Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments
Abstract
1. Introduction
2. Results and Discussion
2.1. GC/MS Analysis of the Neem Oil (Azadirachta indica)
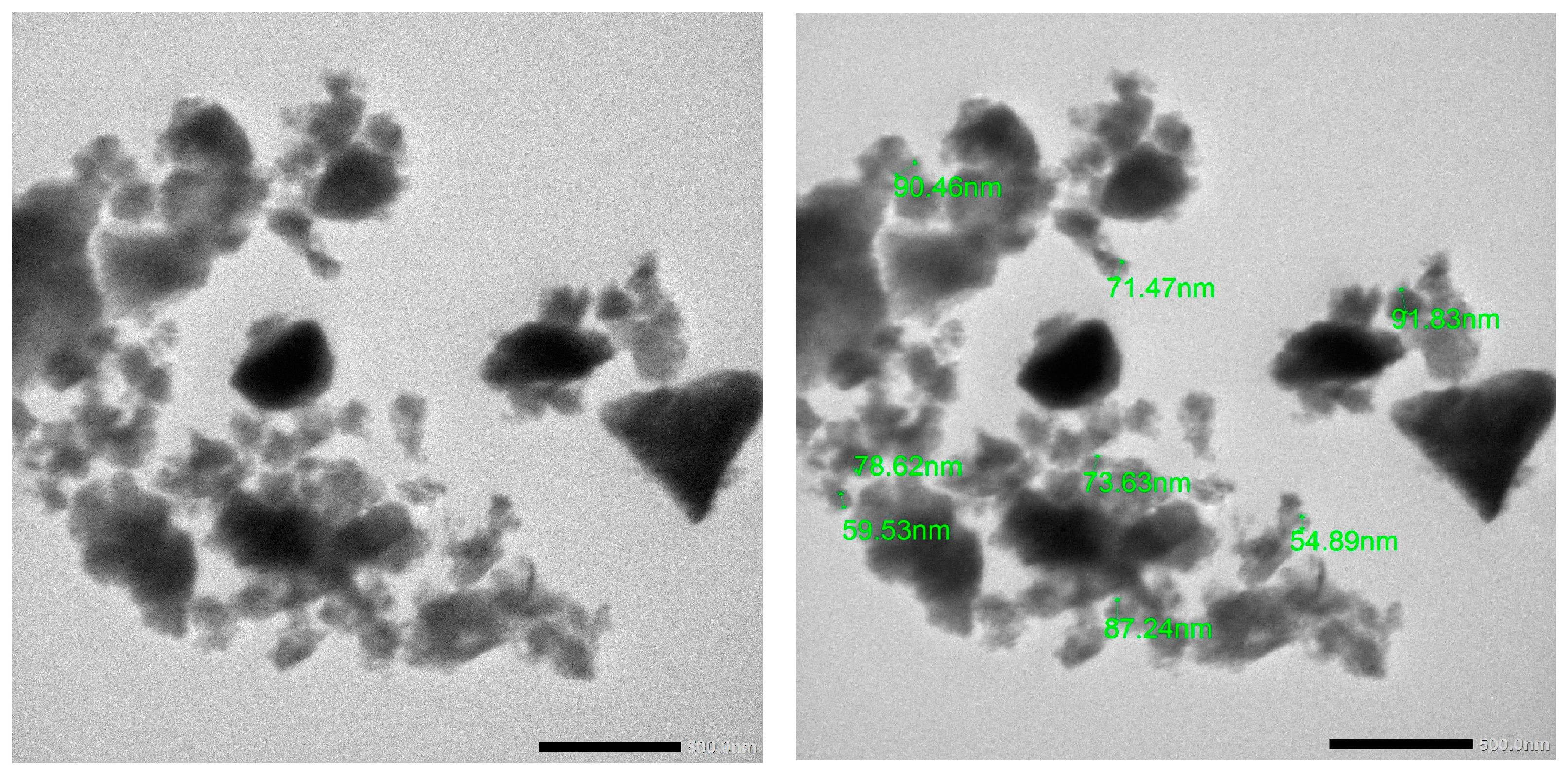
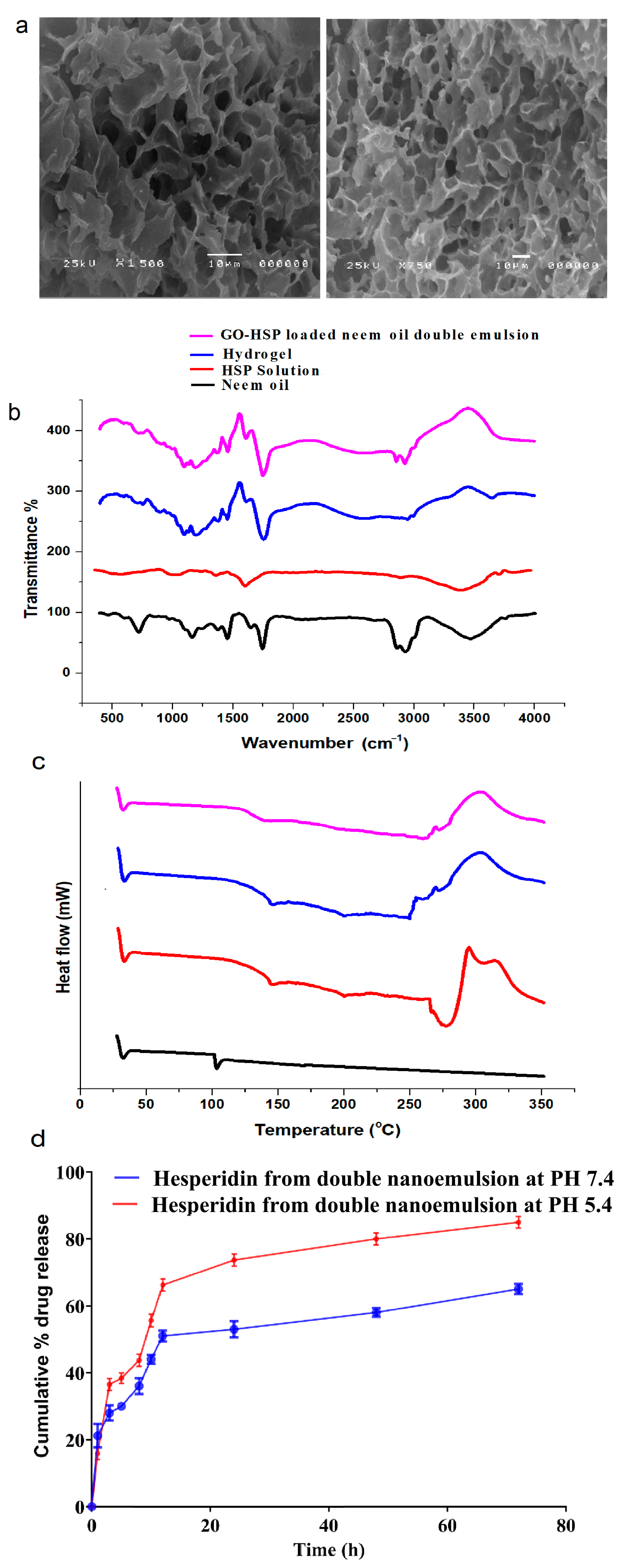
2.2. In Vitro Characterization of Nanoparticles
2.2.1. In Vitro Drug Release Study
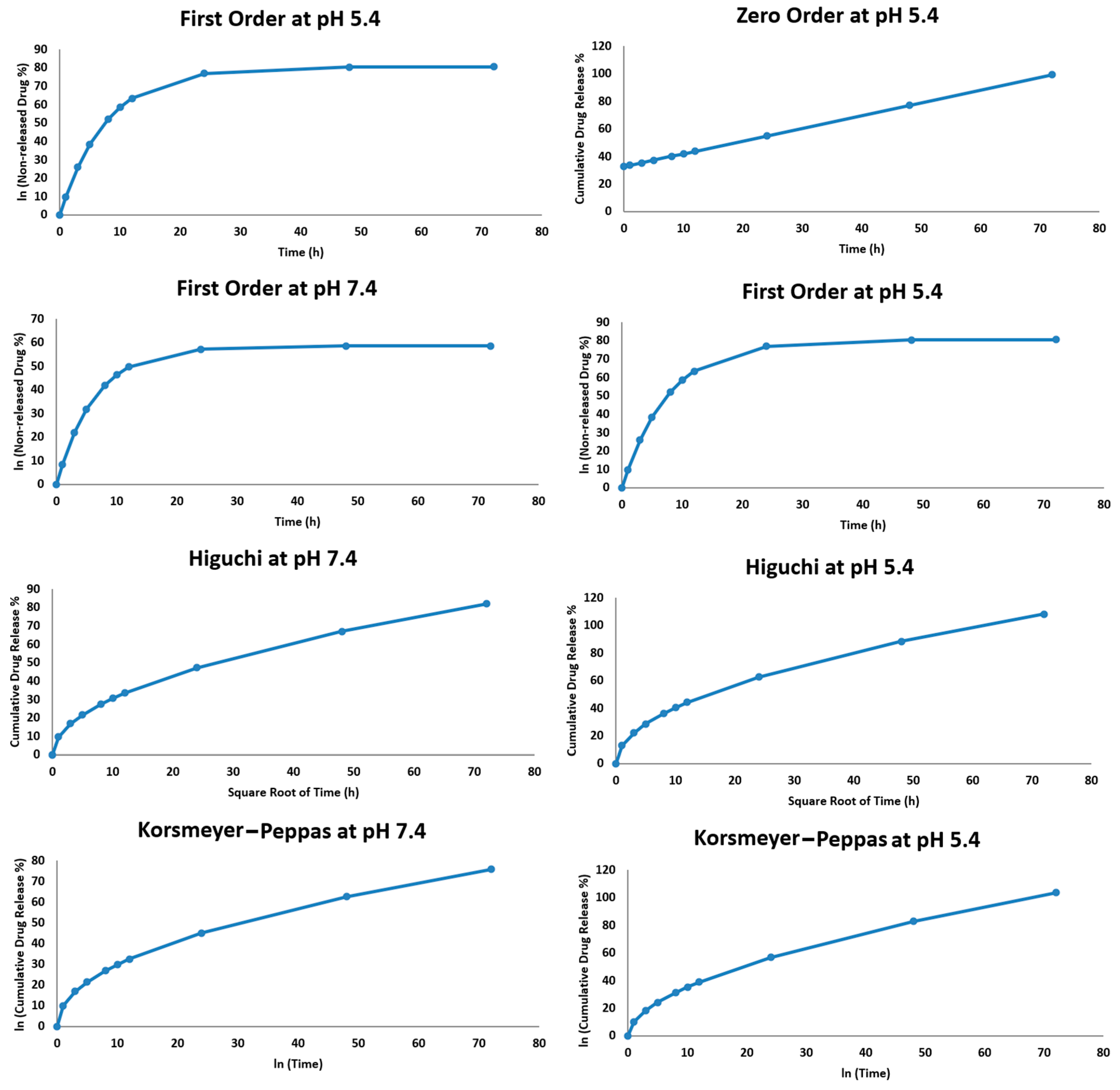
2.2.2. Hesperidin Release Kinetics
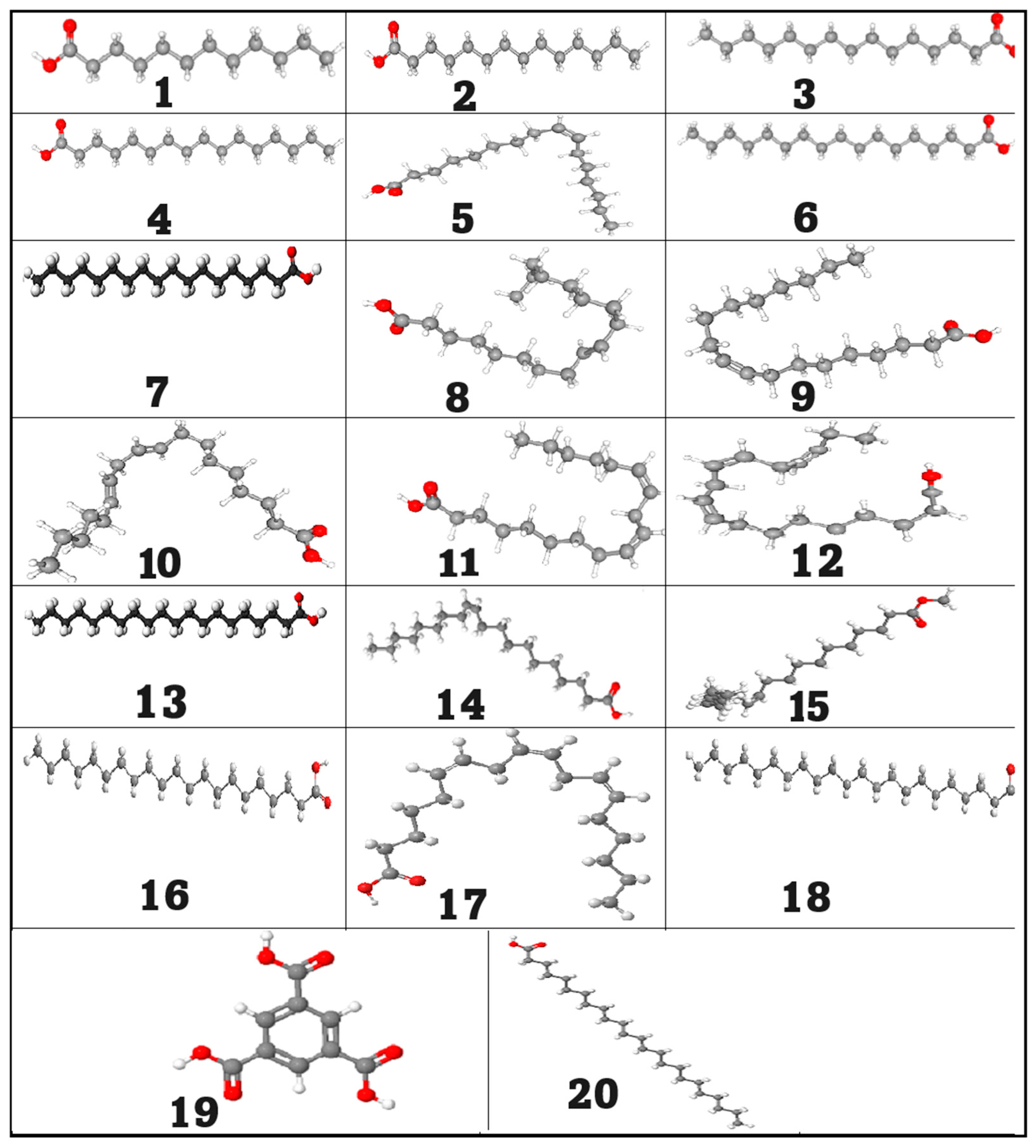
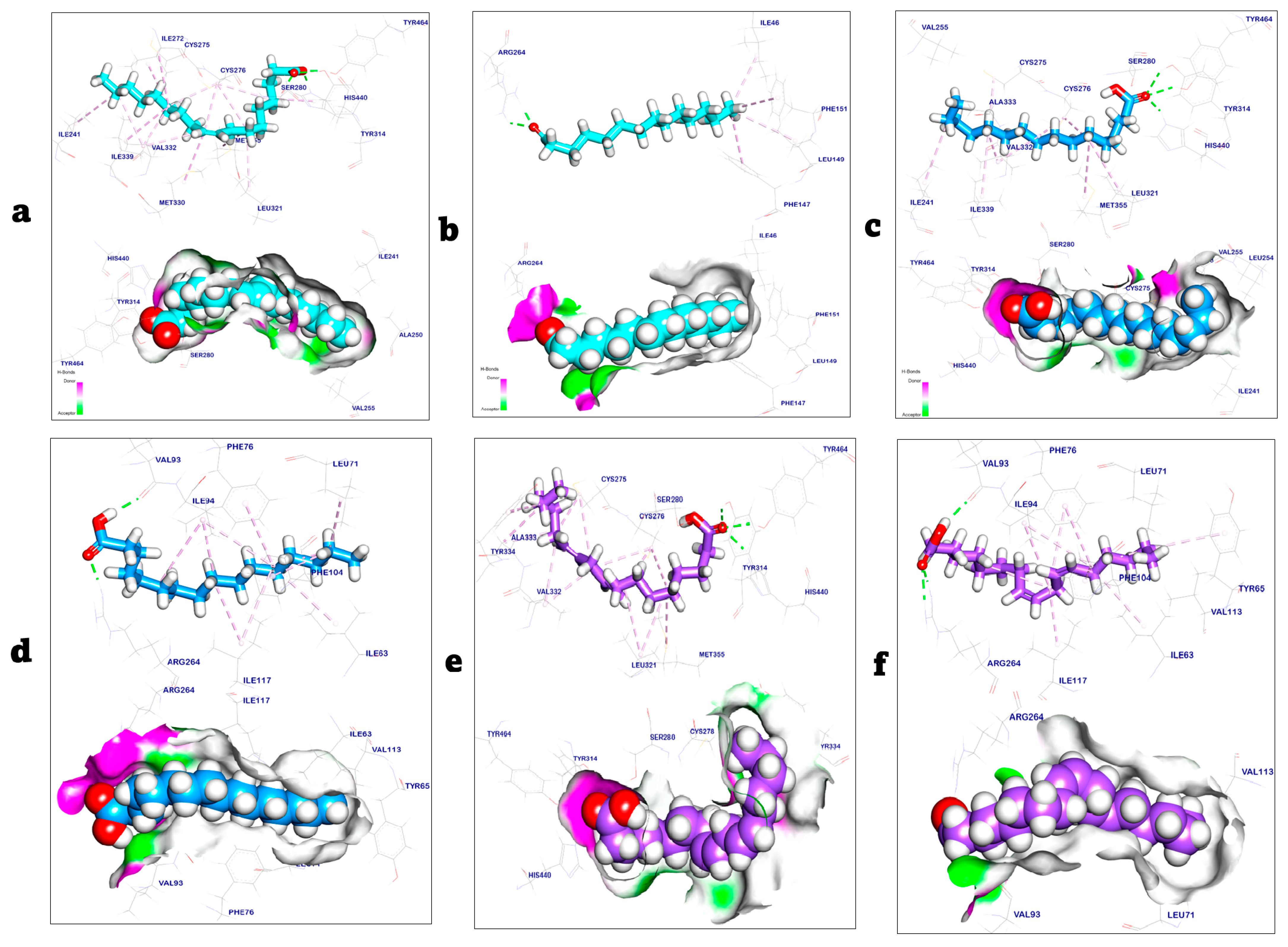
2.3. Molecular Docking Study
2.4. Antioxidant Activity
2.5. Antimicrobial Assay
2.6. Antibiofilm Activity
2.7. Antifungal Activity Against C. albicans
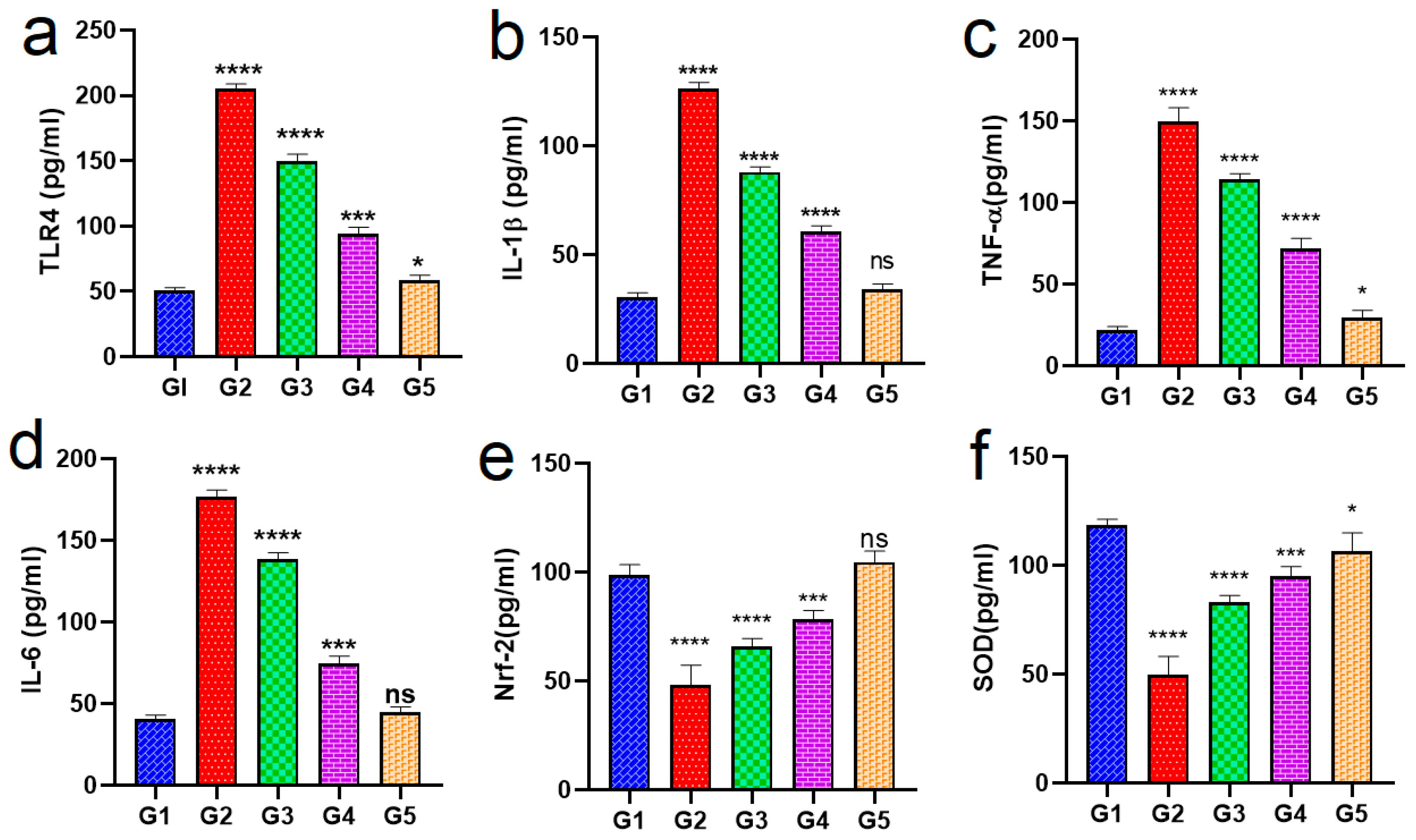
2.8. In Vivo Study
Biochemical Analysis
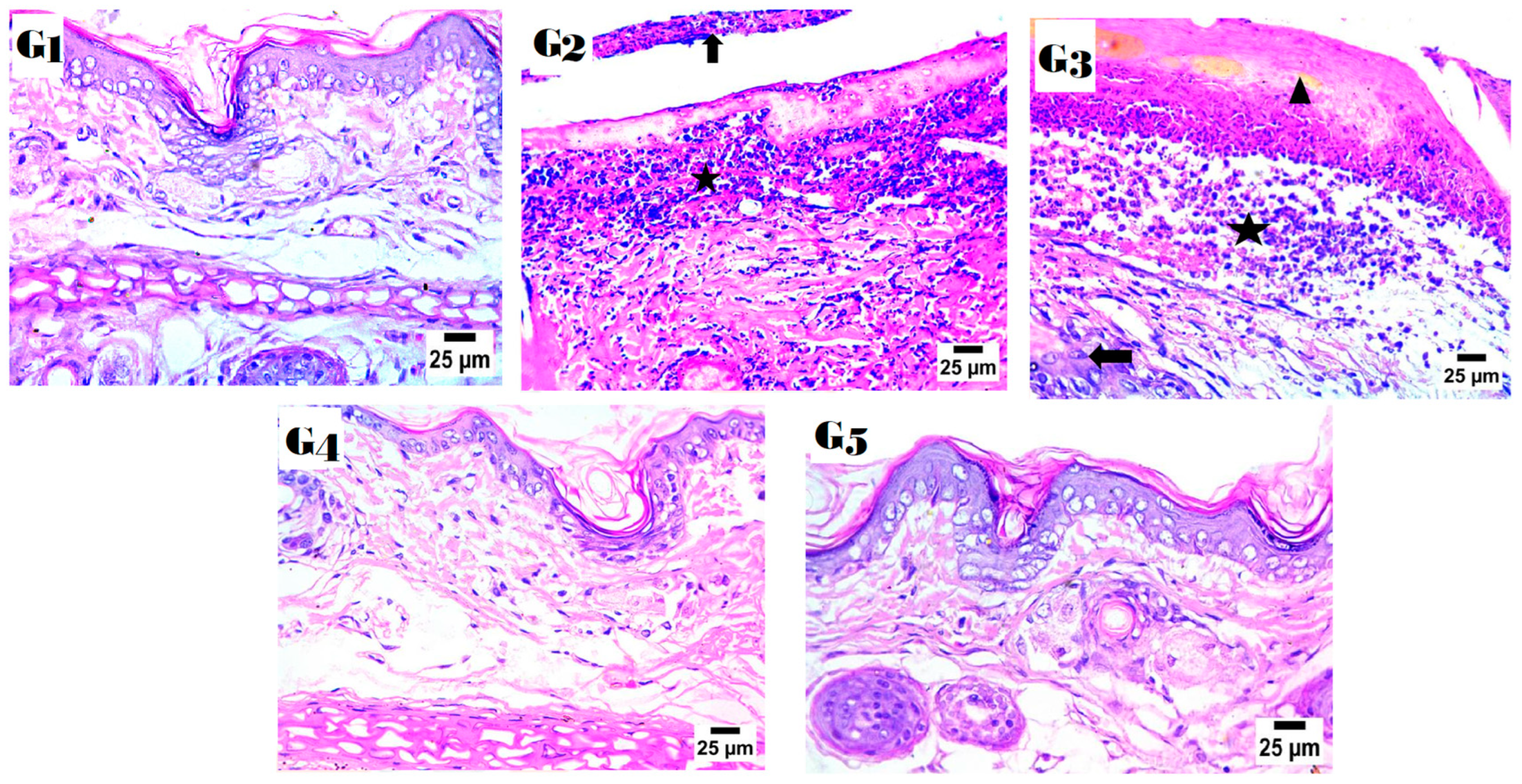
2.9. Histopathology Study
3. Materials and Methods
3.1. Materials and Animals
3.2. GC/MS Analysis of the Neem Oil (Azadirachta indica)
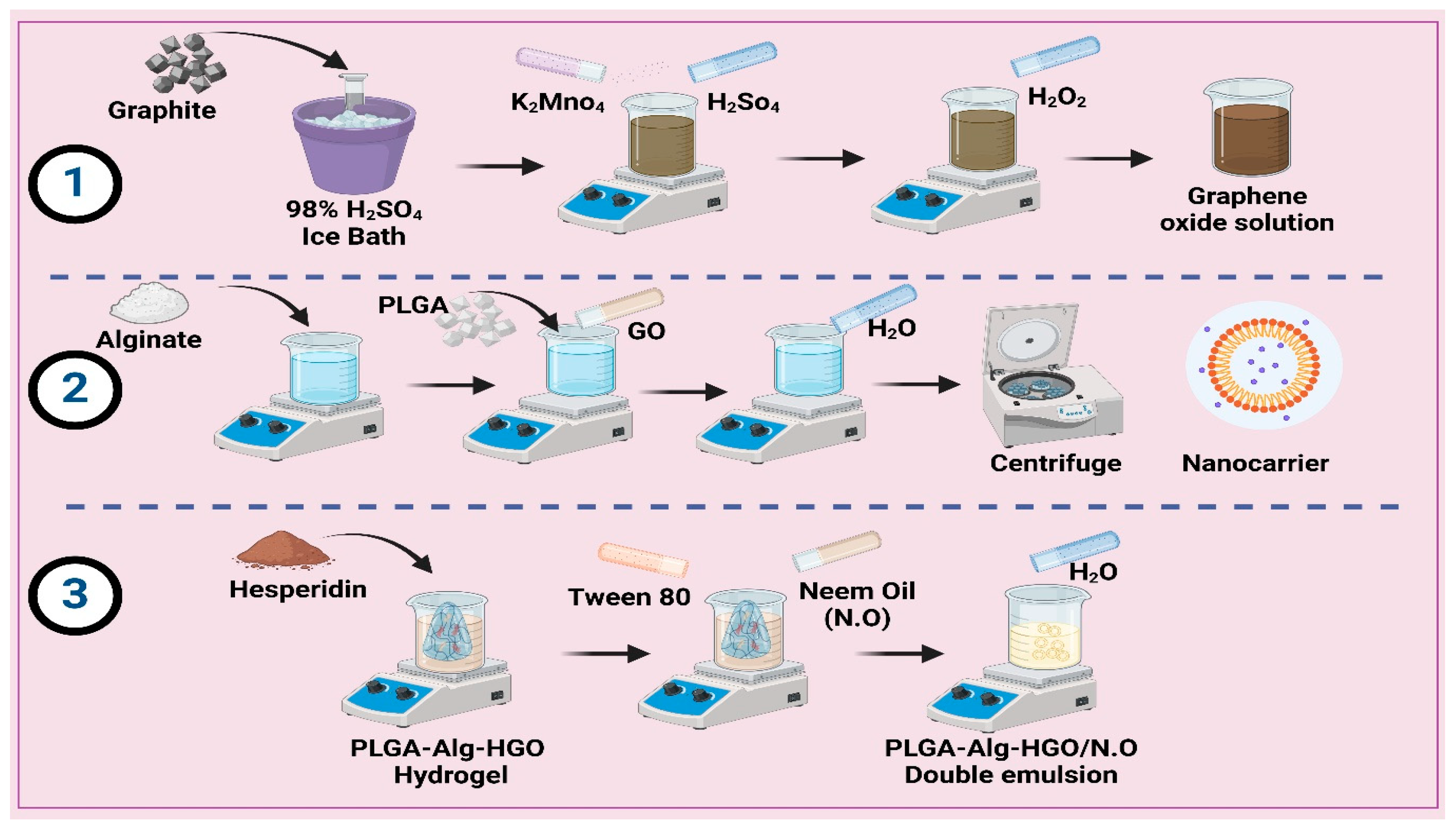
3.3. Preparation of Nanocarrier
3.3.1. Preparation of Graphene Oxide (GO)
3.3.2. Preparation of Polylactic-Co-Glycolic Acid/Alginate Hydrogel (PLGA-Alg-GO)
3.3.3. Preparation of the PLGA-Alg-HGO-Loaded Neem Oil Double Emulsion
3.4. In Vitro Characterization of Nanoparticles
3.5. In Vitro Drug Release Study
3.6. Hesperidin Release Kinetics
3.7. Molecular Docking Study of Neem Oil
3.8. Antioxidant Activity Assay
3.9. Cytotoxicity Study
3.10. Antimicrobial Assay
3.10.1. Antibacterial Assay and Sensitivity
3.10.2. Antibiofilm Assay and Crystal Violet Staining (CVS) Assay
3.11. In Vivo Study
3.11.1. Experimental Design
3.11.2. Biochemical Analysis and Serum Preparation
3.11.3. Histological Study
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| GO | Graphene Oxide |
| HSP | Hesperidin |
| PLGA | Polylactic-Co-Glycolic Acid |
| Alg | Alginate |
| PLGA-Alg | Polylactic-Co-Glycolic Acid/Alginate |
| N.O. | Neem Oil |
| DLS | Dynamic Light Scattering |
| FTIR | Fourier Transform Infrared Spectroscopy |
| TEM | Transmission Electron Microscopy |
| SEM | Scanning Electron Microscopy |
| OM | Otitis Media |
| BCS | Biopharmaceutics Classification System |
| MIC | Minimum Inhibitory Concentration |
| MBC | Minimum Bactericidal Concentration |
| CVS | Crystal Violet Staining Assay |
| LPS | Lipopolysaccharides |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| TNF-α | Tumour Necrosis Factor Alpha |
| IL-1β | Interleukin 1 Beta |
| IL-6 | Interleukin 6 |
| TLR4 | Toll-Like Receptor 4 |
| Nrf-2 | Nuclear Factor Erythroid 2-Related Factor 2 |
| SOD | Superoxide Dismutase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal Bovine Serum |
| ATCC | American Type Culture Collection |
| BHI | Brain–Heart Infusion Broth |
| CHX | Chlorhexidine |
| DMSO | Dimethyl Sulfoxide |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide |
| IP | Intraperitoneal |
| GC/MS | Gas Chromatography-Mass Spectrometry |
| HPLC | High-Performance Liquid Chromatography |
| Mw | Molecular Weight |
| MWCO | Molecular Weight Cut-Off |
| ANOVA | Analysis of Variance |
| PBS | Phosphate-Buffered Saline |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| TLA | Three-Letter Acronym |
| LD | Linear Dichroism |
References
- Schilder, A.G.M.; Chonmaitree, T.; Cripps, A.W.; Rosenfeld, R.M.; Casselbrant, M.L.; Haggard, M.P.; Venekamp, R.P. Otitis Media. Nat. Rev. Dis. Primers 2016, 2, 16063. [Google Scholar] [CrossRef] [PubMed]
- Jackson, E.A.; Geer, K. Acute Otitis Externa: Rapid Evidence Review. Am. Fam. Physician 2023, 107, 145–151. [Google Scholar]
- Wald, E.R. Management of Recurrent Acute Otitis Media. N. Engl. J. Med. 2021, 384, 1859–1860. [Google Scholar] [CrossRef] [PubMed]
- Rettig, E.; Tunkel, D.E. Contemporary Concepts in Management of Acute Otitis Media in Children. Otolaryngol. Clin. N. Am. 2014, 47, 651–672. [Google Scholar] [CrossRef] [PubMed]
- Steele, D.W.; Adam, G.P.; Di, M.; Halladay, C.H.; Balk, E.M.; Trikalinos, T.A. Effectiveness of Tympanostomy Tubes for Otitis Media: A Meta-Analysis. Pediatrics 2017, 139, e20170125. [Google Scholar] [CrossRef]
- Dedhia, K.; Tasian, G.E.; Forrest, C.B. Tympanostomy Tubes or Medical Management for Recurrent Acute Otitis Media. N. Engl. J. Med. 2021, 385, 860–861. [Google Scholar] [CrossRef]
- Pyrzynska, K. Hesperidin: A Review on Extraction Methods, Stability and Biological Activities. Nutrients 2022, 14, 2387. [Google Scholar] [CrossRef]
- Meiyanto, E.; Hermawan, A. Anindyajati Natural Products for Cancer-Targeted Therapy: Citrus Flavonoids as Potent Chemopreventive Agents. Asian Pac. J. Cancer Prev. 2012, 13, 427–436. [Google Scholar] [CrossRef]
- Ermiş, I.S.; Deveci, E.; Aşır, F. Effects of Quince Gel and Hesperidin Mixture on Experimental Endometriosis. Molecules 2023, 28, 5945. [Google Scholar] [CrossRef]
- Taymouri, S.; Hashemi, S.; Varshosaz, J.; Minaiyan, M.; Talebi, A. Fabrication and Evaluation of Hesperidin Loaded Polyacrylonitrile/Polyethylene Oxide Nanofibers for Wound Dressing Application. J. Biomater. Sci. Polym. Ed. 2021, 32, 1944–1965. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Yuan, L.; Chen, X.; Huang, H.; Cheng, F.; Shen, W. Hesperidin Inhibits Colon Cancer Progression by Downregulating SLC5A1 to Suppress EGFR Phosphorylation. J. Cancer 2025, 16, 876–887. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Al-Zuhairy, S.A.S.; El-Nabarawi, M.; Elrefai, M.F.M.; Shoela, M.S.; Hababeh, S.; Nelson, J.; Khalek, M.A.A.; Fady, M.; Elzohairy, N.A.; et al. Enhancing Photothermal Therapy for Antibiofilm Wound Healing: Insights from Graphene Oxide-Cranberry Nanosheet Loaded Hydrogel in Vitro, in Silico, and in Vivo Evaluation. Int. J. Nanomed. 2024, 19, 12999–13027. [Google Scholar] [CrossRef] [PubMed]
- Aryal, A.C.S.; Islam, M.S.; Rani, K.G.A.; Nassar, M.; Rahman, M.M. Hesperidin Improves Wound Healing and Mineralization of Periodontal Ligament Cells in Elevated Glucose Conditions. Curr. Med. Chem. 2025, 32. [Google Scholar] [CrossRef] [PubMed]
- Attia, G.H.; Marrez, D.A.; Mohammed, M.A.; Albarqi, H.A.; Ibrahim, A.M.; El Raey, M.A. Synergistic Effect of Mandarin Peels and Hesperidin with Sodium Nitrite against Some Food Pathogen Microbes. Molecules 2021, 26, 3186. [Google Scholar] [CrossRef]
- Elhabal, S.F.; Abdelaal, N.; Al-Zuhairy, S.A.S.; Mohamed Elrefai, M.F.; Khalifa, M.M.; Khasawneh, M.A.; Elsaid Hamdan, A.M.; Mohie, P.M.; Gad, R.A.; Kabil, S.L.; et al. Revolutionizing Psoriasis Topical Treatment: Enhanced Efficacy Through Ceramide/Phospholipid Composite Cerosomes Co-Delivery of Cyclosporine and Dithranol: In-Vitro, Ex-Vivo, and in-Vivo Studies. Int. J. Nanomed. 2024, 19, 1163–1187. [Google Scholar] [CrossRef]
- Özdemir, S.; Bostanabad, S.Y.; Parmaksız, A.; Canatan, H.C. Combination of St. John’s Wort Oil and Neem Oil in Pharmaceuticals: An Effective Treatment Option for Pressure Ulcers in Intensive Care Units. Medicina 2023, 59, 467. [Google Scholar] [CrossRef] [PubMed]
- Arena, R.; Strazzeri, M.G.; Bianchi, T.; Peghetti, A.; Merli, Y.; Abbenante, D.; Olivari, D. Hypericum and Neem Oil for Dehisced Post-Surgical Wounds: A Randomised, Controlled, Single-Blinded Phase III Study. J. Wound Care 2022, 31, 492–500. [Google Scholar] [CrossRef]
- Fadairo, A.A.; Wong, P.K.; Ip, W.F.; Ghadikolaei, M.A.; Cai, Z.; Ng, K.W.; Lian, Z.D. Impact of Neem Oil Biodiesel Blends on Physical and Chemical Properties of Particulate Matter Emitted from Diesel Engines. Environ. Pollut. 2024, 362, 124972. [Google Scholar] [CrossRef]
- Rasib, I.M.; Jalil, M.J.; Mubarak, N.M.; Azmi, I.S. Hybrid In-Situ and Ex-Situ Hydrolysis of Catalytic Epoxidation Neem Oil via a Peracid Mechanism. Sci. Rep. 2025, 15, 147. [Google Scholar] [CrossRef]
- Rodrigues, M.P.; Astoreca, A.L.; De Oliveira, Á.A.; Salvato, L.A.; Biscoto, G.L.; Keller, L.A.M.; Da Rocha Rosa, C.A.; Cavaglieri, L.R.; De Azevedo, M.I.; Keller, K.M. In Vitro Activity of Neem (Azadirachta Indica) Oil on Growth and Ochratoxin A Production by Aspergillus Carbonarius Isolates. Toxins 2019, 11, 579. [Google Scholar] [CrossRef]
- D’Amora, U.; Dacrory, S.; Hasanin, M.S.; Longo, A.; Soriente, A.; Kamel, S.; Raucci, M.G.; Ambrosio, L.; Scialla, S. Advances in the Physico-Chemical, Antimicrobial and Angiogenic Properties of Graphene-Oxide/Cellulose Nanocomposites for Wound Healing. Pharmaceutics 2023, 15, 338. [Google Scholar] [CrossRef]
- Di Giulio, M.; Zappacosta, R.; Di Lodovico, S.; Di Campli, E.; Siani, G.; Fontana, A.; Cellini, L. Antimicrobial and Antibiofilm Efficacy of Graphene Oxide against Chronic Wound Microorganisms. Antimicrob. Agents Chemother. 2018, 62, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Lemos da Silva, L.A.; de Athayde, A.E.; Moreira, M.A.; Tizziani, T.; Gkionis, S.V.; da Silva, L.V.; Biavatti, M.W.; de Moraes, A.C.R.; dos Santos Nascimento, M.V.P.; Dalmarco, E.M.; et al. Anti-Inflammatory and Anti-Aggregating Effects of Rangpur in the First Trimester of Growth: Ultra-Performance Liquid Chromatography–Electrospray Mass Spectrometry Profile and Quantification of Hesperidin. J. Sci. Food Agric. 2022, 102, 4151–4161. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mao, J.; Guo, Z.; Hu, Y.; Wang, S. Polyvinyl Alcohol/Carboxymethyl Chitosan Hydrogel Loaded with Silver Nanoparticles Exhibited Antibacterial and Self-Healing Properties. Int. J. Biol. Macromol. 2022, 220, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; El-Nabarawi, M.; Elrefai, M.F.M.; Teaima, M.H.; Shoela, M.S.; Khamis, G.M.; Faheem, A.M.; Kholeif, N.A.; Sanad, M.T. Nano-Spanlastics-Loaded Dissolving Microneedle Patches for Ketotifen Fumarate: Advanced Strategies for Allergic Conjunctivitis Treatment and Molecular Insights. Drug Deliv. Transl. Res. 2025, 1–24. [Google Scholar] [CrossRef]
- Mohammed, M.H.H.; Hamed, A.N.E.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S. Chemical Composition and Anti-Proliferative Activities of Hyophorbe Lagenicaulis Aerial Parts and Their Biogenic Nanoparticles Supported by Network Pharmacology Study. S. Afr. J. Bot. 2023, 156, 398–410. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Potential Application of Sucrose Acetate Isobutyrate, and Glyceryl Monooleate for Nanonization and Bioavailability Enhancement of Rivaroxaban Tablets. Pharm. Sci. Adv. 2024, 2, 100015. [Google Scholar] [CrossRef]
- El-Nawawy, T.M.; Adel, Y.A.; Teaima, M.; Nassar, N.N.; Nemr, A.; Al-Samadi, I.; El-Nabarawi, M.A.; Elhabal, S.F. Intranasal Bilosomes in Thermosensitive Hydrogel: Advancing Desvenlafaxine Succinate Delivery for Depression Management. Pharm. Dev. Technol. 2024, 29, 663–674. [Google Scholar] [CrossRef]
- Fathy Elhabal, S.; El-Nabarawi, M.A.; Abdelaal, N.; Elrefai, M.F.M.; Ghaffar, S.A.; Khalifa, M.M.; Mohie, P.M.; Waggas, D.S.; Hamdan, A.M.E.; Alshawwa, S.Z.; et al. Development of Canagliflozin Nanocrystals Sublingual Tablets in the Presence of Sodium Caprate Permeability Enhancer: Formulation Optimization, Characterization, in-Vitro, in Silico, and in-Vivo Study. Drug Deliv. 2023, 30, 2241665. [Google Scholar] [CrossRef]
- Mohammed, M.H.H.; Hamed, A.N.E.; Elhabal, S.F.; Mokhtar, F.A.; Abdelmohsen, U.R.; Fouad, M.A.; Kamel, M.S. Metabolic Profiling and Cytotoxic Activities of Ethanol Extract of Dypsis Leptocheilos Aerial Parts and Its Green Synthesized Silver Nanoparticles Supported by Network Pharmacology Analysis. S. Afr. J. Bot. 2023, 161, 648–665. [Google Scholar] [CrossRef]
- Shoman, N.A.; Saady, M.; Teaima, M.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Merging Konjac Glucomannan with Other Copolymeric Hydrogels as a Cutting-Edge Liquid Raft System for Dual Delivery of Etoricoxib and Famotidine. Drug Deliv. 2023, 30, 2189630. [Google Scholar] [CrossRef] [PubMed]
- Elhabal, S.F.; Abdelmonem, R.; El Nashar, R.M.; Elrefai, M.F.M.; Hamdan, A.M.E.; Safwat, N.A.; Shoela, M.S.; Hassan, F.E.; Rizk, A.; Kabil, S.L.; et al. Enhanced Antibacterial Activity of Clindamycin Using Molecularly Imprinted Polymer Nanoparticles Loaded with Polyurethane Nanofibrous Scaffolds for the Treatment of Acne Vulgaris. Pharmaceutics 2024, 16, 947. [Google Scholar] [CrossRef]
- Yao, C.; Liu, Z.; Yang, C.; Wang, W.; Ju, X.-J.; Xie, R.; Chu, L.-Y. Smart Hydrogels with Inhomogeneous Structures Assembled Using Nanoclay-Cross-Linked Hydrogel Subunits as Building Blocks. ACS Appl. Mater. Interfaces 2016, 8, 21721–21730. [Google Scholar] [CrossRef]
- Al-Shoubki, A.A.; Teaima, M.H.; Abdelmonem, R.; El-Nabarawi, M.A.; Elhabal, S.F. Sucrose Acetate Isobutyrate (SAIB) and Glyceryl Monooleate (GMO) Hybrid Nanoparticles for Bioavailability Enhancement of Rivaroxaban: An Optimization Study. Pharm. Dev. Technol. 2023, 28, 928–938. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi-Ghadi, Z.; Ebrahimnejad, P.; Talebpour Amiri, F.; Nokhodchi, A. Improved Oral Delivery of Quercetin with Hyaluronic Acid Containing Niosomes as a Promising Formulation. J. Drug Target. 2021, 29, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.C.; Paiva-Santos, A.C.; Veiga, F. Liposome-Derived Nanosystems for the Treatment of Behavioral and Neurodegenerative Diseases: The Promise of Niosomes, Transfersomes, and Ethosomes for Increased Brain Drug Bioavailability. Pharmaceuticals 2023, 16, 1424. [Google Scholar] [CrossRef]
- Tong, G.F.; Qin, N.; Sun, L.W. Development and Evaluation of Desvenlafaxine Loaded PLGA-Chitosan Nanoparticles for Brain Delivery. Saudi Pharm. J. 2017, 25, 844–851. [Google Scholar] [CrossRef]
- Sultanova, Z.; Kaleli, G.; Kabay, G.; Mutlu, M. Controlled Release of a Hydrophilic Drug from Coaxially Electrospun Polycaprolactone Nanofibers. Int. J. Pharm. 2016, 505, 133–138. [Google Scholar] [CrossRef]
- Samy, W.; Elnoby, A.; El-Gowelli, H.M.; Elgindy, N. Hybrid Polymeric Matrices for Oral Modified Release of Desvenlafaxine Succinate Tablets. Saudi Pharm. J. 2017, 25, 676–687. [Google Scholar] [CrossRef]
- Fernandes, R.S.; Arribada, R.G.; Silva, J.O.; Silva-Cunha, A.; Townsend, D.M.; Ferreira, L.A.M.; Barros, A.L.B. In Vitro and In Vivo Effect of PH-Sensitive PLGA-TPGS-Based Hybrid Nanoparticles Loaded with Doxorubicin for Breast Cancer Therapy. Pharmaceutics 2022, 14, 2394. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, Y.; Xu, W.; Xiao, S.; Du, S.; Zhou, Y.; Hasan, A. Rheological and Controlled Release Properties of Hydrogels Based on Mushroom Hyperbranched Polysaccharide and Xanthan Gum. Int. J. Biol. Macromol. 2018, 120, 2399–2409. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Branco, F.; Vitorino, R.; Sousa, J.; Pais, A.; Vitorino, C. A Two-Pronged Approach against Glioblastoma: Drug Repurposing and Nanoformulation Design for in Situ-Controlled Release. Drug Deliv. Transl. Res. 2023, 13, 3169–3191. [Google Scholar] [CrossRef]
- Taghe, S.; Mirzaeei, S.; Bagheri, M. Preparation of Polycaprolactone and Polymethacrylate Nanofibers for Controlled Ocular Delivery of Ketorolac Tromethamine: Pharmacokinetic Study in Rabbit’s Eye. Eur. J. Pharm. Sci. 2024, 192, 106631. [Google Scholar] [CrossRef] [PubMed]
- Hernández-ochoa, B.; Navarrete-vázquez, G.; Aguayo-ortiz, R.; Ortiz-ramírez, P.; Morales-luna, L.; Martínez-rosas, V.; González-valdez, A.; Gómez-chávez, F.; Enríquez-flores, S.; Wong-baeza, C.; et al. Identification and in Silico Characterization of Novel Helicobacter Pylori Glucose-6-phosphate Dehydrogenase Inhibitors. Molecules 2021, 26, 4955. [Google Scholar] [CrossRef] [PubMed]
- Mahdizadeh, S.J.; Stier, M.; Carlesso, A.; Lamy, A.; Thomas, M.; Eriksson, L.A. Multiscale In Silico Study of the Mechanism of Activation of the RtcB Ligase by the PTP1B Phosphatase. J. Chem. Inf. Model. 2023, 64, 905–917. [Google Scholar] [CrossRef]
- Abdelfattah, D.S.E.; Fouad, M.A.; Elmeshad, A.N.; El-Nabarawi, M.A.; Elhabal, S.F. Anti-Obesity Effect of Combining White Kidney Bean Extract, Propolis Ethanolic Extract and CrPi3 on Sprague-Dawley Rats Fed a High-Fat Diet. Nutrients 2024, 16, 310. [Google Scholar] [CrossRef]
- Choi, S.-S.; Lee, S.-H.; Lee, K.-A. A Comparative Study of Hesperetin, Hesperidin and Hesperidin Glucoside: Antioxidant, Anti-Inflammatory, and Antibacterial Activities In Vitro. Antioxidants 2022, 11, 1618. [Google Scholar] [CrossRef]
- Jeong, S.J.; Lee, J.S.; Lee, H.G. Nanoencapsulation of Synergistic Antioxidant Fruit and Vegetable Concentrates and Their Stability during in Vitro Digestion. J. Sci. Food Agric. 2020, 100, 1056–1063. [Google Scholar] [CrossRef]
- Kondo, S.; Nishimura, T.; Nishina, Y.; Sano, K. Countercation Engineering of Graphene-Oxide Nanosheets for Imparting a Thermoresponsive Ability. ACS Appl. Mater. Interfaces 2023, 15, 37837–37844. [Google Scholar] [CrossRef]
- Pei, S.; Wei, Q.; Huang, K.; Cheng, H.M.; Ren, W. Green Synthesis of Graphene Oxide by Seconds Timescale Water Electrolytic Oxidation. Nat. Commun. 2018, 9, 145. [Google Scholar] [CrossRef]
- Kaur, B.; Sekhar, V.; Sharma, P.; Malhotra, S.; Jain, A. A Comparative Assessment of Antibacterial Properties of Neem Oil Coated Sutures: An in Vitro Study. J. Indian Soc. Periodontol. 2023, 27, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Won, J.; Monroy, G.L.; Khampang, P.; Barkalifa, R.; Hong, W.; Chaney, E.J.; Aksamitiene, E.; Porter, R.G.; Novak, M.A.; Spillman, D.R.; et al. In Vivo Optical Characterization of Middle Ear Effusions and Biofilms During Otitis Media. JARO J. Assoc. Res. Otolaryngol. 2023, 24, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, Z.; Ghasemi, S.M.; Ahmadi, Y.; Shokri, D. Isolation and Identification of Effective Probiotics on Drug-Resistant Acinetobacter Baumannii Strains and Their Biofilms. Can. J. Infect. Dis. Med. Microbiol. 2024, 2024, 8570521. [Google Scholar] [CrossRef]
- Verma, S.; Bhardwaj, A.; Vij, M.; Bajpai, P.; Goutam, N.; Kumar, L. Oleic Acid Vesicles: A New Approach for Topical Delivery of Antifungal Agent. Artif. Cells Nanomed. Biotechnol. 2014, 42, 95–101. [Google Scholar] [CrossRef]
- Cheng, Z.; Kandekar, U.; Ma, X.; Bhabad, V.; Pandit, A.; Liu, L.; Luo, J.; Munot, N.; Chorage, T.; Patil, A.; et al. Optimizing Fluconazole-Embedded Transfersomal Gel for Enhanced Antifungal Activity and Compatibility Studies. Front. Pharmacol. 2024, 15, 1353791. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Peng, F.; Li, C.; Wang, Q.; Wang, Z.; Hu, L.; Peng, X.; Zhao, G.; Lin, J. Macrophage Membrane-Coated Nanoparticles for the Delivery of Natamycin Exhibit Increased Antifungal and Anti-Inflammatory Activities in Fungal Keratitis. ACS Appl. Mater. Interfaces 2024, 16, 59777–59788. [Google Scholar] [CrossRef]
- Abd-Eldayem, A.M.; Makram, S.M.; Messiha, B.A.S.; Abd-Elhafeez, H.H.; Abdel-Reheim, M.A. Cyclosporine-Induced Kidney Damage Was Halted by Sitagliptin and Hesperidin via Increasing Nrf2 and Suppressing TNF-α, NF-ΚB, and Bax. Sci. Rep. 2024, 14, 7434. [Google Scholar] [CrossRef]
- Ewedah, T.M.; Abdalla, A.; Hagag, R.S.; Elhabal, S.F.; Teaima, M.H.; El-Nabarawi, M.A.; Schlatter, G.; Shoueir, K.R. Enhancing Cellular Affinity for Skin Disorders: Electrospun Polyurethane/Collagen Nanofiber Mats Coated with Phytoceramides. Int. J. Pharm. 2024, 663, 124541. [Google Scholar] [CrossRef]
- Mahfud, M.A.S.; Fitri, A.M.N.; Elim, D.; Sultan, N.A.F.; Saputra, M.D.; Afika, N.; Friandini, R.A.; Himawan, A.; Rahman, L.; Permana, A.D. Combination of Synthetic and Natural Polymers on the Characteristics and Evaluation of Transdermal Hydrogel-Forming Microneedles Preparations Integrated with Direct Compressed Tablets Reservoir Sildenafil Citrate. J. Drug Deliv. Sci. Technol. 2023, 85, 104611. [Google Scholar] [CrossRef]
- Chaudhary, J.P.; Kholiya, F.; Vadodariya, N.; Budheliya, V.M.; Gogda, A.; Meena, R. Carboxymethylagarose-Based Multifunctional Hydrogel with Super Stretchable, Self-Healable Having Film and Fiber Forming Properties. Arab. J. Chem. 2020, 13, 1661–1668. [Google Scholar] [CrossRef]
- Al-Suwayeh, S.A.; Badran, M.M.; Alhumoud, G.O.; Taha, E.I.; Ashri, L.Y.; Kazi, M. Design and Dermatokinetic Appraisal of Lornoxicam-Loaded Ultrafine Self-Nanoemulsion Hydrogel for the Management of Inflammation: In Vitro and in Vivo Studies. Saudi Pharm. J. 2023, 31, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Ben David-Naim, M.; Grad, E.; Aizik, G.; Nordling-David, M.M.; Moshel, O.; Granot, Z.; Golomb, G. Polymeric Nanoparticles of SiRNA Prepared by a Double-Emulsion Solvent-Diffusion Technique: Physicochemical Properties, Toxicity, Biodistribution and Efficacy in a Mammary Carcinoma Mice Model. Biomaterials 2017, 145, 154–167. [Google Scholar] [CrossRef]
- Gönüllü, Ü.; Üner, M.; Yener, G.; Karaman, E.F.; Aydoʇmuş, Z. Formulation and Characterization of Solid Lipid Nanoparticles, Nanostructured Lipid Carriers and Nanoemulsion of Lornoxicam for Transdermal Delivery. Acta Pharm. 2015, 65, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Fioravanti, G.; Galante, A.; Fattibene, P.; Torrieri Di Tullio, L.; Colacicchi, S.; De Thomasis, G.; Perrozzi, F.; De Berardinis, N.; Profeta, G.; Ottaviano, L.; et al. Disentangling the Intrinsic Relaxivities of Highly Purified Graphene Oxide. Nanotechnology 2024, 35, 245101. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Ding, Z.; Zhang, H.; Xia, Y.; Wang, M.; Hu, X.; Xu, X.; Yongzhen, Z. Design and Tribological Performance of Graphene Nanofluids Dispersed by Dragging Effect of Bicomponent Gelator. Nanotechnology 2024, 35, 265709. [Google Scholar] [CrossRef]
- Pal, N.; Banerjee, S.; Roy, P.; Pal, K. Cellulose Nanocrystals-silver Nanoparticles-Reduced Graphene Oxide Based Hybrid PVA Nanocomposites and Its Antimicrobial Properties. Int. J. Biol. Macromol. 2021, 191, 445–456. [Google Scholar] [CrossRef]
- Maulvi, F.A.; Mangukiya, M.A.; Patel, P.A.; Vaidya, R.J.; Koli, A.R.; Ranch, K.M.; Shah, D.O. Extended Release of Ketotifen from Silica Shell Nanoparticle-Laden Hydrogel Contact Lenses: In Vitro and In Vivo Evaluation. J. Mater. Sci. Mater. Med. 2016, 27, 113. [Google Scholar] [CrossRef]
- Qu, J.; Zhang, L.; Chen, Z.; Mao, G.; Gao, Z.; Lai, X.; Zhu, X.; Zhu, J. Nanostructured Lipid Carriers, Solid Lipid Nanoparticles, and Polymeric Nanoparticles: Which Kind of Drug Delivery System Is Better for Glioblastoma Chemotherapy? Drug Deliv. 2016, 23, 3408–3416. [Google Scholar] [CrossRef]
- Yurtdaş Kirimlioğlu, G.; Öztürk, A.A. Levocetirizine Dihydrochloride-Loaded Chitosan Nanoparticles: Formulation and In Vitro Evaluation. Turk. J. Pharm. Sci. 2020, 17, 27–35. [Google Scholar] [CrossRef]
- Brown, P.N.; Turi, C.E.; Shipley, P.R.; Murch, S.J. Comparisons of Large (Vaccinium macrocarpon Ait.) and Small (Vaccinium oxycoccos L., Vaccinium vitis-idaea L.) Cranberry in British Columbia by Phytochemical Determination, Antioxidant Potential, and Metabolomic Profiling with Chemometric Analysis. Planta Med. 2012, 78, 630–640. [Google Scholar] [CrossRef]
- Zaid Alkilani, A.; Abo-Zour, H.; Basheer, H.A.; Abu-Zour, H.; Donnelly, R.F. Development and Evaluation of an Innovative Approach Using Niosomes Based Polymeric Microneedles to Deliver Dual Antioxidant Drugs. Polymers 2023, 15, 1962. [Google Scholar] [CrossRef]
- Kumar, D.; Ladaniya, M.S.; Gurjar, M.; Kumar, S.; Mendke, S. Quantification of Flavonoids, Phenols and Antioxidant Potential from Dropped Citrus Reticulata Blanco Fruits Influenced by Drying Techniques. Molecules 2021, 26, 4159. [Google Scholar] [CrossRef] [PubMed]
- ELhabal, S.F.; El-Nabarawi, M.A.; Hassanin, S.O.; Hassan, F.E.; Abbas, S.S.; Gebril, S.M.; Albash, R. Transdermal Fluocinolone Acetonide Loaded Decorated Hyalurosomes Cellulose Acetate/Polycaprolactone Nanofibers Mitigated Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats. J. Pharm. Investig. 2025, 55, 113–132. [Google Scholar] [CrossRef]
- Hasannejad-Bibalan, M.; Mojtahedi, A.; Biglari, H.; Halaji, M.; Sedigh Ebrahim-Saraie, H. Antibacterial Activity of Tedizolid, a Novel Oxazolidinone against Methicillin-Resistant Staphylococcus Aureus: A Systematic Review and Meta-Analysis. Microb. Drug Resist. 2019, 25, 1330–1337. [Google Scholar] [CrossRef]
- Rodrigues, F.F.; Lino, C.I.; Oliveira, V.L.S.; Zaidan, I.; Melo, I.S.F.; Braga, A.V.; Costa, S.O.A.M.; Morais, M.I.; Barbosa, B.C.M.; da Costa, Y.F.G.; et al. A Clindamycin Acetylated Derivative with Reduced Antibacterial Activity Inhibits Articular Hyperalgesia and Edema by Attenuating Neutrophil Recruitment, NF-ΚB Activation and Tumor Necrosis Factor-α Production. Int. Immunopharmacol. 2023, 122, 110609. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Goo, W.; Karima, G.; Lee, J.H.; Kim, H.D. Polyacrylamide/Gel-Based Self-Healing Artificial Tympanic Membrane for Drug Delivery of Otitis Treatment. Biomater. Res. 2024, 28, 0049. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Pandya, A.K.; Demartis, S.; Domínguez-Robles, J.; Moreno-Castellanos, N.; Li, H.; Gavini, E.; Patravale, V.B.; Donnelly, R.F. Liposome-Loaded Polymeric Microneedles for Enhanced Skin Deposition of Rifampicin. Int. J. Pharm. 2023, 646, 123446. [Google Scholar] [CrossRef]
- Bajorski, P.; Fuji, N.; Kaur, R.; Pichichero, M.E. Window of Susceptibility to Acute Otitis Media Infection. Pediatrics 2023, 151, e2022058556. [Google Scholar] [CrossRef]
- Yoshida, S.; Seki, S.; Sugiyama, T.; Kikuchi, S.; Iino, Y. Clinical Characteristics of Atelectatic Eardrums and Adhesive Otitis Media in Children. Int. J. Pediatr. Otorhinolaryngol. 2022, 159, 111188. [Google Scholar] [CrossRef]
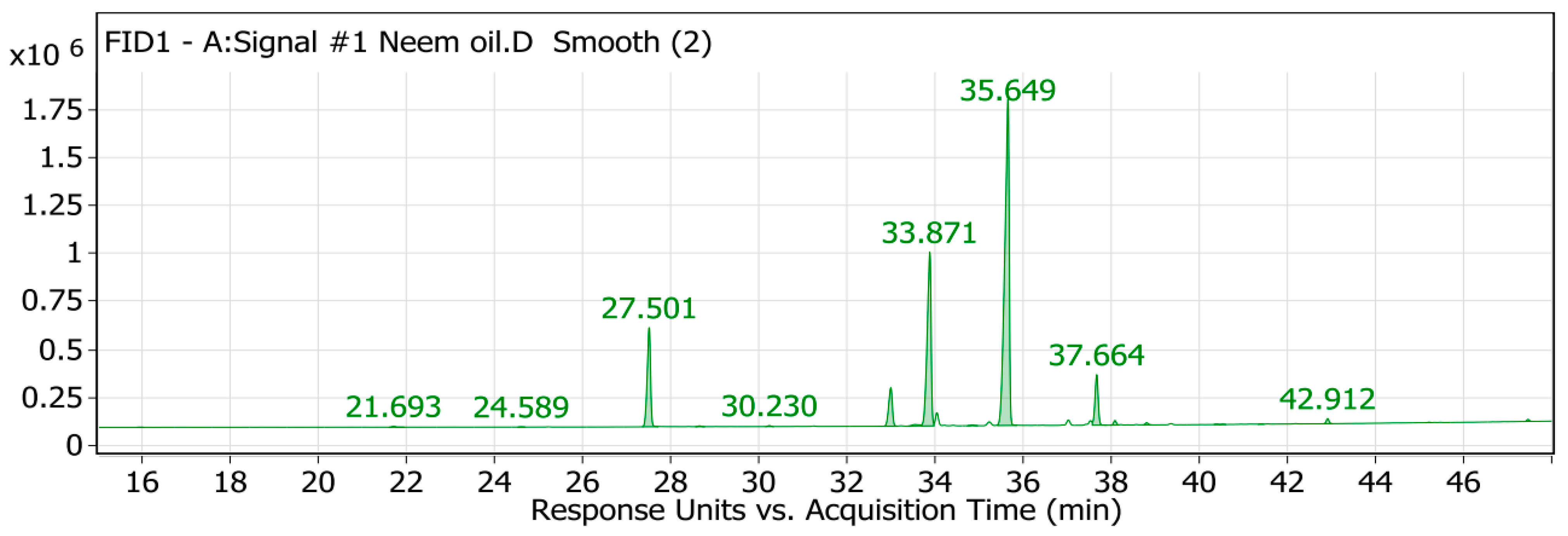


| Peak No. | RT | Identified Compound | Area | Area Sum % | Molecular Formula | Molecular Weight | PubChem CID |
|---|---|---|---|---|---|---|---|
| 4 | 27.5 | Palmitic acid | 2,777,429.22 | 11.85 | C16H32O2 | 256.43 | 985 |
| 7 | 32.99 | Stearic acid | 1,165,425.88 | 4.97 | C18H36O2 | 284.48 | 5281 |
| 9 | 33.87 | Oleic acid | 5,633,160.84 | 24.04 | C18H34O2 | 282.46 | 445639 |
| 11 | 35.65 | Linoleic acid | 11,990,543.3 | 51.18 | C18H32O2 | 280.43 | 5280450 |
| 12 | 37.66 | Linolenic acid | 1,342,567.44 | 5.73 | C18H30O2 | 278.41 | 5280934 |
| 13 | 38.08 | Arachidic acid | 106,312 | 0.45 | C20H40O2 | 312.54 | 10491 |
| 18 | 42.91 | Behenic acid | 114,403.13 | 0.49 | C22H44O2 | 340.6 | 10492 |
| Formula | Particle Size (nm) | Polydispersity Index (nm) | Zeta Potential (mV) | Entrapment Efficiency (EE%) |
|---|---|---|---|---|
| Double emulsion | 168 ± 0.32 | 0.21 ± 0.42 | 37 ± 0.43 | 89.86 ± 0.23 |
| Model | R2 (pH = 5.4) | R2 (pH = 7.4) |
|---|---|---|
| Zero Order | 0.94 | 0.93 |
| First Order | 0.96 | 0.95 |
| Higuchi | 0.98 | 0.99 |
| Korsmeyer–Peppas | 0.97 | 0.98 |
| Targets | Tested Compounds | RMSD Value (Å) | Docking (Affinity) Score (kcal/mol) | Interactions | |
|---|---|---|---|---|---|
| H.B | Pi Interaction | ||||
| PPARα | The co-crystalized ligand | 1.01 | −8.91 | 4 | 13 |
| Palmitic acid | 1.15 | −8.87 | 3 | 8 | |
| Linoleic acid | 1.31 | −9.02 | 3 | 13 | |
| Toll-like receptor 4 | The co-crystalized ligand | 1.23 | −7.02 | 2 | 4 |
| Palmitic acid | 1.32 | −8.75 | 2 | 11 | |
| Linoleic acid | 1.07 | −8.92 | 3 | 8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zuhairy, S.A.K.S.; Elhabal, S.F.; Mohamed Elrefai, M.F.; Hababeh, S.; Nelson, J.; Fady, M.; Elzohairy, N.A.; Ewedah, T.M.; Mousa, I.S.; Hamdan, A.M.E. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals 2025, 18, 381. https://doi.org/10.3390/ph18030381
Al-Zuhairy SAKS, Elhabal SF, Mohamed Elrefai MF, Hababeh S, Nelson J, Fady M, Elzohairy NA, Ewedah TM, Mousa IS, Hamdan AME. Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals. 2025; 18(3):381. https://doi.org/10.3390/ph18030381
Chicago/Turabian StyleAl-Zuhairy, Saeed Abdul Kareem Saeed, Sammar Fathy Elhabal, Mohamed Fathi Mohamed Elrefai, Sandra Hababeh, Jakline Nelson, Marwa Fady, Nahla A. Elzohairy, Tassneim M. Ewedah, Ibrahim S. Mousa, and Ahmed Mohsen Elsaid Hamdan. 2025. "Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments" Pharmaceuticals 18, no. 3: 381. https://doi.org/10.3390/ph18030381
APA StyleAl-Zuhairy, S. A. K. S., Elhabal, S. F., Mohamed Elrefai, M. F., Hababeh, S., Nelson, J., Fady, M., Elzohairy, N. A., Ewedah, T. M., Mousa, I. S., & Hamdan, A. M. E. (2025). Polylactic-Co-Glycolic Acid/Alginate/Neem Oil-Reduced Graphene Oxide as a pH-Sensitive Nanocarrier for Hesperidin Drug Delivery: Antimicrobial and Acute Otitis Media Assessments. Pharmaceuticals, 18(3), 381. https://doi.org/10.3390/ph18030381








