Evaluation of the Anti-Aging Properties of Ethanolic Extracts from Selected Plant Species and Propolis by Enzyme Inhibition Assays and 2D/3D Cell Culture Methods
Abstract
1. Introduction
2. Results
2.1. Cell-Free Enzyme Assays
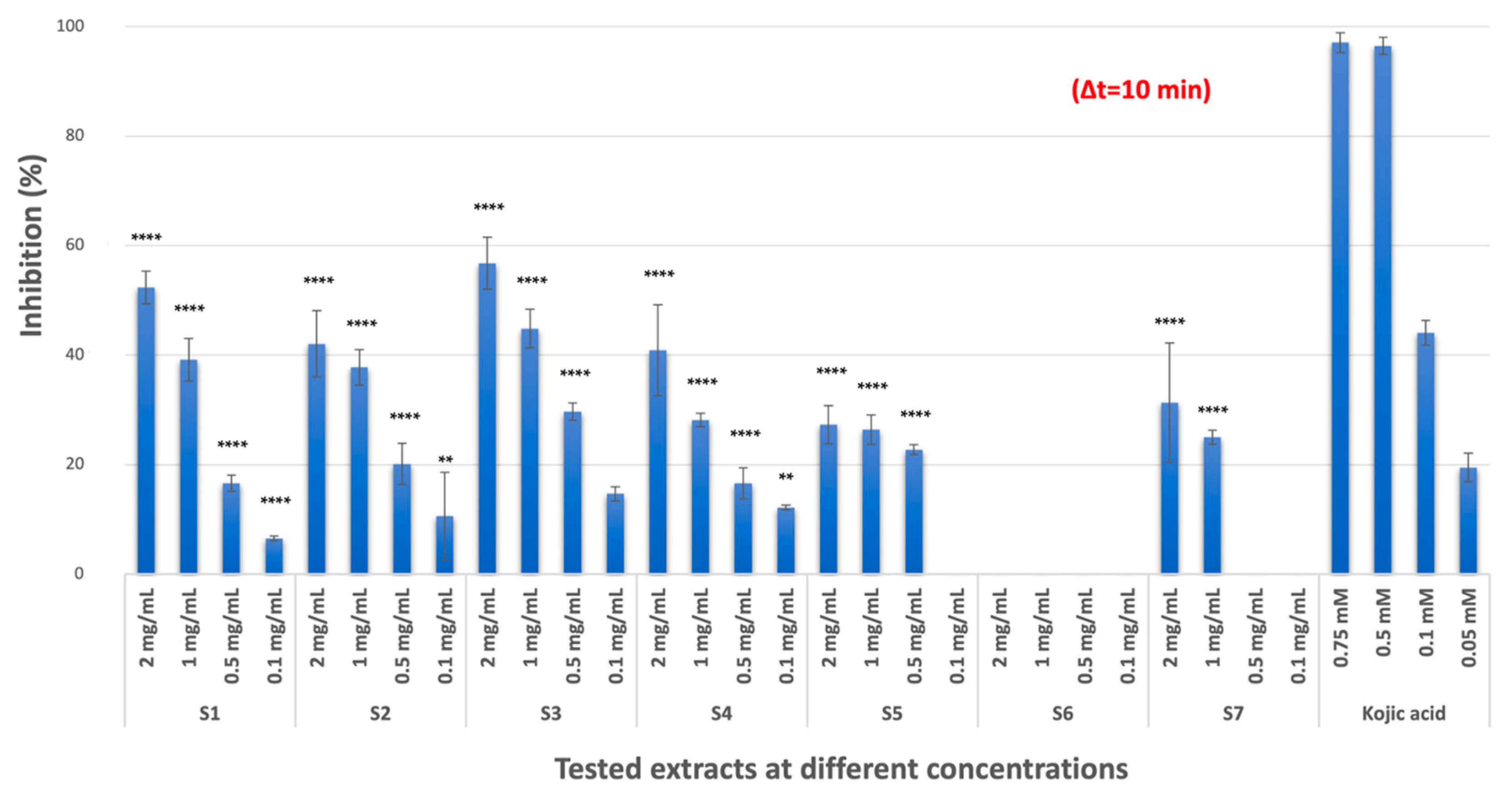
2.1.1. TYR Inhibitory Activity
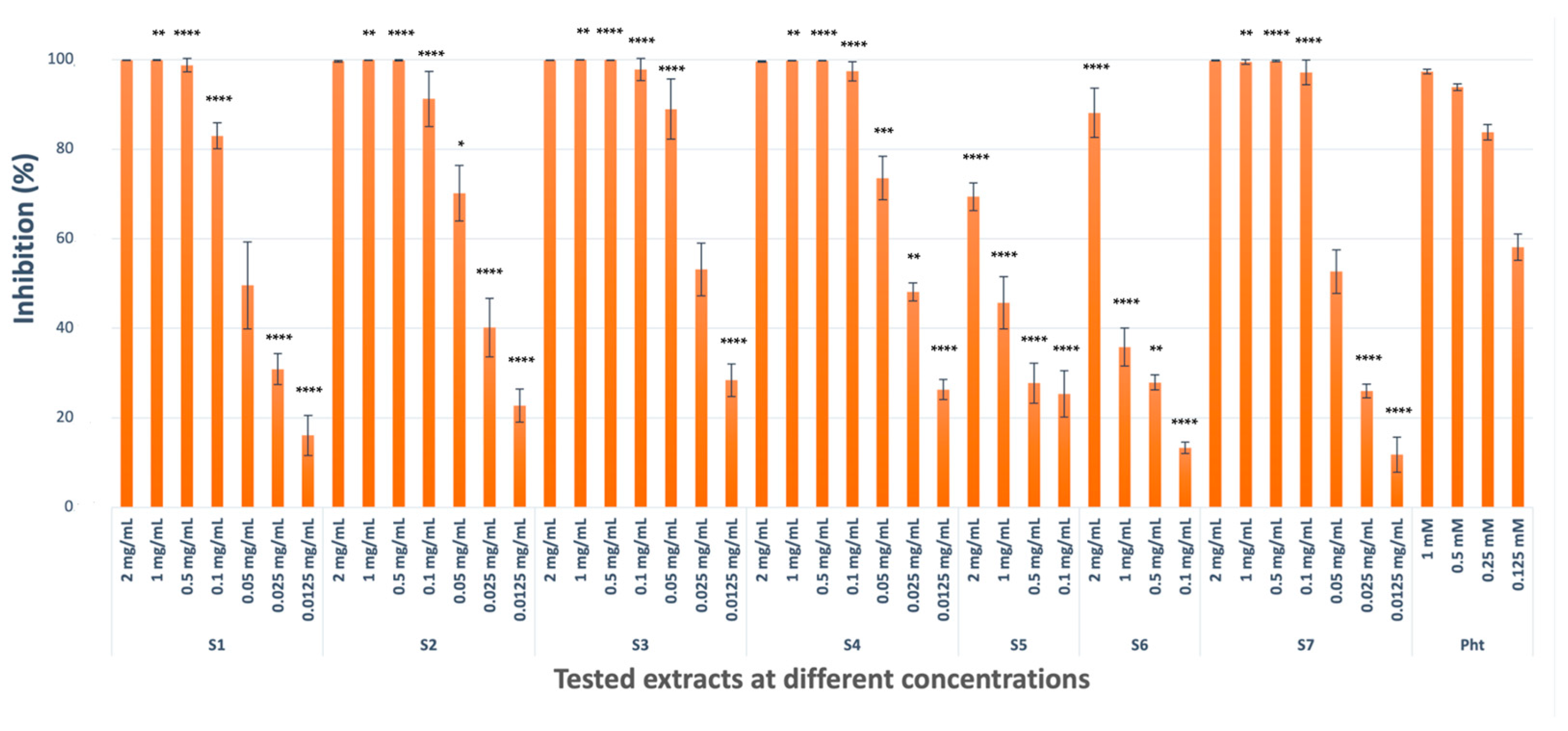
2.1.2. Collagenase Inhibitory Activity
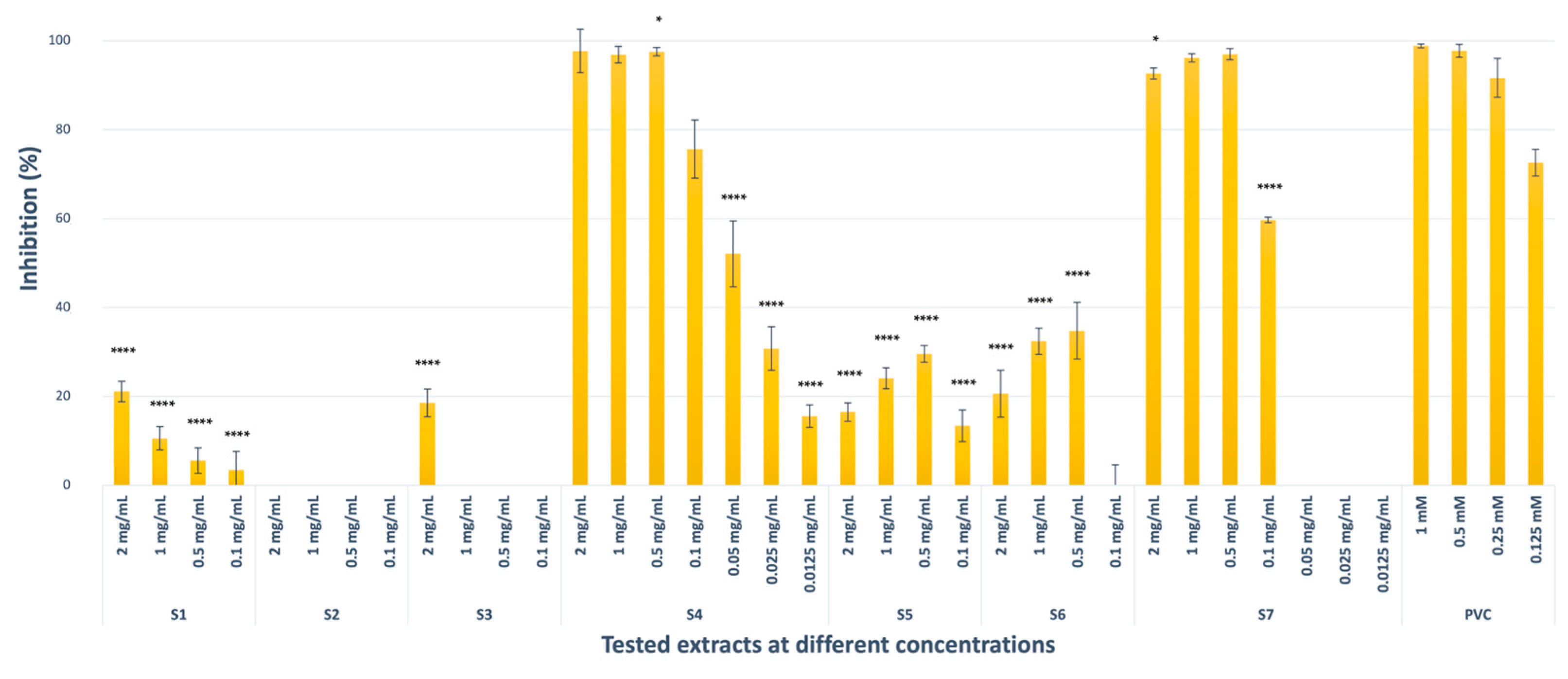
2.1.3. Elastase Inhibitory Activity
2.2. Cell-Based Assays
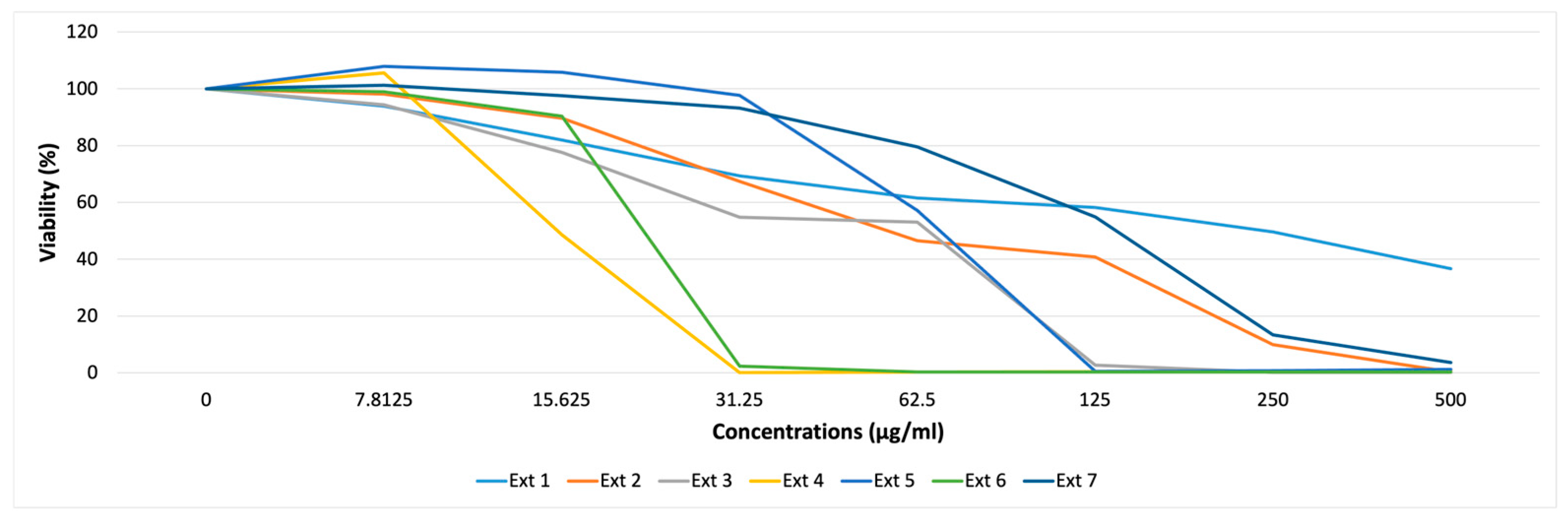
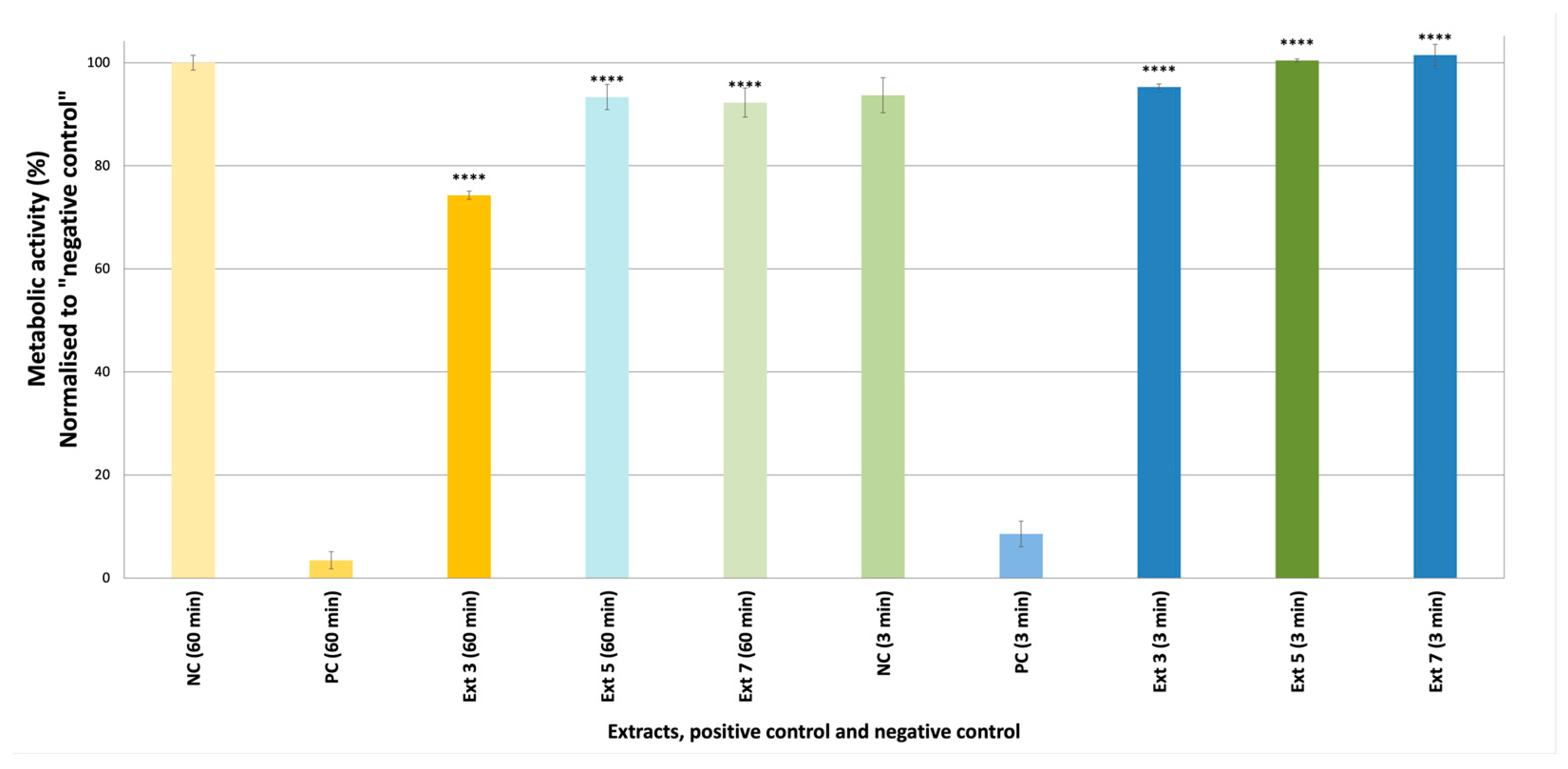
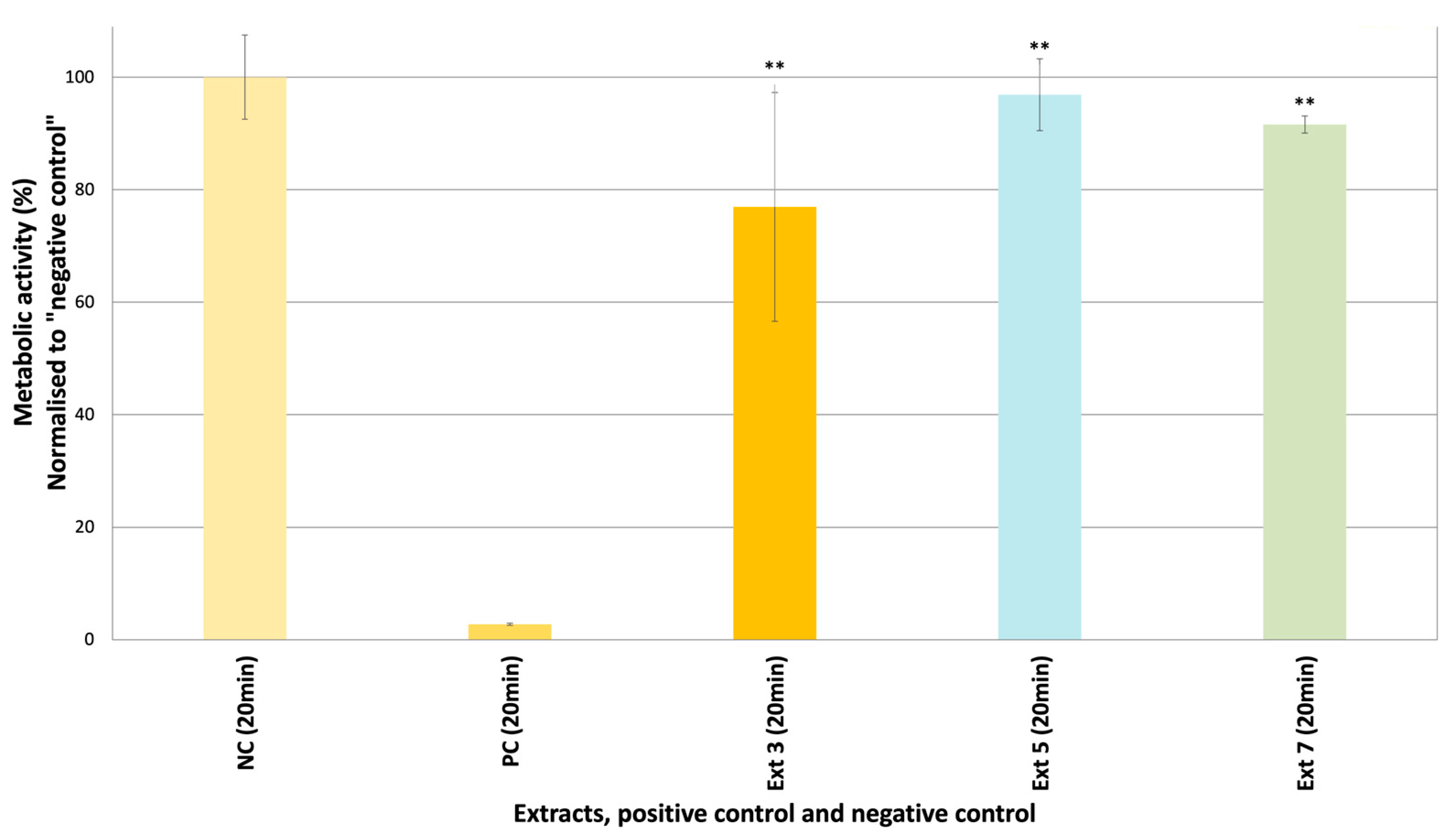
2.2.1. Cytotoxicity Assay
2.2.2. Anti-Inflammatory and Antioxidant Activity
2.2.3. Anti-Inflammatory Activity on Reconstructed Human Epidermis (RHE)
2.2.4. Skin Tanning/Whitening Assay
2.2.5. Skin Irritation/Corrosion Test
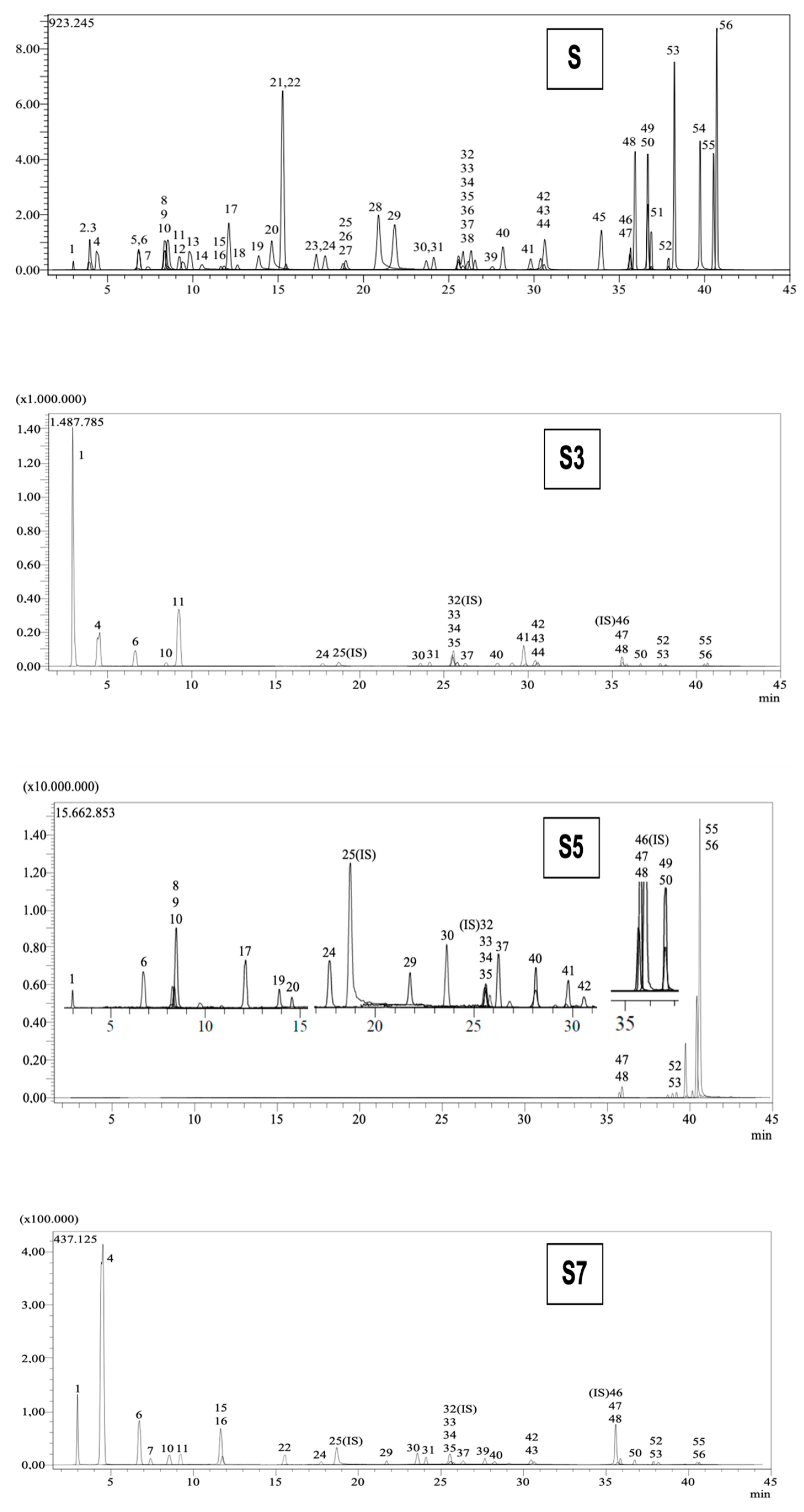
2.3. LC-MS/MS Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Extraction Procedure
4.2. Cell-Free Enzyme Assays
4.2.1. TYR Inhibitory Activity
4.2.2. Collagenase Inhibitory Activity
4.2.3. Elastase Inhibitory Activity
4.3. Cell-Based Assays
4.3.1. Cell Culture
4.3.2. Cytotoxicity Assay
4.3.3. Anti-Inflammatory and Antioxidant Activity
4.3.4. Anti-Inflammatory Activity on Reconstructed Human Epidermis (RHE)
4.3.5. Skin Tanning/Whitening Assay
4.3.6. Skin Irritation Test
4.3.7. Skin Corrosion Test
4.4. Statistical Analyses
4.5. LC-MS/MS Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COX-2 | Cyclooxygenase-2 |
| FBS | Fetal bovine serum |
| HaCaT | Human keratinocyte |
| IBMX | 3-Isobutyl-1-methylxanthine |
| IR | Infrared |
| iNOS | Nitric oxide synthase |
| LPS | Lipopolysaccharide |
| Luc | Luciferase |
| MMPs | Matrix metalloproteinases |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| NO | Nitric oxide |
| NF-κB | Nuclear factor kappa B |
| RHE | Reconstructed human epidermis |
| S.D. | Standard deviation |
| TNFα | Tumor necrosis factor-alpha |
| TYR | Tyrosinase |
| UN-GHS | United Nations Globally Harmonized System |
| WFA | Withaferin A |
References
- Roudsari, M.R.; Karimi, R.; Sohrabvandi, S.; Mortazavian, A.M. Health effects of probiotics on the skin. Crit. Rev. Food Sci. Nutr. 2015, 55, 1219–1240. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S. Tracing skin aging process: A mini-review of in vitro approaches. Biogerontology 2021, 22, 261–272. [Google Scholar] [CrossRef]
- Nema, N.K.; Maity, N.; Sarkar, B.; Mukherjee, P.K. Cucumis sativus fruit-potential antioxidant, anti-hyaluronidase, and anti-elastase agent. Arch. Dermatol. Res. 2011, 303, 247–252. [Google Scholar] [CrossRef]
- Sin, B.Y.; Kim, H.P. Inhibition of collagenase by naturally-occurring flavonoids. Arch. Pharm. Res. 2005, 28, 1152–1155. [Google Scholar] [CrossRef]
- Xu, G.H.; Ryoo, I.J.; Kim, Y.H.; Choo, S.J.; Yoo, I.D. Free radical scavenging and antielastase activities of flavonoids from the fruits of Thuja orientalis. Arch. Pharm. Res. 2009, 32, 275–282. [Google Scholar] [CrossRef]
- Ryu, T.K.; Roh, E.; Shin, H.S.; Kim, J.E. Inhibitory Effect of Lotusine on Solar UV-Induced Matrix Metalloproteinase-1 Expression. Plants 2022, 11, 773. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar] [CrossRef] [PubMed]
- Issa, R.A.; Afifi, F.U.; Amro, B.I. Studying the anti-tyrosinase effect of Arbutus andrachne L. extracts. Int. J. Cosmet. Sci. 2008, 30, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.H.; Liu, W.Y.; Zhu, D.; Cao, Y.L.; Tang, A.F.; Gong, G.M.; Su, H. Natural skin-whitening compounds for the treatment of melanogenesis (Review). Exp. Ther. Med. 2020, 20, 173–185. [Google Scholar] [CrossRef]
- Buche, G.; Laffon, M.; Fougère, L.; Destandau, E. Evaluation and Comparison of Dermo-Cosmetic Activities of Three Oak Species by Targeting Antioxidant Metabolites and Skin Enzyme Inhibitors. Metabolites 2023, 13, 804. [Google Scholar] [CrossRef]
- Global Natural and Organic Cosmetics Market—Analysis By Product Category, Distribution Channel, By Region, By Country (2022 Edition): Market Insights and Forecast with Impact of COVID-19 (2022–2027). Available online: https://www.researchandmarkets.com/reports/5574841/global-natural-and-organic-cosmetics-market#src-pos-2 (accessed on 25 July 2022).
- Cefali, L.C.; Ataide, J.A.; Moriel, P.; Foglio, M.A.; Mazzola, P.G. Plant-based active photoprotectants for sunscreens. Int. J. Cosmet. Sci. 2016, 38, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Chiocchio, I.; Mandrone, M.; Sanna, C.; Maxia, A.; Tacchini, M.; Poli, F. Screening of a hundred plant extracts as tyrosinase and elastase inhibitors, two enzymatic targets of cosmetic interest. Ind. Crop Prod. 2018, 122, 498–505. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Maity, N.; Nema, N.K.; Sarkar, B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine 2011, 19, 64–73. [Google Scholar] [CrossRef]
- Chaiyana, W.; Charoensup, W.; Sriyab, S.; Punyoyai, C.; Neimkhum, W. Herbal Extracts as Potential Antioxidant, Anti-Aging, Anti-Inflammatory, and Whitening Cosmeceutical Ingredients. Chem. Biodivers. 2021, 18, e2100245. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Barthe, M.; Bavoux, C.; Finot, F.; Mouche, I.; Cuceu-Petrenci, C.; Forreryd, A.; Hansson, A.C.; Johansson, H.; Lemkine, G.F.; Thenot, J.P.; et al. Safety Testing of Cosmetic Products: Overview of Established Methods and New Approach Methodologies (NAMs). Cosmetics 2021, 8, 50. [Google Scholar] [CrossRef]
- Sawicka, B.; Hameed, T.S.; Noaema, A.H.; Kiełtyka-Dadasiewicz, A. Safety of Plant Cosmetic Raw Materials. Int. J. Res. Methodol. Soc. Sci. 2016, 2, 6–22. [Google Scholar]
- da Rocha, V.P.M. Study of the Cytotoxicity of Raw Materials of Cosmetic and Topical Pharmaceutical Formulations. Master’s Thesis, Faculdade de Medicina Universidade do Porto, Porto, Portugal, 2015. [Google Scholar]
- Zerbinati, N.; Sommatis, S.; Maccario, C.; Di Francesco, S.; Capillo, M.C.; Grimaldi, G.; Rauso, R.; Herrera, M.; Bencini, P.L.; Mocchi, R. A Practical Approach for the In Vitro Safety and Efficacy Assessment of an Anti-Ageing Cosmetic Cream Enriched with Functional Compounds. Molecules 2021, 26, 7592. [Google Scholar] [CrossRef]
- Deniz, F.S.S.; Orhan, I.E.; Duman, H. Profiling cosmeceutical effects of various herbal extracts through elastase, collagenase, tyrosinase inhibitory and antioxidant assays. Phytochem. Lett. 2021, 45, 171–183. [Google Scholar] [CrossRef]
- Matic, S.; Stanic, S.; Mihailovic, M.; Bogojevic, D. Cotinus coggygria Scop.: An overview of its chemical constituents, pharmacological and toxicological potential. Saudi J. Biol. Sci. 2016, 23, 452–461. [Google Scholar] [CrossRef]
- Antal, D.S.; Ardelean, F.; Jijie, R.; Pinzaru, I.; Soica, C.; Dehelean, C. Integrating Ethnobotany, Phytochemistry, and Pharmacology of Cotinus coggygria and Toxicodendron vernicifluum: What Predictions can be Made for the European Smoketree? Front. Pharmacol. 2021, 12, 662852. [Google Scholar] [CrossRef] [PubMed]
- Akın, A.; Altındişli, A. Emir, Gök Üzüm ve Kara Dimrit Üzüm Çeşitlerinin Çekirdek Yağlarının Yağ Asidi Kompozisyonu ve Fenolik Madde İçeriklerinin Belirlenmesi. Akad. Gıda 2010, 8, 19–23. [Google Scholar]
- Barut, D.; Tekin, H.; Kılıç, İ.H.; Kurt, B.S. Antepfıstığı (Pistacia vera L.) Yumuşak Dış Kabuğunun Kimyasal Bileşimi ve Antioksidan Potansiyelinin Belirlenmesi. Zeugma Biol. Sci. 2021, 2, 20–26. [Google Scholar]
- Greenaway, W.; Scaysbrook, T.; Whatley, F.R. The Composition and Plant Origins of Propolis—A Report of Work at Oxford. Bee World 1990, 71, 107–118. [Google Scholar] [CrossRef]
- Keskin, Ş.; Yatanaslan, L.; Karlıdağ, S. Chemical Characterization of Propolis Samples Collected from Different Provinces of Anatolia. Uludag Bee J. 2020, 20, 81–88. [Google Scholar]
- Khalil, M.L. Biological activity of bee propolis in health and disease. Asian Pac. J. Cancer Prev. 2006, 7, 22–31. [Google Scholar]
- Deniz, F.S.S.; Salmas, R.E.; Emerce, E.; Cankaya, I.I.T.; Yusufoglu, H.S.; Orhan, I.E. Evaluation of collagenase, elastase and tyrosinase inhibitory activities of Cotinus coggygria Scop. through in vitro and in silico approaches. S. Afr. J. Bot. 2020, 132, 277–288. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological insights into the multifaceted biological properties of quinic acid. Biotechnol. Genet. Eng. 2024, 40, 3408–3437. [Google Scholar] [CrossRef]
- Park, Y.; Paing, Y.M.M.; Cho, N.; Kim, C.; Yoo, J.; Choi, J.W.; Lee, S.H. Quinic Acid Alleviates Behavior Impairment by Reducing Neuroinflammation and MAPK Activation in LPS-Treated Mice. Biomol. Ther. 2024, 32, 309–318. [Google Scholar] [CrossRef]
- Ghasemi-Dehnoo, M.; Lorigooini, Z.; Amini-Khoei, H.; Sabzevary-Ghahfarokhi, M.; Rafieian-Kopaei, M. Quinic acid ameliorates ulcerative colitis in rats, through the inhibition of two TLR4-NF-κB and NF-κB-INOS-NO signaling pathways. Immun. Inflamm. Dis. 2023, 11, e926. [Google Scholar] [CrossRef]
- Pero, R.; Lund, H. In vivo treatment of humans with quinic acid enhances DNA repair and reduces the influence of lifestyle factors on risk to disease. Int. J. Biotechnol. Biochem. 2009, 5, 293–305. [Google Scholar]
- Boke Sarikahya, N.; Varol, E.; Sumer Okkali, G.; Yucel, B.; Margaoan, R.; Nalbantsoy, A. Comparative Study of Antiviral, Cytotoxic, Antioxidant Activities, Total Phenolic Profile and Chemical Content of Propolis Samples in Different Colors from Turkiye. Antioxidants 2022, 11, 2075. [Google Scholar] [CrossRef]
- Boyracı, G.M.; Değirmenci, A.; Yıldız, O.; Çelebi, Z.B. Propolis and royal jelly containing skin cream: The evaluation of antioxidant, antihyaluronidase, and antimicrobial activities. Uludag Bee J. 2023, 23, 224–238. [Google Scholar]
- Miguel, M.G.; Antunes, M.D. Is propolis safe as an alternative medicine. J. Pharm. Bioallied Sc. 2011, 3, 479–495. [Google Scholar] [CrossRef]
- Segueni, N.; Akkal, S.; Benlabed, K.; Nieto, G. Potential Use of Propolis in Phytocosmetic as Phytotherapeutic Constituent. Molecules 2022, 27, 5833. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Górecki, M.; Rzepecka-Stojko, A.; Balwierz, R.; Stojko, J. Bee Products in Dermatology and Skin Care. Molecules 2020, 25, 556. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Sun, L.C.; Zhang, H.B.; Gu, C.D.; Guo, S.D.; Li, G.; Lian, R.; Yao, Y.; Zhang, G.Q. Protective effect of acacetin on sepsis-induced acute lung injury via its anti-inflammatory and antioxidative activity. Arch. Pharmacal Res. 2018, 41, 1199–1210. [Google Scholar] [CrossRef]
- Wu, D.D.; Wang, Y.N.; Zhang, H.; Du, M.H.; Li, T.S. Acacetin attenuates mice endotoxin-induced acute lung injury via augmentation of heme oxygenase-1 activity. Inflammopharmacology 2018, 26, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.H.; Gao, Z. Acacetin, a Natural Flavone with Potential in Improving Liver Disease Based on Its Anti-Inflammation, Anti-Cancer, Anti-Infection and Other Effects. Molecules 2024, 29, 4872. [Google Scholar] [CrossRef] [PubMed]
- Sharafan, M.; Malinowska, M.A.; Ekiert, H.; Kwasniak, B.; Sikora, E.; Szopa, A. (Vine Grape) as a Valuable Cosmetic Raw Material. Pharmaceutics 2023, 15, 1372. [Google Scholar] [CrossRef]
- Shay, J.; Elbaz, H.A.; Lee, I.; Zielske, S.P.; Malek, M.H.; Hüttemann, M. Molecular Mechanisms and Therapeutic Effects of (−)- Epicatechin and Other Polyphenols in Cancer, Inflammation, Diabetes, and Neurodegeneration. Oxid. Med. Cell Longev. 2015, 2015, 181260. [Google Scholar] [CrossRef]
- Bai, J.R.; Zhang, Y.S.; Tang, C.; Hou, Y.; Ai, X.P.; Chen, X.R.; Zhang, Y.; Wang, X.B.; Meng, X.L. Gallic acid: Pharmacological activities and molecular mechanisms involved in inflammation-related diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- González-Minero, F.J.; Bravo-Díaz, L. The Use of Plants in Skin-Care Products, Cosmetics and Fragrances: Past and Present. Cosmetics 2018, 5, 50. [Google Scholar] [CrossRef]
- Gǎlbǎu, C.-Ş.; Irimie, M.; Neculau, A.E.; Dima, L.; Pogačnik da Silva, L.; Vârciu, M.; Badea, M. The Potential of Plant Extracts Used in Cosmetic Product Applications—Antioxidants Delivery and Mechanism of Actions. Antioxidants 2024, 13, 1425. [Google Scholar] [CrossRef] [PubMed]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef]
- Leitner, P.D. Identification and Characterization of Anti-inflammatory Compounds from Soil-Algae Extracts. Ph.D. Thesis, Medical University of Innsbruck, Innsbruck, Austria, 2021. [Google Scholar]
- Yilmaz, M.A. Simultaneous quantitative screening of 53 phytochemicals in 33 species of medicinal and aromatic plants: A detailed, robust and comprehensive LC-MS/MS method validation. Ind. Crop Prod. 2020, 149, 112347. [Google Scholar] [CrossRef]
| Codes | TYR Enzyme Inhibition (Inhibition % ± S.D. a) 2 mg/mL b | Collagenase Enzyme Inhibition (Inhibition % ± S.D. a) 2 mg/mL b | Elastase Enzyme Inhibition (Inhibition % ± S.D. a) 2 mg/mL b |
|---|---|---|---|
| S1 | 52.37 ± 2.99 **** (IC50 = 1.70 ± 0.02 mg/mL) | 99.87 ± 0.03 (IC50 = 0.043 ± 0.003 mg/mL) | 21.09 ±2.28 **** |
| S2 | 42.07 ± 6.07 **** | 99.62 ± 0.22 (IC50 = 0.030 ± 0.004 mg/mL) | - c |
| S3 | 56.81 ± 4.77 **** (IC50 = 1.52 ± 0.12 mg/mL) | 99.88 ± 0.01 (IC50 = 0.022 ± 0.001 mg/mL) | 18.54 ± 3.11 **** |
| S4 | 40.89 ± 8.30 **** | 99.57 ± 0.12 (IC50 = 0.025 ± 0.001 mg/mL) | 97.73 ± 4.85 (IC50 = 0.045 ± 0.005 mg/mL) |
| S5 | 27.35 ± 3.46 **** | 69.37 ± 3.07 **** (IC50 = 1.08 ± 0.19 mg/mL) | 16.48 ± 2.07 **** |
| S6 | - | 88.09 ± 5.51 **** (IC50 = 1.11 ± 0.05 mg/mL) | 20.62 ± 5.26 **** |
| S7 | 31.32 ± 10.93 **** | 99.78 ± 0.08 (IC50 = 0.041 ± 0.001 mg/mL) | 92.70 ± 1.24 * (IC50 = 0.076 ± 0.006 mg/mL) |
| REF | 97.08 ± 1.79 d (IC50 = 0.11 ± 0.01 mM) | 97.32 ± 0.52 e (IC50 = 0.103 ± 0.009 mM) | 98.87 ± 0.43 f (IC50 = 0.156 ± 0.03 mM) |
| No | Analytes | RT a | M.I. (m/z) b | F.I. (m/z) c | Ion. Mode | Equation | r2d | Cotinus coggygria Leaf (mg Analyte/g Extract) | Vitis vinifera Seed (mg Analyte/g Extract) | Propolis (mg Analyte/g Extract) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | 3.0 | 190.8 | 93.0 | Neg | y = −0.0129989 + 2.97989× | 0.996 | 129.686 | 11.382 | 0.128 |
| 4 | Gallic acid | 4.4 | 168.8 | 79.0 | Neg | y = 0.0547697 + 20.8152× | 0.999 | 8.048 | 16.901 | N.D. |
| 6 | Protocatechuic acid | 6.8 | 152.8 | 108.0 | Neg | y = 0.211373 + 12.8622× | 0.957 | 3.352 | 2.871 | 0.145 |
| 7 | Catechin | 7.4 | 288.8 | 203.1 | Neg | y = −0.00370053 + 0.431369× | 0.999 | N.D. | 7.603 | N.D. |
| 8 | Gentisic acid | 8.3 | 152.8 | 109.0 | Neg | y = −0.0238983 + 12.1494× | 0.997 | N.D. | N.D. | 0.016 |
| 9 | Chlorogenic acid | 8.4 | 353.0 | 85.0 | Neg | y = 0.289983 + 36.3926× | 0.995 | N.D. | N.D. | 0.019 |
| 10 | Protocatechuic aldehyde | 8.5 | 137.2 | 92.0 | Neg | y = 0.257085 + 25.4657× | 0.996 | 0.016 | 0.215 | 0.357 |
| 11 | Tannic acid | 9.2 | 182.8 | 78.0 | Neg | y = 0.0126307 + 26.9263× | 0.999 | 8.559 | 0.429 | N.D. |
| 15 | Epicatechin | 11.6 | 289.0 | 203.0 | Neg | y = −0.0172078 + 0.0833424× | 0.996 | N.D. | 45.26 | N.D. |
| 16 | Vanilic acid | 11.8 | 166.8 | 108.0 | Neg | y = −0.0480183 + 0.779564× | 0.999 | N.D. | 0.178 | N.D. |
| 17 | Caffeic acid | 12.1 | 179.0 | 134.0 | Neg | y = 0.120319 + 95.4610× | 0.999 | N.D. | N.D. | 0.17 |
| 19 | Vanillin | 13.9 | 153.1 | 125.0 | Poz | y = 0.00185898 + 20.7382× | 0.996 | N.D. | N.D. | 0.054 |
| 20 | Syringic aldehyde | 14.6 | 181.0 | 151.1 | Neg | y = −0.0128684 + 7.90153× | 0.999 | N.D. | N.D. | 0.024 |
| 22 | Epicatechin gallate | 15.5 | 441.0 | 289.0 | Neg | y = −0.0142216 + 1.06768× | 0.997 | N.D. | 2.395 | N.D. |
| 24 | p-Coumaric acid | 17.8 | 163.0 | 93.0 | Neg | y = 0.0249034 + 18.5180× | 0.999 | 0.065 | 0.074 | 0.664 |
| 29 | Salicylic acid | 21.8 | 137.2 | 65.0 | Neg | y = 0.239287 + 153.659× | 0.999 | N.D. | 0.024 | 0.079 |
| 30 | Cyranoside | 23.7 | 447.0 | 284.0 | Neg | y = 0.280246 + 6.13360× | 0.997 | 0.11 | 0.567 | 0.181 |
| 31 | Miquelianin | 24.1 | 477.0 | 150.9 | Neg | y = −0.00991585 + 5.50334× | 0.999 | 0.355 | 0.045 | N.D. |
| 33 | Rutin | 25.6 | 608.9 | 301.0 | Neg | y = −0.0771907 + 2.89868× | 0.999 | 0.085 | 0.017 | 0.038 |
| 34 | isoquercitrin | 25.6 | 463.0 | 271.0 | Neg | y = −0.111120 + 4.10546× | 0.998 | 1.444 | 0.21 | 0.022 |
| 35 | Hesperidin | 25.8 | 611.2 | 449.0 | Poz | y = 0.139055 + 13.2785× | 0.999 | 0.079 | 0.017 | 0.027 |
| 37 | Genistin | 26.3 | 431.0 | 239.0 | Neg | y = 1.65808 + 7.57459× | 0.991 | 0.622 | 0.074 | 0.512 |
| 39 | Ellagic acid | 27.6 | 301.0 | 284.0 | Neg | y = 0.00877034 + 0.663741× | 0.999 | N.D. | 0.279 | N.D. |
| 40 | Cosmosiin | 28.2 | 431.0 | 269.0 | Neg | y = −0.708662 + 8.62498× | 0.998 | 0.457 | 0.059 | 0.403 |
| 41 | Quercitrin | 29.8 | 447.0 | 301.0 | Neg | y = −0.00153274 + 3.20368× | 0.999 | 6.143 | N.D. | 0.021 |
| 42 | Astragalin | 30.4 | 447.0 | 255.0 | Neg | y = 0.00825333 + 3.51189× | 0.999 | 1.569 | 0.53 | 0.024 |
| 43 | Nicotiflorin | 30.6 | 592.9 | 255.0/284.0 | Neg | y = 0.00499333 + 2.62351× | 0.999 | 0.108 | 0.048 | N.D. |
| 44 | Fisetin | 30.6 | 285.0 | 163.0 | Neg | y = 0.0365705 + 8.09472× | 0.999 | 0.013 | N.D. | N.D. |
| 47 | Quercetin | 35.7 | 301.0 | 272.9 | Neg | y = +0.00597342 + 3.39417× | 0.999 | 0.269 | 0.08 | 1.968 |
| 48 | Naringenin | 35.9 | 270.9 | 119.0 | Neg | y = −0.00393403 + 14.6424× | 0.999 | 0.053 | 0.05 | 5.747 |
| 49 | Hesperetin | 36.7 | 301.0 | 136.0/286.0 | Neg | y = +0.0442350 + 6.07160× | 0.999 | N.D. | N.D. | 0.087 |
| 50 | Luteolin | 36.7 | 284.8 | 151.0/175.0 | Neg | y = −0.0541723 + 30.7422× | 0.999 | 0.037 | 0.01 | 0.601 |
| 52 | Kaempferol | 37.9 | 285.0 | 239.0 | Neg | y = −0.00459557 + 3.13754× | 0.999 | 0.029 | 0.019 | 2.005 |
| 53 | Apigenin | 38.2 | 268.8 | 151.0/149.0 | Neg | y = 0.119018 + 34.8730× | 0.998 | 0.006 | 0.003 | 2.471 |
| 54 | Amentoflavone | 39.7 | 537.0 | 417.0 | Neg | y = 0.727280 + 33.3658× | 0.992 | N.D. | N.D. | 0.012 |
| 55 | Chrysin | 40.5 | 252.8 | 145.0/119.0 | Neg | y = −0.0777300 + 18.8873× | 0.999 | 0.04 | 0.015 | 17.078 |
| 56 | Acacetin | 40.7 | 283.0 | 239.0 | Neg | y = −0.559818 + 163.062× | 0.997 | 0.058 | 0.022 | 62.332 |
| Codes | Species Names | Plant Parts | Collection Localities and Dates | Yield (w/w%) |
|---|---|---|---|---|
| S1 | Cotinus coggygria | Commercial leaf extract | Purchased from VemoHerb®, Bulgaria, 2020 | - |
| S2 | Cotinus coggygria | Pedicel | Ankara province, June 2019 | 13.76 |
| S3 | Cotinus coggygria | Folia | Ankara province, November 2019 | 20.77 |
| S4 | Garcinia × mangostana | Pericarpium | Purchased from Thailand, June 2011 | 21.37 |
| S5 | Propolis | Commercial extract | Purchased from Zhejiang Shaoxing Dongling Health Food Co., Ltd., China, 2016 | - |
| S6 | Pistacia vera | Shell | Gaziantep province, 2019 | 11.20 |
| S7 | Vitis vinifera | Seed | Denizli province, 2019 | 2.45 |
| Viability Measured After Exposure Time Points (t = 3 and 60 min) | Prediction to Be Considered |
|---|---|
| STEP 1 | |
| <50% after 3 min exposure | Corrosive |
| ≥50% after 3 min exposure AND <15% after 60 min exposure | Corrosive |
| ≥50% after 3 min exposure AND ≥15% after 60 min exposure | Non-corrosive |
| STEP 2 | |
| <15% after 3 min exposure | Optional Sub-category 1A |
| ≥15% after 3 min exposure | A combination of optional Subcategories 1B and 1C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deniz, F.S.S.; Orhan, I.E.; Filipek, P.A.; Ertas, A.; Gstir, R.; Jakschitz, T.; Bonn, G.K. Evaluation of the Anti-Aging Properties of Ethanolic Extracts from Selected Plant Species and Propolis by Enzyme Inhibition Assays and 2D/3D Cell Culture Methods. Pharmaceuticals 2025, 18, 439. https://doi.org/10.3390/ph18030439
Deniz FSS, Orhan IE, Filipek PA, Ertas A, Gstir R, Jakschitz T, Bonn GK. Evaluation of the Anti-Aging Properties of Ethanolic Extracts from Selected Plant Species and Propolis by Enzyme Inhibition Assays and 2D/3D Cell Culture Methods. Pharmaceuticals. 2025; 18(3):439. https://doi.org/10.3390/ph18030439
Chicago/Turabian StyleDeniz, F. Sezer Senol, Ilkay Erdogan Orhan, Przemyslaw Andrzej Filipek, Abdulselam Ertas, Ronald Gstir, Thomas Jakschitz, and Günther Karl Bonn. 2025. "Evaluation of the Anti-Aging Properties of Ethanolic Extracts from Selected Plant Species and Propolis by Enzyme Inhibition Assays and 2D/3D Cell Culture Methods" Pharmaceuticals 18, no. 3: 439. https://doi.org/10.3390/ph18030439
APA StyleDeniz, F. S. S., Orhan, I. E., Filipek, P. A., Ertas, A., Gstir, R., Jakschitz, T., & Bonn, G. K. (2025). Evaluation of the Anti-Aging Properties of Ethanolic Extracts from Selected Plant Species and Propolis by Enzyme Inhibition Assays and 2D/3D Cell Culture Methods. Pharmaceuticals, 18(3), 439. https://doi.org/10.3390/ph18030439









