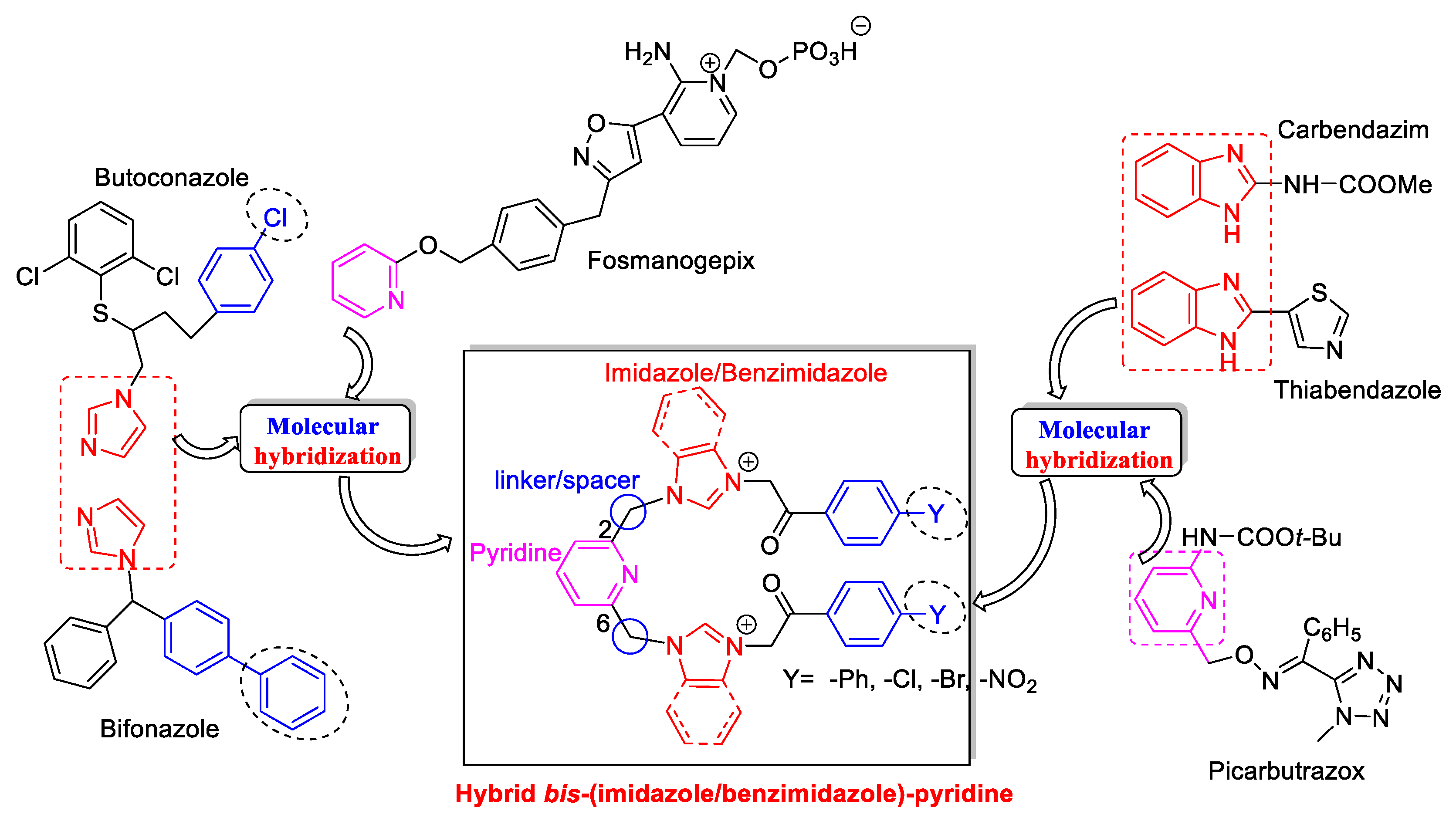
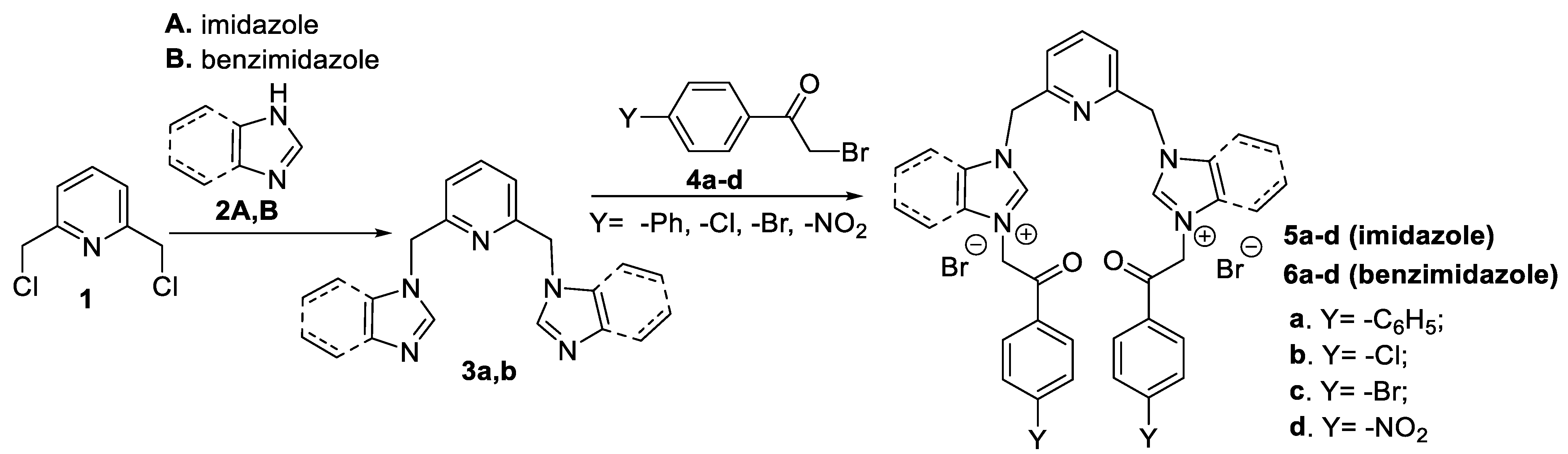
2.1. Design, Mechanism of Action and Synthesis
2.2. Antifungal Results
3. Materials and Methods
3.1. Materials and Measurements
3.2. Antifungal Assay
3.2.1. Antifungal Effect Assessment
3.2.2. Determination of Minimum Inhibitory Concentration
3.3. General Procedure for the Synthesis of Quaternary Salts 5a–d and 6a–d Under US Irradiation
3.4. Spectral Data of Quaternary Salts 5a–d and 6a–d (Figure 3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arastehfar, A.; Lass-Florl, C.; Garcia-Rubio, R.; Daneshnia, F.; Ilkit, M.; Boekhout, T.; Gabaldon, T.; Perlin, D.S. The Quiet and Underappreciated Rise of Drug-Resistant Invasive Fungal Pathogens. J. Fungi 2020, 6, 138. [Google Scholar] [CrossRef]
- Arastehfar, A.; Gabaldón, T.; Garcia-Rubio, R.; Jenks, J.D.; Hoenigl, M.; Salzer, H.J.F.; Ilkit, M.; Lass-Flörl, C.; Perlin, D.S. Drug-Resistant Fungi: An Emerging Challenge Threatening Our Limited Antifungal Armamentarium. Antibiotics 2020, 9, 877. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.; Rodrigues, M.L.; Coelho, C. The Still Underestimated Problem of Fungal Diseases Worldwide. Front. Microbiol. 2019, 10, 214. [Google Scholar] [CrossRef]
- Bongomin, F.; Gago, S.; Oladele, R.O.; Denning, D.W. Global and Multi-National Prevalence of Fungal Diseases—Estimate Precision. J. Fungi 2017, 3, 57. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Antifungal drug resistance: An update. Eur. J. Hosp. Pharm. 2022, 29, 10. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef]
- Lamoth, F.; Lockhart, S.R.; Berkow, E.L.; Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 2018, 73 (Suppl. S1), i4–i13. [Google Scholar] [CrossRef]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://reliefweb.int/report/world/who-releases-first-ever-list-health-threatening-fungi?gad_source=1&gclid=Cj0KCQjwir2xBhC_ARIsAMTXk84wiN-zL56PP58S5yov2g1D-WVbmetUyy6Ara8n1tZEa60QcS3NM6waAkMgEALw_wcB (accessed on 15 November 2024).
- Vanzolini, T.; Magnani, M. Old and new strategies in therapy and diagnosis against fungal infections. Appl. Microbiol. Biotechnol. 2024, 108, 147. [Google Scholar] [CrossRef]
- Donlin, M.J.; Meyers, M.J. Repurposing and optimization of drugs for discovery of novel antifungals. Drug Discov. Today 2022, 27, 2008–2014. [Google Scholar] [CrossRef]
- Mota Fernandes, C.; Dasilva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Poeta, M.D. The future of antifungal drug therapy: Novel compounds and targets. Antimicrob. Agents Chemother. 2021, 65, e01719–e01720. [Google Scholar] [CrossRef]
- Vincent, T.A. Current and future antifungal therapy: New targets for antifungal therapy. Int. J. Antimicrob. Agents 2020, 16, 317–321. [Google Scholar] [CrossRef]
- Sadeghian, S.; Bekhradi, F.; Mansouri, F.; Razmi, R.; Mansouri, S.G.; Poustforoosh, A.; Khabnadideh, S.; Zomorodian, K.; Zareshahrabadi, Z.; Rezai, Z. Imidazole derivatives as novel and potent antifungal agents: Synthesis, biological evaluation, molecular docking study, molecular dynamic simulation and ADME prediction. J. Mol. Struct. 2024, 1302, 137447. [Google Scholar] [CrossRef]
- Peyton, L.R.; Gallagher, S.; Hashemzadeh, M. Triazole antifungals: A review. Drugs Today 2015, 51, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Houst, J.; Spizek, J.; Havlicek, V. Antifungal drugs. Metabolites 2020, 10, 106. [Google Scholar] [CrossRef]
- Campoy, S.; Adrio, J.L. Antifungals. Biochem. Pharmacol. 2017, 133, 86–96. [Google Scholar] [CrossRef]
- Grant, L.M.; Orenstein, R. Treatment of Recurrent Vulvovaginal Candidiasis With Ibrexafungerp. J. Investig. Med. High Impact Case Rep. 2024, 10, 23247096221123144. [Google Scholar] [CrossRef]
- Pan, N.; Wang, N.; An, J.; Liu, C.; Chen, H.; Fei, Q.; Li, P.; Wu, W. Discovery of Novel Compounds for Combating Rising Severity of Plant Diseases Caused by Fungi and Viruses. ACS Omega 2024, 9, 1424–1435. [Google Scholar] [CrossRef]
- Vanreppelen, G.; Wuyts, J.; Van Dijck, P.; Vandecruys, P. Sources of Antifungal Drugs. J. Fungi 2023, 9, 171. [Google Scholar] [CrossRef]
- Huang, Z.X.; Chen, H.J.; Zhang, X.; Wang, R.; Hu, C.; Mao, Z.W. Synthesis and antifungal evaluation of new azole derivatives containing 1,2,3-triazole. RSC Med. Chem. 2025, 16, 791–800. [Google Scholar] [CrossRef]
- Wu, L.L.; Fan, L.X.; Shi, L.J.; Wang, C.X.; Pan, Z.; Xu, C.L.; Yang, G.Y. Synthesis, characterization and antifungal activity of imidazole chitosan derivatives. Carbohydr. Res. 2024, 544, 109238. [Google Scholar] [CrossRef]
- Guin, S.; Alden, K.M.; Krysan, D.J.; Meyers, M.J. Synthesis and Antifungal Activity of Stereoisomers of Mefloquine Analogs. ACS Med. Chem. Lett. 2024, 15, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, N.; Subedi, Y.P.; Shakespear, M.; Grilley, M.; Takemoto, J.Y.; Chang, C.W.T. Synthesis of kanamycin-azole hybrids and investigation of their antifungal activities. Bioorg. Med. Chem. 2024, 114, 117947. [Google Scholar] [CrossRef]
- Litim, B.; Djahoudi, A.; Meliani, S.; Boukhari, A. Synthesis and potential antimicrobial activity of novel α-aminophosphonates derivatives bearing substituted quinoline or quinolone and thiazole moieties. Med. Chem. Res. 2022, 31, 60–74. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, B.; Zhang, B.; Xu, M.; Geng, H.; Zhou, L. New 2-aryl-7,8-dimethoxy-3,4-dihydroisoquinolin-2-ium salts as potential antifungal agents: Synthesis, bioactivity and structure-activity relationships. Sci. Rep. 2017, 7, 7537. [Google Scholar] [CrossRef]
- Léveque, J.M.; Cravotto, G.; Delattre, F.; Cintas, P. Organic Sonochemistry, Challenges and Perspectives for the 21st Century; Springer Nature: Cham, Switzerland, 2018. [Google Scholar]
- Mason, T.J.; Peters, D. Practical Sonochemistry: Power Ultrasound Uses and Applications, 2nd ed.; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Luche, J.L. Synthetic Organic Sonochemistry; Plenum Press: New York, NY, USA, 1998. [Google Scholar]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Savun-Hekimoglu, B. A Review on Sonochemistry and Its Environmental Applications. Acoustics 2020, 2, 766–775. [Google Scholar] [CrossRef]
- Chatel, G. How sonochemistry contributes to green chemistry? Ultrason. Sonochem. 2018, 40, 117–122. [Google Scholar] [CrossRef]
- Lupacchini, M.; Mascitti, A.; Giachi, G.; Tonucci, L.; d’Alessandro, N.; Martinez, J.; Colacino, E. Sonochemistry in non-conventional, green solvents or solvent-free reactions. Tetrahedron 2017, 73, 609–653. [Google Scholar] [CrossRef]
- Cintas, P. Ultrasound and green chemistry—Further comments. Ultrason. Sonochem. 2016, 28, 257–258. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and the environment—Providing a “green” link between chemistry, physics and engineering. Ultrason. Sonochem. 2007, 14, 476–483. [Google Scholar] [CrossRef]
- Zbancioc, G.; Ciobanu, C.I.; Mangalagiu, I.I.; Moldoveanu, C. Ultrasound-Assisted Synthesis of Fluorescent AzatetracyclicDerivatives: An Energy-Efficient Approach. Molecules 2022, 27, 3180. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Mangalagiu, I.I.; Zbancioc, G. Fluorescent Azasteroids through Ultrasound Assisted Cycloaddition Reactions. Molecules 2021, 26, 5098. [Google Scholar] [CrossRef] [PubMed]
- Bejan, V.; Mantu, D.; Mangalagiu, I.I. Ultrasound and microwave assisted synthesis of isoindolo-1,2-diazine: A comparative study. Ultrason. Sonochem. 2012, 19, 999–1002. [Google Scholar] [CrossRef]
- Diaconu, D.; Antoci, V.; Mangalagiu, V.; Amariucai-Mantu, D.; Mangalagiu, I.I. Quinoline—Imidazole/benzimidazole derivatives as dual-/multi- targeting hybrids inhibitors with anticancer and antimicrobial activity. Sci. Rep. 2022, 12, 16988. [Google Scholar] [CrossRef]
- Lungu, C.N.; Bratanovici, B.I.; Grigore, M.M.; Antoci, V.; Mangalagiu, I.I. Hybrid imidazole—Pyridine derivatives: Computational approach to novel anticancer DNA intercalators. Curr. Med. Chem. 2020, 27, 154–169. [Google Scholar] [CrossRef]
- Antoci, V.; Cucu, D.; Zbancioc, G.; Modoveanu, C.; Mangalagiu, V. Bis-(imidazole/benzimidazole)-pyridine derivatives: Synthesis, structure and antimycobacterial activity. Part XII. Future Med. Chem. 2020, 12, 207–222. [Google Scholar] [CrossRef]
- Olaru, A.; Vasilache, V.; Danac, R.; Mangalagiu, I.I. Antimycobacterial activity of nitrogen heterocycles derivatives: 7-(pyridine-4-yl)-indolizine derivatives. Part VII. J. Enzym. Inh. Med. Ch. 2017, 32, 1291–1298. [Google Scholar] [CrossRef] [PubMed]
- Mantu, D.; Antoci, V.; Moldoveanu, C.; Zbancioc, G.; Mangalagiu, I.I. Hybrid imidazole (benzimidazole)/pyridine (quinoline) derivatives and evaluation of their anticancer and antimycobacterial activity. J. Enzyme Inhib. Med. Chem. 2016, 31, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Clinical Standard Laboratory Institute. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts, Approved Guideline; M44; Clinical Standard Laboratory Institute: Wayne, PA, USA, 2023. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, K.S. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Hossain, T.J. Methods for screening and evaluation of antimicrobial activity: A review of protocols, advantages, and limitations. Eur. J. Microbiol. Immunol. 2024, 14, 97–115. [Google Scholar] [CrossRef]
- Kavanagh, A.; Ramu, S.; Gong, Y.; Cooper, M.A.; Blaskovich, M.A.T. Effects of microplate type and broth additives on microdilution MIC susceptibility assays. Antimicrob. Agents Chemother. 2018, 63, e01760-18. [Google Scholar] [CrossRef]
- Osaka, I.; Hefty, P.S. Simple resazurin-based microplate assay for measuring Chlamydia infections. Antimicrob. Agents Chemother. 2013, 57, 2838–2840. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, M. Antifungal agents in wood protection—A review. Molecules 2022, 27, 6392. [Google Scholar] [CrossRef] [PubMed]
- Kathiravan, M.K.; Salake, A.M. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar] [CrossRef] [PubMed]

| Compound | 5a | 5b | 5c | 5d | 6a | 6b | 6c | 6d | |
|---|---|---|---|---|---|---|---|---|---|
| Yield (%) * | TH | 56 | 87 | 84 | 79 | 46 | 71 | 55 | 48 |
| US | 61 | 90 | 86 | 85 | 64 | 73 | 57 | 56 | |
| R.t. (min) ** | TH | 720 | 480 | 600 | 720 | 720 | 480 | 480 | 720 |
| US | 120 | 100 | 100 | 100 | 120 | 120 | 120 | 120 |
| Compound/Strain | Average Diameter of Inhibition Zone (mm) *,** | ||||
|---|---|---|---|---|---|
| CAW | CA10231 | CPW | CP22019 | RH | |
| 5a | 18 ± 1.4 | 23 ± 1.9 | 19 ± 1.82 | 20 ± 1.95 | 18.5 ± 1.8 |
| 5b | 0 | 0 | 0 | 0 | 0 |
| 5c | 7 ± 0.1 | 0 | 0 | 0 | 0 |
| 5d | 0 | 0 | 0 | 0 | 0 |
| 6a | 10 ± 0.8 | 7.5 ± 0.1 | 8.5 ± 0.8 | 10 ± 1.0 | 13 ± 1.1 |
| 6b | 0 | 0 | 0 | 0 | 0 |
| 6c | 7.5 ± 0.03 | 0 | 0 | 0 | 0 |
| 6d | 0 | 0 | 0 | 0 | 0 |
| Std-Flu | 26 ± 2.15 | 24.5 ± 2.1 | 19 ± 1.85 | 18 ± 1.82 | 19 ± 1.75 |
| Std-Nyst | 28 ± 2.18 | 26 ± 2.14 | 25.5 ± 2.05 | 26.5 ± 1.95 | 30 ± 2.6 |
| DMSO | 0 | 0 | 0 | 0 | 0 |
| Compound/Strain | MIC (µg/mL) | ||||
|---|---|---|---|---|---|
| CAW | CA10231 | CPW | CP2019 | RH | |
| 5a | 3.9 | 15.62 | 31.25 | 31.25 | 15.62 |
| 5c | 62.5 | N | N | N | N |
| 6a | 15.62 | 31.25 | 31.25 | 31.25 | 3.9 |
| 6c | 62.5 | N | N | N | N |
| Std-Flu | 0.12 | 0.12 | 0.97 | 7.81 | 0.48 |
| Std-Nyst | 0.24 | 0.24 | 0.97 | 0.24 | 0.12 |
| DMSO | >250 | >250 | 250 | >250 | 125 |
| Compound/Strain | Average Diameter of Inhibition Zone (mm) *,** | |||
|---|---|---|---|---|
| AN | AF | CC | RN | |
| 5a | 12 ± 1.0 | 9 ± 0.07 | 9 ± 0.22 | 8 ± 0.03 |
| 5b | 0 | 0 | 16 ± 1.55 | 0 |
| 5c | 0 | 0 | 8 ± 0.05 | 0 |
| 5d | 0 | 0 | 0 | 0 |
| 6a | 0 | 0 | 8 ± 0.05 | 7 ± 0.1 |
| 6b | 0 | 0 | 0 | 0 |
| 6c | 0 | 0 | 0 | 0 |
| 6d | 0 | 0 | 0 | 0 |
| Std-Flu | 9 ± 0.9 | 0 | 24 ± 2.1 | 0 |
| Std-Nyst | 20.5 ± 1.85 | 13.5 ± 1.24 | 11 ± 1.05 | 0 |
| DMSO | 0 | 0 | 0 | 0 |
| Compound/Strain | MIC (µg/mL) | |||
|---|---|---|---|---|
| AN | AF | CC | RN | |
| 5a | 62.5 | 31.25 | 250 | 250 |
| 6a | N | N | N | 250 |
| Std-Nyst | 0.48 | 0.48 | 125 | >250 |
| DMSO | 0 | 0 | 0 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balaes, T.; Mangalagiu, V.; Antoci, V.; Amariucai-Mantu, D.; Diaconu, D.; Mangalagiu, I.I. Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods. Pharmaceuticals 2025, 18, 495. https://doi.org/10.3390/ph18040495
Balaes T, Mangalagiu V, Antoci V, Amariucai-Mantu D, Diaconu D, Mangalagiu II. Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods. Pharmaceuticals. 2025; 18(4):495. https://doi.org/10.3390/ph18040495
Chicago/Turabian StyleBalaes, Tiberius, Violeta Mangalagiu, Vasilichia Antoci, Dorina Amariucai-Mantu, Dumitrela Diaconu, and Ionel I. Mangalagiu. 2025. "Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods" Pharmaceuticals 18, no. 4: 495. https://doi.org/10.3390/ph18040495
APA StyleBalaes, T., Mangalagiu, V., Antoci, V., Amariucai-Mantu, D., Diaconu, D., & Mangalagiu, I. I. (2025). Hybrid Bis-(Imidazole/Benzimidazole)-Pyridine Derivatives with Antifungal Activity of Potential Interest in Medicine and Agriculture via Improved Efficiency Methods. Pharmaceuticals, 18(4), 495. https://doi.org/10.3390/ph18040495









