Ameliorative Effects of Prunella vulgaris on Lower Urinary Tract Symptoms Induced by Benign Prostatic Hyperplasia in SD Rats via Nitric Oxide and Potassium Channels
Abstract
1. Introduction
2. Results
2.1. PVE Extract Analysis
2.2. PVE Attenuates Prostate Smooth Muscle Contraction Induced by PE
2.3. The Effect of PVE on Inhibiting PE-Induced Prostate Smooth Muscle Contraction Was Blocked by L-NAME and TEA
2.4. Quercetin Inhibits PE-Induced Prostate Smooth Muscle Contraction
2.5. PVE Administration in In Vivo TP-Induced BPH Model
2.6. PVE Alleviates Histologic Changes in the Prostate in In Vivo TP-Induced BPH Model
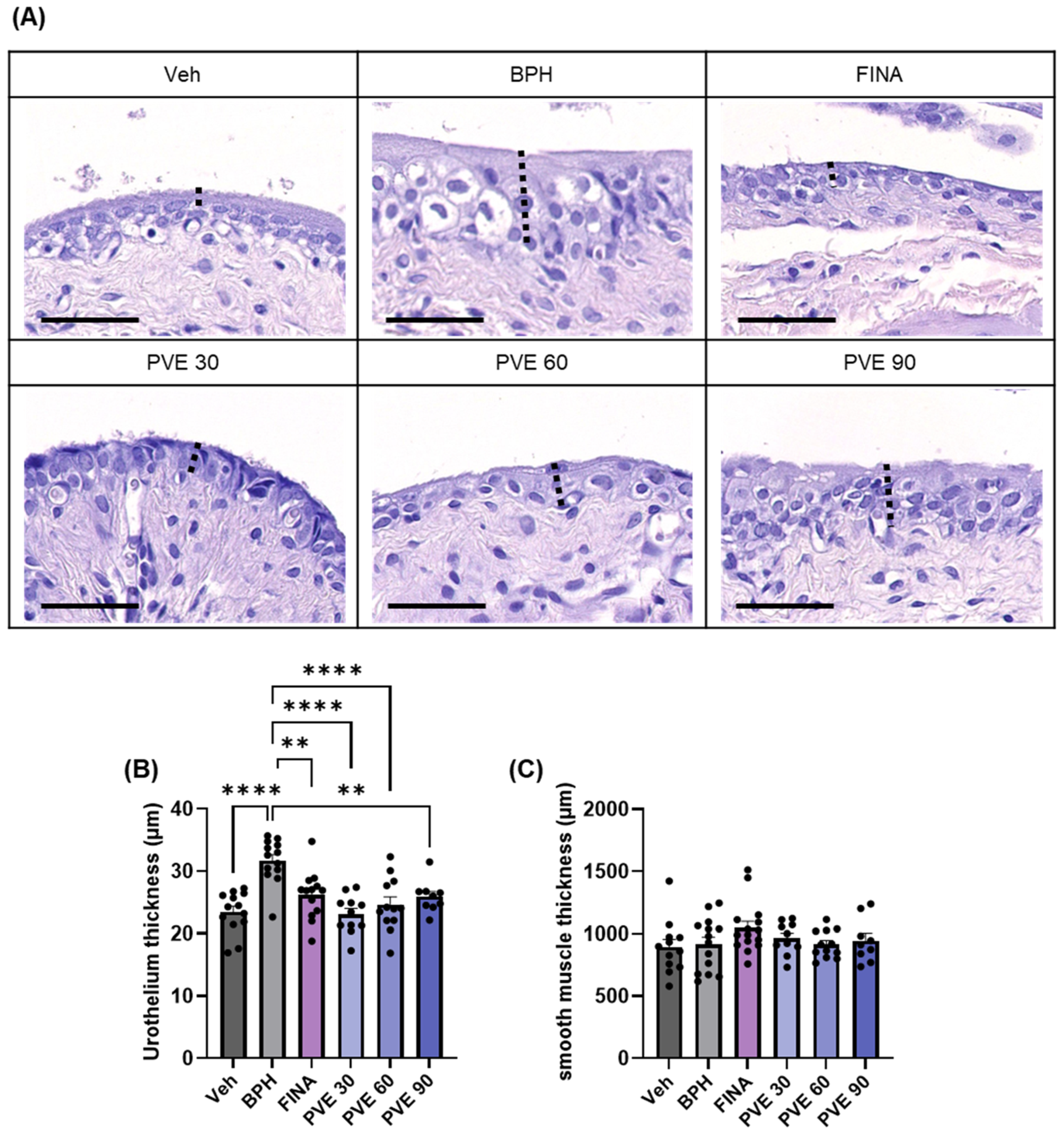
2.7. PVE Alleviates Bladder Dysfunction and Histologic Changes in In Vivo TP-Induced BPH Model
3. Discussion
4. Materials and Methods
4.1. Preparation of PV Extract
4.2. HPLC Analysis
4.3. Experimental Animals
4.4. Preparation of Rat Prostate Smooth Muscle Strips and Measurement of PE-Induced Contraction
4.5. BPH Rat Model and PVE Administration
4.6. Measurement of Intravesical Pressure
4.7. Histology
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BPH | benign prostate hyperplasia |
| LUTS | lower urinary tract symptoms |
| AR | androgen receptors |
| TP | testosterone propionate |
| HPLC | high-performance liquid chromatography |
| H&E | hematoxylin and eosin |
| α1-blockers | alpha1-adrenergic antagonists |
| DHT | dihydrotestosterone |
| PVE | Prunella vulgaris extract |
| UA | ursolic acid |
| PE | phenylephrine |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| L-NAME | Nω-nitro-L-arginine methyl ester hydrochloride |
| TEA | tetraethylammonium |
| TRPC | transient receptor potential cation |
References
- Berry, S.J.; Coffey, D.S.; Walsh, P.C.; Ewing, L.L. The development of human benign prostatic hyperplasia with age. J. Urol. 1984, 132, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Bosch, J.; Tilling, K.; Bohnen, A.; Bangma, C.; Donovan, J. Establishing normal reference ranges for prostate volume change with age in the population-based Krimpen-study: Prediction of future prostate volume in individual men. Prostate 2007, 67, 1816–1824. [Google Scholar] [CrossRef]
- McVary, K.T.; Roehrborn, C.G.; Avins, A.L.; Barry, M.J.; Bruskewitz, R.C.; Donnell, R.F.; Foster, H.E.; Gonzalez, C.M.; Kaplan, S.A.; Penson, D.F. Update on AUA guideline on the management of benign prostatic hyperplasia. J. Urol. 2011, 185, 1793–1803. [Google Scholar] [CrossRef]
- Bushman, W. Etiology, epidemiology, and natural history. Urol. Clin. 2009, 36, 403–415. [Google Scholar] [CrossRef] [PubMed]
- Fusco, F.; Creta, M.; De Nunzio, C.; Iacovelli, V.; Mangiapia, F.; Li Marzi, V.; Finazzi Agrò, E. Progressive bladder remodeling due to bladder outlet obstruction: A systematic review of morphological and molecular evidences in humans. BMC Urol. 2018, 18. [Google Scholar] [CrossRef]
- Lepor, H. Pathophysiology of lower urinary tract symptoms in the aging male population. Rev. Urol. 2005, 7, S3–S11. [Google Scholar]
- Roehrborn, C. Pathology of benign prostatic hyperplasia. Int. J. Impot. Res. 2008, 20, S11–S18. [Google Scholar] [CrossRef] [PubMed]
- McConnell, J.D. The pathophysiology of benign prostatic hyperplasia. J. Androl. 1991, 12, 356–363. [Google Scholar] [CrossRef]
- Foo, K.T. Pathophysiology of clinical benign prostatic hyperplasia. Asian J. Urol. 2017, 4, 152–157. [Google Scholar] [CrossRef]
- Lepor, H. Pathophysiology of benign prostatic hyperplasia in the aging male population. Rev. Urol. 2005, 7, S3–S12. [Google Scholar]
- Yu, Z.J.; Yan, H.L.; Xu, F.H.; Chao, H.C.; Deng, L.H.; Xu, X.D.; Huang, J.B.; Zeng, T. Efficacy and Side Effects of Drugs Commonly Used for the Treatment of Lower Urinary Tract Symptoms Associated With Benign Prostatic Hyperplasia. Front. Pharmacol. 2020, 11, 658. [Google Scholar] [CrossRef] [PubMed]
- Bortnick, E.M.; Simma-Chiang, V.; Kaplan, S.A. Long-term Consequences of Medical Therapy for Benign Prostatic Hyperplasia. Rev. Urol. 2019, 21, 154–157. [Google Scholar]
- Berger, A.P.; Horninger, W.; Bektic, J.; Pelzer, A.; Spranger, R.; Bartsch, G.; Frauscher, F. Vascular resistance in the prostate evaluated by colour Doppler ultrasonography: Is benign prostatic hyperplasia a vascular disease? BJU Int. 2006, 98, 587–590. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, V.; Gauhar, V.; Modi, S.; Somani, B.K. Role of Phytotherapy in the Management of BPH: A Summary of the Literature. J. Clin. Med. 2023, 12, 1899. [Google Scholar] [CrossRef]
- Rasool, R.; Ganai, B.A. Prunella vulgaris L.: A literature review on its therapeutic potentials. Pharmacologia 2013, 4, 441–448. [Google Scholar] [CrossRef][Green Version]
- Pan, J.; Wang, H.; Chen, Y. Prunella vulgaris L.—A Review of its Ethnopharmacology, Phytochemistry, Quality Control and Pharmacological Effects. Front. Pharmacol. 2022, 13, 903171. [Google Scholar] [CrossRef]
- Psotová, J.; Kolář, M.; Soušek, J.; Švagera, Z.; Vičar, J.; Ulrichová, J. Biological activities of Prunella vulgaris extract. Phytother. Res. Int. J. Devoted Pharmacol. Toxicol. Eval. Nat. Prod. Deriv. 2003, 17, 1082–1087. [Google Scholar] [CrossRef]
- Fang, X.; Chang, R.C.-C.; Yuen, W.-H.; Zee, S.Y. Immune modulatory effects of Prunella vulgaris L. Int. J. Mol. Med. 2005, 15, 491–496. [Google Scholar] [CrossRef]
- Li, C.; Huang, Q.; Fu, X.; Yue, X.-J.; Liu, R.H.; You, L.-J. Characterization, antioxidant and immunomodulatory activities of polysaccharides from Prunella vulgaris Linn. Int. J. Biol. Macromol. 2015, 75, 298–305. [Google Scholar] [CrossRef]
- Wang, S.-J.; Wang, X.-H.; Dai, Y.-Y.; Ma, M.-H.; Rahman, K.; Nian, H.; Zhang, H. Prunella vulgaris: A comprehensive review of chemical constituents, pharmacological effects and clinical applications. Curr. Pharm. Des. 2019, 25, 359–369. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Mu, J.; Zhang, Y. Chemical constituents of Prunella vulgaris. J. Environ. Sci. 2013, 25, S161–S163. [Google Scholar] [CrossRef]
- Somova, L.; Nadar, A.; Rammanan, P.; Shode, F. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef]
- Tsai, S.J.; Yin, M.C. Antioxidative and anti-inflammatory protection of oleanolic acid and ursolic acid in PC12 cells. J. Food Sci. 2008, 73, H174–H178. [Google Scholar] [CrossRef]
- Feng, L.; Jia, X.; Zhu, M.-M.; Chen, Y.; Shi, F. Antioxidant activities of total phenols of Prunella vulgaris L. in vitro and in tumor-bearing mice. Molecules 2010, 15, 9145–9156. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Fan, M.; Wedamulla, N.E.; Tang, Y.; Kim, E.-K. Alleviatory effect of isoquercetin on benign prostatic hyperplasia via IGF-1/PI3K/Akt/mTOR pathway. Food Sci. Hum. Wellness 2024, 13, 1698–1710. [Google Scholar] [CrossRef]
- Ghorbanibirgani, A. Efficacy of quercetin in treatment of benign prostatic hyperplasia in a double-blind randomized clinical trial in Iran 2011. Contraception 2012, 85, 321. [Google Scholar] [CrossRef]
- Huang, N.; Hauck, C.; Yum, M.-Y.; Rizshsky, L.; Widrlechner, M.P.; McCoy, J.-A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef] [PubMed]
- Kumbukgahadeniya, P.; Baek, E.-B.; Hong, E.-J.; Song, J.-Y.; Kwak, Y.-G.; Jang, M.-R.; Ji, H.-S.; Kwun, H.-J. Prunella vulgaris Extract Ameliorates Testosterone-Induced Benign Prostatic Hyperplasia by Regulating Androgen Levels, Cell Proliferation, and Apoptosis. Pharmaceuticals 2024, 17, 1516. [Google Scholar] [CrossRef]
- Deng, J.; Li, L.; Yan, J.; Lin, L.-M.; Li, Y.-M.; Lin, Y.; Xia, B.-H. Essential oil from Prunella vulgaris L. as a valuable source of bioactive constituents: In vitro anti-bacterial, anti-viral, immunoregulatory, anti-inflammatory, and chemical profiles. S. Afr. J. Bot. 2022, 151, 614–627. [Google Scholar]
- Wang, Y.; Kunit, T.; Ciotkowska, A.; Rutz, B.; Schreiber, A.; Strittmatter, F.; Waidelich, R.; Liu, C.; Stief, C.; Gratzke, C. Inhibition of prostate smooth muscle contraction and prostate stromal cell growth by the inhibitors of R ac, NSC 23766 and EHT 1864. Br. J. Pharmacol. 2015, 172, 2905–2917. [Google Scholar] [CrossRef]
- Spek, A.; Li, B.; Rutz, B.; Ciotkowska, A.; Huang, R.; Liu, Y.; Wang, R.; Strittmatter, F.; Waidelich, R.; Stief, C.G. Purinergic smooth muscle contractions in the human prostate: Estimation of relevance and characterization of different agonists. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1113–1131. [Google Scholar] [CrossRef]
- Hennenberg, M.; Tamalunas, A.; Wang, Y.; Keller, P.; Schott, M.; Strittmatter, F.; Herlemann, A.; Yu, Q.; Rutz, B.; Ciotkowska, A. Inhibition of agonist-induced smooth muscle contraction by picotamide in the male human lower urinary tract outflow region. Eur. J. Pharmacol. 2017, 803, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hennenberg, M.; Schott, M.; Kan, A.; Keller, P.; Tamalunas, A.; Ciotkowska, A.; Rutz, B.; Wang, Y.; Strittmatter, F.; Herlemann, A. Inhibition of Adrenergic and Non-Adrenergic Smooth Muscle Contraction in the Human Prostate by the Phosphodiesterase 10-Selective Inhibitor TC-E 5005. Prostate 2016, 76, 1364–1374. [Google Scholar] [CrossRef]
- Calmasini, F.B.; Silva, F.H.; Alexandre, E.C.; Rodrigues, R.L.; Barbosa, A.P.L.; Ferrucci, D.L.; Carvalho, H.F.; Anhê, G.F.; Pupo, A.S.; Antunes, E. Implication of Rho-kinase and soluble guanylyl cyclase enzymes in prostate smooth muscle dysfunction in middle-aged rats. Neurourol. Urodyn. 2017, 36, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Han, D.H.; Chae, M.R.; Sung, H.H.; Kang, S.J.; Shin, J.; Lee, S.W. Molecular expression and functional features of Kv7 channels in human prostate smooth muscle. Andrology 2021, 9, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Thébault, S.; Zholos, A.; Enfissi, A.; Slomianny, C.; Dewailly, E.; Roudbaraki, M.; Parys, J.; Prevarskaya, N. Receptor-operated Ca2+ entry mediated by TRPC3/TRPC6 proteins in rat prostate smooth muscle (PS1) cell line. J. Cell. Physiol. 2005, 204, 320–328. [Google Scholar] [CrossRef]
- Asiedu, B.; Anang, Y.; Nyarko, A.; Doku, D.A.; Amoah, B.Y.; Santa, S.; Ngala, R.A.; Asare, G.A. The role of sex steroid hormones in benign prostatic hyperplasia. Aging Male 2017, 20, 17–22. [Google Scholar] [CrossRef]
- Carson III, C.; Rittmaster, R. The role of dihydrotestosterone in benign prostatic hyperplasia. Urology 2003, 61, 2–7. [Google Scholar] [CrossRef]
- Madersbacher, S.; Sampson, N.; Culig, Z. Pathophysiology of benign prostatic hyperplasia and benign prostatic enlargement: A mini-review. Gerontology 2019, 65, 458–464. [Google Scholar] [CrossRef]
- Chapple, C.R. Moving beyond BPH–A contemporary update on male LUTS. Asian J. Urol. 2019, 6, 208. [Google Scholar] [CrossRef]
- Novara, G.; Galfano, A.; Gardi, M.; Ficarra, V.; Boccon-Gibod, L.; Artibani, W. Critical review of guidelines for BPH diagnosis and treatment strategy. Eur. Urol. Suppl. 2006, 5, 418–429. [Google Scholar] [CrossRef]
- Salah Azab, S.; Elsheikh, M.G. The impact of the bladder wall thickness on the outcome of the medical treatment using alpha-blocker of BPH patients with LUTS. Aging Male 2015, 18, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hwang, E.; Lin, P.; Gao, W.; Ngo, H.T.; Yi, T.-H. Prunella vulgaris L. exerts a protective effect against extrinsic aging through NF-κB, MAPKs, AP-1, and TGF-β/Smad signaling pathways in UVB-aged normal human dermal fibroblasts. Rejuvenation Res. 2018, 21, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Dunton, C.L.; Purves, J.T.; Hughes, F.M.; Nagatomi, J. BOO induces fibrosis and EMT in urothelial cells which can be recapitulated in vitro through elevated storage and voiding pressure cycles. Int. Urol. Nephrol. 2021, 53, 2007–2018. [Google Scholar] [CrossRef]
- Subramanian, P.; Anandharamakrishnan, C. Extraction of bioactive compounds. In Industrial Application of Functional Foods, Ingredients and Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2023; pp. 45–87. [Google Scholar]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nirujan, B.R.; Kim, J.; Baek, E.-B.; Kim, K.; Jayathilake, N.J.; Kwak, Y.G.; Jang, M.R.; Ji, H.S.; Kwun, H.-J.; Lee, K.P. Ameliorative Effects of Prunella vulgaris on Lower Urinary Tract Symptoms Induced by Benign Prostatic Hyperplasia in SD Rats via Nitric Oxide and Potassium Channels. Pharmaceuticals 2025, 18, 400. https://doi.org/10.3390/ph18030400
Nirujan BR, Kim J, Baek E-B, Kim K, Jayathilake NJ, Kwak YG, Jang MR, Ji HS, Kwun H-J, Lee KP. Ameliorative Effects of Prunella vulgaris on Lower Urinary Tract Symptoms Induced by Benign Prostatic Hyperplasia in SD Rats via Nitric Oxide and Potassium Channels. Pharmaceuticals. 2025; 18(3):400. https://doi.org/10.3390/ph18030400
Chicago/Turabian StyleNirujan, Beno Ramesh, Jeongsook Kim, Eun-Bok Baek, Kyungmi Kim, Nishani Jayanika Jayathilake, Youn Gil Kwak, Mi Ran Jang, Hyo Seong Ji, Hyo-Jung Kwun, and Kyu Pil Lee. 2025. "Ameliorative Effects of Prunella vulgaris on Lower Urinary Tract Symptoms Induced by Benign Prostatic Hyperplasia in SD Rats via Nitric Oxide and Potassium Channels" Pharmaceuticals 18, no. 3: 400. https://doi.org/10.3390/ph18030400
APA StyleNirujan, B. R., Kim, J., Baek, E.-B., Kim, K., Jayathilake, N. J., Kwak, Y. G., Jang, M. R., Ji, H. S., Kwun, H.-J., & Lee, K. P. (2025). Ameliorative Effects of Prunella vulgaris on Lower Urinary Tract Symptoms Induced by Benign Prostatic Hyperplasia in SD Rats via Nitric Oxide and Potassium Channels. Pharmaceuticals, 18(3), 400. https://doi.org/10.3390/ph18030400





