Rhoifolin Improves Glycometabolic Control in Streptozotocin-Induced Diabetic Rats by Up-Regulating the Expression of Insulin Signaling Proteins and Down-Regulating the MAPK/JNK Pathway
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antioxidant Activity of Rhoifolin
2.2. In Vitro α-Amylase and α-Glucosidase Inhibitory Effects of Rhoifolin
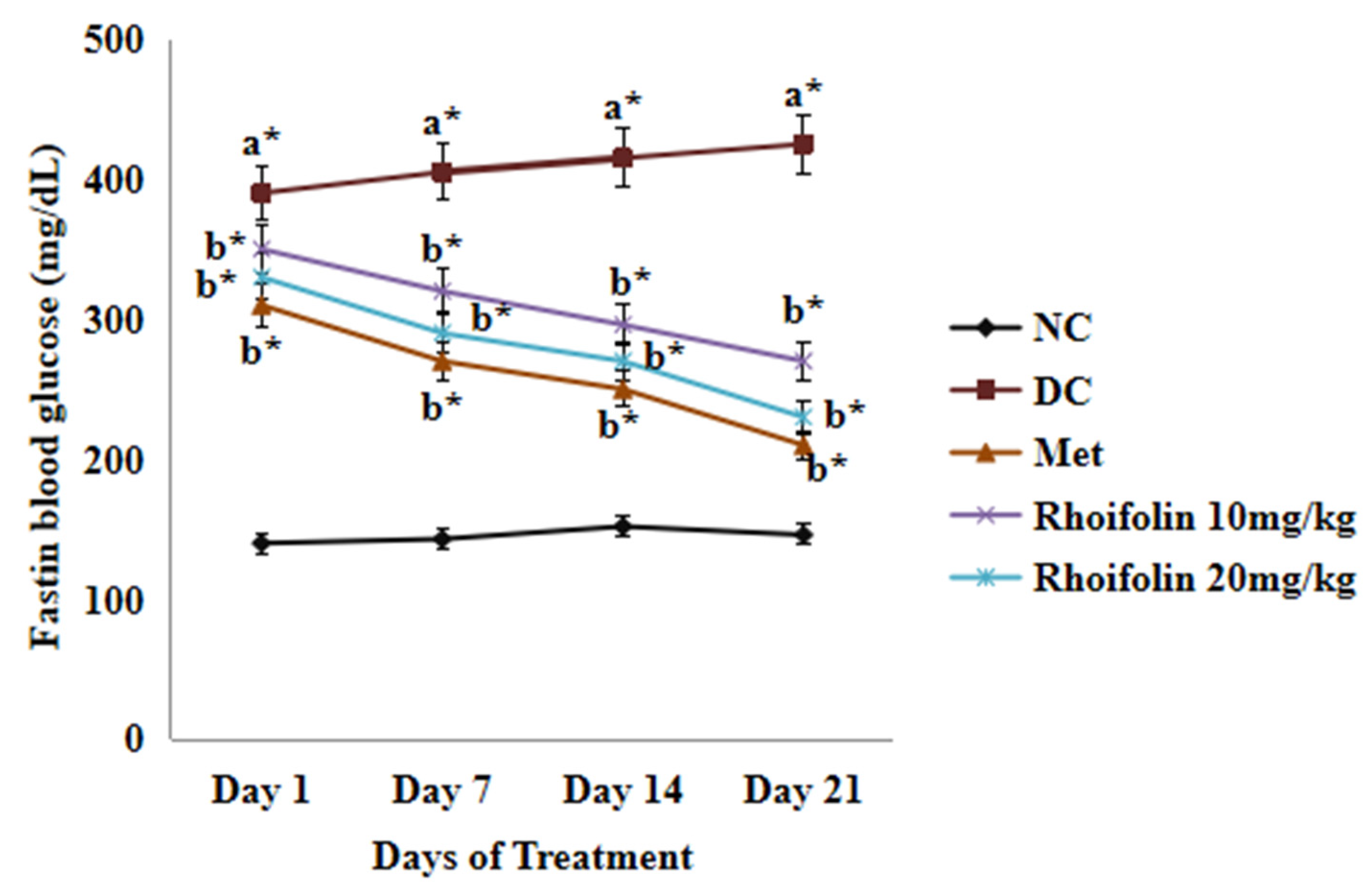
2.3. Effect of Rhoifolin on Fasting Blood Glucose
2.4. Effect of Rhoifolin on Serum Glycometabolic Markers
2.5. Effect of Rhoifolin on Serum Lipid Levels
2.6. Effect of Rhoifolin on Serum Pro-Inflammatory Markers
2.7. Effect of Rhoifolin on Hepatic Oxidant and Antioxidant Markers
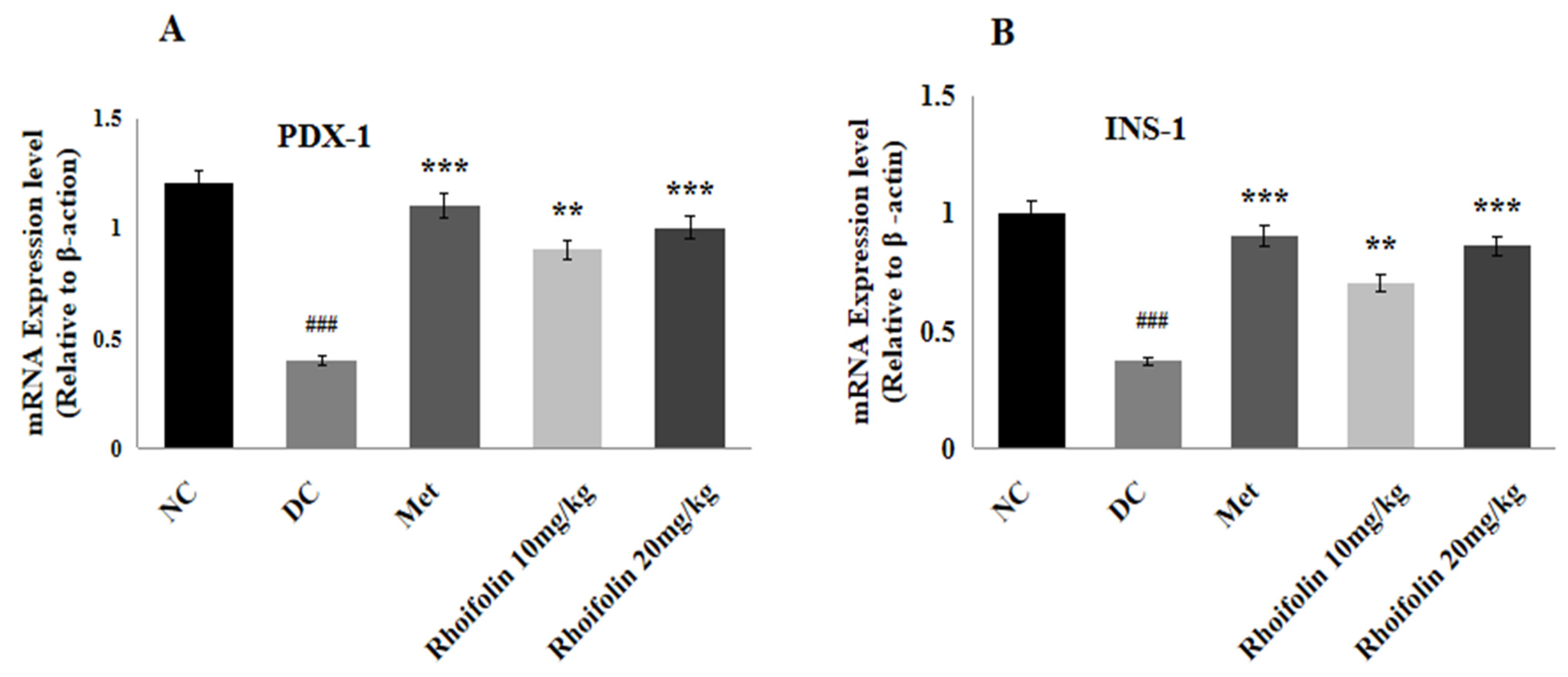
2.8. Effect of Rhoifolin on PDX-1 and INS-1 Gene Expression
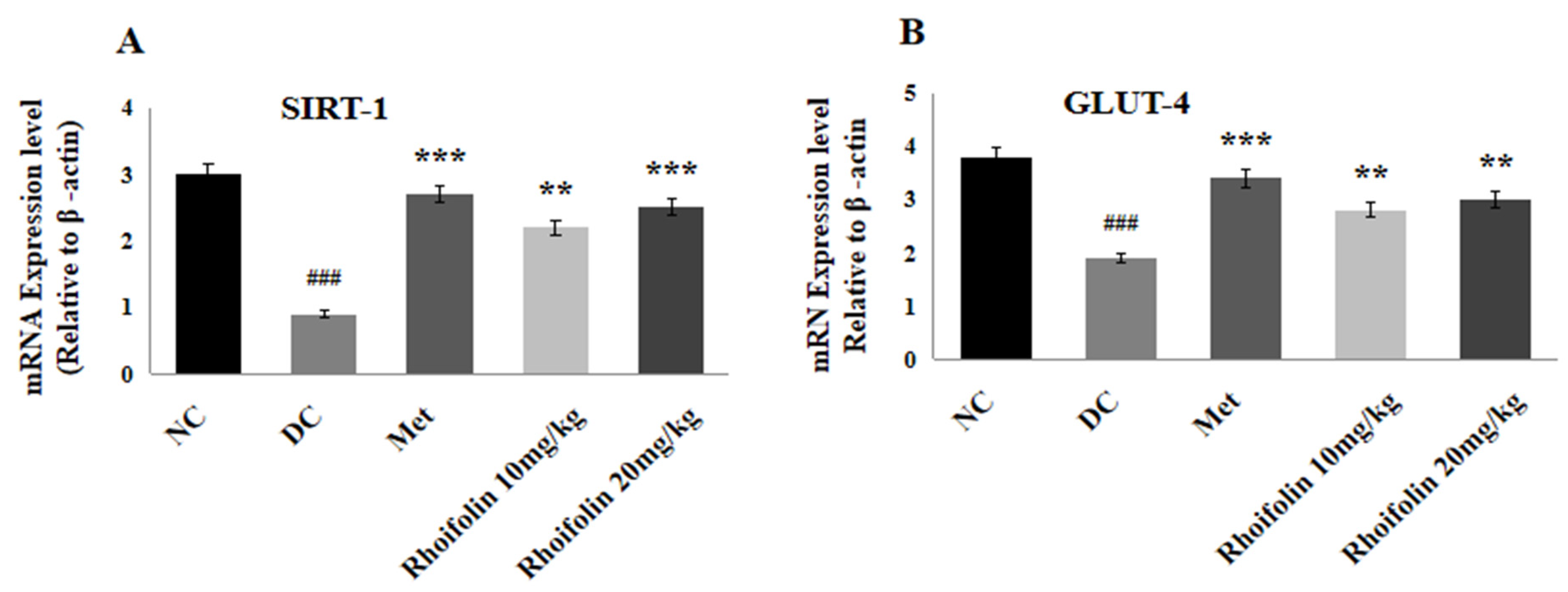
2.9. Effect of Rhoifolin on SIRT-1 and GLUT-4 Expressions in Pancreatic Tissues
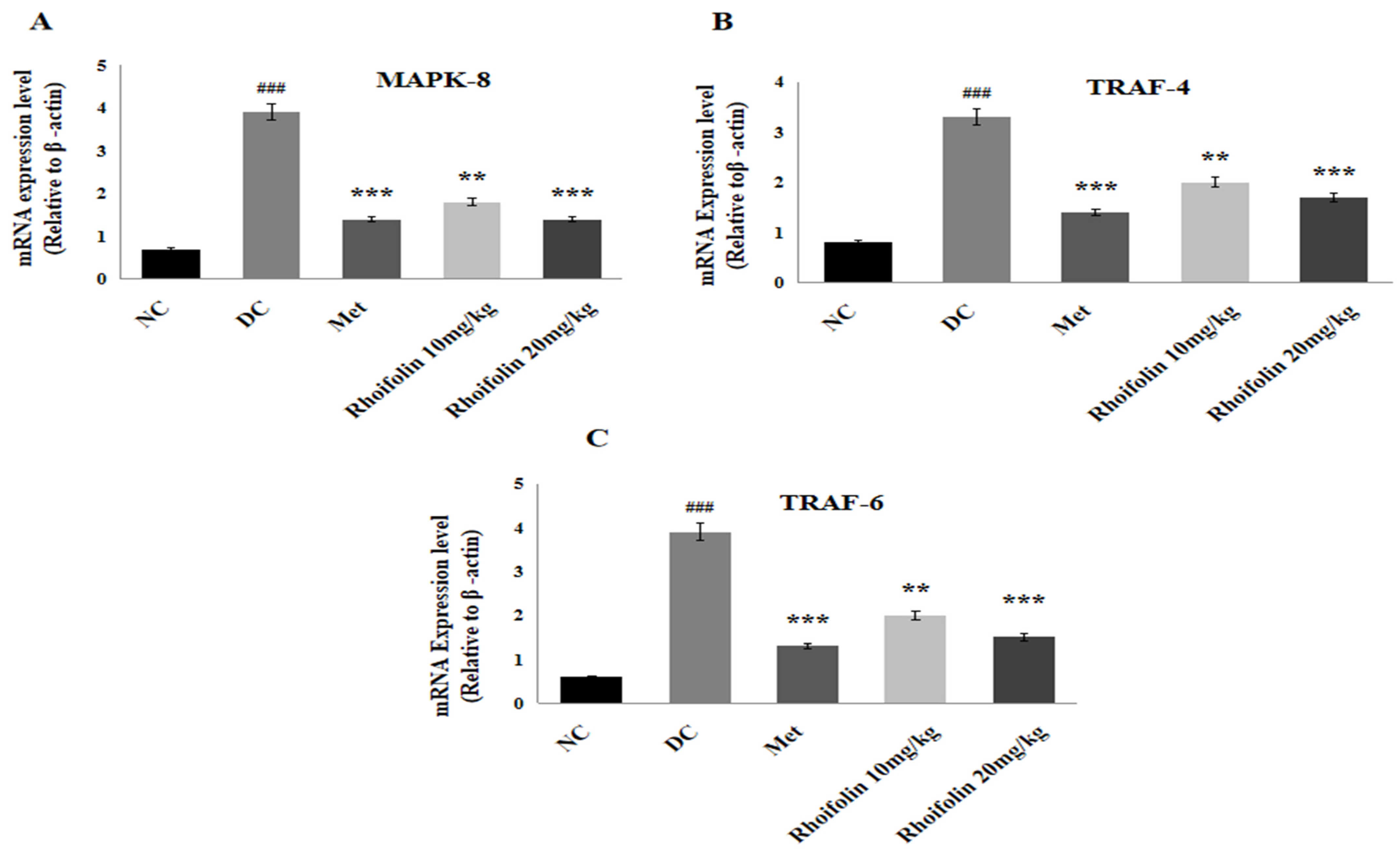
2.10. Effect of Rhoifolin on JNK Signaling from MAPK Family
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Rhoifolin
4.2. Induction of Disease and Treatment Protocol
4.3. Sample Collection
4.4. In Vitro Antioxidant Assays
4.4.1. DPPH Radical-Scavenging Assay
4.4.2. ABTS+ Radical-Scavenging Activity
4.5. In Vitro Alpha-Amylase Assay
4.6. In Vitro Alpha-Glucosidase Inhibition Activity
4.7. Estimation of Fasting Blood Glucose and Determination of Glycemic Parameters
4.8. Estimation of Lipidemic Indicators
4.9. Estimation of Serum Pro-Inflammatory Markers
4.10. Measurement of Hepatic Antioxidant and Oxidant Status
4.11. Real-Time Quantitative PCR for Selected Genes
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yau, J.W.; Rogers, S.L.; Kawasaki, R.; Lamoureux, E.L.; Kowalski, J.W.; Bek, T.; Chen, S.-J.; Dekker, J.M.; Fletcher, A.; Grauslund, J. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, X.; Liu, Z.; Xing, Q.; Liu, R.; Wu, Q.; Hu, Y.; Zhang, J. The signaling pathways of selected traditional Chinese medicine prescriptions and their metabolites in the treatment of diabetic cardiomyopathy: A review. Front. Pharmacol. 2024, 15, 1416403. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- Wei, Y.; Xu, S.; Wu, Z.; Zhang, M.; Bao, M.; He, B. Exploring the causal relationships between type 2 diabetes and neurological disorders using a Mendelian randomization strategy. Medicine 2024, 103, e40412. [Google Scholar] [CrossRef] [PubMed]
- Tarfeen, N.; Nisa, K.U.; Ahmad, M.B.; Waza, A.A.; Ganai, B.A. Metabolic and genetic association of vitamin D with calcium signaling and insulin resistance. Indian J. Clin. Biochem. 2023, 38, 407–417. [Google Scholar] [CrossRef]
- Ma, J.; Nakagawa, Y.; Kojima, I.; Shibata, H. Prolonged insulin stimulation down-regulates GLUT4 through oxidative stress-mediated retromer inhibition by a protein kinase CK2-dependent mechanism in 3T3-L1 adipocytes. J. Biol. Chem. 2014, 289, 133–142. [Google Scholar] [CrossRef]
- Yao, X.; Li, K.; Liang, C.; Zhou, Z.; Wang, J.; Wang, S.; Liu, L.; Yu, C.-L.; Song, Z.-B.; Bao, Y.-L. Tectorigenin enhances PDX1 expression and protects pancreatic β-cells by activating ERK and reducing ER stress. J. Biol. Chem. 2020, 295, 12975–12992. [Google Scholar] [CrossRef]
- Yi, X.; Dong, M.; Guo, N.; Tian, J.; Lei, P.; Wang, S.; Yang, Y.; Shi, Y. Flavonoids improve type 2 diabetes mellitus and its complications: A review. Front. Nutr. 2023, 10, 1192131. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Naqvi, S.A.R.; Hassan, S.U.; Haq, A.U.; Janjua, M.R.S.A.; Mahmoud, M.H.; Batiha, G.E.S.; Rashid, H.; Rahim, M.A.; Rocha, J.M. In vitro and in vivo exploitation of cell stress pathways using methanolic extracts of Phlomis stewartii in diabetic rat’s model. Ind. Crops Prod. 2024, 217, 118861. [Google Scholar] [CrossRef]
- Rao, P.S.; Mohan, G.K. In vitro alpha-amylase inhibition and in vivo antioxidant potential of Momordica dioica seeds in streptozotocin-induced oxidative stress in diabetic rats. Saudi J. Biol. Sci. 2017, 24, 1262–1267. [Google Scholar] [CrossRef]
- Mohamed, J.; Nafizah, A.N.; Zariyantey, A.; Budin, S. Mechanisms of diabetes-induced liver damage: The role of oxidative stress and inflammation. Sultan Qaboos Univ. Med. J. 2016, 16, e132. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Prasad, S.K.; Kumar, R.; Hemalatha, S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac. J. Trop. Biomed. 2012, 2, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Refaat, J.; Desoukey, S.Y.; Ramadan, M.A.; Kamel, M.S. Rhoifolin: A review of sources and biological activities. Int. J. Pharmacogn. 2015, 2, 102–109. [Google Scholar]
- Junejo, J.A.; Zaman, K.; Ali, M.; Rudrapal, M. New flavonoid with antidiabetic and antioxidant potential from Tetrastigma angustifolia (Roxb.) Deb leaves. Braz. J. Pharm. Sci. 2020, 56, e18806. [Google Scholar] [CrossRef]
- Gheda, S.; Naby, M.A.; Mohamed, T.; Pereira, L.; Khamis, A. Antidiabetic and antioxidant activity of phlorotannins extracted from the brown seaweed Cystoseira compressa in streptozotocin-induced diabetic rats. Environ. Sci. Pollut. Res. 2021, 28, 22886–22901. [Google Scholar] [CrossRef]
- Froldi, G. View on Metformin: Antidiabetic and Pleiotropic Effects, Pharmacokinetics, Side Effects, and Sex-Related Differences. Pharmaceuticals 2024, 17, 478. [Google Scholar] [CrossRef]
- Horakova, O.; Kroupova, P.; Bardova, K.; Buresova, J.; Janovska, P.; Kopecky, J.; Rossmeisl, M. Metformin acutely lowers blood glucose levels by inhibition of intestinal glucose transport. Sci. Rep. 2019, 9, 6156. [Google Scholar] [CrossRef]
- Eid, H.M.; Martineau, L.C.; Saleem, A.; Muhammad, A.; Vallerand, D.; Benhaddou-Andaloussi, A.; Haddad, P.S. Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol. Nutr. Food Res. 2010, 54, 991–1003. [Google Scholar] [CrossRef]
- Martiniakova, M.; Sarocka, A.; Penzes, N.; Biro, R.; Kovacova, V.; Mondockova, V.; Sevcikova, A.; Ciernikova, S.; Omelka, R. Protective Role of Dietary Polyphenols in the Management and Treatment of Type 2 Diabetes Mellitus. Nutrients 2025, 17, 275. [Google Scholar] [CrossRef]
- Nagayach, A.; Bhaskar, R.; Ghosh, S.; Singh, K.K.; Han, S.S.; Sinha, J.K. Advancing the understanding of diabetic encephalopathy through unravelling pathogenesis and exploring future treatment perspectives. Ageing Res. Rev. 2024, 100, 102450. [Google Scholar] [CrossRef]
- Haile, H.K.; Fenta, T.G. Magnitude, risk factors and economic impacts of diabetic emergencies in developing countries: A systematic review. PLoS ONE 2025, 20, e0317653. [Google Scholar] [CrossRef]
- Hossain, M.J.; Al-Mamun, M.; Islam, M.R. Diabetes mellitus, the fastest growing global public health concern: Early detection should be focused. Health Sci. Rep. 2024, 7, e2004. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive Compounds in Anti-Diabetic Plants: From Herbal Medicine to Modern Drug Discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Huang, J.; Li, H.; Zhao, D.; Liu, Z.; Zhu, L.; Zhang, Z.; Peng, W. Quercetin: A promising therapy for diabetic encephalopathy through inhibition of hippocampal ferroptosis. Phytomedicine 2024, 126, 154887. [Google Scholar] [CrossRef]
- Alharbi, H.O.A.; Alshebremi, M.; Babiker, A.Y.; Rahmani, A.H. The Role of Quercetin, a Flavonoid in the Management of Pathogenesis Through Regulation of Oxidative Stress, Inflammation, and Biological Activities. Biomolecules 2025, 15, 151. [Google Scholar] [CrossRef]
- Alema, N.M.; Periasamy, G.; Sibhat, G.G.; Tekulu, G.H.; Hiben, M.G. Antidiabetic activity of extracts of Terminalia brownii Fresen. Stem bark in mice. J. Exp. Pharmacol. 2020, 12, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Li, M. The Emerging Role of Flavonoids in the Treatment of Type 2 Diabetes Mellitus: Regulating the Enteroendocrine System. Explor. Res. Hypothesis Med. 2025, 10, 56–68. [Google Scholar] [CrossRef]
- Aamir, K.; Khan, H.U.; Hossain, C.F.; Afrin, M.R.; Jusuf, P.R.; Waheed, I.; Sethi, G.; Arya, A. Arjunolic acid downregulates elevated blood sugar and pro-inflammatory cytokines in streptozotocin (STZ)-nicotinamide induced type 2 diabetic rats. Life Sci. 2022, 289, 120232. [Google Scholar] [CrossRef]
- Rasheed, M.U.; Naqvi, S.A.R.; Al-Asmari, F.; Rahim, M.A.; Ramadan, M.F. Phytochemicals, Health-Promoting Effects, and Enzyme Inhibition Traits of Phlomis stewartii Extracts. Molecules 2024, 29, 1049. [Google Scholar] [CrossRef]
- Khin, P.P.; Lee, J.H.; Jun, H.-S. Pancreatic beta-cell dysfunction in type 2 diabetes. Eur. J. Inflamm. 2023, 21, 1721727X231154152. [Google Scholar] [CrossRef]
- Elamin, N.M.H.; Fadlalla, I.M.T.; Omer, S.A.; Ibrahim, H.A.M. Histopathological alteration in STZ-nicotinamide diabetic rats, a complication of diabetes or a toxicity of STZ. Int. J. Diabetes Clin. Res. 2018, 5, 1–8. [Google Scholar]
- Wang, H.-Y.; Zhao, J.-G.; Wei, Z.-G.; Zhang, Y.-Q. The renal protection of flavonoid-rich ethanolic extract from silkworm green cocoon involves in inhibiting TNF-α-p38 MAP kinase signalling pathway in type 2 diabetic mice. Biomed. Pharmacother. 2019, 118, 109379. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Tang, Z.; Song, Z.; Liu, C.; Wang, C. Total flavonoids of Hippophae rhamnoides L. improves type 2 diabetes symptoms in rats through down-regulating of the DAG/PRKCA/MAPK10/p65/TNF-α signalling pathway. J. Ethnopharmacol. 2024, 318, 116962. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ren, Y.; Lin, D.; Peng, S.; Zhong, B.; Ma, Z. The anti-inflammatory properties of Citrus wilsonii tanaka extract in LPS-induced RAW 264.7 and primary mouse bone marrow-derived dendritic cells. Molecules 2017, 22, 1213. [Google Scholar] [CrossRef]
- Phan, V.K.; Nguyen, T.M.; Minh, C.V.; Nguyen, H.K.; Nguyen, H.D.; Nguyen, P.T.; Nguyen, X.C.; Nguyen, H.N.; Nguyen, X.N.; Heyden, Y.V.; et al. Two new C-glucosyl benzoic acids and flavonoids from Mallotus nanus and their antioxidant activity. Arch. Pharm. Res. 2010, 33, 203–208. [Google Scholar] [PubMed]
- Rao, Y.K.; Lee, M.J.; Chen, K.; Lee, Y.C.; Wu, W.S.; Tzeng, Y.M. Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) Osbeck leaves: Enhanced adiponectin secretion and insulin receptor phosphorylation in 3T3-L1 cells. Evid. Based Complement. Alternat. Med. 2011, 2011, 624375. [Google Scholar] [CrossRef]
- Hao, H.H.; Shao, Z.M.; Tang, D.Q.; Lu, Q.; Chen, X.; Yin, X.X.; Chen, H. Preventive effects of rutin on the development of experimental diabetic nephropathy in rats. Life Sci. 2012, 91, 959–967. [Google Scholar] [CrossRef]
- Eitah, H.E.; Maklad, Y.A.; Abdelkader, N.F.; Gamal El Din, A.A.; Badawi, M.A.; Kenawy, S.A. Modulating impacts of quercetin/sitagliptin combination on streptozotocin-induced diabetes mellitus in rats. Toxicol. Appl. Pharmacol. 2019, 365, 30–40. [Google Scholar] [CrossRef]
- Vanitha, P.; Uma, C.; Suganya, N.; Bhakkiyalakshmi, E.; Suriyanarayanan, S.; Gunasekaran, P.; Ramkumar, K.M. Modulatory effects of morin on hyperglycemia by attenuating the hepatic key enzymes of carbohydrate metabolism and beta-cell function in streptozotocin-induced diabetic rats. Environ. Toxicol. Pharmacol. 2014, 37, 326–335. [Google Scholar] [CrossRef]
- Sharif, H.; Mukhtar, M.; Mustapha, Y.; Baba, G.; Lawal, A. Acute and subchronic toxicity profile of Euphorbia pulcherrima methanol extract on Wistar albino rats. Adv. Pharmaceut. 2015, 2015, 539646. [Google Scholar] [CrossRef]
- Mai, B.; Han, L.; Zhong, J.; Shu, J.; Cao, Z.; Fang, J.; Zhang, X.; Gao, Z.; Xiao, F. Rhoifolin Alleviates Alcoholic Liver Disease In Vivo and In Vitro via Inhibition of the TLR4/NF-κB Signaling Pathway. Front. Pharmacol. 2022, 13, 878898. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Hu, C.; Liu, X.; Lei, L.; He, G.; Xiong, C.; Wu, W. Rhoifolin regulates oxidative stress and proinflammatory cytokine levels in Freund’s adjuvant-induced rheumatoid arthritis via inhibition of NF-κB. Braz. J. Med. Biol. Res. 2020, 53, e9489. [Google Scholar] [CrossRef]
- Kahksha; Alam, O.; Al-Keridis, L.A.; Khan, J.; Naaz, S.; Alam, A.; Ashraf, S.A.; Alshammari, N.; Adnan, M.; Beg, M.A. Evaluation of Antidiabetic Effect of Luteolin in STZ Induced Diabetic Rats: Molecular Docking, Molecular Dynamics, In Vitro and In Vivo Studies. J. Funct. Biomater. 2023, 14, 126. [Google Scholar] [CrossRef]
- Oyedemi, S.O.; Nwaogu, G.; Chukwuma, C.I.; Adeyemi, O.T.; Matsabisa, M.G.; Swain, S.S.; Aiyegoro, O.A. Quercetin modulates hyperglycemia by improving the pancreatic antioxidant status and enzymes activities linked with glucose metabolism in type 2 diabetes model of rats: In silico studies of molecular interaction of quercetin with hexokinase and catalase. J. Food Biochem. 2020, 44, e13127. [Google Scholar] [CrossRef]
- Yung, J.H.M.; Giacca, A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells 2020, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Jubaidi, F.F.; Zainalabidin, S.; Taib, I.S.; Abdul Hamid, Z.; Mohamad Anuar, N.N.; Jalil, J.; Mohd Nor, N.A.; Budin, S.B. The Role of PKC-MAPK Signalling Pathways in the Development of Hyperglycemia-Induced Cardiovascular Complications. Int. J. Mol. Sci. 2022, 23, 8582. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Thotakura, B.; Chandra Sekaran, S.P.; Jyothi, A.K.; Sundaramurthi, I. Naringin (4′,5,7-Trihydroxyflavanone 7-Rhamnoglucoside) Attenuates β-Cell Dysfunction in Diabetic Rats through Upregulation of PDX-1. Cells Tissues Organs 2018, 206, 133–143. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, L.; Qu, R.; Liu, T.; Wang, J.; Tong, Y.; Bei, W.; Guo, J.; Hu, X. Hesperidin activates the GLP-1R/cAMP-CREB/IRS2/PDX1 pathway to promote transdifferentiation of islet α cells into β cells Across the spectrum. Heliyon 2024, 10, e35424. [Google Scholar] [CrossRef]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.-T.; Li, H.-B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.-Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef]
- Ahangarpour, A.; Zamaneh, H.T.; Jabari, A.; Nia, H.M.; Heidari, H. Antidiabetic and hypolipidemic effects of Dorema aucheri hydroalcoholic leave extract in streptozotocin-nicotinamide induced type 2 diabetes in male rats. Iran. J. Basic Med. Sci. 2014, 17, 808. [Google Scholar]
- Umar, U.; Ahmed, S.; Iftikhar, A.; Iftikhar, M.; Majeed, W.; Liaqat, A.; Shahzad, S.; Abbas, M.; Mehmood, T.; Anwar, F. Phenolics Extracted from Jasminum sambac Mitigates Diabetic Cardiomyopathy by Modulating Oxidative Stress, Apoptotic Mediators and the Nfr-2/HO-1 Pathway in Alloxan-Induced Diabetic Rats. Molecules 2023, 28, 5453. [Google Scholar] [CrossRef] [PubMed]
- Feregrino-Pérez, A.A.; Mercado-Luna, A.; Murillo-Cárdenas, C.A.; González-Santos, R.; Chávez-Servín, J.L.; Vargas-Madriz, A.F.; Luna-Sánchez, E. Polyphenolic Compounds and Antioxidant Capacity in Native Maize of the Sierra Gorda of Querétaro. Agronomy 2024, 14, 142. [Google Scholar] [CrossRef]
- Kifle, Z.D.; Debeb, S.G.; Belayneh, Y.M. In Vitro α-Amylase and α-Glucosidase Inhibitory and Antioxidant Activities of the Crude Extract and Solvent Fractions of Hagenia abyssinica Leaves. BioMed Res. Int. 2021, 2021, 6652777. [Google Scholar] [CrossRef]
- Awan, A.M.; Majeed, W.; Muhammad, F.; Faisal, M.N. Acacia Jacquemontii Ethyl Acetate Extract Downregulated the Hyperglycemia Through Its Modulatory Effects on Endogenous Antioxidant, Anti-Inflammatory and Pancreatic β-Cell Regenerative Status in Alloxan Induced Diabetic Rats. Environ. Sci. Pollut. Res. Int. 2022, 29, 52605–52617. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, A.; Aslam, B.; Muhammad, F.; Khaliq, T. Polyherbal formulation Ameliorates diabetes mellitus in alloxan-induced diabetic rats: Involvement of pancreatic genes expression. Pak. Vet. J. 2018, 38, 261–265. [Google Scholar] [CrossRef]



| α-Amylase Inhibition | α-Glucosidase Inhibition | ||||
|---|---|---|---|---|---|
| Conc. (mg/mL) | % Inhibition by Rhoifolin | % Inhibition by Acarbose | Conc. (mg/mL) | % Inhibition by Rhoifolin | % Inhibition by Acarbose |
| 0.2 | 34.5 ± 1.96 d | 42.5 ± 0.16 d’ | 0.2 | 33.2 ± 0.48 d | 40.3 ± 0.73 d’ |
| 0.4 | 43.1 ± 1.27 c | 53.7 ± 2.15 c’ | 0.4 | 46.7 ± 1.63 c | 51.6 ± 1.74 c’ |
| 0.6 | 50.9 ± 2.87 b | 61.3 ± 2.74 bc’ | 0.6 | 53.6 ± 1.97 b | 62.1 ± 2.32 b’ |
| 0.8 | 60.3 ± 3.08 ab | 70.6 ± 3.83 ab’ | 0.8 | 60.1 ± 2.03 ab* | 73.8 ± 3.12 ab’ |
| 1.00 | 65.7 ± 3.26 a* | 79.1 ± 4.21 a’ | 1.00 | 68.4 ± 2.46 a* | 81.5 ± 3.57 a’ |
| IC50 (mg/mL) | 0.58 | 0.35 | IC50 (mg/mL) | 0.54 | 0.37 |
| Groups | Total Cholesterol (mg/dL) | Triglycerides (mg/dL) | High-Density Lipoproteins (mg/dL) | Low-Density Lipoproteins (mg/dL) | Very-Low-Density Lipoproteins (mg/dL) |
|---|---|---|---|---|---|
| Normal control | 76.1 ± 2.16 | 72.6 ± 3.07 | 29.9 ± 1.27 | 32.5 ± 2.02 | 16.5 ± 1.47 |
| Diabetic control | 268.6 ± 6.58 a### | 287.2 ± 5.75 a### | 18.7 ± 1.23 a### | 154.7 ± 3.87 a### | 42.5 ± 2.84 a### |
| Metformin | 104.4 ± 4.67 b*** | 112.2 ± 4.39 b*** | 27.4 ± 2.32 b*** | 54.3 ± 2.68 b*** | 21.2 ± 1.54 b*** |
| Rhoifolin (10 mg/kg) | 149.2 ± 5.03 b*** | 160.6 ± 4.92 b** | 20.9 ± 1.24 b** | 104.6 ± 3.68 b** | 29.3 ± 2.42 b* |
| Rhoifolin (20 mg/kg) | 110.1 ± 4.47 b*** | 128.4 ± 5.31 b*** | 23.9 ± 1.52 b*** | 87.03 ± 2.94 b*** | 24.8 ± 1.94 b*** |
| Groups | TBARS (nmol MDA/mg) | Superoxide Dismutase (U/mg) | Catalase (μmol H2O2/min/mg) | GPx (nmol/min/mg) |
|---|---|---|---|---|
| Normal control | 2.43 ± 0.16 | 6.5 ± 0.40 | 54.1 ± 0.62 | 25.02 ± 0.57 |
| Diabetic control | 5.53 ± 0.36 a### | 3.4 ± 0.36 a### | 28.5 ± 0.40 a### | 13.03 ± 0.24 a### |
| Metformin | 3.20 ± 0.21 b*** | 5.5 ± 0.40 b** | 44.01 ± 2.94 b*** | 20.2 ± 0.82 b*** |
| Rhoifolin (10 mg/kg) | 4.76 ± 0.20 ns | 3.9 ± 0.09 ns | 32.1 ± 2.16 ns | 14.96 ± 0.44 b* |
| Rhoifolin (20 mg/kg) | 4.13 ± 0.26 b** | 4.8 s ± 0.41 b** | 39.3 ± 2.05 b** | 18.3 ± 0.66 b** |
| Gene ID | Sequence (5’ to 3’) | Direction |
|---|---|---|
| INS-1 | ACCCACTGTACCTGGTGTGTG CGGGTCCTCCACTTCACACAC | Forward Reverse |
| PDX-1 | TCGTGAATGGAACCGAGACT TTCATCCACGGGAAAGGGAG | Forward Reverse |
| SIRT-1 | GCAGTAACAGTGACAGTGGC AACTGCCTCTTGATCCCCTC | Forward Reverse |
| GLUT-4 | TTGCCCTTCTGTCCTGAGAG CGCTCTCTTTCCAACTTCCG | Forward Reverse |
| MAPK-8 | GACGACATCGGTCTCTTCG CTGCTGTCAGTATCCGATGC | Forward Reverse |
| TRAF-6 | GCGCCTAGTAAGACAGGACC CACATGCATGCTCTGCGTTT | Forward Reverse |
| TRAF-4 | TCCACACAAGTTCCTGGTGA AGGTGTGGCAGAAGCCGTG | Forward Reverse |
| β-actin | CGAGTACAACCTTCTTGCAGC TATCGTCATCCATGGCGAACTG | Forward Reverse |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehsan, M.; Ahmed, S.; Majeed, W.; Iftikhar, A.; Iftikhar, M.; Abbas, M.; Mehmood, T. Rhoifolin Improves Glycometabolic Control in Streptozotocin-Induced Diabetic Rats by Up-Regulating the Expression of Insulin Signaling Proteins and Down-Regulating the MAPK/JNK Pathway. Pharmaceuticals 2025, 18, 361. https://doi.org/10.3390/ph18030361
Ehsan M, Ahmed S, Majeed W, Iftikhar A, Iftikhar M, Abbas M, Mehmood T. Rhoifolin Improves Glycometabolic Control in Streptozotocin-Induced Diabetic Rats by Up-Regulating the Expression of Insulin Signaling Proteins and Down-Regulating the MAPK/JNK Pathway. Pharmaceuticals. 2025; 18(3):361. https://doi.org/10.3390/ph18030361
Chicago/Turabian StyleEhsan, Maryam, Sibtain Ahmed, Wafa Majeed, Asra Iftikhar, Maryam Iftikhar, Mateen Abbas, and Tahir Mehmood. 2025. "Rhoifolin Improves Glycometabolic Control in Streptozotocin-Induced Diabetic Rats by Up-Regulating the Expression of Insulin Signaling Proteins and Down-Regulating the MAPK/JNK Pathway" Pharmaceuticals 18, no. 3: 361. https://doi.org/10.3390/ph18030361
APA StyleEhsan, M., Ahmed, S., Majeed, W., Iftikhar, A., Iftikhar, M., Abbas, M., & Mehmood, T. (2025). Rhoifolin Improves Glycometabolic Control in Streptozotocin-Induced Diabetic Rats by Up-Regulating the Expression of Insulin Signaling Proteins and Down-Regulating the MAPK/JNK Pathway. Pharmaceuticals, 18(3), 361. https://doi.org/10.3390/ph18030361







