Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives
Abstract
1. Introduction
2. Results
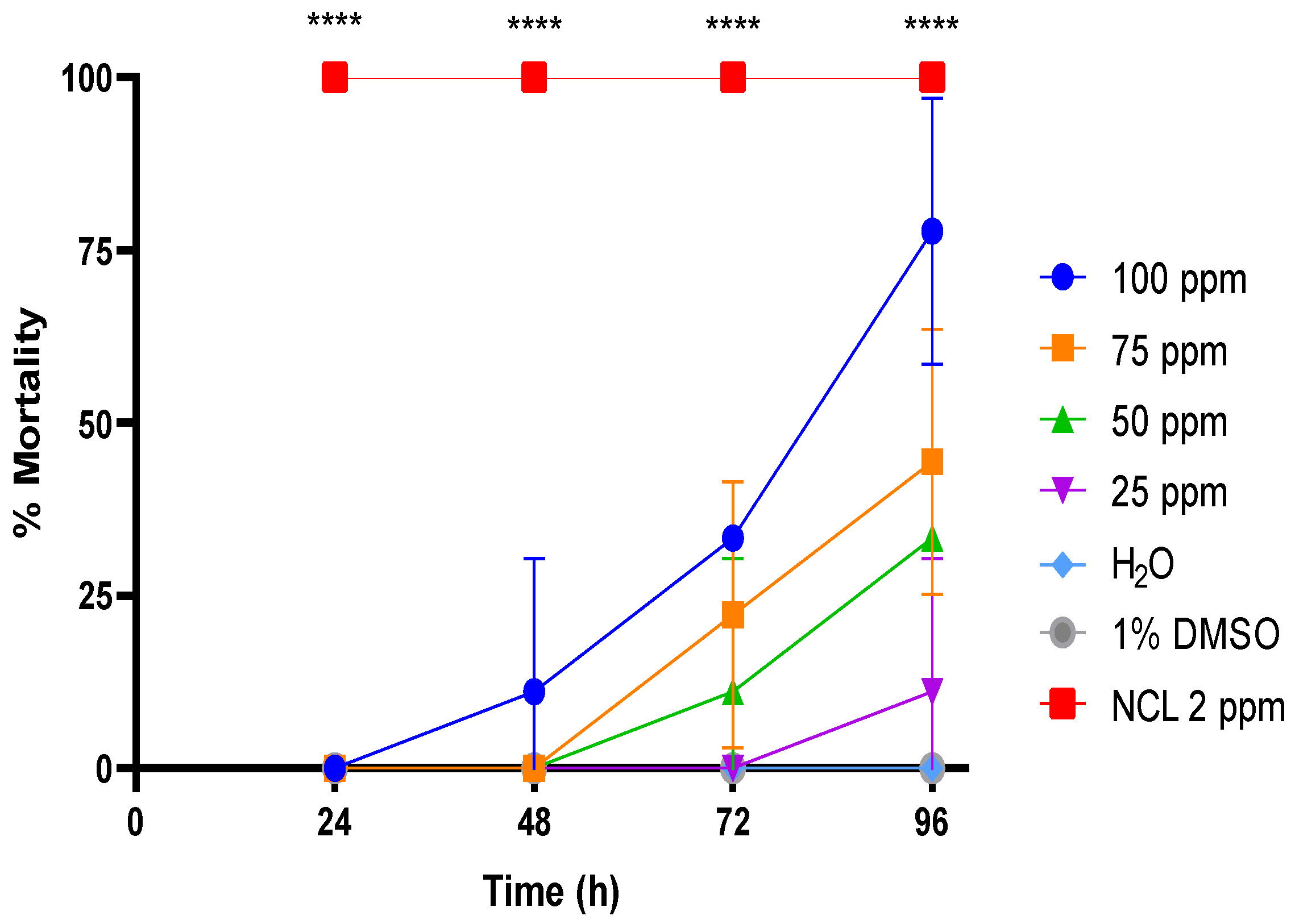
2.1. Moluscicidal Activity
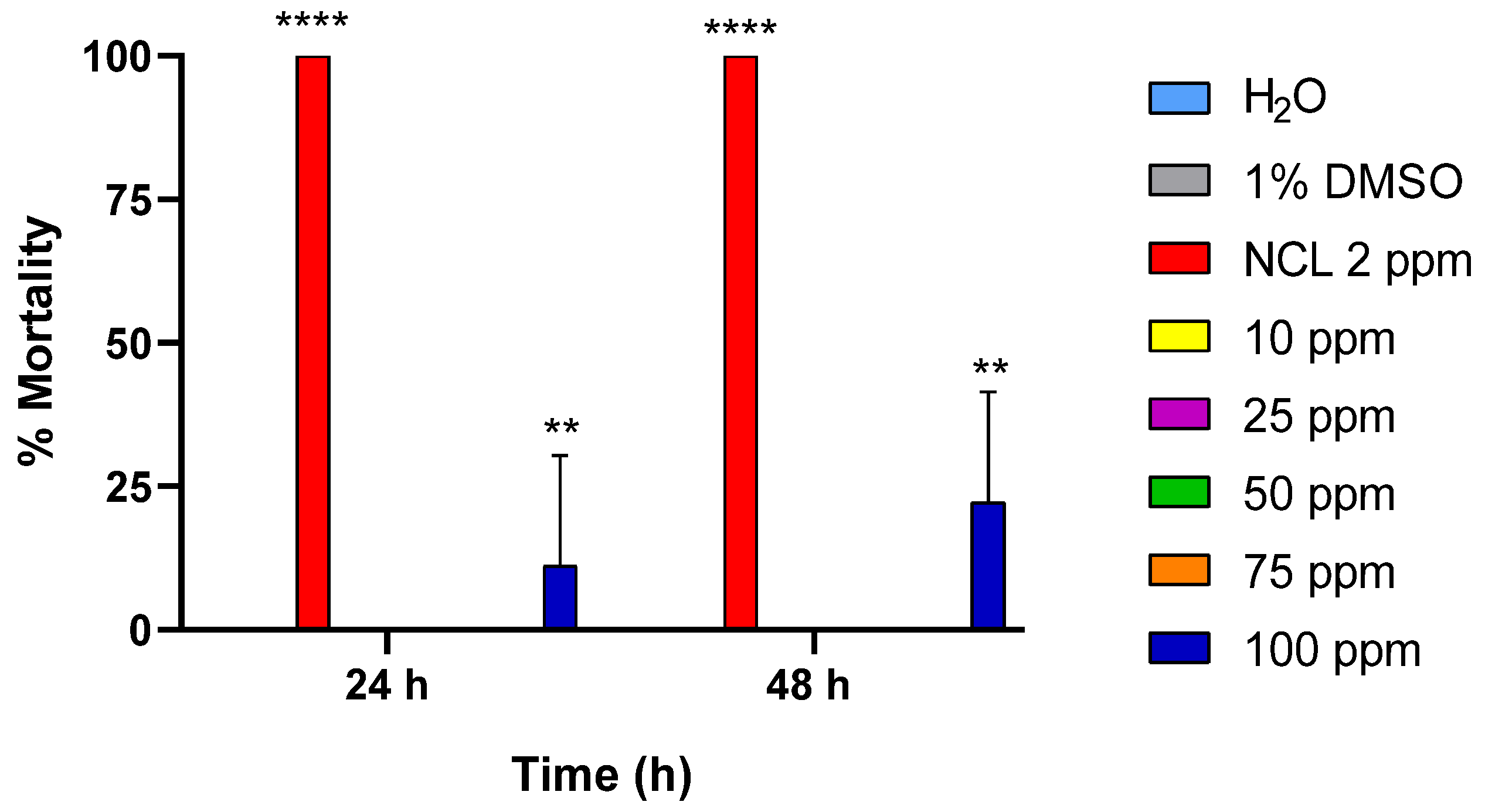
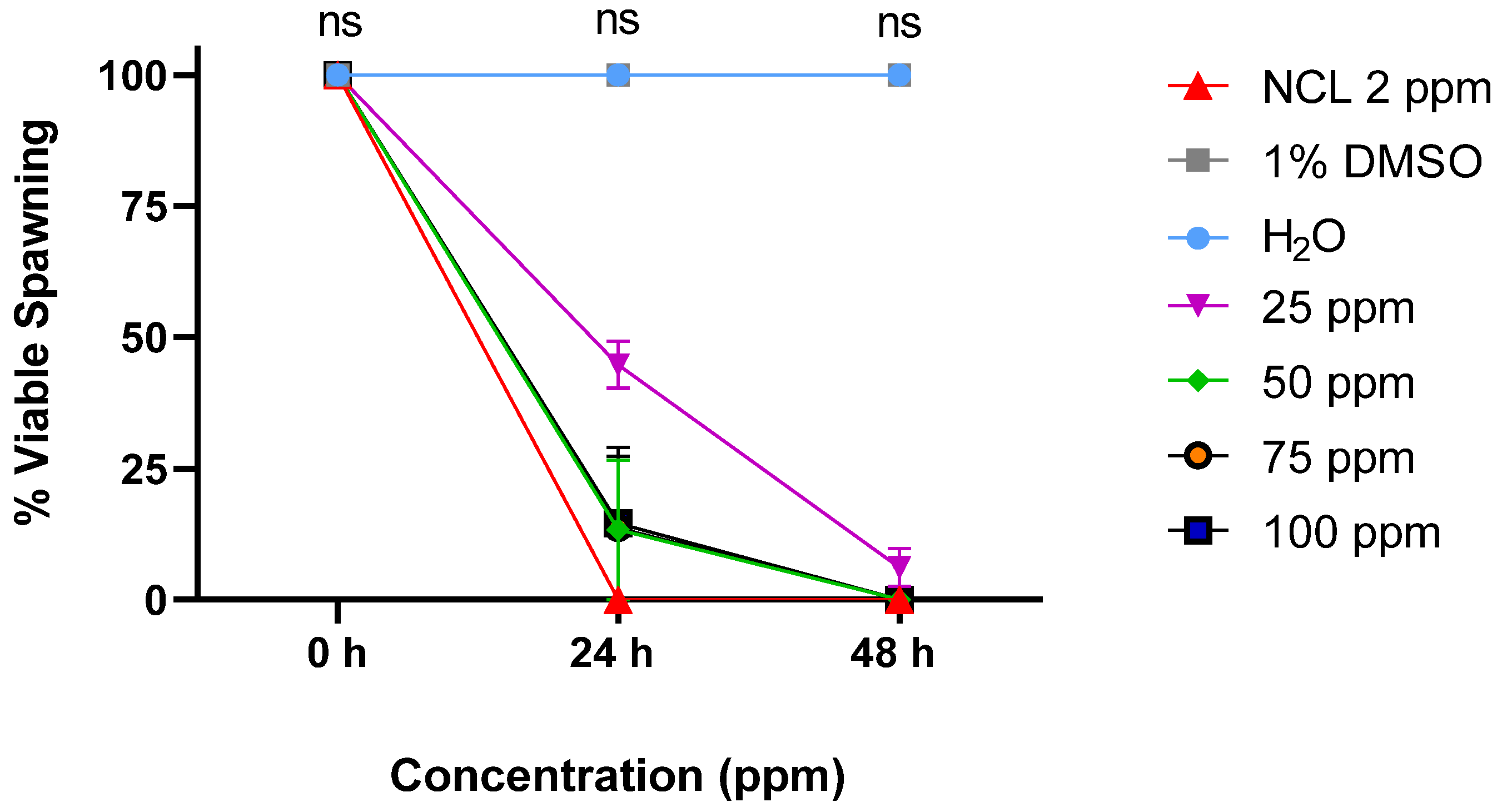
2.2. Embryotoxicity
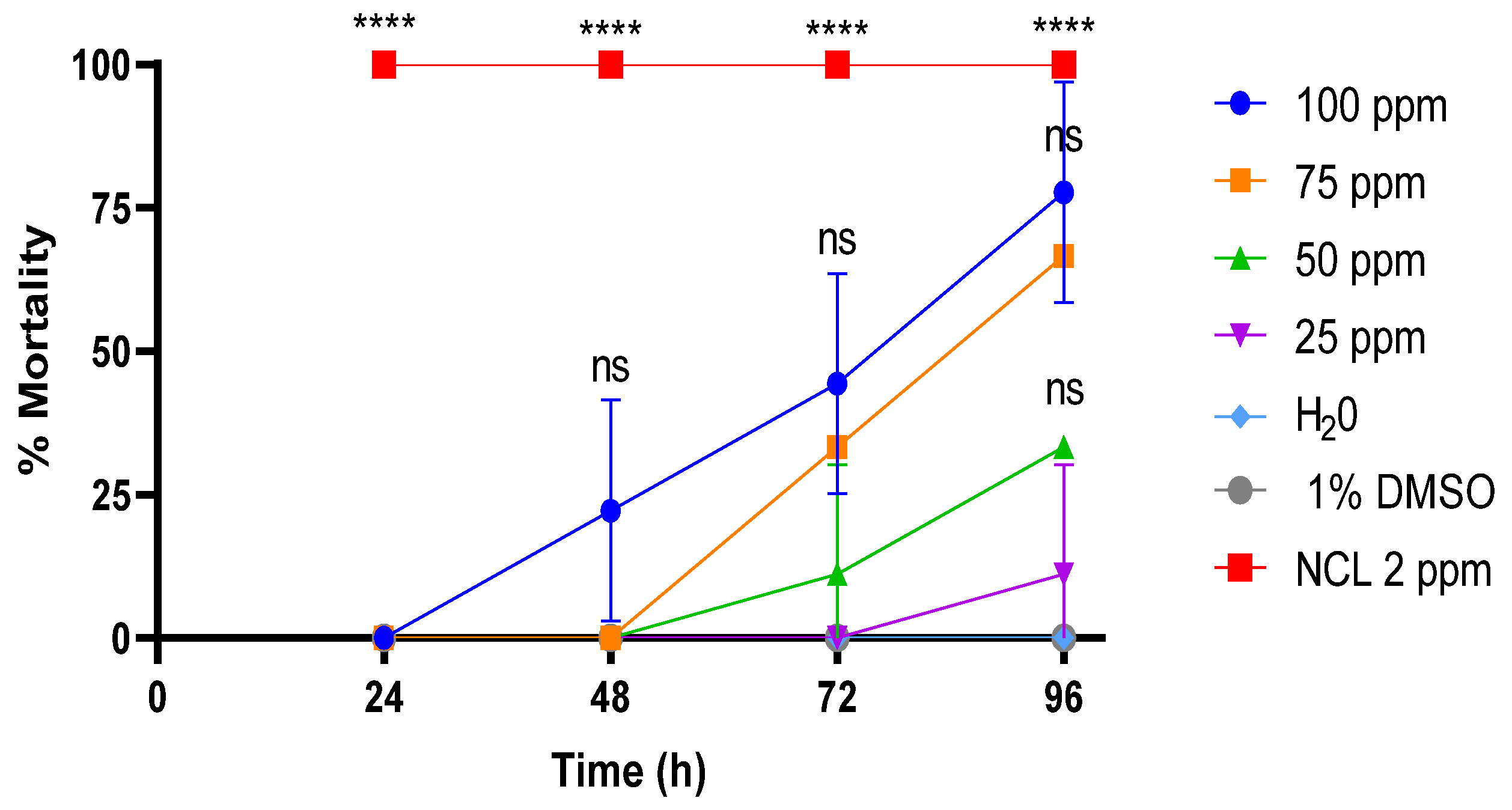
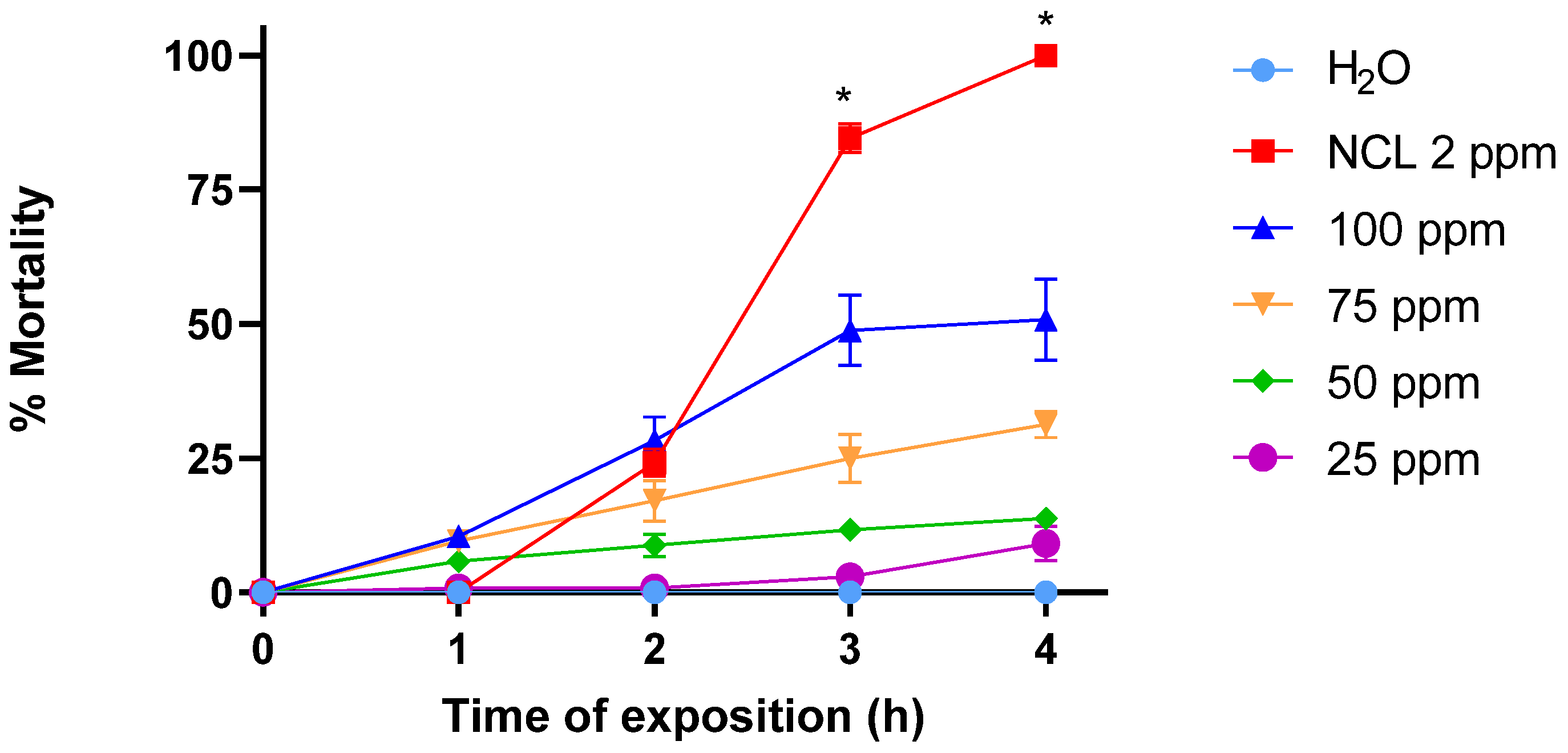
2.3. Cercaricidal Activity
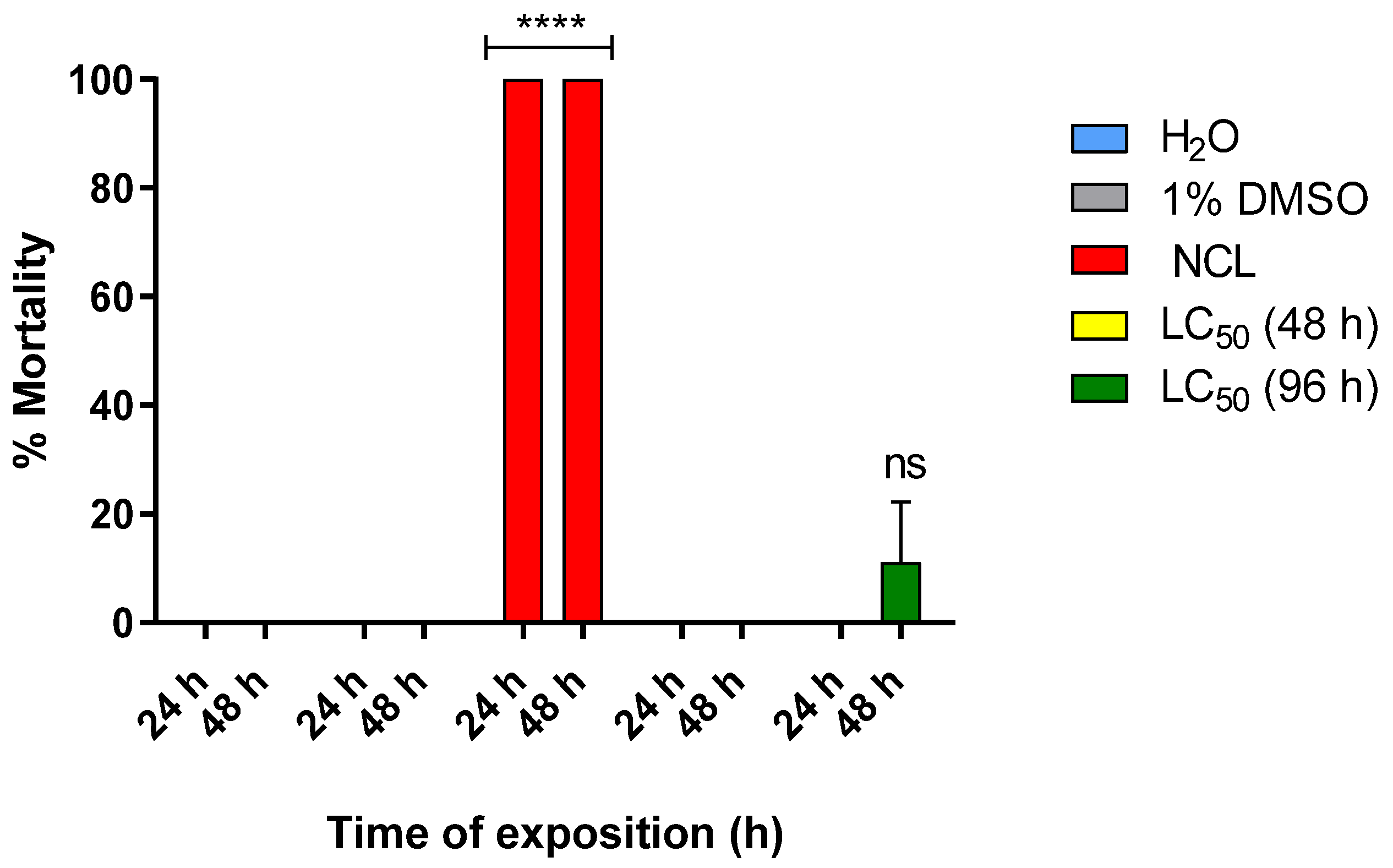
2.4. Toxicity
2.5. In Silico Assay
3. Discussion
4. Material and Methods
4.1. Chemistry
4.2. Bioassays
4.2.1. Molluscicidal Assays
4.2.2. Evaluation of Ovicidal Activity
4.2.3. Evaluation of Cercarial Activity
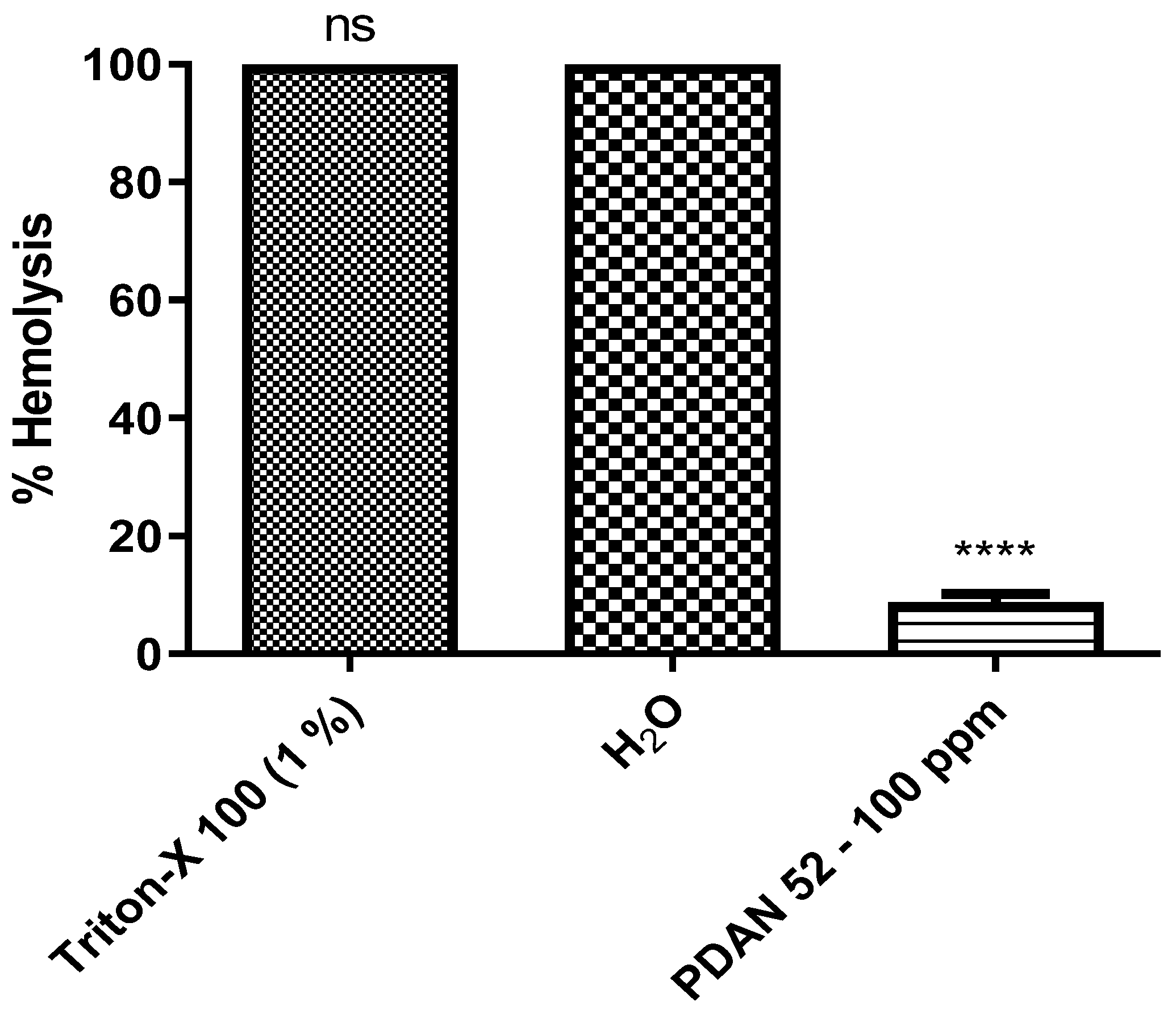
4.2.4. Toxicity Hemocompatibility
4.2.5. Single-Dose Toxicity
4.3. Statistical Analysis
4.4. In Silico Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nelwan, M.L. Schistosomiasis: Life Cycle, Diagnosis, and Control. Curr. Ther. Res. 2019, 91, 5–9. [Google Scholar] [CrossRef] [PubMed]
- World Heath Organization (WHO). Schistosomiasis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/schistosomiasis/ (accessed on 22 June 2023).
- Brasil, Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente, Departamento de Doenças Transmissíveis. Vigilância da Esquistossomose Mansoni: Diretrizes Técnicas [Recurso Eletrônico]; Ministério da Saúde, Secretaria de Vigilância em Saúde e Ambiente, Departamento de Doenças Transmissíveis: Brasília, Brazil, 2024.
- Zacharia, A.; Mushi, V.; Makene, T. A systematic review and meta-analysis on the rate of human schistosomiasis reinfection. PLoS ONE 2020, 15, e0243224. [Google Scholar] [CrossRef] [PubMed]
- Aboagye, I.F.; Addison, A.A. Praziquantel efficacy, urinary and intestinal schistosomiasis reinfection—A systematic review. Pathog. Glob. Health 2022, 117, 623–630. [Google Scholar] [CrossRef]
- Zanardi, V.S.; Barbosa, L.M.; Simões, F.M.; Thiengo, S.C.; Blanton, R.E.; Junior, G.R.; Silva, L.K.; Reis, M.G. Prevalence of Infection of Biomphalaria glabrata by Schistosoma mansoni and the risk of urban Schistosomiasis mansoni in Salvador, Bahia, Brazil. Rev. Soc. Bras. Med. Trop. 2019, 52, e20190171. [Google Scholar] [CrossRef]
- Coelho, P.; Caldeira, R. Critical analysis of molluscicide application in schistosomiasis control programs in Brazil. Infect. Dis. Poverty 2016, 5, 57. [Google Scholar] [CrossRef]
- Leak, T.; Aufderheide, J.; Bergfield, A.; Hubert, T.D. Acute toxicity of the lampricides TFM and niclosamide: Effects on a vascular plant and a chironomid species. J. Great Lakes Res. 2020, 46, 180–187. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, H.; Gao, J.; Jiang, W.; Tian, X.; Xie, Z.; Zhang, T.; Feng, J.; Song, R. Niclosamide exposure disrupts antioxidant defense, histology, and the liver and gut transcriptome of Chinese soft-shelled turtle (Pelodiscus sinensis). Ecotoxicol. Environ. Saf. 2023, 260, 115081. [Google Scholar] [CrossRef]
- Gonzaga, D.T.G.; Oliveira, F.H.; von Ranke, N.L.; Pinho, G.Q.; Salles, J.P.; Bello, M.L.; Rodrigues, C.R.; Castro, H.C.; de Souza, H.V.C.M.; Reis, C.R.C.; et al. Synthesis, Biological Evaluation, and Molecular Modeling Studies of New Thiadiazole Derivatives as Potent P2X7 Receptor Inhibitors. Front. Chem. 2019, 7, 261. [Google Scholar] [CrossRef]
- Bauer, M.; Lautenschlaeger, C.; Kempe, K.; Tauhardt, L.; Schubert, U.S.; Fischer, D. Poly(2-ethyl-2-oxazoline) as alternative for the stealth polymer poly(ethylene glycol): Comparison of in vitro cytotoxicity and hemocompatibility. Macromol. Biosci. 2012, 12, 986–998. [Google Scholar] [CrossRef]
- Coura, J.; Amaral, R. Epidemiological and control aspects of schistosomiasis in Brazilian endemic areas. Mem. Inst. Oswaldo Cruz 2004, 99, 13–19. [Google Scholar] [CrossRef]
- Gurarie, D.; Yoon, N.; Li, E.; Ndeffo-Mbah, M.; Durham, D.; Phillips, A.E.; Aurelio, H.O.; Ferro, J.; Galvani, A.P.; King, C.H. Modelling control of Schistosoma haematobium infection: Predictions of the long-term impact of mass drug administration in Africa. Parasites Vectors 2015, 8, 529. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.A.; Abdel-Latif, E.; El-Mekawy, R.E. Synthesis and molluscicidal activity of some new thiophene, thiadiazole and pyrazole derivatives. Eur. J. Med. Chem. 2009, 44, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- El Shehry, M.F.; Abu-Hashem, A.A.; El-Telbani, E.M. Synthesis of 3-((2,4-dichlorophenoxy)methyl)-1,2,4-triazolo(thiadiazoles and thiadiazines) as anti-inflammatory and molluscicidal agents. Eur. J. Med. Chem. 2010, 45, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- El Shehry, M.F.; Swellem, R.H.; Abu-Bakr, S.M.; El-Telbani, E.M. Synthesis and molluscicidal evaluation of some new pyrazole, isoxazole, pyridine, pyrimidine, 1,4-thiazine and 1,3,4-thiadiazine derivatives incorporating benzofuran moiety. Eur. J. Med. Chem. 2010, 45, 4783–4787. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Metz, P.; Metwally, N.H.; El-Mahrouky, S.F. Synthesis and Molluscicidal Activity of New Cinnoline and Pyrano [2,3-c]pyrazole Derivatives. Arch. Pharm. 2006, 339, 456–460. [Google Scholar] [CrossRef]
- Abdelrazek, F.M.; Michael, F.A.; Mohamed, A.E. Synthesis and Molluscicidal Activity of Some 1,3,4-Triaryl-5-chloropyrazole, Pyrano[2,3-c]pyrazole, Pyrazolylphthalazine and Pyrano[2,3-d]thiazole Derivatives. Arch. Pharm. 2006, 339, 305–312. [Google Scholar] [CrossRef]
- Casertano, M.; Imperatore, C.; Luciano, P.; Aiello, A.; Putra, M.Y.; Gimmelli, R.; Ruberti, G.; Menna, M. Chemical Investigation of the Indonesian Tunicate Polycarpa aurata and Evaluation of the Effects Against Schistosoma mansoni of the Novel Alkaloids Polyaurines A and B. Mar. Drugs 2019, 17, 278. [Google Scholar] [CrossRef]
- Li, T.; Ziniel, P.D.; He, P.; Kommer, V.P.; Crowther, G.J.; He, M.; Liu, Q.; Van Voorhis, W.C.; Williams, D.L.; Wang, M.W. High-throughput screening against thioredoxin glutathione reductase identifies novel inhibitors with potential therapeutic value for schistosomiasis. Infect. Dis. Poverty 2015, 4, 40. [Google Scholar] [CrossRef]
- Ferraro, F.; Corvo, I.; Bergalli, L.; Ilarraz, A.; Cabrera, M.; Gil, J.; Susuki, B.M.; Caffrey, C.R.; Timson, D.J.; Robert, X.; et al. Novel and selective inactivators of Triosephosphate isomerase with anti-trematode activity. Sci. Rep. 2020, 10, 2587. [Google Scholar] [CrossRef]
- EPA. Prevention, Pesticides and Toxic Substances. United States Environmental Protection Agency. 1999. Available online: https://www3.epa.gov/pesticides/chem_search/reg_actions/reregistration/fs_PC-077401_1-Nov-99.pdf (accessed on 11 June 2024).
- Jobin, W.R. Cost of snail control. Am. J. Trop. Med. Hyg. 1979, 28, 142–154. [Google Scholar] [CrossRef]
- Friani, G.; Amaral, A.M.R.; Quinelato, S.; Mello-Silva, C.C.; Golo, P.S. Biological control of Biomphalaria, the intermediate host of Schistosoma spp.: A systematic review. Cienc. Rural 2023, 53, e20210714. [Google Scholar] [CrossRef]
- Pharmacompass. Available online: https://www.pharmacompass.com/price/niclosamide (accessed on 7 February 2025).
- Sigma Aldrich. Available online: https://www.sigmaaldrich.com/BR/pt/search/niclodamide?dym=niclosamide&focus=products&page=1&perpage=30&sort=relevance&term=Niclodamide&type=product (accessed on 7 February 2025).
- Gurgel, P.M.; Navoni, J.Á.; de Morais Ferreira, D.; do Amaral, V.S. Ecotoxicological water assessment of an estuarine river from the Brazilian Northeast, potentially affected by industrial wastewater discharge. Sci. Total Environ. 2016, 572, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Sarkis, J.; Lai, K. Confirmation of a measurement model for green supply chain management practices implementation. Int. J. Prod. Econ. 2008, 111, 261–273. [Google Scholar] [CrossRef]
- Ansari, I.; El-Kady, M.M.; Mahmoud, A.E.D.; Arora, C.; Verma, A.; Rajarathinam, R.; Singh, P.; Verma, D.K.; Mittal, J. Persistent pesticides: Accumulation, health risk assessment, management and remediation: An overview. Desalin. Water Treat. 2024, 317, 100274, ISSN 1944-3986. [Google Scholar] [CrossRef]
- Martins, D.L.; Silva, N.A.A.; Ferreira, V.F.; Rangel, L.S.; Santos, J.A.A.; Faria, R.X. Molluskicidal activity of 3-aryl-2-hydroxy-1,4-naphthoquinones against Biomphalaria glabrata. Acta Trop. 2022, 231, 106414, ISSN 0001-706X. [Google Scholar] [CrossRef]
- Liu, C.; Lou, W.; Zhu, Y.; Nadiminty, N.; Schwartz, C.T.; Evans, C.P.; Gao, A.C. Niclosamide Inhibits Androgen Receptor Variants Expression and Overcomes Enzalutamide Resistance in Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2014, 20, 3198–3210. [Google Scholar] [CrossRef]
- Sobhani, N.; Generali, D.; D’angelo, A.; Aieta, M.; Roviello, G. Current status of androgen receptor-splice variant 7 inhibitor niclosamide in castrate-resistant prostate-cancer. Investig. New Drugs 2018, 36, 1133–1137. [Google Scholar] [CrossRef]
- Parikh, M.; Liu, C.; Wu, C.Y.; Evans, C.P.; Dall’Era, M.; Robles, D.; Lara, P.N.; Agarwal, N.; Gao, A.C.; Pan, C.X. Phase Ib trial of reformulated niclosamide with abiraterone/prednisone in men with castration-resistant prostate cancer. Sci. Rep. 2021, 11, 6377. [Google Scholar] [CrossRef]
- Santos, J.A.A.; Cavalcante, V.P.; Rangel, L.S.; Leite, J.C.V.A.; Faria, R.X. A New Technique Using Low Volumes: A New Technique to Assess the Molluscicidal Activity Using Low Volumes. Evid.-Based Complement. Altern. Med. 2017, 2017, 3673197. [Google Scholar] [CrossRef]
- Silva, Y.R.R.; Silva, L.D.; Rocha, T.L.; Santos, D.B.; Bezerra, J.C.B.; Machado, K.B.; Paula, J.A.M.; Amaral, V.C.S. Molluscicidal activity of Persea americana Mill. (Lauraceae) stem bark ethanolic extract against the snail Biomphalaria glabrata (Say, 1818): A novel plant-derived molluscicide? Ann. Braz. Acad. Sci. 2020, 92, 2–16. [Google Scholar] [CrossRef]
- Araújo, F.d.P.; de Albuquerque, R.D.D.G.; Rangel, L.d.S.; Caldas, G.R.; Tietbohl, L.A.C.; Santos, M.G.; Ricci-Júnior, E.; Thiengo, S.; Fernandez, M.A.; dos Santos, J.A.A.; et al. Nanoemulsion containing essential oil from Xylopia ochrantha Mart. produces molluscicidal effects against different species of Biomphalaria (Schistosoma hosts). Mem. Inst. Oswaldo Cruz 2019, 114, e180489. [Google Scholar] [CrossRef]
- ANVISA; National Health Surveillance Agency. Guide for Conducting Nonclinical Toxicology and Pharmacological Safety Studies Necessary for the Development of Medicine Safety and Efficacy Assessment Management; Version 2; GESEF: Brasília, Brazil, 2013.
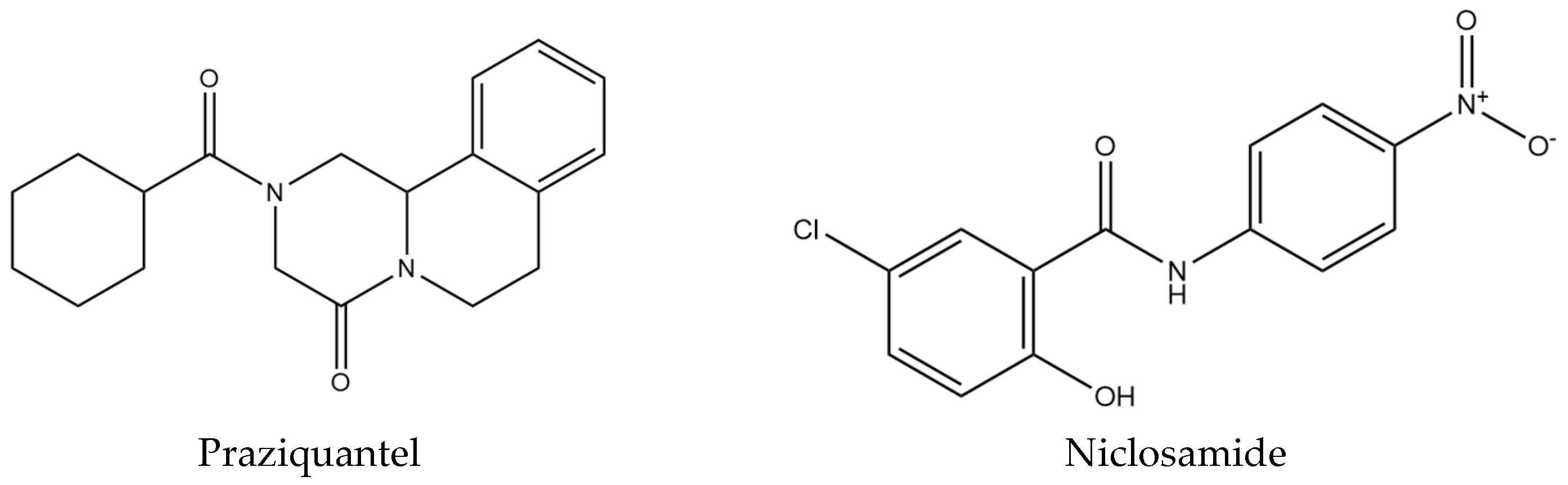
| Compounds | Structure | % Molluscicide Activity in 96 h |
|---|---|---|
| PDAN52 | 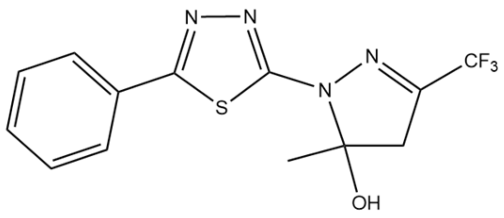 | 63 ± 4 |
| PDAN79 | 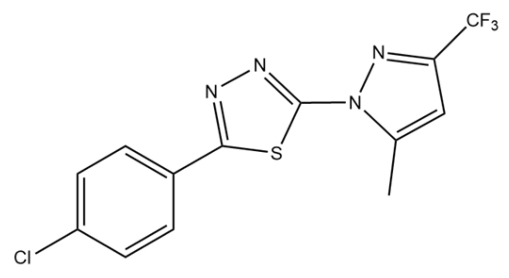 | 12 ± 3 |
| Substance | Molluscicide Activity LC10 (48 h) | Molluscicide Activity LC50 (48 h) | Molluscicide Activity LC90 (48 h) | Molluscicide Activity LC10 (96 h) | Molluscicide Activity LC50 (96 h) | Molluscicide Activity LC90 (96 h) |
|---|---|---|---|---|---|---|
| PDAN52 | 92.4 ± 2.7 ppm | 113.6 ± 8.4 ppm | 142.0 ± 9.2 ppm | 36.1 ± 7.2 ppm | 79.3 ± 7.0 ppm | 99.2 ± 6.5 ppm |
| Substance | Molluscicide Activity LC10 (48 h) | Molluscicide Activity LC50 (48 h) | Molluscicide Activity LC90 (48 h) | Molluscicide Activity LC10 (96 h) | Molluscicide Activity LC50 (96 h) | Molluscicide Activity LC90 (96 h) |
|---|---|---|---|---|---|---|
| PDAN52 | - | - | - | - | 66.7 ± 3.5 ppm | 114.4 ± 3.5 ppm |
| Compound | Cercaricide Activity LC10 (4 h) | Cercaricide Activity LC50 (4 h) | Cercaricide Activity LC90 (4 h) |
|---|---|---|---|
| PDAN52 | 24.2 ± 2.8 ppm | 68.0 ± 5 ppm | 133.4 ± 7.3 ppm |
| Compound | BCF | BD | Aquatic Toxicity | Endocrine Receptor Binding | TOX- Risk | |||
|---|---|---|---|---|---|---|---|---|
| Th_pyr_pIGC50 | Daphnia_LC50 | Minnow_LC50 | Andro_Filter | Estro_Filter | ||||
| NCL | 6.65 | No | 1.968 | 1.752 | 3.612 | Toxic | Nontoxic | 2 |
| PDAN52 | 25.81 | No | 2.426 | 0.057 | 2.564 | Nontoxic | Nontoxic | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva Rangel, L.; Gonzaga, D.T.G.; da Silva, A.C.R.; von Ranke, N.L.; Rodrigues, C.R.; Santos, J.A.A.d.; Boechat, N.; Gomes, K.N.F.; Teixeira, G.P.; Faria, R.X. Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives. Pharmaceuticals 2025, 18, 429. https://doi.org/10.3390/ph18030429
da Silva Rangel L, Gonzaga DTG, da Silva ACR, von Ranke NL, Rodrigues CR, Santos JAAd, Boechat N, Gomes KNF, Teixeira GP, Faria RX. Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives. Pharmaceuticals. 2025; 18(3):429. https://doi.org/10.3390/ph18030429
Chicago/Turabian Styleda Silva Rangel, Leonardo, Daniel Tadeu Gomes Gonzaga, Ana Cláudia Rodrigues da Silva, Natalia Lindmar von Ranke, Carlos Rangel Rodrigues, José Augusto Albuquerque dos Santos, Nubia Boechat, Keyla Nunes Farias Gomes, Guilherme Pegas Teixeira, and Robson Xavier Faria. 2025. "Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives" Pharmaceuticals 18, no. 3: 429. https://doi.org/10.3390/ph18030429
APA Styleda Silva Rangel, L., Gonzaga, D. T. G., da Silva, A. C. R., von Ranke, N. L., Rodrigues, C. R., Santos, J. A. A. d., Boechat, N., Gomes, K. N. F., Teixeira, G. P., & Faria, R. X. (2025). Molluscicidal and Schistosomicidal Activities of 2-(1H-Pyrazol-1-yl)-1,3,4-thiadiazole Derivatives. Pharmaceuticals, 18(3), 429. https://doi.org/10.3390/ph18030429







